Abstract
Lignocellulosic biomass can be a significant source of renewable clean energy with continued improvement in biomass yield and bioconversion strategies. In higher plants, the leaf blade is the central energy convertor where solar energy and CO2 are assimilated to make the building blocks for biomass production. Here we report that introducing the leaf blade development regulator STENOFOLIA (STF), a WOX family transcription factor, into the biofuel crop switchgrass, significantly improves both biomass yield and sugar release. We found that STF overexpressing switchgrass plants produced approximately 2-fold more dry biomass and release approximately 1.8-fold more solubilized sugars without pretreatment compared to controls. The biomass increase was attributed mainly to increased leaf width and stem thickness, which was also consistent in STF transgenic rice and Brachypodium, and appeared to be caused by enhanced cell proliferation. STF directly binds to multiple regions in the promoters of some cytokinin oxidase/dehydrogenase (CKX) genes and represses their expression in all three transgenic grasses. This repression was accompanied by a significant increase in active cytokinin content in transgenic rice leaves, suggesting that the increase in biomass productivity and sugar release could at least in part be associated with improved cytokinin levels caused by repression of cytokinin degrading enzymes. Our study provides a new tool for improving biomass feedstock yield in bioenergy crops, and uncovers a novel mechanistic insight in the function of STF, which may also apply to other repressive WOX genes that are master regulators of several key plant developmental programs.
Author summary
The leaf blade of higher plants serves as a solar panel in which it captures solar energy and carbon dioxide to produce chemical energy in the process of photosynthesis. Thus, the ultimate source of food and feed for most heterotrophic organisms, including humans, comes from the photosynthetic activity of leaves. Plant biomass also promises to be a significant source of transportation fuels which could alleviate some of the stigma of environmental pollution, scarcity and finite resources associated with gasoline. Wider leaf blade may be expected to increase biomass yield and overall plant growth due to larger photosynthetic surface area. Here we introduced a leaf blade outgrowth regulatory factor, STF, from eudicot species into three grasses, switchgrass, Brachypodium and rice, and found that the transgenic plants formed much wider leaves compared to controls. Consequently, transgenic switchgrass plants produced approximately two-fold more total biomass and solubilized sugars without acid pretreatment, demonstrating a novel approach for improving biomass feedstock properties. We also show that the transgenic rice seedlings accumulate the phytohormone cytokinin at higher levels, uncovering a novel mechanism that links STF activity to cytokinin homeostasis. Our work will significantly advance understanding of the mechanistic function of WOX genes in plant development.
Introduction
Plant biomass is an abundant source of renewable energy and biomaterials, and sustainable lignocellulosic fuel ethanol production from biomass feedstocks has a great potential to be exploited as an alternative energy source to meet increasing energy demands worldwide [1]. The United States, for example, has projected to meet approximately 30% of its energy demands by 2030 from such renewable sources [2]. However, apart from the logistics of biomass transportation and processing, significant challenges still persist in biomass feedstock yield and saccharification efficiency. Plant cell wall, the most abundant plant biomass, is composed of cellulose and hemicellulose matrix polysaccharides copolymerized with a phenolic polymer lignin forming a complex crosslink [3–5]. This makes the polysaccharides recalcitrant to enzymatic digestion to soluble sugars (saccharification) for microbial conversion to biofuels [6]. Current biomass conversion technologies utilize acid pretreatment at high temperatures to break apart the lignin polymer and expose the polysaccharides. Such a pretreatment, in addition to cost and environmental pollution, negatively impacts downstream microbial fermentation, reducing the market competitiveness of biofuels. Accordingly, enhancing biomass yield and saccharification efficiency has become a major research focus for the genetic improvement of bioenergy crops. Switchgrass is one of the dedicated bioenergy crops in the USA [7] and research has been intensified in the last few years to increase yield and reduce lignin content in an attempt to improve its feedstock properties [8–10].
The leaf blade is the energy powerhouse of plants where solar energy and CO2 are assimilated to produce the chemical energy used in food, feed and biofuels. Since the leaf blade essentially serves as a solar panel in capturing sunlight, its size and design should have a significant bearing on biomass productivity through increasing photosynthetic efficiency [11–13]. Redesigning the leaf blade is, therefore, potentially a major target for improving biomass feedstock yield. Blade outgrowth is regulated by several antagonistically acting polarity factors that are exclusively expressed either on the upper (adaxial) or lower (abaxial) side of the leaf at least in eudicots. These factors include AS1, AS2, HD ZIP Ⅲ genes and tasiR-ARFs on the adaxial side and KAN, FIL, YAB, miRNA165/6 and ARF3/4 on the abaxial side in Arabidopsis and are required for polarity specification and cell differentiation in their respective domains [14–18]. Extensive studies in Arabidopsis over the past two decades revealed that the combined action of polarity factors and multiple phytohormones is required for the establishment and growth of a determinate bilaterally symmetrical leaf blade from undifferentiated pluripotent cells of the shoot apical meristem (SAM). The leaf marginal meristem (blastozone) has long been recognized as the site of cell proliferation for lateral expansion of the leaf blade after recruitment of leaf founder cells from the SAM and establishment of the leaf primordium [19–21]. However, leaf growth in the proximal-distal (length) direction appears to be to some extent independent from growth in the medial-lateral (width) direction as demonstrated by several genetic mutants affected only in leaf width [22] including the bladeless lam1 mutant of Nicotiana sylvestris and the stenofolia (stf) mutant of Medicago truncatula.
In monocots, Wavy auricle in blade 1 (Wab1) and Liguleless narrow-R (Lgn-R) mutants in maize [23–25] and narrow leaf 1 (nal1) in rice [26] display narrow leaf blades but defects in these mutants appear to include proximal-distal growth as well. On the other hand, the maize, narrowsheath1 (ns1) and narrowsheath2 (ns2) double mutant has a very narrow leaf blade affected in medial-lateral growth [27] without significant defect in leaf length. ns1 and ns2 are duplicate WUSCHEL-related homeobox (WOX) transcription factors homologous to Arabidopsis WOX3/PRS [28]. Mutations in homologous genes, nal2 and nal3 double, also cause narrow leaves in rice [29, 30]. The nal2/3 double mutant displays a pleiotropic phenotype including narrow-curly leaves, more tillers, fewer lateral roots, open spikelets and narrow-thin grains [30], indicating a widespread effect on overall plant development. Auxin transport related genes are found to be altered in expression in the nal2/3 double mutant [30], and the OsWOX3A protein, encoded by NAL2/3, is shown to be involved in negative feedback regulation of GA biosynthesis [31]. Transcriptome analysis in a laser dissected ns1/2 mutant shoot apex in maize also identified changes in hormonal signaling pathways including auxin and jasmonate [32]. However, the actual molecular mechanisms for the function of these WOX genes in blade lateral outgrowth remains unclear.
We cloned the stf and lam1 mutants previously and shown that they are caused by mutations in the same gene that encodes for a putative WUSCHEL-related homeobox (WOX) transcription factor [33] similar to petunia MAW and Arabidopsis WOX1 [34]. STF is expressed at the adaxial-abaxial juxtaposition of the growing leaf primordium that includes the leaf margins and the middle mesophyll, and critically regulates blade outgrowth by activating cell proliferation [33], which was confirmed by Arabidopsis WOX1 [35], suggesting a WUS like function in leaf margins. STF promotes cell proliferation through a transcriptional repression activity [36, 37] that involves the corepressor TOPLESS (MtTPL) [38]. Transcriptional repression activity is a requirement for STF’s blade outgrowth function, and multiple phytohormones including auxin and cytokinin have been proposed to be involved in STF function [33, 39], but the connection between transcriptional repression and activity of hormones has not been firmly established.
Here we report that ectopic expression of STF in three monocot species, switchgrass, Brachypodium and rice leads to improvement in biomass yield. We show that STF directly binds to several regions in the promoters of cytokinin oxidase/dehydrogenase genes and represses their transcription allowing accumulation of active cytokinin pools, highlighting a novel mechanism for WOX-mediated cell proliferation via transcriptional repression.
Results
The Medicago WOX gene STF is a master regulator of plant growth and development required for leaf blade outgrowth, leaf vascular patterning, stem thickness, inflorescence fusion, petal expansion, ovule development and female fertility [33]. Although STF-like sequencers are conserved in eudicots and the early diverging angiosperm Amborella trichopoda [40], obvious STF homologues have not been identified in monocotyledonous plants [33, 38]. We wondered whether STF could be used to modify leaf size and thereby increase vegetative biomass in grasses. To test the hypothesis that the STF gene could be used to manipulate leaf size and improve biomass yield in grasses, we introduced the full-length STF CDS into the bioenergy crop switchgrass (Panicum virgatum L.) as well as two grass models Brachypodium and rice under the control of the maize UBIQUITIN (UBI) promoter by using Agrobacterium-mediated transformation. Meanwhile, we generated UBI::GUS and UBI::GFP transformants as controls. We observed that STF overexpressing transgenic lines in Brachypodium, rice and switchgrass showed significant morphological changes in leaf blade expansion compared with control plants (Fig 1). Each of the STF transformants showed wider leaf blade and thicker stem than controls but also displayed plant height phenotypes depending on STF expression levels. In general, increasing STF expression levels were correlated with increasing phenotypic severity. While modest level of STF expression promoted leaf expansion and stem thickness in all the three species, high level of expression drastically reduced plant height, and caused leaf curling and scattered deformation on the leaf vasculature although the blade still remained wider than controls (Fig 1 and S1 and S2 Figs).
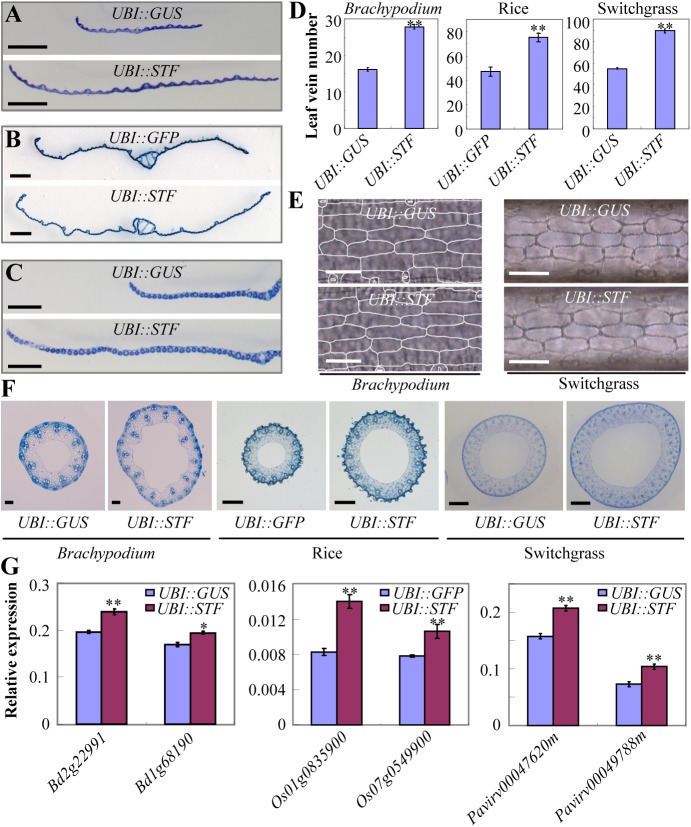
Fig 1. STF overexpression universally increases leaf blade width in grasses.

(A) Phenotypes of STF overexpressing transgenic Brachypodium and GUS expressing control. The right panel shows a close-up of control and STF transgenic leaves from the same position. Bars = 5 cm for plants and 1 cm for leaves. (B) Phenotypes of STF overexpressing rice and GFP transgenic rice used as control with a close-up of equivalent leaves shown on the right. Bars = 10 cm for plants and 1 cm for leaves. (C) Phenotype of STF overexpressing switchgrass and GUS expressing control, the right panel showing close-up of leaves from the same positions. Bars = 10 cm for plants and 1 cm for leaves. All the three STF transgenic grasses showed significantly broader leaves than their respective controls.
In agreement with the morphological changes observed above, histological analysis showed that the cell number as well as the number of vascular bundles were significantly increased in STF overexpressing leaves and culms in all the three monocot plants (Fig 2A–2D and 2F). Cross section through the leaf blade or stem indicated that the number of veins was significantly increased (p < 0.01) in the leaf (Fig 2A–2D) and stems were significantly thicker (Fig 2F) in all the transgenic lines compared with their respective controls. Control plants were transformed with UBI::GUS in Brachypodium and switchgrass, and with UBI::GFP in rice using the same vector pMDC32. Measurement of leaf width in transgenic switchgrass was consistent with the number of veins in quantifying blade lateral expansion (S1 Table). However, examination of leaf epidermal cells in UBI::STF transformants in Brachypodium and switchgrass showed that the cell size was not obviously changed (Fig 2E), suggesting that the wider leaf blade and thicker stem phenotypes were mainly caused by enhanced cell proliferation. Consistent with this, quantitative real time PCR (qRT-PCR) analysis showed that the transcript level of the cell division marker Histone H4 was significantly increased in STF overexpression lines in all the three species (Fig 2G). These results together indicate that ectopic expression of STF in switchgrass, rice and Brachypodium leads to significant increase in plant size primarily through promoting cell proliferation.
Fig 2. Ectopic expression of STF in grasses leads to significant increase in plant size primarily through promoting cell proliferation.
(A-C) Cross section of flag leaves of the control and STF overexpressing Brachypodium (A), rice (B) and switchgrass (C). Bars = 1 mm. (D) Comparison of leaf vein number of the control and STF transgenic Brachypodium, rice and switchgrass. Bars represent means ± SE (n = 7 plants). (E) Micrographs of cleared leaves of STF transgenic Brachypodium and switchgrass. Bars = 100 μm. (F) Cross section of internode Ⅱ (for rice and Brachpodium) and internode Ⅲ (for switchgrass) of STF transgenic Brachypodium, rice and switchgrass. Bars = 100 μm in Brachypodium, 1mm in rice and switchgrass. (G) Transcript level of Histone H4 in STF overexpressing Brachypodium, rice and switchgrass compared to controls. Bd, Brachypodium distachyon; Os, Oryza. Sativa; Pavirv, Panicum virgatum. Bars represent means ± SE of three technical replicates, two biological replicates. The asterisks indicate significant differences (* p<0.05, ** p < 0.01, Student t-test).
To evaluate the impact of STF on biomass production, we measured agricultural traits in the bioenergy crop switchgrass including leaf blade length and width, plant height, internode diameter, tiller number and flowering time (S1 Table), which divided the transgenic switchgrass lines into three categories: Group Ⅰ, Group Ⅱ and Group Ⅲ. Representative lines for each group were shown in Fig 3A. Among these, the transgenic lines with high STF transcript levels (Group Ⅲ) displayed severe morphological alteration including twisted and curled leaf blade, reduced internode and plant height and delayed flowering. The low and moderate STF expressing transgenic lines, Group Ⅰ and Group Ⅱ, respectively, on the other hand, exhibited normal or even slightly enhanced plant height resulting in an overall improved plant stature (Fig 3B). STF transgenic rice and Brachypodium lines also showed a similar dosage-dependent effect on overall growth and development (S1 and S2 Figs).
Fig 3. STF overexpression in switchgrass improves biomass yield and release of solubilized sugars.
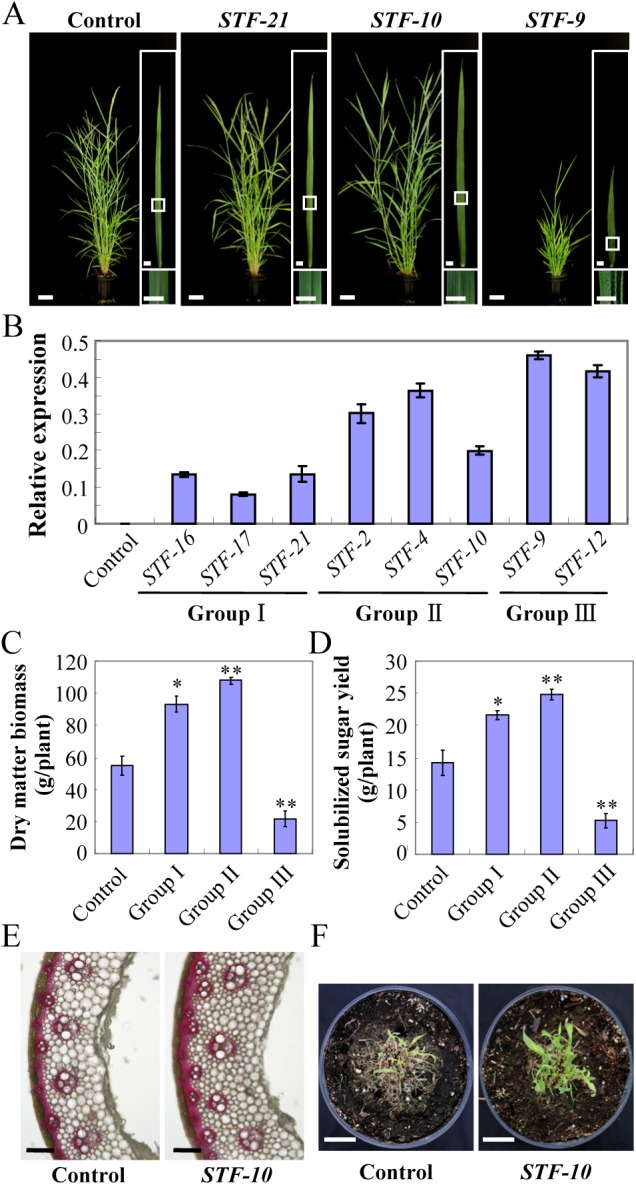
(A) Morphology of switchgrass plants overexpressing different levels of STF at flowering. One representative from each group was shown. Bars = 10 cm for plants, 1cm for leaves. (B) Transcript abundance of STF in transgenic plants revealed by qRT-PCR. UBI::GUS-1 switchgrass plant was used as the control. Bars represent means ± SE of three technical replicates. (C) Comparison of postharvest dry weights of total above-ground biomass of three control (UBI::GUS plants) and three classes of STF overexpressors shown in (B) at maturity. Bars represent means ± SE (n = 3 independent plants for Control, Group Ⅰ, Ⅱ, and 2 plants for group Ⅲ), the asterisks indicate significant differences (*p<0.05, **p < 0.01, Student t-test). (D) Solubilized sugar yield of transgenic switchgrass plants compared to control (UBI::GUS plants) shown in (B). Bars represent means ± SE (n = 3 plants for Control, Group Ⅰ and Ⅱ, 2 plants for group Ⅲ), the asterisks indicate significant differences (*p<0.05, **p < 0.01, Student t-test). (E) Phloroglucinol-HCl staining of lignin in the internode Ⅲ of the control (UBI::GUS-1) and Group Ⅱ STF-10. Bars = 100 μm. (F) Recovery and growth establishment after shoot harvest. The STF transgenic switchgrass (Group Ⅱ STF-10) displays better recovery after cut back compared to the control (UBI::GUS-1). Plants shown were 2 weeks old after cutting. Bars = 5 cm.
To quantitatively determine these improvements, we evaluated total above ground dry biomass yield after maturity. We evaluated three independent transgenic lines in Group Ⅰ (STF-16, 17, and 21) and Group Ⅱ (STF-2, 4, and 10), and two independent lines in Group Ⅲ (STF-9 and 12) using three independent UBI::GUS transformed controls. The average dry weight of Group Ⅰ and Group Ⅱ transgenic switchgrass had a 1.68 and 1.95 fold increase, respectively, in total biomass compared with the controls, whereas strong STF expression in the group Ⅲ transgenic switchgrass led to reduced biomass production lower than controls due to stunted growth (Fig 3C). Further enzymatic hydrolysis of the dry biomass without pretreatment indicated that, excepting the Group Ⅲ transgenic lines, the total amount of solubilized sugar yield of Group Ⅰ and Group Ⅱ had increased by over 1.53 and 1.75-fold per plant, respectively (Fig 3D). This is a significant improvement in the feedstock properties of switchgrass for cellulosic ethanol production because more solubilized sugar implies more ethanol without pretreatment.
Lignin negatively impacts biomass recalcitrance and reduces bioconversion to ethanol. Composition analysis of dried STF transgenic switchgrass was carried out to further assess lignin content and its amenability to biofuel production. Measurement of acid detergent lignin content (ADL) and Phloroglucinol-HCl staining showed no obvious difference in lignin deposition pattern or ADL content in Group Ⅰ and Group Ⅱ transgenic switchgrass compared with the controls, whereas the lignin content in Group Ⅲ transgenic plants was slightly decreased (Fig 3E and S2 Table). Cellulose and hemicellulose analysis also indicated no difference in Group Ⅰ and Group Ⅱ, but slightly decreased in Group Ⅲ (S2 Table). These biochemical analyses indicated that overexpression of STF could significantly increase biomass yield and sugar release without altering relative cell wall components, demonstrating a great potential of STF to developing superior switchgrass varieties for biofuel production. Moreover, we observed that the Group Ⅰ and Group Ⅱ transgenic plants displayed better regenerative capacity in forming new leaves after harvest cutting (Fig 3F), suggesting that weak and moderate level of STF expression is conducive to facilitated switchgrass growth even at early developmental stages.
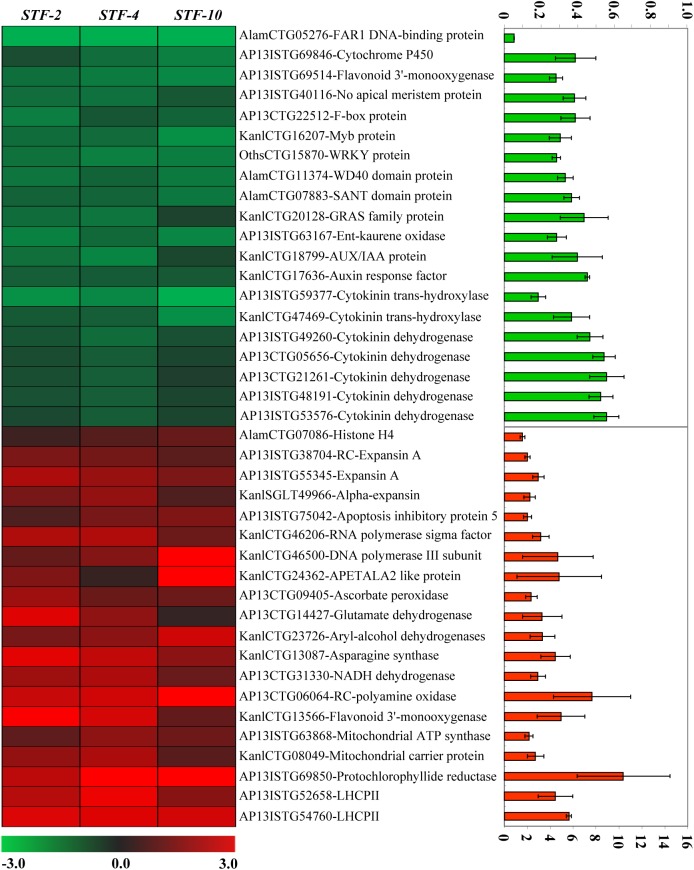
To gain insight into the mechanistic effects of STF on cell proliferation and overall plant development, we performed transcript profiling analysis using the switchgrass Affymetrix GeneChip and compared gene expression between three independent STF overexpressing Group Ⅱ plants and three independent controls transformed with GUS. Profiling analysis was performed in 6–8 cm newly generated tillers approximately 3 weeks after cutting. A total of 886 probes were significantly altered with a 2-fold or more difference compared to controls, in which 665 probes were downregulated (S3 Table) and 221 probes were upregulated (S4 Table), consistent with the primarily transcriptional repression function of STF in its native host. Gene Ontology assignments indicated that a wide range of functional groups were represented in both the upregulated and downregulated genes (S3 Fig). In agreement with the histological observation, this analysis identified genes that are known to be involved in cell proliferation such as Histone H4 and Expansin as upregulated in STF transgenic plants (Fig 4), which were confirmed by qRT-PCR (S4 Fig). The microarray data analysis also revealed that putative cytokinin oxidase/dehydrogenase (CKX) genes were downregulated in STF transformants (Fig 4, Table 1). Cytokinin oxidases/dehydrogenases catalyze the irreversible degradation of cytokinins and play important roles in maintaining cytokinin homeostasis. The phytohormone cytokinin (CK) affects many aspects of plant developmental programs, including a prominent role in the regulation of cell proliferation, plant growth and determination of organ size [41]. It is plausible that increasing the CK levels by reducing expression of CKXs could result in enhanced biomass and grain production as well as increased plant stature [42, 43]. However, several genes involved in auxin signaling/response were also altered in expression (Table 1). The phenotypes of STF transgenic lines in the three grass species and the microarray data prompted us to think that the effect of STF may, at least in part, be connected to cytokinin levels.
Fig 4. A heat map showing some representatives of differentially expressed genes from STF transgenic switchgrass microarray analysis.
Selected genes related to cell division, phytohormones, metabolic processes and transcription factors were shown. Profiling analysis was performed in 6–8 cm newly generated tillers, approximately 3 weeks after cutting, of three independent STF overexpressing Group Ⅱ switchgrass lines and three independent UBI::GUS control lines. The log ratio values (vs control average) were used to construct the heat map with the scale bar at the bottom showing expression intensities. Red colors represent upregulated genes whereas green colors represent downregulated genes. The column on the right shows the fold changes of the selected genes in the STF overexpressing switchgrass plants compared to the controls based on the microarray data. Bars represent means ± SE (n = 3).
Table 1. Hormone-Associated Genes Differentially Expressed in STF Overexpressing Switchgrass.
| Probe sets | Putative annotation | Fold changea | P-valueb | Putative functionc |
|---|---|---|---|---|
| AP13CTG02927_at | 3-β hydroxysteroid dehydrogenase | 0.075 | 0.00228 | Steroid hormones biosynthesis |
| AP13ITG51564_s_at | 3-β hydroxysteroid dehydrogenase | 0.088 | 0.00072 | Steroid hormones biosynthesis |
| AP13ITG59377_s_at | Cytokinin trans-hydroxylase | 0.112 | 0.01188 | Cytokinin biosynthesis |
| AP13ITG69514_s_at | Flavonoid 3'-monooxygenase | 0.282 | 0.02789 | Auxin biosynthesis |
| AP13ITG67646_at | Glutathione s-transferase | 0.324 | 0.00761 | Auxin and cytokinin response |
| KanlowCTG47469_s_at | Cytokinin trans-hydroxylase | 0.366 | 0.01454 | Cytokinin biosynthesis |
| KanlowCTG18799_at | AUX/IAA | 0.397 | 0.01400 | Auxin signaling |
| KanlowCTG17636_x_at | Auxin response factor | 0.452 | 0.00033 | Auxin signaling |
| AP13ITG49260_s_at | Cytokinin dehydrogenase | 0.466 | 0.00279 | Cytokinin degradation |
| AP13ITG48191_s_at | Cytokinin dehydrogenase | 0.525 | 0.00109 | Cytokinin degradation |
| AP13ITG64496_at | Glutathione s-transferase | 2.392 | 0.00712 | Auxin and cytokinin response |
| AP13ITG66839_s_at | ABA induced protein | 2.749 | 0.00380 | ABA response |
| AP13ITG65716_at | Horseradish peroxidase | 3.817 | 0.05530 | Auxin catabolism |
aRelative abundance of transcript in STF overexpression/control (UBI::GUS) switchgrass plants.
bP-value calculated as described in materials and methods.
cCategory of predicted gene function.
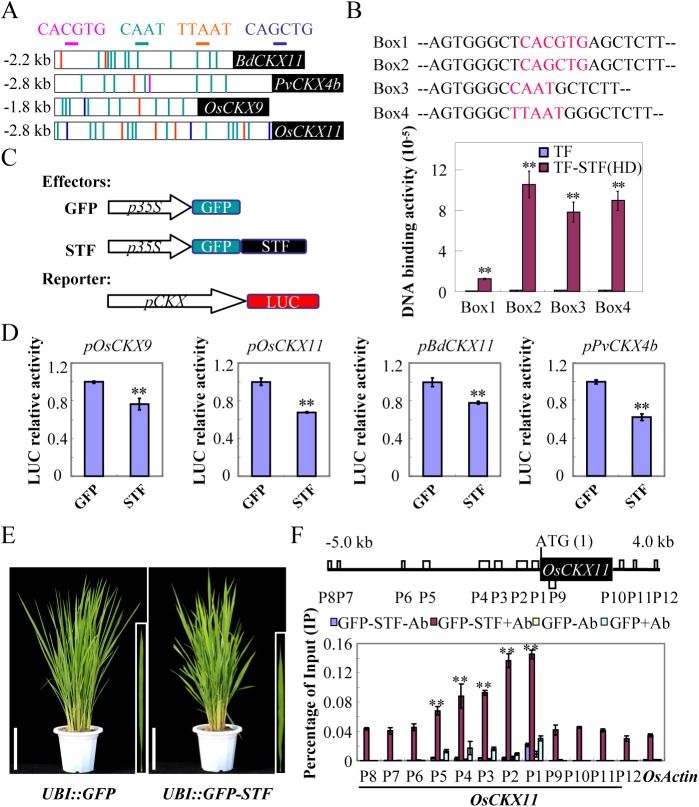
To confirm that overexpression of STF in switchgrass, Brachypodium and rice reduced CKX gene expression, we isolated 9 CKX family members from switchgrass and 11 members from each of Brachypodium and rice and examined their transcript levels by qRT-PCR. We found that the expression levels of 5 out of 9 PvCKX family members were considerably reduced in STF overexpressing switchgrass (Fig 5A). Similarly, the transcript levels of three BdCKX family members in Brachypodium and four OsCKX family members in rice were also found to be significantly reduced in STF transgenic lines compared to the controls (Fig 5B and 5C), indicating that the downregulation of CKXs in transgenic switchgrass microarray was not an isolated event. The CKXs that are repressed by STF across the three species are phylogenetically close to each other except PvCKX6, which appears to have no partners acting in the same way in rice or Brachypodium (S5 Fig). Since CKX enzymes are responsible for cytokinin degradation, we directly measured active and transiently inactive cytokinin levels in the STF overexpressing rice plants, for which optimized methods have been established [44]. We found that the amount of 5 of the 6 CK species measured, iP, isopentenyladenine; iPR, iP riboside; iP9G, iP 9-glucoside; tZ, zeatin; tZ9G, zeatin 9-glucoside, were significantly increased in STF overexpressing rice than the control plants (Fig 5D, S5 Table), suggesting that the repression of CKXs has a biological significance in promoting cytokinin levels. These data are consistent with the cell proliferation promotion activity of STF and suggest that the enhanced cell proliferation in STF transformants could in part be caused by elevated CK levels through repressing the expression of CKX family CK degrading enzymes, providing a novel mechanistic insight for STF molecular function.
Fig 5. Transcript abundance of CKX genes and cytokinin (CK) content in STF overexpressing transgenic grasses.
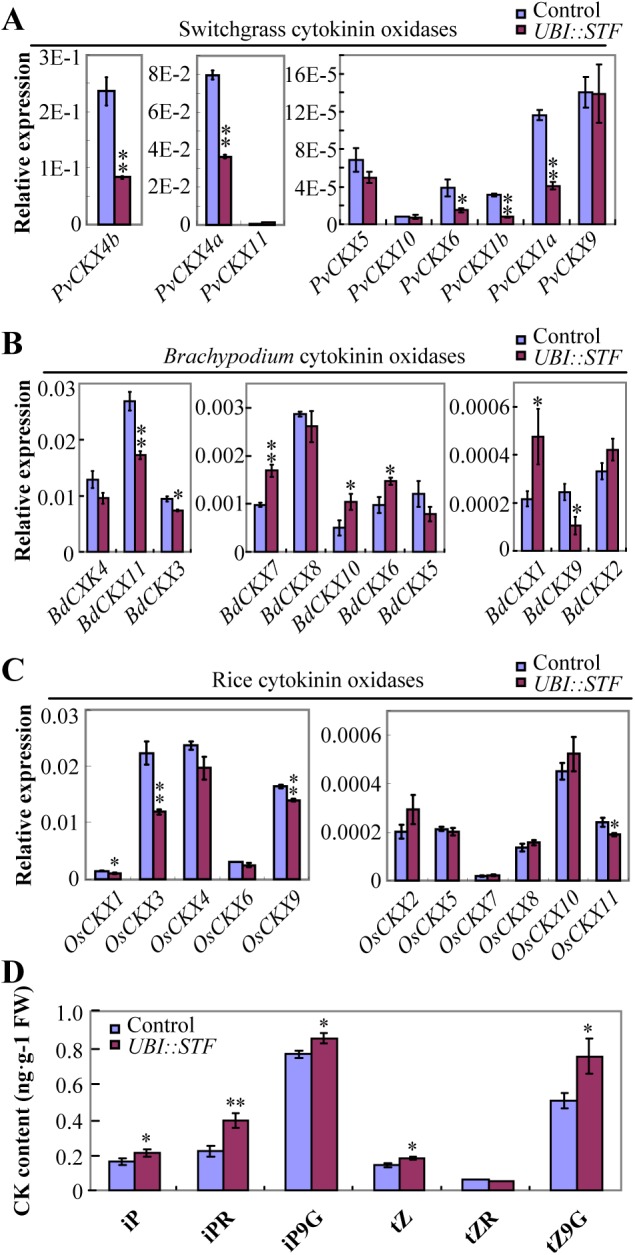
(A) Transcript levels of CKX genes in the 6–8 cm newly generated tillers, approximately 3 weeks after cutting, of control (UBI::GUS) and STF overexpressing switchgrass. Bars represent means ± SE of three technical replicates and two biological replicates. (B) Transcript levels of CKX genes in 4 weeks old seedling of control (UBI::GUS) and STF overexpressing Brachypodium. Bars represent means ± SE of three technical replicates and two biological replicates. (C) Transcript levels of CKX genes in 2 weeks old seedlings of control (UBI::GFP) and STF overexpressing rice. Bars represent means ± SE of three technical replicates and two biological replicates. (D) Comparison of CK content in the top 2 leaves of 2 months old wild type and STF overexpressing rice. iP, isopentenyladenine; iPR, iP riboside; iP9G, iP 9-glucoside; tZ, zeatin; tZR, zeatin riboside; tZ9G, zeatin 9-glucoside. Bars represent means with SE of three technical replicates from three independent wild type and UBI::STF plants. The asterisks indicate significant differences (* p<0.05, ** p < 0.01, Student t-test). FW, fresh weight.
Owing to the fact that STF acts primarily as a transcriptional repressor [36, 38] and specific CKXs are repressed in STF overexpressing switchgrass, Brachypodium and rice transgenic lines, we hypothesized that STF may directly repress the CKX family genes to promote CK activity. To test this hypothesis, we performed three complimentary experiments. First, we tested direct DNA binding of STF to CKX promoters in vitro using DNA binding assay. WOX family proteins have been reported to bind the G-box, the known TAAT motif as well as consensus sequences like CAAT and TTAA [31, 45]. Sequence analysis revealed that these binding sequences were present in the proximal promoter regions of the downregulated CKX family members like OsCKX9 and OsCKX11 in rice, BdCKX11 in Brachypodium and PvCKX4b in switchgrass (Fig 6A). We tested whether the STF protein can directly bind to DNA fragments containing these sequences in vitro. The STF homeodomain region was fused to a Trigger Factor (TF), a molecular chaperone protein that improves protein solubility [46] for expression in E.coli and purified using Profinity IMAC Ni-Charged Resin (BIO-RAD). Our results showed that the recombinant TF fused STF homeodomain protein was able to bind the fragments containing the conserved G-box, TAAT motif and CAAT and TTAA sequences, while binding was not detected by the control TF protein alone (Fig 6B), suggesting that the STF repression of CKXs is mediated by direct binding to their specific promoter sequences.
Fig 6. STF directly binds and represses the expression of several CKX genes in vitro and in vivo.
(A) Schematic presentation of putative STF binding sites in CKX promoters. (B) DNA binding assay corresponding to the putative binding sites of STF. DNA fragments bound to His-TF-STF(HD) fusion protein or His-TF control were quantified by qRT-PCR after elution. Bars represent means ± SE of three technical replicates and two biological replicates. The asterisks indicate significant differences (** p < 0.01, Student t-test). (C) Effector and reporter constructs used in transient dual luciferase assay. (D) Dual luciferase assay showing repression of rice, Brachypodium and switchgrass CKXs by the STF effector construct compared to the GFP control effector construct. Bars represent means ± SE of three technical replicates, and two biological replicates. The asterisks indicate significant differences (** p < 0.01, Student t-test). (E) Phenotype of UBI::GFP-STF T1 transgenic rice plant and UBI::GFP control from which 10-day old T2 seedlings were taken for the ChIP experiment. Bars = 10 cm. (F) ChIP assay showing the association of STF with several regions in the promoter of OsCKX11. The boxes indicate the position of the specific binding sites (P1-P5) and non-specific sites used as control (P6-P12). Bars represent means ± SE of three technical replicates and two biological replicates. The asterisks indicate significant enrichment (** p < 0.01, Student t-test) compared to the OsActin control.
Second, using a well-established transient dual luciferase assay system in rice leaf protoplasts, we found that coexpression of the STF effector protein and the luciferase reporter constructs driven by the 1.8–2.8 kb promoters of OsCKX9 and OsCKX11 (Fig 6C), both downregulated in STF overexpressing rice, resulted in a significant reduction of the luminescence intensity compared with the GFP control effector protein (Fig 6D). Similarly, coexpression of the STF effector with the switchgrass pPvCKX4b and Brachypodium pBdCKX11 reporter constructs significantly reduced luminescence in the rice protoplast luciferase assay (Fig 6D), indicating that STF recognizes these CKX promoters in protoplasts and represses their activity.
Third, we tested direct in vivo binding of STF protein to OsCKX promoters by Chromatin Immunoprecipitation (ChIP) assay. Using UBI::GFP-STF transgenic rice and anti-GFP antibody, we found that the promoter regions of OsCKX9 and OsCKX11 were enriched in the GFP-STF chromatin (Fig 6E and 6F and S6 Fig). We tested five potential binding sites (P1-P5) within 2.8 kb region upstream of the translational start site, three non-specific regions (P6-P8) further upstream, one in the coding region (P9) and three non-specific regions downstream of the translation stop site (P10-P12) of OsCKX11 (Fig 6F). We found that the P1-P5 putative binding regions were significantly enriched compared to the non-specific regions (P6-P12) or the background signal in the OsActin control (Fig 6F), indicating that STF directly binds to multiple regions on CKX promoters, consistent with the DNA binding and dual luciferase assay results. These results together highlight a mechanism by which STF may act to modulate cytokinin levels in leaf development. Our data also demonstrate that ectopically expressing the Medicago WOX gene STF in switchgrass promotes leaf blade outgrowth and biomass accumulation with increased solubilized sugars, suggesting a potential in biomass feedstock improvement.
Discussion
The plant-specific WOX family transcription factors are master regulators of plant growth and development including shoot and root apical meristems, lateral organs, organ size and vasculature [28, 47–53]. The Medicago WOX gene STF and its orthologues are key regulators of leaf blade outgrowth in the medial-lateral direction and floral organ fusion in several eudicot species [33–35, 54]. Here we reported that ectopic expression of STF in switchgrass, Brachypodium and rice universally promotes cell proliferation in vegetative organs leading to wider leaf blades, thicker stems and overall significant increase in total biomass yield. Lignocellulosic biomass has a potential to displace a significant portion of gasoline as a renewable energy source [1, 6, 55], and switchgrass is one of the dedicated bioenergy crops in the United States for lignocellulosic biofuel production [7, 56]. Although a 1.3 billion ton biomass production capacity per annum was projected in the US alone, this estimation takes into account that current biomass production efficiency per unit of land would be at least doubled including in marginal lands that are not in current crop production systems [2], indicating that biomass yield is still a major challenge for sustainable biofuel production. Another major hurdle is bioconversion efficiency imposed by the cell wall lignin polymer. Lignin, a phenolic polymer, is a major component of most plant cell walls which is recalcitrant to saccharification through enzymatic digestion [4, 5]. Lignin forms complex cross-links with two other major cell wall polymers, cellulose and hemicellulose, making these components inaccessible to saccharification without pretreatment with strong acids. This indicates that deconstruction of plant cell walls is a significant challenge and lignin content is an important biomass feedstock trait determining bioconversion efficiency. In fact, reducing lignin content by genetic engineering for feedstock quality improvement is currently a major undertaking in several laboratories. Here we demonstrate that it is possible to contribute to both biomass yield and sugar release in switchgrass and other grasses using the leaf development master regulator STF. Switchgrass Alamo plants overexpressing STF showed approximately a 2-fold increase in above ground total dry weight production and approximately 1.8-fold increase in the release of solubilized sugars (Fig 3), improving biomass feedstock properties without necessarily altering the relative composition of cell wall polymers. Similar successes have been reported in switchgrass using maize Corngrass1 miRNA [10] and rice miRNA156 [9] resulting in improved saccharification efficiency by altering developmental phase changes. Overexpression of a switchgrass ERF gene PvERF001 was also recently reported to increase biomass yield [8], but to our knowledge, this is the first report showing significant improvement in biomass yield and sugar release without pretreatment using a WOX transcription factor in switchgrass, providing a new tool for genetic modification of grasses.
STF and its orthologues are unique among the leaf blade regulators in the sense that they are expressed at the adaxial-abaxial juxtaposition [33–35, 54] unlike the well-studied polarity factors that are axial-specific. In this middle region at the leaf margin and middle mesophyll, STF and Arabidopsis WOX1 promote lateral expansion of the leaf blade by activating cell proliferation [33, 35] analogous to WUS in the SAM [50] and WOX5 in the RAM [48, 57]. STF is clearly shown to affect free auxin and ABA levels by direct measurements but has also been proposed to act as a master switch affecting several developmental programs probably through regulating multiple hormone homeostasis including auxin, cytokinin, and metabolic sugars based on transcriptomic, transgenic and metabolomics data analyses [33, 39]. But the detailed mechanism is unknown. STF physically interacts with the transcriptional co-repressor MtTPL and primarily acts as a transcriptional repressor for its cell proliferation activity in leaf blade outgrowth [36–38]. Since Arabidopsis TPL is known to modulate auxin signaling via interaction with repressive auxin response factors (ARFs) [58], it could be assumed that at least the STF-TPL complex may recruit ARFs to explain the observed effects of STF on auxin levels. However, ARF gene expression was also altered in the Medicago stf mutant microarray data [33], in the current STF transgenic switchgrass microarray, as well as in Arabidopsis wox1/prs double and petunia maw mutants quantified by qRT-PCR [34], suggesting a potential for STF direct effects on auxin signaling/homeostasis, although such direct effects are yet to be shown mechanistically.
Here we demonstrate that STF can directly bind to multiple regions on the promoter of cytokinin oxidases/dehydrogenases (CKXs) in vitro and in vivo, and represses their transcription in transgenic switchgrass, Brachypodium and rice (Figs 5 and 6), providing a novel mechanism to control local cytokinin homeostasis. CKXs are induced by cytokinins in plant tissues and catalyze the degradation of active cytokinins in a feedback inhibition to maintain cytokinin homeostasis [59]. This suggests that STF improves active cytokinin pools by preventing cytokinin degradation through repressing CKX genes (Fig 7). Indeed, direct measurement of active cytokinins and directly convertible cytokinin conjugates confirmed that 5 of the 6 cytokinin species analyzed were significantly increased in STF overexpressing transgenic rice lines. Since cytokinins are major regulators of cell proliferation [60], this finding is consistent with the STF’s role in the promotion of cell proliferation by transcriptional repression activity in leaf blade outgrowth and total biomass accumulation in the native Medicago and transgenic grasses. Activation of cytokinin signaling by WOX genes has been established for WUS in Arabidopsis SAM maintenance through repression of A-type ARRs including ARR5, ARR6, ARR7 and ARR15, which are negative regulators of cytokinin signaling [49, 61]. WOX9/STIP is also reported to act downstream of cytokinin sensing in the Arabidopsis SAM and proposed to activate the A-type ARRs [62]. Rice OsWOX4, that performs an equivalent function to Arabidopsis WUS in shoot meristem maintenance in rice, has also been reported to mimic cytokinin application in inducing calli in transgenic rice [63] although the mechanism is not known. Activation of cytokinin activity by repressing CKXs may thus be yet another mechanism to control cytokinin response, which may also have important implications in SAM maintenance. We checked the effect of STF on CKXs in Medicago truncatula, where STF is native. In the stf mutant microarray, one of only two genes included in the chip (Medtr4g044110) was modestly upregulated at 1.32 and 1.47 times, for 2 different probe sets, in the mutant compared to wild type, the other gene was unchanged at 1.08 [33]. We were able to identify seven definitive CKX-like genes in the annotated version 3.5 of the M. truncatula genome. We tested the expression of all of them by semi quantitative PCR in the unexpanded young leaves of wild type R108 and two stf mutant alleles, stf-1 and stf-2. Our preliminary results showed that one of them (Medtr3g036100) was significantly induced in the mutants, and two others, Medtr4g044110 and Medtr2g039410, were slightly induced, while the other four were basically unchanged (S7 Fig). The Medtr4g044110 weak induction in the mutants is consistent with the stf microarray data. This preliminary observation suggests that the effect on cytokinin activation may be part of the STF function in eudicots. In fact, the first true leaf-like structure with apparent petiole and blade outline was obtained in Nicotiana sylvestris lam1 mutants only after application of auxin and cytokinin together to the shoot apex [33], suggesting that both auxin and cytokinin signaling pathways and/or their crosstalk could be components of the STF function in leaf blade outgrowth.
Fig 7. A simplified hypothetical model depicting STF-mediated leaf blade expansion in transgenic grasses.
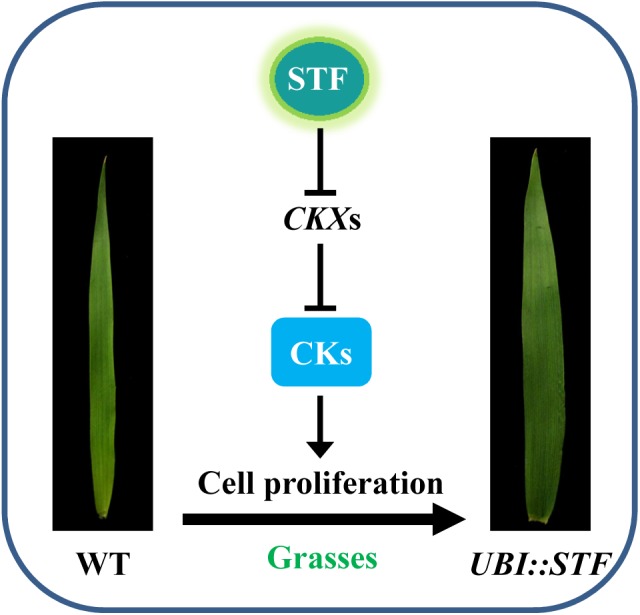
STF transcriptionally represses the expression of cytokinin (CK) degrading cytokinin oxidases/dehydrogenases (CKXs) by directly binding to their promoters. This presumably increases active CK levels in leaf tissues which might contribute to enhanced cell proliferation leading to broader leaf blades.
Nevertheless, STF homologues are not found in monocots [33, 34, 38, 64, 65] and the biological significance of the cytokinin mechanistic model is yet to be tested by the endogenous grass WOX gene(s). In monocots, the WOX3 family is proposed to perform an equivalent function [39]. At least in maize and rice, where information is available, the function of STF/WOX1 appears to be met by the WOX3 family members NS1 and NS2 in maize [27], and NAL2 and NAL3 in rice [29, 30]. In eudicots, one WOX3/PRS and at least one STF/WOX1 genes are present but monocots have at least two WOX3/NS genes instead. Arabidopsis PRS is required for lateral axis-dependent development of flowers, lateral sepals, lateral stamens and leaf stipules [27, 28, 66], but prs mutants do not display narrow leaf blades although PRS is found to redundantly function with WOX1 in leaf blade outgrowth [34, 35]. In M. truncatula and probably other eudicots, the WOX3/LFL role is restricted to flower development [52]. It is likely that WOX1 and WOX3 have separate roles in most eudicots, other than Arabidopsis, and leaf blade development in the medial-lateral axis is governed by the STF/WOX1 family, while redundant WOX3 family genes fulfill this role in monocots. Thus, it is not surprising if some of the mechanisms are conserved between STF/WOX1 and WOX3/NS functions. The actual mechanism of NS and NAL function is unclear but the involvement of hormone signaling, especially auxin, has been predicted from transcriptome analysis [29, 32] and OsWOX3A has been reported to act in GA homeostasis [31]. It would be interesting to see if the ns1/2 and nal2/3 mutants have altered cytokinin activity or CKXs expression levels, and also complement these mutants with STF/WOX1 to understand the extent of mechanistic conservation between monocots and eudicots in lateral outgrowth of the leaf blade.
Our data suggest that promotion of cytokinin activity by direct repression of cytokinin degradation may be a general mechanism for the action of repressive WOX genes that are involved in cell proliferation during meristem maintenance and lateral organ development including WUS, NAL and NS genes. Further experiments are needed to confirm the validity of this hypothesis. Taken together, our results provide a powerful tool for genetic modification of biomass yield and sugar release in perennial and annual grasses, and uncover a novel mechanistic insight directly connecting cytokinin activity and cell proliferation by repressive WOX genes that have far reaching consequences in regulating plant vegetative and reproductive developmental programs.
Materials and methods
Plant materials and growth conditions
Brachypodium distachyon inbred line Bd21-3, Oryza sativa varieties Nipponbare and the lowland-type switchgrass cultivar Alamo were used in this study. Brachypodium and switchgrass plants were grown in the greenhouse under a 26°C/16-h (day) and 23°C/8-h (night) photoperiods with lighting supplied by parabolic aluminized reflector lamps (average 390 μE⁄m2⁄S1). The rice (Oryza sativa L.) plants were cultivated in experiment field and green house in Beijing.
Vector construction and plant transformation
The Maize Ubiquitin promoter from pTCK303 [67] was cloned into pMDC32 Gateway vector substituting 2×35S promoter to generate pMDC32-pUBI destination vector by Hind Ⅲ and Kpn Ⅰ, and STF, GFP and GUS were cloned into pMDC32-pUBI destination vector by using the Gateway system (Invitrogen). To generate the UBI::GFP-STF vector, GFP and STF were cloned separately, with 18 bp overlapping sequence between 3’GFP and 5’STF to acquire the GFP-STF sequence cloned into pMDC32-pUBI destination vector by using the Gateway system (Invitrogen). Constructs were introduced into Agrobacterium tumefaciens by electroporation or the freezing transformation method. A. tumefaciences strain AGL1 was used for Brachypodium, switchgrass and rice transformation as previously described [9, 68, 69].
RNA extraction and quantitative RT-PCR
Total RNA was extracted from 6–8 cm newly generated tillers approximately three weeks after cutting (for switchgrass Microarray and qRT-PCR analysis), 2 weeks old seedlings (for rice qRT-PCR analysis) and 1 month seedling shoots (for Brachypodium qRT-PCR analysis) of UBI::STF, UBI::GUS and UBI::GFP transgenic plants by using TRIzol Reagent (Invitrogen). cDNA was generated by reverse transcription with SuperScript Ⅲ (Invitrogen). Quantitative RT-PCR was performed as previously described [36], with at least 2 biological and 3 technical replicates for both samples and controls. The CKX gene ID and primers used were listed in S6 and S7 Tables.
Histological analysis
Tissue fixation and embedding were performed as described [69]. The tissues were sliced into 10 μm sections with a Leica RM2145 microtome, affixed to microscope slides, and stained with toluidine blue. Cross sections of the flag leaves and internode Ⅱ (for rice and Brachpodium) and internode Ⅲ (for switchgrass) of the controls and STF transformants were stained with phloroglucinol-HCl reagent as previously described [70]. Images were taken under an Olympus BX-51 compound microscope.
Microarray analysis
6–8 cm newly generated tillers, approximately 3 weeks after cutting, of three independent Group Ⅱ UBI::STF transgenic lines (STF-2, 4, and 10) and three independent UBI::GUS lines were used for total RNA extraction and microarray experiment. The Microarray analysis of transgenic switchgrass plants were performed as previously described [9]. Data analysis of differentially expressed probe sets on the chip was performed by associative analysis as described [71].
Quantification of cytokinins
Extraction and determination of CKs from the top two leaves of three UBI::STF transgenic rice lines and wild type at vegetative stage (two months old after planting) were performed by using a polymer monolith microextraction/hydrophilic interaction chromatography/electrospray ionization tandem mass spectrometry method as described [44].
Biofuel property analysis
Total above-ground shoot tissues at flowering stage were harvested for the dry biomass yield analysis and further biofuel property evaluation. Lignin analysis and enzymatic saccharification were performed as previously described [9].
DNA binding assay
The STF cDNA corresponding to the N-terminal and homeodomain (HD) regions with amino acids 1 to 226 was cloned into the E. coli expression vector pCOLD-TF with His tag (Takara, TF indicates Trigger Factor, a molecular chaperone protein of original nuclear pullulan increasing the protein solubility) using EcoR Ⅰ and BamH Ⅰ restriction enzymes. Expression of pCOLD-TF, and pCOLD-TF-STF(HD) in BL21 cells was induced with 0.2 mM isopropyl-1-thio-D-galactopyranoside at 18°C for 16 h. Fusion protein was purified using Profinity IMAC Ni-Charged Resin (BIO-RAD) according to the manufacturer’s protocol and quantified by the Bio-Rad protein assay reagent. DNA binding assay was performed as previous described [72]. The putative STF binding fragments were incubated with purified His-TF alone and with the His-TF-STF(HD) fusion protein, and the DNA binding activity (protein bound DNA) was determined by qRT-PCR after washing and elution. Primers used were listed in S7 Table.
Transient lucifarese assay
The coding sequences of GFP-STF and GFP were cloned into p2GW7 using the Gateway system (Invitrogen) to yield effector plasmids. For the reporter plasmid, a mini 35S promoter [73] with BamH Ⅰ was inserted into the pGreen Ⅱ-0800-Luc vector by exonuclease Ⅲ, to generate the destination vector pGreen Ⅱ-0800-p35S mini-Luc, and the promoter of OsCKX9, OsCKX11, BdCKX11 and PvCKX4b were cloned into pGreen Ⅱ-0800-p35S mini-Luc by restriction digestion to generate the reporter plasmid. Primers used were listed in S7 Table. Transient expression assays were performed in rice protoplasts as previously described [36, 74]. For each transformation, 5 μg of reporter plasmid and 5 μg of effector plasmid were used. Luciferase activities were detected by Dual-Luciferase Reporter Assay System (Promega) as previously described [75].
ChIP assay
The UBI::GFP and UBI::GFP-STF transgenic rice lines were used for ChIP assay according to the method described previously [76] with some modifications. Briefly, 1 g tissue of 10-day-old T2 seedlings per sample was harvested from plants grown in greenhouse. Samples were cross-linked with 1% (v/v) formaldehyde under vacuum for 10 min, quenched with Gly (0.2 M) for 5 min, and then ground to powder in liquid nitrogen. The chromatin complexes were isolated, sonicated and then incubated with polyclonal anti-GFP antibodies (Abcam, AB290). The precipitated DNA was recovered and used as a template for qRT-PCR analysis. The input DNA and no antibody–precipitated DNA were used as positive and negative controls, respectively. The primers used for the ChIP assays were described in S7 Table.
Supporting information
(A) Phenotypes of three classes of STF overexpressing rice lines. The control was transformed in the same way with UBI::GFP. Bars = 10 cm in upper row, 2 cm in middle row and 2 mm in lower row. (B) Transcript abundance of STF in transgenic plants revealed by qRT-PCR. Bars represent means ± SE of three technical replicates.
(TIF)
(A) Phenotypes of STF overexpressing Brachypodium. UBI::GUS transformed Brachypodium was used as the control. Bars = 10 cm. (B) Transcript abundance of STF in transgenic plants revealed by qRT-PCR. Bars represent means ± SE of three technical replicates. (C) Comparison of seed size between STF transgenic and control (UBI::GUS) plants. Bar = 1 mm.
(TIF)
A pie chart representing the distribution of functional classifications of down-regulated (A) and up-regulated (B) probes based on the Gene Ontology Assignments.
(TIF)
Transcript levels of genes encoding putative Histone H4, Expansin A and Alpha-expansin in STF transgenic lines revealed by qRT-PCR. UBI::GUS expressing switchgrass plants were used as controls. Bars represent means ± SE of three technical replicates and two biological replicates. The asterisks indicate significant differences (** means p < 0.01, Student t-test).
(TIF)
Full-length amino acid sequences were aligned using Clustal W and the tree was constructed using MEGA4 with 1000 replicates. Species: Os, Oryza sativa; Bd, Brachypodium distachyon; Pv, Panicum virgatum. The green dots highlight the downregulated CKXs in STF overexpressing grasses.
(TIF)
(A) Schematic representation of the regions of OsCKX9 tested by ChIP experiments. P1-P5 are specific STF binding sites, while P6-P9 are non-specific sites used as control. (B) ChIP assay showing the association of STF with several regions in the promoter of OsCKX9 (P1-P5) compared to background signal (P6-P9) or the OsActin negative control. Bars represent means ± SE of three technical replicates and two biological replicates. The asterisks indicate significant differences (** p < 0.01, Student t-test).
(TIF)
Semi quantitative RT-PCR showing the expression of seven CKX genes in the leaves of four weeks old M. truncatula stf mutants compared to wild type. Numbers on the right show the number of PCR cycles used. * represents a gene that showed weak induction in stf microarray.
(TIF)
Plant height and tiller number of STF transgenic and control switchgrass plants were measured after 4-months of growth in the greenhouse. 7 tillers were used to measure internode length and diameter (internode Ⅱ), leaf blade length and width were measured in the 3rd leaf for each plant. The control represents the average of three independent UBI::GUS transgenic plants. Values are mean ± SE (n = 7). One or two asterisks indicate significance corresponding to *P < 0.05 or **P < 0.01 (Student t-test). G Ⅰ, Ⅱ, Ⅲ indicate group Ⅰ, Ⅱ, Ⅲ respectively.
(DOC)
aIVTDMD, in vitro true dry matter digestibility; bADL, acid detergent lignin. The STF transgenic switchgrass and control plants were harvested after 4-month growth in the greenhouse. Values are mean ± SE (n = 3). One or two asterisks indicate significance corresponding to *P < 0.05 or **P < 0.01 (Student t-test).
(DOC)
Fold change presented as relative abundance of transcript in STF overexpression/control (UBI::STF/UBI::GUS) switchgrass plants. P-value calculated as described in materials and methods. “//”, no significant similarity found.
(DOC)
Fold change presented as relative abundance of transcript in STF overexpression/control (UBI::STF/UBI::GUS) switchgrass plants. P-value calculated as described in materials and methods. “//”, no significant similarity found.
(DOC)
The top two leaf blades of three UBI::STF transgenic rice lines and wild type at vegetative stage (2 months after planting) were collected for Quantification of Cytokinins. iP, isopentenyladenine; iPR, iP riboside; iP9G, iP 9-glucoside; tZ, zeatin; tZR, zeatin riboside; tZ9G, zeatin 9-glucoside. The unit is ng·g-1 FW.
(DOC)
(DOC)
(DOC)
Acknowledgments
We thank K. Chong for providing pTCK303 plasmid and R. Dixon for providing p2GW7 plasmid.
Data Availability
All relevant data are within the paper and its Supporting Information files. Microarray data are available on ArrayExpress (https://www.ebi.ac.uk/arrayexpress/) under accession number E-MTAB-5539.
Funding Statement
This work was supported by: Ministry of Science and Technology of the People’s Republic of China (2015CB150103, 2016YFD0101001, and 2014CB138701) to HL and TL, National Natural Science Foundation of China (31470381) to HL, Chinese Academy of Agricultural Sciences to HL and LN, Samuel Roberts Noble Foundation to ZYW, the National Science Foundation (NSF) grant IOS-1354422 to MT; and the National Institute of Food and Agriculture, U.S. Department of Agriculture, under the Agricultural Experiment Station to MT. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Somerville C, Youngs H, Taylor C, Davis SC, Long SP. Feedstocks for lignocellulosic biofuels. Science. 2010;329(5993):790–2. 10.1126/science.1189268 [DOI] [PubMed] [Google Scholar]
- 2.Perlack RD, Wright LL, Turhollow AF, Graham RL, Stokes BJ, Erbach DC. Biomass as feedstock for a bioenergy and bioproducts industry: The technical feasibility of a billion-ton annual supply: Oak Ridge, TN: US DOE; 2005. [Google Scholar]
- 3.Loque D, Scheller HV, Pauly M. Engineering of plant cell walls for enhanced biofuel production. Curr Opin Plant Biol. 2015;25:151–61. 10.1016/j.pbi.2015.05.018 [DOI] [PubMed] [Google Scholar]
- 4.Pauly M, Keegstra K. Plant cell wall polymers as precursors for biofuels. Curr Opin Plant Biol. 2010;13(3):305–12. 10.1016/j.pbi.2009.12.009 [DOI] [PubMed] [Google Scholar]
- 5.Chen F, Dixon RA. Lignin modification improves fermentable sugar yields for biofuel production. Nat Biotechnol. 2007;25(7):759–61. 10.1038/nbt1316 [DOI] [PubMed] [Google Scholar]
- 6.Carroll A, Somerville C. Cellulosic biofuels. Annu Rev Plant Biol. 2009;60:165–82. 10.1146/annurev.arplant.043008.092125 [DOI] [PubMed] [Google Scholar]
- 7.Schmer MR, Vogel KP, Mitchell RB, Perrin RK. Net energy of cellulosic ethanol from switchgrass. Proc Natl Acad Sci U S A. 2008;105(2):464–9. PubMed Central PMCID: PMCPMC2206559. 10.1073/pnas.0704767105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wuddineh WA, Mazarei M, Turner GB, Sykes RW, Decker SR, Davis MF, et al. Identification and Molecular Characterization of the Switchgrass AP2/ERF Transcription Factor Superfamily, and Overexpression of PvERF001 for Improvement of Biomass Characteristics for Biofuel. Front Bioeng Biotechnol. 2015;3:101 PubMed Central PMCID: PMCPMC4507462. 10.3389/fbioe.2015.00101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu C, Sunkar R, Zhou C, Shen H, Zhang JY, Matts J, et al. Overexpression of miR156 in switchgrass (Panicum virgatum L.) results in various morphological alterations and leads to improved biomass production. Plant Biotechnol J. 2012;10(4):443–52. PubMed Central PMCID: PMCPMC3489066. 10.1111/j.1467-7652.2011.00677.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chuck GS, Tobias C, Sun L, Kraemer F, Li C, Dibble D, et al. Overexpression of the maize Corngrass1 microRNA prevents flowering, improves digestibility, and increases starch content of switchgrass. Proc Natl Acad Sci U S A. 2011;108(42):17550–5. PubMed Central PMCID: PMCPMC3198312. 10.1073/pnas.1113971108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu XG, Long SP, Ort DR. Improving Photosynthetic Efficiency for Greater Yield. In: Merchant S, Briggs WR, Ort D, editors. Annual Review of Plant Biology, Vol 61. Annu Rev Plant Biol. 612010. p. 235–61. [DOI] [PubMed]
- 12.Mathan J. BJ, Ranjan A. Enhancing crop yield by optimizing plant developmental features. Development. 2016;143:3283–94. 10.1242/dev.134072 [DOI] [PubMed] [Google Scholar]
- 13.Braybrook SA, Kuhlemeier C. How a plant builds leaves. Plant Cell. 2010;22(4):1006–18. PubMed Central PMCID: PMCPMC2879743. 10.1105/tpc.110.073924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Husbands AY, Chitwood DH, Plavskin Y, Timmermans MC. Signals and prepatterns: new insights into organ polarity in plants. Genes Dev. 2009;23(17):1986–97. 10.1101/gad.1819909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwakawa H, Ueno Y, Semiarti E, Onouchi H, Kojima S, Tsukaya H, et al. The ASYMMETRIC LEAVES2 gene of Arabidopsis thaliana, required for formation of a symmetric flat leaf lamina, encodes a member of a novel family of proteins characterized by cysteine repeats and a leucine zipper. Plant Cell physiol. 2002;43(5):467–78. [DOI] [PubMed] [Google Scholar]
- 16.McConnell JR, Emery J, Eshed Y, Bao N, Bowman J, Barton MK. Role of PHABULOSA and PHAVOLUTA in determining radial patterning in shoots. Nature. 2001;411(6838):709–13. 10.1038/35079635 [DOI] [PubMed] [Google Scholar]
- 17.Kerstetter RA, Bollman K, Taylor RA, Bomblies K, Poethig RS. KANADI regulates organ polarity in Arabidopsis. Nature. 2001;411(6838):706–9. 10.1038/35079629 [DOI] [PubMed] [Google Scholar]
- 18.Byrne M, Timmermans M, Kidner C, Martienssen R. Development of leaf shape. Curr Opin Plant Biol. 2001;4(1):38–43. [DOI] [PubMed] [Google Scholar]
- 19.Ichihashi Y, Tsukaya H. Behavior of Leaf Meristems and Their Modification. Front Plant Sci. 2015;6:1060 PubMed Central PMCID: PMCPMC4664833. 10.3389/fpls.2015.01060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hagemann W, Gleissberg S. Organogenetic capacity of leaves: The significance of marginal blastozones in angiosperms. Plant Syst evol. 1996;199:121–52. [Google Scholar]
- 21.Wochok RMaZS. Activity of Marginal and Plate Meristems During Leaf Development of Xanthium pennsylvanicum. Am JBot. 1969;56(1):26–30. [Google Scholar]
- 22.Tsukaya H. Mechanism of leaf-shape determination. Annu Rev Plant Biol. 2006;57:477–96. 10.1146/annurev.arplant.57.032905.105320 [DOI] [PubMed] [Google Scholar]
- 23.Hay A, Hake S. The dominant mutant Wavy auricle in blade1 disrupts patterning in a lateral domain of the maize leaf. Plant Physiol. 2004;135(1):300–8. 10.1104/pp.103.036707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moon J, Candela H, Hake S. The Liguleless narrow mutation affects proximal-distal signaling and leaf growth. Development. 2013;140(2):405–12. 10.1242/dev.085787 [DOI] [PubMed] [Google Scholar]
- 25.Lewis MW, Hake S. Keep on growing: building and patterning leaves in the grasses. Curr Opin Plant Biol. 2016;29:80–6. 10.1016/j.pbi.2015.11.008 [DOI] [PubMed] [Google Scholar]
- 26.Jiang D, Fang JJ, Lou LM, Zhao JF, Yuan SJ, Yin L, et al. Characterization of a Null Allelic Mutant of the Rice NAL1 Gene Reveals Its Role in Regulating Cell Division. Plos One. 2015;10(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nardmann J, Ji J, Werr W, Scanlon MJ. The maize duplicate genes narrow sheath1 and narrow sheath2 encode a conserved homeobox gene function in a lateral domain of shoot apical meristems. Development. 2004;131(12):2827–39. 10.1242/dev.01164 [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto N, Okada K. A homeobox gene, PRESSED FLOWER, regulates lateral axis-dependent development of Arabidopsis flowers. Genes Dev. 2001;15(24):3355–64. 10.1101/gad.931001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishiwata A, Ozawa M., Nagasaki H., Kato M., Noda Y., Yamaguchi T., Nosaka M., Shimizu-Sato S., Nagasaki A., Maekawa M., Hirano H.Y., Sato Y. Two WUSCHEL-related homeobox genes, narrow leaf2 and narrow leaf3, control leaf width in rice. Plant Cell Physiol. 2013;54(5):779–92. 10.1093/pcp/pct032 [DOI] [PubMed] [Google Scholar]
- 30.Cho S-H, Yoo S-C, Zhang H, Pandeya D, Koh H-J, Hwang J-Y, et al. The rice narrow leaf2 and narrow leaf3 loci encode WUSCHEL-related homeobox 3A (OsWOX3A) and function in leaf, spikelet, tiller and lateral root development. New phytol. 2013;198(4):1071–84. 10.1111/nph.12231 [DOI] [PubMed] [Google Scholar]
- 31.Cho SH, Kang K, Lee SH, Lee IJ, Paek NC. OsWOX3A is involved in negative feedback regulation of the gibberellic acid biosynthetic pathway in rice (Oryza sativa). J Exp Bot. 2016;67(6):1677–87. PubMed Central PMCID: PMCPMC4783357. 10.1093/jxb/erv559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X, Madi S, Borsuk L, Nettleton D, Elshire RJ, Buckner B, et al. Laser microdissection of narrow sheath mutant maize uncovers novel gene expression in the shoot apical meristem. PLoS Genet. 2007;3(6):e101 10.1371/journal.pgen.0030101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tadege M, Lin H, Bedair M, Berbel A, Wen J, Rojas CM, et al. STENOFOLIA regulates blade outgrowth and leaf vascular patterning in Medicago truncatula and Nicotiana sylvestris. Plant Cell. 2011;23(6):2125–42. PubMed Central PMCID: PMCPMC3160033. 10.1105/tpc.111.085340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vandenbussche M, Horstman A, Zethof J, Koes R, Rijpkema AS, Gerats T. Differential recruitment of WOX transcription factors for lateral development and organ fusion in Petunia and Arabidopsis. Plant Cell. 2009;21(8):2269–83. PubMed Central PMCID: PMCPMC2751957. 10.1105/tpc.109.065862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakata M, Matsumoto N, Tsugeki R, Rikirsch E, Laux T, Okada K. Roles of the middle domain-specific WUSCHEL-RELATED HOMEOBOX genes in early development of leaves in Arabidopsis. Plant Cell. 2012;24(2):519–35. PubMed Central PMCID: PMCPMC3315230. 10.1105/tpc.111.092858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin H, Niu L, McHale NA, Ohme-Takagi M, Mysore KS, Tadege M. Evolutionarily conserved repressive activity of WOX proteins mediates leaf blade outgrowth and floral organ development in plants. Proc Natl Acad Sci U S A. 2013;110(1):366–71. PubMed Central PMCID: PMCPMC3538250. 10.1073/pnas.1215376110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin H, Niu L, Tadege M. STENOFOLIA acts as a repressor in regulating leaf blade outgrowth. Plant Signal Behav. 2013;8(6):e24464 PubMed Central PMCID: PMCPMC3909033. 10.4161/psb.24464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang F, Wang Y, Li G, Tang Y, Kramer EM, Tadege M. STENOFOLIA recruits TOPLESS to repress ASYMMETRIC LEAVES2 at the leaf margin and promote leaf blade outgrowth in Medicago truncatula. Plant Cell. 2014;26(2):650–64. PubMed Central PMCID: PMCPMC3967031. 10.1105/tpc.113.121947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tadege M. WOX3 in the scene: intimacy with hormones. J Exp Bot. 2016;67(6):1605–7. PubMed Central PMCID: PMCPMC4783377. 10.1093/jxb/erw073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soltis DE, Albert VA, Leebens-Mack J, Palmer JD, Wing RA, dePamphilis CW, et al. The Amborella genome: an evolutionary reference for plant biology. Genome Biol. 2008;9(3):402 PubMed Central PMCID: PMCPMC2397498. 10.1186/gb-2008-9-3-402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schaller GE, Street IH, Kieber JJ. Cytokinin and the cell cycle. Curr Opin Plant Biol. 2014;21:7–15. 10.1016/j.pbi.2014.05.015 [DOI] [PubMed] [Google Scholar]
- 42.Ashikari M, Sakakibara H, Lin S, Yamamoto T, Takashi T, Nishimura A, et al. Cytokinin oxidase regulates rice grain production. Science. 2005;309(5735):741–5. 10.1126/science.1113373 [DOI] [PubMed] [Google Scholar]
- 43.Li S, Zhao B, Yuan D, Duan M, Qian Q, Tang L, et al. Rice zinc finger protein DST enhances grain production through controlling Gn1a/OsCKX2 expression. Proc Natl Acad Sci U S A. 2013;110(8):3167–72. PubMed Central PMCID: PMCPMC3581943. 10.1073/pnas.1300359110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Z, Wei F, Feng YQ. Determination of cytokinins in plant samples by polymer monolith microextraction coupled with hydrophilic interaction chromatographytandem mass spectrometry. Anal Methods. 2010;2:1676–85. [Google Scholar]
- 45.Busch W, Miotk A, Ariel FD, Zhao Z, Forner J, Daum G, et al. Transcriptional control of a plant stem cell niche. Dev Cell. 2010;18(5):849–61. 10.1016/j.devcel.2010.03.012 [DOI] [PubMed] [Google Scholar]
- 46.Qing G ML, Khorchid A, Swapna GV, Mal TK, Takayama MM, Xia B, Phadtare S, Ke H, Acton T, Montelione GT, Ikura M, Inouye M. Cold-shock induced high-yield protein production in Escherichia coli. Nat Biotechnol. 2004;22(7):877–82. 10.1038/nbt984 [DOI] [PubMed] [Google Scholar]
- 47.van der Graaff E, Laux T, Rensing SA. The WUS homeobox-containing (WOX) protein family. Genome Biol. 2009;10(12):248 PubMed Central PMCID: PMCPMC2812940. 10.1186/gb-2009-10-12-248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sarkar AK, Luijten M, Miyashima S, Lenhard M, Hashimoto T, Nakajima K, et al. Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature. 2007;446(7137):811–4. 10.1038/nature05703 [DOI] [PubMed] [Google Scholar]
- 49.Leibfried A, To JP, Busch W, Stehling S, Kehle A, Demar M, et al. WUSCHEL controls meristem function by direct regulation of cytokinin-inducible response regulators. Nature. 2005;438(7071):1172–5. 10.1038/nature04270 [DOI] [PubMed] [Google Scholar]
- 50.Mayer KF, Schoof H, Haecker A, Lenhard M, Jurgens G, Laux T. Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell. 1998;95(6):805–15. [DOI] [PubMed] [Google Scholar]
- 51.Hirakawa Y, Kondo Y, Fukuda H. TDIF peptide signaling regulates vascular stem cell proliferation via the WOX4 homeobox gene in Arabidopsis. Plant Cell. 2010;22(8):2618–29. 10.1105/tpc.110.076083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Niu LF, Lin H, Zhang F, Watira TW, Li GF, Tang YH, et al. LOOSE FLOWER, a WUSCHEL-like Homeobox gene, is required for lateral fusion of floral organs in Medicago truncatula. Plant J. 2015;81(3):480–92. 10.1111/tpj.12743 [DOI] [PubMed] [Google Scholar]
- 53.Costanzo E, Trehin C, Vandenbussche M. The role of WOX genes in flower development. Ann Bot. 2014;114(7):1545–53. 10.1093/aob/mcu123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhuang LL, Ambrose M, Rameau C, Weng L, Yang J, Hu XH, et al. LATHYROIDES, encoding a WUSCHEL-related Homeobox1 transcription factor, controls organ lateral growth, and regulates tendril and dorsal petal identities in garden pea (Pisum sativum L.). Mol Plant. 2012;5(6):1333–45. 10.1093/mp/sss067 [DOI] [PubMed] [Google Scholar]
- 55.Demura T, Ye ZH. Regulation of plant biomass production. Curr Opin Plant Biol. 2010;13(3):299–304. 10.1016/j.pbi.2010.03.002 [DOI] [PubMed] [Google Scholar]
- 56.McLaughlin SB, Kszos LA. Development of Switchgrass (Panicum virgatum) as a Bioenergy Feedstock in the United States. Biomass & Bioenergy. 2005;28:515–35. [Google Scholar]
- 57.Pi L, Aichinger E, van der Graaff E, Llavata-Peris CI, Weijers D, Hennig L, et al. Organizer-Derived WOX5 Signal Maintains Root Columella Stem Cells through Chromatin-Mediated Repression of CDF4 Expression. Dev Cell. 2015;33(5):576–88. 10.1016/j.devcel.2015.04.024 [DOI] [PubMed] [Google Scholar]
- 58.Szemenyei H, Hannon M, Long JA. TOPLESS mediates auxin-dependent transcriptional repression during Arabidopsis embryogenesis. Science. 2008;319(5868):1384–6. 10.1126/science.1151461 [DOI] [PubMed] [Google Scholar]
- 59.Schmulling T, Werner T, Riefler M, Krupkova E, Bartrina y Manns I. Structure and function of cytokinin oxidase/dehydrogenase genes of maize, rice, Arabidopsis and other species. J Plant Res. 2003;116(3):241–52. 10.1007/s10265-003-0096-4 [DOI] [PubMed] [Google Scholar]
- 60.Sakakibara H. Cytokinins: activity, biosynthesis, and translocation. Annu Rev Plant Biol. 2006;57:431–49. 10.1146/annurev.arplant.57.032905.105231 [DOI] [PubMed] [Google Scholar]
- 61.Zhao Z, Andersen SU, Ljung K, Dolezal K, Miotk A, Schultheiss SJ, et al. Hormonal control of the shoot stem-cell niche. Nature. 2010;465(7301):1089–92. 10.1038/nature09126 [DOI] [PubMed] [Google Scholar]
- 62.Skylar A, Hong FX, Chory J, Weigel D, Wu XL. STIMPY mediates cytokinin signaling during shoot meristem establishment in Arabidopsis seedlings. Development. 2010;137(4):541–9. 10.1242/dev.041426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ohmori Y, Tanaka W, Kojima M, Sakakibara H, Hirano HY. WUSCHEL-RELATED HOMEOBOX4 is involved in meristem maintenance and is negatively regulated by the CLE gene FCP1 in rice. Plant Cell. 2013;25(1):229–41. PubMed Central PMCID: PMCPMC3584538. 10.1105/tpc.112.103432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tadege M LH, Niu L, Mysore KS. Control of dicot leaf blade expansion by a WOX gene, STF. Plant Signal Behav. 2011;6(11):1861–4. 10.4161/psb.6.11.17761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nardmann J ZR, Durantini D, Kranz E, Werr W. WOX gene phylogeny in Poaceae: A comparative approach addressing leaf and embryo development. Mol Biol Evol. 2007;24(11):2474–84. 10.1093/molbev/msm182 [DOI] [PubMed] [Google Scholar]
- 66.Shimizu R, Ji J, Kelsey E, Ohtsu K, Schnable PS, Scanlon MJ. Tissue specificity and evolution of meristematic WOX3 function. Plant Physiol. 2009;149(2):841–50. 10.1104/pp.108.130765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang Z, Chen C, Xu Y, Jiang R, Han Y, Xu Z, et al. A practical vector for efficient knockdown of gene expression in rice (Oryza sativa L.). Plant Mol Bio Rep. 2004;22(4):409–17. [Google Scholar]
- 68.Alves SC, Worland B, Thole V, Snape JW, Bevan MW, Vain P. A protocol for Agrobacterium-mediated transformation of Brachypodium distachyon community standard line Bd21. Nat Protoc. 2009;4(5):638–49. 10.1038/nprot.2009.30 [DOI] [PubMed] [Google Scholar]
- 69.Lin H, Wang R, Qian Q, Yan M, Meng X, Fu Z, et al. DWARF27, an iron-containing protein required for the biosynthesis of strigolactones, regulates rice tiller bud outgrowth. Plant Cell. 2009;21(5):1512–25. 10.1105/tpc.109.065987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang H, Avci U, Nakashima J, Hahn MG, Chen F, Dixon RA. Mutation of WRKY transcription factors initiates pith secondary wall formation and increases stem biomass in dicotyledonous plants. Proc Natl Acad Sci U S A. 2010;107(51):22338–43. PubMed Central PMCID: PMCPMC3009815. 10.1073/pnas.1016436107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dozmorov I, Centola M. An associative analysis of gene expression array data. Bioinformatics. 2003;19(2):204–11. [DOI] [PubMed] [Google Scholar]
- 72.Yang D, Zhao W, Meng Y, Li H, Liu B. A CIB1-LIKE transcription factor GmCIL10 from soybean positively regulates plant flowering. Sci China Life Sci. 2015;58(3):261–9. 10.1007/s11427-015-4815-6 [DOI] [PubMed] [Google Scholar]
- 73.Lodha M, Marco CF, Timmermans MC. The ASYMMETRIC LEAVES complex maintains repression of KNOX homeobox genes via direct recruitment of Polycomb-repressive complex2. Genes Dev. 2013;27(6):596–601. PubMed Central PMCID: PMCPMC3613607. 10.1101/gad.211425.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bart R, Chern M, Park CJ, Bartley L, Ronald PC. A novel system for gene silencing using siRNAs in rice leaf and stem-derived protoplasts. Plant Methods. 2006;2:13 PubMed Central PMCID: PMCPMC1524957. 10.1186/1746-4811-2-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Asai T, Tena G., Plotnikova J., Willmann M.R., Chiu W.L.,Gomez-Gomez L., Boller T., Ausubel F.M., and Sheen J. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature. 2002;415:977–83. 10.1038/415977a [DOI] [PubMed] [Google Scholar]
- 76.Lu Z, Yu H, Xiong G, Wang J, Jiao Y, Liu G, Jing Y, Meng X, Hu X, Qian Q, Fu X, Wang Y, Li J. Genome-wide binding analysis of the transcription activator ideal plant architecture1 reveals a complex network regulating rice plant architecture. Plant Cell. 2013;25:3743–59. 10.1105/tpc.113.113639 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Phenotypes of three classes of STF overexpressing rice lines. The control was transformed in the same way with UBI::GFP. Bars = 10 cm in upper row, 2 cm in middle row and 2 mm in lower row. (B) Transcript abundance of STF in transgenic plants revealed by qRT-PCR. Bars represent means ± SE of three technical replicates.
(TIF)
(A) Phenotypes of STF overexpressing Brachypodium. UBI::GUS transformed Brachypodium was used as the control. Bars = 10 cm. (B) Transcript abundance of STF in transgenic plants revealed by qRT-PCR. Bars represent means ± SE of three technical replicates. (C) Comparison of seed size between STF transgenic and control (UBI::GUS) plants. Bar = 1 mm.
(TIF)
A pie chart representing the distribution of functional classifications of down-regulated (A) and up-regulated (B) probes based on the Gene Ontology Assignments.
(TIF)
Transcript levels of genes encoding putative Histone H4, Expansin A and Alpha-expansin in STF transgenic lines revealed by qRT-PCR. UBI::GUS expressing switchgrass plants were used as controls. Bars represent means ± SE of three technical replicates and two biological replicates. The asterisks indicate significant differences (** means p < 0.01, Student t-test).
(TIF)
Full-length amino acid sequences were aligned using Clustal W and the tree was constructed using MEGA4 with 1000 replicates. Species: Os, Oryza sativa; Bd, Brachypodium distachyon; Pv, Panicum virgatum. The green dots highlight the downregulated CKXs in STF overexpressing grasses.
(TIF)
(A) Schematic representation of the regions of OsCKX9 tested by ChIP experiments. P1-P5 are specific STF binding sites, while P6-P9 are non-specific sites used as control. (B) ChIP assay showing the association of STF with several regions in the promoter of OsCKX9 (P1-P5) compared to background signal (P6-P9) or the OsActin negative control. Bars represent means ± SE of three technical replicates and two biological replicates. The asterisks indicate significant differences (** p < 0.01, Student t-test).
(TIF)
Semi quantitative RT-PCR showing the expression of seven CKX genes in the leaves of four weeks old M. truncatula stf mutants compared to wild type. Numbers on the right show the number of PCR cycles used. * represents a gene that showed weak induction in stf microarray.
(TIF)
Plant height and tiller number of STF transgenic and control switchgrass plants were measured after 4-months of growth in the greenhouse. 7 tillers were used to measure internode length and diameter (internode Ⅱ), leaf blade length and width were measured in the 3rd leaf for each plant. The control represents the average of three independent UBI::GUS transgenic plants. Values are mean ± SE (n = 7). One or two asterisks indicate significance corresponding to *P < 0.05 or **P < 0.01 (Student t-test). G Ⅰ, Ⅱ, Ⅲ indicate group Ⅰ, Ⅱ, Ⅲ respectively.
(DOC)
aIVTDMD, in vitro true dry matter digestibility; bADL, acid detergent lignin. The STF transgenic switchgrass and control plants were harvested after 4-month growth in the greenhouse. Values are mean ± SE (n = 3). One or two asterisks indicate significance corresponding to *P < 0.05 or **P < 0.01 (Student t-test).
(DOC)
Fold change presented as relative abundance of transcript in STF overexpression/control (UBI::STF/UBI::GUS) switchgrass plants. P-value calculated as described in materials and methods. “//”, no significant similarity found.
(DOC)
Fold change presented as relative abundance of transcript in STF overexpression/control (UBI::STF/UBI::GUS) switchgrass plants. P-value calculated as described in materials and methods. “//”, no significant similarity found.
(DOC)
The top two leaf blades of three UBI::STF transgenic rice lines and wild type at vegetative stage (2 months after planting) were collected for Quantification of Cytokinins. iP, isopentenyladenine; iPR, iP riboside; iP9G, iP 9-glucoside; tZ, zeatin; tZR, zeatin riboside; tZ9G, zeatin 9-glucoside. The unit is ng·g-1 FW.
(DOC)
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files. Microarray data are available on ArrayExpress (https://www.ebi.ac.uk/arrayexpress/) under accession number E-MTAB-5539.