Abstract
Androgen deprivation therapy (ADT) is the most preferred treatment for men with metastatic prostate cancer (PCa). However, the disease eventually progresses and develops resistance to ADT in majority of the patients, leading to the emergence of metastatic castration-resistant prostate cancer (mCRPC). Here, we assessed artesunate (AS), an artemisinin derivative, for its anticancer properties and ability to alleviate resistance to androgen receptor (AR) antagonists. We have shown AS in combination with bicalutamide (Bic) attenuates the oncogenic properties of the castrate-resistant (PC3, 22RV1) and androgen-responsive (LNCaP) PCa cells. Mechanistically, AS and Bic combination inhibits nuclear factor (NF)-κB signaling and decreases AR and/or AR-variant 7 expression via ubiquitin-mediated proteasomal degradation. The combination induces oxidative stress and apoptosis via survivin downregulation and caspase-3 activation, resulting in poly-ADP-ribose polymerase (PARP) cleavage. Moreover, preclinical castrate-resistant PC3 xenograft studies in NOD/SCID mice (n =28, seven per group) show remarkable tumor regression and significant reduction in lungs and bone metastases upon administering AS (50 mg/kg per day in two divided doses) and Bic (50 mg/kg per day) via oral gavage. Taken together, we for the first time provide a compelling preclinical rationale that AS could disrupt AR antagonist–mediated resistance observed in mCRPC. The current study also indicates that the therapeutic combination of Food and Drug Administration–approved AS or NF-κB inhibitors and AR antagonists may enhance the clinical efficacy in the treatment of mCRPC patients.
Introduction
Prostate cancer (PCa) is the second most common cancer in men and the fifth leading cause of cancer-associated death (~6.6% of the total deaths) among men [1]. Although androgen deprivation therapy (ADT) remains the mainstay for the management of metastatic PCa, majority of men develop resistance to primary ADT, leading to an aggressive stage termed “metastatic castration-resistant prostate cancer” (mCRPC). It has been established that CRPC is not an androgen-independent disease; rather, it continues to rely on androgen signaling [2]. Therefore, various second-generation antiandrogen drugs, such as abiraterone acetate [3], [4] and enzalutamide [5], [6], have emerged for the treatment of mCRPC. Increased expression of androgen receptor-variant 7 (AR-V7) has been detected in circulating tumor cells and mCRPC tissues, and is associated with shorter biochemical recurrence, shorter survival, and resistance to ADT [7], [8]. Interestingly, AR-V7 lacks the ligand-binding domain, which is the direct target of enzalutamide and the indirect target of abiraterone, thus maintaining the receptor in constitutively active conformation in a ligand-independent manner [7]. Not surprisingly, most men with mCRPC inevitably develop resistance to ADT, thus necessitating an urgent need for the development of new chemotherapeutic interventions and combinatorial approaches for the treatment of mCRPC [9], [10], [11].
The cross talk between inflammation and cancer progression is a well-established phenomenon and an emerging field of research [12]. Recent studies have shown that the nuclear factor (NF)-κB family of transcription factors is an important player in the development and progression of several human malignancies including mCRPC [13], [14]. It has been known that inflammatory cytokines present in the tumor microenvironment may drive mCRPC by activating NF-κB signaling and upregulating cytokines, thus restricting the efficacy of ADT [15], [16]. Activation of the noncanonical NF-κB pathway (NF-κB2/p52) can enhance expression of AR variants (AR-Vs) including AR-V7, causing resistance to antiandrogens, whereas inhibition of this pathway sensitizes the CRPC cells to antiandrogens by decreasing the expression of AR-Vs [17]. Hence, combination of potent and safe NF-κB inhibitors with antiandrogens might be an effective strategy for the management of CRPC [18].
Artemisinin derivatives (ADs) are semisynthetic compounds derived from Artemisia annua and are approved first-line antimalarial drugs. The clinically important ADs are artesunate (AS), artemether (AM), arteether (AE), and dihydroartemisinin (DHA) [19]. The antiproliferative, antiangiogenic, anti-inflammatory, and antimetastasis properties of these derivatives have been reported in several cancer types including prostate [20], [21], [22], [23]. ADs act through their remarkable endoperoxide bridge, which gets cleaved in the presence of iron to generate cytotoxic free radicals and reactive oxygen species (ROS) [22]. The higher iron requirement and enhanced susceptibility to ROS may damage cancer cells (due to lower expression of antioxidant enzymes in cancer cells as compared to normal cells), elucidating the selective action of artemisinin on cancer cells [21]. Moreover, ADs are also known to inhibit NF-κB signaling, thereby inducing cell cycle arrest [22], [23]. We therefore reasoned that a combinatorial approach using ADs to block NF-κB signaling and antiandrogen could reinstate the responsiveness to AR antagonists in CRPC.
Here, we explored the combinatorial effect of ADs with AR antagonist bicalutamide (Bic) for the treatment of CRPC. We show that ADs enhance the antiproliferative effect of Bic on the castrate-resistant PC3 (AR null), 22RV1 (AR full length and AR-V7 positive), and androgen-responsive LNCaP cells. Interestingly, AS in combination with Bic demonstrated the most effective antiproliferative effect in PC3 cells. This drug combination significantly reduces cell invasion, migration, and foci formation in both androgen-responsive and -nonresponsive cells when compared with individual drug treatments. This is the first report that provides the mechanistic insights into AS and Bic combination–mediated increase in oxidative stress and inhibition of NF-κB signaling, which leads to decrease in AR and/or AR-V7 expression via ubiquitin-mediated proteasomal degradation. Consequently, these cumulative effects of the combinatorial drug treatment lead to enhanced apoptosis through caspase-3 activation, survivin downregulation, and poly-ADP-ribose polymerase (PARP) cleavage. Most importantly, AS and Bic combinatorial therapy in PC3 xenografted mice demonstrates enhanced tumor regression and reduced distant metastases.
Materials and Methods
Cell Lines and Reagents
Prostate cancer cell lines PC3, 22RV1, LNCaP, and PNT2 were procured from the American Type Culture Collection (ATCC) (Manassas, VA) between the years 2011 and 2013 and were maintained as per ATCC recommendation. Frozen stocks in early passages were established immediately after receiving each cell line, and only these early passaged frozen cell lines were used in the study. Cell lines were routinely tested for mycoplasma contamination. The ATCC-recommended medium was supplemented with 10% fetal bovine serum (Thermo-Fisher, Waltham, MA), and cell lines were cultured at 37°C in an incubator with 5% CO2.
Proliferation Assay and Assessment of Drug Synergism
ADs (AS, AE, AM, and DHA) were procured from the malaria research group at CSIR-CDRI, Lucknow, India (Themis/IPCA Pharmaceuticals, Mumbai, India). ADs were used for evaluating the antiproliferative effect on PCa cells. WST-1 assay (Roche, Basel, Switzerland) was performed as described previously [24]. Cells were treated with selected concentration of test drugs or their combinations, and absorbance was measured at 450 nm after incubating cells with WST-1 reagent. IC50 values were calculated by using preprogrammed Logit regression analysis for the dose-response curves. For assessing the synergism between AS and Bic, PC3, 22RV1, and LNCaP cells were treated with serial dilutions of the selected lead combinations and individual drugs for 48 hours. IC50 values were determined, and combination index (CI) of the drug interaction was calculated by following mathematical equation: CI = D1/Dx1 + D2/Dx2. Here, D1 and D2 are the IC50 of drug 1 and drug 2 in the combination respectively, whereas Dx1 and Dx2 are the IC50 values of individual drug 1 and 2, respectively. CI value of less than 1 was considered as synergism, CI equivalent to 1 as additive effect, and CI less than 1 was considered as antagonism. Isobolographic analysis was carried out to understand the nature and quantitative assessment of drugs’ interaction by Loewe's method [25].
Invasion and Migration Assay
Invasion assay was performed with AS and/or Bic (Sigma-Aldrich, St. Louis, MO) treated cells (100,000 cells) along with DMSO control by placing on the Matrigel-coated Boyden chambers (Corning, NY) as described previously [24]. Same protocol was followed for cell migration assay, except cells were plated in the Transwell chambers without Matrigel.
Foci Formation Assay
PCa cells (2000 cells) were plated in the presence of reduced serum (2%) medium in six-well culture dishes. Drugs were replenished every second day, and assay was carried out for 15 days as described before [24]. After staining with crystal violet, foci (>5 μm) were counted under phase contrast Olympus microscope (Tokyo, Japan).
DCFDA Fluorometric Assay
For CM–2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) assay, about 10,000 cells were plated and treated with individual drugs or combinations for 24 hours, followed by treatment with CM-H2DCFDA (10 μM; Sigma Aldrich) in serum-free media for 2 hours after washing with PBS. After lysing the cells, fluorescence readings were taken at an excitation/emission wavelength of 492/517 nm on the BioTek Synergy fluorescence reader (Winooski, VT).
Western Blotting and Immunoprecipitation
Western blotting for all protein samples was performed as previously described [24]. Antibodies [phospho-p65 (#3033), total-p65 (#4764), AR (#5153), β-actin (#4970), survivin (#2808), cleaved-PARP (#9541), Bcl-2 (#2870), and Bcl-xL (#2764)] were procured from Cell Signaling Technology (Danvers, MA). For immunoprecipitation, 22RV1 cells were treated with AS (2.5 μM) and Bic (25 μM) for 20 hours followed by serum starvation for 6 hours. Hereafter, cells were treated with MG132 (20 μM; Sigma-Aldrich) for 4 hours and lysed in cold radioimmunoprecipitation assay buffer. Total cell lysates (~1 mg) were precleared with proteinA/G sepharose beads (Sigma-Aldrich), and immunoprecipitation was performed as previously described [26]. The eluted protein was subjected to immunoblotting with anti-polyubiquitination (BML-PW8805-0500; Enzo Life Sciences, Farmingdale, NY). ImageJ software was used for quantification, and one-tailed Student's t test was employed for statistical significance.
Caspase-3 Colorimetric Assay
Caspase-3 colorimetric assay kit was procured from Invitrogen (Carlsbad, CA). Cells were seeded in a six-well plate and treated with the drugs for 48 hours, and assay was performed according to the manufacturer's protocol.
Luciferase Reporter Assays
The pNiFty-Luc vector (Invivogen, San Diego, CA) and pGL4.10-PSA vector (Promega, Madison, WI) were kind gifts from Dr. Shyam Nyati, University of Michigan, and Dr. Jindan Yu, Northwestern University, USA, respectively. Cells were seeded in a 24-well plate. For NF-κB reporter assay, PC3 cells were transfected with pNiFty-Luc vector and pRL-CMV Luciferase control vector (Promega) using FuGENE-HD transfection reagent (Promega) according to manufacturer's instructions. Cells were treated with individual drugs and combination 24 hours posttransfection. For PSA promoter reporter assay, 22RV1 cells were transfected with pGL4.10-PSA vector and pRL-Null luciferase control vector followed by drug treatment for 24 hours and subsequent R1881 stimulation for 12 hours. Dual-Glo Luciferase Assay was performed according to manufacturer's instructions (Promega) and normalized using Renilla luciferase activity.
Quantitative Real-Time PCR
Total RNA was extracted using TRIzol (Ambion, Waltham, MA) and reverse-transcribed using SuperScript II Reverse-Transcriptase (Invitrogen). qPCRs were performed using SYBR Green Master Mix (Applied Biosystems, Foster City, CA) on the StepOne Plus (Applied Biosystems) following established protocol [26]. Threshold levels for each qPCR were set during the exponential phase using Sequence Detection Software version 1.2.2. The relative expression of the target gene was calculated for each sample using the ΔΔCt method by comparing the mean Ct of the gene to the average Ct of the housekeeping gene. Primer sequences are listed in the Supplementary Table 4.
Immunofluorescence and Immunohistochemistry
LNCaP cells were treated with DHT, AS, Bic, or combination for 24 hours and fixed with 4% paraformaldehyde, and immunofluorescence was performed using primary AR and Alexa Fluor-488 conjugated secondary antibodies (Cell Signaling Technology) as described previously [24]. Immunohistochemical staining for Ki-67 was performed using mouse monoclonal antibody (Cell Signaling Technology) as described earlier [27].
Mouse Xenograft Experiment
Male NOD/SCID mice (6-8 weeks old, Jackson Laboratory, Bar Harbor, ME) were anesthetized using ketamine/xylazine (50 and 5 mg/kg, respectively, intraperitoneal). PC3 cells (1.2 × 106) were suspended in 100 μl of saline with 20% Matrigel (v/v) and implanted subcutaneously on both sides of the dorsal flank (seven per group). Tumor growth was recorded using digital calipers, and volumes were calculated using formula (π/6)*(L × W2) (L = length; W = width). Treatment with AS (50 mg/kg per day in two divided doses) and Bic (50 mg/kg per day) was administered orally once average tumor volume reached ~100 mm3. Spontaneous metastasis in the lungs and bone marrow specimens was analyzed by performing qPCR for human Alu-sequences as described previously [24]. All procedures involving mice were approved by the Committee for the Purpose of Control and Supervision of Experiments on Animals and conform to all regulatory standards.
Statistical Analysis
Logit regression analysis of dose-response curves was performed for the IC50 assessment. Two-way analysis of variance (ANOVA) with Tukey post hoc test using Graphpad Prism was employed for determining the most effective combination for ADs. All values in the study were presented as mean ± S.E.M. The significant differences between the experimental groups were analyzed by Student's t test, and P value of less than 0.05 or 0.01 was considered significant.
Results
AS Sensitizes Castrate-Resistant PCa Cells to Antiandrogen Therapy and Abrogates Their Oncogenic Potential
To investigate the antiproliferative potential of the ADs (AS, AE, AM, and DHA) in PCa cells, we first determined the half maximal inhibitory concentration (IC50) for ADs in castrate-resistant PC3, 22RV1, and androgen-responsive LNCaP cells by using WST-1 reagent. All ADs showed marginal antiproliferative effect (IC50 > 70 μM) in PC3 and 22RV1 cells, whereas LNCaP cells demonstrated higher sensitivity toward AS and DHA (IC50 4.42 and 12.20 μM, respectively) (Supplementary Table S1). Similarly, the IC50 value for Bic was higher in PC3 and 22RV1 cells (80 and 63 μM, respectively) as compared to LNCaP (53 μM) (Supplementary Table S1). The percent inhibition in cell proliferation for PC3, 22RV1, and LNCaP cells was assessed using sub-IC50 concentrations for each derivative (AS, AE, AM, and DHA) either alone or in combination; interestingly, AS and Bic combination showed remarkable response for all three cell lines (Supplementary Table S2). The most effective combination of individual AD and Bic in PC3, 22RV1, and LNCaP cells was determined by employing two-way ANOVA test (Supplementary Table S3). Here, we show that AS at much lower concentration can improve the efficacy of antiandrogen drugs such as Bic. Combinatorial treatment with AS (5 μM) and Bic (50 μM) at much lower concentration than their respective IC50 exhibited the most remarkable inhibition in PC3 (82%, P < 0.0001) and 22RV1 cell proliferation (72%, P < 0.0001) as compared to individual compound, whereas combinatorial treatment of AS (1 μM) and Bic (25 μM) demonstrated ~70% decrease in proliferation in LNCaP cells (P < 0.0001; Figure 1A and Supplementary Figure S1). Moreover, DHA, AE, and AM in combination with Bic also show antiproliferative effect on PC3, 22RV1, and LNCaP cells; however, the effect was more pronounced as compared to individual drug treatments (Supplementary Figure S2, A–C; Supplementary Tables S2–3). Furthermore, the combinatorial treatment of AS (5 μM) and Bic (50 μM) on the immortalized prostate epithelial PNT2 cells failed to show significant antiproliferative effect (Supplementary Figure S2D). Further, to assess the nature of drug interaction between AS and Bic (5 μM AS +50 μM BIC in PC3 and 22RV1 cells; 1 μM AS +25 μM Bic in LNCaP), isobologram analysis by Loewe's method was carried out. The calculated CI values for the selected drug combinations suggest strong synergistic interaction in PC3 (CI = 0.2) and 22RV1 (CI = 0.1), whereas weak synergistic interaction was observed in LNCaP cells (CI = 0.9) (Supplementary Figure S2E).
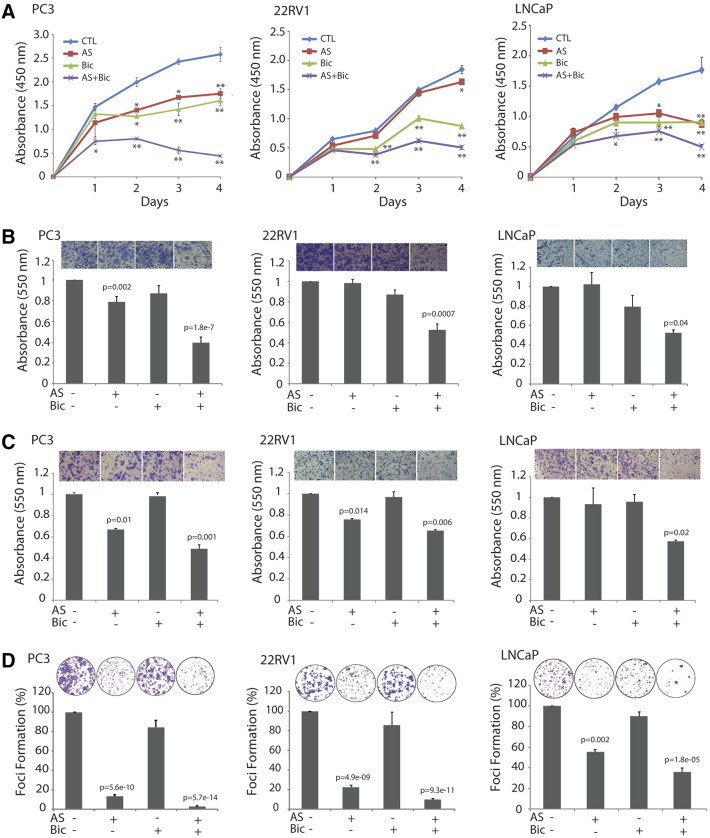
Figure 1.
AS attenuates cell proliferation and abrogates oncogenic properties by sensitizing PCa cells to antiandrogen. (A) Cell proliferation assay using tetrazolium salt–based WST-1 reagent. PC3 and 22RV1 cells were treated with AS (5 μM) and Bic (50 μM) alone or in combination for 24 to 96 hours, whereas LNCaP cells were treated with AS (1 μM) and Bic (25 μM). Error bars in panel A represent mean ± S.E.M. P values were calculated from two-way ANOVA. *P < 0.01 versus control; **P < 0.0001 versus control. P values < 0.05 were considered significant. (B) Boyden chamber Matrigel invasion assay using PC3 and 22RV1 cells treated with AS (2.5 μM) and Bic (25 μM) alone or in combination for 24 hours. LNCaP cells were treated with AS (1 μM) and Bic (12.5 μM). (C) Transwell cell migration assay. All experimental conditions were same as B. (D) Foci formation assay using PC3 and 22RV1 (treated with AS 1.25 μM and Bic 12.5 μM) and LNCaP cells (treated with AS 0.5 μM and Bic 6.25 μM). In panels B to D, error bars represent mean ± S.E.M. P values calculated from two-sided Student's t test. P values < 0.05 were considered significant.
To investigate the effect of AS and Bic alone or in combination on the invasive properties of PCa cells, Boyden chamber Matrigel invasion assay was performed using sublethal concentrations of the drugs. As shown in Figure 1B, Bic (50 μM) alone has no significant effect on cell invasion in PC3 cells, whereas in combination with AS, a significant reduction in cell invasion was observed (P = 1.8e-7). The combinatorial treatment showed remarkable decrease (~60%) in cell invasion of PC3 cells as compared to AS alone (~21%). Similarly, the combinatorial treatment of AS and Bic attenuated cell invasion in 22RV1 (~48% reduction) and LNCaP cells (~49% reduction) (Figure 1B; P = 0.0007 and P = 0.04, respectively), whereas individual drugs have no effect. Next, to explore the effect of AS and Bic combination on cell migration, Transwell migration assay was performed. As anticipated, combinatorial treatment of AS and Bic attenuated migration of PC3, 22RV1, and LNCaP cells. Interestingly, PC3 cells showed most pronounced effect (>50% inhibition) on migration upon treating with AS and Bic (Figure 1C; P = 0.001). Next, we investigated the foci-forming potential of PC3, 22RV1, and LNCaP cells upon AS and Bic treatment. We observed that administration of AS along with Bic vastly reduced the foci-forming property of PC3, 22RV1, and LNCaP cells than treatment with Bic alone. Interestingly, combinatorial treatment of AS and Bic in PC3 cells showed dramatic reduction in foci formation (~97% inhibition, P = 5.7e-14), 22RV1 (~89% inhibition, P = 9.3e-11), and LNCaP cells (~64% inhibition, P = 1.8e-5) as depicted in Figure 1D. Moreover, AS alone could also significantly reduce foci formation in all the three PCa cell lines, whereas the effect was more pronounced in PC3 cells (~86% reduction; P = 5.6e-10; Figure 1D).
AS and Its Combination with Bic Mediate Inhibition of NF-κB Signaling in PCa Cells
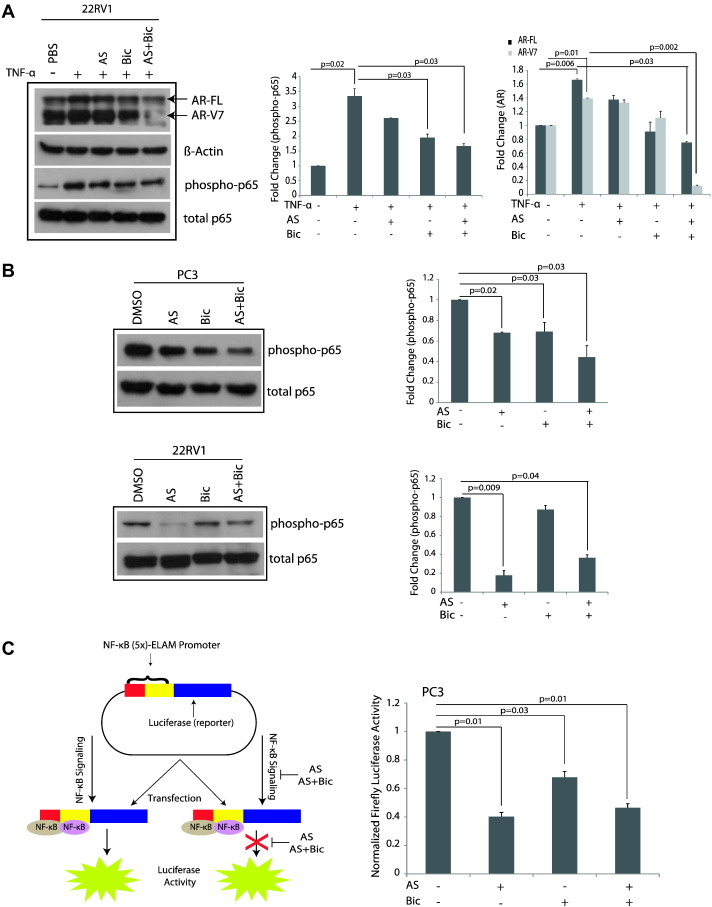
Activation of NF-κB signaling has been known to increase the expression of AR and AR-Vs in mCRPC, and inhibition of NF-κB pathway sensitizes the castrate-resistant PCa cells to antiandrogens [13], [18]. We stimulated 22RV1 cells with tumor necrosis factor alpha (TNF-α), and as anticipated, an upregulation in the NF-κB signaling supported by an increase in phospho-p65 level was observed (Figure 2A; P = 0.02). Further, treatment with AS and Bic combination abrogated this increase significantly (Figure 2A; P = 0.03). Notably, the expression of AR and AR-V7 also increased upon TNF-α stimulation (Figure 2A; P = 0.006 and P = 0.01, respectively), and the combinatorial treatment with AS and Bic significantly downregulated the expression of AR and AR-V7 (Figure 2A; P = 0.03 and P = 0.002, respectively), indicating that NF-κB signaling positively regulated AR and AR-V7 expression. To further elucidate the effect of AS and Bic on NF-κB signaling, we performed immunoblot analysis for phosphorylated NF-κB (pNF-κB or phospho-p65) in PC3 and 22RV1 cells. Interestingly, AS alone or in combination with Bic significantly reduced the pNF-κB levels in PC3 and 22RV1 cells (Figure 2B; P = 0.03 and P = 0.04, respectively). Moreover, LNCaP cells show low levels of NF-κB activity; therefore, we were unable to detect significant pNF-κB protein (data not shown).
Figure 2.
AS inhibits NF-κB signaling and augments sensitivity to AR antagonist in castrate-resistant PCa cells. (A) Immunoblotting for AR, phospho-p65, total p65, and β-actin in 22RV1 cells treated with AS (2.5 μM) and Bic (25 μM) alone or in combination in the presence of TNF-α (10 nM). (B) Immunoblotting for phospho-p65 and total p65 in PC3 and 22RV1 cells treated with AS (2.5 μM) and Bic (25 μM) alone or in combination. (C) Schema showing NF-κB luciferase reporter assay (left panel); NF-κB luciferase reporter assay in PC3 cells treated with AS (2.5 μM) and Bic (25 μM) alone or in combination (right panel). Error bars represent mean ± S.E.M. P values calculated from two-sided Student's t test. P values < 0.05 were considered significant.
Since AS is known to interfere with the canonical NF-κB pathway by targeting RelA/p65 [28], we next determined whether AS treatment alone or in combination with Bic abrogates NF-κB signaling by performing NF-κB reporter assay in PC3 cells. As anticipated, a marked reduction in the NF-κB reporter activity was observed upon AS treatment alone or in combination with Bic (Figure 2C; P = 0.01 for both). Taken together, our results suggest that treatment with AS alone as well as in combination with Bic blocks NF-κB signaling in PC3 cells.
Combinatorial Treatment of AS and Bic Promotes Degradation of AR and AR-Vs via Ubiquitin-Mediated Proteasomal Degradation
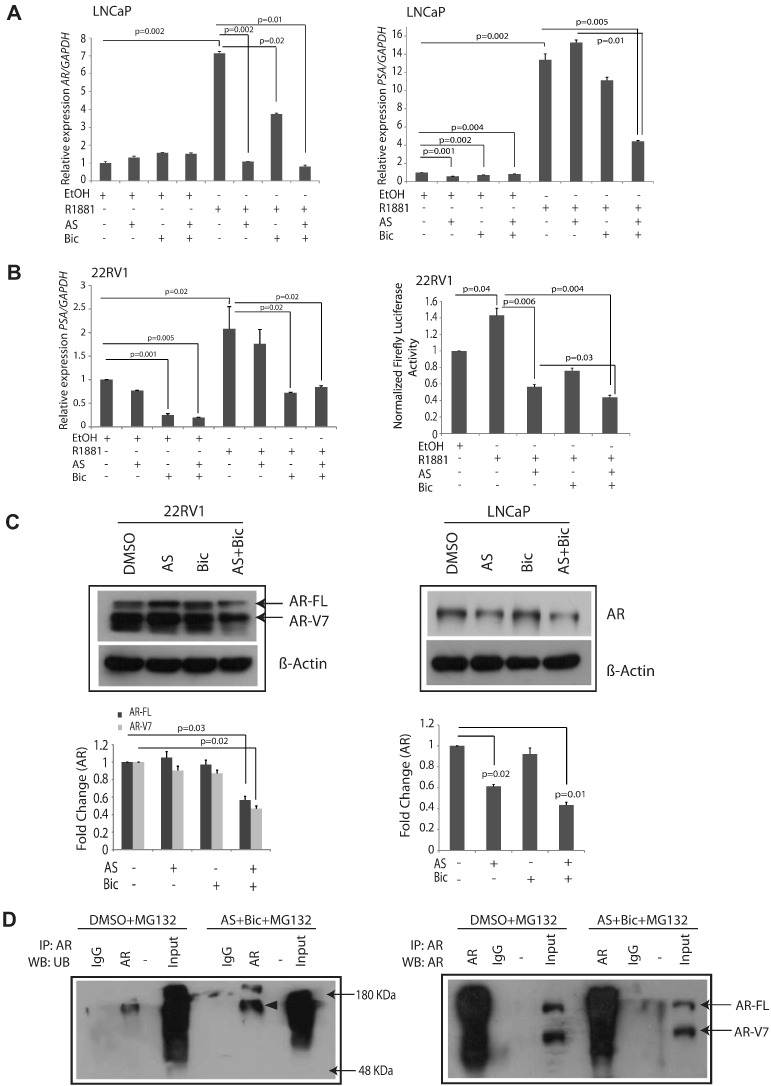
Constitutively active AR and AR-Vs are the key drivers of the AR-regulated transcription in PCa and mCRPC, thus promoting tumor progression and metastases even under castration or ADT conditions. Therefore, we evaluated the effect of AS alone or in combination with Bic on the expression of androgen-driven genes AR and PSA by qPCR using synthetic androgen R1881-stimulated LNCaP cells. As anticipated, R1881-treated LNCaP cells showed significant increase (>six-fold) in AR expression (Figure 3A; P = 0.002). We found a remarkable decrease in AR levels upon treatment of AS with Bic (~89%) compared to Bic alone (47%) (Figure 3A; P = 0.01 and P = 0.02, respectively). Interestingly, AS alone too gave comparable albeit lower reduction in AR levels in androgen-responsive LNCaP cells (P = 0.002). However, no significant decrease in the AR transcript was observed in PC3 and 22RV1 cells upon AS alone or combinatorial treatment with Bic (Supplementary Figure S3, A–B). Next, the effect of AS alone or in combination with Bic on the expression of PSA, an AR target gene in LNCaP cells was evaluated. As expected, R1881 stimulation significantly increased PSA expression (~13-fold), which upon treatment with AS and Bic was significantly reduced (Figure 3A; P = 0.005). Moreover, AS or Bic alone or combined together significantly reduced the basal expression of PSA in LNCaP cells even in the absence of R1881 (Figure 3A; P = 0.001, P = 0.002, and P = 0.004, respectively). Next, we investigated the effect of AS or/and Bic treatment on the expression of PSA in R1881-stimulated 22RV1 cells. As anticipated, a significant decrease in PSA expression was observed in the 22RV1 cells upon combinatorial treatment with AS and Bic (Figure 3B; P = 0.02). Furthermore, a marked reduction in the PSA promoter reporter activity in 22RV1 cells was observed upon treatment with the drug combination (Figure 3B; P = 0.004).
Figure 3.
AS and Bic combinatorial treatment decreases AR and AR-V7 levels via ubiquitin-mediated proteasomal degradation. (A) Quantitative PCR data showing expression of AR and PSA in LNCaP cells treated with AS (1 μM) and Bic (12.5 μM) alone or in combination for 24 hours in the presence or absence of synthetic androgen R1881 (10 nM for 12 hours). (B) qPCR and luciferase reporter assay data for PSA in 22RV1 cells treated with AS (2.5 μM) and Bic (25 μM) alone or in combination for 24 hours in the presence or absence of synthetic androgen R1881 (10 nM for 12 hours). (C) Immunoblotting for AR-FL in 22RV1 and LNCaP cells. Note expression of both AR-FL and AR-V7 in 22RV1 cells. 22RV1 cells were treated with AS (2.5 μM) and Bic (25 μM) alone or in combination, whereas LNCaP cells were treated with AS (1 μM) and Bic (12.5 μM). (D) Immunoprecipitation assay using 22RV1 cells treated with AS (2.5 μM) and Bic (25 μM) in the presence of MG132 (left panel); immunocomplexes were pulled down using AR or IgG antibodies and immunoblotted with an antibody against ubiquitin marks; immunoblotting with an antibody against AR (right panel). Error bars represent mean ± S.E.M. P values were calculated from two-sided Student's t test. P values < 0.05 were considered significant.
To further investigate the effect of AS alone or in combination with Bic on the AR protein level, we performed immunoblotting using 22RV1 and LNCaP cells. A significant reduction in AR full-length (AR-FL ~110 kDa) and AR-V7 expression (~75-80 kDa) was observed (~44% and ~53%, respectively) in 22RV1 cells upon treatment with AS and Bic (Figure 3C; P = 0.03 and P = 0.02, respectively), suggesting that the expression of AR at the transcript level was not affected by the AS and/or Bic treatment, whereas AR protein was downregulated at the posttranscriptional level. Furthermore, LNCaP cells also showed decrease in AR level upon AS treatment alone or in combination with Bic (Figure 3C; ~57% reduction in combination; P = 0.02 and P = 0.01, respectively). To explore the mechanism of AR downregulation upon AS and Bic treatment in PCa cell lines, we next examined the possibility of ubiquitin-mediated AR degradation through 26S proteasome complex [29]. Immunoprecipitation for AR was performed using AS and Bic treated 22RV1 cells. Interestingly, a significant increase in ubiquitination of AR was observed upon AS and Bic combination treatment in the presence of a proteasome inhibitor MG132 as compared to DMSO control (Figure 3D). Furthermore, a remarkable decrease in AR-FL and AR-V7 was observed in AS and Bic treated 22RV1 cells as compared to DMSO alone, suggesting that AS and Bic treatment triggers degradation of AR-FL and AR-Vs through ubiquitin-mediated proteasomal degradation.
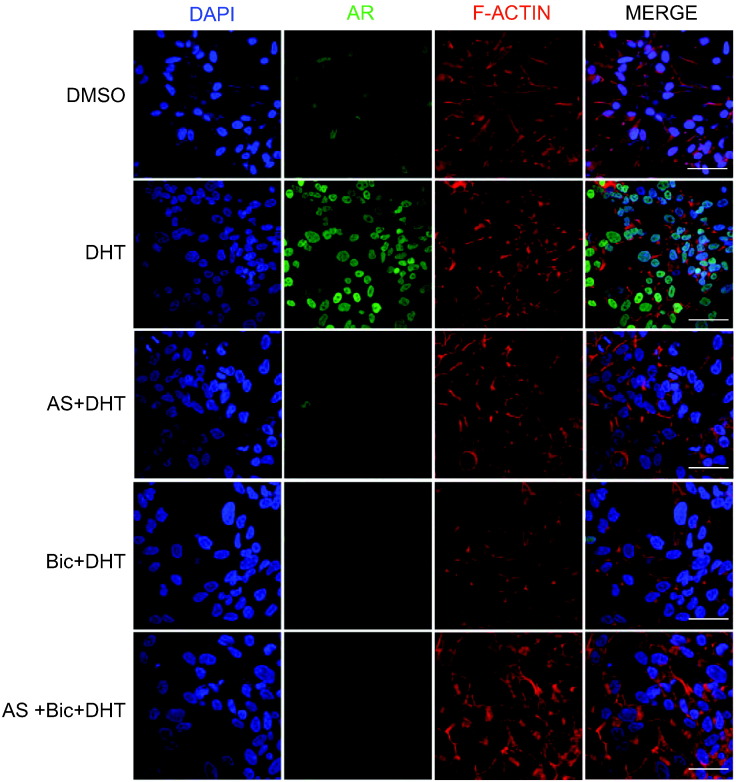
Next, to investigate whether AS treatment alone or in combination with Bic could decrease the expression of AR at the cellular level, we performed immunofluorescence staining for AR in LNCaP cells stimulated with potent androgen dihydrotestosterone (DHT). Interestingly, we observed that AS or Bic alone or in combination could remarkably reduce AR expression in the cytoplasm as well as in the nucleus of the DHT-stimulated LNCaP cells (Figure 4). Taken together, these findings demonstrate that AS sensitizes the PCa cells to Bic treatment by inhibiting NF-κB signaling, thereby resulting in degradation of AR and AR-Vs via ubiquitin-proteasomal complex. Further, AS also potentiates the effect of Bic by decreasing the expression of AR and AR-driven genes in LNCaP cells.
Figure 4.
AS and Bic treatment decreases AR expression in DHT-stimulated LNCaP cells. Immunofluorescence images show AR localization in LNCaP cells treated with AS (1 μM) and Bic (12.5 μM) alone or in combination upon DHT stimulation for 24 hours. Nuclei were stained with DAPI (blue; first panel), AR was immunostained with an antibody against AR (green; second panel), and F-actin filaments were stained with TRITC-Phalloidin (red; third panel); the last panel shows the merged images. Scale bar represents 20 μm.
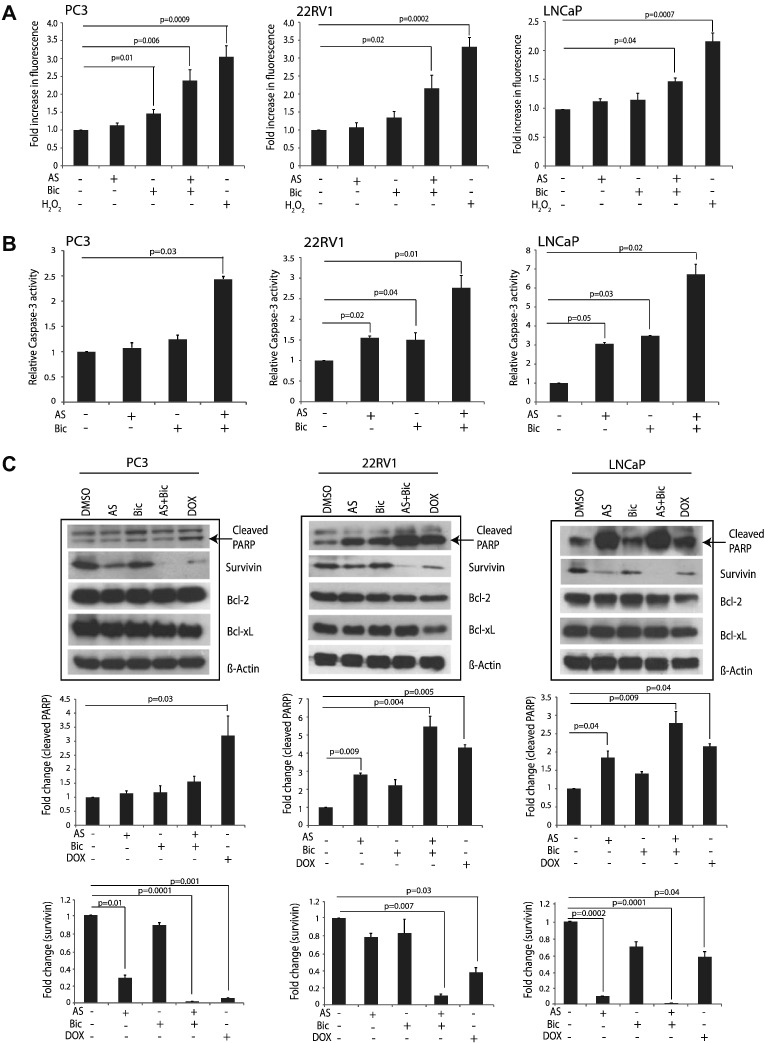
AS and Bic Combination Triggers Apoptosis by Inducing Oxidative Stress and Caspase-3–Mediated PARP Cleavage
Next, we sought to determine whether AS and Bic induced inhibition of NF-κB and AR signaling could trigger apoptosis by generating ROS. Thus, we employed H2DCFDA assay for detecting ROS in AS or Bic treated PCa cells. A significant increase in the generation of ROS was observed in PC3 (>two-fold increase; P = 0.006), 22RV1 (>two-fold increase; P = 0.02), and LNCaP cells (>1.5-fold increase; P = 0.04) upon combinatorial AS and Bic treatment (Figure 5A). Next, we sought to determine whether AS and Bic treatment results in Caspase-3 activation, a known key mediator of apoptosis activated in response to ROS generation and an upstream activator of PARP cleavage. Therefore, colorimetric assay was performed to test Caspase-3 activity in PCa cells treated with AS and Bic alone and/or in combination. Interestingly, the LNCaP cells treated with combination showed highest Caspase-3 activity (six-fold increase), whereas 22RV1 and PC3 cells showed ~2.5-fold increase as compared to DMSO control (Figure 5B; P = 0.02, P = 0.01, and P = 0.03, respectively). Subsequently, we investigated the caspase-3–mediated PARP cleavage by performing immunoblot analysis using AS and Bic treated PCa cells. AS treatment alone could significantly increase PARP cleavage in 22RV1 (~three-fold increase) and LNCaP (~two-fold increase) (Figure 5C; P = 0.009 and P = 0.04, respectively). Intriguingly, the combinatorial effect demonstrated ~five-fold increase in PARP cleavage in 22RV1 cells (P = 0.004) and only ~three-fold increase in LNCaP cells (Figure 5C; P = 0.009). However, no significant change in PARP cleavage was observed in PC3 cells. Moreover, no significant change in antiapoptotic proteins (Bcl-2 and Bcl-xL) was observed in PC3, 22RV1, and LNCaP cells (Figure 5C). Interestingly, treatment with the drug combination led to a significant decrease (~one-fold) in survivin expression in PC3, 22RV1, and LNCaP cells (Figure 5C; P = 0.0001, P = 0.007, and P = 0.0001, respectively). These findings indicate that AS and Bic treatment may trigger oxidative stress by generating ROS, along with induction of caspase-dependent cell death and survivin downregulation, in both castrate-resistant and androgen-responsive PCa cells.
Figure 5.
AS treatment induces apoptosis by eliciting oxidative stress and caspase-3–mediated PARP cleavage. (A) DCFDA-fluorometric assay using PC3 and 22RV1 cells treated with AS (2.5 μM) and Bic (25 μM); LNCaP cells treated with AS (1 μM) and Bic (12.5 μM) for 24 hours. H2O2 (50 μM) treatment for 4 hours was included as a positive control. (B) Colorimetric caspase-3 activity assay in PC3, 22RV1, and LNCaP cells; experimental conditions for drugs treatment were the same as in A. (C) Immunoblotting for cleaved PARP and survivin in PC3, 22RV1, and LNCaP cells. PC3 and 22RV1 were treated with AS (2.5 μM) and Bic (25 μM) alone or in combination, whereas LNCaP cells were treated with AS (1 μM) and Bic (12.5 μM) for 48 hours. Doxorubicin (500 nM for PC3 and 22RV1 cells and 50 nM for LNCaP cells) was used as a positive control. Error bars represent mean ± S.E.M. P values calculated from two-sided Student's t test. P values < 0.05 were considered significant.
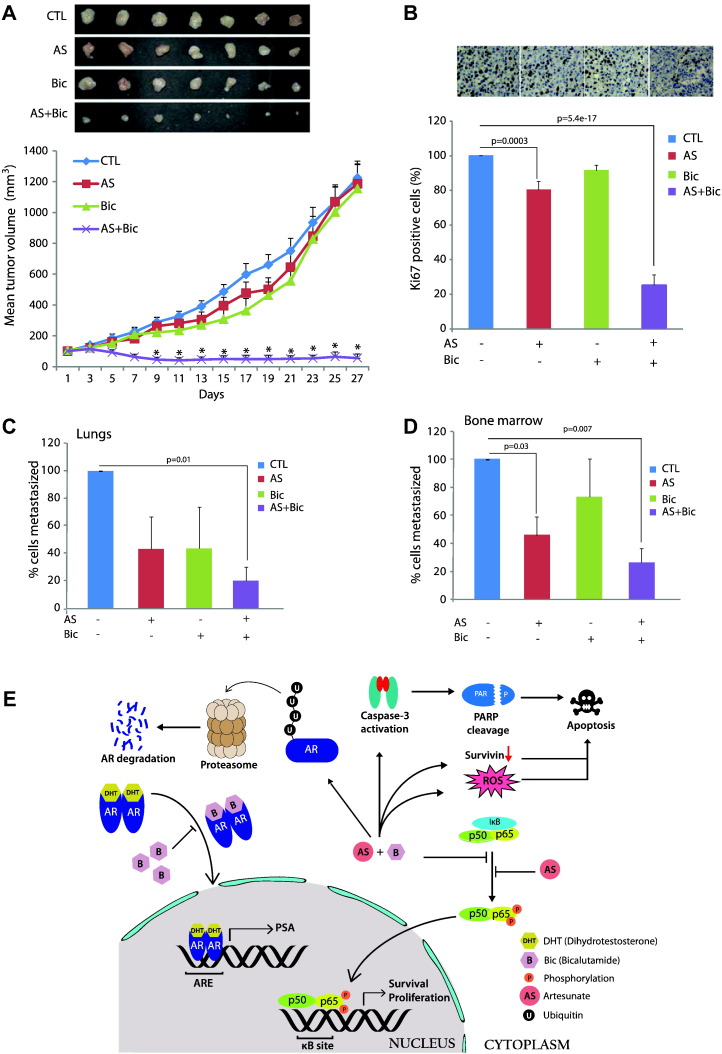
AS and Bic Combination Efficiently Inhibits CRPC Tumor Growth and Distant Metastasis
To investigate the antitumorigenic potential of AS and Bic combination, PC3 xenografts were generated in NOD/SCID mice. Drug treatment was initiated once the tumor volume reached ~100 mm3 in all the experimental groups. Combinatorial treatment of AS (50 mg/kg per day) and Bic (50 mg/kg per day) showed drastic regression (>95% reduction) in tumor growth (Figure 6A; P = 9e-8), whereas monotherapy with either AS or Bic using matched doses (50 mg/kg per day) of the combinatorial therapy failed to exhibit any significant reduction in the tumor burden. To check the cell proliferation index, immunohistochemical staining for Ki-67, a cell proliferation marker, was performed on the tumor samples collected from the xenografted mice. As anticipated, a significant decrease in the Ki-67 positive cells was observed in AS alone (~20% reduction) and in the combinatorial treatment group (~75% reduction) (Figure 6B; P = 0.0003 and P = 5.4e-17, respectively). Spontaneous micrometastases to lungs and bones were investigated by performing qPCR assay for the human Alu-sequences. A significant reduction in the lung metastatic burden was observed in the experimental group that received AS and Bic combinatorial therapy (Figure 6C; P = 0.01). Similarly, significant decrease in the bone metastases was observed in the groups treated with AS alone and in combination with Bic (Figure 6D; P = 0.03 and P = 0.007, respectively). Taken together, our results suggest that AS and Bic combinatorial treatment in castrate-resistant PC3 xenograft–bearing mice demonstrated drastic tumor regression by reinstating sensitivity toward AR antagonists. However, our in vitro data suggest that Bic alone blocks NF-κB signaling in PC3 cells; hence, it could be plausible that Bic in combination with AS regresses tumor growth in AR-null PC3 xenografts by suppressing NF-κB signaling.
Figure 6.
AS and Bic combinatorial treatment attenuates CRPC tumor xenograft growth and distant metastases. (A) Tumor growth of the castrate-resistant PC3 cells xenograft immunodeficient NOD/SCID mice. Mice were treated with AS (50 mg/kg per day in two divided doses) and Bic (50 mg/kg per day) via oral route daily for 14 days. Drug treatment was initiated once average tumor volume in each group reached ~100 mm3. Results are presented as the mean tumor volume ± S.E.M. (B) Immunohistochemical staining of Ki-67 was performed to evaluate the cell proliferation index in the excised tumor specimens. The cells positive for Ki-67 staining were counted manually from 10 different fields for each tumor tissue section. (C) qPCR for human Alu-sequences using genomic DNA extracted from the lungs of the tumor-bearing mice. (D) Same as C, except bone marrow samples were used. Error bars represent mean ± S.E.M. P values calculated from two-sided Student's t test compared with control. *P < 0.0001 versus control. P values < 0.05 were considered significant. (E) Schema showing mechanism of AS-mediated inhibition in NF-κB signaling pathway; consequently, there was a decrease in AR and AR-V7 expression via ubiquitin-mediated proteasomal degradation.
Discussion
ADT has been the main treatment strategy for the metastatic PCa; however, after initial response to antiandrogens, the disease subsequently progresses to a highly aggressive state of mCRPC. Several mechanisms have been proposed for the maintenance of AR activity and resistance to ADT, for example, AR amplification, point mutations or phosphorylation of AR, gain-of-function mutation in androgen-synthesizing enzyme HSD3B1, and expression of constitutively active AR-V7, which are important driving forces in the development of mCRPC [7], [30], [31]. Androgen receptor variants are more frequently expressed in CRPC patients than in patients with hormone-responsive localized PCa. AR antagonist Bic is usually administered in locally advanced nonmetastatic PCa as an adjuvant therapy; however, it fails to be effective upon progression of disease to CRPC [32], [33]. Recent studies demonstrated that AR-mediated signaling and downstream transcriptional activation are critical for the progression of CRPC despite castration [2], which led to the discovery of several novel therapies, namely, enzalutamide, a second-generation selective AR antagonist [5], [6]; abiraterone acetate, a potent cytochrome p450-17A1 (CYP17A1) inhibitor [3], [4]; and sipuleucel-T immunotherapy [34]. Interestingly, many of these agents have been effective as single agents in the pre- and postdocetaxel regimen for mCRPC [35]. A recent study has shown an association between the presence of AR-V7 and resistance to enzalutamide and abiraterone [7]. It is known that treating mCRPC with single-agent therapies in a sequential order eventually fails owing to inherent tumor heterogeneity and development of drug resistance [7], [36]. Significantly, our data demonstrate that AR-FL and AR-V7 expression was remarkably reduced in castrate-resistant PCa cells upon treatment of AS with Bic, suggesting that AS-mediated reduction in AR-V7 in the castrate-resistant PCa cells restored CRPC responsiveness to antiandrogen treatment (Figure 6E). Therefore, our study suggests that employing AS and AR antagonist combination regimens might enhance therapeutic efficacy and abate resistance toward ADT in mCRPC patients.
Several independent studies indicated that NF-κB signaling has an important role in PCa progression and is constitutively activated in CRPC [13], [37], [38]. Nuclear localization of NF-κB has been associated with an increased risk of biochemical relapse [39]. In addition, an increase in the cytoplasmic NF-κB expression has also been associated with shorter time to disease recurrence, increased PSA levels, and disease-associated mortalities [40]. ADs have been effective as anticancer agents and are known to inhibit NF-κB signaling [22], [23], [41]. In this study, we have shown that AS sensitizes castrate-resistant PCa cells to AR antagonist and abrogates their oncogenic potential by inhibiting NF-κB signaling; further, it also results in enhanced cell death possibly due to increase in ROS levels and oxidative stress. Presence of high concentration of intracellular iron in the form of ferritin in cancer cells is the key factor for the ART-mediated potent anticancer activity; it has been shown that Fe(III) binds to transferrin (TF), an iron-transport protein, to form holo-TF, which get transported to TF receptor (TFRC) and subsequently internalized into cancer cells via receptor-mediated endocytosis. Intriguingly, most cancer cells including PCa show elevated levels of TFRC [20], [42], [43], which could be a possible reason for the enhanced sensitivity to ARTs [42], [44], [45]. Furthermore, TFRC expression was found to be upregulated in PCa metastases, whereas PCa cells stimulated with holo-TF show increase in DHA-mediated effects [20].
Our data suggest that combinatorial treatment with AS and Bic reinstates the responsiveness of CRPC cells to the antiandrogen treatment by increasing ubiquitination of AR and its variants (AR-V7), which consequently results into AR degradation by targeting it to the 26S proteasomal complex (Figure 6E). Recently, Jin et al. showed that BMS345541, a specific inhibitor of the NF-κB pathway, sensitizes the CRPC cells to antiandrogens and bortezomib, an inhibitor of the 26S proteasome complex which blocks the NF-κB signaling by degradation of IκB [18]. Another independent study showed Galeterone and 3β-carbamate analog VNPT55 treatment results in decreased AR and AR-V7 expression by implicating Mdm2/CHIP enhanced ubiquitination and targeting them for the proteasomal degradation [46]. In corroboration to these studies, our findings strongly indicate that AS-mediated inhibition of NF-κB signaling pathway restores the responsiveness of the castrate-resistant PCa cells toward AR antagonists by decreasing the expression of AR and its variants. We are also aware that there could be potential cross talk between the AR and NF-κB pathways that may explain some of the discrepancies such as phospho-p65 levels in PC3 (AR-null) and 22RV1 (AR-V7 positive) cells upon AS and Bic treatment.
A single-arm phase II trial with bortezomib in combination with ADT for 3 months showed a rapid clinical response as evidenced by decrease in the PSA level when compared with hormone blockade alone. However, clinical response had a short duration, and bortezomib treatment was associated with significant neural toxicity in those patients [47]. Unlike bortezomib and BMS345541, which are not fully suitable for the clinic due to serious side effects, ADs are the first-line antimalarial drugs recommended by the World Health Organization and are comparatively safe, and no side effects are reported as of yet. To the best of our knowledge, no combinatorial therapy with ADs and AR antagonists is in development phase or in clinical practice for the treatment of mCRPC. In this context, it is encouraging to note that a recent case report on a PCa patient showed that sequential oral administration of A. annua capsule after Bic treatment resulted in significant decrease in the PSA level and tumor remission [36]. Nonetheless, the mechanistic exploration and the rationale supporting this sequential therapy with ADs and Bic were not fully explored. Increased levels of AR and AR-Vs are one of the major mechanisms of ADT resistance in mCRPC. Recently approved second-generation antiandrogens abiraterone and enzalutamide have been reported to increase the expression of AR-Vs in PCa cell lines and clinical settings [7], [48], [49], [50]. Furthermore, AR antagonists currently used for the treatment of PCa do not cause significant apoptosis and may contribute to their failures in the clinic [51].
Intracellular iron in the cancer cells has also been correlated with NF-κB signaling [52]. Furthermore, PC3 cells treated with ferric nitrilotriacetate stimulate the expression of uPA levels by generating intracellular reactive oxygen intermediates and activate NF-κB signaling pathways [53]. Concomitantly, in the current study, we observed high levels of NF-κB in PC3 cells, which were downregulated upon treatment with the drug combination. We also observed that the drug combination mediates apoptosis via caspase-3 activation and PARP cleavage, which corroborates with the previous studies where artemisinin-tagged TF induced apoptosis via mitochondrial pathway along with PARP cleavage [42], [45]. Yet another study explored the possibility of a caspase-independent non-apoptotic cell death which depends on the intracellular levels of iron, termed ferroptosis, wherein ferric ions induce oxidative stress along with cell death [44], [54]. Moreover, generation of free radicals by ADs has been known to trigger oxidative stress and apoptosis [21]. Thus, combinatorial treatment of AS and Bic in the present study induces oxidative stress as well as apoptosis via caspase-3 activation, a key executioner in caspase-mediated cell death [55] and survivin downregulation. Finally, our preclinical mouse studies demonstrated that the combinatorial treatment with AS and Bic significantly attenuated PC3 tumor xenograft growth as well as distant metastases to the lungs and bones, thus indicating that AS restores the responsiveness of the castrate-resistant PCa cells toward AR antagonists. Taken together, our findings strongly recommend the use of targeted therapies against androgen-AR axis and NF-κB signaling pathway, which could provide a novel therapeutic approach for the treatment of mCRPC by alleviating ADT resistance. Overall, this study has high translational relevance and provides strong preclinical rationale for the use of ADs in the treatment of mCRPC and further necessitates the need for well-planned clinical trials using AS and AR antagonists in mCRPC patients.
Authors’ contributions
J. J. N., S. K. P., and B. A. designed the experimental studies. J. J. N., S. K. P., A. Y., and S. G. performed the experiments. J. J. N., S. K. P., A. Y., and B. A. performed statistical analysis and interpreted the data. J. J. N., S. K. P., A. Y., and B. A. performed the PCa xenograft experiments. J. J. N., S. K. P., and B. A. wrote the manuscript. B. A. directed the overall project.
Acknowledgements
B. A. is an Intermediate Fellow of the Wellcome Trust/DBT India Alliance and a Young Investigator of the DST-FAST Track scheme. We thank malaria research group at the CSIR-CDRI, Lucknow (Dr. Saman Habib, Dr. Renu Tripathi, and Dr. Kumkum Srivastava) for providing artemisinin derivatives. We are grateful to Prof. S. Ganesh for providing polyubiquitination antibody and Prof. D. S. Katti for the H2DCFDA reagent. We also thank Dr. Anjali Bajpai for critically reading the manuscript.
Footnotes
Financial support: This work is partially supported by the Wellcome Trust/DBT India Alliance grant (IA/I(S)/12/2/500635 to B.A.) and an intramural funding from the Indian Institute of Technology, Kanpur (PDF-53 to S.K.P.).
Conflict of interest: The authors declare no conflicts of interest or disclosures.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.neo.2017.02.002.
Appendix A. Supplementary data
Supplementary materials.
References
- 1.Ferlay J., Soerjomataram I., Ervik M., Dikshit R., Eser S., Mathers C., Rebelo M., Parkin D.M., Forman D., Bray F. Lyon, France; International Agency for Research on Cancer: 2013. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet] Available from: http://globocan.iarc.fr, accessed on 16/02/2017. [Google Scholar]
- 2.Longo DL. New therapies for castration-resistant prostate cancer. N Engl J Med. 2010;363:479–481. doi: 10.1056/NEJMe1006300. [DOI] [PubMed] [Google Scholar]
- 3.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB, Jr., Saad F. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, Fizazi K, Mainwaring P, Piulats JM, Ng S. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beer TM, Tombal B. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:1755–1756. doi: 10.1056/NEJMc1410239. [DOI] [PubMed] [Google Scholar]
- 6.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 7.Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, Chen Y, Mohammad TA, Fedor HL, Lotan TL. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028–1038. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo Z, Yang X, Sun F, Jiang R, Linn DE, Chen H, Kong X, Melamed J, Tepper CG, Kung HJ. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009;69:2305–2313. doi: 10.1158/0008-5472.CAN-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shtivelman E, Beer TM, Evans CP. Molecular pathways and targets in prostate cancer. Oncotarget. 2014;5:7217–7259. doi: 10.18632/oncotarget.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ateeq B, Kunju LP, Carskadon SL, Pandey SK, Singh G, Pradeep I, Tandon V, Singhai A, Goel A, Amit S. Molecular profiling of ETS and non-ETS aberrations in prostate cancer patients from northern India. Prostate. 2015;75:1051–1062. doi: 10.1002/pros.22989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cookson MS, Lowrance WT, Murad MH, Kibel AS. Castration-resistant prostate cancer: AUA guideline amendment. J Urol. 2015;193:491–499. doi: 10.1016/j.juro.2014.10.104. [DOI] [PubMed] [Google Scholar]
- 12.Crusz SM, Balkwill FR. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol. 2015;12:584–596. doi: 10.1038/nrclinonc.2015.105. [DOI] [PubMed] [Google Scholar]
- 13.Jin R, Yi Y, Yull FE, Blackwell TS, Clark PE, Koyama T, Smith JA, Matusik RJ. NF-κB gene signature predicts prostate cancer progression. Cancer Res. 2014;74:2763–2772. doi: 10.1158/0008-5472.CAN-13-2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L, Shao L, Creighton CJ, Zhang Y, Xin L, Ittmann M, Wang J. Function of phosphorylation of NF-kB p65 ser536 in prostate cancer oncogenesis. Oncotarget. 2015;6:6281–6294. doi: 10.18632/oncotarget.3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keller ET, Chang C, Ershler WB. Inhibition of NFkappaB activity through maintenance of IkappaBalpha levels contributes to dihydrotestosterone-mediated repression of the interleukin-6 promoter. J Biol Chem. 1996;271:26267–26275. doi: 10.1074/jbc.271.42.26267. [DOI] [PubMed] [Google Scholar]
- 16.Sharma J, Gray KP, Harshman LC, Evan C, Nakabayashi M, Fichorova R, Rider J, Mucci L, Kantoff PW, Sweeney CJ. Elevated IL-8, TNF-alpha, and MCP-1 in men with metastatic prostate cancer starting androgen-deprivation therapy (ADT) are associated with shorter time to castration-resistance and overall survival. Prostate. 2014;74:820–828. doi: 10.1002/pros.22788. [DOI] [PubMed] [Google Scholar]
- 17.Nadiminty N, Tummala R, Liu C, Yang J, Lou W, Evans CP, Gao AC. NF-kappaB2/p52 induces resistance to enzalutamide in prostate cancer: role of androgen receptor and its variants. Mol Cancer Ther. 2013;12:1629–1637. doi: 10.1158/1535-7163.MCT-13-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin R, Yamashita H, Yu X, Wang J, Franco OE, Wang Y, Hayward SW, Matusik RJ. Inhibition of NF-kappa B signaling restores responsiveness of castrate-resistant prostate cancer cells to anti-androgen treatment by decreasing androgen receptor-variant expression. Oncogene. 2015;34:3700–3710. doi: 10.1038/onc.2014.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White NJ. Qinghaosu (artemisinin): the price of success. Science. 2008;320:330–334. doi: 10.1126/science.1155165. [DOI] [PubMed] [Google Scholar]
- 20.Morrissey C, Gallis B, Solazzi JW, Kim BJ, Gulati R, Vakar-Lopez F, Goodlett DR, Vessella RL, Sasaki T. Effect of artemisinin derivatives on apoptosis and cell cycle in prostate cancer cells. Anticancer Drugs. 2010;21:423–432. doi: 10.1097/CAD.0b013e328336f57b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crespo-Ortiz MP, Wei MQ. Antitumor activity of artemisinin and its derivatives: from a well-known antimalarial agent to a potential anticancer drug. J Biomed Biotechnol. 2012;2012:247597–247614. doi: 10.1155/2012/247597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Das AK. Anticancer effect of antimalarial artemisinin compounds. Ann Med Health Sci Res. 2015;5:93–102. doi: 10.4103/2141-9248.153609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tran KQ, Tin AS, Firestone GL. Artemisinin triggers a G1 cell cycle arrest of human Ishikawa endometrial cancer cells and inhibits cyclin-dependent kinase-4 promoter activity and expression by disrupting nuclear factor-kappaB transcriptional signaling. Anticancer Drugs. 2014;25:270–281. doi: 10.1097/CAD.0000000000000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tiwari R, Pandey SK, Goel S, Bhatia V, Shukla S, Jing X, Dhanasekaran SM, Ateeq B. SPINK1 promotes colorectal cancer progression by downregulating metallothioneins expression. Oncogenesis. 2015;4:e162. doi: 10.1038/oncsis.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loewe S. The problem of synergism and antagonism of combined drugs. Arzneimittelforschung. 1953;3:285–290. [PubMed] [Google Scholar]
- 26.Ateeq B, Tomlins SA, Laxman B, Asangani IA, Cao Q, Cao X, Li Y, Wang X, Feng FY, Pienta KJ. Therapeutic targeting of SPINK1-positive prostate cancer. Sci Transl Med. 2011;3:72ra17. doi: 10.1126/scitranslmed.3001498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia H, Fleyshman D, Kolesnikova K, Safina A, Commane M, Paszkiewicz G, Omelian A, Morrison C, Gurova K. Expression of FACT in mammalian tissues suggests its role in maintaining of undifferentiated state of cells. Oncotarget. 2011;2:783–796. doi: 10.18632/oncotarget.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hutterer C, Niemann I, Milbradt J, Frohlich T, Reiter C, Kadioglu O, Bahsi H, Zeittrager I, Wagner S, Einsiedel J. The broad-spectrum antiinfective drug artesunate interferes with the canonical nuclear factor kappa B (NF-kappaB) pathway by targeting RelA/p65. Antiviral Res. 2015;124:101–109. doi: 10.1016/j.antiviral.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Lee DK, Chang C. Endocrine mechanisms of disease: expression and degradation of androgen receptor: mechanism and clinical implication. J Clin Endocrinol Metab. 2003;88:4043–4054. doi: 10.1210/jc.2003-030261. [DOI] [PubMed] [Google Scholar]
- 30.Ferraldeschi R, Welti J, Luo J, Attard G, de Bono JS. Targeting the androgen receptor pathway in castration-resistant prostate cancer: progresses and prospects. Oncogene. 2015;34:1745–1757. doi: 10.1038/onc.2014.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang KH, Li R, Kuri B, Lotan Y, Roehrborn CG, Liu J, Vessella R, Nelson PS, Kapur P, Guo X. A gain-of-function mutation in DHT synthesis in castration-resistant prostate cancer. Cell. 2013;154:1074–1084. doi: 10.1016/j.cell.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goa KL, Spencer CM. Bicalutamide in advanced prostate cancer. Drugs Aging. 1998;12:401–422. doi: 10.2165/00002512-199812050-00006. [DOI] [PubMed] [Google Scholar]
- 33.Kawata H, Ishikura N, Watanabe M, Nishimoto A, Tsunenari T, Aoki Y. Prolonged treatment with bicalutamide induces androgen receptor overexpression and androgen hypersensitivity. Prostate. 2010;70:745–754. doi: 10.1002/pros.21107. [DOI] [PubMed] [Google Scholar]
- 34.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 35.Liaw BC, Oh WK. Urological cancer: is docetaxel the 'black widow' of mCRPC drugs? Nat Rev Clin Oncol. 2015;12:316–318. doi: 10.1038/nrclinonc.2015.79. [DOI] [PubMed] [Google Scholar]
- 36.Michaelsen FW, Saeed ME, Schwarzkopf J, Efferth T. Activity of Artemisia annua and artemisinin derivatives, in prostate carcinoma. Phytomedicine. 2015;22:1223–1231. doi: 10.1016/j.phymed.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Jain G, Cronauer MV, Schrader M, Moller P, Marienfeld RB. NF-kappaB signaling in prostate cancer: a promising therapeutic target? World J Urol. 2012;30:303–310. doi: 10.1007/s00345-011-0792-y. [DOI] [PubMed] [Google Scholar]
- 38.Gasparian AV, Yao YJ, Kowalczyk D, Lyakh LA, Karseladze A, Slaga TJ, Budunova IV. The role of IKK in constitutive activation of NF-kappaB transcription factor in prostate carcinoma cells. J Cell Sci. 2002;115:141–151. doi: 10.1242/jcs.115.1.141. [DOI] [PubMed] [Google Scholar]
- 39.Domingo-Domenech J, Mellado B, Ferrer B, Truan D, Codony-Servat J, Sauleda S, Alcover J, Campo E, Gascon P, Rovira A. Activation of nuclear factor-kappaB in human prostate carcinogenesis and association to biochemical relapse. Br J Cancer. 2005;93:1285–1294. doi: 10.1038/sj.bjc.6602851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCall P, Bennett L, Ahmad I, Mackenzie LM, Forbes IW, Leung HY, Sansom OJ, Orange C, Seywright M, Underwood MA. NFkappaB signalling is upregulated in a subset of castrate-resistant prostate cancer patients and correlates with disease progression. Br J Cancer. 2012;107:1554–1563. doi: 10.1038/bjc.2012.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Efferth T, Dunstan H, Sauerbrey A, Miyachi H, Chitambar CR. The anti-malarial artesunate is also active against cancer. Int J Oncol. 2001;18:767–773. doi: 10.3892/ijo.18.4.767. [DOI] [PubMed] [Google Scholar]
- 42.Kelter G, Steinbach D, Konkimalla VB, Tahara T, Taketani S, Fiebig HH, Efferth T. Role of transferrin receptor and the ABC transporters ABCB6 and ABCB7 for resistance and differentiation of tumor cells towards artesunate. PLoS One. 2007;2:e798. doi: 10.1371/journal.pone.0000798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keer HN, Kozlowski JM, Tsai YC, Lee C, McEwan RN, Grayhack JT. Elevated transferrin receptor content in human prostate cancer cell lines assessed in vitro and in vivo. J Urol. 1990;143:381–385. doi: 10.1016/s0022-5347(17)39970-6. [DOI] [PubMed] [Google Scholar]
- 44.Ooko E, Saeed ME, Kadioglu O, Sarvi S, Colak M, Elmasaoudi K, Janah R, Greten HJ, Efferth T. Artemisinin derivatives induce iron-dependent cell death (ferroptosis) in tumor cells. Phytomedicine. 2015;22:1045–1054. doi: 10.1016/j.phymed.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 45.Nakase I, Gallis B, Takatani-Nakase T, Oh S, Lacoste E, Singh NP, Goodlett DR, Tanaka S, Futaki S, Lai H. Transferrin receptor-dependent cytotoxicity of artemisinin-transferrin conjugates on prostate cancer cells and induction of apoptosis. Cancer Lett. 2009;274:290–298. doi: 10.1016/j.canlet.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 46.Kwegyir-Afful AK, Ramalingam S, Purushottamachar P, Ramamurthy VP, Njar VC. Galeterone and VNPT55 induce proteasomal degradation of AR/AR-V7, induce significant apoptosis via cytochrome c release and suppress growth of castration resistant prostate cancer xenografts in vivo. Oncotarget. 2015;6:27440–27460. doi: 10.18632/oncotarget.4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kraft AS, Garrett-Mayer E, Wahlquist AE, Golshayan A, Chen CS, Butler W, Bearden J, Lilly M. Combination therapy of recurrent prostate cancer with the proteasome inhibitor bortezomib plus hormone blockade. Cancer Biol Ther. 2011;12:119–124. doi: 10.4161/cbt.12.2.15723. [DOI] [PubMed] [Google Scholar]
- 48.Antonarakis ES, Nakazawa M, Luo J. Resistance to androgen-pathway drugs in prostate cancer. N Engl J Med. 2014;371:2233–2234. doi: 10.1056/NEJMc1412594. [DOI] [PubMed] [Google Scholar]
- 49.Li Y, Chan SC, Brand LJ, Hwang TH, Silverstein KA, Dehm SM. Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Res. 2013;73:483–489. doi: 10.1158/0008-5472.CAN-12-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mostaghel EA, Marck BT, Plymate SR, Vessella RL, Balk S, Matsumoto AM, Nelson PS, Montgomery RB. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: induction of steroidogenesis and androgen receptor splice variants. Clin Cancer Res. 2011;17:5913–5925. doi: 10.1158/1078-0432.CCR-11-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sadar MD. Small molecule inhibitors targeting the "Achilles' heel" of androgen receptor activity. Cancer Res. 2011;71:1208–1213. doi: 10.1158/0008-5472.CAN_10-3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Torti SV, Torti FM. Iron and cancer: more ore to be mined. Nat Rev Cancer. 2013;13:342–355. doi: 10.1038/nrc3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ornstein DL, Zacharski LR. Iron stimulates urokinase plasminogen activator expression and activates NF-kappa B in human prostate cancer cells. Nutr Cancer. 2007;58:115–126. doi: 10.1080/01635580701308265. [DOI] [PubMed] [Google Scholar]
- 54.Dixon SJ, Stockwell BR. The role of iron and reactive oxygen species in cell death. Nat Chem Biol. 2014;10:9–17. doi: 10.1038/nchembio.1416. [DOI] [PubMed] [Google Scholar]
- 55.Porter AG, Janicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials.