Highlights
-
•
Desmoid type fibromatosis, although benign and rare tumors, pose significant challenge to surgeons due to their high morbidity and recurrence rate.
-
•
After radical resection, closure of the abdominal wall defect requires the surgeońs expertise and an accurate technique.
-
•
Primary abdominal wall reconstruction using onlay mesh achieves high closure rate and low risk of significant complications.
Keywords: Case series, Abdominal wall reconstruction, Desmoid type fibromatosis, Desmoid resection, Surgical technique
Abstract
Background
Abdominal wall desmoid type fibromatosis management has been changing over recent years, from an aggressive approach towards a more conservative one. When radical resection is indicated, the surgical team faces the challenge of abdominal wall reconstruction, for which optimal technique is still debated. The present study reports the experience from a single center with abdominal closures after desmoid type fibromatosis resection.
Material and Methods
Retrospective analysis of patients who underwent abdominal wall closure after sporadic abdominal desmoid type fibromatosis radical resection from 1982 to 2013.
Results
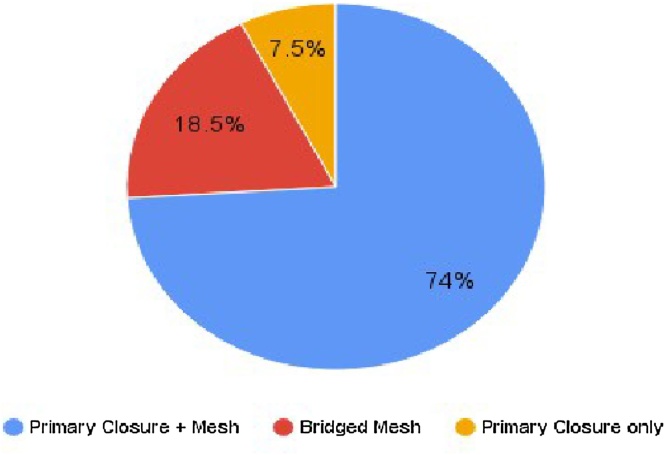
Twenty-seven patients were included, mean tumor diameter was 10 + 5.3 cm, and the main choice of abdominal wall reconstruction was midline closure with anterior rectus sheath relaxing incisions and polypropylene onlay mesh (74% of the cases). Only 7% of the cases required more complex procedures for skin closure. Mean follow-up was 5 years and 89% remained disease-free. No grade 4 or 5 complications were observed.
Conclusion
High midline fascial closure rate can be achieved after resection of abdominal wall desmoid tumor using relaxing incisions and mesh, with low complication rate.
1. Introduction
Desmoid tumors, desmoid type fibromatosis or aggressive fibromatosis are designations of a benign mesenchymal neoplasm with monoclonal proliferation, which belongs to a family of myofibroblastic fibromatosis characterized by aggressive local infiltration of surrounding tissues, with uncertain growth and increased chances of recurrence, despite no metastatic potential [1]. Although first described by MacFarlane in 1832 [2], the designation desmoid was given in 1838 by Muller in Berlin, who first used the term “desmos” relating to the Greek word – which means “similar to tendon” [3].
Such neoplasia is extremely rare, corresponding to 3% of soft-tissue tumors. Incidence is estimated in 2–4 per million population, with 900 new cases per year in the US. Some 70% of patients diagnosed with desmoid type fibromatosis have tumors related to Familial Adenomatous Polyposis (FAP), including a mutation in the APC gene [4]. In most of the patients with sporadic desmoid type fibromatosis (which are not related to FAP), the tumorigenesis is related to endocrine and physiologic factors, including estrogen hormonal stimulus and pregnancy [5], [6], [7]. In those cases, up to 25% of the patients have previous history of trauma or surgical incisions, which are related to the topography of these tumors [1], [8], [9], [10].
Management changed dramatically in the past decade. Surgery was the first line of treatment, consisting of radical therapy with wide resection of the tumor and adjoining tissues. Despite that aggressive approach, recurrence rates stood between 10% and 40%. More recently, additional treatments such as radiotherapy, chemotherapy, hormonal inhibitors and non-hormonal anti-inflammatories have been used as complement or even as the first approach [11], [12], [13], [14], [15], [16], [17], [18]. Whenever radical resection is indicated, the abdominal wall defect closure remains an important challenge to the surgical team and there is no defined consensus regarding the best choice of abdominal wall reconstruction.
Therefore, the aim of this study is to analyze the experience in abdominal wall reconstruction as a result of sporadic desmoid type fibromatosis resection at a single center in São Paulo, Brazil, specifically focusing on the surgical technique.
2. Material and methods
This case series was conducted based on a comprehensive retrospective analysis of charts from patients submitted to radical therapy of abdominal wall desmoid type fibromatosis treated between 1982 and 2013 in the General Surgery Service in the Division of Surgical Clinic III of the Hospital das Clínicas of the University of Sao Paulo, School of Medicine. Local ethical committee approved the study and the research registry number is 1853.
All patients had preoperative abdominal CT scan or magnetic resonance for surgical planning, and underwent operative procedure in a supine position with urinary catheterization and prophylactic antibiotics. The surgery comprised radical resection, with three centimeters free surgical margins, intraoperative frozen section of margins, and further resection in case of residual neoplasm. Despite the period of this cohort, all surgeons involved in the study belonged to Sarcoma and Melanoma Group of the General Surgery Service of Hospital das Clínicas – University of São Paulo. Indications were previously discussed at multidisciplinary meetings. Wide resection was achieved in all procedures, comprising the tumor, all layers of the abdominal wall and healthy adjacent tissues. Even though the period studied was long, the surgical procedure remained the same. All patients were followed in the outpatient clinic after discharge.
3. Results
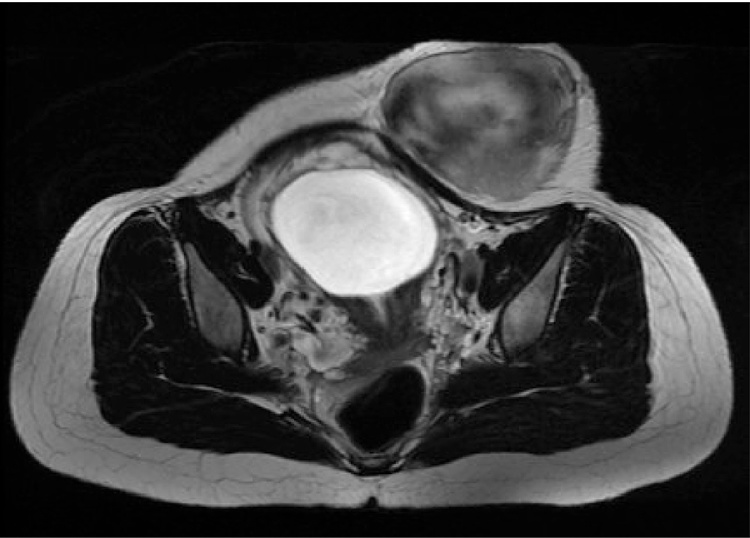
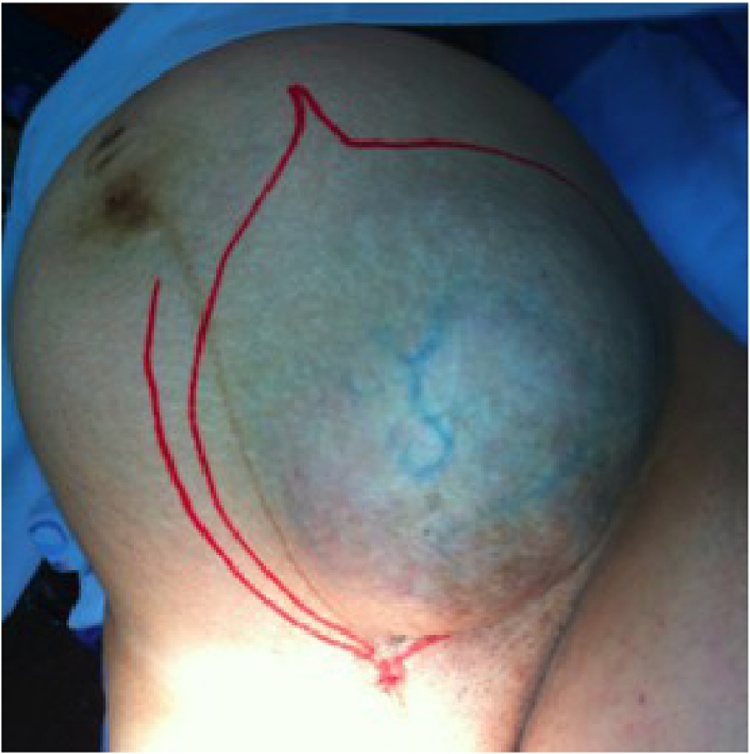
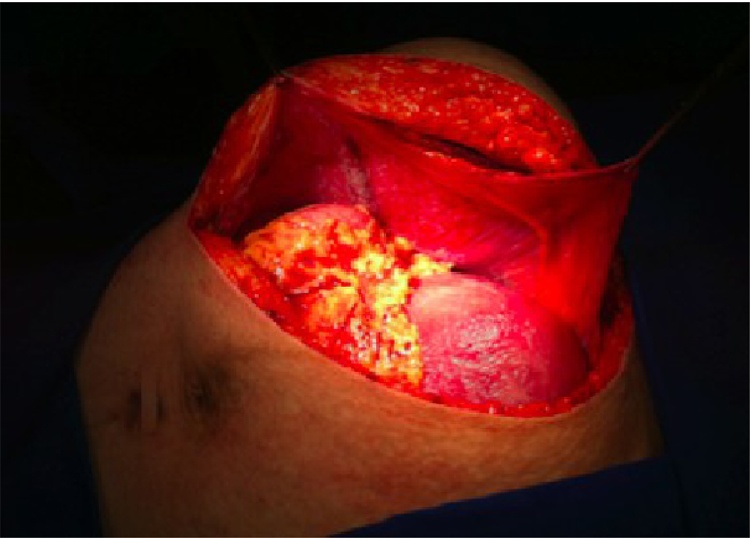
Twenty-seven patients were included in the study. Table 1 summarizes demographic data of the cohort. From the three patients diagnosed during their pregnancies, one was able to start treatment six months after the beginning of gestation, and another had to undergo surgery while in her 16th week of pregnancy, because the tumor's mass effect was compromising the development of the fetus (Fig. 1, Fig. 2, Fig. 3). The third patient underwent tumor resection during her C-section.
Table 1.
Demographic Characteristics of 27 Patients who underwent Abdominal Wall Desmoid Type Fibromatosis Resection.
| Gender | |
| Female | 24 (89%) |
| Male | 3 (11%) |
| Age at diagnosis (mean ± SD) | 34 ± 15 years |
| Resected specimens | |
| Size (mean ± SD) | 10 ± 5,3 cm |
| Weight (mean ± SD) | 558 ± 501 g |
| Pregnancy | |
| Past history | 22 (81%) |
| Diagnosed during pregnancy | 3 (11%) |
| Tumor at previous incision | 18% |
Fig. 1.
Magnetic Resonance in a pregnant woman revealing abdominal wall desmoid tumor.
Fig. 2.
Preoperative planning of skin resection.
Fig. 3.
Surgical incision showing pregnant uterus. The peritoneal surface protects bowel loops from polypropylene mesh repair.
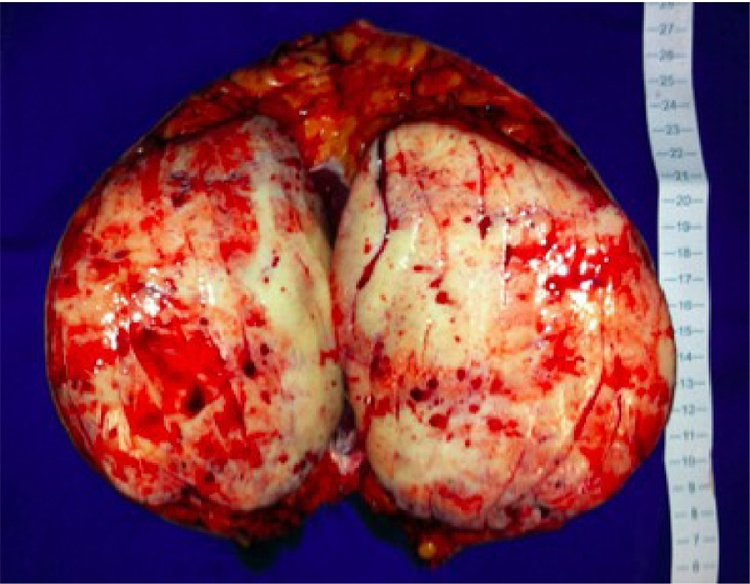
Seven patients had undergone previous abdominal procedures and 18% of those had tumors arising within surgical scars, in line with the desmoid type fibromatosis pathophysiology. Tumor weight varied from 15 to 2.125 g, and tumor diameter from 2 to 25 cm. (Fig. 4).
Fig. 4.
Large abdominal desmoid tumor specimen resected.
3.1. Surgical technique
Resection encompassed removal of the neoplasm, subcutaneous tissue, muscles, peritoneum and, in more superficial lesions, skin parts. After radical resection, the abdominal wall defect was addressed, and surgeons faced two reconstruction scenarios, summarized in Fig. 5. First, and more frequent, were abdominal wall defects that allowed primary closure. In this scenario, the use of relaxing Gibsońs incisions granted successful approach of the wound edges with no tension, followed by a midline fascial closure using polyglactin continuous suture (Fig. 6). In this group, onlay polypropylene mesh placement was frequent. This technique was used in 22 of 27 patients (81,5%).
Fig. 5.
Surgical technique applied for the abdominal wall reconstruction after desmoid type fibromatosis resection (n = 27).
Fig. 6.
Relaxing Gibson incisions on the anterior sheath of the rectus muscle. This technique was used to facilitate primary fascial closure.
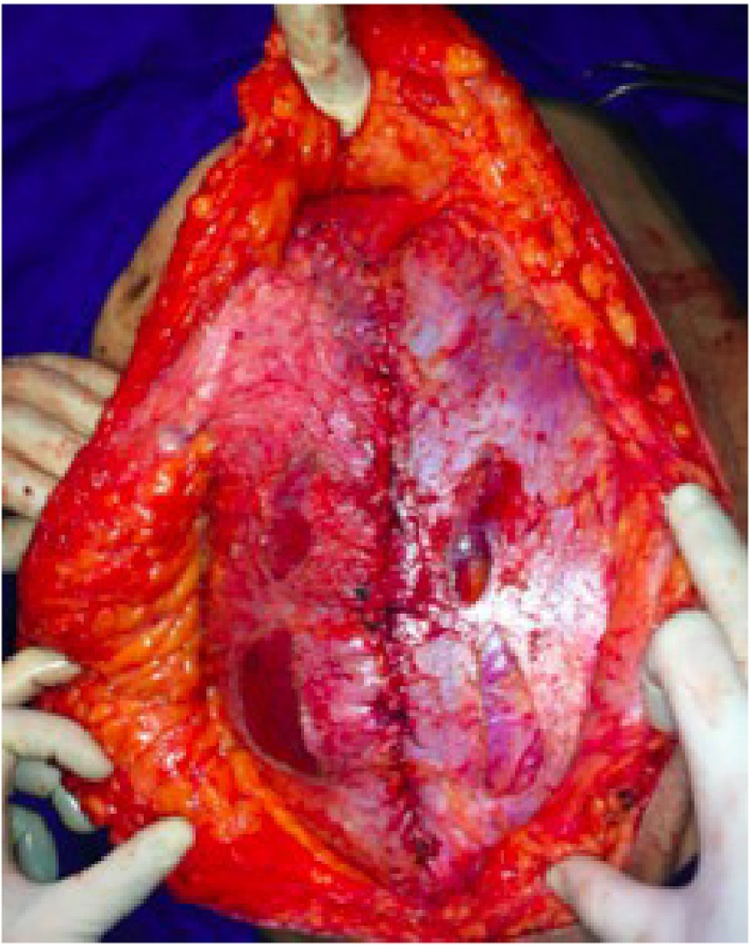
In the remaining cases, edge-to-edge fascial closure and relaxing incisions were not possible, either due to the size or the location of the defect, usually at upper or lower lateral quadrants. In such cases, the technique consisted of using synthetic material that could be in contact with the bowel and, when possible, using the greater omentum as an interface between the mesh and the abdominal organs (Fig. 7).
Fig. 7.
Abdominal wall closure using inlay mesh technique according to prosthetic material.
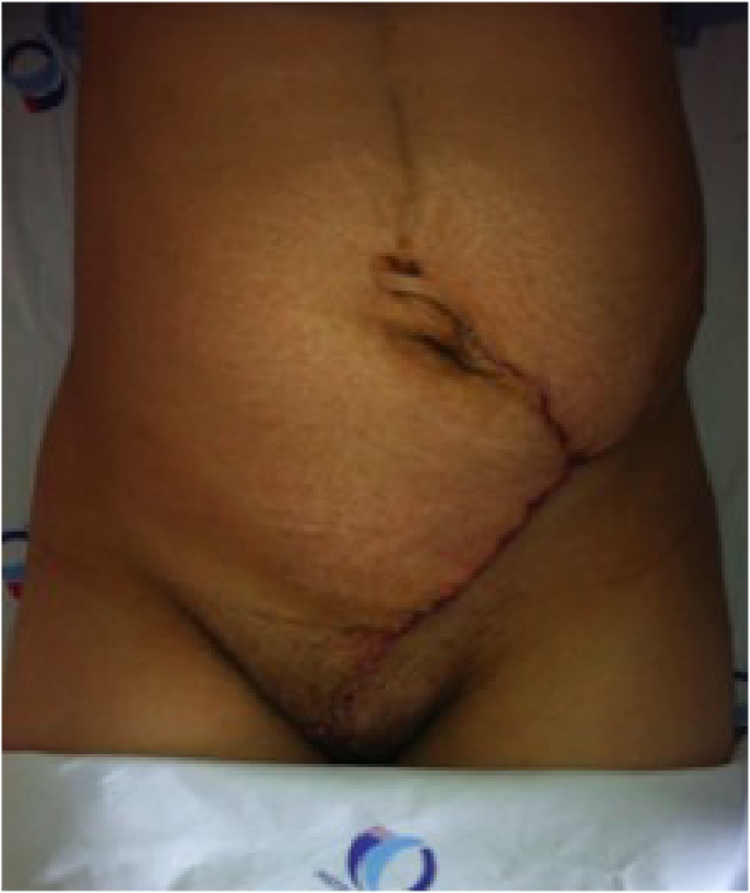
The skin was closed by approaching wound edges in 25 patients (93%). Only two patients required reconstruction by plastic surgeons. One of them required a local advancement flap after a 11 cm tumor excision (Fig. 8), and the other, a deep inferior epigastric perforator (DIEP) flap following the resection of a 6 cm desmoid tumor.
Fig. 8.
Primary skin closure after desmoid tumor resection.
3.2. Complications and follow-up
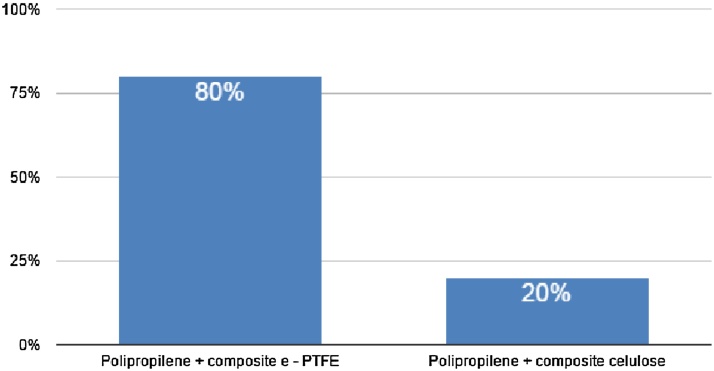
Postoperative complications were classified according to Clavien-Dindo grading system (Table 2). There was one case of small bowel erosion due to intestinal contact with a cellulose-based mesh diagnosed on follow-up. From the two patients who presented incisional hernias as a late complication, one had no mesh placed during the surgery, and the other was morbidly obese. There were no grades 4 and 5 complications.
Table 2.
Postoperative Complications according to Clavien-Dindo Classification.
| Classification | Incidence | Description |
|---|---|---|
| Grade 1 | 5 (18%) | Surgical Site Infection |
| Grade 2 | 1 (4%) | Deep Vein Thrombosis |
| Grade 3 | 2 (7%) | Small Bowel Erosion (1) Evisceration (1). |
After a mean follow up of 5 years (ranging from 20 to 360 months), 24 out of 27 (89%) patients remained disease-free. The other three had desmoid local recurrences with a mean disease-free survival of 22 months. Recurrences were treated by multimodal approach such as hormone or chemotherapy. No patients were lost to follow-up.
4. Discussion
The surgical treatment of desmoid type fibromatosis tumors is still a challenge for surgeons. Although the low incidence, aggressive infiltration and growth towards healthy adjacent tissue implicates in high morbidity associated with radical resection. This represents a major difference when comparing surgical treatment of desmoid tumors to soft tissue sarcomas with well-defined pseudo capsules.
Desmoid type fibromatosis treatment changed during the last decade. Surgical radical therapy used to be the first and unique choice of treatment, despite local relapse or high morbidity. Over the last years, chemotherapy and hormonal adjuncts have been the first option, especially when the location of the neoplasm predicts difficult reconstruction after resection. Even a more conservative approach, such as observation, was advocated. Surgery is now considered a final step to a multimodal approach, after all other options have been exhausted.
When radical resection is indicated, the surgeon will need to close the abdominal wall defect. Our group has preferably chosen the primary fascial closure with onlay polypropylene mesh reinforcement, associated with subcutaneous drainage, as the first choice of reconstruction. However, when the defect is too extensive or the resection margins do not favor primary closure of the wound, alternative options should exist in the surgeon's armamentarium. In such cases, our group prefers to place a synthetic mesh in inlay position to both close the defect and prevent complications such as fistula.
The literature review about desmoid type fibromatosis within the abdominal wall showed no consensus regarding the surgical technique to close the abdominal defects. Table 3 summarizes findings from other studies.
Table 3.
Case Series describing Abdominal Wall Reconstruction after Desmoid Type Fibromatosis Resection.
| Author | N | Diameter (cm) | Complications | Abdominal Wall Reconstruction | Follow-up (mo) |
|---|---|---|---|---|---|
| Sutton [19] | 7 | 11.7 | 0% | 2 polipropylen mesh | 42 |
| Stojadinovic [20] | 39 | 6 | 8% | Midline closure/bridged mesh | 53 |
| Phillips [21] | 23 | 8 | 0% | 2 polipropylen mesh | 39 |
| Bertani [22] | 14 | 4.7 | 0% | bridged mesh | 55 |
| Yezhelyev [23] | 6 | 11 | 20% | Midline closure/Acellular dermal matrix mesh | 28 |
| Catania [24] | 7 | – | 0% | Polipropylen/ePTFE em bridged mesh | 24 |
| Garvey [25] | 37 | 14/16.5 | 42% | Midline closure, onlay polipropylen mesh | 90 |
| Wilkinson [26] | 50 | 8 | 4% | Prosthetic mesh | 72 |
| Couto Netto, 2016 | 27 | 10 | 29% | Midline closure +onlay polipropylen/composite mesh | 59 |
In 1999, Sutton et al. described seven cases of abdominal wall desmoid resection. Reconstruction was performed using two layers of polypropylene mesh, and, when possible, with suturing of the greater omentum to create a “neo-peritoneum”. Surgical specimens had mean size of 11.7 cm. The authors describe 3.5 years of follow-up [19].
The experience from the Memorial Sloan Kettering Cancer Center, described by Stojadinovic et al., regards the treatment of 39 patients whose lesions had a mean size of six centimeters. The authors stated that 10% of the patients had primary closure of their wounds without mesh use while 85% of patients required the use of some prosthesis as an adjunct in the reconstruction procedure. That series, which lasted for 53 months, showed 8% of morbidity, especially wound complications [20].
In 2004, Phillips and his collaborators analyzed the resection of 23 desmoid type fibromatosis within the abdominal wall, 20 of which were primary lesions. Mean tumor size was eight centimeters, and a two-centimeter margin of palpable normal tissue was obtained. Reconstruction entailed using two layers of polypropylene mesh. The series described a mean follow-up of 39 months and no complications [21].
In addition to that, in 2009 Bertani et al. described the experience with 14 patients in the Milan Institute. Tumors had a mean diameter of 4.7 cm and patients were followed by a mean of 55 months. In two cases, the greater omentum was sutured to the margins of the defect to create a “neo-peritoneum”, while in five patients, two layers of mesh were used, one with polyglactin and a superficial one with polipropylen. In the seven remaining cases, lightweight low profile combined e-PTFE mesh was placed after the greater omentum fixation. The series neither describes closure of the midline nor any acute complication, with the exception of two cases of abdominal wall bulging diagnosed during the follow-up [22]. The use of e-PTFE mesh and acellular dermal matrix for abdominal wall closure after desmoid resection was also reported in other series with good results [23], [24].
In 2013, Garvey et al. retrospectively analyzed 164 patients with desmoid tumors, being 47 operated on because of lesions within the abdominal wall. The average size of the specimens were between 14 and 16.6 cm among patients who underwent resection without reconstruction of the abdominal wall and those who needed skin flap reconstruction. Surgical approach was different from the other studies, since component separation was used in 54% of the cases. Midline fascial closure was achieved in 68% of the patients, 10.6% required bridged prosthesis and 38% of them underwent concomitant flap reconstruction for skin replacement and soft-tissue coverage [25].
The large experience with desmoid type fibromatosis treatment from the Royal Marsden Hospital was reported by Wilkinson et al. From 50 patients who underwent surgery, mean tumor diameter was 8 cm and 56% had R1 resection (microscopically involved margins). Mesh was used in 96% of abdominal closures, although there was no description of type of mesh or surgical technique. Morbidity rate was 4% [26].
Apart from abdominal wall reconstruction primary closure and mesh placement, bridged or not, it is also possible to use single-stage myocutaneous free flaps to close the defect. Kadoch et al. described the closure of a 25 × 25 cm abdominal wall defect using a single-stage latissimus dorsi myocutaneous flap in combination with a synthetic mesh with no complications in 15 days of hospitalization [27].
Full-thickness reconstruction of the abdominal wall can also entail using a tensor fascia lata pedicled flap. In 2005, Rifaat et al. described 11 patients in whom the technique was applied without acute complications, except for one case of incisional hernia and one of abdominal wall bulging [28].
Another option to be considered is the use of myofascial flaps with microsurgical end-to-end anastomosis. Brenner and Rammelt reported the management of anterior abdominal wall defects using calcaneus microvascular soft tissue autologous flap. The complications described included hyperkeratosis in the donated area, and one patient required reconstructive plastic surgery [29].
The present study is limited by its retrospective nature and the long period. Potential biases or imprecision could be related to different surgeons performing the procedures, despite sharing the same surgical background, and the 30-year length of the study, during which novel research on management course emerged.
5. Conclusion
After such retrospective analysis, we have concluded that surgical treatment of the desmoid type fibromatosis and reconstruction of the abdominal wall defect is feasible, although challenging. It requires multidisciplinary approach including a surgical team with expertise in resection and reconstruction of the abdominal wall. Despite the need to implement extensive margins, fascial and skin closure can be achieved in the majority of the procedures without the need of complex reconstructions. Our groups suggests that, in light of the results presented, midline fascial closure and adjunct onlay polypropylene mesh placement should be performed whenever possible. Further prospectively designed studies focusing on the surgical technique of abdominal wall reconstruction after desmoid resection should enhance our understanding of the subject.
Conflicts of interest
The authors declare no conflicts of interest.
Sources of funding
The authors state no funding sources.
Ethical approval
Ethical approval was given by Ethical Comission for Research Projects Analysis (CAPPesq) under the reference number 115/16.
Research registration unique identifying number (UIN)
1853.
Process guideline statement
This work has been stated according to the PROCESS guidelines as described in Agha RA, Fowler AJ, Rajmohan S, Barai I, Orgill DP, for the PROCESS Group. Preferred reporting of case series in surgery; the PROCESS guidelines. International Journal of Surgery 36 (2016) 319–323 [30].
Author contributions
SDCN – study design, data collection, data analysis, manuscript writing.
FT – study design, data analysis, manuscript revision.
CAMM – data analysis, manuscript writing.
AA – data collection, data analysis.
EHA – study design, manuscript revision.
EMU – study design, manuscript revision.
Guarantor
Sérgio Dias do Couto Netto, the first author.
References
- 1.Singer S., Maki R.G., O’sullivan B. Cancer Principles and Practice of Oncology. 9th ed. 2011. Soft Tissue Sarcoma. [Google Scholar]
- 2.Mac Farlene J. Glasgow; D Robertson: 1832. Clinical Reports of the Surgical Practice of the Glasgow Royal Infirmary; pp. 63–66. [Google Scholar]
- 3.Muller J. G Reimer; Berlin: 1838. Ueber Den Feiern Bau Und Die Formen Der Krankhaften Geschwulste. [Google Scholar]
- 4.Singer S., Nielsen T., Antonescu C.R. Cancer Principles and Practice of Oncology. 9th ed. 2011. Molecular biology of soft tissue sarcoma. [Google Scholar]
- 5.Durkin A.J., Korkolis D.P., Al-Saif O. Full-Term gestation and transvaginal delivery after wide resection of an abdominal desmoid tumor during pregnancy. J. Surg. Oncol. 2005;89:86–90. doi: 10.1002/jso.20189. [DOI] [PubMed] [Google Scholar]
- 6.Priolli D.G., Martinez C.A.R., Mazzini D.L.S. Desmoid tumor of the abdominal wall during pregnancy: a case report. Rev Bras Ginecol Obstet. 2005;27(5):283–288. [Google Scholar]
- 7.Awwad J., Hammoud N., Farra C. Abdominal wall desmoid during pregnancy: diagnostic challenges. Case Rep. Obstet. Gynecol. 2013;2012:1–4. doi: 10.1155/2013/350894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tejpar S., Nollet F., Li C. Predominance of beta −catenin mutations and beta −catenin dysregulation in sporadic aggressive fibromatosis. Oncogene. 1999;18:6615. doi: 10.1038/sj.onc.1203041. [DOI] [PubMed] [Google Scholar]
- 9.Lazar A.J., Tuvin D., Hajibashi S. Specific mutation in the beta −catenin gene (CTNNB1) correlate with the local recurrence in the sporadic desmoide tumors. Am. J. Pathol. 2008;173:1518–1527. doi: 10.2353/ajpath.2008.080475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network Guidelines in Oncology. Soft Tissue Sarcoma. www.nccn.org.
- 11.Lev D., Kotilingam D., Wei C. Optimizing treatment of desmoid tumors. J. Clin. Oncol. 2007;25:1785–1791. doi: 10.1200/JCO.2006.10.5015. [DOI] [PubMed] [Google Scholar]
- 12.Mullen J.T., DeLaney T.F., Kobayashi W.K. Desmoid tumor: analysis of prognostic factors and outcomes in a surgical series. Ann. Surg. Oncol. 2012;19:4028–4035. doi: 10.1245/s10434-012-2638-2. [DOI] [PubMed] [Google Scholar]
- 13.Bonvalot S., Eldweny H., Haddad V. Extra-abdominal primary fibromatosis: aggressive management could be avoided in a subgroup of patients. EJSO. 2008;34:462–468. doi: 10.1016/j.ejso.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Peng P.D., Hyder O., Mavros M.N. Management and recurrence patterns of desmoids tumors: a multi-institutional analysis of 211 patients. Ann. Surg. Oncol. 2012;19:4036–4042. doi: 10.1245/s10434-012-2634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salas Sébastien, Dufresne Armelle, Bui Binh. Prognostic factors influencing progression-free survival. determined from a series of sporadic desmoid tumors: a wait-and-See policy according to tumor presentation. JCO. 2011;29:3553–3558. doi: 10.1200/JCO.2010.33.5489. [DOI] [PubMed] [Google Scholar]
- 16.Bertani E., Testori A., Chiappa A. Recurrence and prognostic factors in patients with aggressive fibromatosis. The role of radical surgery and its limitations. World J. Surg. Oncol. 2012;10:184–191. doi: 10.1186/1477-7819-10-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mankin H.J., Hornicek F.J., Springfield D.S. Extra-abdominal desmoid tumors: a report of 234 cases. J. Surg. Oncol. 2010;102:380–384. doi: 10.1002/jso.21433. [DOI] [PubMed] [Google Scholar]
- 18.Berri R.N., Baumann D.P., Madewell J.E. Desmoid tumor current multidisciplinary approaches. Ann. Plast. Surg. 2011;67:551–564. doi: 10.1097/SAP.0b013e3182084cf6. [DOI] [PubMed] [Google Scholar]
- 19.Sutton R.J., Thomas J.M. Desmoid tumors of the anterior abdominal wall. Eur. J. Surg. Oncol. 1999;25:398–400. doi: 10.1053/ejso.1999.0664. [DOI] [PubMed] [Google Scholar]
- 20.Stojadinovic A., Hoos A., Karpoff H.M. Soft tissue of the abdominal wall. Arch. Surg. 2001;136:70–79. doi: 10.1001/archsurg.136.1.70. [DOI] [PubMed] [Google Scholar]
- 21.Phillips S.R., Hern R.A., Thomas J.M. Agressive fibromatosis of the abdominal wall, limb and limb girdles. Br. J. Surg. 2004;91:1624–1629. doi: 10.1002/bjs.4792. [DOI] [PubMed] [Google Scholar]
- 22.Bertani E., Chiappa A., Testori A. Desmoid tumors of the anterior abdominal wall: results from a monocentric surgical experience and review of the literature. Ann. Surg. Oncol. 2009;16:1642–1649. doi: 10.1245/s10434-009-0439-z. [DOI] [PubMed] [Google Scholar]
- 23.Yezhelyev M., Deigni O., Losken A. Management of full thickness abdominal wall defects following tumor resection. Ann. Plast. Surg. 2012;69:186–191. doi: 10.1097/SAP.0b013e31821d0715. [DOI] [PubMed] [Google Scholar]
- 24.Catania G., Ruggeri L., Iuppa G. Abdominal wall reconstruction with intraperitoneal prothesis in desmoid tumor surgery. Updates Surg. 2012;64:43–48. doi: 10.1007/s13304-011-0109-0. [DOI] [PubMed] [Google Scholar]
- 25.Garvey P.B., Booth J.H., Baumann D.P. Complex reconstruction of desmoid tumor resection does not increase desmoid tumor recurrence. Am. Coll. Surg. 2013:1–9. doi: 10.1016/j.jamcollsurg.2013.04.038. [DOI] [PubMed] [Google Scholar]
- 26.Wilkinson M.J., Chan K.E., Hayes A.J. Surgical outcomes following resection for sporadic abdominal wall fibromatosis. Ann. Surg. Oncol. 2014;21(July (7)):2144–2149. doi: 10.1245/s10434-014-3618-5. [DOI] [PubMed] [Google Scholar]
- 27.Kadoch V., Bodin F., Himy S. Latissimus dorsi free flap for reconstruction of extensive full −thickness abdominal wall defect.: a case of desmoid tumor. J. Visc. Surg. 2010;147(2):45–48. doi: 10.1016/j.jviscsurg.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rifaat M.A., Gawad W.S.A. The use of tensor fascia lata pedicled flap in reconstructing full thickness abdominal wall defects and groin defects following tumor ablation. J. Egypt Nat. Cancer Inst. 2005;17(3):139–148. [PubMed] [Google Scholar]
- 29.Brenner P., Rammelt S. Abdominal wall and foot reconstruction after extensive desmoid tumor resection with free tissue transfer. Langenbeck’s Arch. Surg. 2002;386:592–597. doi: 10.1007/s00423-002-0277-y. [DOI] [PubMed] [Google Scholar]
- 30.Agha R.A., Fowler A.J., Rajmohan S., Barai I., Orgill D.P., for the PROCESS Group Preferred reporting of case series in surgery; the PROCESS guidelines. Int. J. Surg. 2016;36:319–323. doi: 10.1016/j.ijsu.2016.10.025. [DOI] [PubMed] [Google Scholar]