Abstract
During the 2010 Deepwater Horizon oil well blowout in the Northern Gulf of Mexico (GoM), the application of 6.97 million litres of chemical dispersants was used at the well-head and on the sea surface to promote oil degradation and weathering of the Mississippi Canyon 252 (MC252) crude oil. Chemical dispersants encourage microbial degradation by increasing the surface area of the spilled oil, which also increases its bioavailability. However, the net beneficial effects of using chemical dispersants on spilled oil and their effects on weathering are not completely elucidated in contemporary literature. The use of simulated environmental conditions in replicate laboratory microcosm weathering experiments were employed to study the weathering of oil and the effects of dispersants on oil weathering. Fresh MC252 oil was evaporatively weathered 40% by-weight to approximate the composition of oil seen in surface slicks during the 2010 spill. This surface oil was then well mixed with two types of seawater, autoclaved artificial seawater, the abiotic control, and Gulf of Mexico seawater, the biotic experiment. Four different weathering combinations were tested: 10 mg of oil mixed in 150 ml artificial seawater (OAS) or natural (i.e., GoM) seawater (ON) and 10 mg of oil with dispersant mixed with 150 ml of artificial seawater (OASD) or natural (i.e., GoM) seawater (OND). For the treatments with dispersant (OASD and OND), the dispersant-to-oil ratio (DoR) was 1:20. The experiment was carried out over 28 days with replicates that were sacrificed on Days 0, 0.5, 3, 7, 14, 21 and 28. For the OAS and OASD treatments, abiotic weathering (i.e., evaporation) dominated the weathering process. However, the ON and OND treatments showed a dramatic and rapid decrease in total concentrations of both alkanes and aromatics with biodegradation dominating the weathering process. Further, there were no identifiable differences in the observed weathering patterns between microcosms using oil or oil treated with dispersant. In the biotic weathering microcosms, the relative degree of individual polycyclic aromatic hydrocarbon (PAH) depletion decreases with an increase in rings and within a homolog series (increased alkylation). The n-C17/pristane and n-C18/phytane ratios rapidly decreased compared to the abiotic weathering experiments. The C2-dibenzothiophenes (DBT)/C2-phenanthrenes (D2/P2) and C3-DBTs/C3-phenanthrenes (D3/P3) ratios initially remained constant during the early stages of weathering and then increased with time showing preferential weathering of the sulfur containing compounds compared to similar sized PAH compounds. These ratios in the abiotic microcosms remained constant over 28 days. Additionally, twenty-four quantitative MC252 oil biomarker ratios were evaluated to determine if their usefulness as oil source-fingerprinting tools were compromised after significant weathering and dispersant augmentation.
Keywords: Environmental science, Analytical chemistry
1. Introduction
Crude oil enters marine environments through natural hydrocarbon seeps at a global rate of approximately 700.3 million litres per year (Kvenvolden and Cooper, 2003) (National Research Council, 2003). The Gulf of Mexico (GoM) is an area that has abundant natural oil seepage and, because of this, microbial communities exist in GoM water that specialize in degrading hydrocarbons associated with crude oil (Head et al., 2006) (Hazen et al., 2010). The well blowout that followed the explosion and sinking of the Deepwater Horizon (DWH) drilling rig in 2010 released over 3 million barrels of oil into the GoM (Wade et al., 2016). Chemical dispersants were applied during this response with the goal of minimizing the volume of MC252 oil that could have potentially impacted the coastline by dissipating the oil offshore and encouraging natural biodegradation prior to shoreline oiling (National Commission on the BP Deepwater Horizon Oil Spill and Offshore Drilling, 2011). The application of chemical dispersants during oil spills is traditionally designed to disperse surface oil slicks, reduce oil delivery to shoreline habitats, and increase dissolved oil concentrations in the water column (Lee et al., 1985) (Venosa and Holder, 2007) (Lee et al., 2013). These actions, in turn, increase crude oil bioavailability and thus stimulate the biodegradation and weathering process (Lunel et al., 1996) (Mu et al., 2014) (Prince and Butler, 2014). However, the usefulness of chemical dispersants is routinely disputed because of documented deleterious environmental effects that are concisely summarized by the National Research Council report titled Oil Spill Dispersants: Efficacy and Effects (National Research Council, 2005). The decision to use chemical dispersants during an oil spill does not come lightly and often necessitates making ecological compromises that require an objective evaluation of the benefits versus the possible negative impacts from dispersant usage (Peterson et al., 2012).
Due to the magnitude of the Deepwater Horizon blowout and spill and accompanying large-scale dispersant applications, much interest has been generated concerning the effects of chemical dispersants on the weathering of MC252 oil (Turner et al., 2014). Weathering of spilled crude oil is a combination of chemical, physical and biological processes that includes evaporation, dissolution, emulsification, biodegradation, and photo-oxidation (Wang et al., 1998) (King et al., 2014) (Turner et al., 2014). The extent of the weathering can significantly change the chemical composition of the oil, and therefore changes the toxicity and routes of exposure of oil spilled into marine environments, washed into salt marshes, or deposited in sediments (Tarr et al., 2016). Therefore, it is important to understand the weathering progression of MC252 oil in both the presence and absence of dispersant (National Commission on the BP Deepwater Horizon Oil Spill and Offshore Drilling, 2011).
Laboratory experiments were used to characterize the degradation and weathering of MC252 oil and MC252 oil treated with Corexit 9500 dispersant in artificial sterilized seawater and in natural GoM seawater treatments. The MC252 oil used in these experiments was evaporatively weathered by 40% loss in weight to approximately simulate surface oil composition that was dispersed during the spill. Assessment of the weathering progression was accomplished by quantitating the target alkanes, target PAHs, and their respective depletion percentages over time. Additionally, several hydrocarbon ratios were used to assess how the oil’s composition changed over time. These include the ratios of n-C17/pristane, n-C18/phytane, C2-DBTs/C2-phenanthrenes, C3-DBTs/C3-phenanthrenes, and several forensic biomarker compound ratios (i.e., within the m/z values of 191, 217, 218, and 231) (Overton et al., 1981) (Wang et al., 1994) (Wang et al., 2006) (Stout and Wang, 2007) (Hansen et al., 2007) (Wang et al., 1994).
Our work attempts to answer four key research questions: 1) What are the observable degradation/weathering patterns and depletion percentages of specific alkane and polycyclic aromatic hydrocarbons with and without the addition of dispersant? 2) Does the addition of dispersants impact (either positively or negatively) crude oil degradation? 3) What are the differences between biotic and abiotic degradation? 4) Can certain hydrocarbon ratios be used throughout the weathering process to forensically identify oil, and if so, are there limits to the application of those ratios?
2. Materials and methods
2.1. Solvents, reagents, and chemicals
MC252 source oil was collected by British Petroleum (BP) through a riser vent pipe from the damaged wellhead of the DWH drilling rig in the GoM on May 20, 2010 and was stored at 4 °C in our laboratory. The dispersant Corexit 9500A (Batch # SC0E 2290) was donated by NALCO Company, Asbury, NJ. Artificial seawater was prepared by dissolving 680 g of Instant Ocean (Aquarium System, Inc. Mentor, Ohio) in 18.9 litres of deionized water yielding a specific gravity of 1.022 at 23 °C. All artificial seawater was then automiclaved at 121 °C under pressure for 30 min to ensure all forms of microbial life were eliminated. Natural seawater was collected off of the coast of Grand Isle, Louisiana in June 2012. Dichloromethane (DCM) was purchased from Sigma-Aldrich (>99.9% purity, pesticide grade). The oil analysis standard used for instrument calibration was purchased from Absolute Standards, Hamden, CT. Internal standards (used for quantification) were naphthalene-d8, acenaphthene-d10, chrysene-d12, and perlyene-d12 (AccuStandard, New Haven, CT). The internal standards were bought and stored individually until they were used to create a stock internal standard solution at a concentration of 1000 μg/mL. The surrogate spiking standards (used for method recovery) were 5-alpha androstane (AccuStandard) and phenanthrene-d10 neat (Sigma-Aldrich, St. Louis, MO). The surrogate spiking standards were bought and stored individually until they were used to create a stock surrogate standard solution at a concentration of 20 μg/mL.
2.2. Nutrient solution preparation
Nutrient solution was prepared according to 40 CFR Appendix C to Part 300 section 4 − Bioremediation Agent Effectiveness Test method (The United States Government Publishing Office, 2011). All nutrient chemicals were purchased from Fisher Scientific, Waltham, MA. A nitrogen and phosphorus salt stock solution was made by dissolving Na2HPO4.2H2O (18.40 g) and KNO3 (76.30 g) into 1000 ml distilled water with a final pH of 7.8. A mineral-trace element stock solution was made by adding MgSO4.7H2O (22.50 g), CaCl2.2H2O (27.50 g), FeCl3.6H2O (0.25 g), MnSO2.H2O (30.2 mg), H3Bo3 (57.2 mg), ZnSO4.7H2O (42.8 mg) and (NH4)6Mo7O24.H2O (34.7 mg) into 1000 ml distilled water. The final nutrient solution was then prepared by adding 10 ml of nitrogen and phosphorus salt stock solution and 2 ml of mineral-trace element stock solution into 1000 ml seawater immediately prior to the weathering experiment.
2.3. Oil preparation
Several physical processes, including evaporation, aqueous dissolution, emulsification, and dispersion, concurrently act on the composition and properties of oil when it is released to a marine environment (National Research Council, 2003). Evaporation can eliminate as much as 75% of the oil volume for light crude within a few days and as much as 40% for medium crude oils in the same time period (Fingas, 1997). Surface oil can have heavy losses (i.e., reduced by greater than fifty percent) in volatile and soluble hydrocarbons (≤ n-C17) within the first 25 h after an oil spill (Gros et al., 2014). Based on this information we began this experiment with laboratory weathered MC252 that had been reduced in mass by heating to 27 °C and slowly mixing the oil until 40% of the mass was depleted.
2.4. Oil-dispersant mixture
The oil-dispersant mixture was prepared using a 1:20 DoR ratio (Page et al., 2002). To be specific, 1 ml Corexit 9500A and 20 ml weathered MC252 oil were added to a 40 ml volatile organic analysis (VOA) vial. This solution was then manually agitated to mix the oil and dispersant prior to the weathering experiment.
2.5. Calibration of micro-pipettes
Since we were studying the degradation patterns and depletion percentage of oil and oil-dispersant mixtures in biotic and abiotic aqueous solutions under near ideal degradation conditions, it was necessary to keep the mass of the oil added to each Erlenmeyer flask consistent and accurate. A 50 μl micro-pipette tip was used to transfer approximately 10 mg of oil by mass to the 250 ml Erlenmeyer flask containing 150 ml of either artificial sterile seawater or GoM seawater. The mass of the pipetted oil was calibrated using 10 replicates of the dispersed-oil and oil-only samples with an analytical balance (Table 1). The overall goal was to have a water column concentrations of oil and oil-dispersant mixtures in the microcosm experiments that were similar to conditions reported in DWH spill after the oil had been dispersed (Lee et al., 2013) (Wade et al., 2016). 10 mg of oil or oil-dispersant mixture were added to 150 ml of seawater resulting in an initial concentrations of approximately 67 ppm for each flask. The microcosm weathering experiments were carried out at 25 °C for 28 days on a shaker table set to 100 rpm. Table 2 provides an outline of the results of several oil degradation studies, as well as the parameters used for characterization and analysis of oil and petroleum based compounds, in the recent literature. Our initial concentration was a moderate amount of oil, falling well below some studies and well above other oil degradation studies as outlined in Table 2.
Table 1.
Calibration of dispensed 40% laboratory weathered MC252 oil.
| Test # | Weight of dispersed oil (g) | Weight of oil (g) |
|---|---|---|
| 1 | 0.0102 | 0.0107 |
| 2 | 0.0104 | 0.0110 |
| 3 | 0.0099 | 0.0106 |
| 4 | 0.0101 | 0.0101 |
| 5 | 0.0101 | 0.0102 |
| 6 | 0.0100 | 0.0109 |
| 7 | 0.0095 | 0.0101 |
| 8 | 0.0104 | 0.0099 |
| 9 | 0.0098 | 0.0106 |
| 10 | 0.0102 | 0.0103 |
| Average | 0.0101 | 0.0104 |
Table 2.
Peer-reviewed literature on oil biodegradation in seawater.
| Ref | Microbial Influence | Source of Seawater | Temp °C | Oil (ppm) | Half-Life (days) | Analytes Measured |
|---|---|---|---|---|---|---|
| Siron et al., 1995 | Natural | St Lawrence Estuary | 4 | 67 | 2.1 | Soluble Phenanthrenes |
| Brakstad and Faksness, 2000 | Natural | Trondheim Fjord | 13 | 1000 | 2.6 | nC5-nC9 Alkanes and Aromatics |
| Venosa and Holder, 2007 | Addition from Prince William Sound, AK | Artificial | 20 | 70 | 6 | nC10-nC35 |
| 3 | ||||||
| 5 | 10 | |||||
| 4 | ||||||
| Prince et al., 2007 | Natural | New Jersey | 21 | 70 | 5 | Hydrocarbons greater than nC12 |
| Hazen et al., 2010 | Natural | Gulf of Mexico | 5 | N/A | 3 | nC13-nC26 |
| Campo et al., 2013 | Addition from Macondo Wellhead | Artificial | 25 | 700 | 2 | nC10-nC35 |
| 0.4 | ||||||
| 5 | 12 | |||||
| 2.8 | ||||||
| Prince et al., 2013 | Natural | New Jersey | 8 | 2.5 | 2.5 | nC18 |
| 13 | and Total Hydrocarbons | |||||
| 2.2 | nC18 | |||||
| 11 | and Total Hydrocarbons | |||||
| Prince and Butler, 2014 | Natural | New Jersey | 21 | 2.5 | 8 | Total Hydrocarbons |
| 260 | ||||||
| Wang et al., 2016 | Natural | Gulf of Mexico (deep-water) | 5 | 2 | 2-16 after a several day lag |
Saturated and Aromatic Hydrocarbons |
| Zhuang et al., 2016 | Addition from Macondo Wellhead | Artificial | 25 | 830 | 3 | Total Alkanes |
| 5 | ||||||
| 5 | 25 | |||||
| 11 |
2.6. Weathering condition (OAS, OASD, ON, and OND) set-up
Four experimental treatments, labeled OAS, OASD, ON, and OND, were used to investigate the weathering effects on the chemical composition of surface oil (i.e., 40% evaporatively weathered MC252 oil) in the absence and presence of dispersant. Experiments were initiated by adding the appropriate solutions (Table 3) to each Erlenmeyer flask, and then ensuring the oil-seawater solutions were well mixed using an orbital shaker (New Brunswick Scientific, Edison N.J) with a constant agitation speed of 100 rpm. Microcosms were run in triplicate, and were removed from the orbital shaker on days 0, 0.5, 3, 7, 14, 21, and 28. The entire contents of each microcosm flask were extracted and chemically analyzed.
Table 3.
Weathering treatment set-up.
| Weathering Process | Oil | Nutrient | Aqueous phase |
|---|---|---|---|
| OAS | 10 mg Lab Weathered Oil | Yes | 150 ml Artificial sea water |
| OASD | 10 mg Dispersed Lab Weathered Oil | Yes | 150 ml Artificial sea water |
| ON | 10 mg Lab Weathered Oil | Yes | 150 ml Natural sea water |
| OND | 10 mg Dispersed Lab Weathered Oil | Yes | 150 ml Natural sea water |
2.7. Oil extraction procedure
After the flasks were removed from the orbital shaker, 15 ml aliquots of DCM were added to each Erlenmeyer flask along with 1 mL of surrogate standard solution. They were then mixed manually and allowed to settle. DCM aliquots were removed with 10 ml glass pipettes. The DCM extracts were then transferred through a pre-cleaned, anhydrous Na2SO4 (Sigma-Aldrich) filter into a 250 ml flat-bottom flask. This extraction procedure was repeated two more times for a total of three extractions of each treatment flask, with the surrogate standard solution being added on the first extraction only. Each extraction aliquot was then concentrated to a final volume of 2 mL using a combination of rotary evaporation and nitrogen gas blow-down. One milliliter of this extract was then transferred to a 2 mL amber autosampler GC/MS vial and 10 μL of internal standard solution was added prior to GC/MS analysis. One microliter of these solutions were injected for GC/MS analyses.
2.8. Analyte removal percentages
The analyte removal percentage was calculated by:
% analyte removal = (1-Ci/C0) × 100
where Ci is the analyte concentration at each experimental time period, and C0 is the initial analyte concentration at day 0. Initial and final concentrations were determined by GC/MS analyses of the extracted oil residues. Analyte removal percentage was calculated as a way to determine remediation efficacy (Lin and Mendelssohn, 2009).
2.9. GC/MS and quantitative analysis
Chemical analyses were performed using an Agilent 7890A gas chromatograph (GC) equipped with a Agilent 5975C inert XL mass selective detector (MSD) and fitted with a Zebron-5MS high resolution capillary column (30 m long x 250 mm diameter x 0.25 μm thick film). The carrier gas was ultrahigh purity helium (Air Liquide, Houston, TX) at a constant flow rate of 1 ml min−1. An Agilent 7683B autosampler was used for making splitless injections. The injector port was set at 280 °C and was fitted with an Agilent deactivated borosilicate liner. The oven temperature program was as follows: the initial temperature was set to 60 °C and was held for 3 min; the temperature was then increased to 280 °C at a rate of 5 °C min−1 and held for 3 min. The oven was then heated from 280 °C to 300 °C at a rate of 1.5 °C per min and held at 300 °C for 2 min. The temperature of the MSD interface to MS was set at 300 °C. The mass spectrometer had an ion source temperature of 230 °C, quadrupole temperature of 150 °C, and ionization energy of 70 eV. The MSD was operated in the selective ion monitoring (SIM) mode for quantifying specific alkanes, PAHs and oil biomarkers. Quantitative analysis of targeted petrogenic compounds was performed using average response factors calculated from a five point oil analysis standard curve. The oil analysis standard (Absolute Standards, Hamden, CT) contained saturated alkanes in the range of n-C10 through n-C35 and the unsubstituted parent PAHs with two to six rings. Alkylated PAH homologs were quantified using the response factors generated from the unsubstituted parent PAH compounds as alkylated homolog standards were not commercially available for all targeted compounds of interest.
2.10. Quality assurance/quality control
A two compound surrogate standard was used for calculating extraction efficiencies. 5-alpha androstane was used to determine the recovery of alkanes, and phenanthrene-d10 was used to determine the recovery of PAHs. The acceptable recovery range is 70–120% according to U.S. EPA SW-846 method requirements (Turner et al., 2014) (USEPA, 2016). A commercially-prepared oil analysis standard (Absolute Standards, Hamden, CT), was used to prepare a five-point calibration curve and calculate average response factors for each analyte. The percent relative standard deviation (%RSD, also known as coefficient of variation) was calculated using the average analyte response factor and standard deviation. The acceptable QA/QC limits for the %RSD each analyte was less than 15%. The calibration standards were checked frequently for signs of degradation or evaporation, and replaced if necessary based on daily laboratory QC checks. A continuing calibration standard (i.e., one point of the initial five-point calibration curve) was analyzed with each batch of extracted samples, or during each 12-h period during which analyses were performed. Acceptance criterion for the continuing calibration standard was ±20% of the average relative response factor calculated from the initial five-point curve. An extract of MC252 oil was also analyzed with each sample batch as another QC criteria. The MS was tuned to PFTBA (perfluorotributylamine) before each set of analyses. No samples were analyzed if any of the tune parameters were outside of acceptable limits. All the weathering experiments were conducted in triplicate, and the average values and standard deviations of specific alkanes and PAHs were calculated.
2.11. Diagnostic ratio analysis
The ratios n-C17 heptadecane/pristane (n-C17/pris) and n-C18 octadecane/phytane (n-C18/phy), along with alkylated PAH ratios of C2-dibenzothiophenes (DBT)/C2-phenanthrenes (D2/P2) and C3-DBTs/C3-phenanthrenes (D3/P3) can be used to differentiate physical weathering from microbial weathering. If the normal alkanes n-C17 and n-C18 are degraded faster than their respective isoprenoid hydrocarbon counterparts, this indicates microbial (i.e., biotic) weathering. Moreover, if the isoprenoid pristane is lost faster than phytane, or n-C17 is lost faster than n-C18, this indicates abiotic evaporative weathering. Frequently, natural weathering processes of surface oil includes evaporation, dissolution, and biotic weathering (and photo oxidation to some degree). These ratios, however, can be rapidly degraded to below detection limits (Atlas et al., 2015) and, therefore, have limitations when assessing heavily weathered environmental samples (Overton et al., 1981).
An additional indicator of microbial degradation is the preferential degradation of sulfur containing versus non-sulfur containing alkylated PAHs, specifically, the relative quantities of the C2-DBTs/C2-phenanthrenes and C3-DBTs/C3-phenanthrenes. The degradation of both C2 and C3-DBTs as compared to the degradation of non-sulfur containing alkylated C2 and C3-phenanthrenes (which are of similar molecular weights) is known to follow several bacterial biotransformation pathways (Seo et al., 2009). Therefore, significant ratio changes over time (whether positive or negative) is indicative of microbial degradation and conversely, no change over time is indicative of only physical degradation.
Further, oil source-fingerprinting was accomplished by identifying and measuring peak heights of key forensic and recalcitrant biomarker compounds within MC252 oil (Boehm et al., 1997) (Wang and Fingas, 2003) (Stout and Wang, 2007) (Meyer et al., 2014). Establishing a set of selected biomarker compounds that have quantitative ratios which are unique to MC252 oil is a critical step in the oil source-fingerprinting process (Stout et al., 2016). All oils generally contain the same mix of hydrocarbon compounds, but oils from different sources contain these compounds in varying and distinct quantities. Because of this, oil biomarker compound ratios are used for selective oil source characterization and identification (Stout et al., 2016). Specifically, the extracted ion chromatograms (EIC) were isolated for compounds within the hopanes (m/z 191 EICs), the diasteranes and regular steranes (m/z 217 EICs), the 14β(H)-steranes (m/z 218 EICs), and the tri-aromatic steroids (m/z 231 EICs) families in MC252 unweathered source oil. These compounds have to be identified and their peak heights measured (Table 4). Once these measurements are complete, ratios of biomarker compounds can be generated and statistically compared. Only those ratios that meet the statical criteria set forth by Hansen et al., 2007 are considered to be diagnostically viable. This means that the numerical values from at least 7 replicate GC/MS analyses of the source oil biomarker ratios must meet a specific criteria of < 5% RSD.
Table 4.
List of biomarker analytes found in MC252 with their respective quantitation ions (m/z) and retention times.
| Name | m/z | Ret Time | Name | m/z | Ret Time |
|---|---|---|---|---|---|
| (TC28R) Tricyclic Triterpane (22R) | 191 | 45.32 | (C27dbS) 13beta,17alpha-diacholestane (20S) | 217 | 43.87 |
| (TC28S) Tricyclic Triterpane (22S) | 191 | 45.52 | (C27dbR) 13beta,17alpha-diacholestane (20R) | 217 | 44.37 |
| (TC29R) Tricyclic Triterpane (22R) | 191 | 46.14 | (C27aaS) 5alpha,14alpha,17alpha-cholestane (20S) | 217 | 46.16 |
| (TC29S) Tricyclic Triterpane (22S) | 191 | 46.38 | (C29DbaS) 13beta,17alpha-diaethylcholestane (20S) | 217 | 46.27 |
| (C27Ts) 18alpha(H)-22,29,30-trisnorhopane | 191 | 47.16 | (C27aaR) 5alpha,14alpha,17alpha-cholestane (20R) | 217 | 46.73 |
| (C27Tm) 17alpha(H)-22,29,30-trisnorhopane | 191 | 47.72 | (C29DbaR) 13beta,17alpha-diaethylcholestane (20R) | 217 | 46.82 |
| (C28ab) 17alpha(H), 21beta(H)-28,30-bisnorhopane | 191 | 48.69 | (C28aaS) 5alpha,14alpha,17alpha,24-methylcholestane (20S) | 217 | 47.57 |
| (C25norC29ab) 17alpha(H),21beta(H)-25-norhopane | 191 | 49.06 | (C28bbS) 5alpha,14beta,17beta,24-methylcholestane (20S) | 217 | 47.70 |
| (C29ab) 17alpha(H)21beta(H)-30-norhopane | 191 | 49.77 | (C28bbR) 5alpha,14beta,17beta,24-methylcholestane (20R) | 217 | 47.81 |
| (C29Ts) 18alpha(H)-30-norneohopane | 191 | 49.87 | (C28aaR) 5alpha,14alpha,17alpha,24-methylcholestane (20R) | 217 | 48.27 |
| (C30d) 15alpha-methyl-17alpha(H)-27-norhopane (diahopane) | 191 | 50.10 | (C29aaS) 5alpha,14alpha,17alpha,24-ethylcholestane (20S) | 217 | 48.69 |
| (C29ba) 17beta(H),21alpha(H)-normoretane | 191 | 50.58 | (C29bbR) 5alpha,14beta,17beta,24-ethylcholestane (20R-m/z 217) | 217 | 48.94 |
| (C30 O) 18alpha(H) and 18beta(H) oleanane | 191 | N/A | (C29bbS) 5alpha,14beta,17beta,24-ethylcholestane (20S-m/z 217) | 217 | 49.02 |
| (C30ab) 17alpha(H),21beta(H)-hopane | 191 | 51.18 | (C29aaR) 5alpha,14alpha,17alpha,24-ethylcholestane (20R) | 217 | 49.65 |
| (C30ba) 17beta(H),21alpha(H)-moretane | 191 | 51.87 | (C27bbR) 5alpha,14beta,17beta-cholestane (20R) | 218 | 46.27 |
| (C31abS) 22S-17alpha(H),21beta(H)-30-homohopane | 191 | 52.99 | (C27bbS) 5alpha,14beta,17beta-cholestane (20S) | 218 | 46.39 |
| (C31abR) 22R-17alpha(H),21beta(H)-30-homohopane | 191 | 53.23 | (C28bbR) 5alpha,14beta,17beta,24-methylcholestane (20R) | 218 | 47.70 |
| (C30G) Gammacerane | 191 | N/A | (C28bbS) 5alpha,14beta,17beta,24-methylcholestane (20S) | 218 | 47.81 |
| (C32abS) 22S-17alpha(H),21beta(H)-30-bishomohopane | 191 | 54.55 | (C29bbR) 5alpha,14beta,17beta,24-ethylcholestane (20R-m/z 218) | 218 | 48.95 |
| (C32abR) 22R-17alpha(H),21beta(H)-30-bishomohopane | 191 | 54.90 | (C29bbS) 5alpha,14beta,17beta,24-ethylcholestane (20S-m/z 218) | 218 | 49.04 |
| (C33abS) 22S-17alpha(H),21beta(H)-30-trihomohopane | 191 | 56.50 | (C20TA) C20-Triaromatic Steroids | 231 | 40.65 |
| (C33abR) 22R-17alpha(H),21beta(H)-30-trihomohopane | 191 | 57.03 | (C21TA) C21-Triaromatic Steroids | 231 | 42.13 |
| (C34abS) 22S-17alpha(H),21beta(H)-30-tetrahomohopane | 191 | 58.76 | (SC26TA) C26,20S-Triaromatic Steroids | 231 | 47.63 |
| (C34abR) 22R-17alpha(H),21beta(H)-30-tetrahomohopane | 191 | 59.45 | (RC26TA + SC27TA) C2620R + C27,20S-Triaromatic Steroids | 231 | 48.67 |
| (C35abS) 22S-17alpha(H),21beta(H)-30-pentahomohopane | 191 | 61.17 | (SC28TA) C28,20S-Triaromatic Steroids | 231 | 49.61 |
| (C35abR) 22R-17alpha(H),21beta(H)-30-pentahomohopane | 191 | 62.14 | (RC27TA) C27,20R-Triaromatic Steroids | 231 | 50.12 |
| (RC28TA) C28,20R-Triaromatic Steroids | 231 | 51.40 |
In this study, thirty-two intra-biomarker ratios (within a specific EIC i.e., ratios calculated from the quantity of specific compounds within the hopane, sterane, or triaromatic steroid families) and 14 inter-biomarker quantitative ratios (between different EICs i.e., quantitative ratios selected compounds between the hopane, sterane, or triaromatic steroid families) were examined. After calculating all of these ratios from 15 replicate analyses of the MC252 source oil, it was determined that there were a total of 24 ratios that had a %RSD less than 5%. These included 22 intra- and 2 inter-biomarker ratios (Table 5).
Table 5.
List of biomarker ratios used to identify MC252 based on the critical difference method for biomarker selection.
| Class | Diagnostic Ratio |
|---|---|
| Tri- and Pentacyclic Triterpanes (Hopanes) (m/z 191) | C27Ts/C27Tm |
| C29αβ/C29Ts | |
| C29αβ/C30αβ | |
| C31 ab(S + R)/C32 ab(S + R) + C33 ab(S + R) | |
| C32 ab(S + R)/C31 ab(S + R) + C33 ab(S + R) | |
| C33 ab(S + R)/C31 aB(S + R) + C32 ab(S + R) | |
| Rearranged and Regular 14α(H)- and 14β(H)-Steranes (m/z 217 and 218) | C27D Ba-S/C27D Ba-R |
| C29D Ba-S/C29D Ba-R | |
| C28 aaa-R/C29 aaa-R | |
| C29 aa-S/C29 aa-R | |
| C29 BB-R/C29 BB-S | |
| C29ααS/C29αα(S + R) | |
| C29ββ(R + S)/C29αα(S + R) | |
| C27 BB-R/C27 BB-S | |
| C28 BB-R/C28 BB-S | |
| C29 BB-R/C29 BB-S | |
| C27ββ(R + S)/[C28ββ(R + S) + C29ββ(R + S)] | |
| C28ββ(R + S)/[C27ββ(R + S) + C29ββ(R + S)] | |
| C29ββ(R + S)/[C27ββ(R + S) + C28ββ(R + S)] | |
| Triaromatic Steroids (m/z 231) | C20 TA/C21 TA |
| SC26TA/SC28TA | |
| RC27TA/RC28TA | |
| Inter-Ion Biomarker Ratios (m/z 218/191) | C27ββ(R + S)/C30αβ |
| C29ββ(R + S)/C30αβ |
Quantitative biomarker ratios from GC/MS analyses of the oil in various microcosm samples were then compared to the average (n = 15 in our study, listed in Table 5) MC252 source oil quantitative diagnostic ratios using the critical difference method outlined in Hansen et al., 2007. Once the absolute and critical differences are calculated for each diagnostic ratio in each sample, the oil source-fingerprinting process is completed by determining if the sample is a match, a possible match, or inconclusive. All accepted ratios (those that pass the critical difference) are totaled and divided by 24, which is the number of matching ratios within the sample divided by the total number of selected MC252 ratios used for source fingerprinting in this study and converted into a percentage. This percentage can be used as a ranking/score for assessing source oil match to environmental samples. For this study, MC252 fingerprinting categories were set so that a score of 87%−100% constituted a match, 79%–86% constituted a probable match, and a score < 79% constituted an inconclusive determination. These categories were identified by independently analyzing both fresh and heavily weathered MC252 crude oils. All experimental treatments used MC252 oil and the biomarker ratio analysis provided an idea of how weathering affected the quantitative oil source-fingerprinting results, and therefore was used to establish the fingerprinting categories. Replicate analyses (n = 12) of fresh MC252 source oil resulted in an average score of 93% (±4.0%) using the 24 diagnostic biomarker ratios (including 2 inter-ion ratios). Excluding the 2 inter-ion ratios resulted in an average score of 92% (±4.4%, n = 12). Both of these percentages would constitute a match to MC252 source oil with scores well within the range of 87%–100%. It is important to note that these fingerprinting categories were determined specifically for MC252 oil in this study; therefore, fingerprinting categories for other source oils and studies must be determined independently using the aforementioned critical difference method of biomarker ratio selection as outlined in Hansen et al., 2007.
3. Results and discussion
3.1. Quality assurance/quality control
The average surrogate recoveries for 5-alpha androstane and phenanthrene-d10 were 79% and 72%, respectively for the OAS/OASD spiking experiment, and 77% and 76%, respectively for the ONS/OND spiking experiment. All surrogate recoveries were within the EPA criteria (USEPA, 2016) (Turner et al., 2014). Examination of replicate samples showed that the %RSD were below 20% for quantitative concentrations of all targeted alkanes and PAHs in the various treatment flasks.
3.2. Total alkane concentration change over time
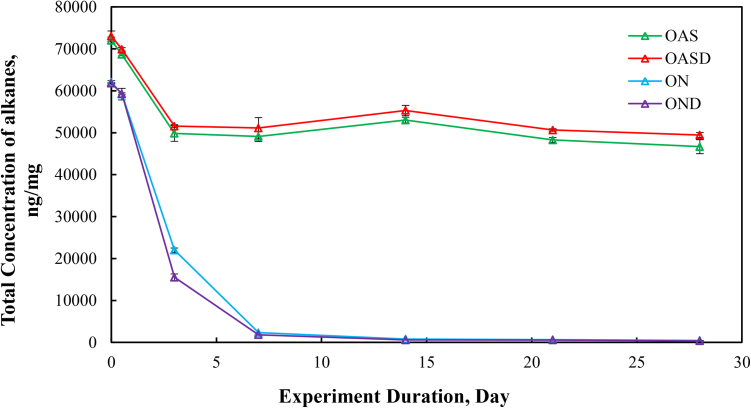
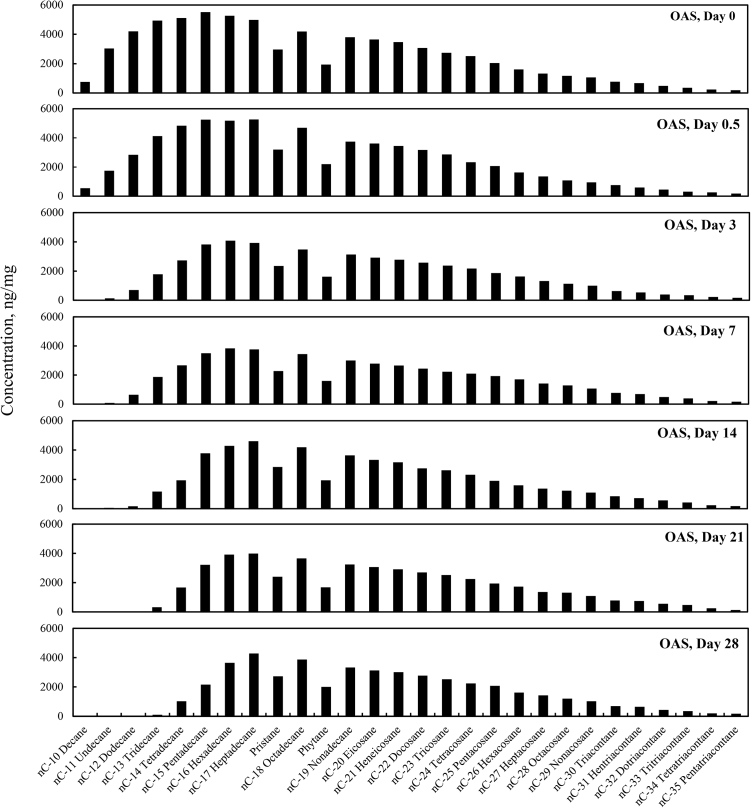
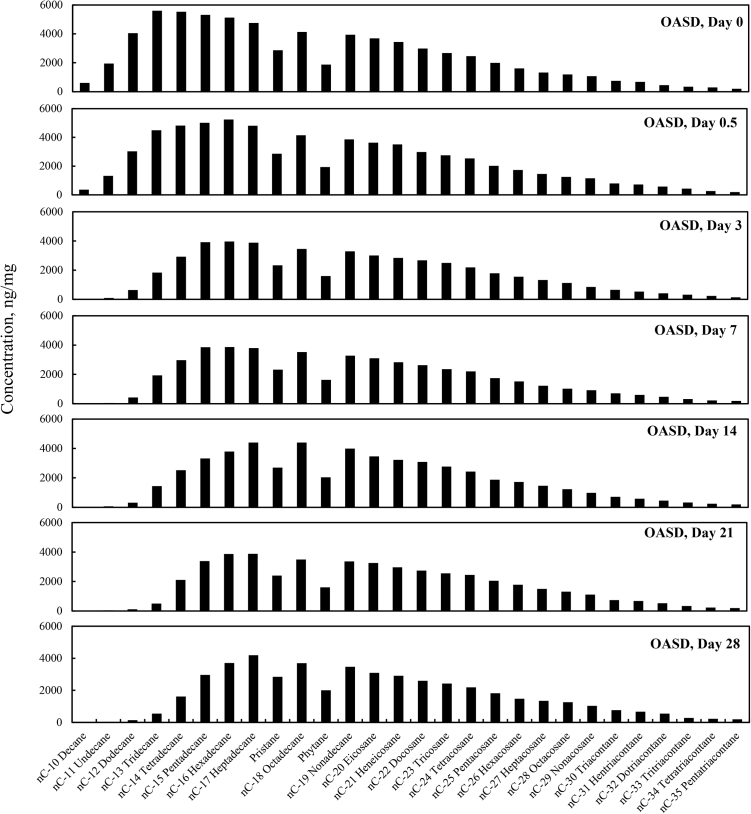
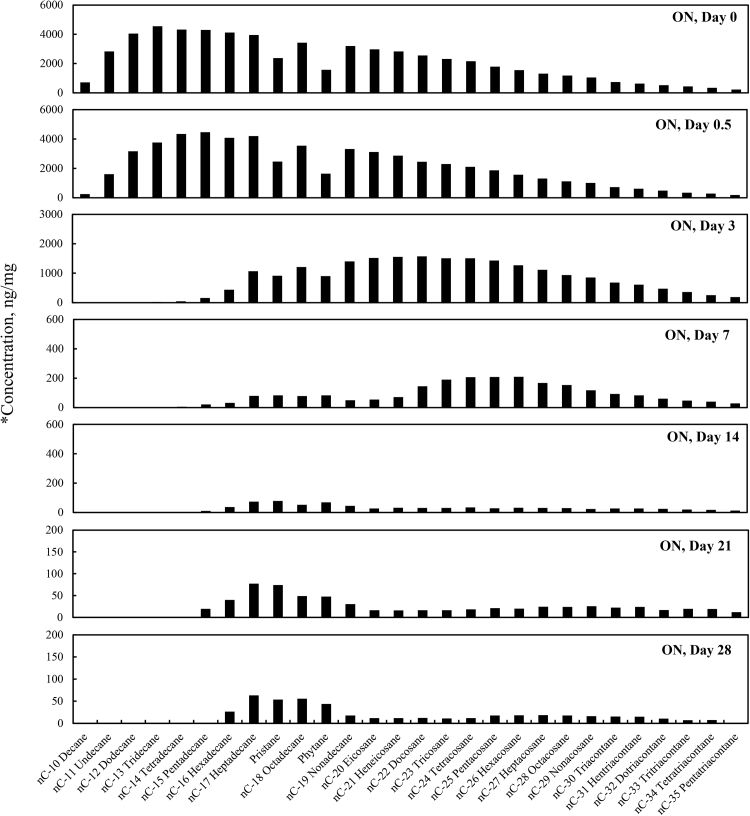
The total alkane concentrations (sum of the target alkane analytes listed in Table 6) for the four different weathering treatments (OAS, OASD, ON and OND) are summarized in Fig. 1. The total alkane concentrations decreased for all four weathering conditions, however there are very distinct differences between the artificial seawater and natural GoM seawater experiments. Since OAS and OASD were abiotic control experiments, evaporative weathering was dominant with virtually no bacterial related oil-degradation. On the contrary, ON and OND treatments used natural seawater as the weathering media, which contained natural bacteria that greatly accelerated oil weathering. Because of this, ON and OND total alkane concentrations showed a dramatic decreasing trend (Fig. 1) over the first week of the experiments. There was no distinctive profile difference between dispersant and non-dispersant treatments with regard to seawater amendments. The OAS and OASD treatments show initial evaporative loss followed by very little profile shift, which can be seen in Fig. 2 and Fig. 3, with the majority of the alkane profile changes happening for compounds n-C10 (decane) to n-C16 (hexadecane) caused by evaporation. Notice that the alkane profile shows no discernable difference between oil and dispersed oil treatments in these artificial seawater microcosms. By examining Fig. 4 and Fig. 5, it is evident that there is a clear difference between the natural seawater and artificial seawater experiments. The natural seawater treatments show significant and rapid degradation of normal alkane concentrations from n-C10 (decane) to n-C35 (pentatriacontane) over the first 3 days and this trend follows the well-established weathering pattern outlined by prior studies (Wang et al., 1994) (Boehm et al., 1997) (Wang et al., 1998) (Stout and Wang, 2007). Half of the target alkanes were lost in under 3 days of weathering.
Table 6.
Target normal alkanes and pristane/phytane with their respective quantitation ion (m/z) and retention times.
| Name | m/z | Ret Time | Name | m/z | Ret Time |
|---|---|---|---|---|---|
| nC10 Decane | 57 | 8.40 | nC22 Docosane | 57 | 36.58 |
| nC11 Undecane | 57 | 11.36 | nC23 Tricosane | 57 | 38.28 |
| nC12 Dodecane | 57 | 14.29 | nC24 Tetracosane | 57 | 39.92 |
| nC13 Tridecane | 57 | 17.09 | nC25 Pentacosane | 57 | 41.50 |
| nC14 Tetradecane | 57 | 19.74 | nC26 Hexacosane | 57 | 43.00 |
| nC15 Pentadecane | 57 | 22.25 | nC27 Heptacosane | 57 | 44.46 |
| nC16 Hexadecane | 57 | 24.60 | nC28 Octacosane | 57 | 45.86 |
| nC17 Heptadecane | 57 | 26.85 | nC29 Nonacosane | 57 | 47.23 |
| Pristane | 57 | 26.98 | nC30 Triacontane | 57 | 48.66 |
| nC18 Octadecane | 57 | 28.98 | nC31 Hentriacontane | 57 | 50.24 |
| Phytane | 57 | 29.16 | nC32 Dotriacontane | 57 | 51.97 |
| nC19 Nonadecane | 57 | 31.02 | nC33 Tritriacontane | 57 | 53.85 |
| nC20 Eicosane | 57 | 32.95 | nC34 Tetratriacontane | 57 | 55.90 |
| nC21 Heneicosane | 57 | 34.81 | nC35 Pentatriacontane | 57 | 58.12 |
Fig. 1.
Concentration of total target alkanes per milligram of weathered oil initially added to the microcosm flask: Four different weathering experiments, oil in artificial seawater (OAS), oil augmented with dispersant in artificial seawater (OASD), oil in natural seawater (ON), and oil augmented with dispersants in natural seawater (OND). The weathering experiments were run for 28 days, and microcosm flasks were extracted at days 0, 0.5, 3, 7, 14, 21 and 28.
Fig. 2.
Concentration profiles of the target normal alkanes, pristane and phytane left in MC252 oil after 0, 0.5, 3, 7, 14, 21, and 28 days of laboratory microcosm weathering using oil in artificial seawater (OAS).
Fig. 3.
Concentration profiles of the target normal alkanes, pristane and phytane left in MC252 oil after 0, 0.5, 3, 7, 14, 21, and 28 days of laboratory microcosm weathering using oil augmented with dispersant in artificial seawater (OASD).
Fig. 4.
Concentration profiles of the target normal alkanes, pristane and phytane left in MC252 oil after 0, 0.5, 3, 7, 14, 21, and 28 days of laboratory microcosm weathering using oil in natural seawater (ON). *Concentration (y-axis) adjusted to show weathering profile.
Fig. 5.
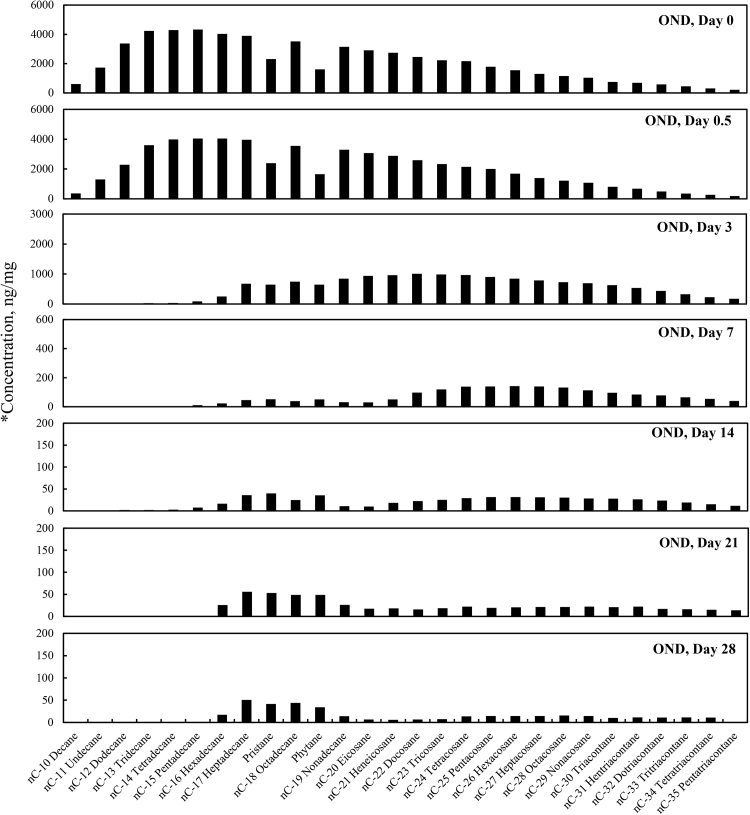
Concentration profiles of the target normal alkanes, pristane and phytane left in MC252 oil after 0, 0.5, 3, 7, 14, 21, and 28 days of laboratory microcosm weathering using oil augmented with dispersant in natural seawater (OND).*Concentration (y-axis) adjusted to show weathering profile.
3.3. Alkane removal percentages
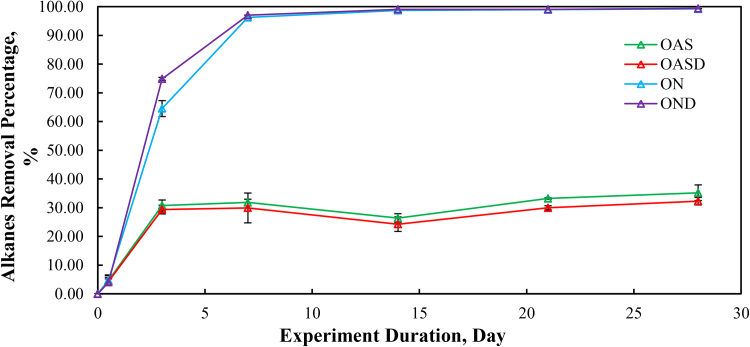
Calculated alkane removal is illustrated in Fig. 6. The ON and OND treatments showed rapid removal from day 0 to day 7. After day 7, the removal percentage stabilized around 99%. However, the evaporation dominated abiotic weathering microcosms, OAS and OASD treatments, showed a much smaller alkane removal percentage, around 30%. The OAS and OASD treatments showed very similar alkane depletions, as did ON and OND treatments. It is important to note that these reductions are measured from the initial MC252 crude that was evaporatively weathered by 40% prior to addition to the microcosm.
Fig. 6.
Percent removal of total target alkanes during the 28 days of laboratory microcosm weathering using oil and artificial seawater (OAS), oil augmented with dispersant and artificial seawater (OASD), oil and natural seawater (ON), and oil augmented with dispersants and natural seawater (OND).
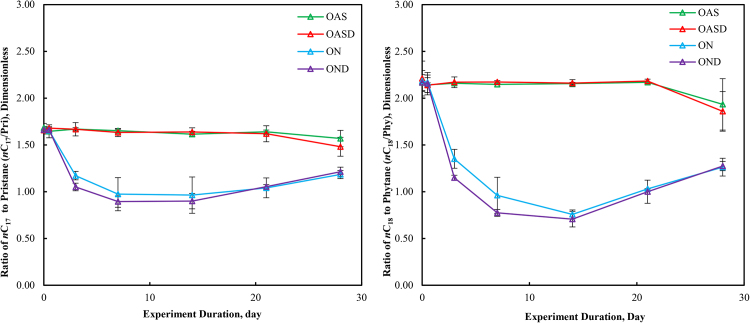
3.4. Ratio of n-C17 to pristane and n-C18 to phytane
The ratio n-C17/pris and n-C18/phy are normally used as an evaporative and biotic weathering indicator (Wang et al., 1994) (Wang et al., 2006) (Turner et al., 2014). In this study, we calculated these two ratios, and the results are summarized in Fig. 7. For OAS and OASD (abiotic treatments), the n-C17/pris and n-C18/phy ratios showed principally constant values over the 28 days of weathering. This is consistent with the notion that most non-biological fate processes (e.g. physical weathering, volatilization, dissolution, etc.) do not produce losses of normal and isoprenoid hydrocarbons at different rates (Wang and Fingas, 2003). Additionally, for ON and OND, the n-C17/pris and n-C18/phy ratios showed a rapidly decreasing trend. This is because biodegradation occurred in the ON and OND treatments, and bacteria prefer normal alkanes relative to the branched isoprenoid alkanes resulting in smaller n-C17/pris and n-C18/phy ratios. After 7 to 14 days of weathering, the quantities of these compounds in the microcosms were so low (over 99% depletion) that the ratios are not predictable of biotic and abiotic weathering.
Fig. 7.
Ratios of nC17/pristane (left) and nC18/phytane (right) over 28 days of laboratory microcosm weathering using the four experimental oil treatments (OAS, OASD, ON, and OND).
Fig. 2 through Fig. 5 show the concentration profiles of specific target alkanes over the course of these 28 day weathering microcosms. As shown in Fig. 2 and Fig. 3, after the initial loss of n-C10 to n-C15 alkanes by day 3 due to evaporative abiotic weathering, the compositions remained relatively stable through 28 days of weathering. However, the biotic weathering microcosm experiments, shown in Fig. 4 and Fig. 5, showed rapid quantitative and compositional changes, and by day 14 the amount of measured alkanes had been reduced by over 99% from the initial day zero.
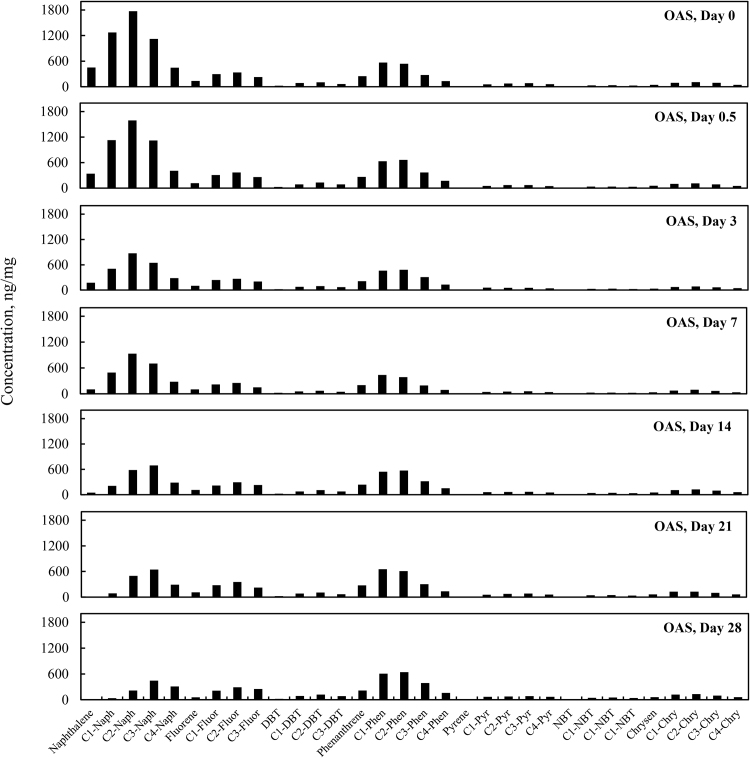
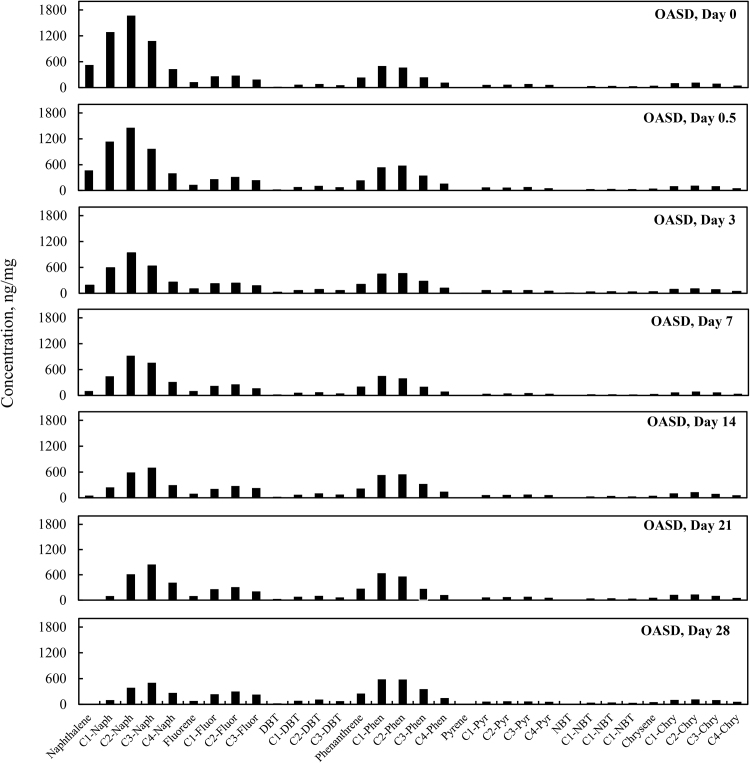
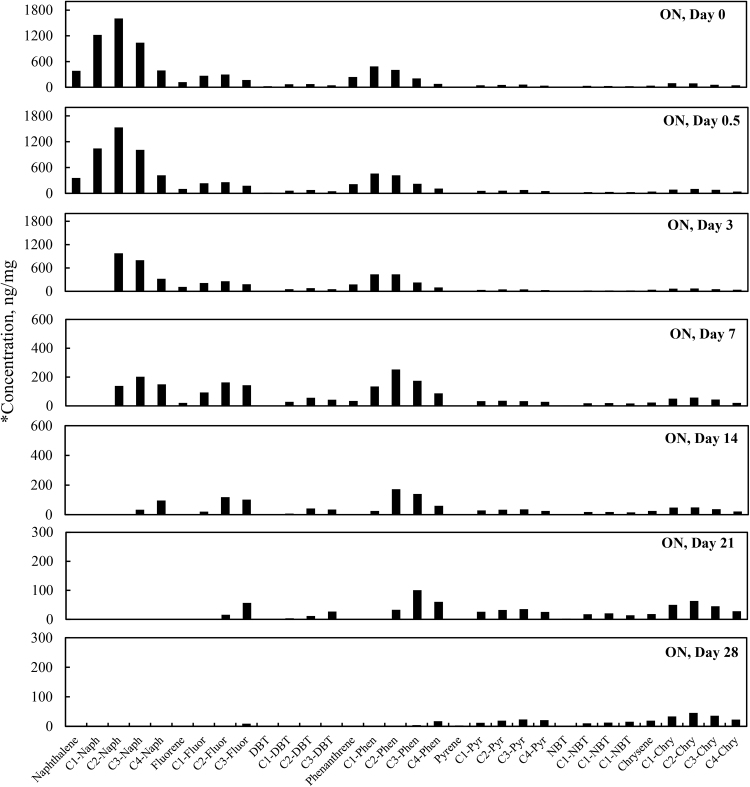
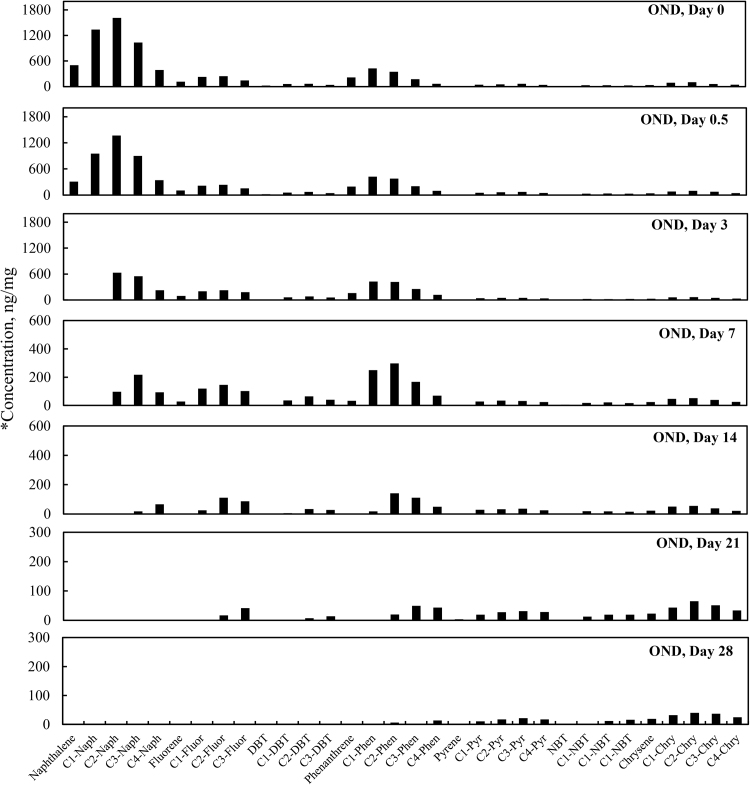
3.5. Total PAHs concentration change over time
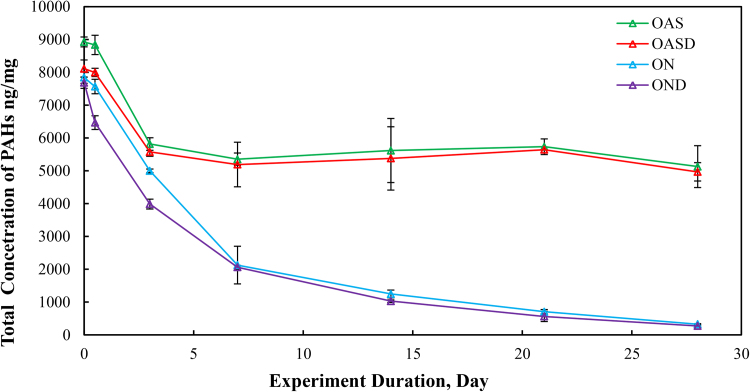
Total PAH concentrations (sum of targeted aromatic analytes listed in Table 7) for the four different weathering treatments (OAS, OASD, ON and OND) are summarized in Fig. 8. The initial total PAH concentrations decreased in Fig. 8 for all four weathering treatments, however there are very distinct differences between biotic and abiotic treatments. The evaporative dominated weathering processes in OAS and OASD treatments showed a similar decreasing trend through the first 3 days of weathering. After that time, the OAS and OASD PAH compounds remained essentially constant. These abiotic experiments were carried out with the same light/dark conditions as was used for the biotic experiments, which implies that any photo-oxidation of the oils during these experiments was insignificant. However, the ON and OND microcosms showed continually decreasing PAH levels through the end of the experiment. Looking at the PAH profiles in Fig. 9 and Fig. 10, along with the quantitative aromatic data in Fig. 8, the only observable change in total targeted PAH concentrations in these abiotic microcosms comes from naphthalene and its alkyl homologs (C1-C4) in the OAS and OASD treatments. Notice that the PAH profiles show no discernable difference between oil and dispersed oil treatments in artificial seawater. However, by examining Fig. 11 and Fig. 12, the biotic ON and OND experiments showed a dramatic decreasing trend in total targeted PAHs during the first week of weathering and then continued a steady decreasing trend over the remaining three weeks for the experiment. This trend follows well-established weathering pattern outlined by prior studies (Wang et al., 1994) (Boehm et al., 1997) (Wang et al., 1998) (Stout and Wang, 2007). As stated previously, the total PAH concentrations in Fig. 8 decreased for all four weathering conditions, however there are very distinct profile differences between artificial seawater (abiotic) and natural GoM seawater (biotic). Since OAS and OASD were abiotic control experiments, evaporative weathering was dominant with no discernable bacterial related oil degradation. Only the lighter molecular weight aromatics and their homologs decreased in concentration. However, the ON and OND treatments used natural seawater as the weathering media, which contained natural bacteria that greatly accelerated PAH weathering, specifically through the naphthalene, fluorene, dibenzothiophene, and phenanthrene PAHs and their alkylated homolog families. Additionally, the profiles of the treatments amended with GoM seawater show preferential degradation of parent and C1 alkyl homologs relative to the higher alkylated homologs within each PAH family. Looking at day 21 for both ON and OND; C3-fluorene, C3-DBT, C3-phenanthrene, and C4-phenanthrene maintain a fairly constant profile from preceding days. PAHs with 4 or more rings showed little degradation over time. ON and OND total PAH concentrations showed a dramatic decreasing trend as seen in Fig. 8 over the course of the experiment with regard to lighter molecular weight PAHs. As with alkanes, there was no discernable difference between dispersant and non-dispersant treatment PAH profiles with regard to seawater amendments.
Table 7.
Target petrogenic polycyclic aromatic hydrocarbons (PAHs) with their respective quantitation ions (m/z) and retention times.
| Name | m/z | Ret time | Name | m/z | Ret time |
|---|---|---|---|---|---|
| Naphthalene | 128 | 12.86 | Anthracene | 178 | 27.98 |
| C1-Naphthalenes | 142 | 16.01 | Fluoranthene | 202 | 33.87 |
| C2-Naphthalenes | 156 | 19.35 | Pyrene | 202 | 34.32 |
| C3-Naphthalenes | 170 | 22.14 | C1-Pyrenes | 216 | 36.09 |
| C4-Naphthalenes | 184 | 25.41 | C2-Pyrenes | 230 | 38.29 |
| Fluorene | 166 | 23.37 | C3-Pyrenes | 244 | 40.72 |
| C1-Fluorenes | 180 | 26.17 | C4-Pyrenes | 258 | 42.40 |
| C2-Fluorenes | 194 | 28.81 | Naphthobenzothiophene | 234 | 38.94 |
| C3-Fluorenes | 208 | 31.04 | C1-Naphthobenzothiophenes | 248 | 40.66 |
| Dibenzothiophene | 184 | 27.19 | C2-Naphthobenzothiophenes | 262 | 42.52 |
| C1-Dibenzothiophenes | 198 | 29.31 | C3-Naphthobenzothiophenes | 276 | 44.70 |
| C2-Dibenzothiophenes | 121 | 31.33 | Benzo[a]Anthracene | 228 | 40.09 |
| C3-Dibenzothiophenes | 226 | 33.54 | Chrysene | 228 | 40.24 |
| Phenanthrene | 178 | 27.77 | C1-Chrysenes | 242 | 42.09 |
| C1-Phenanthrenes | 192 | 30.56 | C2-Chrysenes | 256 | 43.88 |
| C2-Phenanthrenes | 206 | 32.87 | C3-Chrysenes | 270 | 46.16 |
| C3-Phenanthrenes | 220 | 35.10 | C4-Chrysenes | 284 | 47.68 |
| C4-Phenanthrenes | 234 | 37.74 |
Fig. 8.
Concentration of total target polycyclic aromatic hydrocarbons (PAHs) per milligram of weathered oil initially added to the microcosm flask: Four different weathering experiments, oil in artificial seawater (OAS), oil augmented with dispersant in artificial seawater (OASD), oil in natural seawater (ON), and oil augmented with dispersant in natural seawater (OND). The weathering experiments were run for 28 days, and microcosm flasks were extracted at days 0, 0.5, 3, 7, 14, 21 and 28.
Fig. 9.
Concentration profiles of specific petrogenic polycyclic aromatic hydrocarbons (PAHs) left in MC252 oil after 0, 0.5, 3, 7, 14, 21, and 28 days of laboratory microcosm weathering using oil in artificial seawater (OAS).
Fig. 10.
Concentration profiles of specific petrogenic polycyclic aromatic hydrocarbons (PAHs) left in MC252 oil after 0, 0.5, 3, 7, 14, 21, and 28 days of laboratory microcosm weathering using oil augmented with dispersant in artificial seawater (OASD).
Fig. 11.
Concentration profiles of specific petrogenic polycyclic aromatic hydrocarbons (PAHs) left in MC252 oil after 0, 0.5, 3, 7, 14, 21, and 28 days of laboratory microcosm weathering using oil in natural seawater (ON). *Concentration (y-axis) adjusted to show weathering profile.
Fig. 12.
Concentration profiles of specific petrogenic polycyclic aromatic hydrocarbons (PAHs) left in MC252 oil after 0, 0.5, 3, 7, 14, 21, and 28 days of laboratory microcosm weathering using oil augmented with dispersant in natural seawater (OND). *Concentration (y-axis) adjusted to show weathering profile.
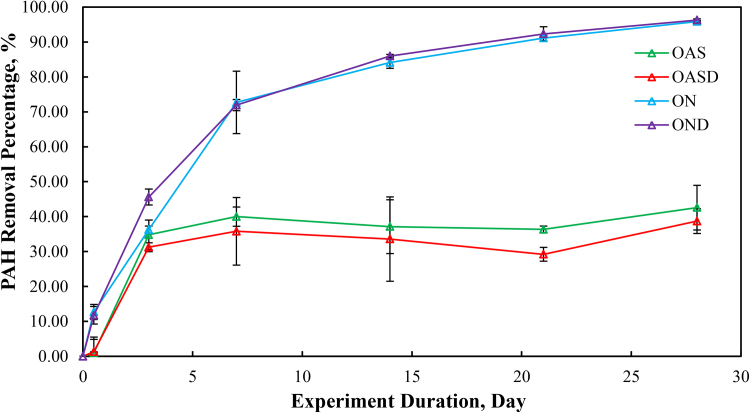
3.6. PAH removal percentages
Calculated PAH removal is illustrated in Fig. 13. The ON and OND treatments showed rapid removal percentages from day 0 to day 14. After day 14, the removal percentage stabilized at approximately 95% removal by day 28. However, the OAS and OASD treatments (abiotic, evaporation-dominated) showed a much lower PAH removal percentage, approximately 35% by day 28. The OAS and OASD treatments had very similar PAH depletion rates among them, as did the ON and OND treatments.
Fig. 13.
Percent removal of total petrogenic PAHs during the 28 days of laboratory microcosm weathering using: oil and artificial seawater (OAS), oil augmented with dispersant and artificial seawater (OASD), oil and natural seawater (ON), and oil augmented with dispersants and natural seawater (OND).
Fig. 9 through Fig. 12 show the profiles of aromatic compound degradation over time. In the abiotic experiments (Fig. 9 and Fig. 10), there is initial rapid loss of the naphthalene family by day 3, but very little degradation thereafter. In Fig. 11 and Fig. 12, there is very rapid loss of the naphthalenes with complete removal of naphthalene and C1-naphthalene by day 3. The compositional loss of the various PAH families follows a trend of losing the parent and C1 alkyl homologs first, followed by loss of the C2, C3 and C4 alkyl homologs in that order. It should be noted that these microcosms were done inside a laboratory and were not exposed to direct sunlight so photo-oxidation dominated weathering processes were not observed. Chrysene and its alkyl homologs were the least affected by weathering in these microcosms.
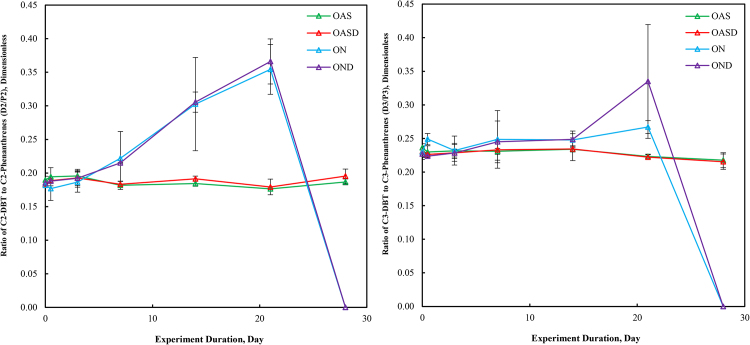
3.7. Ratio of C2-DBTs to C2-phenanthrenes and C3-DBTs to C3-phenanthrenes
Fig. 14 shows the ratios of D2/P2 and D3/P3 from each of the four weathering experiments. Both D2/P2 and D3/P3 remained relatively constant throughout the 28-day abotic experiment for the OAS and OASD treatments, which is consistent with other experimental depletion percentage results in the early stages of weathering (Liu et al., 2012). In GoM seawater treatments (ON and OND), D2/P2 and D3/P3 remained relatively constant through the first three days of weathering, however, the values began to increase in the sampled time periods from day 7 through day 21. This increase is the result of the preferential microbial degradation of the alkylated phenanthrenes relative to the sulfur containing DBTs. The point in Fig. 14 where the ratio falls to zero represents complete degradation of both alkylated DBTs and phenanthrenes by day 28 of the weathering experiment.
Fig. 14.
Changes in the ratios of alkylated C2 Dibenzothiophenes to C2 Phenanthrenes (left) and alkylated C3 Dibenzothiophenes to C3 Phenanthrenes during the 28 days of laboratory microcosm weathering using the four experimental oil treatments (OAS, OASD, ON, and OND).
3.8. Diagnostic ratio analysis
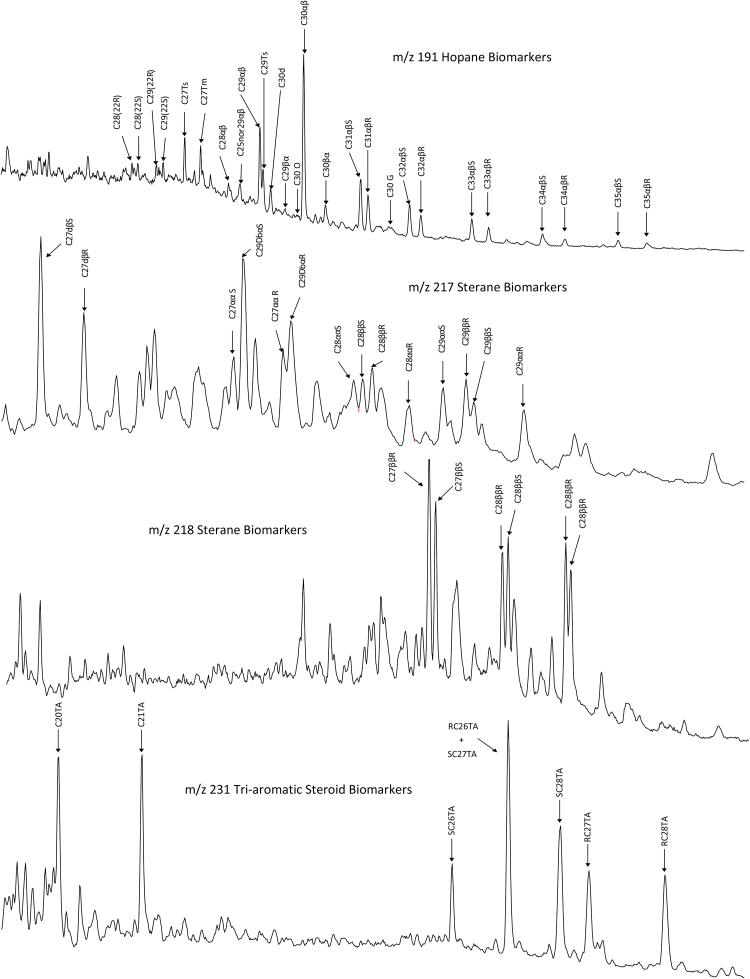
Fig. 15 shows the individual peaks for each of the biomarker compounds used in the ratio analysis by their respective extracted ion chromatograms (EICs m/z 191, 217, 218, and 231). These compounds are labeled by the short abbreviated names given previously on Table 4. These specific oil biomarker compounds were chosen because they are generally resistant to both the initial evaporative weathering as well as traditional weathering and degradation experienced in the environment as well as simulated in our microcosm study (Wang et al., 2006). Of these 53 biomarker compounds, all had consistently stable and quantifiable responses in MC252 source oil except 18alpha(H)/18beta(H)-oleanane (C30 O) and Gammacerane (C30G). None of the MC252 source oil analyses (n = 15) contained identifiable C30 O, and only six samples contained identifiable C30 G (m/z 191, Fig. 15).
Fig. 15.
Chromatograms of MC252 oil biomarker compounds by extracted ion chromatogram (m/z 191, 217, 218, and 231) with individual biomarkers identified.
Twenty-four MC252 ratios met the critical difference criteria discussed in Hansen et al., 2007 and they are listed in Table 5. The 24 identified diagnostic ratios were used to determine if microcosm extracts were a match to unweathered MC252 source oil. All microcosms used 40% evaporatively weathered MC252 oil as the Day 0 starting oil, and the ratio analysis provided an idea of how additional weathering affected the quantitative oil source fingerprinting results over a 28 day laboratory experiment period. On day 0, forensic analyses across all treatments (OAS, OASD, ON, and OND) showed scores of “match” (≥87%) and “probable match” (≥79% and <87%) to the fresh MC252 source oil, with an average score of 87% (±5.7%, n = 12) using all of the 24 biomarker ratios. This average increases to 90% (±6.1, n = 12) when the 2 inter-ion biomarker ratios were not considered. Although this disparity in the inter-ion ratios was not noticed in the fresh MC252 source oil used to test the diagnostic biomarker ratios, there was an obvious shift in the oil source fingerprinting category when the 2 inter-ion biomarker ratios were removed from the percentage calculations. Beginning with 40% weathered oil rendered the inter-biomarker ratios of C27ββ(R + S)/C30αβ and C29ββ(R + S)/C30αβ unusable for source fingerprinting as the 40% evaporative weathering had already shifted these ratios beyond the acceptable range. Consequently, these two inter ion ratios were removed from the calculation of the final score to more accurately represent the source of 40% evaporatively weathered MC252.
For day 28, biomarker weathering across all treatments appeared somewhat divergent. Specifically, both abiotic treatments (OAS: 86% ± 4.5% and OASD: 83% ± 5.2%) retained a higher level of matching diagnostic ratios than did the biotic treatments (ON 80% ± 11.4% and OND 80% ± 6.9%). Because of this, along with observed similar alkane and PAH degradation profiles and similar alkane and PAH removal percentages, artificial and natural treatments were grouped for assessment. The combined OAS and OASD treatments had an average score of 85% (±4.7%, n = 6) on day 28. This score falls short of a “match” but is a fairly high “probable match” for MC252 crude oil for the artificial seawater treatments. This is after 40% initial laboratory weathering by mass, and an additional 28 days of microcosm weathering in sterile artificial seawater. The ON and OND treatments were also combined for diagnostic biomarker ratio analysis based on similar alkane and PAH degradation profiles observed between treatments as well as similar alkane and PAH removal percentages over the course of 28 day microcosm weathering. The ON and OND treatments had a lower match score of 80% (±8.5%, n = 6), also placing them in the “probable match” category. Again, this is after 40% laboratory weathering by mass and an additional 28 days in a microcosm system weathering in natural seawater. In this study, there were fewer matching biomarker ratios for the natural seawater amended microcosms than the artificial seawater amended microcosms, however they were not significantly different from each other (n = 12, p > 0.05, two-tailed t-test). Despite what appeared to be a difference between artificial and natural seawater treatments, there was no significant difference (p > 0.05). This should be further explored using a larger set of samples so that the concept of whether or not a measurable shift in matching diagnostic ratios can be seen in GoM seawater amended microcosms versus sterile, artificial seawater amended microcosms.
It is important to note that chromatographic source fingerprinting data must be assessed with great care and meticulous attention to baseline detail. Fig. 15 shows just how subjective assessment can be based on the shifting baseline visible throughout the four biomarker EICs. For this reason, each individual analyst must be aware of his/her analytical error and/or bias. Choosing where to set the baseline must be standardized internally and monitored with continual relative error assessment QA/QC methodology. Based on the nature of diagnostic ratio biomarker analysis, the response of peak heights rather than peak areas are used. Biomarker ratio consistency is controlled by both respective peak heights and the baseline chosen for peak height measurements. Using peak height data generally gives a more accurate measure of signal intensity for diagnostic ratio purposes. Furthermore, variables such as peak tailing and column overloading are removed from the calculation when using peak height, resulting in more accurate and precise diagnostic ratio analysis.
4. Conclusions
The objective of this research was to determine the impacts of weathering on the chemical composition of oil (i.e., 40% evaporatively weathered MC252 oil) floating on the sea surface over time, both in the absence and presence dispersant. Using MC252 oil with an evaporatively weathered composition similar to that found during the DWH oil spill, we wanted to know first, what are the observable degradation/weathering patterns and depletion percentages of specific alkane and polycyclic aromatic hydrocarbons in well mixed laboratory weathered experiments both with and without the addition of dispersant? Second, does the addition of dispersants impact crude oil degradation (either positively or negatively) in these laboratory weathering microcosm experiments? Third, what are the differences between biotic and abiotic weathering of MC252 oil? And forth, can certain hydrocarbon ratios be used throughout the weathering process to forensically identify MC252 oil, and if so, are there limits to the application of these ratios?
Our conclusions are as follows:
1) For the sterile, artificial seawater systems (OAS and OASD), evaporation dominated the weathering process. Total alkane and PAH concentrations initially decreased slightly and then remained relatively constant over the duration of the experiment. However, when using natural GoM seawater in the microcosms (ON and OND), we observed a dramatic initial decrease in total concentrations, which can be attributed to biotic degradation caused by the natural microbial community in the GoM seawater. The ratio of n-C17/pris and n-C18/phy decreased during the early stages of weathering in the biotic treatments, indicating preferential biotic degradation over evaporative weathering. The D2/P2 and D3/P3 ratios remained constant in OAS and OASD treatments, however, they increased over the course of this study in ON and OND treatments, until both compounds were fully degraded by day 28. This ratio increase is indicative of preferential microbial degradation of alkylated phenanthrenes (non-sulfur containing PAHs) over alkylated DBTs (sulfur containing PAHs) in natural seawater.
2) MC252 oil, even when initially evaporatively weathered by 40%, is rapidly degraded in well-mixed aerobic GoM simulated microcosms. While this was not a kinetic study, well over 50% of the alkane hydrocarbons were lost in less than 3 days, and well over 50% of the aromatic PAHs were loss in less than 7 days. Well-mixed weathered MC252 oil in GoM simulated conditions showed rapid degradation of over >95% of both alkanes and aromatic PAHs over the duration of these experiments.
3) There were no weathering differences observed in the profiles between OAS and OASD. The addition of dispersant, as outlined in this study, had no identifiable influence on 28 day weathering and degradation profiles of the 40% reduced MC252 oil in sterile, artificial seawater. Furthermore, completely different weathering profiles were observed in the biotic microcosms (ON and OND) using natural seawater. The similar weathering and degradation patterns, outlined by each treatment, shows that despite the addition of dispersant, weathering and degradation profiles were not affected within biotic and abiotic seawater amendments. It should be noted that chemical dispersants are used to promote the breaking up of oil slicks into small droplets in order for well mixing of the oil into the seawater column. The goal of this spill technology is to reduce the amount of inshore oiling as well as make oil more available to weathering and degrading processes. The effect of dispersants on biodegradation has become a matter of dispute (Kleindienst et al., 2015) (Zeinstra-Helfrich et al., 2015). There are papers stating that dispersants promote biodegradation while others indicate that dispersants suppress biodegradation (Lindstrom and Braddock, 2002) (Yoshida et al., 2006) (Zahed et al., 2010) (Lee et al., 2013) (Kleindienst et al., 2015). These experiments were designed to help determine if dispersants had any negative effects on natural, oil-degrading bacteria. In these closed system microcosms, we found that the addition of dispersant did not inhibit abiotic or biotic weathering of MC252. It is important to point out that these microcosms were designed to study degradation of oil as it would be found on the sea surface, not the water accommodated oil fractions. Further, the degradation experiments were done under well-mixed conditions to see if the addition of dispersant inhibited oil degradation. We were not testing the efficacy of dispersant use to enhance oil degradation, only testing the possible inhibition of dispersants on the degradation of oil in well-mixed seawater.
4) Various forensic diagnostic biomarker ratios (i.e., compounds within the m/z 191, 217, 218, and 231 EICs) provided data for forensic identification of MC252 crude oil for this and in future studies using the analytical methods found in Hansen et al., 2007. It was speculated that certain inter-ion biomarker ratios could be beneficial in source identification and weathering. However, the inter-biomarker family ratios proved less useful in this forensic oil fingerprinting context (i.e. beginning with 40% evaporatively weathered oil), and therefore, were ultimately removed from the overall final evaluation of matching biomarker ratios. Conversely, they may be useful for following initial fresh oil weathering over time and weathering in different environments. The final percentage for day 28 natural seawater treatments showed that the diagnostic ratios used to source fingerprint oil appeared to only be slightly affected by microbial weathering as compared to the artificial seawater treatments, however, the percentage of matching ratios remained within the “probable match” category for all day 28 samples. The observed difference between artificial and natural treatments (though not significant n = 12, p = 0.34) requires a more robust study to further explore the possible implications of preferential biomarker weathering/shifting as oil degrades.
Declarations
Author contribution statement
Gregory M. Olson: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Heng Gao: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Buffy M. Meyer, M. Scott Miles: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Edward B. Overton: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by the Emergency Response Division of NOAA's Office of Response and Restoration. This work was supported by The Gulf of Mexico Research Initiative.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgments
We would like to thank the Emergency Response Division of NOAA's Office of Response and Restoration. Specifically Dr. Jim Farr for his assistance with the project. We gratefully acknowledge the support of BP and NALCO Company who generously provided the crude oil and dispersant used in this study. We would also like to thank The Gulf of Mexico Research Initiative-Coastal Waters Consortium. Data are publicly available through the Gulf of Mexico Research Initiative Information & Data Cooperative (GRIIDC) at https://data.gulfresearchinitiative.org
References
- Atlas R.M. Oil Biodegradation and Oil-Degrading Microbial Populations in Marsh Sediments Impacted by Oil from the Deepwater Horizon Well Blowout. Environ. Sci. Technol. 2015;49(14):8356–8366. doi: 10.1021/acs.est.5b00413. [DOI] [PubMed] [Google Scholar]
- Boehm P.D. Application of petroleum hydrocarbon chemical fingerprinting and allocation techniques after the Exxon Valdez oil spill. Marine Poll. Bull. 1997;34(8):599–613. [Google Scholar]
- Brakstad O., Faksness L. Biodegradation of water-accommodated fractions and dispersed oil in the seawater column; SPE International Conference on Health, Safety and Environment in Oil and Gas Exploration and Production 2000 - Society of Petroleum Engineers, Stavanger, Norway; 2000. [Google Scholar]
- Campo P., Venosa A.D., Suidan M.T. Biodegradability of Corexit® 9500 and Dispersed South Louisiana Crude Oil at 5 and 25°C. Environ. Sci. Technol. 2013;47:1960–1967. doi: 10.1021/es303881h. [DOI] [PubMed] [Google Scholar]
- Fingas M. Studies on the evaporation of crude oil and petroleum products: I. The relationship between evaporation rate and time. J. Hazard. Mater. 1997;56:227–236. [Google Scholar]
- Gros J. First Day of an Oil Spill on the Open Sea: Early Mass Transfers of Hydrocarbons to Air and Water. Environ. Sci. Technol. 2014;48(16):9400–9411. doi: 10.1021/es502437e. [DOI] [PubMed] [Google Scholar]
- Hansen A.B., Daling P.S., Kienhuis P., Duus R. Emerging CEN Methodology for Oil Spill Identification. In: Wang Z., Stout S., editors. Oil Spill Environmental Forensics. Academic Press; Burlington, MA: 2007. pp. 229–256. [Google Scholar]
- Hazen T.C. Deep-Sea Oil Plume Enriches Indigenous Oil-Degrading Bacteria. Science. 2010;330(6001):204–208. doi: 10.1126/science.1195979. [DOI] [PubMed] [Google Scholar]
- Head I., Jones D., Röling W. Marine microorganisms make a meal of oil. Nature Rev. Microbiol. 2006;4(3):173–182. doi: 10.1038/nrmicro1348. [DOI] [PubMed] [Google Scholar]
- King S.M. Photolytic and photocatalytic degradation of surface oil from the Deepwater Horizon spill. Chemosphere. 2014;95:415–422. doi: 10.1016/j.chemosphere.2013.09.060. [DOI] [PubMed] [Google Scholar]
- Kleindienst S. Chemical dispersants can suppress the activity of natural oil-degrading microorganisms. PNAS. 2015;112(48):14900–14905. doi: 10.1073/pnas.1507380112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvenvolden K., Cooper C. Natural seepage of crude oil into the marine environment. Geo-Mar. Lett. 2003;23(3):140–146. [Google Scholar]
- Lee K., Nedwed T., Prince R.C., Palandro D. Lab tests on the biodegradation of chemically dispersed oil should consider the rapid dilution that occurs at sea. Marine Poll. Bull. 2013;73(1):314–318. doi: 10.1016/j.marpolbul.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Lee K. Microbial Response to Crude Oil and Corexit 9527: SEAFLUXES Enclosure Study. Microb. Ecol. 1985;11(4):337–351. doi: 10.1007/BF02016816. [DOI] [PubMed] [Google Scholar]
- Lindstrom J.E., Braddock J.F. Biodegradation of petroleum hydrocarbons at low temperature in the presence of the dispersant Corexit 9500. Marine Poll. Bull. 2002;44(8):739–747. doi: 10.1016/s0025-326x(02)00050-4. [DOI] [PubMed] [Google Scholar]
- Lin Q., Mendelssohn I.A. Potential of restoration and phytoremediation with Juncus roemerianus for diesel-contaminated coastal wetlands. Ecol. Eng. 2009;35(1):85–91. [Google Scholar]
- Liu Z., Liu J., Zhu Q., Wu W. The weathering of oil after the Deepwater Horizon oil spill: insights from the chemical composition of the oil from the sea surface, salt marshes and sediments. Environ. Res. Lett. 2012;7(3):14. [Google Scholar]
- Lunel T. Shoreline clean up during the Sea Empress incident: the role of surf washing (clay-oil flocculation), dispersants and bioremediation. Proceedings of the Ninteenth Arctic and Marine Oilspill Program (AMOP), Canada, Ottawa, Ontario. 1996 [Google Scholar]
- Meyer B.M., Overton E.B., Turner R. Oil source identification using diagnostic biomarker ratio analyses. International Oil Spill Conference Proceedings. 2014;2014(1):2064–2073. [Google Scholar]
- Mu J. Comparative effects of biological and chemical dispersants on the bioavailability and toxicity of crude oil to early life stages of marine medaka (Oryzias melastigma) Environ. Toxicol. Chem. 2014;33(11):2576–2583. doi: 10.1002/etc.2721. [DOI] [PubMed] [Google Scholar]
- National Commission on the BP Deepwater Horizon Oil Spill and Offshore Drilling . 1st Edition. US Independent Agencies and Commissions; Washington, DC: 2011. Deep Water: The Gulf Oil Disaster and the Future of Offshore Drilling: Report to the President, January 2011. [Google Scholar]
- National Research Council . National Academies Press; Washington, DC: 2003. Committee on oil in the sea III: inputs, fates, and effects. [PubMed] [Google Scholar]
- National Research Council . National Academies Press; Washington, DC: 2005. Committee on understanding oil spill dispersants: efficacy and effects. [Google Scholar]
- Overton E.B. Identification of Petroleum Residue Sources After A Fire and Oil Spill. International Oil Spill Conference Proceedings. 1981;1981(1):541–546. [Google Scholar]
- Page C.A., Bonner J.S., McDonald T.J., Autenrieth R.L. Behavior of a chemically dispersed oil in a wetland environment. Water Res. 2002;36(15):3821–3833. doi: 10.1016/s0043-1354(02)00079-9. [DOI] [PubMed] [Google Scholar]
- Peterson C.H. A tale of two spills: novel science and policy implications of an emerging new oil spill model. Bioscience. 2012;62(5):461–469. [Google Scholar]
- Prince R., Butler J. A protocol for assessing the effectiveness of oil spill dispersants in stimulating the biodegredation of oil. Environ. Sci. Pollut. Res. Int. 2014;21(16):9506–9510. doi: 10.1007/s11356-013-2053-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince R. The primary biodegradation of dispersed crude oil in the sea. Chemosphere. 2013;90(2):521–526. doi: 10.1016/j.chemosphere.2012.08.020. [DOI] [PubMed] [Google Scholar]
- Prince R., Parkerton T., Lee C. The primary aerobic biodegradation of gasoline hydrocarbons. Environ. Sci. Technol. 2007;41(9):3316–3321. doi: 10.1021/es062884d. [DOI] [PubMed] [Google Scholar]
- Seo J.-S., Keum Y.-S., Li Q.X. Bacterial Degradation of Aromatic Compounds. Int. J. Environ. Res. Public Health. 2009;6(1):278–309. doi: 10.3390/ijerph6010278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siron R., Pelletier E., Brochu C. Environmental factors influencing the biodegradation of petroleum hydrocarbons in cold seawater. Arch. Environ. Contam. Toxicol. 1995;28(4):406–416. [Google Scholar]
- Stout S.A., Payne J.R., Emsbo-Mattingly S.D., Baker G. Weathering of field-collected floating and stranded Macondo oils during and shortly after the Deepwater Horizon oil spill. Marine Poll. Bull. 2016;105:7–22. doi: 10.1016/j.marpolbul.2016.02.044. [DOI] [PubMed] [Google Scholar]
- Stout S., Wang Z. Chemical fingerprinting of spilled or discharged petroleum - methods and factors affecting petroleum fingrerprints in the environment. In: Wang Z., Stout S., editors. Oil Spill Environmental Forensics: Fingerprinting and Source Identification. Academic Press; Burlington, MA: 2007. pp. 1–45. [Google Scholar]
- Tarr M. Weathering of oil spilled in the marine environment. Oceanography. 2016;29(3):126–135. [Google Scholar]
- The United States Government Publishing Office . 2011. 40 CFR Appendix C to Part 300 - US Government Publishing Office [Online]https://www.gpo.gov/fdsys/pkg/CFR-2011-title40-vol28/pdf/CFR-2011-title40-vol28-part300-appC.pdf [Accessed 24 October 2016] [Google Scholar]
- Turner R. Distribution and Recovery Trajectory of Macondo (Mississippi Canyon 252) oil in Louisiana Coastal Wetlands. Marine Poll. Bull. 2014;87(1-2):57–67. doi: 10.1016/j.marpolbul.2014.08.011. [DOI] [PubMed] [Google Scholar]
- USEPA . 2016. Test Methods for Evaluating Solid Wastes Physical/Chemical Methods SW-846 [Online]https://www.epa.gov/hw-sw846/sw-846-compendium [Google Scholar]
- Venosa A., Holder E. Biodegradability of dispersed crude oil at two different temperatures. Marine Poll. Bull. 2007;54(5):545–553. doi: 10.1016/j.marpolbul.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Wade T.L. Spatial and temporal distribution of water column total polycyclic aromatic hydrocarbons (PAH) and total petroleum hydrocarbons (TPH) from the Deepwater Horizon (Macondo) incident. Marine Poll. Bull. 2016;103(1-2):286–293. doi: 10.1016/j.marpolbul.2015.12.002. [DOI] [PubMed] [Google Scholar]
- Wang J. Biodegradation of dispersed Macondo crude oil by indigenous Gulf of Mexico microbial communities. Sci. Total Environ. 2016;557-558:453–468. doi: 10.1016/j.scitotenv.2016.03.015. [DOI] [PubMed] [Google Scholar]
- Wang Z. Comparison of oil composition changes due to biodegradation and physical weathering in different oils. J. Chromatogr. A. 1998;809(1-2):89–107. doi: 10.1016/s0021-9673(98)00166-6. [DOI] [PubMed] [Google Scholar]
- Wang Z., Fingas M.F. Development of oil hydrocarbon fingerprinting and identification techniques. Marine Poll. Bull. 2003;47(9-12):423–452. doi: 10.1016/S0025-326X(03)00215-7. [DOI] [PubMed] [Google Scholar]
- Wang Z., Fingas M., Sergy G. Study of 22-Year-Old Arrow Oil Samples Using Biomarker Compounds by GC/MS. Environ. Sci. Technol. 1994;28(9):1733–1746. doi: 10.1021/es00058a027. [DOI] [PubMed] [Google Scholar]
- Wang Z., Stout S.A., Fingas M. Forensic Fingerprinting of Biomarkers for Oil Spill Characterization and Source Identification. Environ. Forensics. 2006;7(2):105–146. [Google Scholar]
- Yoshida A. Microbial responses using denaturing gradient gel electrophoresis to oil and chemical dispersant in enclosed ecosystems. Marine Poll. Bull. 2006;52(1):89–95. doi: 10.1016/j.marpolbul.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Zahed M.A., Aziz H.A., Isa M.H., Mohajeri L. Effect of Initial Oil Concentration and Dispersant on Crude Oil Biodegradation in Contaminated Seawater. Bull. Environ. Contam. Toxicol. 2010;84(4):438–442. doi: 10.1007/s00128-010-9954-7. [DOI] [PubMed] [Google Scholar]
- Zeinstra-Helfrich M., Koops W., Murk A.J. The NET effect of dispersants — a critical review of testing and modelling of surface oil dispersion. Marine Poll. Bull. 2015;100(1):102–111. doi: 10.1016/j.marpolbul.2015.09.022. [DOI] [PubMed] [Google Scholar]
- Zhuang M. Effect of dispersants on the biodegradation of South Louisiana crude oil at 5 and 25 °C. Chemosphere. 2016;144:767–774. doi: 10.1016/j.chemosphere.2015.08.040. [DOI] [PubMed] [Google Scholar]