Abstract
Background
The diagnosis and assessment of COPD rely mainly on the use of spirometry, which is an effort-dependent test and requires good patient cooperation. Impulse oscillometry (IOS) is a non-volitional method that requires less effort and cooperation and presents advantages for geriatric patients. However, the clinical application value of IOS in geriatric patients with COPD remains unclear.
Aim
The aim of this study was to investigate the clinical application value of IOS in geriatric patients with COPD.
Subjects and methods
A total of 234 subjects were retrospectively enrolled in this study, including 133 patients with COPD and 101 healthy volunteers. All the participants underwent IOS and spirometry examination. The data were collected and analyzed in the overall group, the geriatric group (aged ≥65 years), and the advanced elderly group (aged ≥80 years).
Results
1) In COPD patients, a significant increase in respiratory impedance (Z5), resonant frequency (Fres), and respiratory resistance (R5, R20, R5–R20) and a decrease in respiratory reactance (X5) were observed in the overall group, the geriatric group, and the advanced elderly group compared with the healthy control subjects. 2) The IOS parameters correlated well with spirometry in COPD. In particular, R5–R20 showed the best correlation with forced expiratory volume in 1 second (FEV1) in the different age groups. 3) Fres and R5–R20 had the best diagnostic efficiency for COPD. The area under the curve (AUC) values for Fres, expressed by the receiver operating characteristic (ROC) curve, were 0.905, 0.909, and 0.914, for the different age groups, respectively. 4) The optimal cutoff values for Fres to diagnose airflow obstruction from ROC curves was 17.715 in the COPD patients. Its sensitivity and specificity were 0.789 and 0.931, respectively, and the cutoff values were similar in geriatric and advanced elderly patients.
Conclusion
IOS demonstrated good relevance compared with spirometry for geriatric patients with COPD. IOS may serve as an alternative method for spirometry in elderly subjects for the evaluation of the state of COPD.
Keywords: COPD, impulse oscillometry, spirometry, geriatric patients
Introduction
COPD is a type of obstructive lung disorder characterized by airflow limitation that is not fully reversible.1 It is currently the fourth leading cause of death, and the WHO predicts that it will become the third leading cause of death by 2030. Pulmonary function tests are of great significance in the diagnosis and evaluation of COPD, and spirometry is the current gold standard to evaluate airway limitations. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) states that the diagnosis of obstructive lung disease should be made when the postbronchodilator ratio of the forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) is <70%, with the FEV1 percentage of the predicted value used as a classification criterion for the severity of airflow limitation.2
However, the forceful expiratory and inspiratory maneuvers in spirometry require good patient cooperation. The examinations last almost 20–30 minutes and are not well tolerated by most elderly or critically ill patients. Meanwhile, it is easy to produce false-positive results because of the change in bronchomotor tone caused by repeated forced breathing, especially in the elderly. Thus, studies concerning the quality of spirometry in elderly patients showed that only slightly >30% of patients were able to perform spirometry that fulfilled all the quality criteria as defined by European Respiratory Society/American Thoracic Society standards,3,4 and the proportion in the advanced elderly was even lower.5 Furthermore, FEV1 cannot fully assess lung function, particularly the identification of small airway abnormalities. Thus, the maximum midexpiratory flow rate (MMEF) parameter has been investigated as an alternative marker of small airway function, but the parameter is highly variable because of the influence of large airway obstruction.6 Therefore, it is necessary to have other methods to assess lung function in COPD, especially in the elderly, who cannot perform spirometry well, as 23% of the total global disease burden is attributable to disorders in people aged ≥60 years.7
Impulse oscillometry (IOS) has the advantage of being a noninvasive, effort-independent method that can be performed easily in subjects who are unable to undergo spirometry. Importantly, IOS can determine the mechanical properties of the lung and differentiate small airway obstruction from large airway obstruction. It is more sensitive than spirometry and seems to be better able to detect early changes in lung function than spirometry.8 It has been reported that IOS is a good alternative to spirometry and can be used to assess pulmonary function and the effect of maintenance therapy in patients with COPD.8 However, it is difficult to compare IOS measurements with more familiar measurements made under conditions of forced expiration; hence, this technique has not been widely adopted in adult clinical practice. Few studies have assessed the application value of IOS in geriatric patients, especially in advanced aged patients. This study aimed to explore the application value of IOS in COPD patients, especially in geriatric and advanced elderly patients.
Subjects and methods
Subjects
A total of 234 subjects were retrospectively enrolled in this study, including 133 patients with COPD and 101 healthy volunteers, from January 2012 to June 2016 at Peking University First Hospital. In all, 133 COPD cases were diagnosed according to the GOLD criteria, which are based on COPD risk factors, symptoms, and postbronchodilator FEV1/FVC <70%, and patients with asthma, bronchiectasis, pleural effusion, history of exposure to noxious particles (such as founder’s pneumoconiosis, silicosis, and asbestosis) and diseases affecting activity were excluded. The COPD group included 33.1% never-smokers, 42.9% ex-smokers, and 24.1% current smokers; the pack-year expressed by median (interquartile range [IQR]) was 30 (0–40). Different GOLD airflow limitation stages were distinguished as COPD 1–4 according to post-bronchodilator FEV1%predicted. In all, 101 healthy controls included individuals with normal pulmonary function without chronic heart and lung diseases and a recent history of respiratory tract infection. The health control group include 80.2% never-smokers, 6.9% ex-smokers, 12.9% current smokers, the pack years expressed by median (IQR) is 0 (0–0). This study was conducted with approval from the ethics committee of Peking University First Hospital, and all participants provided written informed consent.
Study design
The retrospective study design sought to 1) compare the differences in spirometry parameters and IOS parameters between healthy controls and COPD patients at different COPD stages and assess the correlation of the two parameters; 2) define participants aged ≥65 years as the geriatric group and participants aged <65 years as the non-geriatric group, compare the spirometry and IOS parameters between COPD patients and healthy controls in each age group, and assess the correlation of the two parameters; 3) define participants aged ≥80 years as the advanced elderly group and participants aged between 65 and 80 years as the non-advanced elderly group, compare the spirometry and IOS parameters between COPD patients and healthy controls in each age group, and assess the correlation of the two parameters; and 4) generate the receiver operating characteristic (ROC) curve of the IOS parameters and determine the cutoff values for the IOS parameters to identify COPD patients in the overall group, the geriatric group, and the advanced elderly group.
Measurements
Spirometry and IOS were performed using Master Screen PET (Erich Jaeger, Friedberg, Germany). All the subjects were first examined by IOS to detect respiratory impedance, and then, spirometry was performed before and 15 minutes after inhaling salbutamol (400 μg) from a metered-dose inhaler with a valve-bearing spacer device, following the European Respiratory Society/American Thoracic Society criteria.9,10 Respiratory impedance values at 5 Hz (Z5), respiratory resistance values at 5 and 20 Hz (R5 and R20, respectively), and the values corresponding to the difference between R5 and R20 (R5−R20) were recorded as the values compared with the prediction (%predicted). The reactance at 5 Hz (X5) and the resonant frequency (Fres) were recorded as the measured values. The spirometry parameters observed included FVC, FEV1, FEV1/FVC, and MMEF, which were described as values compared with the prediction (%predicted).
Statistical analysis
Statistical analysis was performed using SPSS version 19.0 software (SPSS Inc., Chicago, IL, USA). The normal dispersal of the values was determined by the Kolmogorov–Smirnov test. Normally distributed data were represented as mean ± standard deviation (SD). The differences between the two groups were compared using Student’s t-test, and differences between multiple groups were compared using analysis of variance (ANOVA) with post hoc tests where appropriate and chi-square tests. Non-normally distributed data were represented as median (IQR), with non-parametric tests used to compare parameters between groups. The relationship between IOS and spirometry measurements was determined by Pearson’s correlation (for normally distributed data) or Spearman’s correlation (for non-normally distributed data) analysis. The diagnostic value of IOS parameters was evaluated by ROC curve analysis, according to which the cutoff was calculated. A two-sided value of P≤0.05 was considered significant.
Results
A total of 133 patients with COPD (including 95 geriatric COPD patients, 41 of whom were advanced elderly COPD patients) and 101 healthy volunteers (including 43 geriatric healthy controls, 26 of whom were advanced elderly healthy controls) were retrospectively enrolled in this study. Their demographic data are summarized in Tables 1–3.
Table 1.
Demographics of all the subjects
| Healthy controls | COPD | COPD 1 | COPD 2 | COPD 3 | COPD 4 | |
|---|---|---|---|---|---|---|
| n | 101 | 133 | 19 | 49 | 38 | 27 |
| Age (years) | 65.07±14.72 | 71.25±11.24 | 79.47±8.90 | 72.96±10.51 | 71.97±10.37 | 61.33±8.50 |
| Male/female | 70/31 | 110/23 | 13/6 | 39/10 | 32/6 | 26/1 |
| BMI (kg/m2) | 24.59±3.26 | 23.59±4.00 | 23.11±3.69 | 25.56±3.67 | 23.07±3.47 | 21.09±3.93 |
Note: Data are expressed as numbers and mean ± standard deviation.
Abbreviation: BMI, body mass index.
Table 2.
Demographics of the geriatric population
| Healthy controls
|
COPD
|
|||
|---|---|---|---|---|
| Non-geriatric | Geriatric | Non-geriatric | Geriatric | |
| n | 58 | 43 | 38 | 95 |
| Age (years) | 54.33±8.83 | 79.56±6.00 | 56.50±5.34 | 77.15±6.57 |
| Male/female | 43/15 | 27/16 | 36/2 | 74/21 |
| BMI (kg/m2) | 24.95±3.05 | 24.11±3.50 | 22.78±4.48 | 23.91±3.77 |
Note: Data are expressed as numbers and mean ± standard deviation.
Abbreviation: BMI, body mass index.
Table 3.
Demographics of the advanced elderly group
| Healthy controls
|
COPD
|
|||
|---|---|---|---|---|
| Non-advanced elderly | Advanced elderly | Advanced elderly | Non-advanced elderly | |
| n | 17 | 26 | 41 | 54 |
| Age (years) | 73.71±4.87 | 83.38±2.59 | 83.29±2.77 | 72.48±4.41 |
| Male/female | 13/4 | 14/12 | 32/9 | 42/12 |
| BMI (kg/m2) | 23.89±2.97 | 24.25±3.85 | 23.66±3.80 | 24.10±3.77 |
Note: Data are expressed as numbers and mean ± standard deviation.
Abbreviation: BMI, body mass index.
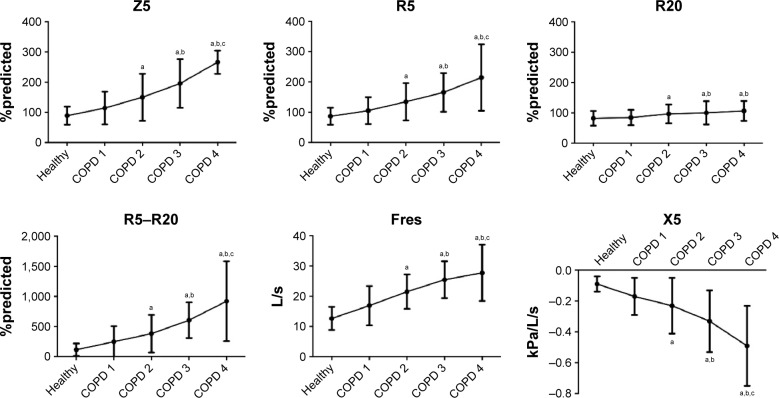
In the COPD patients, a significant increase in Z5, Fres, R5, R20, and R5–R20 and a decrease in X5 were observed compared with the healthy control subjects (Table 4 and Figure 1). Additionally, as the grade of airflow obstruction increased, Z5, Fres, R5, R20, and R5–R20 increased and X5 decreased significantly. The ratio of R5–R20/R5 increased from COPD1 to COPD4 (32.43%, 39.13%, 48.15%, and 56.06%, respectively). The IOS parameters correlated well with the spirometry parameters in COPD, and R5–R20 had the strongest correlation with FVC, FEV1, FEV1/FVC, and MMEF (Table 5).
Table 4.
Comparison of IOS parameters between the healthy control and COPD groups
| Healthy controls | COPD | COPD 1 | COPD 2 | COPD 3 | COPD 4 | |
|---|---|---|---|---|---|---|
| Z5 (%predicted) | 89.24±29.98 | 159.50 (111.30 to 225)a | 114.32±54.47 | 150.22±78.02a | 195.57±80.87a,b | 266.3±138.33a–c |
| Fres (L/s) | 12.62±3.84 | 23.23±7.60a | 16.9±6.52 | 21.48±5.71a | 25.46±6.11a,b | 27.71±9.32a–c |
| X5 (kPa/L/s) | −0.09±0.05 | −0.25 (−0.41 to −0.14)a | −0.17±0.12 | −0.23±0.18a | −0.33±0.2a,b | −0.49±0.26a–c |
| R5 (%predicted) | 86.56±28.39 | 140.50 (106.55 to 185.7)a | 105.25±44.79 | 134.52±61.75a | 165.54±64.12a,b | 214.64±109.68a–c |
| R20 (%predicted) | 82±24.65 | 97.92±33.42a | 84.48±25.25 | 96.55±31.44a | 100.26±38.42a,b | 106.61±32.9a,b |
| R5–R20 (%predicted) | 115.96±103.54 | 458.23 (241.95 to 663.40)a | 248.8±258.35 | 382.9±312.48a | 606.22±295.68a,b | 920.03±664.1a–c |
Notes: Data are expressed as mean ± standard deviation and median (IQR).
P<0.05 compared with the healthy controls.
P<0.05 compared with COPD 1.
P<0.05 compared with COPD 2.
Abbreviations: IOS, impulse oscillometry; Z5, respiratory impedance; Fres, resonant frequency; X5, respiratory reactance; R5–R20, respiratory resistance; IQR, inter-quartile range.
Figure 1.
Changes in IOS parameters with increasing airflow limitation.
Notes: aP<0.05 compared with the healthy controls. bP<0.05 compared with COPD 1. cP<0.05 compared with COPD 2.
Abbreviations: IOS, impulse oscillometry; R5–R20, respiratory resistance; Fres, resonant frequency; X5, respiratory reactance; Z5, respiratory impedance.
Table 5.
Correlation between the IOS and spirometry parameters in COPD
| Z5 (%predicted) | Fres (L/s) | X5 (kPa/L/s) | R5 (%predicted) | R20 (%predicted) | R5–R20 (%predicted) | ||
|---|---|---|---|---|---|---|---|
| FVC (%predicted) | r | −0.450** | −0.361** | 0.415** | −0.416** | −0.173* | −0.491** |
| FEV1 (%predicted) | r | −0.564** | −0.520** | 0.561** | −0.528** | −0.212* | −0.628** |
| FEV1/FVC (%) | r | −0.492** | −0.481** | 0.517** | −0.474** | −0.139 | −0.572** |
| MMEF (%predicted) | r | −0.530** | −0.511** | 0.555** | −0.509** | −0.228** | −0.588** |
Notes:
P<0.05.
P<0.001.
Abbreviations: IOS, impulse oscillometry; Z5, respiratory impedance; Fres, resonant frequency; X5, respiratory reactance; R5–R20, respiratory resistance; FVC, forced vital capacity; r, Pearson’s or Spearman’s correlation coefficient; FEV1, forced expiratory volume in 1 second; MMEF, maximum midexpiratory flow rate.
In the geriatric subjects, a significant increase in Z5, Fres, R5, R20, and R5–R20 and a decrease in X5 were observed in patients with COPD compared with the normal control geriatric group (Table 6). Table 6 also shows a comparison of the IOS parameters between the non-geriatric healthy controls and the non-geriatric COPD subjects, the non-geriatric COPD subjects and the geriatric COPD subjects, and the non-geriatric healthy controls and the geriatric healthy controls. R20 did not differ significantly between the non-geriatric healthy controls and the non-geriatric COPD subjects. The IOS parameters correlated well with spirometry parameters in the geriatric COPD subjects, and R5–R20 had the strongest correlation with FVC, FEV1, and FEV1/FVC (Table 7).
Table 6.
Comparison of the pulmonary function parameters between the groups of 65-year-olds
| Healthy controls
|
COPD
|
|||
|---|---|---|---|---|
| Non-geriatric | Geriatric | Non-geriatric | Geriatric | |
| FVC (%predicted) | 97.24±15.18 | 97.26±20.50 | 66.79±18.90b | 76.40±18.95a,c |
| FEV1 (%predicted) | 95.04±15.16 | 98.6±20.72 | 37.78±20.79b | 54.63±20.73a,c |
| FEV1/FVC (%) | 79.45±5.14 | 77.64±6.34 | 42.69±15.53b | 53.23±12.56a,c |
| MMEF (%predicted) | 74.12±23.08 | 65.78±28.10 | 14.36±11.41b | 19.70±11.18a,c |
| Z5 (%predicted) | 96.37±28.77 | 79.62±29.19d | 215.72±135.42b | 167.97±85.05a,c |
| Fres (L/s) | 12.23±3.83 | 13.14±3.84 | 24.65±8.94b | 22.66±6.96a |
| X5 (kPa/L/s) | −0.09±0.05 | −0.10±0.06 | −0.34±0.28b | −0.29±0.20a |
| R5 (%predicted) | 92.84±29.04 | 78.09±25.43d | 176.56±105.00b | 147.03±66.86a |
| R20 (%predicted) | 90.47±25.86 | 70.58±17.53d | 102.29±32.82 | 96.18±33.67a |
| R5–R20 (%predicted) | 107.22±107.23 | 127.75±98.34 | 658.04±640.25b | 488.01±350.12a |
Notes: Data are expressed as mean ± standard deviation.
P<0.001 compared with the geriatric healthy patients.
P<0.001 compared with the non-geriatric healthy patients.
P<0.05 compared with the non-geriatric COPD patients.
P<0.01 compared with the non-geriatric healthy patients.
Abbreviations: FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; MMEF, maximum midexpiratory flow rate; Z5, respiratory impedance; Fres, resonant frequency; X5, respiratory reactance; R5–R20, respiratory resistance.
Table 7.
Correlation between the IOS and spirometry parameters in geriatric COPD patients
| Z5 (%predicted) | Fres (L/s) | X5 (kPa/L/s) | R5 (%predicted) | R20 (%predicted) | R5–R20 (%predicted) | ||
|---|---|---|---|---|---|---|---|
| FVC (%predicted) | r | −0.373** | −0.253* | 0.345** | −0.337** | −0.197 | −0.390** |
| FEV1(%predicted) | r | −0.527** | −0.479** | 0.514** | −0.499** | −0.293** | −0.551** |
| FEV1/FVC (%) | r | −0.469** | −0.450** | 0.452** | −0.455** | −0.239* | −0.514** |
| MMEF (%predicted) | r | −0.456** | −0.494** | 0.445** | −0.464** | −0.345** | −0.447** |
Notes:
P<0.05.
P<0.001.
Abbreviations: IOS, impulse oscillometry; Z5, respiratory impedance; Fres, resonant frequency; X5, respiratory reactance; R5–R20, respiratory resistance; FVC, forced vital capacity; r, Pearson’s correlation coefficient; FEV1, forced expiratory volume in 1 second; MMEF, maximum midexpiratory flow rate.
In patients of advanced age, a significant increase in Z5, Fres, R5, R20, and R5–R20 and a decrease in X5 were observed in the COPD group compared with the healthy control group (Table 8). Table 8 also shows a comparison of the IOS parameters between the non-advanced elderly healthy controls and the non-advanced elderly COPD subjects, the non-advanced elderly COPD subjects and the advanced elderly COPD subjects, and the non-advanced elderly healthy controls and the advanced elderly healthy controls. IOS parameters showed no statistically significant differences between the non-advanced elderly healthy controls and advanced elderly healthy controls. The IOS parameters correlated well with the spirometry parameters in the advanced elderly COPD subjects, and R5–R20 and X5 were found to be significantly correlated with FEV1 and FEV1/FVC (Table 9).
Table 8.
Comparison of the pulmonary function parameters between the groups of 80-year-olds
| Healthy controls
|
COPD
|
|||
|---|---|---|---|---|
| Non-advanced elderly | Advanced elderly | Non-advanced elderly | Advanced elderly | |
| FVC (%predicted) | 92.76±16.11 | 100.19±22.74 | 74.45±18.06b | 78.97±19.99a |
| FEV1(%predicted) | 93.25±18.77 | 102.10±21.53 | 49.81±19.89b | 60.97±20.33a,c |
| FEV1/FVC (%) | 77.53±7.01 | 77.72±6.01 | 50.41±13.13b | 56.95±10.84a,c |
| MMEF (%predicted) | 65.81±29.60 | 65.76±2,768 | 17.13±10.27b | 22.90±11.55a,c |
| Z5 (%predicted) | 80.64±30.35 | 78.95±2,901 | 189.76±96.32b | 139.26±56.75a,c |
| Fres (L/s) | 13.81±3.94 | 12.70±3.78 | 23.45±7.15b | 21.62±6.64a |
| X5 (kPa/L/s) | −0.10±0.05 | −0.10±0.07 | −0.33±0.22b | −0.23±0.15a,c |
| R5 (%predicted) | 81.04±23.25 | 76.16±2,704 | 165.98±75.20b | 122.07±43.47a,c |
| R20 (%predicted) | 74.12±16.62 | 68.27±1803 | 105.02±36.65b | 84.53±25.29a,c |
| R5–R20 (%predicted) | 125.50±83.07 | 129.22±108.75 | 567.23±396.92b | 383.68±244.33a,c |
Notes: Data are expressed as the mean ± standard deviation.
P<0.001 compared with the advanced elderly healthy patients.
P<0.001 compared with the non-advanced elderly healthy patients.
P<0.05 compared with the non-advanced elderly COPD patients.
Abbreviations: FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; MMEF, maximum midexpiratory flow rate; Z5, respiratory impedance; Fres, resonant frequency; X5, respiratory reactance; R5–R20, respiratory resistance.
Table 9.
Correlation between the IOS and spirometry parameters in advanced elderly COPD patients
| Z5 (%predicted) | Fres (L/s) | X5 (kPa/L/s) | R5 (%predicted) | R20 (%predicted) | R5–R20 (%predicted) | ||
|---|---|---|---|---|---|---|---|
| FVC (%predicted) | r | −0.377* | −0.264 | 0.280 | −0.334* | −0.110 | −0.466** |
| FEV1 (%predicted) | r | −0.615** | −0.532** | 0.560** | −0.558** | −0.286 | −0.639** |
| FEV1/FVC (%) | r | −0.587** | −0.556** | 0.605** | −0.519** | −0.292 | −0.540** |
| MMEF (%predicted) | r | −0.487** | −0.368* | 0.454** | −0.470** | −0.323* | −0.452** |
Notes:
P<0.05.
P<0.001.
Abbreviations: IOS, impulse oscillometry; Z5, respiratory impedance; Fres, resonant frequency; X5, respiratory reactance; R5–R20, respiratory resistance; FVC, forced vital capacity; r, Pearson’s correlation coefficient; FEV1, forced expiratory volume in 1 second; MMEF, maximum midexpiratory flow rate.
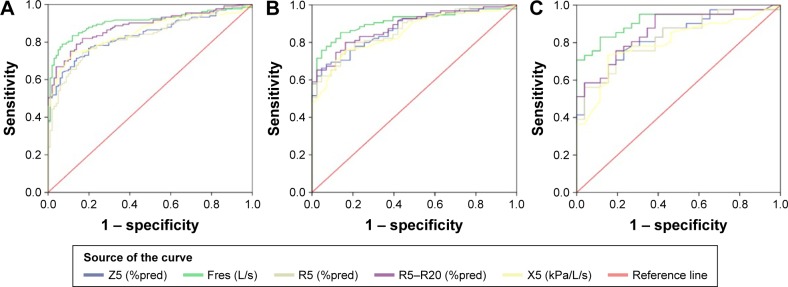
ROC curves were constructed for each of the IOS measurements. The area under the ROC curve for the different grouping categories based on age is shown in Table 10 and Figure 2, and the diagnostic efficiency of IOS parameters for COPD was Fres > R5–R20 > X5 > Z5 > R5 in the overall subjects. Fres and R5–R20 also had the best diagnostic efficiency in the elderly and the advanced elderly COPD subjects. The area under the curve (AUC) values, expressed by the ROC curve, for Fres were 0.905, 0.909, and 0.914 in the overall sample, elderly subjects, and advanced elderly subjects, respectively. The optimal cutoff values for Fres to diagnose airflow obstruction from ROC curves were established in the different age groups, and the cutoff values and their sensitivity, specificity, and Youden’s index are shown in Table 11.
Table 10.
The AUC of IOS parameters in different groups
| IOS parameters | AUC in overall patients | AUC in the geriatric patients | AUC in the advanced elderly patients |
|---|---|---|---|
| Fres (L/s) | 0.905 | 0.909 | 0.914 |
| R5–R20 (%predicted) | 0.883 | 0.885 | 0.863 |
| X5 (kPa/L/s) | 0.854 | 0.850 | 0.800 |
| Z5 (%predicted) | 0.838 | 0.872 | 0.834 |
| R5 (%predicted) | 0.829 | 0.867 | 0.825 |
| R20 (%predicted) | 0.654 | 0.768 | 0.711 |
Abbreviations: ROC, receiver operating characteristic; AUC, area under the curve; IOS, impulse oscillometry; Fres, resonant frequency; R5–R20, respiratory resistance; X5, respiratory reactance; Z5, respiratory impedance.
Figure 2.
ROC curves for COPD in the overall subjects (A), geriatric subjects (B), and advanced elderly subjects (C).
Abbreviations: ROC, receiver operating characteristic; X5, respiratory reactance; R5–R20, respiratory resistance; Fres, resonant frequency; Z5, respiratory impedance.
Table 11.
The cutoff value of Fres and its sensitivity, specificity, and Youden’s index
| Fres cutoff values | Sensitivity | Specificity | Youden’s index | |
|---|---|---|---|---|
| Overall subjects | 17.715 | 0.789 | 0.931 | 0.720 |
| Geriatric subjects | 16.94 | 0.853 | 0.860 | 0.713 |
| Advanced elderly | 16.94 | 0.829 | 0.885 | 0.714 |
Abbreviation: Fres, resonant frequency.
Discussion
Many clinical studies have demonstrated the application value of IOS in COPD, including measuring airway resistance, monitoring drug efficacy, and distinguishing COPD from asthma.11,12 This study compared the spirometry and IOS parameters to explore the application value of IOS in COPD; in particular, we compared the differences in IOS parameters between groups based on age and determined its application value in elderly and advanced elderly patients. Furthermore, this study attempted to identify a cutoff value for IOS to diagnose COPD in patients who failed to undergo spirometry.
COPD is characterized by the incomplete reversibility of airflow limitations; however, it is generally believed that FEV1 is unable to fully evaluate pulmonary function, especially in cases of small airway dysfunction, while IOS can provide more detailed information on pulmonary function, especially on the assessment of peripheral airway conditions, which is the main location of COPD airflow limitation.3,13,14 In this study, FVC, FEV1, FEV1/FVC, and MMEF decreased in COPD patients compared with healthy controls, which reflected airflow limitations. Among the IOS parameters, Z5, Fres, R5, R20, and R5–R20 increased significantly, and the degree of R5–R20 increase was much higher than the degree of R20 increase, which suggested that in COPD, the increase in peripheral airway resistance was predominant with a certain degree of increase in central airway resistance; this was consistent with other research results.15–17 Under physiological conditions, the ratio of peripheral airway resistance to total airway resistance was 10%–25%, but in COPD patients, the percentage of peripheral airway resistance was greatly increased.14 Our results showed that the ratio of R5–R20/R5 increased from healthy controls to COPD 1–4 subjects (17.24%, 32.43%, 39.13%, 48.15%, and 56.06%, respectively). Moreover, X5, the lung compliance index, was significantly decreased, which was consistent with the decrease in lung compliance in COPD patients.
Therefore, IOS can reflect the pathological changes in lung tissue, including airflow limitation, hyperinflation, and lung compliance. R5–R20 reflects peripheral airway resistance, while X5 represents peripheral elastic resistance. Both parameters can evaluate small airway function, and some studies recommend the use of R5–R20 and X5 to evaluate peripheral airway function.6,7
IOS showed a good correlation with spirometry. In all COPD subjects, R5–R20 had a stronger correlation with FVC, FEV1, FEV1/FVC, and MMEF (r=−0.491, −0.628, −0.572, and −0.588, respectively) than X5 (r=0.415, 0.561, 0.517, and 0.555, respectively). Piorunek et al18 showed that the severity of bronchial obstruction, as assessed by spirometry (FEV1%predicted), correlates with the measures obtained using the oscillometric method. The correlation between the FEV1%predicted and oscillometric measurements (R5, R5–R20, and X5) was significant (r=−0.62, −0.80, and 0.75, respectively; P<0.05). The study did not measure Fres, but their results are similar to the correlation results of our study. As described previously, R5–R20 is an index of peripheral airway resistance. In spirometry, MMEF is thought to be an indicator of small airway dysfunction, but the reliability of MMEF has been questioned mainly because a patient’s respiratory forces will gradually decline, while IOS can be unaffected by effort-dependent impedance.9 The correlation between MMEF and R5–R20 was inconsistent in another study,19 possibly because the variability of the measurement was large, while the IOS respiratory system impedance parameters remained fairly constant over 3 months.19
X5 represents lung compliance, which is a measure of how easily the respiratory system can be inflated. It is numerically a negative value, and X5 values that are more negative indicate reduced compliance. The compliance of COPD patients decreased overall, and a previous study has reported results consistent with ours.17 Furthermore, Gong et al20 demonstrated that X5, R5, and Fres are the IOS measurements most closely associated with more traditional measurements of pulmonary function in COPD patients. The associations between FEV1 and the IOS measurements were as follows: X5 (r=0.635), Fres (r=−0.721), and R5 (r=−0.496); these results were consistent with our results. The authors also mentioned that in COPD patients, the IOS reactance measurements were more closely correlated with other pulmonary function measurements rather than with resistance measurements. In addition, it was confirmed that IOS reactance measurements are more informative than resistance measurements regarding the changes in pulmonary mechanics caused by airflow obstruction in COPD patients, and reactance measurements were better than resistance measurements for grading the severity of airflow obstruction in COPD.16,21 These findings are not reflected in our results, possibly because the authors also compared other pulmonary function tests in addition to spirometry. Smith et al suggested COPD classification criteria based on R5 and X5.22
Some studies23,24 have shown that Fres has the strongest correlation with FEV1 and FEV1/FVC. Fres is the point at which the magnitudes of elastance and inertial reactance are equal and opposite, which represents viscous resistance. The AUC showed that Fres had the highest diagnostic value, which is consistent with the results in elderly and advanced elderly COPD patients, which showed that the IOS parameters still had high stability and application value in the elderly. Gong et al25 indicated that IOS indexes would be valuable in diagnosing stage 0 COPD and that IOS parameters (Fres, Z5/pre, R5/pre, R5–R20/pre, and X5) could differentiate the stage 0 group and the control group. The AUC values for the IOS parameters were as follows: Z5/pre > R5/pre > Fres > R5–R20/pre > X5 (0.879, 0.848, 0.820, 0.819, and 0.760, respectively). We also tried to find an IOS parameter to diagnose airflow limitation in individuals who cannot undergo spirometry, and Fres was chosen because of its good AUC value and correlation with FEV1/FVC. The cutoff value of Fres was 17.715 in all COPD patients; sensitivity and specificity were 0.789 and 0.931, respectively; and the cutoff value was similar in the elderly and advanced elderly. These results led us to try to identify independent diagnostic criteria for IOS in a large sample population in China. Although the cutoff value for Fres provided a perspective by which to judge COPD, the Fres parameter itself has its own limitations. Studies have concluded that none of the IOS parameters alone could distinguish healthy individuals from COPD patients.16,26 Fres alone cannot be used to diagnose COPD or distinguish COPD from other pulmonary diseases. Furthermore, we used the postbronchodilator FEV1/FVC ratio to diagnose airflow limitations that are not fully reversible; hence, further studies are needed to determine the application of postbronchodilator IOS parameters for the diagnosis of airflow limitations that are not fully reversible.
Crim et al has shown that despite its numerical significance, age had no significant effect on IOS index.19 Through the comparison of groups based on age, our study showed that geriatric patients can meet the requirements of IOS and that IOS has value in clinical practice for geriatric patients. In geriatric patients, IOS parameters can indicate an increase in resistance and a decrease in compliance in COPD patients compared with healthy controls.
In summary, there was a good correlation between IOS and spirometry parameters, and IOS was found to have good application value in the diagnosis and grading of COPD airflow limitation. Pulmonary function assessment using IOS has not yet been widely carried out in clinical settings, but its non-invasive, simple, force-independent, and repeatable advantages compared with spirometry support its broad application. With an aging society, a technique that caters to the elderly is essential, and further studies are required to explore its application in the elderly.
Conclusion
IOS demonstrated a good correlation with spirometry in COPD patients. IOS had a good application value in the diagnosis and classification of COPD, especially in the elderly, who cannot complete routine pulmonary function tests. This study serves as a good foundation on which to establish independent diagnostic criteria for IOS.
Acknowledgments
The work was supported by the grants from the National Natural Science Foundation of China (30971313, 81141001, 81270114, and 81670043).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet. 2012;379(9823):1341–1351. doi: 10.1016/S0140-6736(11)60968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2016. [Accessed March 7, 2017]. Available from: http://goldcopd.org.
- 3.Tomalak W, Czajkowska-Malinowska M, Radlinski J. Application of impulse oscillometry in respiratory system evaluation in elderly patients. Pneumonol Alergol Pol. 2014;82(4):330–335. doi: 10.5603/PiAP.2014.0041. [DOI] [PubMed] [Google Scholar]
- 4.Czajkowska-Malinowska M, Tomalak W, Radlinski J. Quality of spirometry in the elderly. Pneumonol Alergol Pol. 2013;81(6):511–517. [PubMed] [Google Scholar]
- 5.Pezzoli L, Giardini G, Consonni S, et al. Quality of spirometric performance in older people. Age Ageing. 2003;32(1):43–46. doi: 10.1093/ageing/32.1.43. [DOI] [PubMed] [Google Scholar]
- 6.Abe T, Setoguchi Y, Kono Y, et al. Effects of inhaled tiotropium plus transdermal tulobuterol versus tiotropium alone on impulse oscillation system (IOS)-assessed measures of peripheral airway resistance and reactance, lung function and quality of life in patients with COPD: a randomized crossover study. Pulm Pharmacol Ther. 2011;24(5):617–624. doi: 10.1016/j.pupt.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Prince MJ, Wu F, Guo Y, et al. The burden of disease in older people and implications for health policy and practice. Lancet. 2015;385(9967):549–562. doi: 10.1016/S0140-6736(14)61347-7. [DOI] [PubMed] [Google Scholar]
- 8.Saadeh C, Saadeh C, Cross B, Gaylor M, Griffith M. Advantage of impulse oscillometry over spirometry to diagnose chronic obstructive pulmonary disease and monitor pulmonary responses to bronchodilators: an observational study. SAGE Open Med. 2015;3:2050312115578957. doi: 10.1177/2050312115578957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 10.Oostveen E, MacLeod D, Lorino H, et al.ERS Task Force on Respiratory Impedance Measurements, editors. The forced oscillation technique in clinical practice: methodology, recommendations and future developments. Eur Respir J. 2003;22(6):1026–1041. doi: 10.1183/09031936.03.00089403. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Chen Y, Wang P. Application of impulse oscillometry and bronchial dilation test for analysis in patients with asthma and chronic obstructive pulmonary disease. Int J Clin Exp Med. 2015;8(1):1271–1275. [PMC free article] [PubMed] [Google Scholar]
- 12.Nikkhah M, Amra B, Eshaghian A, et al. Comparison of impulse osillometry system and spirometry for diagnosis of obstructive lung disorders. Tanaffos. 2011;10(1):19–25. [PMC free article] [PubMed] [Google Scholar]
- 13.Frantz S, Nihlén U, Dencker M, Engström G, Löfdahl CG, Wollmer P. Impulse oscillometry may be of value in detecting early manifestations of COPD. Respir Med. 2012;106(8):1116–1123. doi: 10.1016/j.rmed.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 14.McNulty W, Usmani OS. Techniques of assessing small airways dysfunction. Eur Clin Respir J. 2014;17:1. doi: 10.3402/ecrj.v1.25898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Mutairi SS, Sharma PN, Al-Alawi A, Al-Deen JS. Impulse oscillometry: an alternative modality to the conventional pulmonary function test to categorise obstructive pulmonary disorders. Clin Exp Med. 2007;7(2):56–64. doi: 10.1007/s10238-007-0126-y. [DOI] [PubMed] [Google Scholar]
- 16.Kolsum U, Borrill Z, Roy K, et al. Impulse oscillometry in COPD: identification of measurements related to airway obstruction, airway conductance and lung volumes. Respir Med. 2009;103(1):136–143. doi: 10.1016/j.rmed.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 17.Pisi R, Aiello M, Zanini A, et al. Small airway dysfunction and flow and volume bronchodilator responsiveness in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2015;10:1191–1197. doi: 10.2147/COPD.S82509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piorunek T, Kostrzewska M, Cofta S, et al. Impulse oscillometry in the diagnosis of airway resistance in chronic obstructive pulmonary disease. Adv Exp Med Biol. 2015;838:47–52. doi: 10.1007/5584_2014_49. [DOI] [PubMed] [Google Scholar]
- 19.Crim C, Celli B, Edwards LD, et al. Respiratory system impedance with impulse oscillometry in healthy and COPD subjects: ECLIPSE baseline results. Respir Med. 2011;105(7):1069–1078. doi: 10.1016/j.rmed.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Gong SG, Yang WL, Zheng W, Liu JM. Evaluation of respiratory impedance in patients with chronic obstructive pulmonary disease by an impulse oscillation system. Mol Med Rep. 2014;10(5):2694–2700. doi: 10.3892/mmr.2014.2528. [DOI] [PubMed] [Google Scholar]
- 21.Di Mango AM, Lopes AJ, Jansen JM, Melo PL. Changes in respiratory mechanics with increasing degrees of airway obstruction in COPD: detection by forced oscillation technique. Respir Med. 2006;100(3):399–410. doi: 10.1016/j.rmed.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Smith HJ, Eichler R, Vogel J, et al. Technical adaptation of impulse oscillometry to special research conditions. Pneumologie. 1997;51(suppl 2):465–468. [PubMed] [Google Scholar]
- 23.Wang M, Niu S, Li Y, Zhang Z, Bai C. The diagnostic value of total respiratory impedance by impulse oscillometry in chronic obstructive lung disease. Chin Med J (Engl) 1999;112(11):982–984. [PubMed] [Google Scholar]
- 24.Xi Z, Zhang Z, Cheng K, et al. The diagnostic utility of the Macao predictive values of impulse oscillometry for chronic obstructive pulmonary disease in patients over 45 years old. Zhonghua Jie He He Hu Xi Za Zhi. 2016;39(1):41–46. doi: 10.3760/cma.j.issn.1001-0939.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Gong SG, Yang WL, Liu JM, Liu WZ, Zheng W. Change in pulmonary function in chronic obstructive pulmonary disease stage 0 patients. Int J Clin Exp Med. 2015;8(11):21400–21406. [PMC free article] [PubMed] [Google Scholar]
- 26.Kanda S, Fujimoto K, Komatsu Y, Yasuo M, Hanaoka M, Kubo K. Evaluation of respiratory impedance in asthma and COPD by an impulse oscillation system. Intern Med. 2010;49(1):23–30. doi: 10.2169/internalmedicine.49.2191. [DOI] [PubMed] [Google Scholar]