Abstract
Background
The aim of our study was revised as follows: to clarify the postoperative complications of multifunctional central venous ports and the risk factors for such complications to promote the safe use of the PowerPort system in the hospital.
Methods
The study group comprised 132 patients in whom implantable central venous access ports (PowerPortⓇ) were placed in our hospital from March 2014 through December 2015. The approach used for port placement was the subclavian vein in 43 patients (33%), the internal jugular vein in 87 patients (66%), and the femoral vein in 2 patients (1%).
Results
Postoperative complications occurred in 8 patients (6%). The catheter was removed because of infection in 4 patients and catheter kinking in 1 patient. Port extravasation occurred in 3 patients. No patient had catheter pinch-off. The mean operation time was 74 min (range, 32 to 171). No patients had intraoperative bleeding or pneumothorax. Benign disease was a risk factor for postoperative complications (p = 0.009).
Conclusion
PowerPort is a multifunctional port. Benign disease was a risk factor for postoperative complications. Because many types of subcutaneously implanted ports are used in our hospital, we had to inform the hospital staff about the functions of PowerPort.
Keywords: Power port, Multifunctional port, Implantation central venous access port
Highlights
-
•
Implanted central venous access ports have been used for chemotherapy and nutritional therapy.
-
•
Postoperative complications occurred in 8 patients (6%).
-
•
Benign disease was a risk factor for postoperative complications.
1. Introduction
The usage of implantable central venous ports has increased year by year because of progress in chemotherapy, leading to increased numbers of patients who receive long-term treatment, and because repeated administration of continuous intravenous infusions make it difficult to obtain peripheral vascular access [1], [2], [3], [4], [5], [6], [7], [8], [9]. Home parenteral nutrition is now readily initiated in patients who require long-term central venous nutrition because of difficulty in oral or enteral nutrition, such as those with terminal cancer or short bowel syndrome, thereby eliminating the need for long-term hospitalization and allowing early return to work. In addition, the risk of extravasation has decreased even in patients in whom peripheral venous access is difficult to obtain, thereby allowing drugs to be safely and reliably administered intravenously [10], [11]. PowerPort is a multifunctional central venous access port that can be used to obtain blood samples, perform imaging examinations such as contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI), and administer anticancer therapy, high calorie infusion solutions, and blood transfusions. The aim of our study was to determine whether PowerPort is understood and used safely in our hospital. We studied patients in whom a new multifunctional central venous port was used and clarified the usefulness of this port and risk factors for postoperative complications.
2. Material and methods
The study group comprised 132 patients in whom PowerPortⓇ implantable ports were surgically placed in our hospital from March 2014 through December 2015 (Table 1). There were 61 men (46%) and 71 women (54%), with a mean age of 64.5 years (range, 20 to 90) and a mean body mass index of 21 kg/m2 (range, 11 to 35). The ports were placed to provide treatment in 90 patients (68%) and nutrition in 42 (32%). The underlying diseases were digestive tract disorders in 70 patients (53%), medical disorders in 26 (20%), gynecological disorders in 17 (13%), mammary disorders in 13 (10%), urological disorders in 4 (3%), and others in 2. The approach used was the subclavian vein in 43 patients (33%), the internal jugular vein in 87 (66%), and the femoral vein in 2 (1%). As for central venous access and port placement, the approach was basically made from the right internal jugular vein under superficial ultrasonic guidance. However, the approach was determined on the basis of patient's condition, the lesion site, and skills of physicians in each department, and a port was placed in the precordial region. If approaches from the subclavian vein and the internal jugular vein were difficult, an approach from the femoral vein was selected, and the port was placed in the lower abdomen. After port insertion, oral antimicrobial agents were given for 3 days to prevent infection. In our hospital, clinical residents and surgeons who have practiced for 5 years are in charge of central venous port placement. Practical training in surgical techniques is included in educational programs in our university hospital. We take time to educate them. Therefore, the duration of surgery became relatively long. The used port was a PowerPort device (Bard X-Port isp, Medicon, Inc., Osaka, Japan). The presence of port infection was evaluated on the basis of abscess culture tests, serum culture tests, the detection of pathogens on culture tests after removal of the port or catheter, and the exclusion of other diagnoses on heat-source testing.
Table 1.
Patients characteristics in our study.
| n = 132(%) | ||
|---|---|---|
| Sex | Male: Female | 61 (46%): 71 (54%) |
| Age | 63.5 (±12) | |
| BMI(kg/m2) | 21.0(±4.5) | |
| Purpose | Chemotherapy: Nutrition | 90 (68%): 42 (32%) |
| Operation time(min) | 74.2 (±26.4) | |
| Period(median,methods) | 17.0 (8–29) | |
2.1. Usage
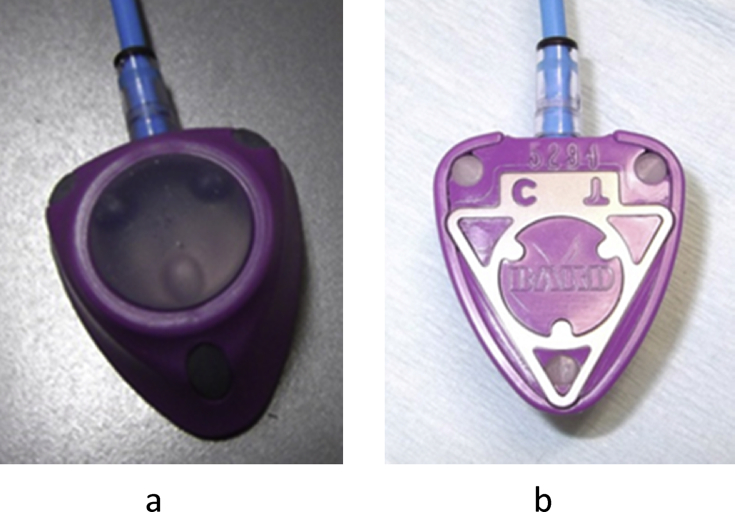
Since January 2014, all implantable central venous access ports used in our hospital were switched to PowerPort devices (Fig. 1a and b). To confirm the details of PowerPort device insertion, the date of insertion, the insertion site, and the name of the surgeon were recorded in an electronic medical record. In addition, frontal plain chest radiographs were taken after port placement to confirm the port (Fig. 2). To facilitate understanding of PowerPort devices by the staff in our hospital, we conducted awareness and usage educational sessions on PowerPort, which were sponsored by the Therapy Safety Promotion Office, and prepared an original manual for usage in our hospital. As for the procedure at the time of usage, each patient's port certificate and bracelet were confirmed, and the medical staff then confirmed that the port was a PowerPort device on the basis of medical records and chest radiographic images. At the time of contrast-enhanced CT scanning, a pressure-resistant PowerLoc® port access needle (C.R. BARD, Salt Lake City, Utah, USA) was used with PowerPort. In addition to anticancer therapy and venous nutrition, blood collection, transfusion, contrast-enhanced CT, and contrast-enhanced MRI were performed.
Fig. 1.
Appearance of PowerPort. PowerPort is characterized by 3 points at the septum of the insertion site on the front (1a) and back (1b) of the device.
Fig. 2.
Chest Radiographic Images.
2.2. Primary and secondary outcomes
The evaluated variables were defined as concurrent diseases and the treatment regimens, the results of imaging studies performed during the follow-up period, and early and late postoperative complications derived from the outpatient and inpatient medical records since March 1, 2014 or from the time of initial presentation to our hospital. The primary endpoint was postoperative complications during the follow-up period. The types and incidences of postoperative complications and risk factors for postoperative complications were analyzed by univariate analysis. Secondary endpoints were the safe use of the PowerPort system in the hospital and early and late postoperative complications.
2.3. System
To ensure that the PowerPort system was understood and used correctly in our hospital, we held meetings with the department in charge of using this system. To confirm that the PowerPort system was used safely, we obtained data on all patients who underwent port insertion and followed up the patients by sharing electronic medical records. Conventional port systems were not fully managed. Many types of ports and different surgical techniques were used previously, thereby precluding a direct comparison with our results. Patients who received systemic anticancer agents using a port and those who required nutritional management were included in the study. Patients were excluded from the study if they gave no informed consent for port usage. Overall, 132 patients had no port dysfunction before and after treatment although port-related troubles occurred. The limitation of our study was a small sample size. Further studies of a larger number of patients may be needed to confirm the usefulness of the PowerPort system and risk factors for postoperative complications.
3. Results
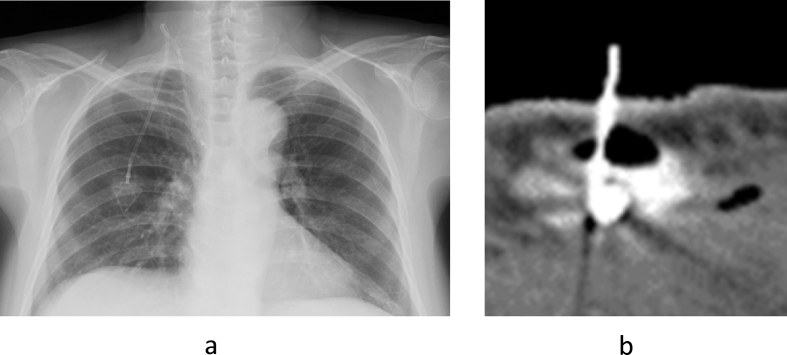
The median observation period after surgery was 17 months (range, 8 to 29). Postoperative complications developed in 8 patients (6%). In 4 patients the port was removed because of infection. Catheter kinking occurred in 1 patient in whom a right internal jugular vein approach was used for port placement. The port was removed and then replaced. Port extravasation occurred in 3 patients (Fig. 3a and b). In all 3 patients, port placement was performed to provide nutrition, and the ports were used after confirming reverse blood flow. There was no catheter pinch-off (Table 2).
Fig. 3.
Patients with Postoperative Complications 3a. Catheter kinking on a chest radiograph (▲) 3b. Port extravasation during contrast-enhanced CT of the chest (▲▲). The puncture needle was located outside the port and surrounded by air.
Table 2.
Patients with postoperative complications.
| Case | Sex | Age | Disease | purpose | Operation time(min) | Port site | complication | Occurrece (days) |
|---|---|---|---|---|---|---|---|---|
| 1 | Female | 44 | Breast cancer | Chemotherapy | 171 | Rt.internal jugular vein | infection | 90 |
| 2 | Male | 50 | Crohn disease | Nutrition | 44 | Rt.subclavian vein | infection | 21 |
| 3 | Male | 36 | Ulcerative colitis | Nutrition | 89 | Lt.internal jugular vein | Infection | 150 |
| 4 | Male | 61 | Rectal cancer | Chemotherapy | 62 | Lt. subclavian vein | Infection | 28 |
| 5 | Female | 69 | Colon cancer | Chemotherapy | 150 | Rt.internal jugular vein | Catheter kink | 25 |
| 6 | Female | 66 | Ovarian cancer | Nutrition | 147 | Rt.subclavian vein | Port extravasationa | 9 |
| 7 | Female | 68 | Dermatom yosistis | Nutrition | 148 | Rt.internal jugular vein | Port extravasationa | 35 |
| 8 | Male | 81 | Esophageal cancer | Nutrition | 160 | Rt.internal jugular vein | Port extravasationa | 81 |
Reverse blood flow was confirmed before using the port.
Early complications were defined as complications that occurred within 30 days after surgery and comprised infection in 2 patients, catheter kinking in 1 patient, and port leakage in 1 patient. Late complications were defined as complications that occurred 30 or more days after surgery and comprised infection in 2 patients and port leakage in 2 patients. In our study, no patient had port-associated thrombosis, occlusion, dislodgement, migration, breakage, or rupture. Only 1 patient had catheter kinking. Port infection occurred in 4 patients, all of whom had local infection. The port was promptly removed after infection in all of these patients.
The mean operation time was 74 min (range, 32–171). No patient had intraoperative bleeding or pneumothorax. Benign disease was a risk factor for postoperative complications (p = 0.009) (Table 3). Postoperative complications developed in 3 patients with benign disease (30%). One patient had Crohn's disease, in 1 had ulcerative colitis, and 1 had dermatomyositis. One month after insertion, postoperative complications occurred in 37.5% of the patients, and the rate of port patency calculated with the Kaplan-Meier method was 62.5%. After 3 months, postoperative complications occurred in 12.5% of the patients, and the rate of port patency was 87.5%. Because the follow-up period was short, the 1-year survival rate was 94%. In our hospital, residents and surgeons who worked for 5 years are in charge of inserting central venous ports. Practical training in surgical techniques is included in educational programs in our university hospital. We take time to educate them. Therefore, the duration of surgery became relatively long. As for sample size, about 200 more patients may be required for a significance test. We are planning to study more patients and perform a retrospective statistical analysis. The following sentences were added to the Results section.
Table 3.
Postoperative complications.
| Factors | Complications(n = 8) | No commplications(n = 124) | p = value | |
|---|---|---|---|---|
| Sex | Male: Female | 4: 4 | 57: 67 | 0.8248 |
| Age | <65:≧65 | 4: 4 | 62: 62 | 0.9999 |
| BMIkg/m2 | <25: ≧25 | 7: 1 | 98: 26 | 0.9019 |
| Purpose | Chemotherapy: Nutrition | 5: 3 | 85: 39 | 0.7254 |
| Port site | Internal jugular vein: Subclavian vein | 5: 3 | 82: 40 | 0.7170 |
| Disease | Malignant neoplasm: Benign | 5: 3 | 117: 7 | 0.0009 |
| Operation time(min) | <90: ≧90 | 5: 3 | 98: 26 | 0.5131 |
The causative organisms of port infection were Staphylococcus epidermidis in 1 patient, Staphylococcus capitis in 1 patient, and Staphylococcus aureus in 1 patient. The cultures in 1 patient were negative. As for the treatment of infection, the port was removed promptly after confirming symptoms such as fever, pain, and edema. Oral antimicrobial agents were given for 3–5 days. After port removal, the effect of antimicrobial agents was assessed on the basis of the results of cultures obtained from the port and catheter, and the antimicrobial agents were modified if necessary.
3.1. Statistical analysis
Patient data are expressed as means ± SD. Comparisons between 2 groups were done with unpaired t-tests. Incidences were compared with the use of chi-square tests with Yates' correction. Statistical significance was evaluated with the Mann-Whitney U test. Port patency rates were calculated with the Kaplan-Meier method.
4. Discussion
In our study, complications developed in 8 patients (6%) after PowerPort placement. The port was removed and replaced because of infection in 4 patients. Catheter kinking occurred in 1 patient, and the catheter was replaced. Port extravasation was detected in 3 patients. Benign disease was a risk factor for postoperative complications. Progress in chemotherapy and an increased number of drugs that can be selected have prolonged the duration of chemotherapy and increased the number of doses administered, thereby increasing the number of ports required to reliably obtain peripheral venous access. In some patients with unresectable or recurrent cancer, the gastrointestinal tract cannot be used as a nutritional route because of intestinal disease. The dissemination of home-based parenteral nutrition has contributed to improving patients' quality of life. However, in our study benign disease was a risk factor for postoperative complications. This was probably because the daily intravenous administration of high-calorie infusion solutions, frequent port punctures, and long-term treatment are associated with a high risk of infection, including decreased disinfectant activity. The extravascular extravasation of anticancer agents can damage the soft tissue around veins and cause redness, edema, pain, sacculation, ulceration, and necrosis. In the United States, the incidence of extravascular extravasation of intravenously administered necrotic anticancer agents is estimated to range from 0.1% to 6.5%. Therefore, the placement of venous access ports has been recommended to reliably obtain venous access ports and decrease the risk of extravascular extravasation [12]. The internal jugular vein and veins of the upper arm and the forearm have been advocated for catheter placement. An approach from the internal jugular vein is free of the risk of catheter pinch-off, but may require the creation a long subcutaneous tunnel for passage of the catheter at the time of port placement. Although the risks of catheter kinking and other complications remain to some extent, a bigger concern is that the large surgical invasion leads to a poor esthetic outcome of the neck caused by subcutaneous placement of the catheter [13]. As compared with conventional venous access, the advantages of central venous access ports include less pain caused by frequent punctures, freedom from the need for fluid lines, less vascular pain, the ability to infuse highly irritating drugs (e.g., anticancer agents), a low incidence of infection, and reliable venous puncture. On the other hand, central venous access ports have some disadvantages, such as esthetic problems and serious complications (e.g., catheter pinch-off, infection, thrombus formation, catheter obstruction, and port extravasation) [14]. In all 3 patients with port extravasation in our study, ports were placed to perform nutritional therapy, frequent port punctures were required, and the ports were used after confirming reverse blood flow. Patients and medical staff members should therefore confirm methods for puncture at a certain interval. If necessary, the appropriate puncture site should be reconfirmed on superficial ultrasonography.
A previous study reported that catheter rupture occurred when an implantable port that could not withstand the injection pressure was used to perform contrast-enhanced CT [15]. The Food and Drug Administration in the United States recommended that implantable ports able to withstand the pressure created by rapid injection of contrast media, should be developed, and PowerPort ports that permit the rapid injection of contrast media were launched [16]. Recent studies have reported on the safety and usage of ports that can be used to rapidly inject contrast media. PowerPort devices were not associated with any adverse events at the time of contrast-enhanced imaging, and the incidences of adverse events such as catheter obstruction and infection were similar to those of other types of ports. PowerPort ports can thus be used safely [17], [18], [19].
Although PowerPort devices have many advantages, the features of PowerPort ports should be differentiated from those of the many other types of ports that have been used in our hospital. In our hospital, central venous access ports are placed in about 100 patients per year. In some of the patients, ports other than PowerPort devices have been used, and performing contrast-enhanced imaging via ports that are not designed to be used for contrast-enhanced CT carries the risk of accidents. We have therefore used PowerPort devices in all patients in whom implantable ports have been placed in our hospital since January 2014. At the time of introduction, we conducted adequate educational sessions and prepared an original manual to facilitate the understanding of PowerPort devices by our hospital staff.
It is important to acknowledge that postoperative complications can occur even if puncture is performed under ultrasound guidance [20]. After puncture, catheter-related infection, thrombus formation, and catheter pinch-off occur at certain rates. The early management of complications is essential before they become serious. Appropriate countermeasures against such complications must therefore be clearly defined. Catheter-related infections are often caused by thrombus formation and bacterial contamination of infusion solution via the catheter insertion sites, connection sites, or three-way stopcocks. Long-term catheter placement is associated with an increased incidence of thrombus formation. One study reported that thrombus formation occurred in 84% of patients in whom a catheter was placed for 10 months or longer [21]. Scolapios et al. reported that 11 (5%) of 225 patients with port placement died of catheter-related sepsis. They strongly recommended that appropriate action and early catheter removal should be performed in a timely manner in patients suspected to have catheter-related infection [22]. Catheter pinch-off syndrome is a troublesome problem that occurs when a catheter placed along the subclavian vein route gets caught between the clavicle and the first rib. The incidence of port catheter rupture has been reported to range from 1.1% to 2.1% [23]. No port catheter rupture occurred in our study. Fisher et al. reported that serious complications such as arrhythmias, sepsis, and cardiac puncture can occur in about 71% of patients in whom catheter fragments are released [24].
Careful screening of risk factors for port-related complications can decrease patients' discomfort associated with port insertion and management and provide appropriate treatment, permitting the maintenance of a better quality of life and allowing medical staff members to offer safe treatment.
Ethical approval
This study has been approved by Institutional Review Board for Observation and Epidemiological Study of Kitasato University, and all the subjects gave informed consent. (B16-99).
Sources of funding
None to declare.
Author contribution
All the authors, Takatoshi Nakamura, Jiichiro Sasaki, Yasushi Asari, Takeo Sato, Shinzo Torii, and Masahiko Watanabe, contributed to the study design, data collections, data analysis and writing of the manuscript.
Conflicts of interest
None to declare.
Guarantor
Takatoshi Nakamura, First Author.
Masahiko Watanabe, Senior Author.
Trial registry number
N/A.
References
- 1.Kock H.J., Pietsch M., Krause U. Implantable vascular access systems: experience in 1500 patients with totally implantable central venous port systems. World J. Surg. 1998;22:12–16. doi: 10.1007/s002689900342. [DOI] [PubMed] [Google Scholar]
- 2.Marcy P.Y., Magne N., Castadot P. Radiological and surgical placement of ports devices: a 4-year institutional analysis of procedure performance, quality of life and cost in breast cancer patients. Breast Cancer Res. Treast. 2005;92:61–67. doi: 10.1007/s10549-005-1711-y. [DOI] [PubMed] [Google Scholar]
- 3.Sakamoto N., Arai Y., Kakeuchi Y. Ultrasound-guided radiological placement of central venous port via the subclavian vein: a retrospective analysis of 500 cases at a single institute. Cardiovasc. Interv. Radiol. 2010;33:989–994. doi: 10.1007/s00270-010-9841-y. [DOI] [PubMed] [Google Scholar]
- 4.Hsieh C.C., Weng H.H., Huang W.S. Analysis of risk factors for central venous port failure in cancer patients. World J. Gastroenterol. 2009;15:4709–4714. doi: 10.3748/wjg.15.4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vescia S., Baumgratner A.K., Jacobs V.R. Management of venous port systems in oncology: a review of current evidence. Ann. Oncol. 2008;19:9–15. doi: 10.1093/annonc/mdm272. [DOI] [PubMed] [Google Scholar]
- 6.Niederhuber J.E., Ensminger W., Gyves J.W. Totally implanted venous and arterial access system to replace external catheters in cancer treatment. Surgery. 1982;92:706–712. [PubMed] [Google Scholar]
- 7.Agha R.A., Fowler A.J., Rammohan S. The PROCESS Statement: preferred Reporting of case series in surgery. Int. J. Surg. 2016;36:319–323. doi: 10.1016/j.ijsu.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 8.Silberzweig J.E., Sacks D., Khorsandi A.S. Reporting standards for central venous access. J. Vasc. Interv. Radiol. 2000;11:391–400. doi: 10.1016/s1051-0443(07)61435-3. Technology assessment committee. [DOI] [PubMed] [Google Scholar]
- 9.Lewis C.A., Allen T.E., Burke D.E. Quality improvement guidelines for central venous access. J. Vasc. Interv. Radiol. 2011;14:231–235. [PubMed] [Google Scholar]
- 10.Matsushima H., Adachi T., Iwata T. Analysis of the outcomes in central venous access port implantation performed by residents via the internal juglar vein and subclavian vein. J. Surg. Educ. 2016:1–7. doi: 10.1016/j.jsurg.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Tabatabaie O., Kasumova G.G., Eskander,et al M.F. Totally implantable venous access devices: a reviw of complications and management strategies. Am. J. Clin. Oncol. 2017;40:94–105. doi: 10.1097/COC.0000000000000361. [DOI] [PubMed] [Google Scholar]
- 12.Herts B.R., O'Malley C.M., Wirth S.I. Power injection of contrast media using central venous catheters: feasibility, safety, and efficacy. Am. J. Roentgenol. 2001;176:447–453. doi: 10.2214/ajr.176.2.1760447. [DOI] [PubMed] [Google Scholar]
- 13.Macdonald S., Watt A.J. Comparison of technical success and outcome of tunneled catheters inserted via the jugular and subclavian approaches. J. Vasc. Intev Radiol. 2000;11:225–231. doi: 10.1016/s1051-0443(07)61470-5. [DOI] [PubMed] [Google Scholar]
- 14.Kawamura J., Nagayama S., Nomura A. Long-term outcomes of peripheral arm ports implanted in patients with colorectal cancer. Int. J. Clin. Oncol. 2008;13:349–354. doi: 10.1007/s10147-008-0766-2. [DOI] [PubMed] [Google Scholar]
- 15.Smith L.H. Implanted ports, computed tomography, port injectors, and catheter rupture. Clin. J. Oncol. Nurs. 2008;5:809–812. doi: 10.1188/08.CJON.809-812. [DOI] [PubMed] [Google Scholar]
- 16.Johson K.A. Power injectable portal systems. J. Radiol. Nurs. 2009;28:27–31. [Google Scholar]
- 17.Goltz J.P., Noack C., Pertritsh B. Totally implantable venous power ports of the forearm and the chest: initial clinical experience with port devices approved for high-pressure injections. Br. J. Radiol. 2012;85:966–972. doi: 10.1259/bjr/33224341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alexander M.D., Morrison H.L. Power-injectable ports: safety during placement, therapeutic use, and contrast administration during computed tomography procedures. J. Vasc. Access. 2012;13:432–437. doi: 10.5301/jva.5000074. [DOI] [PubMed] [Google Scholar]
- 19.Teichgraber U.K., Nagel S.N., Kausche S. Clinical benefit of power-injectable port systems: a prospective observational study. Eur. J. Radiol. 2012;81:528–533. doi: 10.1016/j.ejrad.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 20.Randolph A.G., Cook D.J., Gonzales C.A. Ultrasound guidance for placement of central venous catheters: a meta-analysis of the literature. Crit. Care Med. 1996;24:2053–2058. doi: 10.1097/00003246-199612000-00020. [DOI] [PubMed] [Google Scholar]
- 21.Lorch H., Zwaan M., Kagel C. Central venous access ports placed by interventional radiologists: experience with 125 consecutive patients. Cardiovasc. Intervnt. Radiol. 2001;24:180–184. doi: 10.1007/s002700001721. [DOI] [PubMed] [Google Scholar]
- 22.Scolapios J.S., Fleming C.R., Kelly D.G. Survival of home parenteral nutrition-treated patients: 20 years of experience at the Mayo Clinc. Mayo Clinc Proc. 1999;74:217–222. doi: 10.4065/74.3.217. [DOI] [PubMed] [Google Scholar]
- 23.Hinke D.H., Zandt-Stastny D.A., Goodman L.R. Pinch-off syndrome: a complication of implantable subclavian venous access devices. Radiology. 1990;177:353–356. doi: 10.1148/radiology.177.2.2217768. [DOI] [PubMed] [Google Scholar]
- 24.Fisher R.G., Ferreyro R. Evaluation of current techniques for nonsurgical removal of intravascular iatrogenic foreign bodies. Am. J. Roentgenol. 1978;130:541–548. doi: 10.2214/ajr.130.3.541. [DOI] [PubMed] [Google Scholar]