Abstract
Patient: Male, 31
Final Diagnosis: Fibrolamellar hepatocellular carcinoma
Symptoms: Encephalopathy
Medication:—
Clinical Procedure: —
Specialty: Gastroenterology and Hepatology
Objective:
Rare disease
Background:
Hyperammonemic encephalopathy is a potentially fatal condition that may progress to irreversible neuronal damage and is usually associated with liver failure or portosystemic shunting. However, other less common conditions can lead to hyperammonemia in adults, such as fibrolamellar hepatocellular carcinoma. Clinical awareness of hyperammonemic encephalopathy in patients with normal liver function is paramount to timely diagnosis, but understanding the underlying physiopathology is decisive to initiate adequate treatment for complete recovery.
Case Report:
A 31-year-old male with fibrolamellar carcinoma and peritoneal carcinomatosis presented with rapid onset hyperammonemic encephalopathy. Despite usual treatment for hepatic encephalopathy, his hyperammonemia was aggravated. A physiopathological pathway to encephalopathy resulting from hepatocellular dysfunction or portosystemic shunting was suspected and proper treatment was initiated, which resulted in complete remission of encephalopathy. Thus, we propose there is a physiopathology path to hyperammonemic encephalopathy in non-cirrhotic patients with fibrolamellar carcinoma independent of ornithine transcarbamylase (OTC) mutation. An ornithine metabolism imbalance resulting from overexpression of Aurora Kinase A as a result of a single, recurrent heterozygous deletion on chromosome 19, common to all fibrolamellar carcinomas, can lead to a c-Myc and ornithine decarboxylase overexpression that results in ornithine transcarboxylase dysfunction with urea cycle disorder and subsequent hyperammonemia.
Conclusions:
The identification of a physiopathological pathway allowed adequate medical treatment and full patient recovery from severe hyperammonemic encephalopathy.
MeSH Keywords: Ammonia; Brain Diseases, Metabolic; Liver Neoplasms; Ornithine Carbamoyltransferase Deficiency Disease
Background
Protein digestion results in the production of amino acids, which are metabolized by the liver by oxidative deamination or transamination into ammonia, which are then converted to urea and excreted by the kidneys. Any disturbance of this cycle may lead to hyperammonemia and as a result, hyperammonemic encephalopathy (HE).
There are three well stablished physiopathological pathways that lead to disruption of the nitrogen excretion cycle. An excessive nitrogen load, for instance in the case of gastrointestinal bleeding or urinary diversion, may oversaturate the hepatic metabolization capacity. Another pathway disruption is an inability of the urea cycle to metabolize a normal nitrogen load, as in hepatic failure or in specific enzymes deficiencies. Additionally, the nitrogen load coming from intestinal protein digestion may bypass the liver, which occurs with portosystemic shunting [1].
The most common cause of hyperammonemic encephalopathy is liver failure. Hepatocellular dysfunction and portosystemic shunting are the most important causes of inability of the urea cycle to properly excrete nitrogen metabolites resulting in hyperammonemia in patients with cirrhosis [2]. Nevertheless, there are many non-hepatic causes of HE related to inborn errors of metabolism, such as ornithine transcarbamylase (OTC) mutation.
We present the case of non-cirrhotic patient with fibrolamellar hepatocellular carcinoma (FHC) who developed hyperammonemia and encephalopathy despite having normal hepatocellular function. A physiopathological path involving an overexpression of Aurora Kinase A (AURKA) due to a heterozygous deletion on chromosome 19 (which is present in every FHC) is proposed.
Case Report
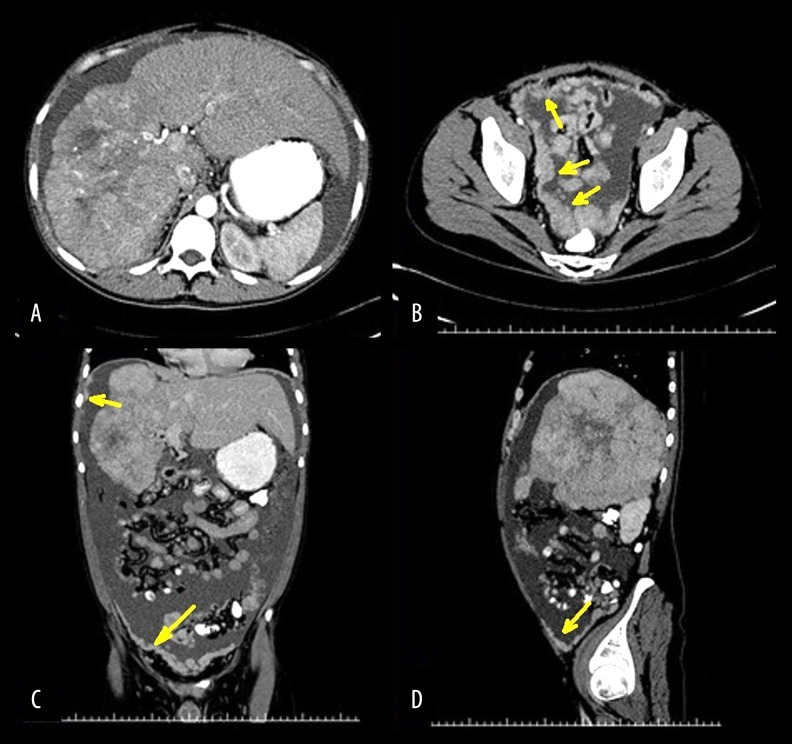
A 31-year-old male with no comorbidities and no risk factor for chronic hepatic disease presented with a history of three weeks of abdominal circumference enlargement. A computed tomography (CT) disclosed a large 18 cm hepatic mass occupying the right liver, as well as ascites and signs of carcinomatosis (Figure 1). Serum bilirubin level was 0.9 mg/dL (reference interval: 0.2–1.0 mg/dL), INR was 1.34 (reference interval: 0.8–1.2). He had normal hepatic enzymes at presentation. There were no stigmata of chronic hepatic disease at physical examination, other than ascites, that could be explained by peritoneal carcinomatosis.
Figure 1.
Computed tomography (yellow arrows: peritoneal seedings). (A) Axial plane, 18 cm hepatic mass on the right lobe with tumor thrombosis of the right portal and right hepatic veins and compensatory hypertrophy of the left hepatic lobe. (B) Axial plane, peritoneal carcinomatosis on the pelvis. (C) Coronal plane, hepatic tumor and peritoneal carcinomatosis. (D) Sagittal plane, hepatic tumor occupying the entire right lobe and peritoneal carcinomatosis.
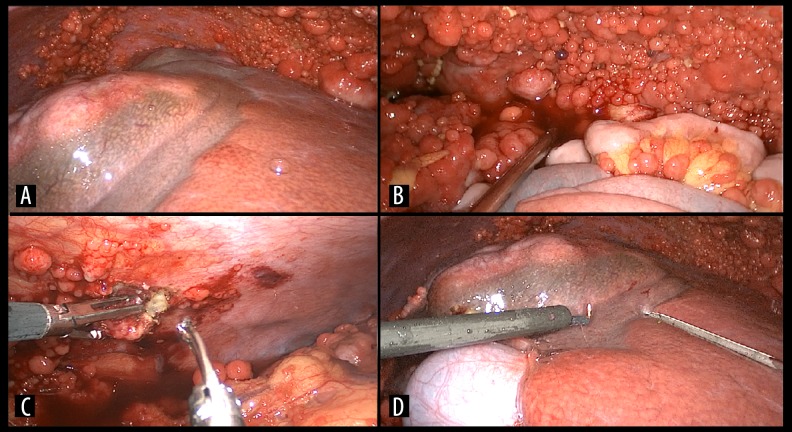
The patient underwent diagnostic laparoscopy and biopsy of the hepatic tumor, and peritoneal implants were obtained (Figure 2). A large hepatic mass was found on the right liver with signs of rupture. Ascites and carcinomatosis were also present. The left lobe was enlarged probably due to compensatory hypertrophy as a result of the right portal vein tumor thrombosis. The non-tumoral liver was normal, with no signs suggesting chronic hepatic disease
Figure 2.
Laparoscopy. (A) Hepatic tumor and diffuse peritoneal neoplastic implants, normal aspect of the non-tumoral liver and hypertrophy of the left hepatic lobe. (B) Peritoneal carcinomatosis on the pelvis. (C) Biopsy of a peritoneal seeding. (D) Biopsy of the hepatic tumor.
The patient’s postoperative period was uneventful and the patient was dismissed from the hospital two days after the procedure. Pathology identified hepatic fibrolamellar hepatocellular carcinoma (FHC) with metastatic peritoneal seeding. Immunohistochemistry examination determined a profile consistent with FHC (Table 1).
Table 1.
Immunohistochemistry profile of the hepatic tumor.
| Antibody | Clone | Interpretation |
|---|---|---|
| Ki 67 | 30–9 | Positive in 70% |
| Polyclonal CEA | Polyclonal positive membranous | Positive, membranous staining |
| CAM 5.2 | CAM 5.2 | Moderately positive, diffuse cytoplasmatic |
| Glypican 3 | GC33 | Positive, multifocal |
| CK19 | RCK108 | Negative |
| CK7 | SP52 | Positive, intense |
| Hepatocyte | OCH1E5 | Intense positive, granular staining |
One week after surgery, he was admitted to the hospital due to vague symptoms such as fatigue and mild abdominal pain. Moderate ascites had returned. He presented no signs of infection or dehydration. Laboratory tests disclosed normal liver function, with serum bilirubin 0.8 mg/dL (reference interval: 0.2–1.0 mg/dL), INR 1.16 (reference interval: 0.8–1.2), serum venous lactate 11 mg/dL (reference interval: 6.3–18.9 mg/dL). One day after admission, he presented with lethargy and confusion and was admitted to the ICU.
A head MRI disclosed no secondary malignancies, no signs of intracranial hypertension, and no other significant findings. Blood ammonia level was 204 mcmol/L (reference interval: 9–30 mcmol/L). Usual treatment for hepatic encephalopathy was initiated with neomycin, lactulose, and L-ornithine-L-aspartate. A continuous 18-hour electroencephalography disclosed lowered basic rhythm, frequent diffuse triphasic waves, and sporadic outbreaks of moderate amplitude theta waves, consistent with HE.
Despite initial therapy, blood ammonia level increased to 280 mcmol/L and the patient presented with neurological deterioration and coma. He required endotracheal intubation and mechanical ventilation. Hemodiafiltration and proper parenteral nutrition were initiated. Despite all procedures to treat HE and continuous hemodiafiltration, his serum ammonia level reached 312 mcmol/L.
At this time, as treatment was not achieving the therapeutic results expected, another physiopathological cause other than the usual excessive nitrogen load, hepatic dysfunction, and portosystemic shunting to HE was suspected. In fact, the reticulocyte production index was 0.5% (reference interval: 0.5–2.5%), lactic dehydrogenase was 280 IU/L (reference interval: 105–333 IU/L) and the hematocrit was stable, suggesting there was no hemolysis secondary to portosystemic shunting. Moreover, hepatic function laboratory tests were still between normal range and the compensatory hypertrophy of the left lobe as a result of tumor thrombosis of the right portal vein suggested healthy non-tumoral hepatic parenchyma.
This led to the investigation of less common causes of hyperammonemia, such as a urea cycle disorder. Since ornithine transcarbamylase (OTC) deficiency is the most common urea cycle enzymatic defect associated with late onset HE in adults without hepatic dysfunction, this diagnosis was suspected. As urinalysis disclosed very elevated orotic acid (10 mmol/mol of creatinine, reference interval: 0.4–1.2 mmol/mol of creatinine) and plasma amino acid chromatography revealed reduced citruline (3.0 mcmol/L, reference interval 16–51 mcmol/L), very low arginine (15.5 mcmol/L, reference interval 43–407 mcmol/L) and reduced ornithine (19.0 mcmol/L, reference interval 15.0–80.0 mcmol/L), confirming OTC deficiency, treatment with sodium benzoate (3 g) and arginine (3 g) administered every four hours via nasogastric tube was promptly initiated. A multi-gene panel genetic testing for inborn errors of metabolism was performed and no mutations were observed (Figure 3). OTC gene was tested and was not mutated.
Figure 3.

List of genes related to inborn errors of metabolism tested. OTC was tested and no mutation was found.
Within 18 hours, blood ammonia level had reduced to 107 mcmol/L. As the conscious level improved, the endotracheal tube was removed. The patient was dismissed from the intensive care unit one day after, with no clinical signs of HE. After treatment was initiated, there were no more clinical episodes of encephalopathy and the patient’s neurological examinations were normal. Before hospital discharge, the patient had chemotherapy treatment with a multikinase inhibitor (sorafenib) and GEMOX (gemcitabine-oxaliplatin). After two months on chemotherapeutic treatment, an abdominal CT disclosed stable disease.
Three months after the introduction of sodium benzoate and arginine, and adequate diet, without any other medical treatment for HE (i.e., neomycin, lactulose, and L-ornithine-L-aspartate were discontinued), even with ongoing chemotherapy treatment, there were no clinical signs of encephalopathy and the patient’s ammonia blood levels ranged between 40 and 60 mcmol/L.
Discussion
As hyperammonemic encephalopathy may be present even in patients with normal hepatic function, a high level of suspicion of encephalopathy is paramount in order to reach a timely diagnosis and to not miss the possibility of reversion and cure.
Clinical presentation can be very variable and symptoms are usually episodic. Initial signs of HE may be inversion of sleep pattern, mild confusion, lethargy, and personality changes. There may be asterixis. However, HE can develop into somnolence, disorientation, marked confusion, and even coma. If left untreated, it may lead to intracranial hypertension, seizures, and death [2].
Early diagnosis relies on measuring plasmatic ammonia level in suspected patients. Although plasmatic ammonia levels do not always correlate directly with the degree of encephalopathy, subsequent dosages may be used to monitor treatment response, and clinical improvement usually accompanies decreasing levels of blood ammonemia [2,3].
Image studies such as CT and magnetic resonance imaging (method of choice) may help stablishing diagnosis. Typical findings of hyperammonemia are hyperintense lesions in the globus pallidus [4,5]. Other image study modalities, such as magnetic resonance spectroscopy, single-photon emission CT, and positron emission tomography may add some information on the degree of impairment of cerebral water hemostasis, metabolic changes, brain edema, and astrocyte dysfunction and contribute to assessment of the severity and prognosis of neurological impairment [5–7].
Another useful diagnostic modality is the electroencephalography. Common findings are abnormal power of theta activity in mild or latent encephalopathy and low mean dominant frequency identified by biphasic power spectrum (delta and theta peaks) or high power delta activity in more severe cases [8–11].
As long as the diagnosis HE persists, proper treatment must be initiated, and it depends of identifying and understanding the myriad of conditions and physiopathological pathways that may lead to HE in patients with normal liver function.
Fibrolamellar hepatocellular carcinoma is a rare tumor of unknown etiology that almost always arises in non-cirrhotic livers of young adults without chronic viral hepatitis. It was first described in 1956 by Edmonson as a subtype of hepatocellular carcinoma [12]. Since 2009, this specific type of hepatic tumor has been associated with hyperammonemia in the literature [13–17].
There is no consensus in the literature about the exact causative physiopathological mechanism of hyperammonemia in patients with FHC. Some authors propose that a portosystemic shunt resulting from large tumors occupying significant portions of the liver may impair the hepatic capacity of nitrogen waste clearance [13,14].
However, despite the fact that our patient had an 18 cm tumor occupying almost the entire right hepatic lobe with tumor thrombosis of the right portal hepatic veins, the left hepatic lobe was substantially enlarged probably as a result of compensatory hypertrophy. This compensatory hypertrophy of the left liver along with normal reticulocyte production index, serum lactic dehydrogenase, unconjugated bilirubin, and stable hematocrit suggested no significant portosystemic shunt bypassing the liver. It also demonstrated regenerative capacity of the non-tumoral liver that requires healthy parenchyma. Pathological examination of the liver from biopsies that were performed during the exploratory laparoscopy showed no signs of chronic hepatic disease and there were no laboratory or physical signs of hepatic failure. All these factors raise questions regarding why the liver lost only the capacity to perform nitrogen waste clearance and no other energy consuming metabolic pathways were affected.
Another explanation to hyperammonemia in patients with hepatic cancer is chemotherapy-related toxicity [15,17,18]. Several chemotherapy agents have been associated with HE, such as oxaliplatin, vincristine, 5-fluorouracil, cyclophosphamide, methotrexate, etoposide, and gemcitabine [15]. Nevertheless, our patient had never received chemotherapy before developing HE.
As previously reported in the literature, a more convincing explanation to the development of HE in patients with FHC is a disorder in the urea cycle [15–17]. The urea cycle (also known as ornithine cycle) was described by Krebs and Henseleit in 1932, being the first metabolic cycle discovered [19]. It is responsible for converting waste nitrogen from protein digestion and protein catabolism into urea, which is than excreted by the kidneys. The urea cycle consists of five consecutive reactions, two mitochondrial and three cytosolic, and converts one amino group from ammonia, two molecules of nitrogen from ornithine, and one molecule from aspartate to urea. Two transporters (citrin and ornithine transporter-1) and a cofactor enzyme (N-acetyl glutamate synthetase) are also involved in the urea cycle [20]. Therefore, a total of eight enzymes are involved is this metabolic cycle:
– N-acetylglutamate synthetase (NAGS);
– Carbamoyl phosphate synthetase (CPS1);
– Citrin (aspartate glutamate translocase);
– Argininosuccinate synthetase (ASS);
– Argininosuccinate lyase (ASL);
– Arginase (ARG);
– Ornithine translocase (ORNT1);
– Ornithine transcarboxylase (OTC).
Disorders in the urea cycle are deficiencies of any of these factors and result in the accumulation of ammonia and other precursor metabolites. In fact, OTC deficiency has been previously associated with FHC [15–17,21]. However, the specific reason for the suppression of OTC enzyme activity or for a deficiency of ornithine availability and consequent decreased ornithine cycle functioning has never been determined [15,22]. The answer to that question probably began to be solved in 1972 by Weber and colleagues [23]. They demonstrated in rats a decrease in OTC activity reaching 1% of that observed in normal livers of control animals parallel to the increase in the hepatomas growth rate. This decrease had a close link to the increase in hepatomas growth rates, thus providing support to the Molecular Correlation Concept described by Weber and Lea [24]. A decrease in the urea cycle functioning determined by the reduction on OTC activity results in a decline in the utilization of aspartate and carbamoyl phosphate that could be spared for biosynthesis of DNA and RNA and thus become a biological advantage to the hepatic tumors.
It was also demonstrated that, despite the fact that OTC is not a rate-limiting enzyme of the urea cycle, its metabolic location in the cycle puts it in competition with other enzymes that use ornithine as a substrate. This has been shown to be the case with ornithine decarboxylase (ODC) [23].
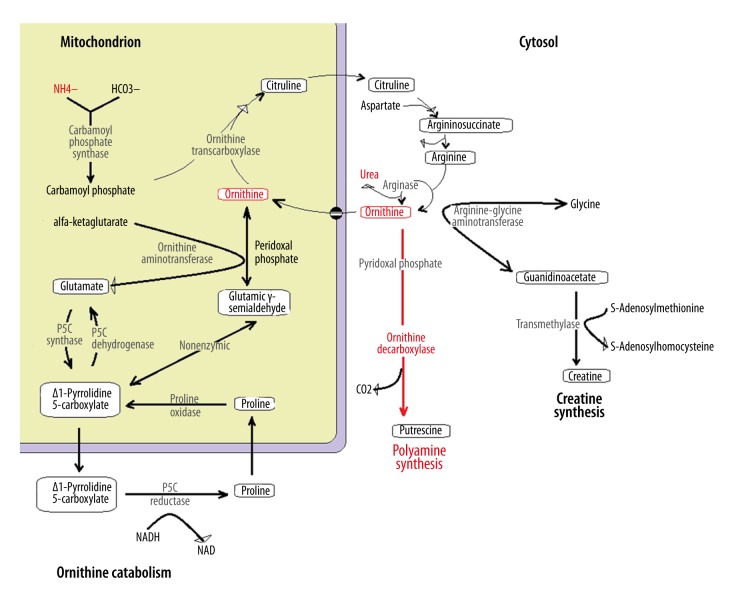
Ornithine decarboxylase catalyzes the decarboxylation of ornithine, which is the first rate-limiting and committed step in polyamines synthesis, particularly for putrescine, spermine, and spermidine molecules that are indispensable for mammalian cell growth. They are key substrates for DNA stabilization and repair and are important antioxidants. So, ornithine decarboxylase activity is essential for cell growth and the reduction of this enzyme may induce apoptosis in DNA damaged cells [25]. Weber et al. also demonstrated an imbalance in the activities and ratios of OTC and ODC. While in normal rat livers the OTC activity is very high compared to ODC, in hepatomas there is a proportional reduction on OTC parallel to increase in ODC activity [23]. Thus, the ratio of activity of ODC/OTC rise along with the increase in hepatomas growth rates, resulting in an augmented utilization of ornithine for polyamine synthesis and the resulting nucleic acid biosynthesis by hepatoma cells. This imbalance becomes progressively increased parallel to the tumor growth rate, as showed by the Molecular Correlation Concept of an imbalance in enzymatic activities in opposing and competing metabolic pathways involved in tumor growth [26]. Therefore, in contrast to recent publications on the association of FHC and HE that focused on a probable paraneoplastic effect of the tumor on the direct reduction on the activity of OTC, the hypothesis is that an augmented activation of ODC results in the consumption of ornithine, and resulting urea cycle disturbance (Figure 4). However, the reason for this ODC activation is still missing in this clinical scenario.
Figure 4.
Ornithine metabolism.
This takes us back to the characteristics of the FHC itself. The etiology of this tumor is unknown and there is limited data on its pathophysiology. Elevations on blood alpha fetoprotein in patients with FHC are less likely to occur than in patients with traditional hepatocellular carcinoma and the genome sequencing of such tumors (mutations, pathways and structural variants) have demonstrated that it is in fact a distinct disease [27,28].
The analysis of fresh frozen specimens of FHC has demonstrated the presence of a single, recurrent heterozygous deletion of chromosome 19 that resulted in a functional chimeric protein of the heat shock protein DNAJB1 and a catalytic subunit of protein kinase A, PRKACA [27–29]. This finding was very sensitive and specific (100% of the FHC presented this chimeric transcript) for this tumor [28–30].
The lack of a high background of mutations throughout the genome of this tumor and the absence of an identifiable second hit mutation necessary to carcinogenesis suggests that the chimeric DNAJB1-PRKACA kinase is necessary and probably sufficient for the tumorigenesis of FHC [26]. The most probable molecular pathway involved is that of the DNAJB1-PRKACA chimera, which results in changes of AURKA expression within the tumor [30,31]. Transcriptome sequencing has demonstrated increased expression of AURKA (a known oncogene) in FHC samples [31].
It has been previously demonstrated that AURKA accumulations in the nucleus of hepatocellular carcinomas upregulates c-Myc transcription by binding to its promoter, which contains a high conserved CCCTCCCCA in the NHE region of CpG islands [32]. C-Myc is a regulator gene that codes a multifunctional nuclear phosphoprotein that is a transcription factor of paramount importance to cell cycle progression, apoptosis, and cellular transformation. It was first described in patients with Burkitt lymphoma [33]. A persistent expression of c-Myc leads to over-expression of many genes involved in cellular proliferation, resulting in carcinogenic effects [32]. AURKA and c-Myc mediate each other’s expression at the transcriptional level, affecting cellular proliferation, growth, and ATP production, and playing an important role in the carcinogenesis of hepatic tumors [32]. So, increased expression of AURKA correlates with that of c-Myc.
Finally, one of the c-Myc oncogene targets is ODC [34]. Overexpression of ODC secondary to c-Myc signaling resulting in increased polyamines has been observed in many tumors samples and is one of the first and most important carcinogenic steps in a variety of cancers [35].
To summarize, a recurrent heterozygous deletion of chromosome 19 common to all FHCs results in an increased expression of AURKA. This leads to an overexpression of c-Myc that upregulates ODC function that consumes ornithine in polyamines synthesis. The consequence is a reduction on intracellular ornithine bioavailability and consequent decreased urea cycle functioning resulting in HE.
In our particular patient, a multi-gene panel genetic testing for inborn errors of metabolism was performed, including the OTC gene, and no pathological variants were observed (Figure 3). He also presented very elevated plasmatic histidine level (451.0 mcmol/L, reference interval 6.0–250.0 mcmol/L) and low serine plasmatic level (19.0 mcmol/L, reference interval 20.0–120.0 mcmol/L) that could be indicative of ODC overexpression.
As the fibrolamellar hepatocellular carcinoma presents increased expression of AURKA, the chemotherapeutic regimen option for this patient was sorafenib (a multikinase inhibitor) and GEMOX. Two months after beginning chemotherapy, a CT of the abdomen and thorax disclosed stable disease. Four months after the acute encephalopathy episode and treatment with sodium benzoate and arginine was introduced, ammonia blood levels remained low and there were no other signs of HE.
Conclusions
Hyperammonemic encephalopathy in patients with normal hepatocellular function is a potentially fatal complication of rapidly growing liver tumors. A high level of suspicion is necessary to prompt diagnosis, and adequate treatment relies on the proper understanding of the physiopathological mechanism involved in order to reach complete remission of encephalopathy. We advise that all patients with large hepatic tumors should have ammonia blood level dosed at initial evaluation and repeatedly during treatment. We propose that an overexpression of AURKA, which occurs in all FHCs, is the initial key event that results in ornithine metabolism imbalance that leads to a urea cycle disorder resulting in hyperammonemia and encephalopathy.
Acknowledgments
We want to thank Valdemir Carlos Moraes de Menezes and Erica Santos Flora da Silva for the continuous and relentless support.
Footnotes
Statement
All authors declare that they have no conflicts of interest.
References:
- 1.Eggert T, McGlynn KA, Duffy A, et al. Fibrolamellar hepatocellular carcinoma in the USA, 2000–2010: A detailed report on frequency, treatment and outcome based on the Surveillance, Epidemiology, and End Results database. United European Gastroenterol J. 2013;1:351–57. doi: 10.1177/2050640613501507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hawkes ND, Thomas GAO, Jurewicz A, et al. Non-hepatic hyperammonaemia: An important, potentially reversible cause of encephalopathy. Postgrad Med J. 2001;77:717–22. doi: 10.1136/pmj.77.913.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Green A. When and how should we measure plasma ammonia? Ann Clin Biocehm. 1988;25:199–209. doi: 10.1177/000456328802500301. [DOI] [PubMed] [Google Scholar]
- 4.Bathla G, Hegde AN. MRI and CT appearances in metabolic encephalopathies due to systemic diseases in adults. Clin Radiol. 2013;68:545–54. doi: 10.1016/j.crad.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 5.Huda A, Gupta RK, Rajakumar N, Thomas MA. Role of magnetic resonance in understanding the pathogenesis of hepatic encephalopathy. Magn Reson Insights. 2008;2:109–22. doi: 10.4137/mri.s973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chavarria L, Alonso J, García-Martínez R, et al. Brain magnetic resonance spectroscopy in episodic hepatic encephalopathy. J Cereb Blood Flow Metab. 2013;33:272–77. doi: 10.1038/jcbfm.2012.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarma MK, Huda A, Nagarajan R, et al. Multi-dimensional MR spectroscopy: Towards a better understanding of hepatic encephalopathy. Metab Brain Dis. 2011;26:173–84. doi: 10.1007/s11011-011-9250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montagnese S, Balistreri E, Schiff S, et al. Covert hepatic encephalopathy: Agreement and predictive validity of different indices. World J Gastroenterol. 2014;20:15756–62. doi: 10.3748/wjg.v20.i42.15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van der Rijt CC, Schalm SW, De Groot GH, De Vlieger M. Objective measurement of hepatic encephalopathy by means of automated EEG analysis. Electroencephalogr Clin Neurophysiol. 1984;57:423–26. doi: 10.1016/0013-4694(84)90071-3. [DOI] [PubMed] [Google Scholar]
- 10.Amodio P, Marchetti P, Del Piccolo F, et al. Spectral versus visual EEG analysis in mild hepatic encephalopathy. Clin Neurophysiol. 1999;110:1334–44. doi: 10.1016/s1388-2457(99)00076-0. [DOI] [PubMed] [Google Scholar]
- 11.Weissenborn K, Ennen JC, Schomerus H, et al. Neuropsychological characterization of hepatic encephalopathy. J Hepatol. 2001;34:768–73. doi: 10.1016/s0168-8278(01)00026-5. [DOI] [PubMed] [Google Scholar]
- 12.Edmonson HA. Differential diagnosis of tumors and tumor-like lesions of liver in infancy and childhood. AMA J Dis Child. 1956;91:168–86. doi: 10.1001/archpedi.1956.02060020170015. [DOI] [PubMed] [Google Scholar]
- 13.Berger C, Dimanti P, Hermida L, et al. Encefalopatía hiperamoniémica y hepatocarcinoma fibrolamelar. Medicina (Buenos Aires) 2012;72:425–27. [in Portuguese] [PubMed] [Google Scholar]
- 14.Sethi S, Tageja N, Singh J, et al. Hyperammonemic encephalopathy: A rare presentation of fibrolamellar hepatocellular carcinoma. Am J Med Sci. 2009;338:522–24. doi: 10.1097/MAJ.0b013e3181bccfb4. [DOI] [PubMed] [Google Scholar]
- 15.Chapuy CI, Sahai I, Sharma R, et al. Hyperammonemic encephalopathy associated with fibrolamellar hepatocellular carcinoma: Case report, literature review, and proposed treatment algorithm. Oncologist. 2016;21:514–20. doi: 10.1634/theoncologist.2015-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bender HU, Staudigl M, Schmid I, Führer M. Treatment of paraneoplastic hyperammonemia in fibrolamellar hepatocellular carcinoma with oral sodium phenylbutyrate. J Pain Symptom Manage. 2015;49:e8–10. doi: 10.1016/j.jpainsymman.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Sulaiman RA, Geberhiwot T. Fibrolamellar hepatocellular carcinoma mimicking ornithine transcarbamylase deficiency. JIMD Rep. 2014;16:47–50. doi: 10.1007/8904_2014_318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan JS, Harding CO, Blanke CD. Postchemotherapy hyperammonemic encephalopathy emulating ornithine transcarbamoylase (OTC) deficiency. South Med J. 2008;101:543–45. doi: 10.1097/SMJ.0b013e31816bf5cc. [DOI] [PubMed] [Google Scholar]
- 19.Kinne-Saffran E, Kinne RK. Vitalism and synthesis of urea. From Friedrich Wöhler to Hans A. Krebs. Am J Nephrol. 1999;19:290–94. doi: 10.1159/000013463. [DOI] [PubMed] [Google Scholar]
- 20.Machado MC, Pinheiro da Silva F. Hyperammonemia due to urea cycle disorders: A potentially fatal condition in the intensive care setting. J Intensive Care. 2014;2:22. doi: 10.1186/2052-0492-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Upadhyay R, Bleck TP, Busl KM. Hyperammonemia: What urea-lly need to know: Case report of severe noncirrhotic hyperammonemic encephalopathy and review of the literature. Case Rep Med. 2016;2016:8512721. doi: 10.1155/2016/8512721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malouf GG, Job S, Paradis V, et al. Transcriptional profiling of pure fibrolamellar hepatocellular carcinoma reveals an endocrine signature. Hepatology. 2014;59:2228–37. doi: 10.1002/hep.27018. [DOI] [PubMed] [Google Scholar]
- 23.Weber G, Queener SF, Morris HP. Imbalance in ornithine metabolism in hepatomas of different growth rates as expressed in behavior of L-ornithine carbamyl transferase activity. Cancer Res. 1972;32:1933–40. [PubMed] [Google Scholar]
- 24.Weber G, Lea MA. The molecular correlation concept of neoplasia. Adv Enzyme Regul. 1966;4:115–45. doi: 10.1016/0065-2571(66)90011-2. [DOI] [PubMed] [Google Scholar]
- 25.Pendeville H, Carpino N, Marine JC, et al. The ornithine decarboxylase gene is essential for cell survival during early murine development. Mol Cell Biol. 2001;21:6549–58. doi: 10.1128/MCB.21.19.6549-6558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferdinandus JA, Morris HP, Weber G. Behavior of opposing pathways of thymidine utilization in differentiating, regenerating, and neoplastic liver. Cancer Res. 1971;31:550–56. [PubMed] [Google Scholar]
- 27.Darcy DG, Chiaroni-Clarke R, Murphy JM, et al. The genomic landscape of fibrolamellar hepatocellular carcinoma: Whole genome sequencing of ten patients. Oncotarget. 2015;6:755–70. doi: 10.18632/oncotarget.2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Honeyman JN, Simon EP, Robine N, et al. Detection of a recurrent DNAJB1-PRKACA chimeric transcript in fibrolamellar hepatocellular carcinoma. Science. 2014;343:1010–14. doi: 10.1126/science.1249484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graham RP, Jin L, Knutson DL, et al. DNAJB1-PRKACA is specific for fibrolamellar carcinoma. Mod Pathol. 2015;28:822–29. doi: 10.1038/modpathol.2015.4. [DOI] [PubMed] [Google Scholar]
- 30.Lim IIP, Greene-Colozzi EA, Murphy JM, et al. DNAJB1-PRKACA chimera increases Aurora kinase A expression in fibrolamellar hepatocellular carcinoma. AACR 106th Annual Meeting; 2015; April 18–22, 2015. [Google Scholar]
- 31.Simon EP, Freije CA, Farber BA, et al. Transcriptomic characterization of fibrolamellar hepatocellular carcinoma. Proc Natl Acad Sci USA. 2015;112:E5916–25. doi: 10.1073/pnas.1424894112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu L, Han H, Tian Y, et al. Aurora kinase A mediates c-myc’s oncogenic effects in hepatocellular carcinoma. Mol Carcinog. 2015;54:1467–79. doi: 10.1002/mc.22223. [DOI] [PubMed] [Google Scholar]
- 33.Finver SN, Nishikura K, Finger LR, et al. Sequence analysis of the MYC oncogene involved in the t(8;14)(q24;q11) chromosome translocation in a human leukemia T-cell line indicates that putative regulatory regions are not altered. Proc Natl Acad Sci USA. 1988;85:3052–56. doi: 10.1073/pnas.85.9.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nilsson JA, Keller UB, Baudino TA, et al. Targeting ornithine decarboxylase in Myc-induced lymphomagenesis prevents tumor formation. Cancer Cell. 2005;7:433–44. doi: 10.1016/j.ccr.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 35.Mohammed A, Janakiram NB, Madka V, et al. Eflornithine (DFMO) prevents progression of pancreatic cancer by modulating ornithine decarboxylase signaling. Cancer Prev Res (Phila) 2014;7:1198–209. doi: 10.1158/1940-6207.CAPR-14-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]