Abstract
Objective
To investigate the associations among salivary bacteria, oral emanations of volatile sulfur compounds, and academic-related chronic stress in healthy male subjects.
Materials and methods
Seventy-eight healthy male undergraduate dental students were classified as stressed or not by evaluation of burnout, a syndrome attributed to academic-related chronic stress. This evaluation was carried out using the Maslach Burnout Inventory—Student Survey questionnaire. Oral emanations of hydrogen sulfide, methyl mercaptan, and dimethyl sulfide were measured using an Oral Chroma™ portable gas chromatograph. The amounts in saliva of total bacteria and seven bacteria associated with halitosis were quantified by qPCR. The in vitro production of H2S by S. moorei and/or F. nucleatum was also measured with the Oral Chroma™ instrument.
Results
The stressed students group showed increased oral emanations of hydrogen sulfide and dimethyl sulfide, together with higher salivary Solobacterium moorei levels (p < 0.05, Mann Whitney test). There were moderate positive correlations between the following pairs of variables: Fusobacterium nucleatum and S. moorei; F. nucleatum and hydrogen sulfide; Tannerella forsythia and F. nucleatum; T. forsythia and S. moorei. These correlations only occurred for the stressed group (p < 0.05, Spearman correlation). The in vitro experiment demonstrated that S. moorei increased H2S production by F. nucleatum (p < 0.05, ANOVA and Tukey’s test).
Conclusion
The increased amount of S. moorei in saliva, and its coexistence with F. nucleatum and T. forsythia, seemed to be responsible for increased oral hydrogen sulfide in the healthy male stressed subjects.
Introduction
Halitosis can be a physiological and/or a pathological condition [1]. In both cases, it is characterized by malodorous gases released from the mouth and/or the nose, and can originate in the oral cavity, the respiratory tract, the stomach, systemically, or due to various combinations of these sources [2].
Oral malodor, or “morning breath”, is common on awakening, due to the physiological reduction in salivary flow during sleep and the lack of physiological oral cleansing promoted by the facial muscles, resulting in increased microbial metabolic activity [3]. This condition is transient, disappearing following normal oral hygiene after awakening. However, pathological halitosis is more intense, persistent, and offensive, requiring treatment to eliminate its cause, and can be due to extra- or intra-oral factors [4]. Physiological halitosis can be persistent, but may not be offensive to others, while the pathological condition is offensive and can continue while its source exists [2].
It is known that anaerobic bacteria can degrade sulfur-containing amino acids, producing the volatile sulfur compounds (VSCs) that are the major causative agents of oral malodor [5]. The main VSCs involved are hydrogen sulfide (H2S), methyl mercaptan (CH3SH), and dimethyl sulfide ((CH3)2S) [6].
Gram-negative anaerobic periodontopathogenic bacteria such as Porphyromonas gingivalis, Tannerella forsythia, Treponema denticola, and Fusobacterium nucleatum are some of the most important VSCs producers [7, 8]. Furthermore, it has been reported that bacteria of the Actinomyces and Veillonella genera, especially the commensal bacteria Actinomyces odontolyticus (Gram-positive) and Veillonella dispar (Gram-negative), are associated with malodor formation [9]. Higher levels of Solobacterium moorei, a Gram-positive bacterium, have also been found in the tongue coatings and saliva of patients with halitosis [10–12].
It has been shown that anxiogenic experimental situations [13, 14] and academic examinations [15] can lead to increased oral H2S production in healthy subjects, demonstrating a positive influence of anxiety and psychological stress on oral VSCs emanation. In these cases, the VSCs production may not necessarily be considered halitosis, but when associated with other factors, such as poor oral hygiene or periodontal diseases, increased production of these compounds may cause halitosis.
Although the relationship between psychological stress and VSCs production has been reported [13–15], the mechanisms involved remain unclear. Some studies have demonstrated that stress-related substances can affect the growth of several periodontopathogens [16,17], upregulate the expression of virulence and oxidative stress genes in P. gingivalis [18], and increase H2S and CH3SH production by F. nucleatum [19].
Therefore, we hypothesized that the alteration of saliva composition by academic-related chronic stress could create a favorable environment for bacterial VSCs production, resulting in increases of VSCs in these subjects. The aim of the present study was to evaluate the relationships among the quantity of bacteria in saliva, oral emanation of VSCs, and academic-related chronic stress in healthy male subjects.
Materials and methods
Clinical study
Experimental design
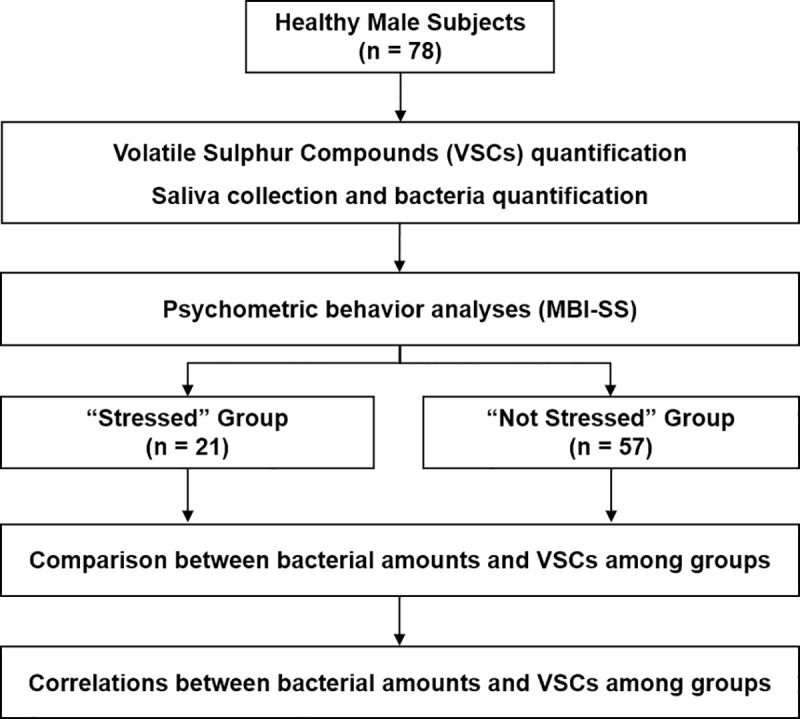
In order to avoid the influence of the menstrual cycle, only male volunteers were invited to participate in the present study. Seventy-eight healthy male subjects provided saliva for quantification of bacterial species producing VSCs. The VSCs (H2S, CH3SH, and (CH3)2S) were also quantified in the oral breath. The volunteers were classified as either “Stressed” (n = 21) or “Not stressed” (n = 57) using psychometric behavior analysis (Maslach Burnout Inventory Student Survey questionnaire; MBI-SS), a validated tool for determination of a state of academic-related chronic stress. Comparisons between oral VSC and salivary bacterial levels, correlations among bacterial species and between bacterial species and oral VSC emanation were made (Fig 1).
Fig 1. Flowchart of experimental design.
Participants
Selection was made of seventy-eight male undergraduate students at Piracicaba Dental School, aged between 18 and 24 years and in good oral and systemic health. Clinical evaluation was made of the tongue coating (measured using an index described elsewhere [19]), caries, third molar eruption, dental plaque and gingival indexes, and probing depth. Exclusion criteria were a tongue coating index equal to or greater than one, plaque and bleeding index values higher than 10%, presence of periodontal pockets, defective restorations, prostheses, caries, third molars in eruption, smoking habit, and systemic disease. These criteria were implemented to ensure that all the volunteers had good oral and systemic health and that chronic academic stress was the distinguishing factor between the groups. This study was approved by the Research Ethics Committee of Piracicaba Dental School, University of Campinas, Piracicaba, Brazil (protocol numbers #108/2007 and #147/2014), and the volunteers provided written informed consents.
Clinical data (collection of saliva and VSCs quantification)
Clinical data were obtained between 7:00 and 8:00 a.m. in the second semester of the year, on three different days (0, 7, and 15) within a 15-day period. These dates were scheduled after stressful events such as seminars, exams, or any kind of academic work that might cause worry in the students concerning their approval in the disciplines. These precautions were taken in order to ensure that the stress conditions were strictly chronic, since acute stressful circumstances (such as biochemistry exams or an anxiogenic experimental situation) have been previously reported as being capable of increasing VSCs production [13, 15]. The averages of the three collections were used, since this short period was not sufficient to observe significant changes in oral mature biofilms in young adults [20].
The subjects were instructed to refrain from using any oral rinse or breath freshener for a week, avoid eating spicy foods or those containing onion or garlic for 24 h, and not to practice their oral hygiene routines, use scented cosmetics or after-shave lotions, or eat and drink (including water) for 8 h before the experiment [13].
The unstimulated saliva collection was carried out as recommended elsewhere [13, 16]. The subjects were instructed to avoid swallowing for 5 min. Subsequently, the total volume of saliva was placed in a plastic tube kept on ice. Immediately after collection, the saliva was homogenized using a vortex mixer and stored in a freezer at -70°C until analysis.
Oral breath samples (1 mL of mouth air) were obtained with disposable plastic syringes, after 1 min of keeping the mouth closed. The VSCs measurements were performed by injecting 0.5 mL of the sample into an Oral Chroma™ (Ability, Osaka, Japan) portable gas chromatograph, which separated and quantified (in ppb) H2S, CH3SH, and (CH3)2S.
Salivary bacteria quantification
Quantitative microbial analyses were performed with saliva samples collected as described above from each volunteer on the three collection days. Quantitative real-time polymerase chain reactions (qPCR) were carried out in order to obtain total bacteria counts and quantify the main oral bacterial species that produced VSCs. A 520 μL volume of saliva was centrifuged at 13,523 g for 10 min at 4°C. The supernatant was discarded and the pellet was used for DNA extraction using a genomic DNA detection kit (PureLinkTM Genomic DNA Mini Kit, Invitrogen, Carlsbad, CA, USA). The protocol was performed according to the manufacturer’s instructions, with minor modification of the lysis time (Proteinase K and PurelinkTM Genomic Lysis/Binding Buffer step), which was changed to 2 h. The final elution was made using 25 μL of elution buffer. Following extraction, the DNA concentration was determined using a small volume PICOPET01 spectrophotometer (Picodrop Ltd., Alpha Biotech Ltd., Killearn, Glasgow, Scotland).
All species-specific primers for A. odontolyticus, T. forsythia, T. denticola, P. gingivalis, and total bacterial counts targeted the 16SrRNA gene [21–25]. Since the Fusobacterium and Veillonella genera have high levels of genotypic similarity among their species, primers for F. nucleatum and V. dispar targeted the rpoB gene, which is a species-specific gene [26, 27]. The S. moorei primer targeting the 16SrRNA gene was designed using the Primer 3 software [28]. All primers were tested for specificity using the NCBI BLAST database [29]. Table 1 shows the sequences of primers used in this study.
Table 1. Species-specific primers used in the real-time PCR.
| Bacteria | Primer sequences (5' → 3') | References | |
|---|---|---|---|
| A. odontolyticus | F | CTTTGGGATAACGCCGGGAAAC | [21] |
| R | CTACCCGTCAAAGCCTTGGT | ||
| F. nucleatum | F | ACCTAAGGGAGAAACAGAACCA | [26] |
| R | CCTGCCTTTAATTCATCTCCAT | ||
| P. gingivalis | F | ACCTTACCCGGGATTGAAATG | [24] |
| R | CAACCATGCAGCACCTACATAGAA | ||
| S. moorei | F | CTCAACCCAATCCAGCCACT | Designed in this study |
| R | TATTGGCTCCCCACGGTTTC | ||
| T. forsythia | F | AGCGATGGTAGCAATACCTGTC | [22] |
| R | TTCGCCGGGTTATCCCTC | ||
| T. denticola | F | CCGAATGTGCTCATTTACATAAAGGT | [23] |
| R | GATACCCATCGTTGCCTTGGT | ||
| Total bacterial counts | F | TGGAGCATGTGGTTTAATTCGA | [25] |
| R | TGCGGGACTTAACCCAACA | ||
| V. dispar | F | AACGCGTTGAAATTCGTCATGAAC | [27] |
| R | GTGTAACAAGGGAGTACGGACC |
The qPCR reaction was carried out in a total volume of 10 μL, containing 5 μL of SYBR® Select Master Mix (Thermo Fisher Scientific, Waltham, USA), 2 μL of DNA template, and 1 μL of primer pair solution (300 nM/reaction). For each run, DEPC-treated water (Thermo Fisher Scientific, Waltham, USA) was used as the negative control and melting peaks were used to determine the speciFIcity of the PCR. Amplification of the extracted DNA template was performed using a real-time PCR system (Step One Plus®, Thermo Fisher Scientific, Waltham, USA), with an initial incubation of 2 min at 50°C and 2 min at 95°C, followed by 40 cycles of 15 s at 95°C and 30 s at 60°C.
The absolute quantification of the target bacteria was performed by comparing the Ct values of the saliva samples with Ct values from a standard curve (102–108 CFU/mL) constructed using pure cultures of the bacterial species studied [30]. Calculations were carried out to obtain the number of bacteria per mL of saliva (cells/mL).
Psychometric behavior analyses (MBI-SS)
Following data collection, the volunteers were classified into two groups: G1 - “Stressed” or G2 - “Not stressed”. Stress was evaluated by quantification of burnout syndrome, a state associated with long-term work-related mental exhaustion, accompanied by diminished interest and disengagement [31], which is directly related to perceived stress [32]. The volunteers were invited to answer the MBI-SS questionnaire (S1 and S2 Figs), which is used to detect burnout syndrome in undergraduate dental students [31, 32]. This syndrome is triggered by academic-related chronic stress and it is characterized by a high degree of emotional exhaustion, cynicism, and low academic efficacy [31].
The questionnaire consists of 15 questions divided into three categories: exhaustion (5 items), cynicism (4 items), and efficacy (6 items). All questions are scored on a 7-point frequency rating scale ranging from 0 (never) to 6 (every day). This evaluation has a three-dimensional burnout concept, with high scores for emotional exhaustion and cynicism and low scores for academic efficacy indicating a high degree of burnout. Classification of the volunteers was performed according to the sum of scores for each category (range 0–90 points). The volunteers were considered as “stressed” when the scores sum was above the third quartile, and as “not stressed” when the scores sum was below the third quartile [31].
Clinical statistical analysis
The sample size was calculated a priori, considering a difference of 30% between the means of the two groups, and a standard deviation of 20% of the mean. In this scenario, a sample size of 12 volunteers in both groups provides 99% of power, considering 5% for alpha. The study included 78 volunteers (21 in a stressed condition and 57 control subjects), representing almost all the male undergraduate students enrolled at the faculty during the study period. The data distribution was tested using the Shapiro-Wilk test. Non-normally distributed data (bacterial quantification and clinical VSCs determination) were analyzed using the Mann-Whitney U test. Spearman correlation coefficients (rS) were calculated for VSCs production and bacterial quantification, with rS values of 0.2–0.4, 0.4–0.8, and >0.8 considered to indicate weak, moderate, and strong correlations, respectively [33]. For bacterial quantification, the data were expressed as percentages representing the proportion of each species relative to the total quantification. The significance level was set at 5%, and all analyses were performed using GraphPad Prism v. 6.0 for Windows statistical software (GraphPad Software Inc., Los Angeles, USA).
In vitro study
Bacteria and culture conditions
S. moorei (DSM 22971) was kept on Bacto™ Tryptic Soy Agar (TSA; Difco, Le Pont de Claix, France) supplemented with sheep blood (7% v/v). F. nucleatum (NCTC 11326) was kept on TSA supplemented with 0.2% Bacto™ Yeast Extract (YE; Difco, Le Pont de Claix, France), 5 μg/mL of hemin (HE; Sigma, Poole, UK), 1 μg/mL of menadione (ME; Sigma, Poole, UK), and 5% (v/v) sheep blood. The cultures were grown under anaerobic conditions (10% CO2, 10% H2, 80% N2) in an anaerobic chamber (MiniMacs Anaerobic Workstation, Don Whitley Scientific, Shipley, UK) at 37°C. For the VSCs assays, S. moorei was cultured in Bacto™ Tryptic Soy Broth (TSB; Difco, Le Pont de Claix, France), while F. nucleatum was cultured in TSB supplemented with yeast extract, hemin, and menadione.
In vitro VSCs assay
An in vitro VSCs assay was carried out to test the hypothesis that S. moorei and F. nucleatum cultures in combination could produce higher amounts of VSCs, compared to the individual cultures. This assay was performed as described previously [7]. Briefly, S. moorei and F. nucleatum were cultured in broth until reaching the logarithmic phase (0.5 < optical density < 1.0) and were then centrifuged at 2876 g for 5 min. The pellets were washed and the bacterial inoculum was prepared in phosphate buffer saline (PBS; pH 7.7) at an optical density of 0.1 at 660 nm.
The following suspensions were kept in 20 mL headspace vials and incubated at 37°C for 1 h: 1) S. moorei—100 μL of S. moorei inoculum + 885 μL of PBS + 15 μL of 33 mM L-cysteine (Sigma, Poole, UK); 2) F. nucleatum—100 μL of F. nucleatum inoculum + 885 μL of PBS + 15 μL of 33 mM L-cysteine; 3) S. moorei + F. nucleatum—100 μL of S. moorei inoculum + 100 μL of F. nucleatum inoculum + 785 μL of PBS + 15 μL of 33 mM L-cysteine.
After incubation, the reaction was stopped by the addition of 500 μL of 3 M phosphoric acid during 10 min. A 1 mL volume of the headspace gas was sampled and the H2S production was measured using the Oral Chroma™ system, as described in Section 2.1.3. Under these experimental conditions, CH3SH and (CH3)2S were not detectable. The experiment was carried out using nine replicates.
In vitro statistical analyses
Group comparisons were performed by ANOVA followed by Tukey's multiple comparisons test. The significance level was set at 5% and the analyses were performed using GraphPad v. 4.0 for Windows statistical software (GraphPad Software Inc., Los Angeles, USA).
Results
The subjects were allocated to the “Stressed” and “Not stressed” groups based on the MBI-SS results (clinical data are available as supporting information–S1 File). The production of H2S, CH3SH, and (CH3)2S by volunteers in the two groups is shown in Fig 2.
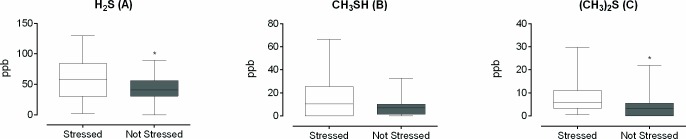
Fig 2.
Box plots of (A) H2S, (B) CH3SH, and (C) (CH3)2S produced by the volunteers in the “Stressed” and “Not stressed” groups. Box plot explanation: upper horizontal line of box, maximum; lower horizontal line of box, minimum; horizontal bar within box, median; upper horizontal bar outside box, 75th percentile; lower horizontal bar outside box, 25th percentile. Mann-Whitney test, * p < 0.05.
The “Stressed” group presented higher levels of H2S (p = 0.03) and (CH3)2S (p = 0.004), compared to the “Not stressed” group. There was no significant difference between the groups for CH3SH production.
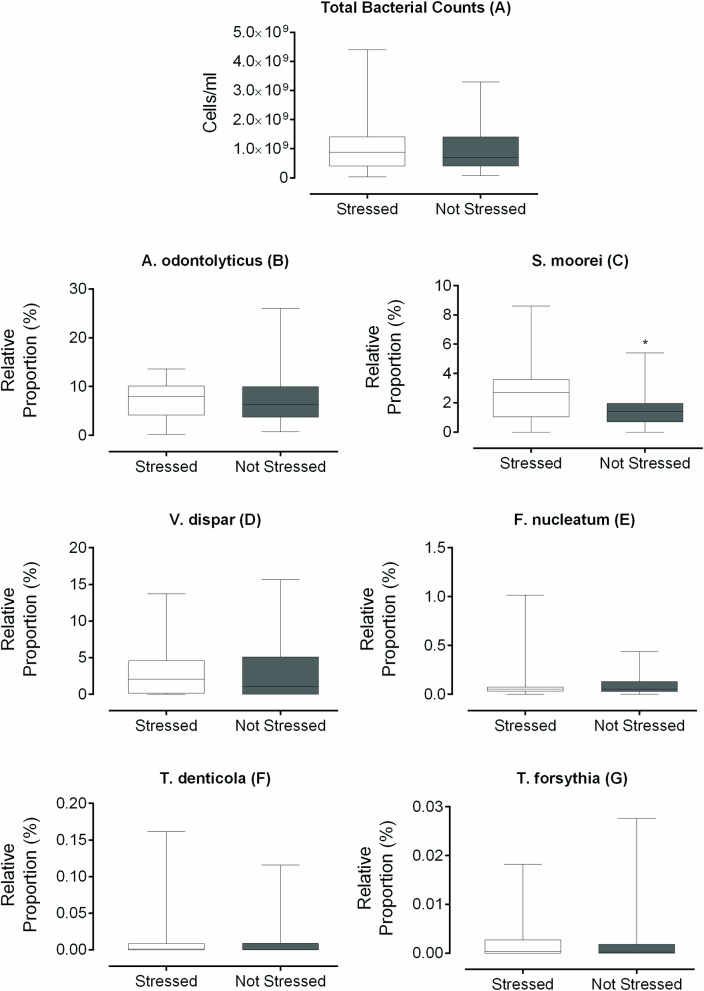
The total bacterial counts and the relative proportions of bacterial species producing VSCs, including A. odontolyticus, F. nucleatum, S. moorei, T. denticola, T. forsythia, and V. dispar, in saliva samples of volunteers from the “Stressed” and “Not stressed” groups, are shown in Fig 3.
Fig 3.
Box plots of (A) total bacterial counts, and the relative proportions (percentage of the total bacteria) of (B) A. odontolyticus, (C) S. moorei, (D) V. dispar, (E) F. nucleatum, (F) T. denticola, and (G) T. forsythia in the saliva of volunteers from the “Stressed” and “Not stressed” groups. Box plot explanation: upper horizontal line of box, maximum; lower horizontal line of box, minimum; horizontal bar within box, median; upper horizontal bar outside box, 75th percentile; lower horizontal bar outside box, 25th percentile. Mann-Whitney test, * p < 0.05.
There was a statistically significant difference between the groups for salivary S. moorei (p = 0.01). For both groups, the saliva samples were negative for P. gingivalis.
Table 2 shows the relationship between the VSCs values and the amounts of bacterial in saliva (Spearman correlation test).
Table 2. Correlation coefficients (rS) between H2S, CH3SH, and (CH3)2S and the relative proportions of A. odontolyticus, F. nucleatum, S. moorei, T. denticola, T. forsythia, and V. dispar in the saliva of subjects from the “Stressed” and “Not stressed” groups.
| Stressed | Not Stressed | |||||
|---|---|---|---|---|---|---|
| H2S | CH3SH | (CH3)2S | H2S | CH3SH | (CH3)2S | |
| A. odontolyticus | -0.18 | 0.00 | 0.40 | 0.15 | 0.15 | 0.05 |
| F. nucleatum | 0.51* | 0.53* | 0.29 | -0.01 | -0.09 | -0.02 |
| S. moorei | 0.13 | 0.51* | 0.42 | 0.25 | 0.25 | 0.16 |
| T. denticola | -0.22 | -0.05 | 0.18 | -0.13 | -0.03 | -0.01 |
| T. forsythia | 0.38 | 0.24 | 0.23 | -0.01 | -0.19 | 0.06 |
| V. dispar | 0.00 | 0.01 | -0.01 | -0.07 | 0.08 | -0.29 |
Spearman´s correlation test,
* p < 0.05.
There were moderate positive correlations between F. nucleatum and H2S (p = 0.006), F. nucleatum and CH3SH (p = 0.01), and S. moorei and CH3SH (p = 0.008), but only in the “Stressed” group (Table 2).
The data obtained (Fig 3 and Table 2) suggested that S. moorei and F. nucleatum could have been responsible for the increased VSCs production in the “Stressed” group (Fig 2). Therefore, a Spearman correlation test was performed in order to determine the relationships between the relative proportions of S. moorei or F. nucleatum and the relative proportions of other bacterial species, for the “Stressed” and “Not stressed” groups. The results are shown in Table 3.
Table 3. Correlation coefficients (rS) between the relative proportions of S. moorei or F. nucleatum and the relative proportions of A. odontolyticus (Ao), T. denticola (Td), T. forsythia (Tf), and V. dispar (Vd), for the “Stressed” and “Not stressed” groups.
| Stressed | Not stressed | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ao | Fn | Td | Tf | Vd | Ao | Fn | Td | Tf | Vd | |
| S. moorei | 0.46 * | 0.59 * | 0.36 | 0.53 * | 0.16 | 0.61 * | 0.08 | 0.18 | 0.09 | 0.35 |
| F. nucleatum | 0.20 | - | 0.09 | 0.55 * | 0.31 | 0.10 | - | 0.11 | 0.25 | -0.17 |
Spearman´s correlation test
* p < 0.01.
There were moderate positive correlations between A. odontolyticus and S. moorei for the subjects of the “Stressed” (p = 0.02) and “Not stressed” (p < 0.001) groups. On the other hand, moderate positive correlations between F. nucleatum and S. moorei (p = 0.02), T. forsythia and S. moorei (p = 0.02), and T. forsythia and F. nucleatum (p = 0.04) were only found for the “Stressed” group.
Based on these clinical findings, an in vitro experiment was carried out to demonstrate that S. moorei and F. nucleatum could interact and produce greater amounts of H2S, compared to production by the individual bacteria. The results are shown in Fig 4.
Fig 4. Means and standard deviations for the in vitro production of H2S (ppb) by S. moorei (Sm), F. nucleatum (Fn) and the H2S production by Sm and Fn when they were grown together (Sm & Fn).
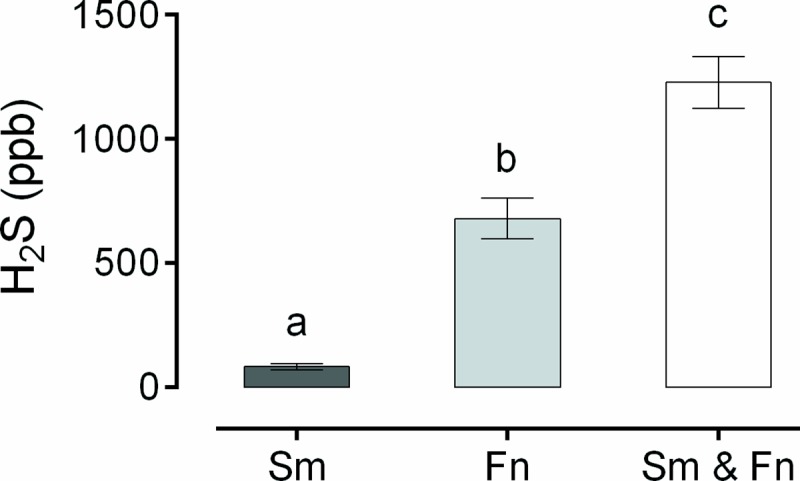
Different letters indicate statistically significant differences between groups (ANOVA and Tukey’s test, p < 0.05).
The H2S production obtained for the co-culture of S. moorei and F. nucleatum was significantly higher than the H2S production values for S. moorei or F. nucleatum grown individually (ANOVA and Tukey’s test, p < 0.0001).
Discussion
Halitosis is characterized by an emanation of malodorous compounds from the oral cavity [1]. The main gases involved are H2S, CH3SH, and (CH3)2S, which are produced by anaerobic microorganisms during the degradation of sulfur-containing proteins [5]. It has been shown that psychological stress and anxiety are able to increase the oral VSCs emanation [13–15], but the mechanisms involved are not still understood.
In the present study, we found increased oral production of H2S and (CH3)2S, together with a higher proportion of salivary S. moorei, in the case of the stressed subjects (Figs 2 and 3). In addition, correlations were observed between oral VSCs and F. nucleatum and S. moorei (Table 2), and among S. moorei, F. nucleatum, and T. forsythia (Table 3). We believe that these data indicate that the increase in H2S production in stressed subjects can be attributed to the coexistence of these bacteria in the oral cavity, especially considering S. moorei and F. nucleatum, since these bacteria produced higher amounts of H2S when cultured together, as demonstrated in vitro (Fig 4).
Oral VSCs emanations can be affected by many factors, such as periodontal diseases [34], presence of tongue coating [35], menstrual cycle and gender [15, 16], systemic diseases [35], and drinking [36] and eating habits [37]. In the present study, the effects of other factors, apart from stress, that could influence VSCs production were controlled, since the volunteers were all fasted non-alcoholic and non-smoker men with good oral and systemic health, as assessed during clinical examination. Therefore, the observed effects on VSCs production could not be explained by negligent oral hygiene.
Moreover, the MBI-SS questionnaires showed that 21 subjects had burnout syndrome. Since burnout syndrome is directly related to the perceived chronic stress [32], we assumed that chronic stress was the distinguishing factor between the groups in this study, with the “Stressed” group presenting higher amounts of H2S in the oral cavity. It is important to note that VSCs levels could be even higher in patients with oral disorders facing stressful conditions.
In previous work by our group, it was found that anxiogenic experimental situations [13, 14] and academic exams [15] resulted in increased VSCs production in healthy undergraduate students. Other studies have also reported that academic stress impaired oral health, with academic exams being implicated in plaque accumulation and gingival inflammation [34, 38].
However, none of these studies explained how these oral alterations occur. Here, we demonstrated that subjects with academic-related chronic stress presented higher proportions of S. moorei in the saliva and greater oral VSCs production. This finding is in agreement with the literature, since this anaerobic bacterium is related to halitosis, due to its higher prevalence in the tongue coating of patients with this disease [10, 39].
Saliva bacterial composition reflects the overall condition of the oral cavity [40], and a previous study also reported significant correlations between salivary S. moorei and VSCs production [12]. Furthermore, our results show that an increased relative proportion of S. moorei in saliva could be associated with greater VSCs production in stressed healthy undergraduate students.
It was reported elsewhere that S. moorei seemed to be a low VSCs producer, compared to other oral microorganisms, and was unable to produce CH3SH [41]. Here, we found a moderate positive correlation between S. moorei and CH3SH in stressed subjects, which suggests that the presence of a stress condition might be important for it. We also found that S. moorei did not produce CH3SH in vitro, even when exposed to different culture conditions (data not shown). Therefore, future studies are necessary to understand the possible correlation of S. moorei with CH3SH.
The relative proportion of F. nucleatum showed no significant difference between the “Stressed” and “Not stressed” groups, but presented moderate positive correlations with H2S and CH3SH levels, only in stressed subjects. These results are in accordance with the literature, since F. nucleatum has a moderate ability to produce H2S, but is one of the leading producers of CH3SH [7, 41]. Therefore, our findings indicate that F. nucleatum may have been responsible for higher levels of these gases in the “Stressed” group, with increased production possibly being due to a direct effect of stress in stimulation of VSCs production by F. nucleatum.
Another mechanism that could explain the increase in VSCs production by F. nucleatum is the ability of S. moorei to interact with other bacteria, including species of Fusobacterium, as suggested by other authors since these two bacteria were found to cause endodontic infection [42], wound infection [43], septic pulmonary embolization [44], and periodontitis [45].
In agreement with this hypothesis, S. moorei was found in higher amounts in the “Stressed” group and presented a moderate positive correlation with the presence of F. nucleatum. Moreover, S. moorei was correlated with increased production of CH3SH in the “Stressed” group, even though it is not able to produce this gas.
We believe that these bacteria may be responsible for increased VSCs production in stressed subjects, as S. moorei and F. nucleatum were correlated with each other and with VSCs production in subjects with stress. Based on these findings, we performed an in vitro experiment showing that the co-culture of S. moorei and F. nucleatum resulted in H2S levels that were 1.6-fold higher than the sum of the H2S production by these bacteria cultured individually.
Previously, it was shown that although Gram-positive microorganisms are weak producers of VSCs, they play an important role in malodor formation, because they are responsible for the first enzymatic step (deglycosylation) required for the subsequent degradation of proteins by Gram-negative species [46]. In light of this information, we suggest that S. moorei and F. nucleatum may interact, participating in different steps of VSCs production, with S. moorei (Gram-positive) being responsible for deglycosylation, and F. nucleatum and T. forsythia (Gram-negative) acting in subsequent protein degradation, resulting in raised VSCs levels in stressed subjects. However, there is little information in the literature concerning biochemical studies of bacterial communities and VSCs production, and further work is required to understand these interactions.
The relationship between psychological stress and the development of infectious diseases has been studied. Recently, the concept of “microbial endocrinology” was introduced, with it being shown that microorganisms can interact with stress-related substances and react by altering their metabolism, virulence, and growth profile [47]. Adrenaline and noradrenaline have been shown to affect the growth of periodontal microorganisms [17]. Moreover, cortisol, noradrenaline, and adrenaline were able to reduce the growth of F. nucleatum and Porphyromonas endodontalis, while increasing the production of H2S by these bacteria [19]. These studies corroborate our findings, showing that VSCs levels could be altered even when bacterial loads are not altered. In the present study, F. nucleatum levels were not altered, but this bacterium showed correlations with the levels of H2S and CH3SH in stressed subjects.
The concentrations of stress-related substances in saliva may be altered under conditions of academic-related chronic stress, creating a favorable environment for the growth of S. moorei and its interaction with other bacteria species, such as F. nucleatum, resulting in an increase in VSCs production in the oral cavity. Another possibility is that stress-related substances might not affect bacterial growth or interaction, but upregulate the genes involved in the production of VSCs, as shown in previous work, where it was found that adrenaline and noradrenaline upregulated the expression of virulence and oxidative stress genes of P. gingivalis [18]. These genes may be related to β-galactosidases (deglycosylating enzymes) [46] or METase enzymes, which are responsible for CH3SH formation [48]. Future studies should be conducted in order to confirm the interactions between stress substances and bacterial VSCs production.
T. forsythia was found in very low relative proportion in both “Stressed” and “Not stressed” groups. However, this species showed moderate positive correlation with S. moorei and F. nucleatum, only in stressed subjects, suggesting that it might be related to them under stress conditions.
T. forsythia has been found in greater proportion in subjects with halitosis [11], but it is rarely found in healthy subjects [49]. To our knowledge, no other study has demonstrated any correlation among S. moorei, F. nucleatum, and T. forsythia, and additional studies are necessary to elucidate these relationships.
S. moorei was also moderately correlated with A. odontolyticus in both “Stressed” and “Not stressed” groups, coexisting with this bacterium in saliva samples of these volunteers. These correlations suggest that these bacteria might interact with each other, while stress was not able to stimulate this phenomenon and did not influence VSCs production.
Volunteers of the “Stressed” group showed increased oral emanation of (CH3)2S. The production of this gas has been reported to be associated with extra-oral halitosis [35], and its presence in the oral cavity could also be associated with its release from blood to saliva. The bacterial species evaluated here do not usually produce detectable levels of (CH3)2S, as reflected by the absence of correlation between the bacteria and (CH3)2S production. However, it is possible that other bacteria could be responsible for its production. Pseudomonas aeruginosa has been identified as a producer of this gas [50], but was not evaluated in the present study, because this bacterium is not associated with halitosis.
Conclusions
Academic-related chronic stress conditions can stimulate the production of H2S in healthy males with no diagnosis of halitosis, by increasing levels of S. moorei in the presence of F. nucleatum. T. forsythia may also participate in this process. The findings indicate that these bacteria are important contributors to increased H2S emanations due to emotional changes in male subjects. The reason for increased (CH3)2S production in such individuals remains unclear.
Supporting information
English version of the Maslach Burnout Inventory Student Survey questionnaire.
(DOC)
Portuguese version of the Maslach Burnout Inventory Student Survey questionnaire.
(DOC)
Clinical parameters obtained in the present study.
(XLSX)
Data Availability
All relevant data are within the paper and Supporting Information.
Funding Statement
Financial support was provided by the São Paulo Research Foundation (FAPESP, grant #2011/50419-2). Bruno Dias Nani acknowledges the scholarship provided by FAPESP (grant #2013/26691-0). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Yaegaki K, Coil JM. Examination, classification, and treatment of halitosis; clinical perspectives. J Can Dent Assoc. 2000;66(5):257–61. [PubMed] [Google Scholar]
- 2.Aydin M, Harvey-Woodworth CN. Halitosis: a new definition and classification. Br Dent J. 2014;217(1):E1 10.1038/sj.bdj.2014.552 [DOI] [PubMed] [Google Scholar]
- 3.Scully C, Greenman J. Halitosis (breath odor). Periodontol 2000. 2008;48:66–75. 10.1111/j.1600-0757.2008.00266.x [DOI] [PubMed] [Google Scholar]
- 4.Harvey-Woodworth CN. Dimethylsulphidemia: the significance of dimethyl sulphide in extra-oral, blood borne halitosis. Br Dent J. 2013;214(7):E20 10.1038/sj.bdj.2013.329 [DOI] [PubMed] [Google Scholar]
- 5.Persson S, Edlund MB, Claesson R, Carlsson J. The formation of hydrogen sulfide and methyl mercaptan by oral bacteria. Oral Microbiol Immunol. 1990;5(4):195–201. [DOI] [PubMed] [Google Scholar]
- 6.Van den Velde S, van Steenberghe D, Van Hee P, Quirynen M. Detection of odorous compounds in breath. J Dent Res. 2009;88(3):285–9. 10.1177/0022034508329741 [DOI] [PubMed] [Google Scholar]
- 7.Salako NO, Philip L. Comparison of the use of the Halimeter and the Oral Chroma™ in the assessment of the ability of common cultivable oral anaerobic bacteria to produce malodorous volatile sulfur compounds from cysteine and methionine. Med Princ Pract. 2011;20(1):75–9. 10.1159/000319760 [DOI] [PubMed] [Google Scholar]
- 8.Kurata H, Awano S, Yoshida A, Ansai T, Takehara T. The prevalence of periodontopathogenic bacteria in saliva is linked to periodontal health status and oral malodour. J Med Microbiol. 2008;57(Pt 5):636–42. 10.1099/jmm.0.47706-0 [DOI] [PubMed] [Google Scholar]
- 9.Washio J, Sato T, Koseki T, Takahashi N. Hydrogen sulfide-producing bacteria in tongue biofilm and their relationship with oral malodour. J Med Microbiol. 2005;54(Pt 9):889–95. 10.1099/jmm.0.46118-0 [DOI] [PubMed] [Google Scholar]
- 10.Haraszthy VI, Gerber D, Clark B, Moses P, Parker C, Sreenivasan PK, et al. Characterization and prevalence of Solobacterium moorei associated with oral halitosis. J Breath Res. 2008;2(1):017002. [DOI] [PubMed] [Google Scholar]
- 11.Kazor CE, Mitchell PM, Lee AM, Stokes LN, Loesche WJ, Dewhirst FE, et al. Diversity of Bacterial Populations on the Tongue Dorsa of Patients with Halitosis and Healthy Patients. Journal of clinical microbiology. 2003;41(2):558–63. 10.1128/JCM.41.2.558-563.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vancauwenberghe F, Dadamio J, Laleman I, Van Tornout M, Teughels W, Coucke W, et al. The role of Solobacterium moorei in oral malodour. J Breath Res. 2013;7(4):046006 10.1088/1752-7155/7/4/046006 [DOI] [PubMed] [Google Scholar]
- 13.Calil CM, Marcondes FK. Influence of anxiety on the production of oral volatile sulfur compounds. Life Sci. 2006;79(7):660–4. 10.1016/j.lfs.2006.02.010 [DOI] [PubMed] [Google Scholar]
- 14.Lima PO, Calil CM, Marcondes FK. Influence of gender and stress on the volatile sulfur compounds and stress biomarkers production. Oral Dis. 2013;19(4):366–73. 10.1111/odi.12011 [DOI] [PubMed] [Google Scholar]
- 15.Queiroz CS, Hayacibara MF, Tabchoury CP, Marcondes FK, Cury JA. Relationship between stressful situations, salivary flow rate and oral volatile sulfur-containing compounds. Eur J Oral Sci. 2002;110(5):337–40. [DOI] [PubMed] [Google Scholar]
- 16.Calil CM, Lima PO, Bernardes CF, Groppo FC, Bado F, Marcondes FK. Influence of gender and menstrual cycle on volatile sulphur compounds production. Archives of oral biology. 2008;53(12):1107–12. Epub 2008/08/12. 10.1016/j.archoralbio.2008.06.008 [DOI] [PubMed] [Google Scholar]
- 17.Roberts A, Matthews JB, Socransky SS, Freestone PP, Williams PH, Chapple IL. Stress and the periodontal diseases: effects of catecholamines on the growth of periodontal bacteria in vitro. Oral Microbiol Immunol. 2002;17(5):296–303. [DOI] [PubMed] [Google Scholar]
- 18.Graziano TS, Closs P, Poppi T, Franco GC, Cortelli JR, Groppo FC, et al. Catecholamines promote the expression of virulence and oxidative stress genes in Porphyromonas gingivalis. J Periodontal Res. 2013. [DOI] [PubMed] [Google Scholar]
- 19.Calil CM, Oliveira GM, Cogo K, Pereira AC, Marcondes FK, Groppo FC. Effects of stress hormones on the production of volatile sulfur compounds by periodontopathogenic bacteria. Braz Oral Res. 2014;28. [DOI] [PubMed] [Google Scholar]
- 20.Jakubovics NS. Intermicrobial Interactions as a Driver for Community Composition and Stratification of Oral Biofilms. J Mol Biol. 2015;427(23):3662–75. 10.1016/j.jmb.2015.09.022 [DOI] [PubMed] [Google Scholar]
- 21.Yang R, Zou J, Li JY. [Study of the relationship between oral Actinomyces and childhood caries]. Hua Xi Kou Qiang Yi Xue Za Zhi. 2007;25(6):568–70. [PubMed] [Google Scholar]
- 22.Kuboniwa M, Amano A, Kimura KR, Sekine S, Kato S, Yamamoto Y, et al. Quantitative detection of periodontal pathogens using real-time polymerase chain reaction with TaqMan probes. Oral Microbiol Immunol. 2004;19(3):168–76. 10.1111/j.0902-0055.2004.00135.x [DOI] [PubMed] [Google Scholar]
- 23.Suzuki N, Yoshida A, Nakano Y. Quantitative analysis of multi-species oral biofilms by TaqMan Real-Time PCR. Clin Med Res. 2005;3(3):176–85. PubMed Central PMCID: PMCPMC1237159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saito T, Inagaki S, Sakurai K, Okuda K, Ishihara K. Exposure of P. gingivalis to noradrenaline reduces bacterial growth and elevates ArgX protease activity. Arch Oral Biol. 2011;56(3):244–50. 10.1016/j.archoralbio.2010.09.014 [DOI] [PubMed] [Google Scholar]
- 25.Yang S, Lin S, Kelen GD, Quinn TC, Dick JD, Gaydos CA, et al. Quantitative multiprobe PCR assay for simultaneous detection and identification to species level of bacterial pathogens. J Clin Microbiol. 2002;40(9):3449–54. PubMed Central PMCID: PMCPMC130696. 10.1128/JCM.40.9.3449-3454.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park SN, Lim YK, Kook JK. Development of quantitative real-time PCR primers for detecting 42 oral bacterial species. Arch Microbiol. 2013;195(7):473–82. 10.1007/s00203-013-0896-4 [DOI] [PubMed] [Google Scholar]
- 27.Igarashi E, Kamaguchi A, Fujita M, Miyakawa H, Nakazawa F. Identification of oral species of the genus Veillonella by polymerase chain reaction. Oral Microbiol Immunol. 2009;24(4):310–3. 10.1111/j.1399-302X.2009.00513.x [DOI] [PubMed] [Google Scholar]
- 28.Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, et al. Primer3—new capabilities and interfaces. Nucleic Acids Res. 2012;40(15):e115 PubMed Central PMCID: PMCPMC3424584. 10.1093/nar/gks596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ye J, McGinnis S, Madden TL. BLAST: improvements for better sequence analysis. Nucleic Acids Res. 2006;34(Web Server issue):W6–9. PubMed Central PMCID: PMCPMC1538791. 10.1093/nar/gkl164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Casarin RC, Ribeiro EeP, Mariano FS, Nociti FH, Casati MZ, Gonçalves RB. Levels of Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, inflammatory cytokines and species-specific immunoglobulin G in generalized aggressive and chronic periodontitis. J Periodontal Res. 2010;45(5):635–42. 10.1111/j.1600-0765.2010.01278.x [DOI] [PubMed] [Google Scholar]
- 31.Schaufeli W, Martínez I, Pinto A, Salanova M, Bakker A. Burnout and engagement in university students. J Cross Cult Psychol. 2015;33(5):464–81. Epub September 2002. [Google Scholar]
- 32.Mafla AC, Villa-Torres L, Polychronopoulou A, Polanco H, Moreno-Juvinao V, Parra-Galvis D, et al. Burnout prevalence and correlates amongst Colombian dental students: the STRESSCODE study. Eur J Dent Educ. 2015;19(4):242–50. 10.1111/eje.12128 [DOI] [PubMed] [Google Scholar]
- 33.Zou KH, Tuncali K, Silverman SG. Correlation and simple linear regression. Radiology. 2003;227(3):617–22. 10.1148/radiol.2273011499 [DOI] [PubMed] [Google Scholar]
- 34.Johannsen A, Bjurshammar N, Gustafsson A. The influence of academic stress on gingival inflammation. Int J Dent Hyg. 2010;8(1):22–7. 10.1111/j.1601-5037.2009.00397.x [DOI] [PubMed] [Google Scholar]
- 35.Tangerman A, Winkel EG. Intra- and extra-oral halitosis: finding of a new form of extra-oral blood-borne halitosis caused by dimethyl sulphide. J Clin Periodontol. 2007;34(9):748–55. 10.1111/j.1600-051X.2007.01116.x [DOI] [PubMed] [Google Scholar]
- 36.Rosenberg M, Knaan T, Cohen D. Association among bad breath, body mass index, and alcohol intake. J Dent Res. 2007;86(10):997–1000. 10.1177/154405910708601015 [DOI] [PubMed] [Google Scholar]
- 37.Suarez F, Springfield J, Furne J, Levitt M. Differentiation of mouth versus gut as site of origin of odoriferous breath gases after garlic ingestion. Am J Physiol. 1999;276(2 Pt 1):G425–30. [DOI] [PubMed] [Google Scholar]
- 38.Deinzer R, Granrath N, Spahl M, Linz S, Waschul B, Herforth A. Stress, oral health behaviour and clinical outcome. Br J Health Psychol. 2005;10(Pt 2):269–83. 10.1348/135910705X26858 [DOI] [PubMed] [Google Scholar]
- 39.Haraszthy VI, Zambon JJ, Sreenivasan PK, Zambon MM, Gerber D, Rego R, et al. Identification of oral bacterial species associated with halitosis. J Am Dent Assoc. 2007;138(8):1113–20. [DOI] [PubMed] [Google Scholar]
- 40.Mager DL, Haffajee AD, Socransky SS. Effects of periodontitis and smoking on the microbiota of oral mucous membranes and saliva in systemically healthy subjects. J Clin Periodontol. 2003;30(12):1031–7. 10.1046/j.0303-6979.2003.00418.x [DOI] [PubMed] [Google Scholar]
- 41.Stephen AS, Naughton DP, Pizzey RL, Bradshaw DJ, Burnett GR. In vitro growth characteristics and volatile sulfur compound production of Solobacterium moorei. Anaerobe. 2014;26:53–7. 10.1016/j.anaerobe.2014.01.007 [DOI] [PubMed] [Google Scholar]
- 42.Schirrmeister JF, Liebenow AL, Pelz K, Wittmer A, Serr A, Hellwig E, et al. New bacterial compositions in root-filled teeth with periradicular lesions. J Endod. 2009;35(2):169–74. 10.1016/j.joen.2008.10.024 [DOI] [PubMed] [Google Scholar]
- 43.Zheng G, Summanen PH, Talan D, Bennion R, Rowlinson MC, Finegold SM. Phenotypic and molecular characterization of Solobacterium moorei isolates from patients with wound infection. J Clin Microbiol. 2010;48(3):873–6. PubMed Central PMCID: PMCPMC2832436. 10.1128/JCM.01381-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin CA, Wijesurendra RS, Borland CD, Karas JA. Femoral vein thrombophlebitis and septic pulmonary embolism due to a mixed anaerobic infection including Solobacterium moorei: a case report. J Med Case Rep. 2007;1:40 PubMed Central PMCID: PMCPMC1929108. 10.1186/1752-1947-1-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong BK, McGregor NR, Butt HL, Knight R, Liu LY, Darby IB. Association of clinical parameters with periodontal bacterial haemolytic activity. J Clin Periodontol. 2016;43(6):503–11. 10.1111/jcpe.12554 [DOI] [PubMed] [Google Scholar]
- 46.Sterer N, Rosenberg M. Streptococcus salivarius promotes mucin putrefaction and malodor production by Porphyromonas gingivalis. J Dent Res. 2006;85(10):910–4. 10.1177/154405910608501007 [DOI] [PubMed] [Google Scholar]
- 47.Freestone PP, Sandrini SM, Haigh RD, Lyte M. Microbial endocrinology: how stress influences susceptibility to infection. Trends Microbiol. 2008;16(2):55–64. 10.1016/j.tim.2007.11.005 [DOI] [PubMed] [Google Scholar]
- 48.Nakano Y, Yoshimura M, Koga T. Methyl mercaptan production by periodontal bacteria. Int Dent J. 2002;52 Suppl 3:217–20. [DOI] [PubMed] [Google Scholar]
- 49.Tanaka M, Yamamoto Y, Kuboniwa M, Nonaka A, Nishida N, Maeda K, et al. Contribution of periodontal pathogens on tongue dorsa analyzed with real-time PCR to oral malodor. Microbes Infect. 2004;6(12):1078–83. 10.1016/j.micinf.2004.05.021 [DOI] [PubMed] [Google Scholar]
- 50.Zscheppank C, Wiegand HL, Lenzen C, Wingender J, Telgheder U. Investigation of volatile metabolites during growth of Escherichia coli and Pseudomonas aeruginosa by needle trap-GC-MS. Anal Bioanal Chem. 2014;406(26):6617–28. 10.1007/s00216-014-8111-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
English version of the Maslach Burnout Inventory Student Survey questionnaire.
(DOC)
Portuguese version of the Maslach Burnout Inventory Student Survey questionnaire.
(DOC)
Clinical parameters obtained in the present study.
(XLSX)
Data Availability Statement
All relevant data are within the paper and Supporting Information.