Abstract
Purpose: To compare the T staging of potentially resectable esophageal cancer using free-breathing radial VIBE (r-VIBE) and breath-hold Cartesian VIBE (C-VIBE), with pathologic confirmation of the T stage. Materials and Methods: Fifty patients with endoscopically proven esophageal cancer and indeterminate T1/T2/T3 stage by CT scan were examined on a 3-T scanner. The MRI protocol included C-VIBE at 150 seconds post–IV contrast, immediately followed by a work-in-progress r-VIBE with identical spatial resolution (1.1 mm × 1.1 mm × 3.0 mm). Two independent readers assigned a T stage on MRI according to the 7th edition of UICC-AJCC TNM Classification, and postoperative pathologic confirmation was considered the gold standard. Interreader agreement was also calculated. Results: The T staging agreement between both VIBE techniques and postoperative pathologic T staging was 52% (26/50) for C-VIBE, 80% (40/50) for r-VIBE for reader 1, and 50% (25/50), 82% (41/50) for reader 2, respectively. For the esophageal cancer with invading lamina propria, muscularis mucosae, or submucosa (T1 stage), r-VIBE achieved 86% (12/14) agreement for both readers 1 and 2. For invasion of muscularis propria (T2 stage), r-VIBE achieved 83% (25/30) for both readers 1 and 2, whereas for the invasion of adventitia (T3 stage), r-VIBE could only achieve agreement in 50% (3/6) and 67% (4/6) for readers 1 and 2, respectively. Conclusion: Contrast-enhanced free-breathing r-VIBE is superior to breath-hold CVIBE in T staging of potentially resectable esophageal cancer, especially for T1 and T2.
Introduction
Esophageal cancer remains one of the most lethal cancers [1]. It is listed as the sixth most common cancer, and it constitutes 5% to 7% of gastrointestinal cancers [2], [3]. In the management of patients with early-stage (T1 and T2) esophageal cancer, the longest disease-free survival could be achieved by surgical resection. Surgery is the first treatment for early (T1 and T2) esophageal cancer. More advanced (T3 and T4) patients may benefit from neoadjuvant chemoradiation prior to resection. Studies have shown that, in such cases, these patients have better survival than T3 patients undergoing resection alone [4]. Lund et al. [5] reported a 36% 5-year survival for T1 following surgical resection, and 5-year survival was much lower in patients with T2 and T3, with reported survival of 21% and 8%, respectively [4]. Therefore, preoperative T staging of esophageal cancer is critical in patient management and in determining survival.
One of the major challenges in determining the optimum treatment for patients with esophageal cancer is lack of precise preoperative staging guidelines/recommendations. The currently available noninvasive staging techniques include computed tomography (CT) of the chest and abdomen and endoscopic ultrasound. The reported overall T staging accuracy of CT is 50% to 80% as compared with 72% to 85% for endoscopic ultrasound [6], [7]. Fludeoxyglucose F 18 positron emission tomography has limitations in the preoperative staging of early esophageal cancer due to its lower spatial resolution but may be useful in detecting T4 stage [8], [9], [10], [11]. Although staging accuracy of endoscopic ultrasound is higher than both CT and positron emission tomography, it is considered invasive, is operator dependent, and may be of limited value in patients with nontraversable lesions.
Riddell et al. reported that a high-resolution T2-weighted fast spin-echo technique showed in exquisite detail the esophagus and posterior mediastinal structures on two cadavers who were imaged by 1.5 T magnetic resonance imaging (MRI) [12]. Another study using the same technique in 39 patients with esophageal cancer reported that this technique had high accuracy in differentiating between T2 and T3 disease but with a tendency to overstage T1 tumors [13]. The utility of conventional gradient echo T1-weighted sequences was explored because these sequences are faster than conventional spin-echo T2-weighted sequences and allow better contrast resolution after injection of gadolinium contrast [14], [15]. However, individual layers of the esophageal wall could still not be well identified. This is likely due to the posterior location of the esophagus in the mediastinum, in addition to motion artifacts resulting from breathing, heartbeat, swallowing, peristalsis, and magnetic susceptibility artifacts. These artifacts may result in poor delineation of the different layers of the esophageal wall on T1-weighted imaging.
Contrast-enhanced T1 W MRI is valuable in staging of esophageal cancer. However, obtaining motion artifact–free images of the esophagus with high spatial resolution is a challenge for conventional breath-hold Cartesian T1 W 3D gradient recalled echo sequences such as volumetric interpolated breath-hold examination (C-VIBE) [16]. For example, higher spatial resolution normally requires longer breath-hold capability, which is quite challenging for some patients, often resulting in poor image quality. The recently developed free-breathing radial VIBE (r-VIBE) sequence uses a stack-of-stars data acquisition scheme, which is less sensitive to motion compared with the Cartesian acquisition scheme in the conventional VIBE sequence [16], [17], [18], [19], [20]. Fujinaga et al. [16] concluded that free-breathing r-VIBE and breath-hold r-VIBE (and breath-hold C-VIBE) have similar high image quality in the liver. Azevedo et al. [21] showed that the free-breathing r-VIBE sequence is feasible for abdominal MRI and may be applicable in imaging of patients who are unable to suspend respiration, especially in children. Chandarana et al. [22] demonstrated that r-VIBE has better image quality and superior lesion conspicuity compared with C-VIBE in pediatric patients undergoing contrast-enhanced abdominopelvic MRI at 3 T.
The aim of this study was to compare the accuracy of free-breathing radial VIBE (r-VIBE) and breath-hold Cartesian VIBE (C-VIBE) in T staging of patients with potentially resectable esophageal cancer undergoing preoperative MRI at 3 T.
Materials and Methods
Patients
Fifty consecutive patients (mean age, 57.4 ± 9.9 years; range, 31-78 years; 40 men, 10 women) with preoperative biopsy pathologically confirmed esophageal cancer by endoscopy and potentially resectable staging of T1/T2/T3 by CT scan were included. All patients had undergone an esophageal MR study between July 2014 and January 2015. Surgery was performed within 7 days after MRI. The institutional review waived the requirement of informed patient consent in this retrospective review of clinically indicated MRI studies.
Magnetic Resonance Imaging
All examinations were acquired on a 3-T MR scanner (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany) with an anterior 18-element body coil and posterior 32-element spine coil array. Raceanisodamine hydrochloride (6542) with a dose of 10 mg was injected intramuscularly 15 to 20 minutes before MRI to decrease peristalsis. An MR-compatible automated injector pump (Spectris Solaris EP; Medrad, Indianola, PA) was used to administer the contrast agent through a 20-gauge antecubital intravenous site. After injection of 0.1 mmol/kg body weight of a macrocyclic Gd-DOTA contrast agent (Dotarem; Guerbet, Paris, France) at 2.5 ml/s, a 30-ml saline chaser at the same injection rate followed. The routine MRI protocol covers the chest, with sequences including axial fat suppression (FS) T1-weighted imaging, axial, coronal, and sagittal T2-weighted imaging, axial diffusion-weighted imaging (DWI) with the b value of 0 and 700 s/mm2, and axial postcontrast FS T1-weighted imaging in the arterial and venous phases. After the routine MRI protocol, a breath-hold C-VIBE sequence and a work-in-progress r-VIBE sequence with radial data sampling were acquired. Both sequences had identical spatial resolution (1.1 mm × 1.1 mm × 3.0 mm). Detailed sequence parameters are listed in Table 1. r-VIBE and C-VIBE sequences were sequentially acquired in the delayed phase which started 80 and 450 seconds after contrast injection, respectively. All studies were considered diagnostic, and no patients were excluded due to technical limitations.
Table 1.
Sequence Parameters of Conventional VIBE and Radial VIBE
| Parameters | Breath-Hold Cartesian VIBE | Free-Breathing Radial VIBE |
|---|---|---|
| FOV (mm3) | 266 × 360 × 192 | 280 × 280 × 192 |
| Resolution (mm3) | 1.1 × 1.1 × 3.0 | 1.1 × 1.1 × 3.0 |
| TR/TE (ms) | 5.32/2.46 | 3.68/1.71 |
| Flip angle (degree) | 12 | 13 |
| Fat suppression | Two-point Dixon | None |
| Applicable phases | Delayed phase | Delayed phase |
| Acquisition time (s) | 9 | 354 |
| Radial views | n/a | 1704 |
T Staging and Image Quality Evaluation
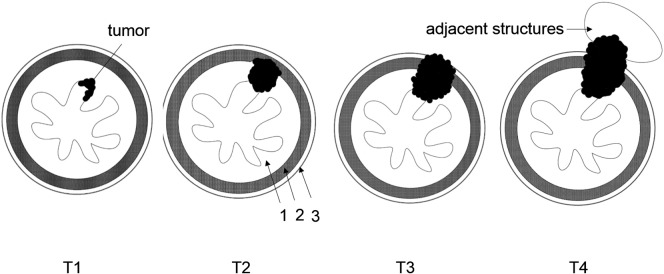
The C-VIBE (four subsets: water, fat, in-phase, and out-phase) and r-VIBE images were evaluated independently by two blinded radiologists with 8 and 6 years of experience in body MRI. The radiologists were aware of the diagnosis of esophageal cancer but were unaware of the patients' pathologic T staging. In the evaluation of C-VIBE images, the water images were reviewed for invasion of mucosa and muscularis propria, where the water, fat, in-phase, and out-phase subset images were reviewed side-by-side for the invasion of or breaking through adventitia. The C-VIBE and r-VIBE images were assessed in two different sessions 2 weeks apart to minimize recall bias. Each reader assessed image quality (1 = poor, 2 = acceptable, 3 = average, 4 = good, 5 = excellent) for both C-VIBE and r-VIBE (Figure 1). Radiologists also documented the extent of involvement of the esophageal wall layers, and T staging was reported according to the staging criteria for EC [13], [23] (Figure 2). These include T1: tumor invades mucosa with lower-signal tumor and high-signal mucous layer remaining ring-like intact; T2: tumor invades muscularis propria with lower-signal tumor interrupting the high-signal mucous layer and invading muscularis propria, but without breaking through muscularis propria; T3: tumor invades adventitia with tumor, and mucous layer and muscularis propria are blurred; and T4: tumor invades adjacent structures with lower-signal tumor invading adjacent structures. T staging using the r-VIBE and C-VIBE images was compared to postoperative pathologic T staging.
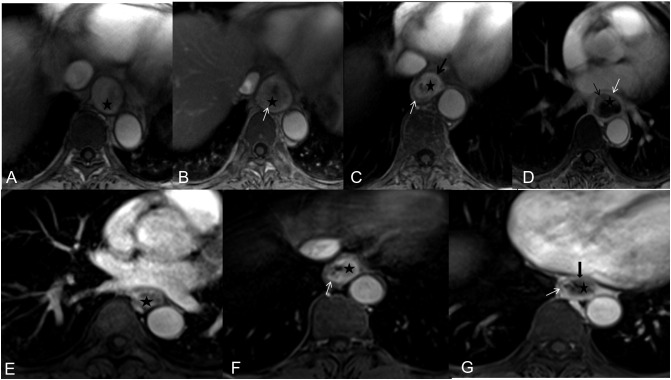
Figure 1.
Examples of image quality (1 = poor, 2 = acceptable, 3 = average, 4 = good, 5 = excellent) for both C-VIBE (E-G: score 2-4) and r-VIBE (A-D: score 2-5).  = tumor, mucosa (black arrow), muscularis propria (white arrow), and interrupted muscularis propria (thick black arrow).
= tumor, mucosa (black arrow), muscularis propria (white arrow), and interrupted muscularis propria (thick black arrow).
Figure 2.
T staging criteria of contrast-enhanced radial VIBE for esophageal cancer. 1, mucosa; 2, muscularis propria; 3, adventitia.
Statistical Analysis
Statistical analyses were performed using SPSS 18.0, and P﹤.05 was considered statistically significant. The interreader agreement for r-VIBE and C-VIBE, and the T staging agreement between both VIBE techniques and postoperative pathologic T staging were analyzed by a Kappa test. Agreement in terms of Kappa values was as follows: <0.40 is poor agreement, 0.41 to 0.75 is moderate/good agreement, and >0.75 is excellent agreement. Patients were stratified into early (T1 and T2 tumors) and advanced (T3 and T4 tumors), and the staging accuracy was calculated.
Results
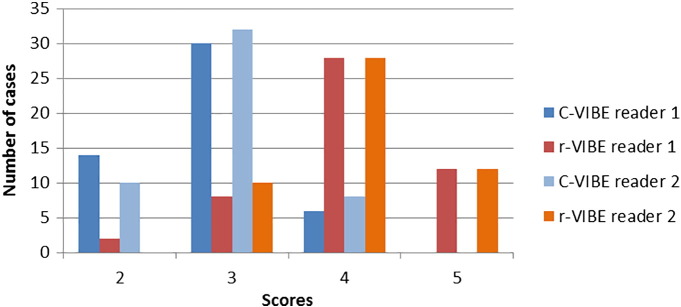
The patient population consisted of 50 individuals (mean age, 57.4 ± 9.9 years; range, 31-78 years; 40 men, 10 women). Postoperative pathology showed esophageal squamous cell carcinoma in 42 cases and adenocarcinoma in eight cases. Fourteen cases were T1, 30 cases were T2, 6 cases were T3, and no case was T4. For reader 1, image quality was considered as good or excellent in 6/50 cases and 40/50 cases for C-VIBE and r-VIBE, respectively. For reader 2, image quality was considered as good or excellent in 8/50 cases and 40/50 cases for C-VIBE and r-VIBE, respectively (Figure 3). The interreader agreement was excellent for both r-VIBE (Kappa = 0.933, P﹤0.001) and good for C-VIBE (Kappa = 0.704, P﹤0.001). The T staging agreement was good between r-VIBE and postoperative pathologic T staging for both readers, and poor between C-VIBE and postoperative pathologic T staging for both readers (Table 2).
Figure 3.
Scatter diagram of image quality for C-VIBE and r-VIBE (n = 50).
Table 2.
T Staging Agreement between Both VIBEs and Postoperative Pathologic T Staging
| C-VIBE |
r-VIBE |
||||
|---|---|---|---|---|---|
| Reader 1 | Reader 2 | Reader 1 | Reader 2 | ||
| Pathology | Kappa | 0.268 | 0.179 | 0.641 | 0.624 |
| P | .003 | .055 | .000 | .000 | |
The T staging agreement between both VIBE techniques and postoperative pathologic T staging was as follows: 52% (26/50) for C-VIBE and 80% (40/50) for r-VIBE for reader 1, and 50% (25/50) for C-VIBE and 82% (41/50) for r-VIBE for reader 2, respectively (Figure 4, Figure 5, Figure 6). Comparison between radiologic and pathologic T staging for both readers using C-VIBE is shown in Table 3. Accuracy ranged between 33% and 60% for the two readers. Comparison between radiologic and pathologic T staging for both readers using r-VIBE is shown in Table 4. Accuracy ranged between 50% and 86% for the two readers.
Figure 4.
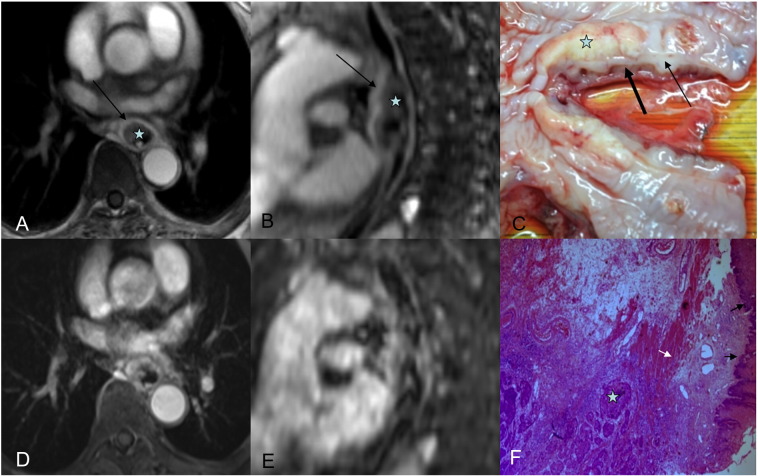
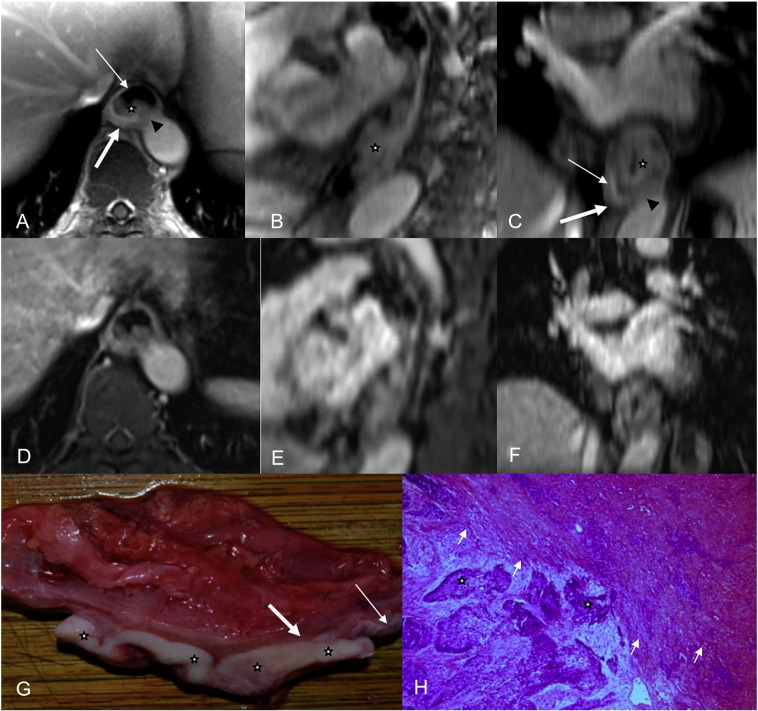
(A and B) Axial radial VIBE and reformatted sagittal images in a 65-year-old female esophageal cancer patient,  = tumor. (A) Image obtained with contrast-enhanced radial VIBE shows mucous layer remains ring-like intact (black arrow), and MRI staging is T1. (D and E) Axial conventional VIBE and reformatted sagittal images; both tumor and mucous layer are blurred. (C) On a photograph of EC, tumor is seen as a yellowish solid mass, mucous layer (thin black arrow) is not interrupted completely, and muscularis propria is intact (thick black arrow). (F) Tumor invades submucosa (white arrow), and muscularis propria is intact (black arrow) via a hematoxylin and eosin (H&E)–stained section at ×100.
= tumor. (A) Image obtained with contrast-enhanced radial VIBE shows mucous layer remains ring-like intact (black arrow), and MRI staging is T1. (D and E) Axial conventional VIBE and reformatted sagittal images; both tumor and mucous layer are blurred. (C) On a photograph of EC, tumor is seen as a yellowish solid mass, mucous layer (thin black arrow) is not interrupted completely, and muscularis propria is intact (thick black arrow). (F) Tumor invades submucosa (white arrow), and muscularis propria is intact (black arrow) via a hematoxylin and eosin (H&E)–stained section at ×100.
Figure 5.
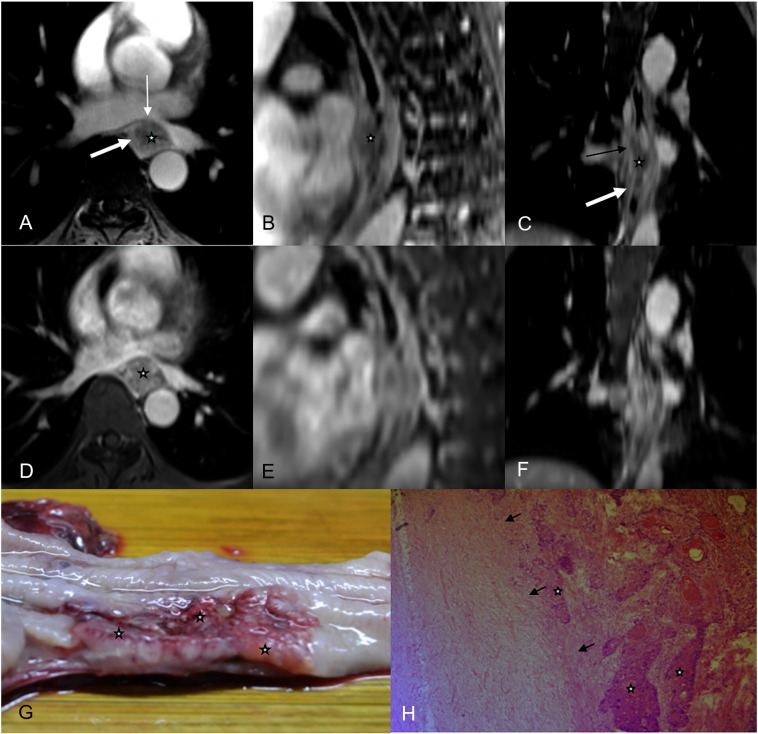
(A-C) Axial radial VIBE, reformatted sagittal and coronal images in a 63-year-old male esophageal cancer patient,  = tumor, thin white arrow = mucous layer, and thick white arrow means muscularis propria. (A-C) Images obtained with contrast-enhanced radial VIBE show that tumor invades muscularis propria (arrowhead), and MRI staging is T2. (D-F) Axial conventional VIBE, reformatted sagittal and coronal images, tumor, mucous layer, and muscularis propria are blurred. (G) On a photograph of EC, tumor is seen as a white solid mass, mucous layer (thin white arrow) is interrupted, and muscularis propria (thick white arrow) is invaded. (H) Tumor invades muscularis propria (white arrow) via an H&E-stained section at ×100.
= tumor, thin white arrow = mucous layer, and thick white arrow means muscularis propria. (A-C) Images obtained with contrast-enhanced radial VIBE show that tumor invades muscularis propria (arrowhead), and MRI staging is T2. (D-F) Axial conventional VIBE, reformatted sagittal and coronal images, tumor, mucous layer, and muscularis propria are blurred. (G) On a photograph of EC, tumor is seen as a white solid mass, mucous layer (thin white arrow) is interrupted, and muscularis propria (thick white arrow) is invaded. (H) Tumor invades muscularis propria (white arrow) via an H&E-stained section at ×100.
Figure 6.
(A-C) Axial radial VIBE, reformatted sagittal and coronal images in 47-year-old male esophageal cancer patients,  = tumor, thick arrow = muscularis propria. (A-C) Images obtained with contrast-enhanced radial VIBE show that tumor invades adventitia (thin arrow), and MRI staging is T3. (D-F) Axial conventional VIBE, reformatted sagittal and coronal images, tumor, mucous layer, and muscularis propria are blurred. (G) On a photograph of EC, tumor is seen as a solid mass, mucous layer is interrupted, and muscularis propria and adventitia are invaded. (H) Tumor invades adventitia (black arrow) via an H&E-stained section at ×100.
= tumor, thick arrow = muscularis propria. (A-C) Images obtained with contrast-enhanced radial VIBE show that tumor invades adventitia (thin arrow), and MRI staging is T3. (D-F) Axial conventional VIBE, reformatted sagittal and coronal images, tumor, mucous layer, and muscularis propria are blurred. (G) On a photograph of EC, tumor is seen as a solid mass, mucous layer is interrupted, and muscularis propria and adventitia are invaded. (H) Tumor invades adventitia (black arrow) via an H&E-stained section at ×100.
Table 3.
Comparison between Preoperative Breath-Hold C-VIBE T Staging and Postoperative Pathologic T Staging (n = 50)
| Preoperative C-VIBE T Staging | Postoperative Pathologic T Staging |
|||||||
|---|---|---|---|---|---|---|---|---|
| T1 |
T2 |
T3 |
T4 |
|||||
| Reader 1 | Reader 2 | Reader 1 | Reader 2 | Reader 1 | Reader 2 | Reader 1 | Reader 2 | |
| T1 | 8/14 | 5/14 | 5/30 | 4/30 | 0 | 0 | 0 | 0 |
| T2 | 4/14 | 6/14 | 15/30 | 18/30 | 1/6 | 2/6 | 0 | 0 |
| T3 | 2/14 | 3/14 | 8/30 | 7/30 | 3/6 | 2/6 | 0 | 0 |
| T4 | 0 | 0 | 2/30 | 1/30 | 2/6 | 2/6 | 0 | 0 |
| Accuracy (%) | 57 (8/14) | 36 (5/14) | 50 (15/30) | 60 (18/30) | 50 (3/6) | 33 (2/6) | 0 | 0 |
Table 4.
Preoperative Free-Breathing r-VIBE T Staging and Postoperative Pathologic T Staging (n = 50)
| Preoperative r-VIBE T Staging | Postoperative Pathologic T Staging |
|||||||
|---|---|---|---|---|---|---|---|---|
| T1 |
T2 |
T3 |
T4 |
|||||
| Reader 1 | Reader 2 | Reader 1 | Reader 2 | Reader 1 | Reader 2 | Reader 1 | Reader 2 | |
| T1 | 12/14 | 12/14 | 3/30 | 2/30 | 2/6 | 2/6 | 0 | 0 |
| T2 | 2/14 | 2/14 | 25/30 | 25/30 | 1/6 | 0 | 0 | 0 |
| T3 | 0 | 0 | 2/30 | 3/30 | 3/6 | 4/6 | 0 | 0 |
| T4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Accuracy (%) | 86 (12/14) | 86 (12/14) | 83 (25/30) | 83 (25/30) | 50 (3/6) | 67 (4/6) | 0 | 0 |
Cases were stratified into two groups based on pathologic T stage. The first included early disease (T1 and T2), and the second included more advanced disease (T3 and T4). Accuracy for each reader in assigning the cases into the two main groups is shown in Table 5. Both readers had higher accuracy for T1/T2 stage using r-VIBE compared to C-VIBE. For T3/T4, reader 1 had lower accuracy using r-VIBE compared to C-VIBE, whereas accuracy for reader 2 was equal for both techniques.
Table 5.
Differentiation of T1-T2 and T3-T4 by C-VIBE and r-VIBE Using the Postoperative Pathologic T Staging (n = 50) as Gold Standard
| Preoperative VIBEs T Staging | Postoperative Pathologic T Staging |
|||
|---|---|---|---|---|
| T1-T2 |
T3-T4 |
|||
| Reader 1 | Reader 2 | Reader 1 | Reader 2 | |
| C-VIBE: T1/T2 | 32 | 33 | 1 | 2 |
| C-VIBE: T3/T4 | 12 | 11 | 5 | 4 |
| C-VIBE: accuracy (%) | 72 (32/44) | 75 (33/44) | 83 (5/6) | 67 (4/6) |
| r-VIBE: T1/T2 | 42 | 41 | 3 | 2 |
| r-VIBE: T3/T4 | 2 | 3 | 3 | 4 |
| r-VIBE: accuracy (%) | 95 (42/44) | 93 (41/44) | 50 (3/6) | 67 (4/6) |
Discussion
To our knowledge, this is the first report of feasibility and clinical application of radial 3D fat-suppressed T1-weighted postcontrast acquisition in esophageal cancer. Our study showed that contrast-enhanced free-breathing r-VIBE is superior to conventional contrast-enhanced breath-hold C-VIBE technique for the accuracy of early (T1/T2) staging of esophageal cancer. In all cases, image quality of r-VIBE was higher than that of C-VIBE, with minimal artifacts reported by both readers.
The main distinct advantage of r-VIBE is a significantly lower sensitivity to motion. The phase error introduced by motion in Cartesian sampling as used in C-VIBE is eliminated when sampling k-space along radial spokes, each with different readout direction. Therefore, motion artifacts present in the images as radially oriented streaks or mild blurring. Furthermore, the overlap of the radial spokes in the center of k-space has a time-averaging effect that additionally reduces the sensitivity to motion and flow [24]. As we observed, r-VIBE acquisition results in significantly lower pulsation artifacts compared to breath-hold C-VIBE. These results are consistent with published studies which have demonstrated that radial schemes can improve image quality for 3D gradient echo of abdominopelvic imaging, pediatric imaging, and head and neck imaging [25], [26].
For the T1 stage esophageal cancer, r-VIBE achieved 86% (12/14) agreement for both reader 1 and 2. However, in two cases, both readers overcalled mucosal interruption and called these cases T2. For invasion of muscularis propria (T2), r-VIBE achieved 83% (25/30) for both reader 1 and 2. Careful evaluation of the three cases for reader 1 and two cases for reader 2 of underestimated staging (T2- > T1) showed that this staging is interfered by the hyperintense vessel close to the esophagus, where the hyperintense vessel was considered as enhanced mucosa. Considering the different enhancement property of muscularis propria and mucosa, the underestimated staging could be corrected by using a dynamic free-breathing golden-angle radial sparse technique (GRASP) with higher temporal resolution. For the overestimated staging (T2- > T3), in two cases for reader 1 and three cases for reader 2, this overstaging might be due to the current protocol limitation (such as the spatial resolution, slice thickness with 3 mm, contrast) in depicting the very thin adventitia. Therefore, an r-VIBE protocol with higher in-plane spatial resolution and thin slice thickness might improve the accuracy of staging this case.
In the evaluation of invasion of the adventitia (T3 stage), r-VIBE correctly staged the tumor in 50% (3/6) and 67% (4/6) for reader 1 and 2, respectively. For all the remaining cases, the tumor was underestimated by r-VIBE. A possible explanation for tumor underestimation and overestimation is limited spatial resolution of r-VIBE that may lead to suboptimum tumor definition. A higher spatial resolution r-VIBE might be helpful for assessing invasion of the adventitia.
It is very important to accurately perform staging pretreatment, differentiating T1 to T2 from T3 to T4 of esophageal cancer. In the current study, we stratified patients into two groups based on the T stage. The early group included T1 and T2, whereas the advanced group included T3 and T4. This stratification is important because early stages are treated surgically, whereas late stages are treated by radiation and/or chemotherapy. r-VIBE showed higher accuracy of identification of T1-T2 as compared to C-VIBE which overestimated the T1 to T2 and T3 to T4 with 12 and 11 cases for reader 1 and reader 2, respectively. Increasing motion artifacts on C-VIBE may result in blurring of the esophageal contour and overestimating the T stage. T3 staging, however, was still limited for reader 1. This may be due to sample size limitations. The major obstacle is that the very thin adventitia is not easily visible in the current delayed-phase r-VIBE and C-VIBE protocol. With dynamic imaging after contrast administration, it might be possible to improve the contrast of the adventitia during the time course of contrast uptake. Therefore, a higher spatial and temporal resolution/dynamic T1 W gradient recalled echo protocol (such as GRASP) might improve the T3 staging.
The interreader agreement was excellent for r-VIBE (Kappa = 0.933, P﹤.001) and good for C-VIBE (Kappa = 0.704, P﹤.001), which reveals that r-VIBE delivers a reliable and consistent result over C-VIBE. The reason might be that r-VIBE is less sensitive to motion.
It should be noted that the scan time for r-VIBE (354 seconds) is much longer than breath-hold C-VIBE (9 seconds). Therefore, image quality and signal-to-noise ratio comparisons between the two techniques may be difficult. However, the aim of this study was to evaluate the accuracy of T staging of both techniques instead of signal-to-noise ratio. For the breath-hold C-VIBE, the acquisition time is limited by the patient's breath-hold capability. In this study, with the introduction of CAIPIRIHNA parallel imaging [27], the 9-second breath-hold C-VIBE is optimized for its routine usage. For the r-VIBE acquisition, 1704 radial views were used, which are not well optimized. Our recent and preliminary evaluations show that images acquired using only 600 radial views (~2-minute acquisition time) are, in fact, sharper than those reconstructed from 1704 views due to reduced motion blurring in the shorter acquisition time.
The limitations of this study included only six cases with T3 and no cases with T4. Because these cases were clinically indicated, none of the advanced T4 stages were indicated for surgery as the treatment of choice is neoadjuvant chemoradiation. Secondly, the acquisition of r-VIBE is static, which is lacking the illustration of contrast enhancement property of different layers; however, the contrast between different layers of the esophagus wall is expected to be improved by the high temporal resolution of improved r-VIBE. For example, future studies utilizing GRASP could potentially be performed [16], [20]. Thirdly, nodal staging was not included in this preliminary study. The area of coverage in this study was approximately 6 cm due to technique limitation, so it was inadequate to assess for N stages.
A further limitation is that the parameters TR/TE/FA are not the same for both VIBEs. TR/TE is configured to minimize scan time with acceptable image quality, and C-VIBE has been optimized for its image quality. Additional restrictions on TR apply because the Dixon method is used for the fat suppression in C-VIBE. r-VIBE is configured with minimized acquisition time for each view. The TE setting in r-VIBE was approximating the out-of-phase condition, and there was a potential that the contour of the tumor and the esophagus was misdiagnosed due to a hypointense band by partial volume effect. It should be noted that the surrounding fat may also help to improve the T-stage of T3/T4a tumors. Therefore, the evaluation of T3/T4a tumors considers the Dixon fat, out-phase, and in-phase images. However, in all our T3/T4a cases, there is no fat surrounding, but it is worth to further evaluate it by including more T3/T4a patients.
Conclusion
Contrast-enhanced r-VIBE showed a promising sight for esophageal cancer staging not only for the esophageal cancer in mucosa but also invasion of muscularis propria. It outperformed the C-VIBE for T1/T2 staging, while being limited in T3 staging.
Footnotes
Conflict of Interest: All authors declare that there is no conflict of interest.
This study was supported by special funding of the Henan Health Science and Technology Innovation Talent Project (No.201004057).
Contributor Information
Jinrong Qu, Email: qjryq@126.com.
Hailiang Li, Email: doctorhnchr@126.com.
References
- 1.Onbas O, Eroglu A, Kantarci M, Polat P, Alper F, Karaoglanoglu N, Okur A. Preoperative staging of esophageal carcinoma with multidetector CT and virtual endoscopy. Eur J Radiol. 2006;57:90–95. doi: 10.1016/j.ejrad.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–2252. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 3.Dumont P, Wihlm JM, Hentz JG, Roeslin N, Lion R, Morand G. Respiratory complications after surgical treatment of esophageal cancer. A study of 309 patients according to the type of resection. Eur J Cardiothorac Surg. 1995;9:539–543. doi: 10.1016/s1010-7940(05)80001-6. [DOI] [PubMed] [Google Scholar]
- 4.Schrager JJ, Tarpley JL, Smalley WE, Austin MT, Pearson AS. Endoscopic ultrasound: impact on survival in patients with esophageal cancer. Am J Surg. 2005;190:682–686. doi: 10.1016/j.amjsurg.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Lund O, Hasenkam JM, Aagaard MT, Kimose HH. Time-related changes in characteristics of prognostic significance in carcinomas of the oesophagus and cardia. Br J Surg. 1989;76:1301–1307. doi: 10.1002/bjs.1800761227. [DOI] [PubMed] [Google Scholar]
- 6.Lightdale CJ, Kulkarni KG. Role of endoscopic ultrasonography in the staging and follow-up of esophageal cancer. J Clin Oncol. 2005;23:4483–4489. doi: 10.1200/JCO.2005.20.644. [DOI] [PubMed] [Google Scholar]
- 7.Choi J, Kim SG, Kim JS, Jung HC, Song IS. Comparison of endoscopic ultrasonography (EUS), positron emission tomography (PET), and computed tomography (CT) in the preoperative locoregional staging of resectable esophageal cancer. Surg Endosc. 2010;24:1380–1386. doi: 10.1007/s00464-009-0783-x. [DOI] [PubMed] [Google Scholar]
- 8.Flamen P, Lerut A, Van Cutsem E, De Wever W, Peeters M, Stroobants S, Dupont P, Bormans G, Hiele M, De Leyn P. Utility of positron emission tomography for the staging of patients with potentially operable esophageal carcinoma. J Clin Oncol. 2000;18:3202–3210. doi: 10.1200/JCO.2000.18.18.3202. [DOI] [PubMed] [Google Scholar]
- 9.Flamen P, Van Cutsem E, Lerut A, Cambier JP, Haustermans K, Bormans G, De Leyn P, Van Raemdonck D, De Wever W, Ectors N. Positron emission tomography for assessment of the response to induction radiochemotherapy in locally advanced oesophageal cancer. Ann Oncol. 2002;13:361–368. doi: 10.1093/annonc/mdf081. [DOI] [PubMed] [Google Scholar]
- 10.Weber WA, Ott K, Becker K, Dittler HJ, Helmberger H, Avril NE, Meisetschläger G, Busch R, Siewert JR, Schwaiger M. Prediction of response to preoperative chemotherapy in adenocarcinomas of the esophagogastric junction by metabolic imaging. J Clin Oncol. 2001;19:3058–3065. doi: 10.1200/JCO.2001.19.12.3058. [DOI] [PubMed] [Google Scholar]
- 11.van Westreenen HL, Westerterp M, Bossuyt PM, Pruim J, Sloof GW, van Lanschot JJ, Groen H, Plukker JT. Systematic review of the staging performance of 18F-fluorodeoxyglucose positron emission tomography in esophageal cancer. J Clin Oncol. 2004;22:3805–3812. doi: 10.1200/JCO.2004.01.083. [DOI] [PubMed] [Google Scholar]
- 12.Riddell AM, Davies DC, Allum WH, Wotherspoon AC, Richardson C, Brown G. High-resolution MRI in evaluation of the surgical anatomy of the esophagus and posterior mediastinum. AJR Am J Roentgenol. 2007;188:W37–W43. doi: 10.2214/AJR.05.1795. [DOI] [PubMed] [Google Scholar]
- 13.Riddell AM, Allum WH, Thompson JN, Wotherspoon AC, Richardson C, Brown G. The appearances of oesophageal carcinoma demonstrated on high-resolution, T2-weighted MRI, with histopathological correlation. Eur Radiol. 2007;17:391–399. doi: 10.1007/s00330-006-0363-6. [DOI] [PubMed] [Google Scholar]
- 14.Nakashima A, Nakashima K, Seto H, Kakishita M. Normal appearance of the esophagus in sagittal section; measurement of the anteroposterior diameter with ECG gated MR imaging. Radiat Med. 1996;14:77–80. [PubMed] [Google Scholar]
- 15.Nakashima A, Nakashima K, Seto H, Kakishita M, Sakamoto T, Yamada A, Fujimaki M. Thoracic esophageal carcinoma: evaluation in the sagittal section with magnetic resonance imaging. Abdom Imaging. 1997;22:20–23. doi: 10.1007/s002619900132. [DOI] [PubMed] [Google Scholar]
- 16.Fujinaga Y, Ohya A, Tokoro H, Yamada A, Ueda K, Ueda H, Kitou Y, Adachi Y, Shiobara A, Tamaru N. Radial volumetric imaging breath-hold examination (VIBE) with k-space weighted image contrast (KWIC) for dynamic gadoxetic acid (Gd-EOB-DTPA)-enhanced MRI of the liver: advantages over Cartesian VIBE in the arterial phase. Eur Radiol. 2014;24:1290–1299. doi: 10.1007/s00330-014-3122-0. [DOI] [PubMed] [Google Scholar]
- 17.Vigen KK, Peters DC, Grist TM, Block WF, Mistretta CA. Undersampled projection-reconstruction imaging for time-resolved contrast-enhanced imaging. Magn Reson Med. 2000;43:170–176. doi: 10.1002/(sici)1522-2594(200002)43:2<170::aid-mrm2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 18.Song HK, Dougherty L. Dynamic MRI with projection reconstruction and KWIC processing for simultaneous high spatial and temporal resolution. Magn Reson Med. 2004;52:815–824. doi: 10.1002/mrm.20237. [DOI] [PubMed] [Google Scholar]
- 19.Block K, Chandarana H, Milla S, Bruno M, Mulholland T, Fatterpekar G, Hagiwara M, Grimm R, Geppert C, Kiefer B. towards routine clinical use of radial stack-of-stars 3D gradient-echo sequences for reducing motion sensitivity. J Korean Soc Magn Reson Med. 2014;18:87–106. [Google Scholar]
- 20.Fujinaga Y, Kitou Y, Ohya A, Adachi Y, Tamaru N, Shiobara A, Ueda H, Nickel MD, Maruyama K, Kadoya M. Advantages of radial volumetric breath-hold examination (VIBE) with k-space weighted image contrast reconstruction (KWIC) over Cartesian VIBE in liver imaging of volunteers simulating inadequate or no breath-holding ability. Eur Radiol. 2015 doi: 10.1007/s00330-015-4103-7. [DOI] [PubMed] [Google Scholar]
- 21.Azevedo RM, de Campos RO, Ramalho M, Heredia V, Dale BM, Semelka RC. Free-breathing 3D T1-weighted gradient-echo sequence with radial data sampling in abdominal MRI: preliminary observations. AJR Am J Roentgenol. 2011;197:650–657. doi: 10.2214/AJR.10.5881. [DOI] [PubMed] [Google Scholar]
- 22.Chandarana H, Block KT, Winfeld MJ, Lala SV, Mazori D, Giuffrida E, Babb JS, Milla SS. Free-breathing contrast-enhanced T1-weighted gradient-echo imaging with radial k-space sampling for paediatric abdominopelvic MRI. Eur Radiol. 2014;24:320–326. doi: 10.1007/s00330-013-3026-4. [DOI] [PubMed] [Google Scholar]
- 23.Talsma K, van Hagen P, Grotenhuis BA, Steyerberg EW, Tilanus HW, van Lanschot JJ, Wijnhoven BP. Comparison of the 6th and 7th editions of the UICC-AJCC TNM classification for esophageal cancer. Ann Surg Oncol. 2012;19:2142–2148. doi: 10.1245/s10434-012-2218-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chandarana H, Block TK, Rosenkrantz AB, Lim RP, Kim D, Mossa DJ, Babb JS, Kiefer B, Lee VS. Free-breathing radial 3D fat-suppressed T1-weighted gradient echo sequence: a viable alternative for contrast-enhanced liver imaging in patients unable to suspend respiration. Investig Radiol. 2011;46:648–653. doi: 10.1097/RLI.0b013e31821eea45. [DOI] [PubMed] [Google Scholar]
- 25.Wu X, Raz E, Block TK, Geppert C, Hagiwara M, Bruno MT, Fatterpekar GM. Contrast-enhanced radial 3D fat-suppressed T1-weighted gradient-recalled echo sequence versus conventional fat-suppressed contrast-enhanced T1-weighted studies of the head and neck. AJR Am J Roentgenol. 2014;203:883–889. doi: 10.2214/AJR.13.11729. [DOI] [PubMed] [Google Scholar]
- 26.Block KTCH, Fatterpekar G, Hagiwara M, Milla S, Mulholland T, Bruno M, Geppert C, Sodickson DK. clinical head-to-toe imaging. Vol. 5. 2013. improving the robustness of clinical T1-weighted MRI using radial VIBE; pp. 6–11. [Google Scholar]
- 27.Breuer FA, Blaimer M, Mueller MF, Seiberlich N, Heidemann RM, Griswold MA, Jakob PM. Controlled aliasing in volumetric parallel imaging (2D CAIPIRINHA) Magn Reson Med. 2006;55:549–556. doi: 10.1002/mrm.20787. [DOI] [PubMed] [Google Scholar]