Abstract
Background
The aims of this study are to investigate the difference between the diaphragm thickness at end expiration and the thickness at total lung capacity (TLC), and to examine differences in inspiratory muscle function between stroke patients and healthy individuals.
Material/Methods
Forty-five stroke patients and 49 healthy volunteers were included in this study. Diaphragm thickness was measured at end expiration and at TLC by ultrasonography. The maximal inspiratory pressure (MIP), peak inspiratory flow (PIF), vital capacity (VC), and inspiratory muscle endurance (IME) were assess to evaluate inspiratory muscle function.
Results
In stroke patients, the diaphragm was significantly thinner on the affected side than the less affected side at end expiration and at TLC. The change between the thickness at end expiration and at TLC were also significant on both sides. Between groups, the difference in diaphragm thickness at end expiration was not significant, but at TLC, the diaphragms were significantly thicker in healthy individuals than on either side in stroke patients, and the change in diaphragm thickness was significantly greater for healthy individuals. Inspiratory muscle functions were also significantly greater in healthy individuals. MIP, PIF, and VC were positively correlated with the change in thickness in healthy individuals, and MIP was positively correlated with the change in thickness and IME in stroke patients.
Conclusions
Stroke patients showed decreases in the thickening ability of the diaphragm at TLC and in inspiratory muscle function. The change between the diaphragm thickness at end expiration and at TLC was positively correlated with MIP, PIF, and VC.
MeSH Keywords: Breathing Exercises, Diaphragm, Inspiratory Capacity, Stroke, Ultrasonography
Background
Stroke disabilities result from decreased muscle function and limitations of activity. Motor weakness is one of the common disabilities of stroke survivors, and is the most prevalent disabilities after stroke. Hemiplegia and hemiparesis are the most common motor-related problems and are the primary indications for rehabilitation [1]. Consequences of hemiparesis may include abnormalities in muscular tonus, as well as postural and motor control that lead to inadequate functioning of the entire body and could compromise voluntary motor function [2]. The physical capacity of stroke patients are, on average, reduced by 40% compared to healthy individuals and these reductions are often a result of a decrease of muscle thickness and muscle weakness that then causes a decrease in motor function [3,4]. The prominent changes in stroke patients are decreased muscle strength and voluntary contraction of some muscle groups, these changes are a result of impaired motor unit and muscular changes such as atrophy [5].
The inspiratory muscles morphologically and functionally belong to the skeletal muscles group [6]. After a stroke, the diaphragm, which is one of the major inspiratory muscles, tends to atrophy due to central nervous system impairments [7]. The atrophy of the diaphragm results in a decrease of exercise ability related to muscle fatigue [8]. In stroke patients, the structure of the diaphragm can show displacement and resulting changes in excursion [9]. Pinheiro et al. reported that inspiratory muscle strength in stroke patients reached half the level of healthy individuals and this decrease in inspiratory muscle function can cause difficulties in functional locomotion [10].
Maximal inspiratory pressure (MIP) is an indicator that is widely used and is clinically related to the effect of inspiratory muscle training. Measurement is of the volitional force output of the inspiratory muscles working in synergy, and is an established and reliable measure of global inspiratory muscle strength [11,12]. MIP is significantly decreased in stroke patients compared with healthy individuals [13]. Stroke patients with respiratory muscle weakness show fatigue and dyspnea in conditions of higher effort demands [14], which in turn can interfere with the performance of activities of daily livings [15]. Peak inspiratory flow (PIF), at a given volume, depends on the airway caliber as well as the strength and speed of shortening of the inspiratory muscles [16]. The inspiratory muscle training device, an electronic inspiratory loading device, can provide automatically processed information for the MIP, PIF, and vital capacity (VC) during breathing tasks [17].
Inspiratory muscle endurance (IME) measures the fatigue of inspiratory muscles, especially in the diaphragm. IME is an important respiratory value that is affected by exercise ability (capacity, endurance, tolerance) in stroke patients [18]. Changes in respiratory muscular strength and function were investigated in several studies [19–21] that consistently reported that the paretic hemi-diaphragm was associated with decreases in diaphragmatic movement during spontaneous breathing. However, it is not clear that atrophy of the diaphragm and decreases in MIP, PIF, and VC in stroke patients is related to IME. Although measuring IME is helpful for expectations of inspiratory muscle function, strength, and the effect of inspiratory muscle training, the studies on diaphragm thickness and IME in stroke patients compared with healthy individuals is limited.
Rehabilitative ultrasound image (RUSI) allows the visualization of muscle contractions; and has been shown to reliably assess diaphragm thickness [22]. Two-dimensional B-mode ultrasound of the diaphragm is a feasible, relatively inexpensive, radiation-free imaging modality that has advantages over video fluoroscopy. We used B-mode ultrasonography to study the structure and function of the diaphragm, measuring the thickness of the muscles and the thickening ratio between at end expiration and at TLC.
Therefore the purpose of this study was to investigate the diaphragm thickness between at end expiration and at TLC, and the differences in MIP, PIF, VC, and IME in stroke patients compared to healthy individuals of the same age. Our hypothesis was that the thickness of the diaphragm visualized by the RUSI will be thinner at the hemiplegic side than at the unaffected side and in healthy adults. Second, MIP, PIF, VC, and IME will be relatively decreased compared to healthy individuals.
Material and Methods
Participants
Forty-five post-stroke patients and the 49 healthy individuals from the M Rehabilitation Hospital in Seoul, Republic of Korea, volunteered and participated in this study. A physical assessment and interview were initially conducted with all individuals to collect anthropometric and demographic data (gender, age, height, weight, body mass index (BMI), affected side, onset time of stroke, and use of medications). A summary of the general characteristics of study participants is described in Table 1. A questionnaire was administered to gather information related to the work environment, smoking history, recent respiratory infections, and the presence or absence of lung and heart disease. The data were used as parameters for both the analyses and interpretation of the inspiratory muscle function tests.
Table 1.
General characteristics of participants.
| Parameters | Stroke patients (n=45) | Healthy subject (n=49) | t (p)-values |
|---|---|---|---|
| Age, years | 49.51 (12.48) | 48.15 (3.59) | −.706 (.482) |
| Height, cm | 166.46 (10.01) | 166.30 (6.84) | −.090 (.929) |
| Weight, kg | 64.12 (11.47) | 62.91 (10.34) | −.526 (.600) |
| BMI | 23.49 (3.05) | 22.61 (2.43) | −1.513 (.134) |
| Gender; Male/Female, % | 31/14 (68.9/31.1) | 26/23 (53.1/46.9) | 1.600 (0.206) |
| Paretic side; Right/Left, % | 20/25 (44.4/55.6) | ||
| Etiology; Infarction/Hemorrhage, % | 20/25 (44.4/55.6) | ||
| Post stroke duration, days | 198.91 (182.55) | ||
| MMSE-K, score | 27.51 (2.13) |
Values are mean (SD). BMI – body mass index; MMSE-K – Mini Mental Status Evaluation-Korean; SD – standard deviation.
The inclusion criteria were as follows: no history of cardiovascular or respiratory problems, first episode of unilateral stroke with hemiparesis during the previous six months, mini mental status evaluation score ≥24, no facial palsy, no receptive aphasia, and no prior thoracic or abdominal surgery. The exclusion criteria were as follows: use of medications that could affect neuromuscular control or provoke drowsiness, evidence of constructive or restrictive lung disease based on lung volumes, history of asthma, and use of psychotropic drugs or alcohol abuse. The participants gave signed consent after receiving verbal and written information about the study. The study’s protocol was approved by the institutional review board of the Myungji Choonhey Rehabilitation Hospital in Seoul. (MJCHIRB-2015-02). The protocol of this study was approved by World Health Organization International Clinical Trials Registry Platform (KCT0001883).
Diaphragm thickness
Real-time B-mode (MYSONO US, Samsung Medicine, Seoul, Korea) ultrasonography was used to measure the diaphragm thickness at the zone of apposition during inspiration or end expiration using the intercostal approach with 10-MHz linear transducer. With the study participant in the supine position, the transducer was placed on the chest wall at approximately the anterior axillary line, just cephalad to the lower costal margin. With the transducer spanning/perpendicular to two ribs, the diaphragm can be visualized as a hypoechoic layer of muscle encased in two hyperechoic layers of connective tissue (the parietal pleura and the peritoneum), deep to the intercostal muscles connecting the two ribs [23]. This technique was previously reported to be reliable, with intra-class correlation coefficients ranging from 0.89 to 0.98 for intra-rater and inter-rater reliability [13].
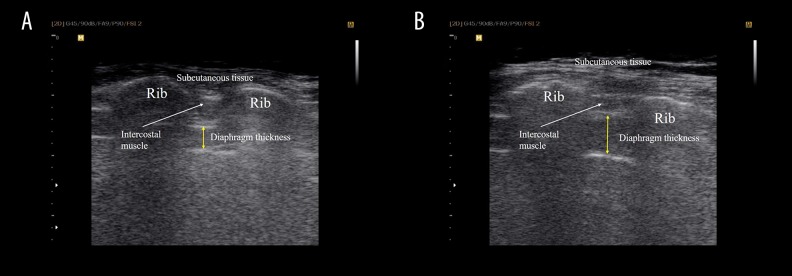
Diaphragm thickness was measured in two different conditions (Figure 1). Measurements of diaphragm thickness at end expiration were obtained, that is the patient was instructed to perform breathing to TLC and then to exhale to end expiration. Measurements of the diaphragm thickness at TLC were gained by asking the participants to take a maximal inspiration starting from the end of a residual volume. Measurements were taken on three different images to calculate the average thickness of the diaphragm. On each frozen B-mode image, the diaphragm thickness was measured from the middle of the pleural line to the middle of the peritoneal line [22]. The analysis of all images was performed using Sante DICOM Viewer FREE 4.0.14 to determine the thickness of diaphragm at end expiration and TLC.
Figure 1.
Ultrasonographic view of the diaphragm over the intercostal space. (A) Rehabilitative ultrasound imaging of diaphragm thickness at end expiration, deep to the intercostal muscle layer and ribs. (B) Rehabilitative ultrasound imaging of diaphragm thickness at total lung capacity, deep to the intercostal muscle layer and ribs
Inspiratory muscle functions
An electronic inspiratory loading device (PowerBreathe K5, 2010, HaB International Ltd, UK) was used to provide automatically processed information on MIP during loaded breathing tasks and inspiratory muscle strength was assessed by measuring the PIF and VC [17]. Participants were comfortably seated on a chair with their feet on the ground, their back unsupported, and their trunk at a 90° angle to their hips. To record MIP, participants were instructed to perform inspirations against an obstructed airway at near-RV. A conventional mouthpiece and nose clip were used to prevent leakage of air. The pressure, which was sustained for 2–3 seconds, was recorded. Participants could choose their own breathing frequency, but were instructed to perform forceful and deep inspirations followed by complete expirations. Complete expiration was indicated by an acoustic signal provided by the handheld loading device upon cessation of flow. Participants were given strong verbal encouragement but no visual feedback. They performed a minimum of three efforts and the value used was the best of these; participants were allowed to practice twice, and immediately afterwards were asked to repeat the trial until three acceptable measurements were obtained [24]. Measurements were chosen acceptable if they were maintained without an air leak for the duration of at least one second and if two readings were taken with a maximum difference of 10%. Between the measurements, 20 minutes of break time was given to participants to minimize the bias for the MIP. The highest MIP values were recorded for analyses. The device provides automatically processed information on inspiratory muscle function, PIF, and VC during loaded breathing tasks.
IME was measured by using the electronic inspiratory loading device (PowerBreathe K5, 2010, HaB International Ltd, UK) to provide automatically processed information during loaded breathing tasks. The threshold was used to assess IME. IME was assessed by using the 2-minute incremental load method, following proposed protocols [25]. Participants were instructed to breathe during the two minutes at 30% of MIP, and subsequently, external weights of 10% of MIP were added every two minutes. The test was completed when participants were unable to lift the weight or had to stop because of severe dyspnea. IME was determined by using maximal load in centimeters of water sustained for at least one minute. The test also was performed with a nose clip and with participants comfortably positioned and seated on a chair with their feet on the ground, backs unsupported, and trunk at a 90° angle in relation to the hips. They were instructed to keep their heart rates and O2 saturation levels as close as possible to their physiologic values. Signals from the pneumotachograph were captured electronically using J-Lab software version 5.22.1.50 (Cardinal Health GmbH, Hoechberg, Germany).
Statistical analysis
PASW statistics 18.0 (SPSS) was used to analyze data. Results were considered significant at a p-value of <0.05. Chi-square tests were used for categorical variables. The diaphragm thickness (cm) at end expiration and at TLC and thickening ratio (%) within group were assessed with paired t-tests for the diaphragm, independent t-test was used to compare the variables between groups. MIP, PIF, VC, and IME were assessed using independent t-test to compare the difference between groups. The Pearson’s correlation analysis was used to evaluate correlation between thickening ratio for diaphragm thickness and inspiratory muscle functions.
Results
General characteristics between stroke patients and healthy individuals that had no statistical differences were age, height, weight, BMI, and gender. Within the stroke patient group (Table 2), the diaphragm thickness between the affected and the less affected side was significantly different at end expiration (p<0.05), at TLC (p<0.001), and the change value between at end expiration and at TLC (p<0.001). Between groups, diaphragm thickness of the affected side during contraction (p<0.001) and the change value (p<0.001) for stroke patients were significantly different than for healthy individuals. The less affected side in stroke patients showed the same results during contraction (p<0.01) and for the change value (p<0.001).
Table 2.
Comparison of diaphragm thickness between stroke patients and healthy subjects.
| Stroke patients (n=45) | Healthy subjects (n=49) | Between groups | |||||
|---|---|---|---|---|---|---|---|
| Affected side | Less affected side | Dominant side | Affected side | Less affected side | |||
| Diaphragm thickness (cm) | At end expiration | 0.21 (0.02) | 0.22 (0.02) | p<0.05 | 0.21 (0.05) | p>0.05 | p>0.05 |
| At TLC | 0.40 (0.13) | 0.54 (0.17) | p<0.001 | 0.65 (0.14) | p<0.001 | p<0.001 | |
| Change in thickness | 0.19 (0.12) | 0.32 (0.17) | p<0.001 | 0.44 (0.12) | p<0.001 | p<0.001 | |
Values are mean (SD). TLC – total lung capacity.
MIP between stroke patients and healthy participants (Table 3) was significantly different (p<0.001); PIF and VC showed the same results (p<0.01, p<0.01, respectively). IME was significantly different in healthy participants compared to stroke patients (p<0.001).
Table 3.
Comparison of inspiratory muscle function between stroke patients and healthy subjects.
| Stroke patients (n=45) | Healthy subjects (n=49) | p-value | |
|---|---|---|---|
| Maximal inspiratory pressure (cm/H20) | 49.73 (18.30) | 77.05 (25.67) | p<0.000 |
| Peak inspiratory flow (L/s) | 3.16 (0.72) | 3.60 (0.63) | p<0.001 |
| Vital capacity (L) | 1.99 (0.69) | 2.36 (0.38) | p<0.001 |
| Inspiratory muscle endurance (cm/H20) | 13.17 (5.86) | 26.63 (11.06) | p<0.000 |
Values are mean (SD).
The diaphragm thickening ratio between at end expiration and at TLC (Table 4) was positively correlated with MIP, PIF, and VC (r=0.315, p<0.05; r=0.342, p<0.05; r=0.307, p<0.05, respectively) in healthy participants, and positively correlated with MIP (r=0.565, p<0.001) in stroke patients. Within inspiratory muscle functions, MIP showed positive correlation with IME (r=0.769, p<0.001) in stroke patients, but no significant correlations in healthy participants.
Table 4.
Correlation between thickness change ratio and inspiratory muscle function.
| Stroke patients (n=45) | Healthy subjects (n=49) | |||||||
|---|---|---|---|---|---|---|---|---|
| MIP | PIF | VC | IME | MIP | PIF | VC | IME | |
| Change in thickness |
r=0.565 p<0.001 |
r=0.125 p>0.05 |
r=−0.135 p>0.05 |
r=0.262 p>0.05 |
r=0.315 p<0.05 |
r=0.342 p<0.05 |
r=0.307 p<0.05 |
r=0.030 p>0.05 |
| MIP |
r=0.235 p>0.05 |
r=−0.135 p>0.05 |
r=0.769 p<0.001 |
r=0.140 p>0.05 |
r=0.201 p>0.05 |
r=0.073 p>0.05 |
||
| PIF |
r=−0.016 p>0.05 |
r=0.214 p>0.05 |
r=0.217 p>0.05 |
r=0.164 p>0.05 |
||||
| VC |
r=−0.058 p>0.05 |
r=−0.124 p>0.05 |
||||||
Thickness change ratio is the ratio of diaphragm thickness at end expiration to the diaphragm thickness at TLC. TLC – total lung capacity; MIP – maximal inspiratory pressure; PIF – peak inspiratory flow; VC – vital capacity; IME – inspiratory muscle endurance.
Discussion
In recent years, as technology has progressed and image resolution has improved markedly, B-mode ultrasonography has become more readily available. The advantages of ultrasonography over other imaging modalities include portability, relatively low cost, and absence of contraindications. This study investigated the differences between stroke patients and healthy individuals for diaphragm thickness at end expiration and at TLC. Also, we compared the MIP, PIF, VC, and IME to confirm the differences for inspiratory muscle functions between stroke patients and healthy individuals
Diaphragm thickness
Our results showed that the diaphragm thickness at end expiration was significantly decreased in the affected side compared to the less affected side within the stroke patient group. Compared to healthy individuals group, however, there was no significant difference based on side. Also, the diaphragm thickness at TLC was significantly different in the affected side within the stroke patient group; there are also significant differences between healthy participants and stroke patients. Our hypothesis was that stroke patients would have thinner diaphragm thickness compared to healthy individuals, because the diaphragm is one of the musculoskeletal muscles [6] and the diaphragm tends to atrophy due to impairments in the central nervous system [7]. Contrary to our hypotheses, our study results showed that stroke patients had comparable diaphragm thickness at end expiration to that of healthy individuals. This is similar to results that found normal diaphragm structure, diaphragm function, and respiratory drive in patients with COPD [26].
However, the diaphragm thickness at TLC in stroke patients was significantly thinner in the affected side compared to the less affected side, and compared with healthy individuals. Jung et al. examined diaphragmatic motion via M-mode ultrasonography and a correlation with pulmonary function in stroke patients [19]. Their results showed that all stroke patients had restrictive pulmonary dysfunction. Compared to the control participants, stroke patients exhibited a significant unilateral reduction in motion on the hemiplegic side, particularly during volitional breathing. Reductions in diaphragmatic motion and pulmonary function can occur in stroke patients. Thus, this should be assessed prior to the initiation of rehabilitation therapy, and ultrasonography would be helpful to assess this. Park et al. studied the diaphragm excursion via ultrasonography in stroke patients with dysphagia and determined whether they present reduced diaphragm excursion during voluntary cough compared with stroke patients without dysphagia and healthy participants [20]. They examined the diaphragm motions via ultrasonography during quiet breathing, deep breathing, and voluntary coughing, and reported that stroke patients with dysphagia had decreased diaphragm excursion and compromised respiratory function during voluntary coughing. Our results are consistent with these studies with regard to the diaphragm thickness at TLC and the change value compared to the thickness at end expiration. These findings are clinically significant because the diaphragm thickness was different compared to the less affected side, and compared to healthy individuals. In addition, stroke may include abnormalities in postural and motor control, which could lead to inadequate functioning of the whole body [2].
In assessing the diaphragm, it is important to negate the influence of gravity on diaphragm movement and raise the sensitivity of the evaluation in subtle weakness which are not observable in the seated or standing position [27]. Using B mode ultrasonography, Gottesman and McCool compared 12 patients who had diaphragm paralysis with 15 control participants and found that the patient group had both decreased thickness and a change in the thickening ratio [28]. De Bruin et al. examined the thickness and thickening ratio in boys with Duchenne muscular dystrophy and found evidence of increased thickness, possibly representing pseudo-hypertrophy, but decreased contractility [29]. These studies used supine position for measuring the diaphragm thickness to increase sensitivity of the thickness quantifying.
Inspiratory muscle function
Enright et al. investigated the respiratory muscle, lung function, and diaphragm thickness in adults with cystic fibrosis [30]. To estimate the inspiratory muscle function, they used the MIP, sustained MIP, and pulmonary function (RV, VC, and TLC). They reported that MIP was relatively well preserved in adults with cystic fibrosis, although there was a relationship between the loss of inspiratory muscle work capacity, physical activity status, and pulmonary functions. Another study evaluated the diaphragm via ultrasonography and reported the correlation between diaphragmatic motion and pulmonary function [19]. Left diaphragmatic motion during deep breathing correlated positively with forced vital capacity and forced expiratory volume in one second. Hiwatani et al. evaluated diaphragm thicknesses during respiration by ultrasonography and compared respiratory functions in patients with amyotrophic lateral sclerosis (ALS) [31]. Their conclusion was that the diaphragm thickness and thickening ratio were significantly reduced in ALS patients and VC was also reduced. Inspiratory muscle training (IMT) has been used to increase muscle strength and improve respiratory function in patients with COPD and emphysema [15,32]. One study reported that IMT decreased dyspnea and resulted in better tolerance in performing activities of daily living [15]. In addition, IMT positively influenced functional performance and the quality of life of cardiac disease patients [32]. The results of our study revealed the MIP, PIF, VC, and IME, which are clinically relevant indicator of inspiratory muscle function, were significantly lower in stroke patients than in healthy individuals. In addition, the diaphragm thickening ratio between at end expiration and at TLC was positively correlated with MIP in stroke patients, and MIP, PIF, and VC were positively correlated in healthy individuals. This study confirmed that the diaphragm thickness of the affected side was decreased in stroke patients compared to healthy individuals, and the inspiratory muscle functions were significantly reduced, especially MIP and IME. Thus diaphragm strengthening exercise is needed for chronic stroke patients because the diaphragm is one of the trunk muscles and an important inspiratory muscles for improving exercise capacity.
Our study had several limitations that should be investigated in future research. First, lung flow and volume were measured in the sitting position [24,25] and ultrasonography measurements were performed in a supine position [23]. In the supine position, the diaphragm thickness can be measured without gravity but the maximal inspiration ability can be influenced by position, so inspiratory muscle functions were evaluated in the sitting posture. The correlations of diaphragm measurements and lung volumes could be affected by this bias. However, we decided to use this sitting technique because we wanted to test the relationships of measurements performed under different conditions. Second, we measured the diaphragm thickness only on the dominant side in healthy participants. Several authors have compared side-to-side hemi-diaphragm motion in healthy control participants [32,33], and showed large variability in side-to-side hemi-diaphragm motion in the normal individual; unequal movement of both side of the diaphragm is usual and unlikely to be of significance unless one excursion is at least twice as great as the other.
Conclusions
We found that stroke patients had decreased thickening ability of the diaphragm at TLC, especially in the affected side compared to healthy individuals, and inspiratory muscle functions were reduced. The change value of the diaphragm thickening between at end expiration and at TLC was correlated with MIP, PIF, and VC in healthy individuals; and in stroke patients the MIP was correlated with IME. Future study is needed to investigate the relationship between the diaphragm thickening ratio, the inspiratory muscle function, and the functional abilities of stroke patients.
Footnotes
Source of support: Departmental sources
References
- 1.Andrews AW, Bohannon RW. Distribution of muscle strength impairments following stroke. Clin Rehabil. 2000;14:79–87. doi: 10.1191/026921500673950113. [DOI] [PubMed] [Google Scholar]
- 2.Nelles G, Spiekermann G, Jueptner M, et al. Reorganization of sensory and motor systems in hemiplegic stroke patients. A positron emission tomography study. Stroke. 1999;30:1510–16. doi: 10.1161/01.str.30.8.1510. [DOI] [PubMed] [Google Scholar]
- 3.Macko RF, Smith GV, Dobrovolny CL, et al. Treadmill training improves fitness reserve in chronic stroke patients. Arch Phys Med Rehabil. 2001;82:879–84. doi: 10.1053/apmr.2001.23853. [DOI] [PubMed] [Google Scholar]
- 4.Macko RF, Benvenuti F, Stanhope S, et al. Adaptive physical activity improves mobility function and quality of life in chronic hemiparesis. J Rehabil Res Dev. 2008;45:323–28. doi: 10.1682/jrrd.2007.02.0025. [DOI] [PubMed] [Google Scholar]
- 5.Lieber RL, Steinman S, Barash IA, Chambers H. Structural and functional changes in spastic skeletal muscle. Muscle Nerve. 2004;29:615–27. doi: 10.1002/mus.20059. [DOI] [PubMed] [Google Scholar]
- 6.Kraemer WJ, Adams K, Cafarelli E, et al. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2002;34:364–80. doi: 10.1097/00005768-200202000-00027. [DOI] [PubMed] [Google Scholar]
- 7.Jandt SR, Caballero RM, Junior LA, Dias AS. Correlation between trunk control, respiratory muscle strength and spirometry in patients with stroke: An observational study. Physiother Res Int. 2011;16:218–24. doi: 10.1002/pri.495. [DOI] [PubMed] [Google Scholar]
- 8.Davis RT, 3rd, Bruells CS, Stabley JN, et al. Mechanical ventilation reduces rat diaphragm blood flow and impairs oxygen delivery and uptake. Crit Care Med. 2012;40:2858–66. doi: 10.1097/CCM.0b013e31825b933a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Similowski T, Catala M, Rancurel G, Derenne JP. Impairment of central motor conduction to the diaphragm in stroke. Am J Respir Crit Care Med. 1996;154:436–41. doi: 10.1164/ajrccm.154.2.8756819. [DOI] [PubMed] [Google Scholar]
- 10.Pinheiro MB, Polese JC, Faria CD, et al. Inspiratory muscular weakness is most evident in chronic stroke survivors with lower walking speeds. Eur J Phys Rehabil Med. 2014;50:301–7. [PubMed] [Google Scholar]
- 11.Romer LM, McConnell AK. Inter-test reliability for non-invasive measures of respiratory muscle function in healthy humans. Eur J Appl Physiol. 2004;91:167–76. doi: 10.1007/s00421-003-0984-2. [DOI] [PubMed] [Google Scholar]
- 12.Larson JL, Covey MK, Vitalo CA, et al. Maximal inspiratory pressure. Learning effect and test-retest reliability in patients with chronic obstructive pulmonary disease. Chest. 1993;104:448–53. doi: 10.1378/chest.104.2.448. [DOI] [PubMed] [Google Scholar]
- 13.Lanini B, Bianchi R, Romagnoli I, et al. Chest wall kinematics in patients with hemiplegia. Am J Respir Crit Care Med. 2003;168:109–13. doi: 10.1164/rccm.200207-745OC. [DOI] [PubMed] [Google Scholar]
- 14.Nachtmann A, Siebler M, Rose G, et al. Cheyne-Stokes respiration in ischemic stroke. Neurology. 1995;45:820–21. doi: 10.1212/wnl.45.4.820. [DOI] [PubMed] [Google Scholar]
- 15.Reid WD, Samrai B. Respiratory muscle training for patients with chronic obstructive pulmonary disease. Phys Ther. 1995;75:996–1005. doi: 10.1093/ptj/75.11.996. [DOI] [PubMed] [Google Scholar]
- 16.Fry DL, Hyatt RE. Pulmonary mechanics. A unified analysis of the relationship between pressure, volume and gasflow in the lungs of normal and diseased human subjects. Am J Med. 1960;29:672–89. doi: 10.1016/0002-9343(60)90100-5. [DOI] [PubMed] [Google Scholar]
- 17.Lee KB, Kim MK, Jeong JR, Lee WH. Reliability of an electronic inspiratory loading device for assessing pulmonary function in post-stroke patients. Med Sci Monit. 2016;22:191–96. doi: 10.12659/MSM.895573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao Y, Luo M, Wang J, Luo H. Inspiratory muscle training for the recovery of function after stroke. Cochrane Database Syst Rev. 2012;5:CD009360. doi: 10.1002/14651858.CD009360.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jung KJ, Park JY, Hwang DW, et al. Ultrasonographic diaphragmatic motion analysis and its correlation with pulmonary function in hemiplegic stroke patients. Ann Rehabil Med. 2014;38:29–37. doi: 10.5535/arm.2014.38.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park GY, Kim SR, Kim YW, et al. Decreased diaphragm excursion in stroke patients with dysphagia as assessed by M-mode sonography. Arch Phys Med Rehabil. 2015;96:114–21. doi: 10.1016/j.apmr.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 21.Cohen E, Mier A, Heywood P, et al. Diaphragmatic movement in hemiplegic patients measured by ultrasonography. Thorax. 1994;49:890–95. doi: 10.1136/thx.49.9.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boon AJ, Harper CJ, Ghahfarokhi LS, et al. Two-dimensional ultrasound imaging of the diaphragm: quantitative values in normal subjects. Muscle Nerve. 2013;47:884–89. doi: 10.1002/mus.23702. [DOI] [PubMed] [Google Scholar]
- 23.Wait JL, Nahormek PA, Yost WT, Rochester DP. Diaphragmatic thickness-lung volume relationship in vivo. J Appl Physiol (1985) 1989;67:1560–68. doi: 10.1152/jappl.1989.67.4.1560. [DOI] [PubMed] [Google Scholar]
- 24.Neder JA, Andreoni S, Lerario MC, Nery LE. Reference values for lung function tests. II. Maximal respiratory pressures and voluntary ventilation. Braz J Med Biol Res. 1999;32:719–27. doi: 10.1590/s0100-879x1999000600007. [DOI] [PubMed] [Google Scholar]
- 25.Martyn JB, Moreno RH, Pare PD, Pardy RL. Measurement of inspiratory muscle performance with incremental threshold loading. Am Rev Respir Dis. 1987;135:919–23. doi: 10.1164/arrd.1987.135.4.919. [DOI] [PubMed] [Google Scholar]
- 26.Topeli A, Laghi F, Tobin MJ. The voluntary drive to breathe is not decreased in hypercapnic patients with severe COPD. Eur Respir J. 2001;18:53–60. doi: 10.1183/09031936.01.00014101. [DOI] [PubMed] [Google Scholar]
- 27.Baria MR, Shahgholi L, Sorenson EJ, et al. B-mode ultrasound assessment of diaphragm structure and function in patients with COPD. Chest. 2014;146:680–85. doi: 10.1378/chest.13-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gottesman E, McCool FD. Ultrasound evaluation of the paralyzed diaphragm. Am J Respir Crit Care Med. 1997;155:1570–74. doi: 10.1164/ajrccm.155.5.9154859. [DOI] [PubMed] [Google Scholar]
- 29.De Bruin PF, Ueki J, Bush A, Khan Y, et al. Diaphragm thickness and inspiratory strength in patients with Duchenne muscular dystrophy. Thorax. 1997;52:472–75. doi: 10.1136/thx.52.5.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Enright S, Chatham K, Ionescu AA, et al. The influence of body composition on respiratory muscle, lung function and diaphragm thickness in adults with cystic fibrosis. J Cyst Fibros. 2007;6:384–90. doi: 10.1016/j.jcf.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Hiwatani Y, Sakata M, Miwa H. Ultrasonography of the diaphragm in amyotrophic lateral sclerosis: clinical significance in assessment of respiratory functions. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14:127–31. doi: 10.3109/17482968.2012.729595. [DOI] [PubMed] [Google Scholar]
- 32.Suga K, Tsukuda T, Awaya H, et al. Impaired respiratory mechanics in pulmonary emphysema: evaluation with dynamic breathing MRI. J Magn Reson Imaging. 1999;10:510–20. doi: 10.1002/(sici)1522-2586(199910)10:4<510::aid-jmri3>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 33.Gierada DS, Curtin JJ, Erickson SJ, et al. Diaphragmatic motion: fast gradient-recalled-echo MR imaging in healthy subjects. Radiology. 1995;194:879–84. doi: 10.1148/radiology.194.3.7862995. [DOI] [PubMed] [Google Scholar]