Abstract
Purpose
Glucocorticoids (GCs) are often administered prior to any chemotherapeutics to prevent the secondary effects of anticancer agents. Glucocorticoid receptors (GRs) are expressed in several types of cancer cells, particularly in several histological types of breast cancer. Activation of GRs is not associated with any specific cellular response. Both proapoptotic and antiapoptotic responses have been observed, depending on the study or the type of breast cancer cells. Therefore, it is of relevance to investigate the possible modulation of apoptotic effect of chemotherapeutic agents when cancerous cells have previously been exposed to GCs.
Methods
In vitro cell growth was assayed by counting MCF-7 cells upon exposure to epirubicin (25 nM), 5-fluorouracil (5-FU) (15 µM), and paclitaxel (15 nM), either with or without prior exposure to the GC dexamethasone (Dex) (100 nM).
Results
Following preexposure to Dex, the antiapoptotic activity of paclitaxel was significantly reduced by 8.5% (p<0.05), but the activities of epirubicin and 5-FU remained unaltered.
Conclusion
In light of the finding that the response of MCF-7 cells pretreated with Dex was significantly reduced, we recommend that the function of GCs should be defined more precisely if they are to be used in conjunction with chemotherapy.
Keywords: dexamethasone, 5-fluorouracil, epirubicin, paclitaxel, MCF-7, chemotherapy
Introduction
Glucocorticoids (GCs) are steroid hormones that are critically involved in regulating and resolving inflammatory processes in mammals. They are also involved in many other essential processes, including cellular metabolism, differentiation, apoptosis, and immune response.1 Clinically, GCs are used to treat allergic reactions and inflammatory or autoimmune diseases, to reduce soft tissue edema after solid organ transplantation, and to eliminate malignant lymphoid cells by triggering apoptotic cell death.2 Various doses of GCs, most commonly dexamethasone (Dex), are frequently administered throughout the course of chemotherapeutic treatment for solid tumors in order to reduce toxicity and to protect normal tissue from the negative effects of continued exposure to genotoxic drugs. Dex is particularly effective in preventing chemotherapy-related hyperemesis.3–5 First-line chemotherapy for early and advanced stage breast cancers is based mainly on anthracyclines (doxorubicin or epirubicin), cyclophosphamide, 5-fluorouracil (5-FU) (Merck, Darmstadt, Germany), and taxanes (primarily paclitaxel).6,7 Taxanes, such as paclitaxel, are among the most common chemotherapeutic agents currently used for the treatment of breast cancer. Use of taxanes was initially limited because of hypersensitivity reactions, but once these could be adequately managed, largely by premedication with GC, taxane chemotherapy became part of the standard breast cancer treatment in most Western countries.4
Glucocorticoid receptors (GRs) have been identified in several types of cancerous cells, including breast cancer cell lines such as MCF-7.8–11 Exposure of these receptors – functional in MCF-7 cells – to Dex has been reported to inhibit cell proliferation.12 Other in vitro studies have shown that GCs inhibit the growth of estrogen receptor (ER) α-positive (ERα-positive) (eg, MCF-7) cells by arresting cell cycle in G0/G1 phase. In contrast, proliferation of ERα-negative (eg, MDA-MB-231) cells is not inhibited by treatment with GCs, suggesting that GCs inhibit the proliferation of breast cancer cells via the ERα signaling pathway.13 GCs and mineralocorticoids can also cross talk with progesterone receptors to induce a progesterone-like effect in breast cancer.14 However, Dex treatment of MCF-7 cells has also been reported to promote cell proliferation by upregulating c-Myc, which is induced by the promotion of NFκB transcriptional activity.12,15 Loss of GR activation has been observed in BRCA1-mutated breast tissue.9,11 While the presence of GRs in specific breast cancer cell lines has been clearly established, their activation is associated with multiple and opposite effects. GCs exert an antiapoptotic effect. This antiapoptotic effect was studied further and could be mediated by the induction of the expression of other genes frequently associated with protection from cell apoptosis, such as Bcl-XL, BAk, SGK-1, and MKP-1. Concomitantly, Dex also reinforces its survival effect by downregulation of proapoptotic genes.16–18 Dex induces the expression of genes associated with protection against cell apoptosis. GRs disrupt p53-mediated regulation of cell survival. Loss of p53 activity has been linked with a range of human cancers. P53 mediates cell apoptosis in case of DNA damage or hypoxia.19
The majority of chemotherapy patients receive a pre-administration of Dex. We deemed that this sequential treatment (ie, administration of Dex followed by a chemotherapeutic agent) warranted further assessment. The present study aimed to investigate what influence Dex treatment, prior to the administration of a chemotherapeutic agent (5-FU, epirubicin, or paclitaxel), had on the proliferation of MCF-7 cells.
Materials and methods
Cell culture
MCF-7 cells obtained from (ATCC Bioresource Centre, Manassas, VA, USA), were maintained at 37°C in a humidified cell incubator with a 5% CO2 atmosphere. Cells were cultured in Dulbecco’s Modified Essential Medium (DMEM) containing phenol red and supplemented with 10% fetal bovine serum (FBS; Life Technologies, Paisley, UK), 2 mM l-glutamine, and 1% penicillin–streptomycin (all from Life Technologies). For the experimental procedures, cells were seeded in DMEM (phenol red-free) supplemented with 10% charcoal-stripped FBS and 100 nM 17β-estradiol.
Measurement of cell culture growth by cell counting
MCF-7 cells were plated at a density of 15,000 cells/mL in 12-well dishes and then incubated at 37°C with a 5% CO2 atmosphere. The following day, the cell cultures were given fresh medium, with or without Dex (control [CT]), at a concentration of 100 nM for 30 min. The cells were then incubated for 4 hours in a medium containing 15 nM paclitaxel (Sigma-Aldrich, St. Louis, MO, USA) or 15 µM 5-FU (Merck). Cells were treated with epirubicin hydrochloride (Tocris Bioscience, Bristol, UK) by culturing the cells for 3 days in the presence of 25 nM of the drug. Measurement of cell proliferation was performed 3 days after the drug treatments. Cells were dislodged from the surface of the culture vessel by treatment with 1 mL of trypsin–EDTA solution (Sigma-Aldrich) for 2 min. After vigorous pipetting, the number of cells in the suspension was determined using an electronic cell counter (model Z1 Coulter counter; Beckman Coulter, Fullerton, CA, USA). The cell counter did not count nonviable cells and only recorded the number of live cells. An equivalent number of cells were counted for each experiment regardless of the treatment conditions.
Statistical analysis
SigmaPlot® 11 software (Systat Software Inc., Chicago, IL, USA) was used for the statistical analyses. Parametric analysis was performed by Student’s t-test. A p-value <0.05 was considered statistically significant.
Results
Cell numbers decreased by more than half (p<0.001) when we incubated MCF-7 with 100 nM Dex for 30 min compared to untreated (CT) cells (Figure 1). No significant difference was seen between the samples exposed to epirubicin or to Dex and then epirubicin (Figure 2). This was also the case for the samples treated with 5-FU or Dex followed by 5-FU (Figure 3). By contrast, a statistically significant difference (p=0.038) was noted between samples treated with paclitaxel or with Dex followed by paclitaxel (Figure 4). Preexposure of MCF-7 cells to Dex before paclitaxel treatment reduced the cytotoxic effect of paclitaxel by 8.5%.
Figure 1.
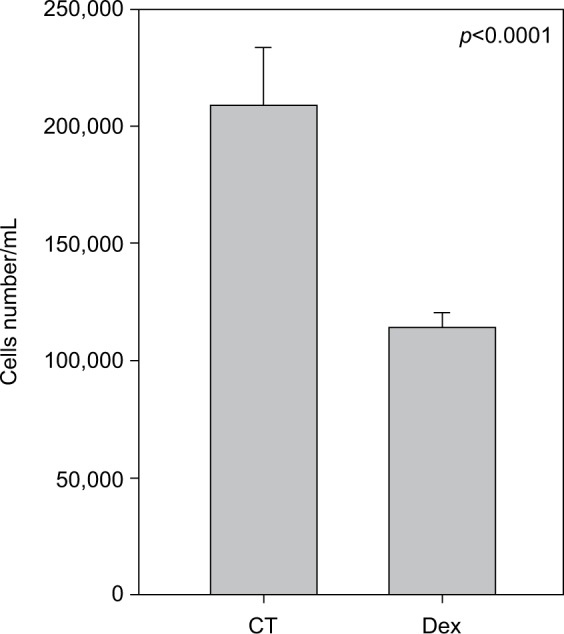
Untreated and dexamethasone (Dex) controls. MCF-7 cells were either untreated (control [CT]) or treated with 100 nM of Dex. The graph indicates the number of cells/mL 3 days after treatment.
Figure 2.
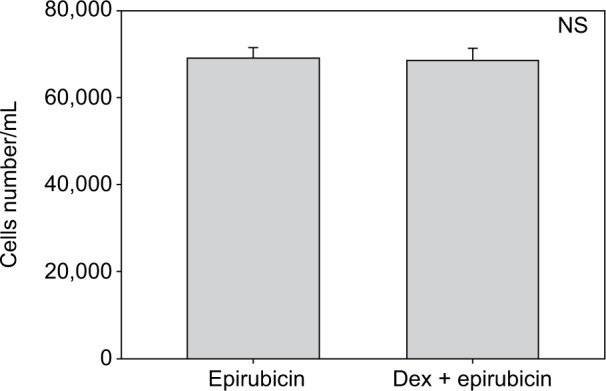
Epirubicin with dexamethasone (Dex) pretreatment. MCF-7 cells were pretreated with 100 nM of Dex prior to administration of 25 nM of epirubicin. The graph indicates the number of cells/mL 3 days after treatment. No significant difference was discernible between the samples treated with Dex alone or Dex in combination with epirubicin (n=4). Error bars indicate standard deviation.
Abbreviation: NS, nonsignificant.
Figure 3.
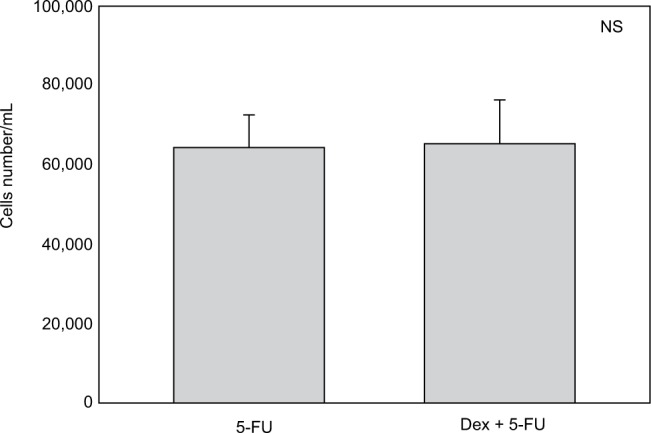
5-Fluorouracil (5-FU) with dexamethasone (Dex) pretreatment. MCF-7 cells were pretreated with 100 nM of Dex prior to administration of 15 µM of 5-FU. The graph indicates the number of cells/mL 3 days after treatment. No significant difference was discernible between the samples treated with Dex alone or Dex in combination with 5-FU (n=4). Controls not shown (as for Figure 1). Error bars indicate standard deviation.
Abbreviation: NS, nonsignificant.
Figure 4.
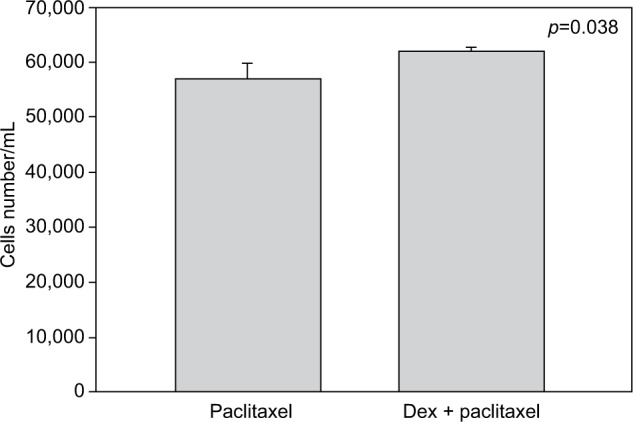
Paclitaxel with dexamethasone (Dex) pretreatment. MCF-7 cells were pretreated with 100 nM of Dex prior to administration of 15 nM of paclitaxel. The graph indicates the number of cells/mL 3 days after treatment. A significant difference was discernible between the samples treated with Dex alone or Dex in combination with paclitaxel (n=4). Error bars indicate standard deviation.
Discussion
GCs have been reported to have multiple modes of action, thus explaining their proapoptotic and antiapoptotic effects on ER-positive or ER-negative breast cancer cells, respectively. As GCs are administered systematically with epirubicin- and paclitaxel-based chemotherapies, the consequences of GC exposure in terms of the chemotherapeutic effect warranted further investigation.
We chose to study the apoptotic effects of epirubicin, 5-FU, and paclitaxel when MCF-7 cells were preexposed to Dex because they are the three main drugs currently used in routine chemotherapy. Since the anthracyclines doxorubicin and epirubicin have similar mechanisms of action, we did not perform additional testing with doxorubicin. In this study, we demonstrated that the apoptotic activity of paclitaxel was significantly reduced (by 8.5%) when MCF-7 cells were preexposed to Dex. No significant effect of Dex was seen with exposure to the chemotherapeutic agent 5-FU or epirubicin. Under in vitro conditions, paclitaxel enhances the polymerization of tubulin so as to form stable microtubules. It also interacts directly with microtubules and protects them from depolymerization by cold or calcium, both of which can readily depolymerize microtubules in the absence of the drug. Paclitaxel arrests cells at the G2–M phase of the cell cycle by the stabilization of spindle microtubules. However, the exact mechanism by which paclitaxel induces apoptosis is not clear.20 An explanation could be that taxanes act more than docetaxel by phosphorylation of bcl2, thus abrogating the normal antiapoptotic function of bcl2.21
Wu et al17,18 demonstrated that pretreatment of breast cancer cell lines by Dex inhibits chemotherapy-induced apoptosis in a GR-dependent manner and that it is associated with transcriptional induction of at least two genes identified in our screening – SGK-1 and MKP-1.
By contrast, there was no difference in cell proliferation following treatment with 5-FU and epirubicin. Their mechanisms of action, however, are different from that of paclitaxel. 5-FU needs to be converted at the nucleotide level in order to exert its effect. It can be incorporated into RNA, leading to interference with the maturation of nuclear RNA. Its conversion to 5-fluoro-2′deoxy-5′monophosphate, which leads to inhibition of thymidylate synthase and subsequently DNA synthesis, is however considered to be its main mechanism of action.15,22 Epirubicin is an anthracycline antibiotic; its antitumor activity is caused by its ability to interfere with transcription, resulting in inhibition of RNA synthesis.23
Under in vitro conditions, several, at times antagonistic, actions of GCs have been described.2,3,8–14 This underscores the relevance of a direct study of preexposure to GCs prior to chemotherapy. In our study, the results were dependent on the specific chemotherapeutic agent being administered, which may be explained by the different cytotoxic mechanisms of these chemotherapeutics. However, the result of this study is not sufficient to warrant changing the current therapeutic approach. Other factors most certainly influence the tumor response in vivo. Indeed, it has been proposed that the peritumoral stroma and cancer-associated fibroblasts play an important role in breast tumorigenesis.24 The observed decrease in the efficacy of paclitaxel on MCF-7 cells previously exposed to Dex is, however, not sufficient to warrant changing the current therapeutic approach. Indeed, it has been proposed that the peritumoral stroma and cancer-associated fibroblasts play an important role in breast tumorigenesis.17,24 For example, in tumors that exhibit an aggressive metastatic phenotype, such as luminal HER2-positive and triple-negative, metallo-proteinase-2 (MMP-2) expression is significantly increased.25 Dex could be acting at this level, by alleviating the effect of MMP-2.19,26 More recently, we demonstrated frequent expression of GRs in breast cancer-associated fibroblasts. The presence of GRs within breast tumors and the adjacent peritumoral stroma must be taken into account when GCs are prescribed to cancer patients. As shown in this study, GCs could substantially decrease the cellular response(s) caused by exposure to chemotherapeutics. The use of GCs other than Dex could be a solution. Recently, Orqueda et al showed that the rigid steroid 21-hydroxy-6,19-epoxyprogesterone (21OH-6, 19OP), a selective GR ligand, behaves as a dissociated GC that retains anti-inflammatory action without affecting the apoptotic process triggered by chemotherapeutic drugs. Indeed, contrary to Dex, 21OH-6, 19OP neither reverts the paclitaxel-induced caspase-3 activity nor induces the antiapoptotic Bcl-X(L) gene expression in murine tumor mammary epithelial cells.27
Conclusion
Since GCs affect the behavior of numerous cancer cell types, their function should be defined more precisely if they are to be used in conjunction with chemotherapy. Indeed, in this study we demonstrated a decrease in the response of MCF-7 cells to paclitaxel when they had been pretreated with Dex. Use of other antiemetogenic molecules should be considered in light of these results.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Rosen J, Miner JN. The search for safer glucocorticoid receptor ligands. Endocr Rev. 2005;26(3):452–464. doi: 10.1210/er.2005-0002. [DOI] [PubMed] [Google Scholar]
- 2.Kofler R. The molecular basis of glucocorticoid-induced apoptosis of lymphoblastic leukemia cells. Histochem Cell Biol. 2000;114(1):1–7. doi: 10.1007/s004180000165. [DOI] [PubMed] [Google Scholar]
- 3.Evans-Storms RB, Cidlowski JA. Delineation of an antiapoptotic action of glucocorticoids in hepatoma cells: the role of nuclear factor-kappaB. Endocrinology. 2000;141(5):1854–1862. doi: 10.1210/endo.141.5.7466. [DOI] [PubMed] [Google Scholar]
- 4.Bernard-Marty C, Cardoso F, Piccart MJ. Use and abuse of taxanes in the management of metastatic breast cancer. Eur J Cancer. 2003;39(14):1978–1989. doi: 10.1016/s0959-8049(03)00538-0. [DOI] [PubMed] [Google Scholar]
- 5.Micha JP, Rettenmaier MA, Brown JV, 3rd, et al. A randomized controlled pilot study comparing the impact of aprepitant and fosaprepitant on chemotherapy induced nausea and vomiting in patients treated for gynecologic cancer. Int J Gynecol Cancer. 2016;26(2):389–393. doi: 10.1097/IGC.0000000000000593. [DOI] [PubMed] [Google Scholar]
- 6.Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies—improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol. 2015;26(8):1533–1546. doi: 10.1093/annonc/mdv221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Senkus E, Kyriakides S, Ohno S, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v8–v30. doi: 10.1093/annonc/mdv298. [DOI] [PubMed] [Google Scholar]
- 8.Skor MN, Wonder EL, Kocherginsky M, et al. Glucocorticoid receptor antagonism as a novel therapy for triple-negative breast cancer. Clin Cancer Res. 2013;19(22):6163–6172. doi: 10.1158/1078-0432.CCR-12-3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lien HC, Lu YS, Cheng AL, et al. Differential expression of glucocorticoid receptor in human breast tissues and related neoplasms. J Pathol. 2006;209(3):317–327. doi: 10.1002/path.1982. [DOI] [PubMed] [Google Scholar]
- 10.Buxant F, Engohan-Aloghe C, Noël JC. Estrogen receptor, progesterone receptor, and glucocorticoid receptor expression in normal breast tissue, breast in situ carcinoma, and invasive breast cancer. Appl Immunohistochem Mol Morphol. 2010;18(3):254–257. doi: 10.1097/PAI.0b013e3181c10180. [DOI] [PubMed] [Google Scholar]
- 11.Vilasco M, Communal L, Hugon-Rodin J, et al. Loss of glucocorticoid receptor activation is a hallmark of BRCA1-mutated breast tissue. Breast Cancer Res Treat. 2013;142(2):283–296. doi: 10.1007/s10549-013-2722-8. [DOI] [PubMed] [Google Scholar]
- 12.Buxant F, Kindt N, Laurent G, Noël JC, Saussez S. Antiproliferative effect of dexamethasone in the MCF-7 breast cancer cell line. Mol Med Rep. 2015;12(3):4051–4054. doi: 10.3892/mmr.2015.3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lippman M, Bolan G, Huff K. The effects of glucocorticoids and progesterone on hormone-responsive human breast cancer in long-term tissue culture. Cancer Res. 1976;36(12):4602–4609. [PubMed] [Google Scholar]
- 14.Leo JC, Guo C, Woon CT, Aw SE, Lin VC. Glucocorticoid and mineralocorticoid cross talk with progesterone receptor to induce focal adhesion and growth inhibition in breast cancer cells. Endocrinology. 2004;145(3):1314–1321. doi: 10.1210/en.2003-0732. [DOI] [PubMed] [Google Scholar]
- 15.Khan S, Lopez-Dee Z, Kumar R, Ling J. Activation of NFkB is a novel mechanism of pro-survival activity of glucocorticoids in breast cancer cells. Cancer Lett. 2013;337(1):90–95. doi: 10.1016/j.canlet.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 16.Schorr K, Furth PA. Induction of bcl-xL expression in mammary epithelial cells is glucocorticoid-dependent but not signal transducer and activator of transcription 5-dependent. Cancer Res. 2000;60(21):5950–5953. [PubMed] [Google Scholar]
- 17.Wu W, Chaudhuri S, Brickley DR, Pang D, Karrison T, Conzen SD. Microarray analysis reveals glucocorticoid-regulated survival genes that are associated with inhibition of apoptosis in breast epithelial cells. Cancer Res. 2004;64(5):1757–1764. doi: 10.1158/0008-5472.can-03-2546. [DOI] [PubMed] [Google Scholar]
- 18.Wu W, Pew T, Zou M, Pang D, Conzen SD. Glucocorticoid receptor-induced MAPK phosphatase-1 (MPK-1) expression inhibits paclitaxel-associated MAPK activation and contributes to breast cancer cell survival. J Biol Chem. 2005;280(6):4117–4124. doi: 10.1074/jbc.M411200200. [DOI] [PubMed] [Google Scholar]
- 19.Rutz HP. Effects of corticosteroid use on treatment of solid tumours. Lancet. 2002;360(9349):1969–1970. doi: 10.1016/S0140-6736(02)11922-2. [DOI] [PubMed] [Google Scholar]
- 20.Gotaskie GE, Andreassi BF. Paclitaxel: a new antimitotic chemotherapeutic agent. Cancer Pract. 1994;2(1):27–33. [PubMed] [Google Scholar]
- 21.Haldar S, Basu A, Croce CM. Bcl2 is the guardian of microtubule integrity. Cancer Res. 1997;57(2):229–233. [PubMed] [Google Scholar]
- 22.Pinedo HM, Peters GF. Fluorouracil: biochemistry and pharmacology. J Clin Oncol. 1988;6(10):1653–1664. doi: 10.1200/JCO.1988.6.10.1653. [DOI] [PubMed] [Google Scholar]
- 23.Wang F, Liu X, Liu C, Liu Z, Sun L. Effects of antibiotic antitumor drugs on nucleotide levels in cultured tumor cells: an exploratory method to distinguish the mechanisms of antitumor drug action based on targeted metabolomics. Acta Pharm Sin B. 2015;5(3):223–230. doi: 10.1016/j.apsb.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo H, Tu G, Liu Z, Liu M. Cancer-associated fibroblasts: a multifaceted driver of breast cancer progression. Cancer Lett. 2015;361(2):155–163. doi: 10.1016/j.canlet.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 25.Catteau X, Simon P, Noël JC. Stromal expression of matrix metalloproteinase 2 in cancer-associated fibroblasts is strongly related to human epidermal growth factor receptor 2 status in invasive breast carcinoma. Mol Clin Oncol. 2016;4(3):375–378. doi: 10.3892/mco.2015.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J, Zhang H, Su C, et al. Dexamethasone ameliorates H2S-induced acute lung injury by alleviating matrix metalloproteinase-2 and -9 expression. PLoS One. 2014;9(4):e94701. doi: 10.1371/journal.pone.0094701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orqueda AJ, Dansey MV, Español A, et al. The rigid steroid 21-hydroxy-6,19-epoxyprogesterone (21OH-6,19OP) is a dissociated glucocorticoid receptor modulator potentially useful as a novel coadjuvant in breast cancer chemotherapy. Biochem Pharmacol. 2014;89(4):526–535. doi: 10.1016/j.bcp.2014.04.006. [DOI] [PubMed] [Google Scholar]


