Abstract
We recently found that NFATc1, a member of the NFAT family and a key regulator of the immune response, could induce bladder carcinogenesis and cancer progression. In this study, we immunohistochemically stained for NFATc1 in upper urinary tract urothelial carcinoma (UUTUC) specimens and paired nonneoplastic urothelial tissues. NFATc1 was positive in 51 [52%; 40 (40%) weak (1+), 9 (9%) moderate (2+), and 2 (2%) strong (3+)] of 99 UUTUCs, which was significantly higher than in benign urothelium [30 (36%) of 83; 28 (34%) weak and 2 (2%) moderate] (0 vs 1+/2+/3+, P = .038; 0/1+ vs 2+/3+, P = .023). There were no significant associations between NFATc1 expression pattern and tumor grade or pT stage. However, the positive rates of NFATc1 expression tended to be higher in renal pelvic tumors (60%) than in ureteral tumors (42%; P = .080) as well as in pN+ tumors (75%) than in pN0 tumors (49%; P = .089). Kaplan-Meier and log-rank tests revealed that moderate (2+) to strong (3+) NFATc1 expression correlated with lower progression-free survival (P = .032) and cancer-specific survival (P = .005) rates in the 99 cases. Patients with high (2+/3+) NFATc1 muscle-invasive tumor (n = 9) also had a significantly higher risk of cancer-specific mortality (P = .021) compared to those with low (0/1+) NFATc1 muscle-invasive tumor (n = 53). Thus, compared with nonneoplastic urothelium, a significant increase in the expression of NFATc1 in UUTUC was seen, implying the involvement of NFATc1 signals in the development of UUTUC. The current results further suggest that NFATc1 overexpression serves as a predictor of poor prognosis in patients with UUTUC.
Introduction
Upper urinary tract urothelial carcinoma (UUTUC) is a relatively rare genitourinary malignancy accounting for less than 10% of urothelial cancers but is often aggressive with poor prognosis compared with bladder cancer [1], [2], [3]. Indeed, more than half of UUTUC patients versus only up to 25% of bladder cancer patients present with an invasive disease [2], [4]. In addition, presumably due to its lower incidence compared with that of bladder cancer, the pathogenesis of UUTUC remains far from being fully understood, while some of molecular or genetic factors are similar to those associated with bladder cancer [5], [6]. Notably, although histopathological features, such as tumor grade and stage, lymphovascular invasion, surgical margin status, and the presence of concomitant carcinoma in situ, are reliable prognosticators, none of molecular markers for UUTUC have been confirmed to be useful for daily clinical decision making.
NFAT, a family of transcription factors consisting of five members [i.e., NFATc1 (also known as NFAT2), NFATc2 (NFAT1), NFATc3 (NFAT4), NFATc4 (NFAT3), NFAT5], was initially identified as a regulator of T-cell activation [7], and its regulatory role has been extensively studied in immune cells [8]. Emerging evidence has also suggested the involvement of NFAT signaling in the outgrowth of hematological malignancies as well as several types of solid tumors [9], [10]. In a sarcoma model, NFATc1 and NFATc2 were shown to function as an oncogene and a tumor suppressor, respectively [11].
Recently, we found that immunosuppressants, including cyclosporine A and tacrolimus, inhibited bladder tumorigenesis and tumor growth via downregulation of NFATc1 expression in urothelial cells [12], [13]. NFATc1 knockdown in bladder cancer lines also resulted in significant reduction of cell viability and invasion, suggesting its direct regulation of tumor growth without involvement of immune system [12]. In these studies [12], [13], we immunohistochemically demonstrated that NFATc1 expression was considerably elevated in bladder cancer specimens compared with nonneoplastic urothelial tissues and that patients with NFATc1-positive muscle-invasive bladder tumor had a significantly higher risk of disease progression after radical cystectomy. However, to the best of our knowledge, little is known about the functional role of NFAT signaling in the development and progression of UUTUC. The current study aims to determine the expression status of NFATc1 in UUTUCs and corresponding nonneoplastic urothelial tissues as well as to assess prognostic significance of NFATc1 expression.
Materials and Methods
Patients and Tissue Samples
UUTUC tissue microarray (TMA) was constructed with spotted triplicate tumor samples and paired normal-appearing urothelial tissues from 99 patients who underwent radical nephroureterectomy performed at Osaka General Medical Center, Osaka, Japan, as described previously [14]. Appropriate approval was obtained from the institutional review board before construction and use of the TMA. Clinicopathological characteristics of these 99 patients were previously summarized [14], [15]. These patients included 60 males and 31 females, with a median (range) age of 71 (48-87) years at the time of surgery and a median (range) follow-up of 47 (2-173) months after surgery. These also included 45 renal pelvic tumors and 50 ureteral tumors (4 cases with tumors at both sites), 15 low-grade urothelial carcinomas and 84 high-grade urothelial carcinomas, 37 non–muscle-invasive tumors (pTa or pT1) and 62 muscle-invasive tumors (pT2, pT3, or pT4), 84 pN0 tumors and 12 pN+ tumors (3 cases of pNx tumors), and 4 pM1 tumors. During follow-up, metachronous or synchronous recurrence in the lower urinary tract was observed in 32 patients. Tumor progression defined as the development of non–lower urinary tract lesions, including recurrence at the nephroureterectomy site and lymph node or visceral metastasis, was seen in 38 patients.
Immunohistochemistry
Immunohistochemical staining was performed on the sections (5 μm thick) from the UUTUC TMA using a primary antibody to NFATc1 (clone 7A6; dilution 1:50; Santa Cruz Biotechnology), as we described previously [10], [12], [13]. All the stains were manually scored by a single pathologist (H.M.) who was blinded to sample identity. The German Immunoreactive Score (range: 0-12) calculated by multiplying the percentage of immunoreactive cells (0% = 0; 1%-10% = 1; 11%-50% = 2; 51%-80% = 3; 81%-100% = 4) by staining intensity (0, negative; 1, weak; 2, moderate; 3, strong) was considered negative (0; 0-1), weakly positive (1+; 2-4), moderately positive (2+; 6-8), or strongly positive (3+; 9-12).
Statistical Analysis
The chi-square test was used to evaluate the association between categorized variables. The survival rates were determined using the Kaplan-Meier method, and comparison was made by the log-rank test. In addition, the Cox proportional hazards model was used to determine statistical significance of prognostic indicators in a multivariate setting. P values less than .05 were considered statistically significant.
Results
Expression of NFATc1 in Tumors Versus Matched Normal Tissues
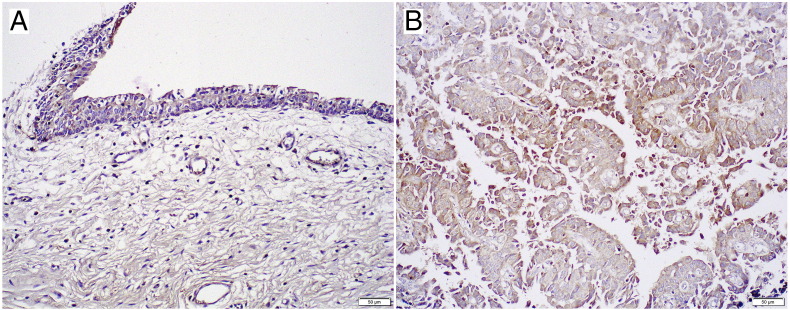
We immunohistochemically stained for NFATc1 in 99 UUTUC samples and corresponding 83 normal-appearing urothelial tissues. Positive signals for NFATc1 were detected predominantly in the cytoplasm of benign and malignant epithelial cells (Figure 1).
Figure 1.
Immunohistochemistry of NFATc1 in UUTUC specimens. Cytoplasmic signals are seen in nonneoplastic urothelium (A) and urothelial tumor (B) (original magnification: ×200).
Table 1 summarizes the status of NFATc1 expression in nonneoplastic urothelium versus urothelial tumor tissues. NFATc1 was positive in 30 (36%) of 83 benign urothelial tissue samples [28 (34%) weak (1+), 2 (2%) moderate (2+), and 0 (0%) strong (3+)] and 51 (52%) of 99 UUTUCs [40 (40%) weak (1+), 9 (9%) moderate (2+), and 2 (2%) strong (3+)]. Thus, the levels of NFATc1 expression were significantly elevated in tumor than in nonneoplastic urothelium (0 vs 1+/2+/3+, P = .038; 0/1+ vs 2+/3+, P = .023).
Table 1.
NFATc1 Expression in Nonneoplastic Urothelium Versus Urothelial Neoplasm Tissue Specimens
| Tissue | n | NFATc1 Expression |
P Value |
||||
|---|---|---|---|---|---|---|---|
| 0 (%) | 1+ (%) | 2+ (%) | 3+ (%) | 0 vs 1+/2+/3+ | 0/1+ vs 2+/3+ | ||
| Normal | 83 | 53 (64) | 28 (34) | 2 (2) | 0 (0) | .038 | .023 |
| Tumor | 99 | 48 (48) | 40 (40) | 9 (9) | 2 (2) | ||
Correlations of NFATc1 Expression with Clinicopathological Characteristics of UUTUCs
Next, we analyzed the correlations between the status of NFATc1 expression in UUTUCs and the clinicopathological profile available for our patient cohort (Table 2). Six (40%) of 15 low-grade versus 45 (54%) of 84 high-grade UUTUCs (P = .332) and 16 (43%) of 37 non–muscle-invasive versus 35 (56%) of 62 muscle-invasive UUTUCs (P = .203) were immunoreactive for NFATc1. However, the positive rate of NFATc1 expression tended to be higher (P = .089) in pN+ tumors (75%) than in pN0 tumors (49%). Additionally, NFATc1 positivity was marginally increased (P = .080) in renal pelvic tumors (60%) compared with ureteral tumors (42%). There were no strong correlations of the status of NFATc1 expression with distant metastasis (data not shown), the laterality of the tumors, or gender of the patients.
Table 2.
Correlations between NFATc1 Expression and Clinicopathological Profile of the Patients
| Parameter | n | NFATc1 Expression |
P Value |
P Value |
|||
|---|---|---|---|---|---|---|---|
| 0 (%) | 1+ (%) | 2+ (%) | 3+ (%) | 0 vs 1+/2+/3+ | 0/1+ vs 2+/3+ | ||
| Gender | .203 | .383 | |||||
| Male | 60 | 26 (43) | 26 (43) | 7 (12) | 1 (2) | ||
| Female | 39 | 22 (56) | 14 (36) | 2 (5) | 1 (3) | ||
| Laterality | .729 | .430 | |||||
| Right | 43 | 20 (47) | 17 (40) | 5 (12) | 1 (2) | ||
| Left | 56 | 28 (50) | 23 (41) | 4 (7) | 1 (2) | ||
| Tumor site | .080⁎ | .622⁎ | |||||
| Renal pelvis | 45 | 18 (40) | 23 (51) | 3 (7) | 1 (2) | ||
| Ureter | 50 | 29 (58) | 15 (30) | 5 (10) | 1 (2) | ||
| Both | 4 | 1 (25) | 2 (50) | 1 (25) | 0 (0) | ||
| Tumor grade | .332 | .137 | |||||
| Low grade | 15 | 9 (60) | 6 (40) | 0 (0) | 0 (0) | ||
| High grade | 84 | 39 (46) | 34 (40) | 9 (11) | 2 (2) | ||
| Pathologic stage | .203† | .163† | |||||
| pTa | 19 | 10 (53) | 7 (37) | 1 (5) | 1 (5) | ||
| pT1 | 18 | 11 (61) | 7 (39) | 0 (0) | 0 (0) | ||
| NMI (pTa + pT1) | 37 | 21 (57) | 14 (38) | 1 (3) | 1 (3) | ||
| pT2 | 8 | 2 (25) | 4 (50) | 2 (25) | 0 (0) | ||
| pT3 | 48 | 23 (48) | 19 (40) | 5 (10) | 1 (2) | ||
| pT4 | 6 | 2 (33) | 3 (50) | 1 (17) | 0 (0) | ||
| MI (pT2 + pT3 + pT4) | 62 | 27 (44) | 26 (42) | 8 (13) | 1 (2) | ||
| Lymph node involvement | .089‡ | .115‡ | |||||
| pN0 | 84 | 43 (51) | 33 (39) | 6 (7) | 2 (2) | ||
| pN1-3 | 12 | 3 (25) | 6 (50) | 3 (25) | 0 (0) | ||
| pNx | 3 | 2 (67) | 1 (33) | 0 (0) | 0 (0) | ||
NMI, non–muscle-invasive; MI, muscle-invasive.
Renal pelvis versus ureter.
NMI versus MI.
pN0 versus pN1-3.
Correlations of NFATc1 Expression with Patient Outcomes
We then performed Kaplan-Meier analysis coupled with the log-rank test to assess the prognostic values of NFATc1 expression in UUTUCs. There were no statistically significant differences in tumor progression (P = .390; Figure 2A) or cancer-specific mortality (P = .318; Figure 2B) between NFATc1 positivity and negativity. However, patients with high (2+/3+) NFATc1 tumor had a significantly higher risk of tumor progression (P = .032; Figure 3A) or cancer-specific mortality (P = .005; Figure 3B) compared to those with low (0/1+) NFATc1 tumor. In 62 patients with muscle-invasive tumor, moderate (2+) to strong (3+) NFATc1 expression was also strongly associated with a lower cancer-specific survival rate (P = .024) but not with a lower progression-free survival rate (P = .118). In addition, NFATc1 levels were not significantly associated with tumor recurrence in the bladder (0 vs 1+/2+/3+: P = .525; 0/1+ vs 2+/3+: P = .277).
Figure 2.
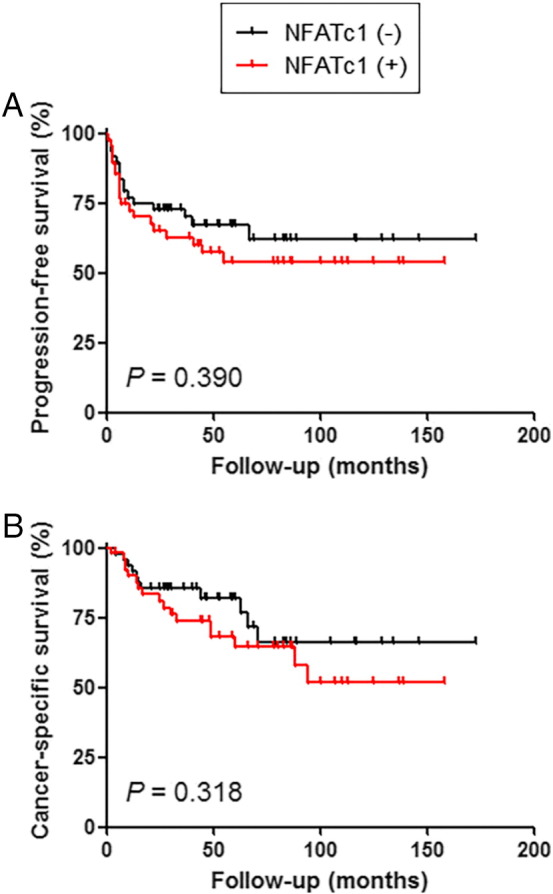
Progression-free survival (A) or cancer-specific survival (B) in patients with UUTUC according to the positivity of NFATc1 (0 vs 1+/2+/3+).
Figure 3.
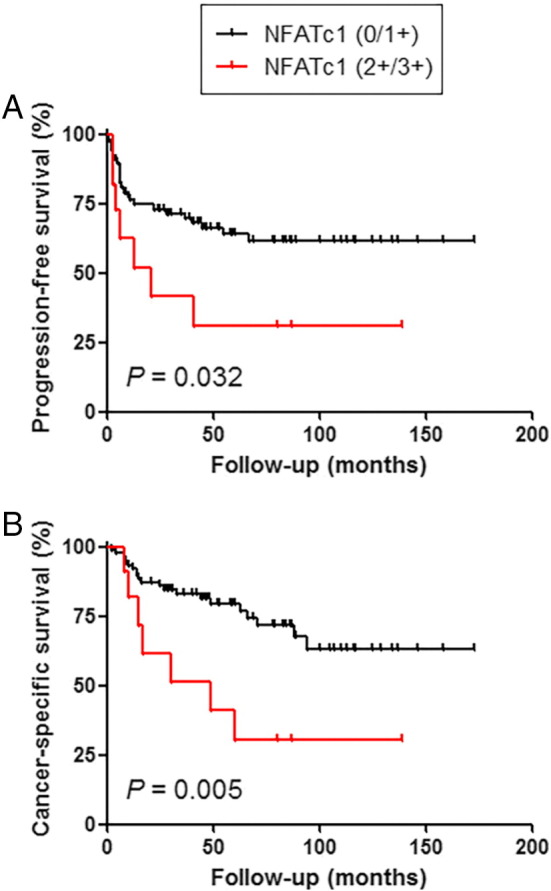
Progression-free survival (A) or cancer-specific survival (B) in patients with UUTUC according to the status of NFATc1 expression (0/1+ vs 2+/3+).
To determine whether NFATc1 status was an independent prognosticator in patients with UUTUC, multivariate analysis was performed with the Cox model (Table 3). In the entire cohort of the patients, tumor grade, pT stage, or lymph node involvement, but not the level of NFATc1 expression, was found to correlate with tumor progression and/or cancer-specific mortality. Similarly, in patients with muscle-invasive UUTUC, NFATc1 status (0/1+ vs 2+/3+) was not an independent predictor of tumor progression or cancer-specific mortality.
Table 3.
Univariate and Multivariate Analysis of Progression-Free Survival and Cancer-Specific Survival in Patients with UUTUC
| Parameter | Progression-Free Survival |
Cancer-Specific Survival |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate |
Multivariate |
Univariate |
Multivariate |
|||||||||
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| All cases (n = 99) | ||||||||||||
| Tumor grade | 4.442 | 1.066-18.507 | .256 | 5.099 | 1.180-22.038 | .029 | 7.207 | 0.976-53.207 | .053 | 6.115 | 0.815-45.884 | .078 |
| pT stage | 2.962 | 1.923-4.562 | <.001 | 2.630 | 1.728-4.003 | <.001 | 3.340 | 1.987-5.616 | <.001 | 3.325 | 1.810-6.110 | <.001 |
| pN stage | 4.397 | 2.065-9.364 | <.001 | 3.163 | 1.262-7.931 | .014 | 2.981 | 1.263-7.035 | .013 | 0.802 | 0.294-2.190 | .667 |
| NFATc1⁎ | 3.492 | 1.113-10.958 | .032 | 0.718 | 0.264-1.954 | .516 | 6.068 | 1.712-21.515 | .005 | 1.923 | 0.783-4.722 | .154 |
| pT2-4 cases (n = 62) | ||||||||||||
| Tumor grade | 2.635 | 0.974-7.133 | .056 | 10.139 | 1.324-77.627 | .026 | 2.384 | 0.876-6.484 | .089 | 5.541 | 0.738-41.616 | .096 |
| pT stage | 2.522 | 1.195-5.322 | .015 | 1.956 | 0.945-4.045 | .071 | 2.108 | 0.987-4.502 | .054 | 1.970 | 0.826-4.698 | .126 |
| pN stage | 3.244 | 1.510-6.968 | .003 | 3.917 | 1.478-10.384 | .006 | 1.768 | 0.747-4.187 | .195 | 1.086 | 0.385-3.065 | .876 |
| NFATc1⁎ | 2.327 | 0.806-6.711 | .118 | 0.665 | 0.240-1.846 | .434 | 6.068 | 1.712-21.515 | .024 | 1.943 | 0.793-4.761 | .146 |
HR, hazard ratio; CI, confidence interval.
0/1+ versus 2+/3+.
Discussion
An increasing, yet limited, amount of evidence has indicated that NFAT signaling plays an important role in the development and progression of solid tumors [9], [10], [11]. We have also recently reported preclinical findings suggesting that NFATc1 promotes urothelial tumorigenesis [13] and bladder cancer cell growth [12]. In the present study, we immunohistochemically determined the expression levels of NFATc1 in a set of TMA consisting of 99 nephroureterectomy specimens. We obtained some results similar to previous observations in bladder cancer tissue samples and others dissimilar to them.
In accordance with our previous findings in bladder specimens [13], NFATc1 expression was found to be considerably elevated in UUTUCs compared with nonneoplastic urothelial tissues. In contrast, there were no significant differences in NFATc1 levels between low-grade versus high-grade UUTUCs or between non–muscle-invasive versus muscle-invasive UUTUCs. Although bladder papillary urothelial neoplasms of low malignant potential, very low grade tumors that are neither benign nor intrinsically malignant, had considerably lower levels of NFATc1 expression compared with bladder urothelial carcinomas (low grade + high grade) [13], no papillary urothelial neoplasms of low malignant potential in the upper urinary tract were included in the TMA used for this study. In addition, a similar trend showing a higher cytoplasmic NFATc1 positivity in lymph node–positive tumors was seen between cystectomy (P = .088 by chi-square test [12]) and nephroureterectomy (current study) cases. The current results thus support the role of NFATc1 as an oncogenic molecule in urothelial tumor outgrowth.
NFATc1 positivity in muscle-invasive bladder cancer was shown to correlate with the risk of tumor progression after radical cystectomy [12]. Correspondingly, patients with strongly (2+/3+) NFATc1-positive UUTUC were found to have significantly higher risks of disease progression and cancer-specific mortality. Strong NFATc1 expression was also associated with a lower cancer-specific survival, but not a lower progression-free survival, in those with muscle-invasive UUTUC. Accordingly, NFATc1 expression appears to precisely predict the prognosis of patients with UUTUC. However, multivariate analysis failed to reveal the values of NFATc1 expression in UUTUC as an independent prognosticator. Further studies are required to determine the clinicopathological and prognostic significance of NFATc1 expression in UUTUC in larger patient cohorts.
Using the same set of TMA, we previously demonstrated marginal increases in the positivity of two proteins [i.e., androgen receptor (AR), estrogen receptor–β] in ureteral tumors compared with renal pelvic tumors, while there were no significant differences in the expression of other three proteins (i.e., estrogen receptor–α, glucocorticoid receptor, progesterone receptor) [15]. A significant increase in the positive rate of AR expression in ureteral tumors (77.5%) versus renal pelvic tumors (34.9%) was indeed reported [16]. Moreover, AR immunohistochemically stained by the same groups was shown to be more highly expressed in bladder tumors than in UUTUCs (e.g., 42% in bladder tumors [17] vs 28% in ureteral tumors or 11% in renal pelvic tumors [15], 25% in bladder tumors [18] vs 16% in renal pelvic tumors [18]). In contrast, we here showed that ureteral tumors (42%) were inclined to exhibit a lower positive rate of NFATc1 expression compared with renal pelvic tumors (60%). Interestingly, the positive rate of NFATc1 expression in muscle-invasive bladder cancers was even lower (22%) [12]. The underlying reasons for these findings in AR as well as NFATc1 remain uncertain. However, differences in tissue preservation among bladder, ureteral, and renal pelvic tumors due to their anatomic locations and/or thickness of the specimens around the tumors (e.g., time to complete tissue fixation) might have had an impact on the immunoreactivity.
It has been documented that, in immune cells, NFATc1 is located in their cytoplasms in a hyperphosphorylated state and translocates into the nuclei upon cell stimulation via dephosphorylation [8]. Nonetheless, subcellular localization of NFATc1 detected by immunohistochemical staining in tissue specimens appears to be cancer type dependent. For instance, predominant nuclear or cytoplasmic expression was seen in hepatocellular carcinoma [19] or prostatic adenocarcinoma [10]/subcutaneous T-cell lymphoma [20], respectively. Meanwhile, both nuclear and cytoplasmic signals were detected in not only pancreatic [21] or lung [22] carcinomas but also bladder urothelial neoplasms [12], [13]. By contrast, our current immunohistochemistry for NFATc1 in the UUTUC TMA exclusively stained the cytoplasm of benign and malignant urothelial cells. It has been implied that “fresh” tissue is required for detecting nuclear NFATc1 signals in tumor cells by immunohistochemistry [20].
In conclusion, we observed a significant increase in the expression of NFATc1 in UUTUC compared with nonneoplastic urothelium included in the same surgical specimens. These findings support preclinical evidence suggesting the involvement of NFATc1 signals in the initiation of urothelial cancer. Moreover, strong NFATc1 expression was significantly associated with worse patient outcomes. Therefore, NFATc1 overexpression may serve as a reliable prognosticator in patients with UUTUC. Further assessments of NFATc1 functions are necessary for determining its biological significance in the development and progression of UUTUC.
Conflict of Interest
The authors have no conflicts of interest to disclose.
Acknowledgement
This work was partially supported by a grant (16K20152 to T.K.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1.Munoz JJ, Ellison LM. Upper tract urothelial neoplasms: incidence and survival during the last 2 decades. J Urol. 2000;164(5):1523–1525. [PubMed] [Google Scholar]
- 2.Margulis V, Shariat SF, Matin SF, Kamat AM, Zigeuner R, Kikuchi E, Lotan Y, Weizer A, Raman JD, Wood CG. Outcomes of radical nephroureterectomy: a series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer. 2009;115(6):1224–1233. doi: 10.1002/cncr.24135. [DOI] [PubMed] [Google Scholar]
- 3.Raman JD, Messer J, Sielatycki JA, Hollenbeak CS. Incidence and survival of patients with carcinoma of the ureter and renal pelvis in the USA, 1973-2005. BJU Int. 2011;107(7):1059–1064. doi: 10.1111/j.1464-410X.2010.09675.x. [DOI] [PubMed] [Google Scholar]
- 4.Babjuk M, Böhle A, Burger M, Capoun O, Cohen D, Compérat EM, Hernández V, Kaasinen E, Palou J, Rouprêt M. EAU guidelines on non–muscle-invasive urothelial carcinoma of the bladder: update 2016. Eur Urol. 2017;71(3):447–461. doi: 10.1016/j.eururo.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 5.Catto JW, Yates DR, Rehman I, Azzouzi A, Patterson J, Sibony M, Cussenot O, Hamdy F. Behavior of urothelial carcinoma with respect to anatomical location. J Urol. 2007;177(5):1715–1720. doi: 10.1016/j.juro.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 6.Colin P, Koenig P, Ouzzane A, Berthon N, Villers A, Biserte J, Rouprét M. Environmental factors involved in carcinogenesis of urothelial cell carcinomas of the upper urinary tract. BJU Int. 2009;104(10):1436–1440. doi: 10.1111/j.1464-410X.2009.08838.x. [DOI] [PubMed] [Google Scholar]
- 7.Shaw JP, Utz PJ, Durand DB, Toole JJ, Emmel EA, Crabtree GR. Identification of a putative regulator of early T cell activation genes. Science. 1988;241(4862):202–205. doi: 10.1126/science.3260404. [DOI] [PubMed] [Google Scholar]
- 8.Macian F. NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol. 2005;5(6):472–484. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- 9.Qin JJ, Nag S, Wang W, Zhou J, Zhang WD, Wang H, Zhang R. NFAT as cancer target: mission possible? Biochim Biophys Acta. 2014;1864(2):297–311. doi: 10.1016/j.bbcan.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawahara T, Kashiwagi E, Ide H, Li Y, Zheng Y, Ishiguro H, Miyamoto H. The role of NFATc1 in prostate cancer progression: cyclosporine A and tacrolimus inhibit cell proliferation, migration, and invasion. Prostate. 2015;75(6):573–584. doi: 10.1002/pros.22937. [DOI] [PubMed] [Google Scholar]
- 11.Robbs BK, Cruz AL, Werneck MB, Mognol GP, Viola JP. Dual roles for NFAT transcription factor genes as oncogenes and tumor suppressors. Mol Cell Biol. 2008;28(23):7168–7181. doi: 10.1128/MCB.00256-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawahara T, Kashiwagi E, Ide H, Li Y, Zheng Y, Miyamoto Y, Netto GJ, Ishiguro H, Miyamoto H. Cyclosporine A and tacrolimus inhibit bladder cancer growth through down-regulation of NFATc1. Oncotarget. 2015;6(3):1582–1593. doi: 10.18632/oncotarget.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawahara T, Kashiwagi E, Li Y, Zheng Y, Miyamoto Y, Netto GJ, Ishiguro H, Miyamoto H. Cyclosporine A and tacrolimus inhibit urothelial tumorigenesis. Mol Carcinog. 2016;55(2):161–169. doi: 10.1002/mc.22265. [DOI] [PubMed] [Google Scholar]
- 14.Munari E, Fujita K, Faraj S, Chaux A, Gonzalez-Roibon N, Hicks J, Meeker A, Nonomura N, Netto GJ. Dysregulation of mammalian target of rapamycin pathway in upper tract urothelial carcinoma. Hum Pathol. 2013;44(12):2668–2676. doi: 10.1016/j.humpath.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Kashiwagi E, Fujita K, Yamaguchi S, Fushimi H, Ide H, Inoue S, Mizushima T, Reis LO, Sharma R, Netto GJ. Expression of steroid hormone receptors and its prognostic significance in urothelial carcinoma of the upper urinary tract. Cancer Biol Ther. 2016;17(11):1188–1196. doi: 10.1080/15384047.2016.1235667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shyr CR, Chen CC, Hsieh TF, Chang CH, Ma WL, Yeh S, Messing E, Li TH, Li FY, Chang C. The expression and actions of androgen receptor in upper urinary tract urothelial carcinoma (UUTUC) tissues and the primary cultured cells. Endocrine. 2013;43(1):191–199. doi: 10.1007/s12020-012-9762-4. [DOI] [PubMed] [Google Scholar]
- 17.Miyamoto H, Yao JL, Chaux A, Zheng Y, Hsu I, Izumi K, Chang C, Messing EM, Netto GJ, Yeh S. Expression of androgen and oestrogen receptors and its prognostic significance in urothelial neoplasm of the urinary bladder. BJU Int. 2012;109(11):1716–1726. doi: 10.1111/j.1464-410X.2011.10706.x. [DOI] [PubMed] [Google Scholar]
- 18.Williams EM, Higgins JP, Sangoi AR, McKenney JK, Troxell ML. Androgen receptor immunohistochemistry in genitourinary neoplasms. Int Urol Nephrol. 2015;47(1):81–85. doi: 10.1007/s11255-014-0834-7. [DOI] [PubMed] [Google Scholar]
- 19.Wang S, Kang X, Cao S, Cheng H, Wang D, Geng J. Calcineurin/NFATc1 pathway contributes to cell proliferation in hepatocellular carcinoma. Dig Dis Sci. 2012;57(12):3184–3188. doi: 10.1007/s10620-012-2255-8. [DOI] [PubMed] [Google Scholar]
- 20.Tauro S, MacCallum S, Groves MJ, Rojnuckarin P, Assanasen T, Feldman AL, Robson A, Marschalkó M, Kini H, Alzolibani AA. Immunohistochemical localization of cellular NFATc1 does not predict clinical responses to ciclosporin in subcutaneous panniculitis-like T-cell non-Hodgkin lymphoma. Br J Dermatol. 2010;162(4):887–889. doi: 10.1111/j.1365-2133.2009.09616.x. [DOI] [PubMed] [Google Scholar]
- 21.Buchholz M, Schatz A, Wagner M, Michl P, Linhart T, Adler G, Gress TM, Ellenrieder V. Overexpression of c-myc in pancreatic cancer caused by ectopic activation of NFATc1 and the Ca2+/calcineurin signaling pathway. EMBO J. 2006;25(15):3714–3724. doi: 10.1038/sj.emboj.7601246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen ZL, Zhao SH, Wang Z, Qiu B, Li BZ, Zhou F, Tan XG, He J. Expression and unique functions of four nuclear factor of activated T cells isoforms in non-small cell lung cancer. Chin J Cancer. 2011;30(1):62–68. doi: 10.5732/cjc.010.10156. [DOI] [PMC free article] [PubMed] [Google Scholar]