Abstract
Legionella pneumophila is a common, usually underreported and undiagnosed cause of community acquired pneumonia which can lead to significant morbidity and mortality. Diffuse alveolar hemorrhage rarely have been associated with legionella infection. We present a 61-year-old man with hypertension, diabetes mellitus and obesity admitted with severe acute respiratory distress syndrome. He was found to have Legionella pneumonia with associated diffuse alveolar hemorrhage diagnosed with bronchoscopic sequential bronchoalveolar lavage. He was successfully managed with antibiotics, lung protective strategies and intravenous pulse dose steroids. This patient highlights the unusual association of Legionella infection and diffuse alveolar hemorrhage. Additionally, the case re-enforces the need for early and aggressive evaluation and management of patients presenting with pneumonia and progressive hypoxia despite adequate treatment.
Keywords: Diffuse alveolar hemorrhage, Legionella, Pneumonia, Acute respiratory distress syndrome
1. Introduction
Legionella pneumophila, an aerobic gram-negative intracellular bacillus is a common cause of community acquired pneumonia (CAP) that can cause mild illness in 2–6% of cases [1]. Incidence of severe legionella infection leading to hospitalization is increasing in United States [2]. Eight to 20% of cases requires admission to the intensive care unit (ICU) with a case fatality rate of 5–25% in immunocompetent patients [3]. Legionella pneumophila triggers an immune-mediated reaction that can lead to severe CAP and acute respiratory distress syndrome (ARDS). Diffuse alveolar damage (DAH) in ARDS leading to direct leakage of red blood cells into alveolar spaces results in DAH which is a potentially life-threatening condition. Disorders associated with DAH are broadly divided into immune mediated and non-immune mediated. Pulmonary infections are an uncommon cause of non-immune-mediated DAH. In immunocompromised patients, the main infectious diseases associated with DAH are cytomegalovirus, adenovirus, invasive aspergillosis, mycoplasma, and strongyloides. In immunocompetent patients, Influenza A, dengue, leptospirosis, malaria, and Staphylococcus aureus are the most frequently reported infections associated with DAH [4].
2. Case presentation
A 61-year-old male was admitted to the ICU with three-day of progressive dyspnea associated with acute cough, productive of yellowish sputum and fever. He denied constitutional or gastrointestinal symptoms, chest pain, hemoptysis, exposure to birds, recent travel or sick contacts. His medical history was significant for hypertension, dyslipidemia, diabetes mellitus type 2, and obesity. Medications included olmesartan, amlodipine, hydrochlorothiazide, glyburide-metformin, pioglitazone and simvastatin. He had no surgeries and was a retired military officer. He denied smoking, use of illicit drugs and drank alcohol socially.
On presentation, the patient was in moderate to severe respiratory distress. He was febrile to 102.7 F, tachycardic with a pulse rate of 123 per/minute, tachypneic with a respiratory rate of 25 per/minute, and blood pressure of 154/74 mmHg. He was hypoxic with oxygen saturation of 80% on ambient air which improved to 96% with noninvasive positive pressure ventilation and FIO2 of 0.7. Exam was significant for use of accessory respiratory muscles and bibasilar crackles. Cardiac, abdominal, neurological and skin examination were unrevealing. Initial laboratory showed leukocytosis, acute kidney injury, hyponatremia and rhabdomyolysis (See Table 1).
Table 1.
Pertinent laboratory parameters.
| Laboratory parameters | On Admission | Day 6 | On Discharge |
|---|---|---|---|
| Hemoglobin (g/dl) | 10.2 | 9 | 10.9 |
| Hematocrit (%) | 31.8 | 29.9 | 33.5 |
| White Blood Cell (WBC/ul) | 18 | 18.5 | 16.5 |
| Platelet (per/ul) | 179 | 310 | 161 |
| Sodium (mEq/L) | 132 | 138 | 137 |
| Blood Urea Nitrogen (mg/dL) | 40 | 37 | 22 |
| Creatinine (mg/dL) | 1.6 | 1.7 | 1 |
| Creatinine kinase (unit/L) | 462 | 79 |
Initial chest radiograph (CXR) and computed tomography (CT) of the chest showed extensive left lower lobe alveolar infiltrates (Fig. 1, Fig. 2).
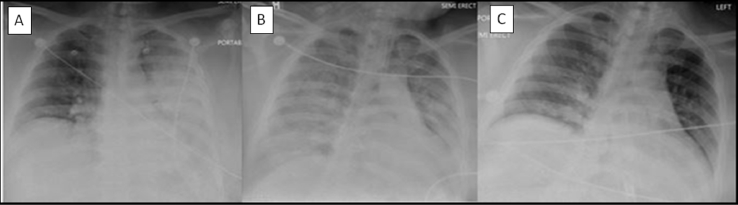
Fig. 1.
Chest radiograph A/P view (A): On admission, showing left side dense infiltrate (B): Hospital day 4 showing worsening bilateral infiltrates (C): Hospital day 14 showing radiological resolution of infiltrates.
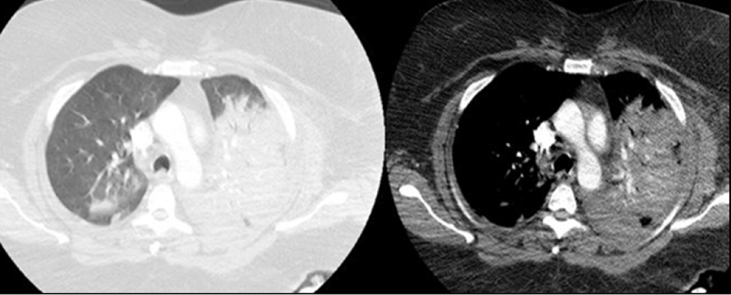
Fig. 2.
Chest CT on admission showing dense consolidation in left side and mild infiltrate in right.
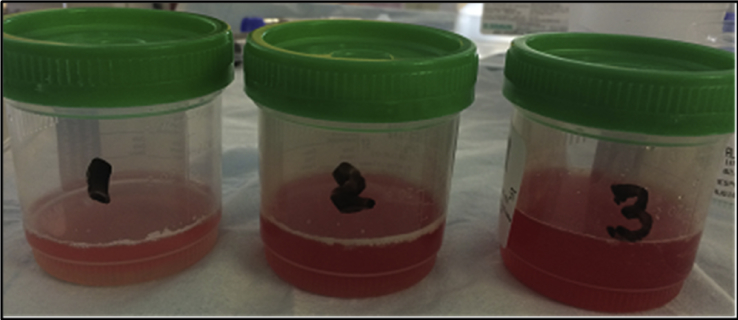
Patient's clinical condition continued to deteriorate, he required intubation and mechanical ventilation on day 6 for severe ARDS (PAO2/FIO2 of 84). Piperacillin-tazobactim, vancomycin and azithromycin were started on admission, and Levofloxacin was added on day 5 when result for urine legionella antigen was positive. Blood and urine cultures, mycoplasma antibody and urine streptococcus pneumonia antigen were negative. He received ventilator management based on ARDSnet protocol and fluid restriction [5]. Fiberoptic bronchoscopy (FFB) with bronchoalveolar lavage (BAL) was performed to evaluate for associated infections or non-infectious process; lavage became progressively hemorrhagic, with no evidence of local bleeding. A rising RBC count in sequential BAL aliquots from same location was consistent with DAH (Fig. 3).
Fig. 3.
FOB-BAL with sequential lavage showing progressively increasing hemorrhagic fluid.
BAL cultures were negative for viral, mycobacterial and fungal infections, cell counts were predominant neutrophils, and cytology was negative for malignant cells. Evaluation for common causes of DAH including connective tissue diseases were performed (Table 2).
Table 2.
Pertinent work up for Diffuse Alveolar Hemorrage.
| Antinuclear antibody (ANA) | Negative |
| Anti Jo-1 antibody | Negative |
| Centromere antibody | Negative |
| Anti Ribosomal P antibody | Negative |
| Anti DNA antibody | Negative |
| Smooth muscle antibody | Negative |
| Anti Scleroderma 70 antibody | Negative |
| Myeloperoxidase | Negative |
| Proteinase 3 antibody | Negative |
| Cold Agglutinin antibody | Negative |
| Rheumatoid Factor | Weakly positive |
| Anti- Cyclic Citrullinated antibody | Negative |
| HIV | Negative |
| Mycoplasma Pneumonia antibody | Negative |
| Legionella antigen, urine | Positive |
| Blood, urine and respiratory culture | Negative |
| BAL cultures and acid fast bacilli stains | Negative |
Considering severity of illness and presence of DAH, pulse steroid with one-gram methylprednisolone for three days followed by prednisone 1 mg/kg were given. Patient conditions steadily improved, with no evidence of hemoptysis or severe drop in hemoglobin. A final diagnosis of Legionella pneumonia associated with DAH and severe ARDS was made. Patient was liberated for ventilator after 7 days, antibiotics were deescalated to Levofloxacin to complete 21 days, steroids were tapered off and he was discharged home after three weeks of hospitalization.
3. Discussion
Legionellosis refers to clinical syndromes caused by bacteria of the genus Legionella. Legionnaires' disease refers to the pneumonic form of legionellosis [6]. Globally, most cases relate to Legionella pneumophila, with serogroup 1 being the most virulent and most common cause of legionella disease [7]. The yearly incidence is associated with climate changes, most cases been sporadic and transmitted by inhalation of aerosols, micro aspiration of contaminated water or direct contact with surgical wounds. Presentation of Legionnaires' disease ranges from mild to severe and it is characterized by an incubation period of 2–14 days and multi-systemic manifestations. Presence of gastrointestinal and neurological symptoms in patients with pneumonia suggest Legionnaires' disease. Risk factors include chronic lung disease, smoking, old age, use of glucocorticoids, malignancies and organ transplant recipients. Radiological findings are nonspecific, with unilateral or bilateral infiltrates commonly reported [8]. First-line diagnostic test is urinary antigen while culture of the lower respiratory tract remains the gold standard [9]. Direct nucleic acid amplification by polymerase chain reaction of Legionella using respiratory, urine and serum specimens is currently the diagnostic method of choice [10]. Treatment with macrolides-azithromycin or quinolones-levofloxacin is considered highly effective and most patients respond within three days of treatment [11].
Progression of infiltrates despite appropriate therapy is common as radiographic improvement lags days behind clinical improvement. However, worsening pulmonary involvement with clinical deterioration should raise the suspicion for complications of pneumonia, associated conditions or other diagnosis.
DAH is a life-threatening condition with a mortality rate of 20–50% [12]. It is characterized by a distinct clinic-pathologic syndrome of pulmonary hemorrhage that originates from the pulmonary microcirculation leading to a clinical constellation of hemoptysis, anemia, diffuse radiographic pulmonary infiltrates, and hypoxemic respiratory failure. Constitutional symptoms like fever, chest pain, cough, and dyspnea have been reported. Hemoptysis can be absent in one-third of the patients [13].
Etiology of DAH can be divided into immune and non-immune mediated causes. Immune mediated include antineutrophilic cytoplasm associated vasculitis, connective tissue diseases (i.e. systemic lupus erythematosus, rheumatoid arthritis, inflammatory myopathies), antiphospholipid antibody syndrome, Henoch-Schonlein purpura, cryoglobulinemic vasculitis, Behcet disease, lung transplant rejection, hypocomplementemic urticarial vasculitis, drug-induced vasculitis and bone marrow transplantation. Non immune mediated causes include cardiac etiologies, medications, ARDS, idiopathic pulmonary hemosiderosis, coagulopathy, radiation and occupational exposure, crack cocaine inhalation and infectious etiologies [14].
Infectious etiologies may affect immunocompetent or immunodeficient patients [4]. The most common infections associated with DAH in immunocompetent patients are influenza A, dengue, leptospirosis, malaria, and staphylococcus aureus infections. DAH associated with Legionella have been rarely reported [15]. Risk factors for legionella in our patient was age and diabetes.
Radiological findings in DAH are nonspecific, with bilateral consolidation, ground-glass opacity or septal thickening with occasional crazy-paving pattern been the most commonly described [16], [17]. This features can be indistinguible from findings in pneumonic process. Radiological distribution and temporal evolution of hemorrhage with the radiologic manifestations of underlying pulmonary disease are key features to suspect DAH [16]. DAH must be distinguished from localized pulmonary hemorrhage with diffuse aspiration of blood due to tumors or localized infections [18]. Bronchoscopy with BAL allows early diagnosis of DAH, evaluation of hemoptysis, and assist in excluding infectious or non-infectious etiologies such as alveolar proteinosis, eosinophilic pneumonias among others [19], [20], [21]. Histopathology of DAH includes presence of intra-alveolar red blood cells and fibrin, with accumulation of hemosiderin-laden macrophages, which may take 2–3 days to accumulate. One of three histologic patterns, pulmonary capillaritis, bland pulmonary hemorrhage and diffuse alveolar damage are associated with DAH with pulmonary capillaritis been the most frequently described [22]. Diffuse alveolar damage in ARDS leading to direct leakage of red blood cells into alveolar spaces is the suggested reason for DAH in legionella infection [23].
Acute management of DAH involves respiratory support, identification and correction of coagulopathy, and diagnosis and treatment of underlying etiology. In immune-mediated cases, immunosuppressive therapy is paramount. Intravenous methylprednisolone up to 500 mg every 6 hours for 4–5 days is recommended by most experts, although lower doses seem to have similar efficacy, followed by a gradual taper to maintenance doses of oral steroids [24]. Based on the severity of the disease, underlying etiology and organ involvement, plasma exchange or other immunosuppressive drugs such as cyclophosphamide, azathioprine, methotrexate, mycophenolate mofetil, etanercept may be indicated [25], [26], [27].
Treatment of non-immune mediated causes of DAH is targeted to management of the underlying etiology as immunosuppressive treatment could be deleterious [12]. Use of immunosuppressive therapy in patient with infections-related DAH should be individualized and used mainly for patients receiving appropriate antibiotics with deteriorating clinical course like our patient. We assumed that our patient developed pulmonary capillaritis due to infectious process, could not exclude infection–mediated immune disorder and extrapolated data from treatment of immune-mediated DAH to offer steroid therapy.
Legionella pneumonia associated with DAH is rare, but should be considered in the diagnostic workup because of the obvious therapeutic implications. On review of the English literature, we found two cases reported of DAH due to legionella infection [15]. In our patient, we excluded other etiologies of DAH to a reasonable degree and he was treated successfully with high dose steroids and quinolones for legionella infection with favorable outcome. Immunosuppressive therapy for patients with DAH associated with infections is not well standardized and should be carefully individualized.
4. Conclusion
This case highlights the association of Legionella infection with DAH, both of which are potentially serious and occasionally fatal conditions. Clinicians caring for this patients should consider DAH in those patients with pneumonia with worsening clinic-radiological features especially if they develop anemia and or hemoptysis. Early diagnosis and intervention is vital as these patients might require high dose steroids in addition to the routine antibiotics management for favorable outcome.
Informed consent
Written consent was obtained from the next of kin for publication of this case report and accompanying images.
Competing interests
The author(s) of the manuscript declare that there is no conflict of interest regarding the publication of this paper.
Availability of data and materials
Not applicable.
Authors contributions
M K searched the literature and wrote the manuscript. GDF conceived and edited the manuscript. GDF supervised the patient treatment, critically revised and edited the manuscript. RP and BB were involved in patient care. All authors have made significant contributions to the manuscript and have reviewed it before submission. All authors have confirmed that the manuscript is not under consideration for review at any other Journal. All authors have read and approved the final manuscript.
Funding
No funding was provided for the production of this case report.
Acknowledgements
Not applicable.
Biographies
MK is a Fellow in Pulmonary Medicine at the Department of Medicine, Bronx Lebanon Hospital Center.
RP is a second year resident at the Department of Medicine, Bronx Lebanon Hospital Center.
BB is a Fellow in Pulmonary Medicine at the Department of Medicine, Bronx Lebanon Hospital Center.
GDF is Chief of Pulmonary and Critical Care Division and an Attending physician at the Department of Medicine, Bronx Lebanon Hospital Center.
Contributor Information
Muhammad Kashif, Email: drkashif178@gmail.com.
Ravi Patel, Email: rpatel5@bronxleb.org.
Bharat Bajantri, Email: BBanjant@bronxleb.org.
Gilda Diaz-Fuentes, Email: gfuentes@bronxleb.org.
List of abbreviations
- CAP
Community acquired pneumonia
- ARDS
Acute respiratory distress syndrome
- DAH
Diffuse alveolar hemorrhage
- BAL
Bronchoalveolar lavage
References
- 1.Cillóniz C., Torres A., Niederman M., van der Eerden M., Chalmers J., Welte T. Community-acquired pneumonia related to intracellular pathogens. Intensive Care Med. 2016;42(9):1374–1386. doi: 10.1007/s00134-016-4394-4. [DOI] [PubMed] [Google Scholar]
- 2.Marston B.J., Plouffe J.F., File T.M., Jr. Incidence of community-acquired pneumonia requiring hospitalization: results of a population-based active surveillance study in ohio. Arch. Intern. Med. 1997;157(15):1709–1718. [PubMed] [Google Scholar]
- 3.Mandell L.A., Bartlett J.G., Dowell S.F., File T.M., Jr., Musher D.M., Whitney C. Update of practice guidelines for the management of community-acquired pneumonia in immunocompetent adults. Clin. Infect. Dis. 2003;37(11):1405–1433. doi: 10.1086/380488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.von Ranke F.M., Zanetti G., Hochhegger B., Marchiori E. Infectious diseases causing diffuse alveolar hemorrhage in immunocompetent patients: a state-of-the-art review. Lung. 2013;191(1):9–18. doi: 10.1007/s00408-012-9431-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The acute respiratory distress syndrome network. N. Engl. J. Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 6.Cunha B.A., Burillo A., Bouza E. Legionnaires' disease. Lancet. 2016;387(10016):376–385. doi: 10.1016/S0140-6736(15)60078-2. [DOI] [PubMed] [Google Scholar]
- 7.Stout J.E., Yu V.L. Legionellosis. N. Engl. J. Med. 1997;337(10):682–687. doi: 10.1056/NEJM199709043371006. [DOI] [PubMed] [Google Scholar]
- 8.Tan M.J., Tan J.S., Hamor R.H., File T.M., Jr., Breiman R.F. The radiologic manifestations of Legionnaire's disease. The Ohio community-based pneumonia incidence study group. Chest. 2000;117(2):398–403. doi: 10.1378/chest.117.2.398. [DOI] [PubMed] [Google Scholar]
- 9.Jarraud S., Descours G., Ginevra C., Lina G., Etienne J. Identification of legionella in clinical samples. Methods Mol. Biol. 2013;954:27–56. doi: 10.1007/978-1-62703-161-5_2. [DOI] [PubMed] [Google Scholar]
- 10.Phin N., Parry-Ford F., Harrison T., Stagg H.R., Zhang N., Kumar K. Epidemiology and clinical management of Legionnaires' disease. Lancet Infect. Dis. 2014;14(10):1011–1021. doi: 10.1016/S1473-3099(14)70713-3. [DOI] [PubMed] [Google Scholar]
- 11.Kuzman I., Soldo I., Schonwald S., Culig J. Azithromycin for treatment of community acquired pneumonia caused by Legionella pneumophila: a retrospective study. Scand. J. Infect. Dis. 1995;27(5):503–505. doi: 10.3109/00365549509047054. [DOI] [PubMed] [Google Scholar]
- 12.de Prost N., Parrot A., Cuquemelle E., Picard C., Antoine M., Fleury-Feith J. Diffuse alveolar hemorrhage in immunocompetent patients: etiologies and prognosis revisited. Respir. Med. 2012;106(7):1021–1032. doi: 10.1016/j.rmed.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 13.Lara A.R., Schwarz M.I. Diffuse alveolar hemorrhage. Chest. 2010;137(5):1164–1171. doi: 10.1378/chest.08-2084. [DOI] [PubMed] [Google Scholar]
- 14.Krause M.L., Cartin-Ceba R., Specks U., Peikert T. Update on diffuse alveolar hemorrhage and pulmonary vasculitis. Immunol. Allergy Clin. North Am. 2012;32(4):587–600. doi: 10.1016/j.iac.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sundar K.M., Pearce M.J. Diffuse alveolar hemorrhage due to Legionella pneumonia. Sarcoidosis Vasc. Diffuse Lung Dis. 2004;21(2):158–159. [PubMed] [Google Scholar]
- 16.Lichtenberger Iii J.P., Digumarthy S.R., Abbott G.F., Shepard J.-A.O., Sharma A. Diffuse pulmonary hemorrhage: clues to the diagnosis. Curr. Probl. Diagn. Radiol. 2014;43(3):128–139. doi: 10.1067/j.cpradiol.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Rossi S.E., Erasmus J.J., Volpacchio M., Franquet T., Castiglioni T., McAdams H.P. “Crazy-paving” pattern at thin-section CT of the lungs: radiologic-pathologic overview. Radiographics. 2003;23(6):1509–1519. doi: 10.1148/rg.236035101. [DOI] [PubMed] [Google Scholar]
- 18.Primack S.L., Miller R.R., Muller N.L. Diffuse pulmonary hemorrhage: clinical, pathologic, and imaging features. AJR Am. J. Roentgenol. 1995;164(2):295–300. doi: 10.2214/ajr.164.2.7839958. [DOI] [PubMed] [Google Scholar]
- 19.Schnabel A., Holl-Ulrich K., Dalhoff K., Reuter M., Gross W.L. Efficacy of transbronchial biopsy in pulmonary vaculitides. Eur. Respir. J. 1997;10(12):2738–2743. doi: 10.1183/09031936.97.10122738. [DOI] [PubMed] [Google Scholar]
- 20.Colby T.V., Fukuoka J., Ewaskow S.P., Helmers R., Leslie K.O. Pathologic approach to pulmonary hemorrhage. Ann. Diagn Pathol. 2001;5(5):309–319. doi: 10.1053/adpa.2001.27923. [DOI] [PubMed] [Google Scholar]
- 21.Travis W.D., Colby T.V., Lombard C., Carpenter H.A. A clinicopathologic study of 34 cases of diffuse pulmonary hemorrhage with lung biopsy confirmation. Am. J. Surg. Pathol. 1990;14(12):1112–1125. doi: 10.1097/00000478-199012000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Baumgartner M., Ashton R. Diffuse alveolar hemorrhage. South Med. J. 2011;104(4):274–275. doi: 10.1097/SMJ.0b013e3182126d6d. [DOI] [PubMed] [Google Scholar]
- 23.Escuissato D.L., Warszawiak D., Marchiori E. Differential diagnosis of diffuse alveolar haemorrhage in immunocompromised patients. Curr. Opin. Infect. Dis. 2015;28(4):337–342. doi: 10.1097/QCO.0000000000000181. [DOI] [PubMed] [Google Scholar]
- 24.Ioachimescu O.C., Stoller J.K. Diffuse alveolar hemorrhage: diagnosing it and finding the cause. Cleve Clin. J. Med. 2008;75(4) doi: 10.3949/ccjm.75.4.258. 258, 60, 64–5 passim. [DOI] [PubMed] [Google Scholar]
- 25.Frankel S.K., Schwarz M.I. The pulmonary vasculitides. Am. J. Respir. Crit. Care Med. 2012;186(3):216–224. doi: 10.1164/rccm.201203-0539CI. [DOI] [PubMed] [Google Scholar]
- 26.Cartin-Ceba R., Diaz-Caballero L., Al-Qadi M.O., Tryfon S., Fervenza F.C., Ytterberg S.R. Diffuse alveolar hemorrhage secondary to antineutrophil cytoplasmic antibody-associated vasculitis: predictors of respiratory failure and clinical outcomes. Arthritis Rheumatol. 2016;68(6):1467–1476. doi: 10.1002/art.39562. [DOI] [PubMed] [Google Scholar]
- 27.Yates M., Watts R.A., Bajema I.M., Cid M.C., Crestani B., Hauser T. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann. Rheum. Dis. 2016;75(9):1583–1594. doi: 10.1136/annrheumdis-2016-209133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.