Abstract
Tobacco and alcohol use are linked behaviors that individually and synergistically increase the risk for negative health consequences. This study was a two-group, randomized clinical trial evaluating the efficacy of a behavioral intervention, “Motivation And Problem Solving Plus” (MAPS+), designed to concurrently address smoking cessation and the reduction of at-risk drinking. Targeted interventions may promote coaction, the likelihood that changing one behavior (smoking) increases the probability of changing another behavior (alcohol use). Puerto Ricans (N=202) who were smokers and at-risk drinkers were randomized to standard MAPS treatment focused exclusively on smoking cessation (S-MAPS), or MAPS+, focused on cessation and at-risk drinking reduction. Drinking outcomes included: number of at-risk drinking behaviors, heavy drinking, binge drinking, and drinking and driving. MAPS+ did not have a significant main effect on reducing at-risk drinking relative to S-MAPS. Among individuals who quit smoking, MAPS+ reduced the number of drinking behaviors, the likelihood of meeting criteria for heavy drinking relative to S-MAPS, and appeared promising for reducing binge drinking. MAPS+ did not improve drinking outcomes among individuals who were unsuccessful at quitting smoking. MAPS+ showed promise in reducing at-risk drinking among Puerto Rican smokers who successfully quit smoking, consistent with treatment enhanced coaction. Integrating an alcohol intervention into cessation treatment did not reduce engagement in treatment, or hinder cessation outcomes, and positively impacted at-risk drinking among individuals who quit smoking. Findings of coaction between smoking and drinking speak to the promise of multiple health behavior change interventions for substance use treatment and chronic disease prevention.
Keywords: smoking, at-risk drinking, coaction, multiple health risk behaviors, Latinos
1. Introduction
Smoking and problematic alcohol use are both major risk factors for death and chronic disease. For example, almost one-third of all cancers and cardiovascular disease in the U.S. are directly attributable to smoking1,2 and 10% of deaths are attributable to excessive alcohol use 3,4. Not only are tobacco and alcohol use major public health problems individually, they are clustered within individuals5 and the simultaneous use of both substances synergistically increases the risk for chronic disease and mortality6-8. Moreover, these negative consequences are not limited to only heavy use. Light smoking increases cancer risk as does “at-risk” drinking9. At-risk drinking, as defined by the Institute of Medicine and the National Institute on Alcohol Abuse and Alcoholism, is characterized by engaging in chronic moderate or high levels of use and/or frequent binge drinking, and is related to numerous negative health and social consequences10-12.
Fortunately, the risk for various diseases and other negative health consequences declines following smoking cessation and the reduction of alcohol use13-15. Thus, a critical strategy for chronic disease prevention is to reduce the use of these two substances. National recommendations include integrating screening and treatment of tobacco use and at-risk drinking into health-related settings12,16. Further, because the clustering of smoking and drinking increases disease risk, treatment costs, and public health burden, there is an urgent need for interventions designed to change multiple risk factors17. Some research suggests that multiple risk behavior interventions are cost effective, efficacious, and well received18,19. In addition, research on multiple health risk behaviors is increasingly emphasizing the study of coaction, defined as the likelihood that change in one behavior increases the probability of change in a second behavior20,21. Importantly, coaction is more likely to occur in the context of targeted interventions addressing the behaviors of interest, indicating that coaction can be induced via treatment. However, few studies have evaluated coaction between smoking and at-risk drinking either via treatment or as part of the natural history of change in these behaviors22-25.
Combined treatments for smoking and alcohol use have been primarily conducted among individuals who were in treatment for alcohol abuse/dependence, with smoking as a secondary target of treatment26. However, the few studies that have evaluated interventions that add treatment for nondependent alcohol use to smoking cessation treatment have yielded promising results for both smoking and drinking outcomes27,28. Given that approximately half of all smokers attempt to quit each year29, introducing alcohol risk reduction into smoking cessation treatments could be an effective approach to increasing the impact of substance use treatment and chronic disease prevention efforts.
Motivational enhancement and problem solving/coping skills training are empirically supported treatments for both smoking and problematic alcohol use16,30,31, and recommendations have been made for the integration of these approaches32,33. Motivation And Problem Solving (MAPS) is an intervention that combines attributes of both approaches16,34,35 to address the consideration, initiation, and maintenance of behavior change36. MAPS utilizes a Wellness Program that is developed in collaboration with the participant. The Wellness Program addresses treatment goals related to behavior change as well as other salient concerns for the participant such as mood and contextual factors36. Compared to approaches that emphasize stages or phases of change37, MAPS conceptualizes motivation for behavior change and maintenance as a dynamic and fluid process that varies from moment to moment depending on both individual and contextual factors36. Randomized clinical trials have demonstrated that MAPS and its precursors are effective interventions for improving smoking related outcomes including promoting a quit attempt, cessation, and relapse prevention38-40.
The current study evaluated the efficacy and coaction potential of using MAPS to address both smoking cessation and reduction of at-risk alcohol use among Puerto Rican smokers who were also at-risk drinkers. Like the general population of the U.S., tobacco and alcohol use are major public health problems in Puerto Rico (PR). Although the adult prevalence of smoking in PR is lower (14.8%) than the prevalence of smoking in the U.S. (18.1%)41,42, three (heart disease, cancer and cerebrovascular disease) of the five leading causes of death in PR are associated with smoking43. Similarly, although Puerto Ricans living in PR are less likely to drink than are either the general population or Latinos in the U.S., those who do drink are more likely to be binge drinkers44. Thus, reducing both smoking and drinking is crucial to disease prevention in this population.
A standard MAPS treatment (S-MAPS) focused on smoking cessation alone was compared to an enhanced MAPS intervention (MAPS+) that addressed both smoking cessation and the reduction of at-risk drinking behaviors. MAPS+ was hypothesized to be more effective than S-MAPS at reducing at-risk drinking and to produce greater coaction such that individuals who quit smoking would be more likely to also reduce at-risk drinking behaviors. Similar to coaction metrics in previous research23, in our study coaction was evidenced by individuals indicating change in a second behavior (e.g. at-risk drinking) after successfully changing an initial behavior (e.g. smoking). S-MAPS and MAPS+ were hypothesized to be equally effective with respect to smoking cessation.
2. Methods
2.1. Participants
The study was a two-group randomized clinical trial (RCT) conducted among 202 Puerto Rican smokers who were attempting to quit smoking and who were also at-risk drinkers. Inclusion criteria were: current daily smoker interested in quitting smoking in the next 30 days, aged ≥ 18 years, resident of PR, having a working telephone number and home address, no other household members enrolled in the study, and meeting at least one at-risk drinking criterion in the past 30 days [average of ≥ 2 drinks per day (males) or ≥ 1 drink per day (females); two or more occasions of consuming ≥ 5 drinks (males) or ≥ 4 drinks (females); or one or more occasions of driving after consuming ≥ 3 drinks]11,12. Exclusion criteria were: currently pregnant, currently incarcerated, or having a score of ≥16 on the Alcohol Use Disorders Identification Test45, which indicated a probable alcohol use disorder and the need for more than brief counseling. Major causes of ineligibility included not meeting criteria for at-risk drinking (37%) or scoring 16 or above on the AUDIT (62%). Individuals excluded from the study due to high AUDIT scores were given referral for more intensive alcohol treatment.
2.2. Procedures
This trial was registered on the United States National Institutes of Health Clinical Trials Registry, and was approved by the Institutional Review Board.
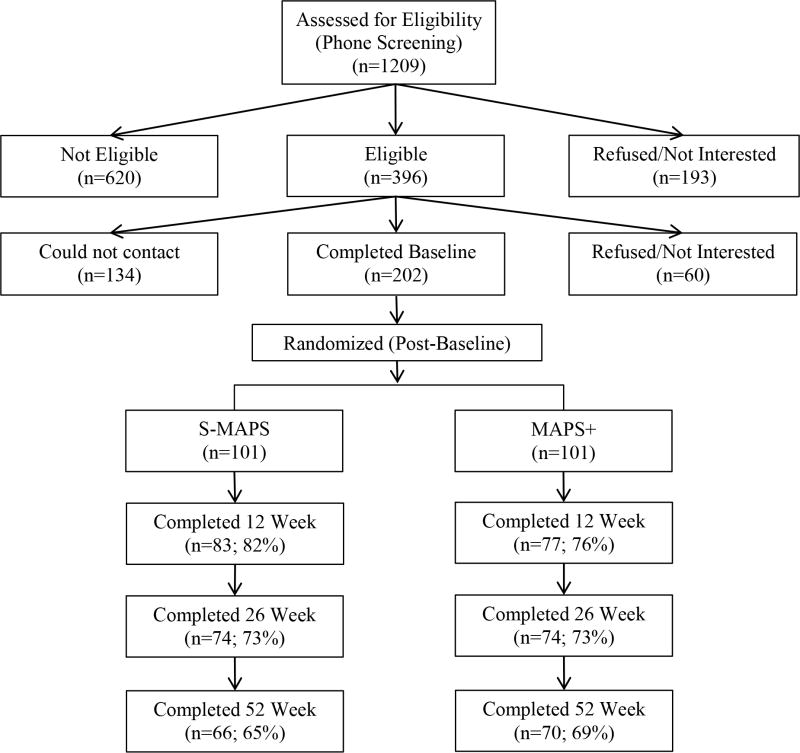
Potential participants were recruited through the Puerto Rico Quitline (PRQ) and via local newspaper advertisements in PR. Individuals were screened for eligibility by phone, and eligible participants were scheduled for a Baseline assessment call. All individuals who were ineligible, withdrew from the study, or chose not to participate, remained eligible for free smoking cessation treatment from the PRQ. Baseline assessments were conducted following verbal informed consent procedures (i.e. audiotaped, encrypted, and stored in password protected files). Enrollment spanned September 2009 through May 2011. After the Baseline assessment call, participants were randomly assigned to S-MAPS or MAPS+ using a computer-generated form of adaptive randomization called minimization46,47. Compared to techniques such as stratification, minimization results in a better group balance with respect to participant characteristics. Minimization also provides for balanced treatment groups throughout the randomization process and extended accrual periods. The variables used for minimization randomization were: age, gender, cigarettes/day, drinks/day and depressive symptoms (as measured by The Center for Epidemiologic Studies Depression Scale48. Blinded follow-up assessments were conducted via telephone at weeks 12, 26 and 52 after Baseline. Study procedures were conducted in Spanish. Different personnel functioned as counselors and assessors. Participant's compensation (gift cards up to $100) was for data collection only and was not associated with treatment. Figure 1 shows participant flow.
Figure 1.
CONSORT flowchart of recruitment, randomization, attendance and follow-ups. S-MAPS = Standard Motivation And Problem Solving treatment for smoking cessation; MAPS + = Motivation And Problem Solving for smoking cessation plus at-risk alcohol use
Counselor selection criteria and training were consistent with several major national clinical trials evaluating motivational interviewing-based approaches and with real world quitline counselors49,50. Five counselors provided the treatments. All counselors had a masters or doctoral degree in counseling or psychology, at least two years of clinical experience, and received approximately 40 hours of training in tobacco cessation and MAPS.
2.3. Interventions
All participants received Spanish-language self-help materials addressing alcohol and tobacco use51,52 and up to seven telephone counseling calls, which lasted approximately 20-30 minutes each, and occurred within 26 weeks of the Baseline assessment. The sessions were usually completed over the six month time frame. Sessions typically occurred more often in the beginning as participants attempted to set a quit date, with more time elapsing between sessions as the treatment progressed. For participants willing to set a quit date, a typical call schedule included two pre-quit calls, one call on the quit day, and four calls post-quit day. However, is important to highlight that MAPS is characterized by a patient-centered approach, and as such, the timing of each call was flexible, based on the participant's individual needs and negotiated together by the participant and counselor.
In brief, S-MAPS utilized motivational enhancement techniques to increase motivation to change smoking behavior and enhance participant's self-efficacy to make a quit attempt35. In addition, S-MAPS utilized skills training and problem solving techniques to assist participants in initiating and maintaining their quit attempts, and in recovering from lapses16,34. In the S-MAPS group, the Wellness Program addressed treatment goals related to smoking abstinence as well as other relevant issues for the participant. Importantly, this treatment approach underscore participant's language to help guide the course of the treatment from moment-to-moment and determine when to shift from a motivational to a skill-building focus, and vice versa (for details see Vidrine et al., 2013)36.
The content of MAPS+ was identical to S-MAPS with respect to smoking cessation. Additionally, MAPS+ incorporated discussion of at-risk drinking and the counselor encouraged the inclusion of reducing at-risk drinking as a goal in the Wellness Program. Typically, the introduction of alcohol use in MAPS+ occurred in relation to participant's smoking patterns (e.g. contexts in which they smoke, past relapses). If alcohol use was not brought up by the participant, the counselor would note that drinking is a common antecedent of smoking and would inquire about how drinking might influence the participant's smoking in order to introduce the topic. In MAPS+, the topic of at-risk drinking was raised in each session by the counselor if the participant themselves did not raise it. In S-MAPS, the counselor never raised the issue of at-risk drinking, but would be responsive to its discussion as a trigger for smoking (similarly to other triggers like stress, etc.) if the issue was raised by the participant.
To ensure treatment fidelity and monitor drift from the protocol, all counseling sessions were recorded, and two randomly selected sessions per month per counselor were coded using a modified version of the Motivational Interviewing Treatment Integrity Code 3.1.1(MITI)53. Additionally, the supervisor assessed intervention fidelity by assuring that the counselor raised the discussion of at-risk drinking only in the MAPS+ sessions.
2.4. Measures
2.4.1. Demographic and tobacco-related variables at baseline
Demographic and tobacco-related variables collected at Baseline included age, gender, partner status, education, employment status, income, daily smoking rate, and time to first cigarette upon awakening. Daily smoking rate and time to first cigarette comprised the Heaviness of Smoking Index (HSI)54.
2.4.2. Drinking Outcomes
Drinking outcomes included indicators of at-risk drinking within the past 30 days. Specifically, to assess heavy drinking, participants were asked to indicate how many drinks they consumed on average each day of the week. To assess binge drinking, participants were asked how many times they consumed four or more drinks (for females) and five or more drinks (for males) in one occasion. To assess drinking and driving, they were asked how many times they drove after consuming three or more drinks. Raw data were categorized to identify respondents that met criteria for one or more at-risk drinking indicators, as defined in the eligibility criteria. For instance, primary drinking outcomes were: (a) number of at-risk drinking behaviors for which the person met criteria; (b) average number of drinks per day; (c) number of binge drinking episodes; and, (d) number of drinking and driving episodes. Secondary drinking outcomes were dichotomous indicators of whether the person met criteria (yes/no) for: (a) overall at-risk drinking (meeting criteria for either heavy drinking, binge drinking, or drinking and driving); (b) heavy drinking - an average of ≥ 2 drinks per day (males) or ≥ 1 drink per day (females); (c) binge drinking - two or more occasions of consuming ≥ 5 drinks (males) or ≥ 4 drinks (females); and (d) drinking and driving - one or more occasions of driving after consuming ≥ 3 drinks.
Drinking outcomes were measured at each follow-up (weeks 12, 26 and 52).
2.4.3. Smoking Outcomes
Smoking status was defined as a seven-day point prevalence smoking abstinence, indicated by a self-report of not smoking during the previous 7 days. Smoking outcomes were measured at each follow-up (weeks 12, 26 and 52).
2.5. Data Analysis
Descriptive statistics, chi-square tests, and t-tests were used to describe the sample and examine between-group differences in Baseline demographic characteristics, tobacco use, and at-risk drinking behaviors. Because all of the outcome measures were repeated over time and correlated within subjects, the data analytic approach utilized generalized linear mixed model (GLMM) regression55,56. For continuous outcomes, linear mixed models (LMMs) were fitted. For binary outcomes, GLMM with logit link and binomial variance functions were used. All tests were two-sided.
To examine treatment effects at the end of the intervention and beyond, the main effect of treatment condition (S-MAPS vs. MAPS+) on each of the primary and secondary at-risk drinking outcomes over time (i.e., weeks 26 and 52) was tested after adjusting for time, demographics [age, gender, partner status, education, employment and income], smoking status (at week 12) and the baseline measure of the specific outcome being analyzed (e.g., average drinks/day). Additionally, to evaluate the effect of treatment on coaction between smoking and alcohol use, the interaction between treatment and smoking status at week 12 was tested to prospectively predict the reduction of at-risk drinking overtime (i.e., weeks 26 and 52) after adjusting for time, the same covariates, treatment, and week 12 smoking status.
To evaluate treatment effects on smoking outcomes over time, analyses included treatment condition (S-MAPS vs. MAPS+) as the predictor, adjusted for time, demographics, and baseline HSI. An intent-to-treat imputation procedure was utilized for smoking outcomes whereby participants lost to follow-up were treated as smokers. This approach was compared with a “Completers only” analyses.
3. Results
3.1. Participants characteristics and follow-up rates
There were no group differences in demographics, tobacco use, or at-risk drinking behaviors at Baseline (Table 1). Follow-up rates were 79% (n = 160) at week 12, 73% (n = 148) at week 26, and 67% (n = 136) at week 52. Over 68% of the sample completed all seven counseling calls, and 82.1% completed at least 4 of the 7 calls. Follow-up rates and completion of counseling sessions did not differ by treatment group (Figure 1; X2=4.65, df (3); p < .05).
Table 1.
Baseline participant characteristics by treatment group
| Variable | Total (N= 202) | S-MAPS (n=101) | MAPS+ (n=101) | p |
|---|---|---|---|---|
| Demographics | ||||
| Age, years (M±SD) | 43.59 (11.8) | 42.97 (11.7) | 44.21 (11.9) | 0.46 |
| Gender, n (%) | 0.89 | |||
| Female | 93 (46.0) | 47 (46.5) | 46 (45.5) | |
| Male | 109 (54.0) | 54 (53.5) | 55 (54.5) | |
| Partner status, n (%) | 0.89 | |||
| Married/Living with partner | 81 (40.1) | 41 (40.6) | 40 (39.6) | |
| Not living with partner | 121 (59.9) | 60 (59.4) | 61 (60.4) | |
| Education, n (%) | 1.00 | |||
| ≤ High School | 64 (31.7) | 32 (31.7) | 32 (31.7) | |
| > High School | 138 (68.3) | 69 (68.3) | 69 (68.3) | |
| Employment status, n (%) | 0.20 | |||
| Employed | 115 (56.9) | 62 (61.4) | 53 (52.5) | |
| Unemployed | 87 (43.1) | 39 (38.6) | 48 (47.5) | |
| Household income, n (%) | 0.34 | |||
| <$24,000/year | 133 (65.8) | 64 (64.0) | 69 (70.4) | |
| ≥$24,000/year | 65 (34.2) | 36 (36.0) | 29 (29.6) | |
| Tobacco use | ||||
| Cigarettes per day (M±SD) | 16.82 (8.6) | 16.62 (9.0) | 17.02 (8.3) | 0.74 |
| Smokes ≤ 5 minutes after waking, n (%) | 72 (35.64) | 36 (35.6) | 36 (35.6) | 1.00 |
| At-risk drinking behaviors | ||||
| Primary Outcomes [M±SD] | ||||
| Heavy drinking (average drinks per day) | 2.63 (2.2) | 2.56 (2.1) | 2.70 (2.3) | 0.65 |
| Binge drinking (episodes in past 30 days) | 6.46 (6.4) | 6.75 (6.5) | 6.16 (6.3) | 0.51 |
| Drinking and driving (episodes in past 30 days) | 2.43 (4.5) | 2.78 (5.2) | 2.07 (3.6) | 0.26 |
| Secondary Outcomes [(meeting criteria), n (%)] | ||||
| Heavy drinking | 147 (72.8) | 76 (75.3) | 71 (70.3) | 0.43 |
| Binge drinking | 176 (87.1) | 89 (88.1) | 87 (86.1) | 0.67 |
| Drinking and driving | 89 (44.1) | 43 (42.6) | 46 (45.5) | 0.67 |
Note: S-MPAS = Standard Motivation And Problem Solving; MAPS+ = Enhanced Motivation And Problem Solving.
3.2. Drinking Outcomes
The main effect of treatment condition was not statistically significant for any of the primary or secondary at-risk drinking outcomes (Table 2). However, the interaction between treatment condition and smoking status at week 12 (coaction) was significant for multiple at-risk drinking outcomes (Table 2). Specifically, the treatment by smoking status interaction significantly predicted the primary outcomes of number of at-risk drinking behaviors and number of binge drinking episodes, and the secondary outcomes of meeting criteria for heavy drinking and binge drinking (Table 2).
Table 2.
Effects of treatment on drinking outcomes overtime
| Overall At-risk Drinking | Heavy Drinking | Binge Drinking | Drinking & Driving | |
|---|---|---|---|---|
| Primary Outcomes | B [CI] | B [CI] | B [CI] | B [CI] |
|
| ||||
| Main Treatment Effect^ | −0.01 [−0.27, 0.24] | 0.04 [−0.26, 0.34] | 0.50 [−0.49, 1.49] | −0.14 [−0.63, 0.35] |
| Coaction Effect | ||||
| Treatment*Smoking Status (week 12)^^ | −0.72 [−1.30, 0.14]** | −0.33 [−1.02, 0.35] | −2.51 [−4.77, −0.26]* | −0.84 [−2.03, 0.34] |
|
| ||||
| Secondary Outcomes | OR (CI) | OR (CI) | OR (CI) | OR (CI) |
|
| ||||
| Main Treatment Effect^ | 1.05 (0.46, 2.39) | 0.78 (0.27, 2.29) | 1.15 (0.54, 2.45) | 0.72 (0.29, 1.79) |
| Coaction Effect | ||||
| Treatment*Smoking Status (week 12)^^ | 0.22 (0.03, 1.44) | 0.03 (0.002, 0.49)** | 0.12 (0.02, 0.71)* | 0.71 (0.07, 6.72) |
Note: S-MAPS is the reference category; 1=meeting criteria for the specific at-risk behavior
Demographic covariates are age, gender, partner status, education, employment and income
Baseline alcohol = baseline (pre-treatment) value for the specific alcohol outcome variable being analyzed
Boldface indicates statistical significance (*p < .05, **p < .01)
Adjusted for time, demographics, week 12 smoking status & baseline alcohol
Adjusted for time, demographics, treatment, week 12 smoking status & baseline alcohol
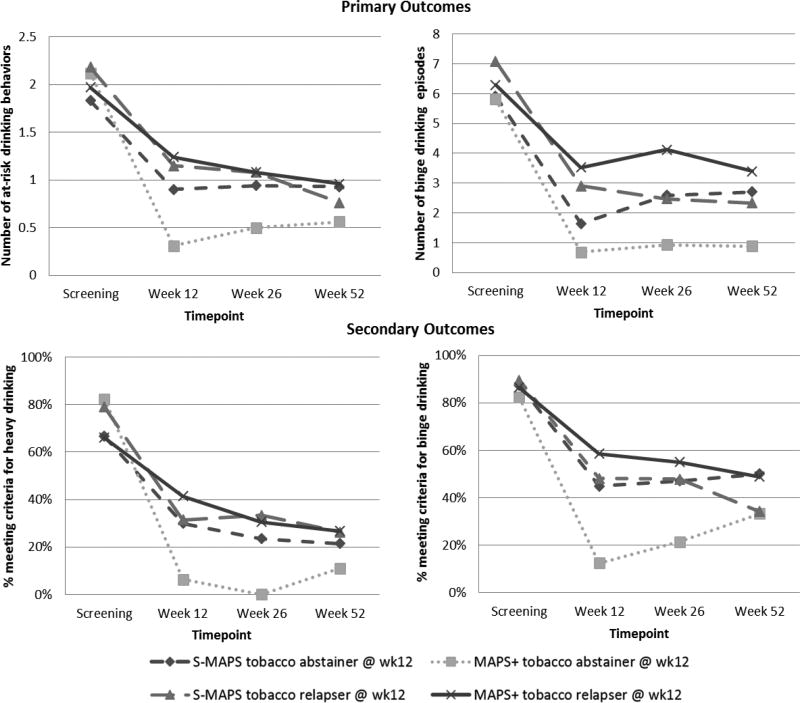
Follow-up analyses of the significant interactions revealed that MAPS+ increased the likelihood of reducing various at-risk drinking behaviors compared to S-MAPS, but only among smokers who successfully quit, as predicted by the coaction hypothesis. Figure 2 illustrates the reduction of at-risk alcohol drinking patterns for the treatment conditions by smoking status. For smoking abstainers at week 12, MAPS+ was significantly more efficacious than S-MAPS in reducing the number of at-risk drinking behaviors (B= −0.560; 95% CI = −1.067, − 0.053; p = 0.031) and the odds of meeting criteria for heavy drinking (OR= 0.042; 95% CI = 0.003, 0.579; p = 0.018), and approached significance for reducing the odds of meeting criteria for binge drinking (OR= 0.214; 95% CI = 0.043, 1.060; p = 0.059), but was not statistically significant in reducing the number of binge drinking episodes (OR= −1.42; 95% CI = −3.40, 0.56; p = 0.159). Among smokers at week 12, MAPS+ did not reduce the total number of at-risk drinking behaviors (B= 0.158; 95% CI = −0.126, 0.442; p = 0.276), number of binge drinking episodes (B= 1.092; 95% CI = −0.015, 2.199; p = 0.053), the likelihood of meeting criteria for heavy drinking (OR= 1.513; 95% CI = 0.490, 4.667; p = 0.471), or the likelihood of meeting criteria for binge drinking (OR= 1.842; 95% CI = 0.802, 4.233; p = 0.150).
Figure 2.
At-risk drinking behaviors by treatment group and smoking status.
3.3. Smoking Outcomes
As expected, there were no effects of treatment condition on smoking abstinence overtime or at any single time point when examining intent-to-treat or completers only analyses, including after controlling for demographics and HSI (all p's > .05).
4. Discussion
MAPS+, a theoretically and empirically-based intervention to simultaneously treat smoking and at-risk alcohol use, showed promise in reducing at-risk drinking behaviors among Puerto Rican smokers, but only among those individuals who were able to quit smoking. These effects are consistent with treatment enhanced coaction. Among those individuals who quit smoking, MAPS+ reduced the overall number of at-risk drinking behaviors and the likelihood of meeting criteria for heavy drinking relative to S-MAPS, and appeared promising for reducing binge drinking, although the treatment effect was not significant. MAPS+ did not improve drinking outcomes among individuals who were unsuccessful at quitting smoking.
Contrary to our hypotheses, MAPS+ did not have a significant main effect on reducing at-risk drinking relative to S-MAPS. Notably, both groups showed substantial declines in at-risk drinking behaviors from screening to week 12 and those declines were sustained across the entire one year follow-up period. The findings demonstrating considerable reductions in drinking behaviors in both treatment groups is consistent with results from Kahler et al.27, one of the few other trials explicitly designed to address drinking in the context of a smoking cessation intervention. Together, these results suggest that participants receiving smoking cessation treatments may learn and apply principles and skills for behavior change that apply across behaviors, or that such participants may be motivated for changing multiple behaviors (at least with respect to smoking and drinking). Importantly, however, the current study found that the reductions in at-risk drinking differed among those who continued to smoke and those who successfully quit only when treatment specifically addressed the reduction of drinking. That is, there was evidence for MAPS+ induced coaction related to successful smoking cessation and declines in at-risk drinking, but not coaction related to abstinence from smoking per se57,58. These findings are consistent with previous research indicating that higher levels of coaction typically occur among participants receiving treatments designed to promote multiple behavior change compared to usual care or control groups21,23, and indicate that further research is needed to elucidate the natural history of alcohol use among smokers attempting to quit.
Our findings uniquely contribute to the literature26,59-61 by suggesting that concurrent treatment for at-risk alcohol use in the context of smoking cessation treatment may facilitate coaction. Given that coaction has typically been examined in the context of stage-based interventions, this study also contributes to the dearth of research addressing coaction in the context of other theoretically-driven approaches21,23. MAPS-based interventions specifically target behavior change in an integrated and holistic way by addressing substance use within the context of general life stressors and regardless of the individual's motivation to change36. By taking a broad, holistic approach to treatment, MAPS-based interventions may be a particularly appropriate approach for addressing multiple risk factors simultaneously. The results also indicate that integrating an alcohol intervention into smoking cessation treatment does not undermine smoking outcomes (or improve them either)26,27. Given the phone-based nature of the intervention, our findings are consistent with current recommendations that quitlines should offer alcohol counseling to smokers who exhibit hazardous drinking62, and particularly so among successful quitters.
4.1. Strengths and Limitations
To the best of our knowledge, this research is the first randomized controlled trial evaluating a combined behavioral intervention for at-risk drinking and smoking cessation among a Latino group, and one of the few addressing at-risk drinking in the context of a smoking cessation treatment, rather than focusing on smoking cessation among alcohol dependent individuals. Long term follow up, and satisfactory follow-up rates were also strengths.
Findings should be interpreted in light of some limitations. Results may not be generalizable to individuals from other racial/ethnic groups and/or dependent drinkers. Applicability to Puerto Ricans living in the U.S. as well as other Latino subgroups also awaits verification. Also, the sample size may be considered small and precluded formal examination of the mechanisms of coaction effects. Finally, this study examined self-reported abstinence from both tobacco and alcohol63,64; thus, future studies should utilize additional methodologies including biochemical markers or other corroborating data sources19,65.
4.2. Conclusion
The present study addresses the need for treatments targeted at changing multiple addictive behaviors (i.e., smoking and at-risk alcohol use), and does so through telephone counseling, a population-based delivery mechanism that is ubiquitous throughout the U.S. and Puerto Rico. Findings suggest that integrating an alcohol intervention into smoking cessation treatment does not reduce engagement in smoking cessation treatment, does not hinder smoking cessation outcomes, and can positively impact at-risk drinking among individuals who successfully quit smoking. Findings of coaction between smoking and drinking speak to the promise of multiple health behavior change interventions for substance use treatment and chronic disease prevention19,20.
Highlights.
Study tested the efficacy of concurrently treating smoking and at-risk drinking
Motivation And Problem Solving (MAPS+) was the enhanced intervention in the study
There was no main effect of treatment on at-risk drinking or smoking outcomes
Smoking status moderated the effect of MAPS+ on several at-risk drinking behaviors
Findings are consistent with treatment enhanced coaction for smoking and drinking
Acknowledgments
The authors are grateful to the Puerto Rico Department of Health, Telemedik (San Juan, PR), and all study personnel for their invaluable contributions during the implementation of the trial, including Carlos A. Mazas, Luz M. Mejia, Patricia Figueroa, Erica Cantú, Gabrielle Ruiz-Tudo, María Salazar, Rocio Tharp, Araceli Flores, Jasmín Berríos, María Arredondo, Devin Olivares, Thelma Majalca and Armando Morin. The content of this article has been partially presented in professional organizations and forums.
Role of Funding Sources
This project was supported by grants from the National Cancer Institute at the National Institutes of Health [U54 CA096297/CA096300 (DWW & ECD)], a Diversity Supplement to R25T CA57730 (VCF), U54CA153505 (DWW), CA016672; the National Center for Research Resources (UL1 TR000371); a CDC Cooperative Agreement to The Puerto Rico Department of Health (5U58DP001998), and an American Cancer Society's Mentored Research Scholar Grant [MRSG-15-018-01-CPPB (VCF)]. These organizations had no further role in study design; in the collection, analysis and interpretation of the data; in the writing of the report; or in the decision to submit the article for publication. The content of this work is solely the responsibility of the authors and do not necessarily represent the official views of the sponsoring organizations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
VCF, ECDT, LRR, YL, YTS, & DWW were investigators on the grant and contributed significantly to the conceptualization and design of the study. VCF and WAC assisted with study implementation. LG and MC completed data management and analyses. YL consulted on data analyses. VCF and DWW conceptualized the overall analytic plan and drafted the manuscript. All authors revised drafts of the manuscript and approved the final article.
Conflict of Interest
The authors have declared no conflicts of interest or financial disclosures related to this article.
References
- 1.Ries LG, Eisner MP, Kosary CL, et al. SEER Cancer Statistics Review, 1975-2001. National Cancer Institute; Bethesda, MD: 2004. [Google Scholar]
- 2.Burns DM. Epidemiology of smoking-induced cardiovascular disease. Prog. Cardiovasc. Dis. 2003;46:11–29. doi: 10.1016/s0033-0620(03)00079-3. [DOI] [PubMed] [Google Scholar]
- 3.Boffetta P, Hashibe M. Alcohol and cancer. Lancet Oncol. 2006;7:149–156. doi: 10.1016/S1470-2045(06)70577-0. [DOI] [PubMed] [Google Scholar]
- 4.Stahre M, Roeber J, Kanny D, Brewer RD, Zhang X. Contribution of excessive alcohol consumption to deaths and years of potential life lost in the United States. Prev. Chronic Dis. 2014;11:E109. doi: 10.5888/pcd11.130293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falk DE, Yi HY, Hiller-Sturmhofel S. An epidemiologic analysis of co-occurring alcohol and tobacco use and disorders: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Alcohol Res Health. 2006;29(3):162–171. [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor B, Rehm J. When risk factors combine: the interaction between alcohol and smoking for aerodigestive cancer, coronary heart disease, and traffic and fire injury. Addict. Behav. 2006 Sep;31(9):1522–1535. doi: 10.1016/j.addbeh.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Pelucchi C, Gallus S, Garavello W, Bosetti C, La Vecchia C. Cancer risk associated with alcohol and tobacco use: focus on upper aero-digestive tract and liver. Alcohol Research & Health : The Journal of the National Institute on Alcohol Abuse and Alcoholism. 2006;29(3):193–198. [PMC free article] [PubMed] [Google Scholar]
- 8.Hart CL, Davey Smith G, Gruer L, Watt GC. The combined effect of smoking tobacco and drinking alcohol on cause-specific mortality: a 30 year cohort study. BMC Public Health. 2010;10:789. doi: 10.1186/1471-2458-10-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bjartveit K, Tverdal A. Health consequences of smoking 1-4 cigarettes per day. Tob. Control. 2005 Oct;14(5):315–320. doi: 10.1136/tc.2005.011932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NIAAA . The physicians’ guide to helping patients with alcohol problems Vol NIH publication No. 95-3769. USDHHS, PHS, National Institutes of Health; Bethesda, MD: 1995. [Google Scholar]
- 11.National Institute of Health . NIAAA Council approves definition of binge drinking. Vol No. 04-5346. NIH Publication; Bethesda, MD: 2004. p. 3. [Google Scholar]
- 12.Institute of Medicine . In: Broadening the base of treatment for alcohol problems: A report of a study by a commitee of The Institute of Medicine. Division of Mental Healh and Behavioral Medicine, editor. National Academy Press; Washington, DC: 1990. [Google Scholar]
- 13.Hayes RB, Bravo-Otero E, Kleinman DV, et al. Tobacco and alcohol use and oral cancer in Puerto Rico. Cancer Causes Control. 1999 Feb;10(1):27–33. doi: 10.1023/a:1008876115797. [DOI] [PubMed] [Google Scholar]
- 14.Bosetti C, Garavello W, Gallus S, La Vecchia C. Effects of smoking cessation on the risk of laryngeal cancer: An overview of published studies. Oral Oncol. 2006;42:866–872. doi: 10.1016/j.oraloncology.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Bjartveit K, Tverdal A. Health consequences of sustained smoking cessation. Tob. Control. 2009 Jun;18(3):197–205. doi: 10.1136/tc.2008.026898. [DOI] [PubMed] [Google Scholar]
- 16.Fiore MC, Jaen CR, Baker TB, et al. Clinical practice guideline. U.S. Department of Health and Human Services, Public Health Service; Rockville, MD: 2008. Treating tobacco use and dependence: 2008 update. [Google Scholar]
- 17.Pronk NP, Peek CJ, Goldstein MG. Addressing multiple behavioral risk factors in primary care. A synthesis of current knowledge and stakeholder dialogue sessions. Am. J. Prev. Med. 2004 Aug;27(2 Suppl):4–17. doi: 10.1016/j.amepre.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 18.Emmons KM, McBride CM, Puleo E, et al. Project PREVENT: a randomized trial to reduce multiple behavioral risk factors for colon cancer. Cancer Epidemiol. Biomarkers Prev. 2005 Jun;14(6):1453–1459. doi: 10.1158/1055-9965.EPI-04-0620. [DOI] [PubMed] [Google Scholar]
- 19.Prochaska JJ, Spring B, Nigg CR. Multiple health behavior change research: an introduction and overview. Prev. Med. 2008 Mar;46(3):181–188. doi: 10.1016/j.ypmed.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prochaska JO. Multiple Health Behavior Research represents the future of preventive medicine. Prev. Med. 2008 Mar;46(3):281–285. doi: 10.1016/j.ypmed.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 21.Johnson SS, Paiva AL, Mauriello L, Prochaska JO, Redding C, Velicer WF. Coaction in multiple behavior change interventions: Consistency across multiple studies on weight management and obesity prevention. Health Psychol. 2014 May;33(5):475–480. doi: 10.1037/a0034215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lipschitz JM, Paiva AL, Redding CA, Butterworth S, Prochaska JO. Co-occurrence and coaction of stress management with other health risk behaviors. J. Health Psychol. 2013 doi: 10.1177/1359105313506026. [DOI] [PubMed] [Google Scholar]
- 23.Paiva AL, Prochaska JO, Yin HQ, et al. Treated individuals who progress to action or maintenance for one behavior are more likely to make similar progress on another behavior: coaction results of a pooled data analysis of three trials. Prev. Med. 2012 May;54(5):331–334. doi: 10.1016/j.ypmed.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.deRuiter WK, Cairney J, Leatherdale ST, Faulkner GE. A longitudinal examination of the interrelationship of multiple health behaviors. Am. J. Prev. Med. 2014 Sep;47(3):283–289. doi: 10.1016/j.amepre.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 25.Funderburk JS, Maisto SA, Sugarman DE, Wade M. The covariation of multiple risk factors in primary care: a latent class analysis. J. Behav. Med. 2008 Dec;31(6):525–535. doi: 10.1007/s10865-008-9176-1. [DOI] [PubMed] [Google Scholar]
- 26.Prochaska JJ, Delucchi K, Hall SM. A meta-analysis of smoking cessation interventions with individuals in substance abuse treatment or recovery. J. Consult. Clin. Psychol. 2004 Dec;72(6):1144–1156. doi: 10.1037/0022-006X.72.6.1144. [DOI] [PubMed] [Google Scholar]
- 27.Kahler CW, Metrik J, LaChance HR, et al. Addressing heavy drinking in smoking cessation treatment: a randomized clinical trial. J. Consult. Clin. Psychol. 2008 Oct;76(5):852–862. doi: 10.1037/a0012717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ames SC, Pokorny SB, Schroeder DR, Tan W, Werch CE. Integrated smoking cessation and binge drinking intervention for young adults: A pilot efficacy trial. Addict. Behav. 2014 May;39(5):848–853. doi: 10.1016/j.addbeh.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention Quitting smoking among adults-United States, 2001-2010. MMWR. 2011;60(44):1513–1519. [PubMed] [Google Scholar]
- 30.Whitlock EP, Polen MR, Green CA, Orleans T, Klein J. Behavioral counseling interventions in primary care to reduce risky/harmful alcohol use by adults: a summary of the evidence for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2004;140(7):557–568. doi: 10.7326/0003-4819-140-7-200404060-00017. [DOI] [PubMed] [Google Scholar]
- 31.Moyer A, Finney JW, Swearingen CE, Vergun P. Brief interventions for alcohol problems: a meta-analytic review of controlled investigations in treatment-seeking and non-treatment-seeking populations. Addiction. 2002 Mar;97(3):279–292. doi: 10.1046/j.1360-0443.2002.00018.x. [DOI] [PubMed] [Google Scholar]
- 32.Baer JS, Kivlahan DR, Donovan DM. Integrating skills training and motivational therapies. Implications for the treatment of substance dependence. J. Subst. Abuse Treat. 1999 Jul-Sep;17(1-2):15–23. doi: 10.1016/s0740-5472(98)00072-5. [DOI] [PubMed] [Google Scholar]
- 33.Constantino MJ, DeGeorge J, Dadlani MB, Overtree CE. Motivational interviewing: a bellwether for context-responsive psychotherapy integration. J. Clin. Psychol. 2009 Nov;65(11):1246–1253. doi: 10.1002/jclp.20637. [DOI] [PubMed] [Google Scholar]
- 34.Witkiewitz K, Marlatt GA. Relapse prevention for alcohol and drug problems: that was Zen, this is Tao. Am. Psychol. 2004 May-Jun;59(4):224–235. doi: 10.1037/0003-066X.59.4.224. [DOI] [PubMed] [Google Scholar]
- 35.Miller W, Rollnick S. Motivational Interviewing Preparing People to Change Addictive Behavior. Guilford Press; New York: 2002. [Google Scholar]
- 36.Vidrine JI, Reitzel LR, Figueroa PY, et al. Motivation and Problem Solving (MAPS): Motivationally Based Skills Training for Treating Substance Use. Cogn Behav Pract. 2013;20:501–516. doi: 10.1016/j.cbpra.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prochaska JO, DiClemente CC, Norcross JC. In search of how people change. Applications to addictive behaviors. Am. Psychol. 1992 Sep;47(9):1102–1114. doi: 10.1037//0003-066x.47.9.1102. [DOI] [PubMed] [Google Scholar]
- 38.Reitzel LR, Irvin Vidrine J, Businelle MS, et al. Preventing postpartum smoking relapse among diverse low income women: a randomized clinical trial. Nicotine Tob Res. 2010 Feb;12(4):326–335. doi: 10.1093/ntr/ntq001. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wetter DW, Mazas C, Daza P, et al. Reaching and treating Spanish-speaking smokers through the National Cancer Institute's Cancer Information Service. A randomized controlled trial. Cancer. 2007 Jan 15;109(2 Suppl):406–413. doi: 10.1002/cncr.22360. [DOI] [PubMed] [Google Scholar]
- 40.McClure JB, Westbrook E, Curry SJ, Wetter DW. Proactive, motivationally enhanced smoking cessation counseling among women with elevated cervical cancer risk. Nicotine Tob Res. 2005 Dec;7(6):881–889. doi: 10.1080/14622200500266080. [DOI] [PubMed] [Google Scholar]
- 41.Centers for Disease Control and Prevention Current Cigarette Smoking among Adults - United States, 2011. MMWR. 2012;61(44):889–894. [PubMed] [Google Scholar]
- 42.Centers for Disease Control and Prevention Current Cigarette Smoking Among Adults — United States, 2005–2012. MMWR. 632014:29–34. [Google Scholar]
- 43.Instituto de Estadísticas de Puerto Rico . In: Nuevas estadisticas de mortalidad, 2000-2008. Departamento de Salud de Puerto Rico, editor. San Juan, PR: 2010. [Google Scholar]
- 44.Chartier K, Caetano R. Ethnicity and Health Disparities in Alcohol Research. Alcohol Res. Health. 2010;33(1-2):152–160. [PMC free article] [PubMed] [Google Scholar]
- 45.Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. The Alcohol Use Disorders Identification Test: Guidelines for use in primary health care. 2nd ed. World Health Organization; Geneva: 2001. [Google Scholar]
- 46.Chow SC, Lui JP. Design and analysis of clinical trials: Concepts and methodologies. John Wiley and Sons; New York: 2004. [Google Scholar]
- 47.Pocock SJ. Statistical and ethical issues in monitoring clinical trials. Stat. Med. 1993 Aug;12(15-16):1459–1469. doi: 10.1002/sim.4780121512. discussion 1471-1455. [DOI] [PubMed] [Google Scholar]
- 48.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psych Meas. 1977;1(3):385–401. [Google Scholar]
- 49.Miller WR, Zweben A, DiClemente CC, Rychtarik RG. Motivational enhancement therapy manual: A clinical research guide for therapists treating individuals with alcohol abuse and dependence. U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health; Rockville, MD: 1995. Vol NIH Publication No. 94-3723. [Google Scholar]
- 50.Centers for Disease Control and Prevention . Telephone Quitlines: A Resource for Development, Implementation, and Evaluation. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: 2004. [Google Scholar]
- 51.U.S. Department of Health and Human Services . Guia para dejar de fumar. National Cancer Institute; 2002. Vol NIH Publication: 02-3001. [Google Scholar]
- 52.Sánchez-Craig M. Drinking Alcohol: A Guide for Evaluating and Changing Drinking Patterns. In: The Addiction Research Foundation, editor. Group Health Cooperative of Puget Sound; Toronto, Ontario: [Google Scholar]
- 53.Moyers TB, Martin T, Manuel JK, Miller WR. The Motivational Interviewing Treatment Integrity (MITI) scale. Version 2.0. Center on Alcoholism, Substance Abuse and Addictions, University of New Mexico; Albuquerque, NM: 2003. [Google Scholar]
- 54.Borland R, Yong HH, O'Connor RJ, Hyland A, Thompson ME. The reliability and predictive validity of the Heaviness of Smoking Index and its two components: findings from the International Tobacco Control Four Country study. Nicotine Tob. Res. 2010 Oct;12(Suppl):S45–50. doi: 10.1093/ntr/ntq038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verbeke G, Molenberghs G. Linear mixed models for longitudinal data. Springer-Verlag; New York: 2000. [Google Scholar]
- 56.McCulloch CE, Searle SR. Generalized, Linear, and Mixed Models. John Wiley & Sons, Inc.; New York: 2001. [Google Scholar]
- 57.Karlamangla A, Zhou K, Reuben D, Greendale G, Moore A. Longitudinal trajectories of heavy drinking in adults in the United States of America. Addiction. 2006 Jan;101(1):91–99. doi: 10.1111/j.1360-0443.2005.01299.x. [DOI] [PubMed] [Google Scholar]
- 58.Unger JB. Stages of change of smoking cessation: relationships with other health behaviors. Am Jn Prev Med. 1996 Mar-Apr;12(2):134–138. [PubMed] [Google Scholar]
- 59.Fu SS, Kodl M, Willenbring M, et al. Ethnic differences in alcohol treatment outcomes and the effect of concurrent smoking cessation treatment. Drug Alcohol Depend. 2008 Jan 1;92(1-3):61–68. doi: 10.1016/j.drugalcdep.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kalman D, Hayes K, Colby SM, Eaton CA, Rohsenow DJ, Monti PM. Concurrent versus delayed smoking cessation treatment for persons in early alcohol recovery. A pilot study. J. Subst. Abuse Treat. 2001 Apr;20(3):233–238. doi: 10.1016/s0740-5472(00)00174-4. [DOI] [PubMed] [Google Scholar]
- 61.Bobo JK, McIlvain HE, Lando HA, Walker RD, Leed-Kelly A. Effect of smoking cessation counseling on recovery from alcoholism: findings from a randomized community intervention trial. Addiction. 1998 Jun;93(6):877–887. doi: 10.1046/j.1360-0443.1998.9368779.x. [DOI] [PubMed] [Google Scholar]
- 62.Toll BA, Cummings KM, O'Malley SS, et al. Tobacco quitlines need to assess and intervene with callers' hazardous drinking. Alcohol. Clin. Exp. Res. 2012 Sep;36(9):1653–1658. doi: 10.1111/j.1530-0277.2012.01767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sobell LC, Sobell MB. Alcohol consumption measures. 2nd ed. U.S. Department for Health and Human Services, National Institute on Alcohol Abuse and Alcoholism; Bethesda: 2003. Vol NIH Publication No. 03-3745. [Google Scholar]
- 64.Connor Gorber S, Schofield-Hurwitz S, Hardt J, Levasseur G, Tremblay M. The accuracy of self-reported smoking: a systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine Tob. Res. 2009 Jan;11(1):12–24. doi: 10.1093/ntr/ntn010. [DOI] [PubMed] [Google Scholar]
- 65.Del Boca FK, Darkes J. The validity of self-reports of alcohol consumption: state of the science and challenges for research. Addiction. 2003 Dec;98(Suppl 2):1–12. doi: 10.1046/j.1359-6357.2003.00586.x. [DOI] [PubMed] [Google Scholar]