Abstract
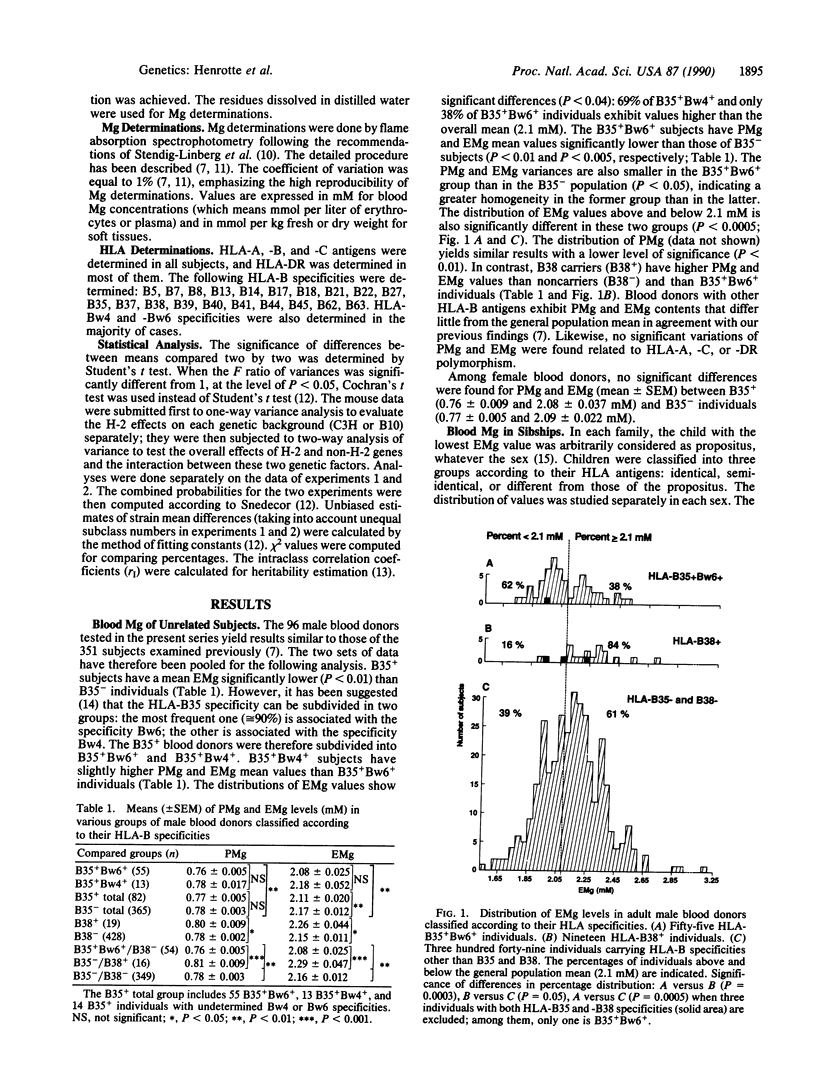
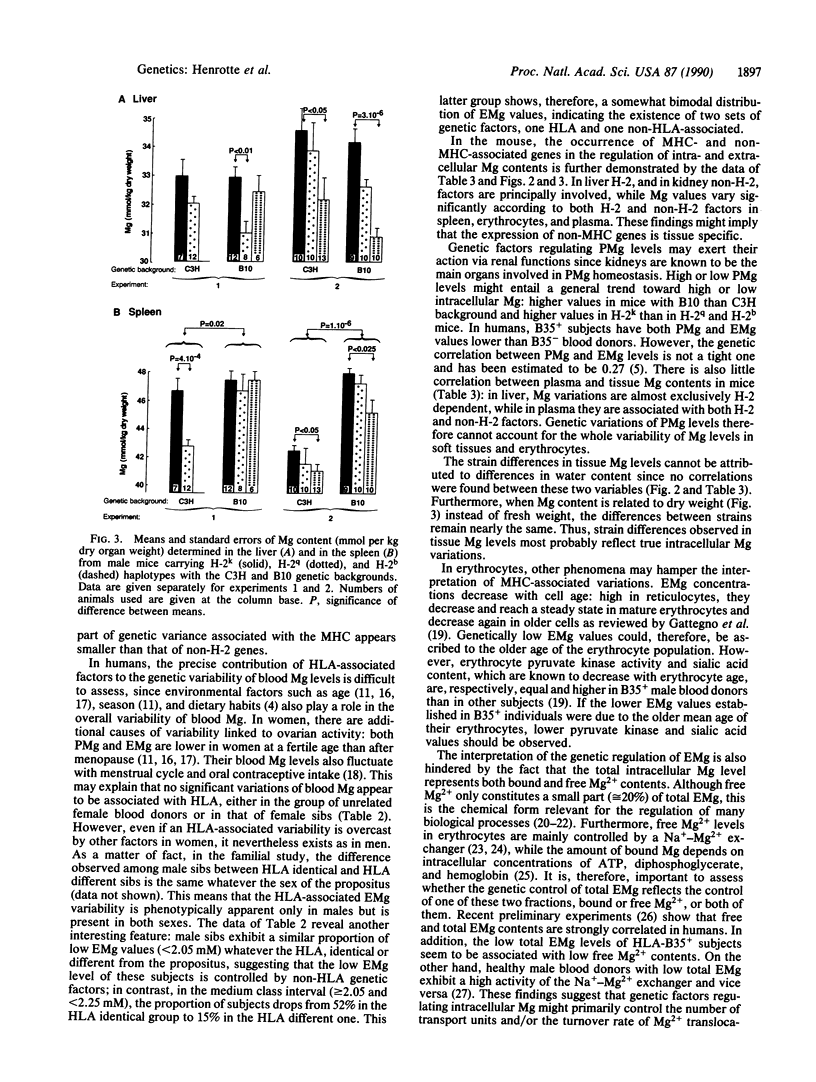
Erythrocyte and plasma magnesium (EMg, PMg) levels have been shown to be genetically controlled in human and mouse. The possible association of these genetic factors with the major histocompatibility complex (MHC) (HLA and H-2) was investigated. Among unrelated adult male blood donors, HLA-B35 carriers have PMg (P less than 0.01) and EMg (P less than 0.0005) levels lower than those of noncarriers, while HLA-B38 carriers exhibit a significant (P less than 0.05) increase of both PMg and EMg levels when compared to the other individuals. Furthermore, HLA identical sibs have EMg values more similar than those of HLA different sibs. In the mouse, erythrocyte (P less than 0.001), plasma (P less than 0.001), liver (P less than 0.03), and spleen (P less than 0.04) Mg contents vary significantly according to H-2, with higher values being found in H-2k than in H-2q or H-2b congenic strains. However, non-MHC genes also have an influence on erythrocyte (P less than 10(-10], plasma (P less than 10(-10], spleen (P less than 10(-5], and kidney (P less than 10(-6] Mg contents as shown by differences between H-2 identical strains, which differ by the C3H and B10 genetic backgrounds. In conclusion, genetic factors controlling intra- and extracellular Mg levels are composed of at least three components: MHC (HLA and H-2)-associated genes, non-MHC genes, and tissue factors modulating the respective importance of the first two sets of factors. The mechanisms underlying this genetic system are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blazsek I., Mathé G. Zinc and immunity. Biomed Pharmacother. 1984;38(4):187–193. [PubMed] [Google Scholar]
- Brinley F. J., Jr Calcium and magnesium transport in single cells. Fed Proc. 1973 Jul;32(7):1735–1739. [PubMed] [Google Scholar]
- Bryan C. F., Leech S. H. The immunoregulatory nature of iron. I. Lymphocyte proliferation. Cell Immunol. 1983 Jan;75(1):71–79. doi: 10.1016/0008-8749(83)90306-4. [DOI] [PubMed] [Google Scholar]
- Darlu P., Michotte Y., Defrise-Gussenhoven E., Henrotte J. G. The inheritance of plasma and red blood cell magnesium and zinc levels studied from twin and family data. Acta Genet Med Gemellol (Roma) 1981;30(1):67–75. doi: 10.1017/s0001566000006632. [DOI] [PubMed] [Google Scholar]
- Darlu P., Rao D. C., Henrotte J. G., Lalouel J. M. Genetic regulation of plasma and red blood cell magnesium concentrations in man. I. Univariate and bivariate path analyses. Am J Hum Genet. 1982 Nov;34(6):874–887. [PMC free article] [PubMed] [Google Scholar]
- Dausser J., Heurotte J. G. HLA and Bw35. Possible influence of Mg metabolism. Tissue Antigens. 1982 Aug;20(2):81–85. doi: 10.1111/j.1399-0039.1982.tb00330.x. [DOI] [PubMed] [Google Scholar]
- David V., Papadopoulos P., Yaouanq J., Blayau M., Abel L., Zappone E., Perichon M., Drysdale J., Le Gall J. Y., Simon M. Ferritin H gene polymorphism in idiopathic hemochromatosis. Hum Genet. 1989 Jan;81(2):123–126. doi: 10.1007/BF00293887. [DOI] [PubMed] [Google Scholar]
- DeGoey S. R., Moore S. B., Watts S. K., Radue V. A., Testin J. Heterogeneity of HLA-BW35 based on the diallelic BW4-BW6 system. Mayo Clin Proc. 1979 Aug;54(8):527–530. [PubMed] [Google Scholar]
- Flatman P. W., Lew V. L. Magnesium buffering in intact human red blood cells measured using the ionophore A23187. J Physiol. 1980 Aug;305:13–30. doi: 10.1113/jphysiol.1980.sp013346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Féray J. C., Garay R. An Na+-stimulated Mg2+-transport system in human red blood cells. Biochim Biophys Acta. 1986 Mar 27;856(1):76–84. doi: 10.1016/0005-2736(86)90012-x. [DOI] [PubMed] [Google Scholar]
- Galland L. Magnesium and immune function: an overview. Magnesium. 1988;7(5-6):290–299. [PubMed] [Google Scholar]
- Gattegno L., Henrotte J. G., Benbunan M. Content of red blood-cell sialic acid in BW 35 blood donors. Relation to magnesium concentration and pyruvate kinase activity. Carbohydr Res. 1985 Oct 1;142(1):115–122. doi: 10.1016/s0008-6215(00)90738-9. [DOI] [PubMed] [Google Scholar]
- Gupta C., Goldman A. H-2 histocompatibility region: influence on the murine glucocorticoid receptor and its response. Science. 1982 May 28;216(4549):994–996. doi: 10.1126/science.7079749. [DOI] [PubMed] [Google Scholar]
- Gupta R. K., Benovic J. L., Rose Z. B. The determination of the free magnesium level in the human red blood cell by 31P NMR. J Biol Chem. 1978 Sep 10;253(17):6172–6176. [PubMed] [Google Scholar]
- Günther T., Vormann J. Mg2+ efflux is accomplished by an amiloride-sensitive Na+/Mg2+ antiport. Biochem Biophys Res Commun. 1985 Jul 31;130(2):540–545. doi: 10.1016/0006-291x(85)90450-4. [DOI] [PubMed] [Google Scholar]
- Henrotte J. G., Colombani J., Pineau M., Dausset J. Role of H-2 and non-H-2 genes in the control of blood magnesium levels. Immunogenetics. 1984;19(5):435–448. doi: 10.1007/BF00364646. [DOI] [PubMed] [Google Scholar]
- Henrotte J. G. Genetic regulation of blood and tissue magnesium content in mammals. Magnesium. 1988;7(5-6):306–314. [PubMed] [Google Scholar]
- Henrotte J. G., Hannoun C., Benech A., Dausset J. Relationship between postvaccinal anti-influenza antibodies, blood magnesium levels, and HLA antigens. Hum Immunol. 1985 Jan;12(1):1–8. doi: 10.1016/0198-8859(85)90249-6. [DOI] [PubMed] [Google Scholar]
- Henrotte J. G., Santarromana M., Pla M. Genetic factors regulating zinc concentrations in mice spleen and liver: relationship with the H-2 complex. Immunogenetics. 1987;25(6):408–410. doi: 10.1007/BF00396108. [DOI] [PubMed] [Google Scholar]
- Henrotte J. G. The variability of human red blood cell magnesium level according to HLA groups. Tissue Antigens. 1980 May;15(5):419–430. doi: 10.1111/j.1399-0039.1980.tb00204.x. [DOI] [PubMed] [Google Scholar]
- Iványi P. Some aspects of the H-2 system, the major histocompatibility system in the mouse. Proc R Soc Lond B Biol Sci. 1978 Jun 5;202(1146):117–158. doi: 10.1098/rspb.1978.0060. [DOI] [PubMed] [Google Scholar]
- Lowenstein F. W., Stanton M. F. Serum magnesium levels in the United States, 1971-1974. J Am Coll Nutr. 1986;5(4):399–414. doi: 10.1080/07315724.1986.10720143. [DOI] [PubMed] [Google Scholar]
- Page E., Polimeni P. I. Magnesium exchange in rat ventricle. J Physiol. 1972 Jul;224(1):121–139. doi: 10.1113/jphysiol.1972.sp009884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stendig-Lindberg G., Penciner J., Rudy N., Wacker W. E. Comparison of diluents for serum magnesium estimation by atomic absorption spectrophotometry. Magnesium. 1984;3(1):50–56. [PubMed] [Google Scholar]



