Abstract
Exercise can enhance learning and memory and produce resistance against stress-related psychiatric disorders such as depression and anxiety. In rats, these beneficial effects of exercise occur regardless of exercise controllability: both voluntary and forced wheel running produce stress-protective effects. The mechanisms underlying these beneficial effects of exercise remain unknown. The mammalian target of rapamycin (mTOR) is a translation regulator important for cell growth, proliferation, and survival. mTOR has been implicated in enhancing learning and memory as well as antidepressant effects. Moreover, mTOR is sensitive to exercise signals such as metabolic factors. The effects of exercise on mTOR signaling, however, remain unknown. The goal of the present study was to test the hypothesis that exercise, regardless of controllability, increases levels of phosphorylated mTOR (p-mTOR) in brain regions important for learning and emotional behavior. Rats were exposed to 6 weeks of either sedentary (locked wheel), voluntary, or forced wheel running conditions. At 6 weeks, rats were sacrificed during peak running and levels of p-mTOR were measured using immunohistochemistry. Overall, both voluntary and forced exercise increased p-mTOR-positive neurons in the medial prefrontal cortex, striatum, hippocampus, hypothalamus, and amygdala compared to locked wheel controls. Exercise, regardless of controllability, also increased numbers of p-mTOR-positive glia in the striatum, hippocampus, and amygdala. For both neurons and glia, the largest increase in p-mTOR positive cells was observed after voluntary running, with forced exercise causing a more modest increase. Interestingly, voluntary exercise preferentially increased p-mTOR in astrocytes (GFAP+), while forced running increased p-mTOR in microglia (CD11+) in the inferior dentate gyrus. Results suggest that mTOR signaling is sensitive to exercise, but subtle differences exist depending on exercise controllability. Increases in mTOR signaling could contribute to the beneficial effects of exercise on cognitive function and mental health.
INTRODUCTION
Exercise can enhance learning and memory [1–4] and reduce the incidence of stress-related psychiatric disorders such as depression [5–11] and anxiety [12–14]. In rats, these beneficial effects of exercise occur regardless of exercise controllability. Both voluntary wheel running [15–18] and forced treadmill training [19–21] improve aspects of cognition, and both voluntary and forced wheel running produce protective effects against the development of stress-induced anxiety- and depression-like behavior [22]. Identification of the mechanisms underlying these beneficial effects of exercise could lead to novel therapeutic approaches.
The mammalian target of rapamycin (mTOR), a serine/threonine kinase important for cell growth, proliferation, and survival [23], has been increasingly implicated in cognitive function [24–27]. For example, learning transiently increases p-mTOR in the hippocampus [28, 29] and blockade of mTOR signaling with Rapamycin impairs hippocampus-dependent learning in tasks such as inhibitory avoidance [30], and both voluntary [31, 32] and forced [33] exercise enhance learning in this same task. Considering that mTOR activates proteins involved in synaptic protein synthesis such as ribosomal S6 kinase 1 (RS6K1) and eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1) [34, 35], enhanced synaptic plasticity could contribute to the beneficial effects of mTOR on cognitive function. Indeed, mTOR can increase dendritic arborization in the hippocampus via calmodulin-dependent protein kinase IIα (CaMKIIα) [27] and downstream activation of RS6K1 has been reported to enhance dendritic arborization in the PFC [36]. Interestingly, Rapamycin has been recently reported to block the facilitation of hippocampal long-term potentiation provided by an enriched environment [37], suggesting that mTOR signaling could be critical for beneficial effects of environmental manipulations on cognitive function.
In addition to improving cognitive function, mTOR has also been implicated in providing antidepressant effects [36, 38–40]. For example, inhibition of mTOR signaling in the prefrontal cortex (PFC) with Rapamycin can block the antidepressant effects of low-dose ketamine [40]. Moreover, inactivation of RS6K1, a protein that can increase dendritic arborization, in the PFC can cause depressive-like behavior in the absence of external stressors [36].
It may not be a coincidence that the beneficial effects of mTOR signaling seem to parallel the broad benefits of exercise. In fact, mTOR is a compelling candidate for mediating the cognitive benefits and anti-depressant effects of exercise. mTOR signaling is sensitive to metabolic signals such as glucose [41] and amino acids [42–44]; availability of which are increased in circulation and the brain during exercise [45–47]. Additionally, mTOR signaling is also stimulated by factors such as glutamate, tumor necrosis factorα (TNF α) and receptor activator of nuclear factor kappa-B ligand (RANKL) [46, 48, 49], as well as by growth factors such as brain-derived neurotrophic factor (BDNF) [50] and insulin-like growth factor-1 (IGF-1) [51, 52]. The growth factors BDNF and IGF-1 are increased by exercise [53, 54] and are thought to be important for the enhanced plasticity [53], cognitive benefits [55] and antidepressant effects [56] conferred by exercise. BDNF signaling, for example, can lead to phosphorylation of mTOR through the PI3K/AKT intracellular cascade [48, 57–59]. Thus, it is possible that mTOR signaling represents a common downstream target of exercise signals important for beneficial effects of exercise [60].
Although it has been previously reported that forced treadmill training increases mTOR signaling in the brain [61–63], these prior studies focused principally on the hippocampus. This attention to the hippocampus is logical due to the consistent beneficial effects of treadmill training on hippocampal-dependent memory [33, 64, 65]. However, forced treadmill training has produced inconsistent anxiolytic and antidepressant effects in rodents (for a review, [66]). Voluntary wheel running, on the other hand, produces well known antidepressant and anxiolytic effects [6, 13, 66–69], and both voluntary and forced wheel running increase resistance against the depression- and anxiety-like behavioral effects of stress [22]. The effects of exercise on mTOR signaling in brain regions involved in emotional regulation beyond the hippocampus; however, remain unknown.
The goal of the current experiment was to test the hypothesis that both voluntary and forced wheel running increase phosphorylation of mTOR in brain regions implicated in cognitive function and emotional behavior. Forced exercise was controlled by a motorized wheel driven by software designed to rotate the wheel in a pattern that closely resembles natural running behavior [22]. Immunohistochemistry was utilized to allow quantification of both neurons and glia in specific brain subregions, as p-mTOR in these cell types could have different effects on neural circuit function and behavior.
MATERIALS AND METHODS
Subjects
A total of 23, young adult (between P42-P49 upon arrival), male Fischer 344 rats (Harlan SPF, Indianapolis, IN., USA) were pair housed in Nalgene Plexiglas cages (45 cm × 25.2 cm × 14.7 cm) in a humidity-controlled environment at a temperature of 22°C. The rats were maintained on a 12:12 hour light:dark cycle with the lights on 07:00 −19:00 h. All rats had ad libitum access to water and food (lab chow). Rats were weighed weekly and acclimated to these housing conditions for 7 days prior to any experimental manipulation. Precautions were taken to minimize animal discomfort during all procedures. The University of Colorado Boulder Animal Care and Use Committee approved all experimental protocols.
Exercise Protocols
Rats were randomly assigned to one of three conditions: locked wheel (n = 8), voluntary running wheel (n = 8), or forced running wheel (n = 7). Voluntary and forced running protocols were carried out as previously described [22, 70]. To minimize non-running behaviors in the forced wheels (e.g. tumbling, hanging onto wheel rungs), experience with voluntary wheel running is required. Motorized wheels belonging to rats in the forced exercise group were therefore removed from the motor and all rats in the voluntary and forced wheel running conditions were allowed voluntary access to their individual wheels for 5 consecutive active (dark) cycles prior to the start of differential group treatment. Wheels belonging to rats in the forced wheel running group were reconnected to motors following these 5 nights of voluntary running, and all subsequent running by the forced exercise group was controlled by the motor. Rats were transported 5 nights a week from their home cages to their assigned locked, freely mobile or motorized running wheels (1.1 m circumference; Lafayette Instruments, Lafayette, IN., USA). The forced wheels were driven by a motor controlled by the Activity Wheel Monitor software (Lafayette Instruments, Lafayette, IN., USA) according to a protocol pre-programmed by the experimenters based on previous analysis of F344 rats’ natural voluntary running behavior [22]. In order to minimize tumbling in the forced wheel, the motorized wheels slowly increased in speed during the first few days of forced running, and running speeds in the forced wheel running group were kept below 17 m/min for the duration of the experiment. Due to the slower speeds run by the forced exercise rats, the average distance run per day of rats in the forced wheel running condition was less than that of the voluntary wheel condition [70]. Nevertheless, the pattern of exercise, namely the duration of rest periods (range 0.33–30 min) and running bout length (average 2.04±1.95 minutes), were carefully matched between voluntary and forced wheel running conditions [22, 70]. Stress-protective [22] and rewarding [70] effects of this forced running pattern have been previously described. Rats in the locked, freely-mobile, and motorized groups were confined to their wheels for their assigned running periods, which was the entire 12h duration of the active cycle. We have previously observed that rats eat and drink equal amounts regardless of exercise condition [22]. A food tray and water bottle mounted on the side of the wheel allowed access to food and water while rats were confined to the wheel. Locked, freely-mobile, and motorized wheel conditions continued for 5 nights / week for 6 weeks following the initial 5 nights of voluntary running. The Lafayette Activity Wheel Monitor software automatically recorded wheel revolutions. The analyses of voluntary and forced running patterns of these rats in addition to body weight data have been previously published [70].
Single p-mTOR Immunohistochemistry
On the last night of the experiment, rats were transported to their locked, freely-mobile or motorized wheel as usual and were allowed to run at least 2 hours prior to being removed from the wheels and deeply anesthetized with sodium pentobarbital. Two hours was required to perfuse all the rats, thus rats remained in their wheels between 2 – 4 hours prior to sacrifice. Times prior to sacrifice were counterbalanced between groups. Rats were perfused transcardially with cold saline, followed by 300–400 ml of 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB). Brains were extracted, post-fixed in PFA overnight, and then transferred to 30% sucrose solution for 3 days. Brains were then flash frozen in isopentane with dry ice and stored at −70°C. Brains were sliced on a cryostat at 35 µm in coronal sections. Brain slices were stored in cryoprotectant at −20°C prior to staining. All tissue from each brain region was processed in 25-well staining dishes so that direct group comparisons could be made with three tissue sections per rat. The number of rats per group differed slightly (from n=6 to n=8) within and between brain regions due to tissue damage incurred during slicing or IHC.
Immunohistochemistry (IHC) was performed as previously described [6, 70–72] on brain sections containing (from rostral to caudal) PFC (3.7mm to 1.70mm rostral from Bregma), striatum (1.6mm to 0.2mm rostral from Bregma), hippocampus/amygdala (−2.12mm to −4.52mm caudal from Bregma) and hypothalamus (−0.20mm to −3.30mm caudal from Bregma). Sections were rinsed for 10 min 3 times in 0.01 M tris-buffered saline (TBS) followed by a 40 min incubation in a solution of 0.3% hydrogen peroxide, 3.75% Triton-X in 0.01 M TBS. Sections were incubated for 48 h at 4°C in blocking solution containing 2.5% Triton X, 5% normal goat serum (NGS), and rabbit anti-p-S2448-mTOR (catalog number 49F9, Cell Signaling, Danvers, MA) at 1:1000. Our study focused on phosphorylated S2448 mTOR (p-mTOR), as this is the phosphorylation site that has been shown to activate proteins downstream of mTOR such as RS6K1 [40, 73, 74]. The specificity of the chosen antibody has been demonstrated by western blot [75, 76]. This antibody has also been used by others to quantify p-mTOR in brain [77, 78], including after treadmill training [62].
Incubation in primary antisera was followed by another series of washes for 10 minutes 3 times in 0.01 M phosphate buffed saline (PBS) with 0.75% Triton-X and 5% NGS. The sections were incubated at room temperature (RT) for 120 min in blocking solution containing a 1:200 dilution of biotinylated goat anti-rabbit IgG (Jackson Immunoresearch, West Grove, PA). The brain sections were rinsed for 10 minutes 3 times in 0.01 M PBS. Sections were then incubated with avidin-biotin-horseradish peroxidase complexes (ABC; Vecastain Elite ABC kit, Vector Laboratories, Burlingame, CA) in PBS containing 0.5% Triton X for 2 h. After 3 washes for 10 min with 0.1 M PB, sections were placed in a solution containing 3,3’-diaminobenzidene tetrahydrochloride (DAB) and glucose oxidase in PBS for 10 min. The peroxidase reaction was started by addition of glucose solution and reacted for ~15 min, yielding a dark brown reaction product. The reaction was stopped by 3 rinses for 10 min in PBS. Stained sections were mounted onto gelatin coated, glass slides and air-dried overnight. Slide-mounted sections were dehydrated in a series of alcohols, rinsed in Histoclear, and cover-slipped with Permount.
Double Fluorescent IHC
Tissue slices from the hippocampus and amygdala from a subset of rats with numbers of p-mTOR-positive glia closest to their group’s mean were used to identify the type of neuroglia expressing p-mTOR in these regions (n=2 / group), because the highest number of p-mTOR-positive glia were observed in these regions. The brain sections were washed in 0.01 M phosphate buffer saline (PBS) for 10 min 3 times, followed by a 1 h incubation in a blocking solution of 5% normal goat serum (NGS) in PBS. Sections were incubated for 48 h at 4°C in blocking solution containing rabbit anti-p-mTOR (catalog number 49F9, Cell Signaling, Danvers, MA) at 1:500 and then a solution containing rat monoclonal GFAP (marker of astrocytes; catalog number G3893, Sigma-Aldrich, St Louis, MO) or rat monoclonal CD11 (OX42) (marker of microglia; catalog number 554859, BD Pharmingen, San Jose, CA) at 1:500. The next steps of the procedure were completed under low light. The brain sections were washed again in 0.01 M phosphate buffer saline (PBS) for 10 min 3 times, followed by a 2 h incubation at RT in a solution of goat anti-mouse Alexa Fluor 488 IgG (catalog number A11001, BD Pharmingen, San Jose, CA) at 1:400. Then, a 2 h incubation at RT in a solution of goat anti-rabbit Alexa Fluor 594 IgG at 1:400 (catalog number A11007, BD Pharmingen, San Jose, CA). The reaction was stopped by rinses in PBS. The coverslips were mounted using ProlongGold and sealed with clear nail polish.
Quantification of immunohistochemistry
Images of the prelimbic (PL) and infralimbic (IL) PFC, dorsal lateral (DLS) and dorsal medial (DMS) striatum, nucleus accumbens core (NAcC) and nucleus accumbens shell (NAcS), central (CeA) and basolateral (BLA) amygdala, superior and inferior dentate gyrus (DG), cornu ammonis (CA1, CA2, CA3), median preoptic nucleus (MnPO), medial preoptic area (MPO), dorsomedial hypothalamus (DMH), ventromedial hypothalamus (VMH), and paraventricular nucleus (PVN) were captured digitally at 40X on an Olympus BX51 microscope. Quantification of neurons, glia, and unbiased densitometry was done with VisioPharm Integrator System software by multiple experimenters blind to treatment condition of the rats. Pearson’s correlation resulted in an inter-rater reliability of 0.8 (p<0.0001).
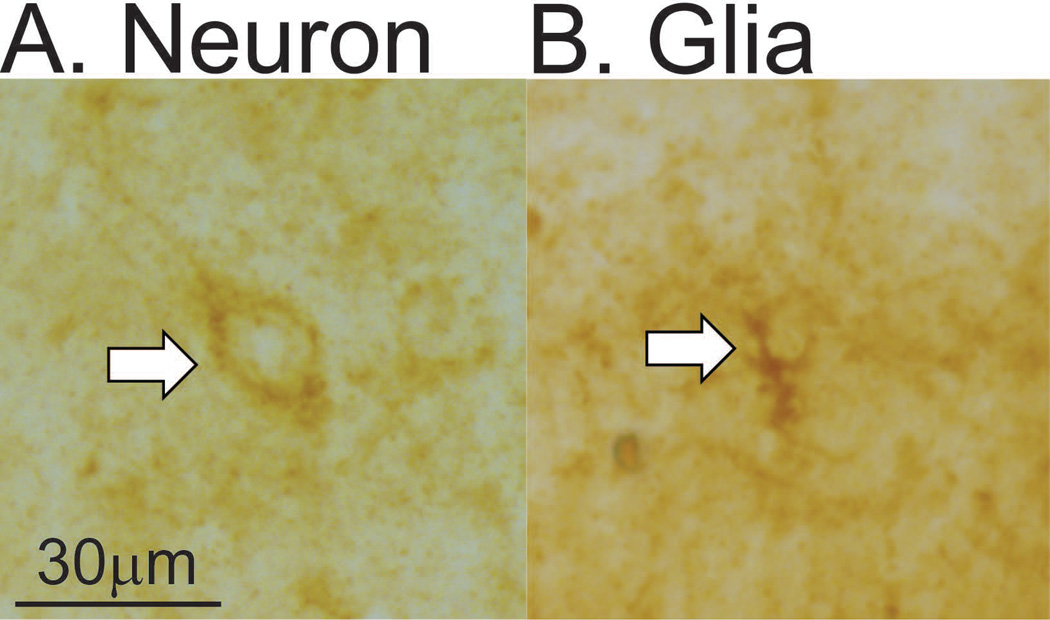
Cell counts and densitometry values were collected from 3 tissue sections and averaged for each region. The number of hemispheres quantified in each rat differed slightly due to damage incurred during slicing or IHC. Only neurons and glia staining dark brown were counted as p-mTOR-positive cells and cells that did not lie completely within the region of interest or were out of focus were not counted. Neurons and glia were distinguished based on criteria including number of processes and clarity of nucleus. Representative photomicrograms of a neuron and glia are provided in Figure 1A and 1B, respectively. Previous studies have also documented p-mTOR-positive glia based on morphology [79]. The cell counts based on morphology were confirmed by the double-immunofluorescence for p-mTOR and glial markers GFAP and CD11, which yielded p-mTOR-positive glial counts that did not differ from the total number of p-mTOR-positive glia observed using morphological identification (p>0.05 in the DG and BLA). Densitometry values collected with Visiopharm measure the average intensity of all pixels in the region of interest and serves as an unbiased measure because it cannot be influenced by the observer. A 450 µm by 450 µm square was drawn around the region of interest and quantification of single p-mTOR took place within that area. All quantification was performed with the same size region of interest and converting quantification to cells per unit area did not impact the results. A Zeiss 700 laser scanning confocal microscope coupled to Axiovision BIO software was used to capture double-fluorescent images of the inferior DG and BLA. The number of single-labeled astrocytes (GFAP+) and microglia (CD11+), as well as double-labeled GFAP/p-mTOR and CD11/p-mTOR glia were quantified with Zeiss software.
Figure 1.
Quantification parameters of neurons and glia. A) Representative photomicrogram of a p-mTOR-positive neuron. B) Representative photomicrogram of a p-mTOR-positive glia.
Statistical Analyses
One-way ANOVA was used to analyze group differences in expression of p-mTOR in neuron and glial cells, unbiased densitometry, and numbers of CD11, GFAP, and double-labeled cells. Analyses were followed by Fisher’s protected least significant difference (PLSD) post hoc tests when appropriate. Regression analyses were completed in each region test the hypothesis that average weekly running distance or running distance on the last night of running prior to sacrifice are related to levels of p-mTOR. Group differences were considered different when p ≤ 0.05.
RESULTS
Voluntary exercise had minimal effects on mTOR signaling in the PFC
Neurons
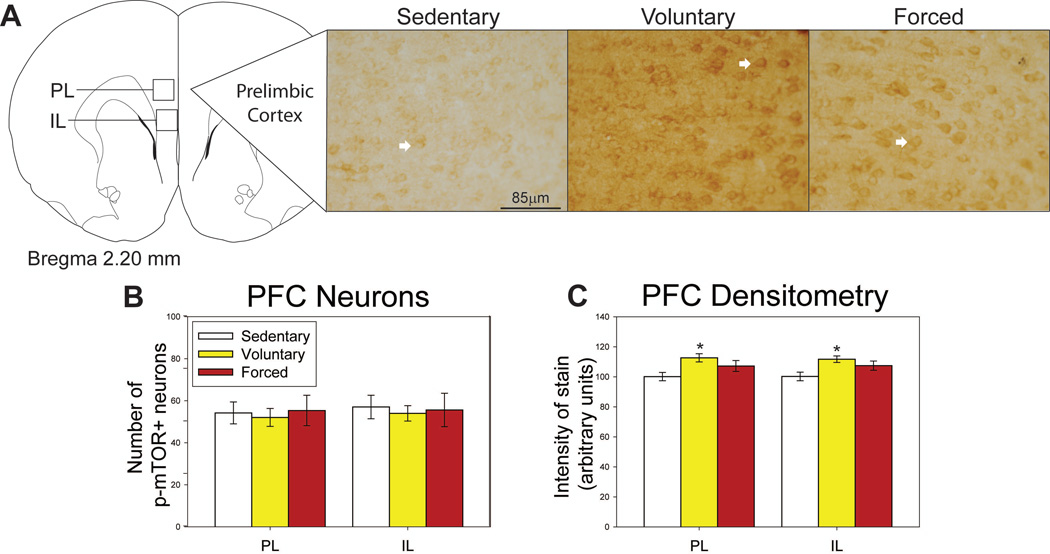
Regions where p-mTOR was assessed in the PFC, as well as representative photomicrographs, are shown in Figure 2A. Neither voluntary nor forced exercise increased the number of p-mTOR-positive neurons in the PL (F(2,20) = 0.090; p=0.914; Figure 2B) or IL (F(2,20) = 0.033; p=0.967; Figure 2B). Average weekly distance ran by the voluntary running group negatively correlated with the number of p-mTOR-positive neurons in the PL (R = −0.720; F(1,6) = 6.468; p= 0.0439) and the IL (R = −0.845; F(1,6) = 15.007; p=0.0082; data not shown).
Figure 2.
Effects of exercise on p-mTOR in the prefrontal cortex (PFC). A) Brain atlas diagram modified from Paxinos and Watson (2006) and representative photomicrograms depicting p-mTOR staining in the prelimbic (PL) of sedentary (left), voluntary run (middle) and forced run (right) groups. White arrows indicate examples of p-mTOR-positive neurons. B) Exercise does not alter the number of p-mTOR-positive neurons in either the PL or the infralimbic (IL) cortices. C) Voluntary exercise increases the intensity of stain in the PL and IL compared to sedentary rats. *p<0.01 relative to sedentary rats. Bars represent group means ± SEM.
Densitometry
Despite the lack of significant differences in p-mTOR-positive cell counts, voluntary exercise significantly increased the intensity of p-mTOR stain as measured by densitometry in both the PL (F(2,20) = 4.715; p=0.0226) and IL (F(2,20) = 4.920; p=0.0197; see Figure 2C for post-hoc analyses). p-mTOR-positive glia were not observed in the PFC. No significant correlations between running distance and intensity of p-mTOR stain in the PFC were found.
Exercise increased numbers of p-mTOR-positive neurons and glia in the striatum
Neurons
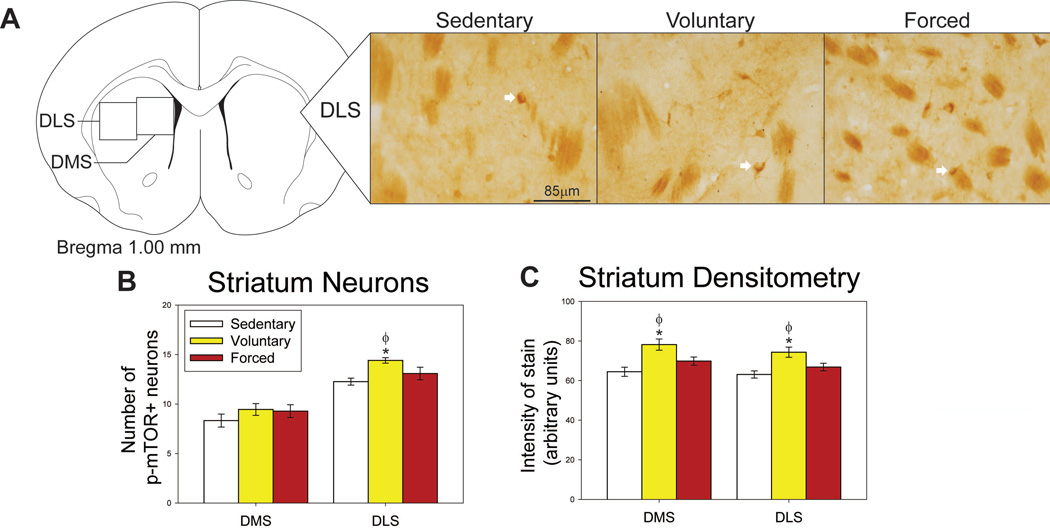
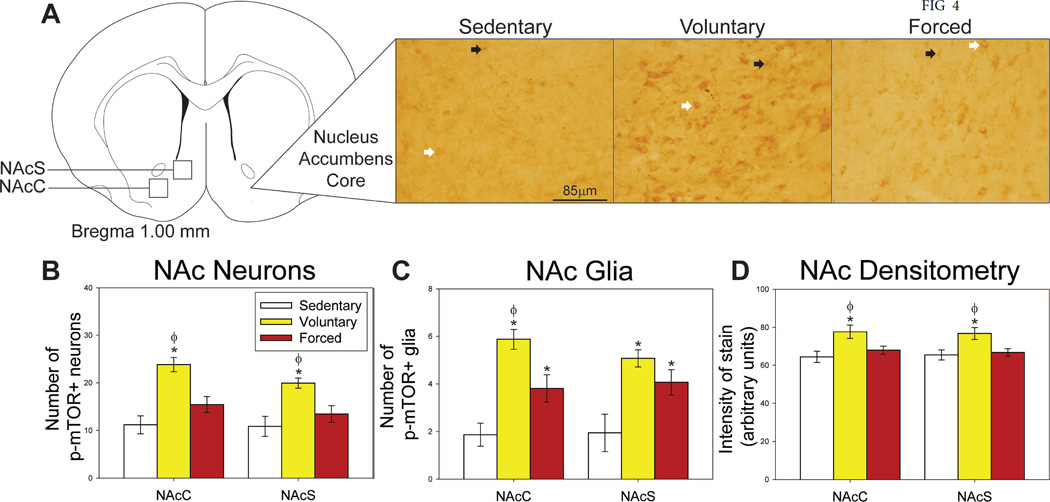
Regions where p-mTOR was assessed in the dorsal and ventral striatum, as well as representative photomicrographs, are shown in Figures 3A and 4A. Group differences in the number of p-mTOR-positive neurons were observed in the DLS (F(2,19) = 0.917; p=0.04168; Figure 3B), NAcC (F(2,19) = 15.191; p=0.0001; Figure 4B) and NAcS (F(2,19) = 8.488; p = 0.0023; Figure 4B). Post-hoc analyses reveal that only voluntary exercise increased the number of p-mTOR-positive neurons in these areas compared to the sedentary group (p<0.05; Figure 3B & Figure 4B). The numbers of p-mTOR-positive neurons in the forced wheel running group differed from neither the voluntary nor locked wheel conditions in the DMS. Average weekly voluntary running distance positively correlated with the number of p-mTOR-positive neurons in the DLS (R = 0.718; F(1,6) = 6.369; p=0.0451), but negatively correlated with neuron counts in the NAcC (R = −0.950; F(1,6) = 56.034; p=0.0003). The distance run on the night prior to sacrifice predicted the number of p-mTOR-positive neurons in the DMS (R = 0.772; F(1,6) = 8.832; p=0.0249) in the voluntary running group, but not the forced running group.
Figure 3.
Effect of exercise on p-mTOR in the dorsal striatum. A) Brain atlas diagram modified from Paxinos and Watson (2006) and representative photomicrograms depicting p-mTOR staining in the dorsal lateral striatum (DLS) of sedentary (left), voluntary run (middle) and forced run (right) groups. White arrows indicate examples of p-mTOR-positive neurons. B) Voluntary exercise increases p-mTOR-positive neurons in the DLS. C) Voluntary exercise increases the intensity of p-mTOR stain compared to both sedentary and forced exercise groups in the dorsal medial striatum (DMS) and DLS. * p<0.05 relative to locked group; Φ p<0.05 relative to forced run group. Bars represent group means ± SEM.
Figure 4.
Effects of exercise on p-mTOR in the nucleus accumbens (NAc) A) Brain atlas diagram modified from Paxinos and Watson (2006) and representative photomicrograms depicting p-mTOR staining in the nucleus accumbens core (NAcC) of sedentary (left), voluntary run (middle) and forced run (right) groups. White arrows indicate examples of p-mTOR-positive neurons; black arrows indicate examples of p-mTOR-positive glia. B) Voluntary, but not forced exercise increases p-mTOR-positive neurons compared to sedentary and forced run groups in the NAcC and the nucleus accumbens shell (NAcS). C) Both voluntary and forced exercise increase p-mTOR-positive glia compared to sedentary controls in the NAcC and NAcS. In the NAcC, voluntary exercise also increases the number of p-mTOR-positive glia compared to the forced exercise condition. D) Densitometry results show that only voluntary exercise increases the intensity of stain compared to sedentary and forced wheel running conditions in the NAcC and NAcS. * represents p<0.05 relative to sedentary rats; Φ represents p<0.05 relative to forced run group. Bars represent group means ± SEM.
Glia
Very few p-mTOR-positive glia were observed in the dorsal striatum. In the ventral striatum, both voluntary and forced exercise increased p-mTOR-positive glia in the NAcC (F(2,18) =16.281; p<0.0001; Figure 4C) and NAcS (F(2,18) = 8.153; p=0.0030; Figure 4C), with voluntary exercise providing the largest increase in the NAcC (p<0.05 compared to locked and forced exercise conditions; Figure 4C). Average weekly voluntary running distance negatively correlated with numbers of p-mTOR- positive glia in the NAcC (R = −0.829; F(1,6) = 13.195; p=0.0109) and NAcS (R = − 0.829; F(1,6) = 13.195; p=0.0109).
Densitometry
Densitometry, which does not discriminate between neurons and glia, revealed group differences in intensity of p-mTOR staining in the DMS (F(2,19) = 8.047; p=0.0029; Figure 3C), DLS (F(2,19) = 7.017; p=0.0050; Figure 3C), NAcC (F(2,18) = 5.220; p=0.0163; Figure 4D) and NacS (F(2,18) = 5.353; p=0.0150; Figure 4D). Voluntary exercise increased the intensity of p-mTOR stain in these regions above both sedentary and forced exercise conditions (p< 0.05; Figure 3C & Figure 4D). Average weekly distance run by the voluntary running group predicted intensity of stain in both the DMS (R = 0.715; F(1,6) = 6.259; p=0.0464) and DLS (R = 0.720; F(1,6) = 6.466; p=0.0439).
Exercise increased p-mTOR in the hippocampus and amygdala
Neurons
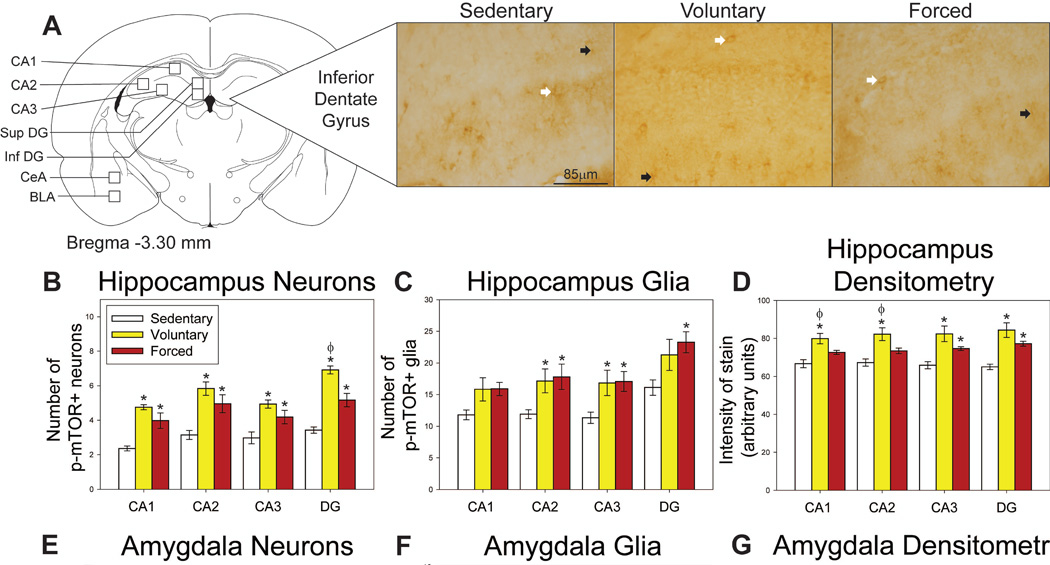
Regions where p-mTOR was assessed in the hippocampus and amygdala, as well as representative photomicrographs, are shown in Figure 5A. Both voluntary and forced exercise increased p-mTOR-positive neurons in the CA1 (F(2,20) = 18.257; p<0.0001; Figure 5B), CA2 (F(2,20) = 12.442; p=0.0003; Figure 5B), CA3 (F(2,20) = 9.689; p=0.0011; Figure 5B) and DG (F(2,20) = 44.085, p<0.0001; Figure 5B). Voluntary exercise elicited the largest increase in p-mTOR-positive neurons in the DG (p<0.05 compared to sedentary and forced exercise conditions; Figure 5B). Group differences in p-mTOR-positive neurons were also observed in the BLA (F(2,20) = 4.975; p=0.0176; Figure 5E), but not in CeA (F(2,20) = 1.849; p=0.1833; Figure 5E). In the BLA, voluntary running increased p-mTOR-positive neuron counts compared to the locked wheel locked wheel condition (p<0.05; Figure 5E).
Figure 5.
Effects of exercise on p-mTOR in the hippocampus and amygdala. A) Brain atlas diagram modified from Paxinos and Watson (2006) and representative photomicrograms depicting p-mTOR staining in the dentate gyrus (DG) of sedentary (left), voluntary run (middle) and forced run (right) groups. White arrows indicate examples of p-mTOR-positive neurons; black arrows indicate examples of p-mTOR-positive glia. B) Both voluntary and forced exercise increase p-mTOR-positive neurons compared to sedentary rats in the cornu ammonis (CA1, CA2, CA3) and DG regions of the hippocampus. Voluntary exercise also increases p-mTOR-positive neurons in the DG compared to the forced wheel running condition. C) Both voluntary and forced exercise increase p-mTOR-positive glia compared to sedentary controls in the CA2 and CA3. Forced exercise increases counts of p-mTOR-positive glia in the DG compared to sedentary rats. D) Voluntary exercise increases the intensity of p-mTOR stain compared to sedentary and forced exercise conditions in the CA1 and CA2. Both voluntary and forced exercise increase the intensity of stain compared to sedentary controls in the CA3 and DG. E) Voluntary exercise increases p-mTOR-positive neurons in the basolateral amygdala (BLA) compared to sedentary rats. F) Both voluntary and forced exercise increase p-mTOR-positive glia in the BLA and central amygdala (CeA) compared to sedentary controls. G) Both voluntary and forced exercise increase the intensity of stain in the BLA and CeA relative to sedentary controls, with voluntary providing a larger increase compared to the forced wheel running condition. * represents p<0.05 relative to sedentary rats; Φ represents p<0.05 relative to forced run group. Bars represent group means ± SEM.
Glia
Quantification of the p-mTOR-positive glia revealed a trend for group differences in the CA1 (F(2,20) = 3.183; p=0.0631; Figure 5C), and significant differences in p-mTOR-positive glia in CA2 (F(2,20) = 4.060, p=0.0331; Figure 5C), CA3 (F(2,20) = 4.371; p=0.0226; Figure 5C) and DG (F(2,20) = 3.875; p=0.0378; Figure 5C). Post-hoc analyses revealed that both voluntary and forced exercise significantly increased the number of p-mTOR-positive glia in CA2 and CA3 compared to locked wheel controls (p<0.05; Figure 5C), while only forced exercise increased p-mTOR-positive glia in DG relative to the sedentary condition (p<0.05; Figure 5C). Both voluntary and forced exercise increased p-mTOR-positive glia in the BLA (F(2,20) = 7.280; p=0.0042; Figure 5F) and CeA (F(2,20) = 3.612; p=0.0458; Figure 5F).
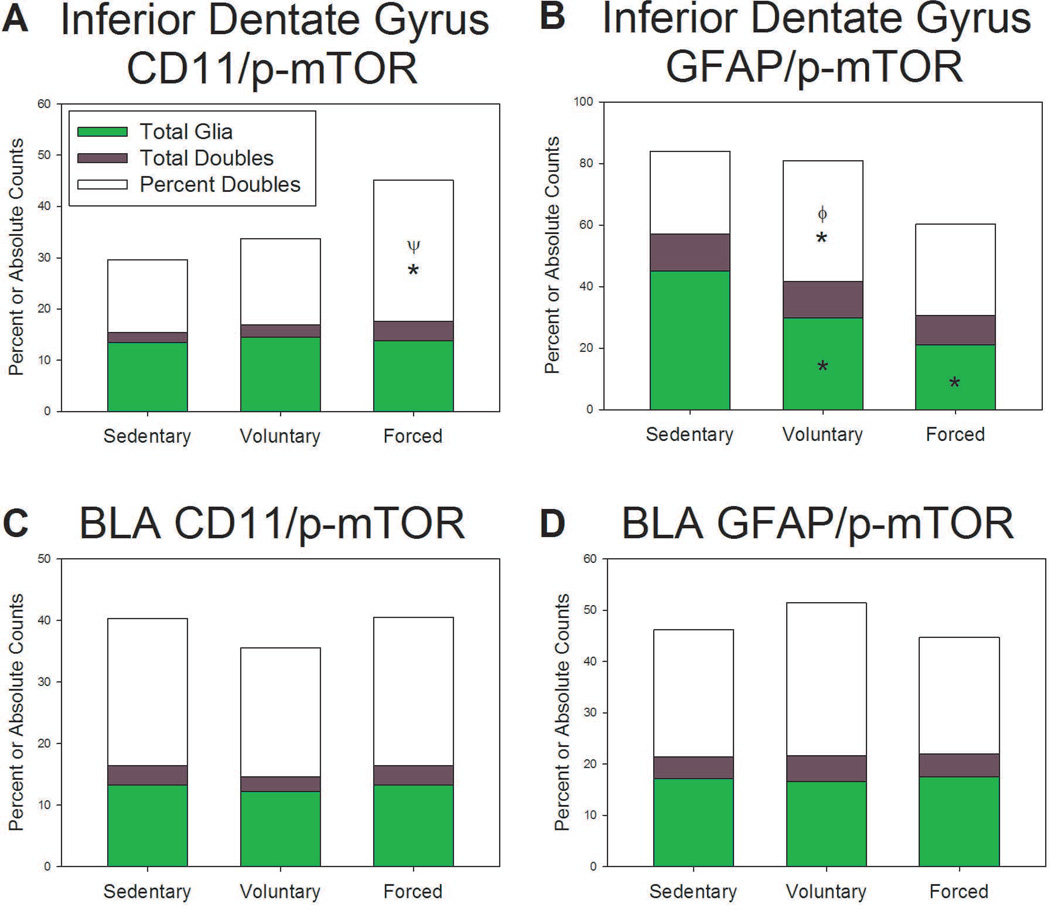
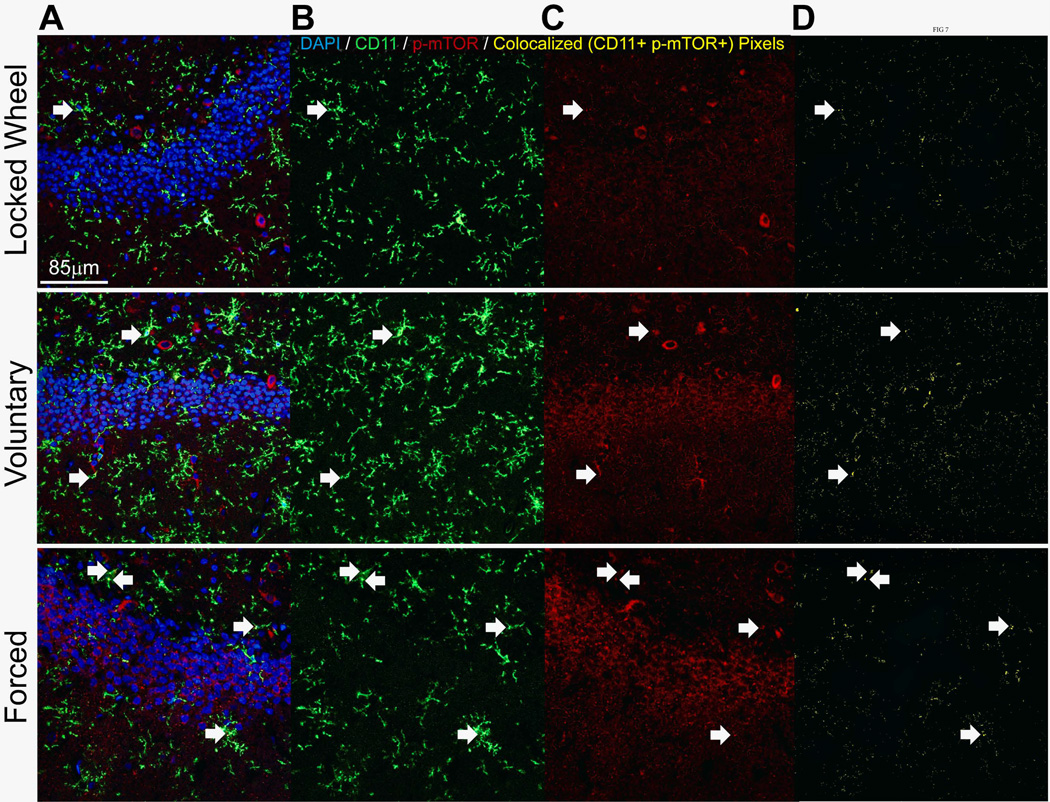
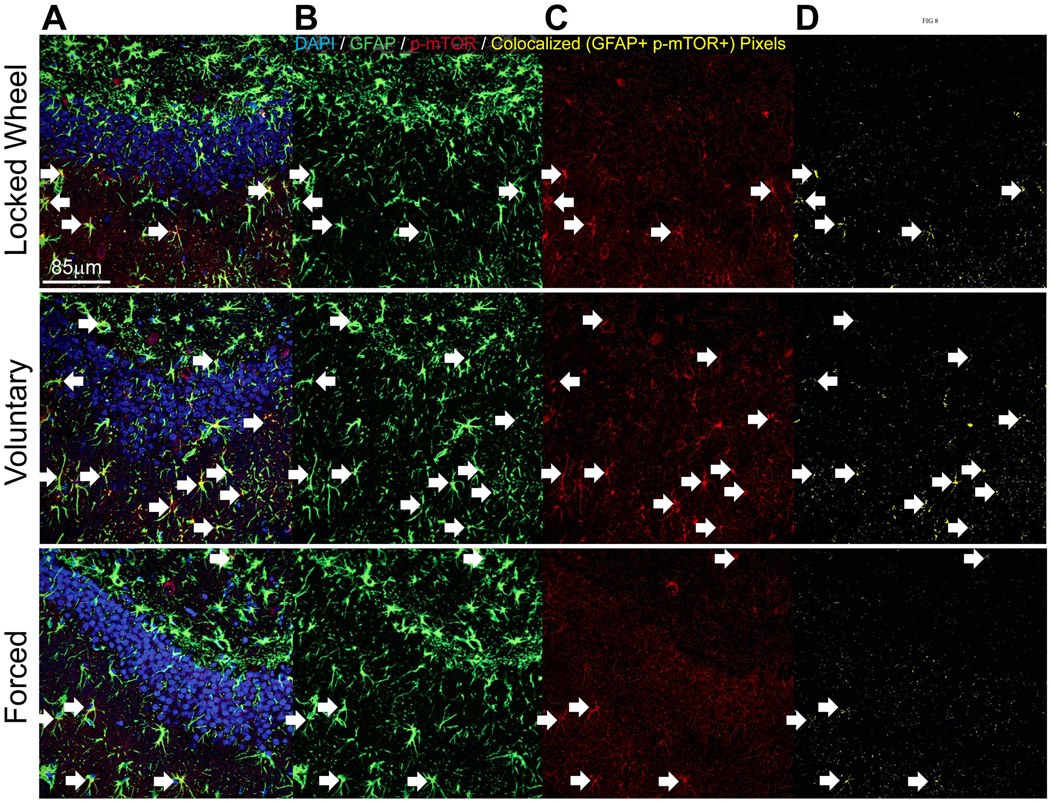
Double immunofluorescence was completed to assess the relative expression of p-mTOR in microglia (CD11-positive) and astrocytes (GFAP-positive). There was a significant effect of exercise on percent glia double-labeled with CD11 and p-mTOR in the DG (F(2,3) = 52.678; p=0.0046; Figure 6A), with a significant increase in the forced exercise condition compared to both voluntary and locked wheel groups (p<0.05; Figure 6A). Exercise also significantly affected the percentage of glia double-labeled with GFAP and p-mTOR in the DG (F(2,3) = 12.929; p=0.0335; Figure 6B), with voluntary exercise providing the largest increase compared to both forced exercise and locked wheel conditions (p<0.05; Figure 6B). The DG was also remarkable for a significant effect of exercise on the total number of GFAP-positive glia (F(2,3) = 9.945; p=0.0475; Figure 6B). Both voluntary and forced running reduced the number of GFAP-positive glia (p<0.05; Figure 6B). In the BLA, both microglia and astrocytes were observed to express p-mTOR. Exercise did not significantly change counts of GFAP-positive, CD11-positive, or the percent of p-mTOR-positive/glia-positive double-labeled cells in the BLA (Figures 6C and 6D). Representative confocal images of CD11-positive glia and GFAP-positive glia are shown in Figure 7 and Figure 8, respectively.
Figure 6.
Effects of exercise on glial expression of p-mTOR in the hippocampus and amygdala A) Forced exercise increases the percent of CD11 positive cells expressing p-mTOR compared to sedentary and voluntary exercise conditions in the dentate gyrus (DG). B) Only voluntary exercise increases the percent of GFAP positive cells expressing p-mTOR compared to sedentary and forced wheel running conditions in the inferior dentate gyrus. Both voluntary and forced decrease GFAP-positive cells compared to sedentary controls in the DG. C,D) There were no significant changes in the proportion of CD11 or GFAP positive cells expressing p-mTOR in the basolateral amygdala (BLA). * p<0.05 relative to sedentary rats; Φ represents p<0.05 relative to forced run group; Ψ represents p<0.05 relative to voluntary run group. Bars represent group means ± SEM.
Figure 7.
Representative confocal images of CD11 (green) / p-mTOR (red) double immunofluorescence in the dentate gyrus of sedentary (top), voluntary run (middle) and forced run (bottom) groups. Bar A: Overlay. Bar B: CD11 only. Bar C: p-mTOR only. Bar D: co-localized pixels only. White arrows indicate cells that were counted as double labels.
Figure 8.
Representative confocal images of GFAP (green) / p-mTOR (red) double immunofluorescence in the dentate gyrus of sedentary (top), voluntary run (middle) and forced run (bottom) groups. Bar A: Overlay. Bar B: CD11 only. Bar C: p-mTOR only. Bar D: co-localized pixels only. White arrows indicate cells that were counted as double labels.
Densitometry
Unbiased densitometry was consistent with neuron and glia data. Voluntary and forced wheel running increase intensity of stain in CA1 (F(2,20) = 9.737; p=0.0011; Figure 5D), CA2 (F(2,20) = 9.811; p=0.0011; Figure 5D), CA3 (F(2,20) = 9.256; p=0.0015; Figure 5D), and DG (F(2,20) = 15.331; p<0.0001; Figure 5D) compared to locked wheel controls. The distance run by the forced running group on the last day negatively correlated to the intensity of p-mTOR stain in CA1 (R = −0.850; F(1,5) = 13.044; p=0.0154) and CA2 (R = −0.758; F(1,5) = 6.751; p=0.0483). Both voluntary and forced exercise increase the intensity of stain in the BLA (F(2,20) = 15.733; p<0.0001; Figure 5G) and CeA (F(2,20) = 14.385; p=0.0001; Figure 5D). Voluntary exercise caused the largest increase in densitometry in the CeA (p<0.05 compared to sedentary and forced exercise conditions; Figure 5G). No significant correlations were found in the amygdala.
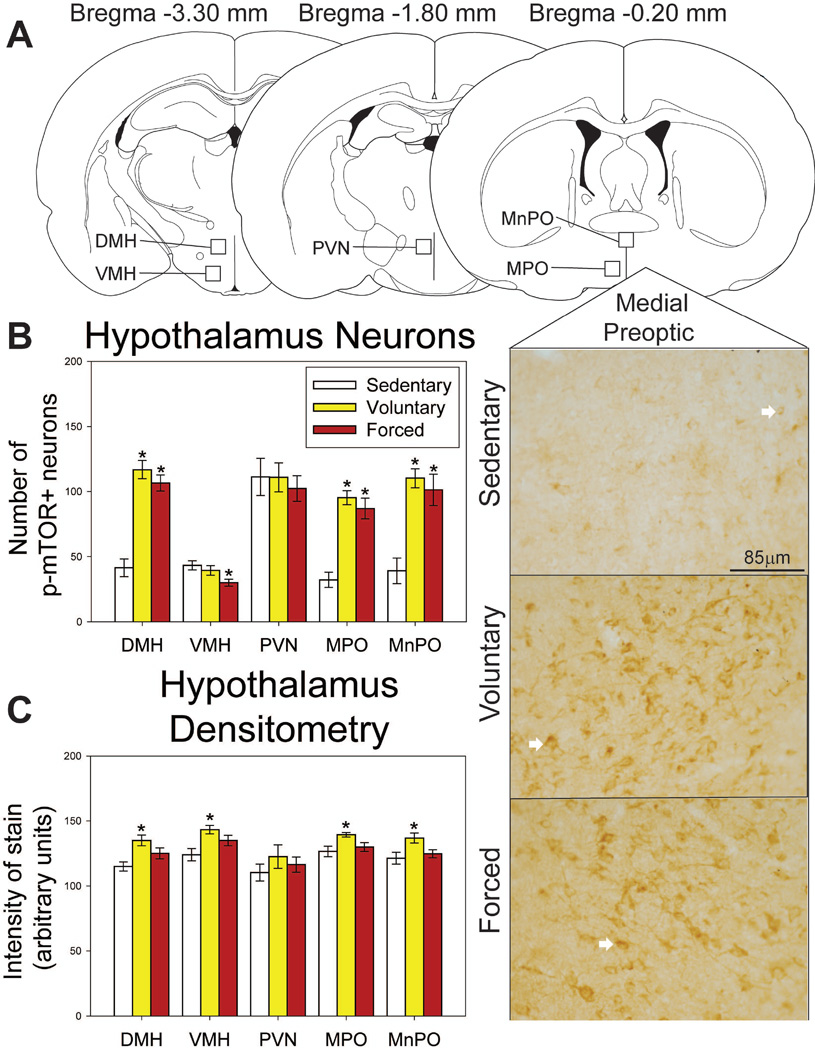
Both voluntary and forced exercise increased p-mTOR-positive neurons in the hypothalamus
Neurons
Regions where p-mTOR was assessed in the hypothalamus, as well as representative photomicrographs, are shown in Figure 9A. Both voluntary and forced wheel running increase p-mTOR-positive neurons in the MnPO (F(2,19) = 17.306; p<0.0001; Figure 7B), MPO (F(2,19) = 32.023; p<0.0001; Figure 9B), and DMH (F(2,19) = 36.373; p<0.0001; Figure 9B). Group differences were observed in the VMH (F(2,20) = 3.649; p=0.0446; Figure 9B), where forced exercise reduces the number of p-mTOR-positive neurons compared to the sedentary group (p<0.05; Figure 9B). No group differences were observed in the PVN (F(2,20) = 0.227; p=0.7987). Average weekly voluntary running distance predicts number of p-mTOR-positive neurons in the MnPO (R = 0.725; F = 7.047; p=0.0378).
Figure 9.
Effects of exercise on p-mTOR in the hypothalamus. A) Brain atlas diagram modified from Paxinos and Watson (2006) and representative photomicrograms depicting p-mTOR staining in the medial preoptic area (MPO) of sedentary (left), voluntary run (middle) and forced run (right) groups. White arrows indicate examples of p-mTOR-positive neurons. B) Both voluntary and forced exercise increase the number of p-mTOR-positive neurons compared to locked wheel controls in the dorsomedial hypothalamus (DMH), MPO, and median preoptic nucleus (MnPO). Forced wheel running decreases p-mTOR-positive cells in the ventromedial hypothalamus (VMH) compared to sedentary controls. C) Voluntary exercise increases intensity of stain compared to locked wheel controls in the DMH, VMH, MPO and MnPO. Forced exercise does not differ from either condition (p>0.05). * represents p<0.01 relative to sedentary rats. Bars represent group means ± SEM.
Densitometry
Group differences in intensity of staining were also observed in the MnPO (F(2,20) = 4.381; p=0.0273; Figure 9C), MPO (F(2,20) = 4.602; p=0.0235; Figure 9C), VMH (F(2,20) = 6.135; p=0.0088; Figure 9C), and DMH (F(2,19) = 6.955; p=0.0054; Figure 9C). p-mTOR-positive glia were not observed in the hypothalamus.
DISCUSSION
Here we report the novel findings that wheel running increases mTOR signaling in brain regions involved in cognition and emotional behavior. Increases in p-mTOR immunoreactivity following exercise were observed in almost every brain region investigated. Combined with prior reports of increased mTOR signaling in peripheral tissues following exercise [80, 81], these data suggest that mTOR signaling could be a fundamental and ubiquitous cellular target of physical activity. Given the emerging data supporting a role for mTOR in neural plasticity [27, 82], cognitive function [24–26] and emotional regulation [36, 40], increases in mTOR signaling following exercise could contribute to the broad beneficial impact of exercise on brain health, cognition, and emotional behavior.
It is not necessarily surprising that both voluntary and forced exercise increase mTOR signaling. Signals that lead to the phosphorylation of mTOR would not be expected to be sensitive to exercise controllability. Indeed, both voluntary and forced exercise increase BDNF [83–85] and nutrient availability [41–44], which can each lead to mTOR phosphorylation [45–47, 50]. Despite the similarities in signaling mechanisms, voluntary running elicited a bigger increase in mTOR signaling in many regions than forced exercise. Differences in running distance or stress between the voluntary and forced running groups could account for these differences in mTOR signaling. Voluntary running rats ran a greater distance over the course of the study compared to forced wheel running rats [70]. However, average weekly distance run was only positively correlated with p-mTOR in the dorsal striatum and MnPO. In fact, running distance was negatively correlated with p-mTOR in other regions, such as the IL, NAc, CA1 and CA2. These regions might be expected to be particularly sensitive to stress; therefore, stress could contribute to the negative correlations and the differences between voluntary and forced running in these areas. In support of this, prior work indicates that excessive voluntary running can elicit signs of chronic stress [86], such as gastric ulceration and morphologic changes in the hippocampus [87]. We have observed that forced wheel running can also elicit signs of chronic stress [22], despite its rewarding [70] and stress-protective [22] effects. Since reductions in mTOR have been reported following stressor exposure [88, 89], it is possible that the negative correlations between running and p-mTOR observed in some brain regions, as well as the more modest effect of forced wheel running on p-mTOR, are due to the potential stress effects of excessive or forced exercise. In these regions, then, overall levels of p-mTOR would reflect a competition between factors signaling an increase in p-mTOR and the potentially inhibitory influence of stress. Consistent with this interpretation is the observation that in brain regions in which p-mTOR was negatively correlated to voluntary running distance, forced wheel running either did not increase p-mTOR or elicited a more modest increase. However, the possibility that the 5 days of voluntary wheel access prior to beginning forced wheel running could have influenced the results in the forced wheel running group cannot be ruled out.
Another remaining question is whether the increase in p-mTOR observed in the brains of exercising rats represents levels of p-mTOR elicited by the acute running bout, or an accumulation of p-mTOR or mTOR due to the history of repeated nightly exercise. Time-course data for the activation of p-mTOR in response to exercise does not exist at this time; however, previous data acquired after acute activation of mTOR signaling with inhibitory avoidance training show that the highest levels of phosphorylation occur 0–6 hours after acute manipulation and return to baseline levels within 9 hours [28]. Moreover, previous work in muscle indicates an equivalent increase in p-mTOR in response to an acute exercise bout following either 10 or 20 days of prior exercise training which suggests a lack of compounding effects of exercise history on tissue-p-mTOR [90]. The levels of p-mTOR observed in the current study therefore most likely represent an increase in mTOR activity elicited by the acute exercise bout on the night of sacrifice. Levels of p-mTOR in regions in which weekly running distance was positively correlated with p-mTOR; however, could have been influenced by an increase in mTOR protein availability prior to acute exercise. In other regions, it remains unknown whether repeated nightly exercise is required to enable the increase in p-mTOR elicited by an acute exercise bout.
mTOR signaling in the PFC has been especially implicated in antidepressant effects. The rapid antidepressant effects of ketamine, for example, are dependent on mTOR signaling in the medial PFC [40]. Exercise can reduce the incidence of depression [91] and can be used on its own [92–95] or as an adjunct to typical antidepressants [96, 97] for the treatment of depression. Similarly, both voluntary and forced wheel running result in an antidepressant-like effect in the shuttle box escape task [22]. In the PFC; however, neither voluntary nor forced wheel running increased the number of p-mTOR-positive neurons and only voluntary wheel running increased p-mTOR staining intensity. The fact that mTOR signaling in the PFC is minimally sensitive to exercise is consistent with prior data suggesting that mechanisms outside of the PFC are involved in the antidepressant effects of exercise [22, 98]. In fact, negative correlations in the voluntary running group were found between average weekly running distance and p-mTOR-positive neurons in the PFC. Interestingly, rats in the voluntary wheel running group that ran more than 5000m per week had lower levels of p-mTOR-positive neurons than locked wheel controls. Increases in mTOR signaling in areas outside of the PFC, such as the amygdala, hippocampus, or striatum, could lead to neuroplastic adaptations in circuits contributing to the stress-protective, antidepressant, and anxiolytic effects of exercise.
Increased mTOR signaling in the dorsal and ventral striatum could contribute to the adaptations in locomotor behavior [99] and reward pathway sensitivity [100–102] known to occur following habitual physical activity. It is somewhat surprising that exercise did not increase p-mTOR in the DMS, especially considering that exercise elicits other adaptations in this region including changes in mRNA levels of dopamine and opioid receptors [72, 103], 5-HT2C receptors [104], and increases in ΔFosB [70]. However, there were positive correlations between distance run and numbers of p-mTOR-positive neurons in the DMS and DLS of voluntarily running rats.
Both voluntary and forced wheel running resulted in large increases in p-mTOR in the hippocampus. Interestingly, BDNF, which is increased in the hippocampus following both voluntary [53, 54, 105] and forced [84, 85] exercise, can increase mTOR signaling through trkB receptors [84]. The exercise-induced increase in BDNF in the hippocampus could, therefore, contribute to the particularly robust increase in mTOR signaling observed in this region. These data are also consistent with mTOR being important for the beneficial effects of exercise on hippocampal learning and memory. For example, mTOR signaling has been reported to be necessary for learning of the inhibitory avoidance hippocampal-dependent task [30], and both voluntary [31, 32] and forced [33] exercise enhance performance in this same task. Additionally, blockade of mTOR with Rapamycin prevents the acquisition of memory necessary for novel object recognition [30] which is another hippocampal-dependent task in which performance is enhanced by voluntary [106] and forced [107] exercise.
Large increases in p-mTOR were observed in the hypothalamus, where both forced and voluntary exercise increased p-mTOR-positive neurons in the MPO, MnPO, and DMH. The increase in p-mTOR in hypothalamic regions could be related to these regions’ role in thermoregulation [108, 109]. Indeed, mTOR signaling in the hypothalamus has been shown modulate thermoregulation in rats [110] and exercise has been shown to both increase basal temperature and alter thermoregulatory response to acute stimuli in rats [111–113]. The positive correlation between running distance and p-mTOR-positive cells in the MnPO could be a result of increased demands on the thermoregulatory system with increasing running distance.
The majority of p-mTOR was observed in neurons. However, the hippocampus and amygdala contained an appreciable number of p-mTOR-positive glia, overall numbers of which in the hippocampus were sensitive to exercise. Double fluorescent IHC revealed that the p-mTOR-positive glia observed in the hippocampus and amygdala included a mix of both astrocytes (GFAP+) and microglia (CD11+), with a tendency toward a greater number of p-mTOR-positive astrocytes. Interestingly, however, while voluntary wheel running increased the percentage of astrocytes expressing p-mTOR in the DG, forced wheel running increased the percentage of p-mTOR-positive microglia. These data suggest that, while both forms of exercise increase mTOR signaling in neurons and glia, the type of glia sensitive to exercise may differ depending on whether exercise is voluntary or forced. This is important, as mTOR could support very different functions depending on type of glial cell. For example, astrocytes (GFAP+) release glutamate [114] as well as BDNF [115]. Proliferation of astrocytes due to mTOR signaling could thus further activate mTOR in neurons providing enhanced synaptic plasticity and dendritic arborization [23, 27, 114]. mTOR signaling in astrocytes also leads to upregulation of glutamate transporter 1 and can reduce glutamate neurotoxicity [116], therefore potentially increasing the longevity of neurons. In microglia (CD11/OX42+), mTOR signaling is increased in response to oxidative stress, hypoxia, and cytokines including TNFα [49] and IL-1β, and modulates the production of nitric oxide [117]. mTOR signaling could also contribute to activation of microglia, as blockade of mTOR with rapamycin decreases numbers of activated microglia [118, 119]. Therefore, increases in p-mTOR-positive astrocytes in the voluntary exercise condition could contribute to the beneficial effects of exercise on neuronal health and plasticity, whereas an increase of p-mTOR-positive microglia following forced wheel running could be a consequence of, or contributor to, cellular stress and inflammation. This difference could be driven by the greater stress response elicited by forced, relative to voluntary, exercise [22]. Additional experiments are required for further speculation on the effects of voluntary and forced exercise on mTOR signaling in glia.
Exercise activates mTOR in brain regions involved in cognition and emotion. mTOR signaling in these regions could contribute to the beneficial effects of exercise on cognitive function, emotional behavior, and brain health. Whether mTOR activation is required for the cognitive or stress-buffering effects of exercise remains unknown. Future experiments utilizing mTOR inhibitors are warranted to uncover whether mTOR signaling is required for the broad beneficial effects of exercise.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Voss MW, et al. Exercise, brain, and cognition across the life span. J Appl Physiol (1985) 2011;111(5):1505–1513. doi: 10.1152/japplphysiol.00210.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomez-Pinilla F, Hillman C. The influence of exercise on cognitive abilities. Compr Physiol. 2013;3(1):403–428. doi: 10.1002/cphy.c110063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scudder MR, et al. Tracking the relationship between children’s aerobic fitness and cognitive control. Health Psychol. 2016;35(9):967–978. doi: 10.1037/hea0000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donnelly JE, et al. Physical Activity, Fitness, Cognitive Function, and Academic Achievement in Children: A Systematic Review. Med Sci Sports Exerc. 2016;48(6):1223–1224. doi: 10.1249/MSS.0000000000000966. [DOI] [PubMed] [Google Scholar]
- 5.Solberg LC, Horton TH, Turek FW. Circadian rhythms and depression: effects of exercise in an animal model. Am J Physiol. 1999;276(1 Pt 2):R152–R161. doi: 10.1152/ajpregu.1999.276.1.R152. [DOI] [PubMed] [Google Scholar]
- 6.Greenwood BN, et al. Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. J Neurosci. 2003;23(7):2889–2898. doi: 10.1523/JNEUROSCI.23-07-02889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng H, et al. Beneficial effects of exercise and its molecular mechanisms on depression in rats. Behav Brain Res. 2006;168(1):47–55. doi: 10.1016/j.bbr.2005.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crabbe JB, Smith JC, Dishman RK. Emotional & electroencephalographic responses during affective picture viewing after exercise. Physiol Behav. 2007;90(2–3):394–404. doi: 10.1016/j.physbeh.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Blumenthal JA, et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med. 2007;69(7):587–596. doi: 10.1097/PSY.0b013e318148c19a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooney GM, et al. Exercise for depression. Cochrane Database Syst Rev. 2013;(9):CD004366. doi: 10.1002/14651858.CD004366.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takacs J. [Regular physical activity and mental health. The role of exercise in the prevention of, and intervention in depressive disorders] Psychiatr Hung. 2014;29(4):386–397. [PubMed] [Google Scholar]
- 12.Herring MP, O’Connor PJ, Dishman RK. The effect of exercise training on anxiety symptoms among patients: a systematic review. Arch Intern Med. 2010;170(4):321–331. doi: 10.1001/archinternmed.2009.530. [DOI] [PubMed] [Google Scholar]
- 13.Sciolino NR, Holmes PV. Exercise offers anxiolytic potential: a role for stress and brain noradrenergic-galaninergic mechanisms. Neurosci Biobehav Rev. 2012;36(9):1965–1984. doi: 10.1016/j.neubiorev.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powers MB, Asmundson GJ, Smits JA. Exercise for Mood and Anxiety Disorders: The State-of-the Science. Cogn Behav Ther. 2015;44(4):237–239. doi: 10.1080/16506073.2015.1047286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fordyce DE, Wehner JM. Physical activity enhances spatial learning performance with an associated alteration in hippocampal protein kinase C activity in C57BL/6 and DBA/2 mice. Brain Res. 1993;619(1–2):111–119. doi: 10.1016/0006-8993(93)91602-o. [DOI] [PubMed] [Google Scholar]
- 16.Ahmadiasl N, Alaei H, Hanninen O. Effect of exercise on learning, memory and levels of epinephrine in rats’ hippocampus. J Sports Sci Med. 2003;2(3):106–109. [PMC free article] [PubMed] [Google Scholar]
- 17.van Praag H, et al. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25(38):8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van der Borght K, et al. Exercise improves memory acquisition and retrieval in the Y-maze task: relationship with hippocampal neurogenesis. Behav Neurosci. 2007;121(2):324–334. doi: 10.1037/0735-7044.121.2.324. [DOI] [PubMed] [Google Scholar]
- 19.Ang ET, et al. Alterations in spatial learning and memory after forced exercise. Brain Res. 2006;1113(1):186–193. doi: 10.1016/j.brainres.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 20.Alaei H, et al. Daily running promotes spatial learning and memory in rats. J Sports Sci Med. 2007;6(4):429–433. [PMC free article] [PubMed] [Google Scholar]
- 21.Berchtold NC, Castello N, Cotman CW. Exercise and time-dependent benefits to learning and memory. Neuroscience. 2010;167(3):588–597. doi: 10.1016/j.neuroscience.2010.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenwood BN, et al. Exercise-induced stress resistance is independent of exercise controllability and the medial prefrontal cortex. Eur J Neurosci. 2013;37(3):469–478. doi: 10.1111/ejn.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall MN. mTOR-what does it do? Transplant Proc. 2008;40(10 Suppl):S5–S8. doi: 10.1016/j.transproceed.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 24.Graber TE, McCamphill PK, Sossin WS. A recollection of mTOR signaling in learning and memory. Learn Mem. 2013;20(10):518–530. doi: 10.1101/lm.027664.112. [DOI] [PubMed] [Google Scholar]
- 25.Bergeron Y, et al. mTOR signaling contributes to motor skill learning in mice. Front Mol Neurosci. 2014;7:26. doi: 10.3389/fnmol.2014.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su ZW, et al. Postnatal high-protein diet improves learning and memory in premature rats via activation of mTOR signaling. Brain Res. 2015;1611:1–7. doi: 10.1016/j.brainres.2015.01.052. [DOI] [PubMed] [Google Scholar]
- 27.Sosanya NM, et al. Mammalian Target of Rapamycin (mTOR) Tagging Promotes Dendritic Branch Variability through the Capture of Ca2+/Calmodulin-dependent Protein Kinase II alpha (CaMKIIalpha) mRNAs by the RNA-binding Protein HuD. J Biol Chem. 2015;290(26):16357–16371. doi: 10.1074/jbc.M114.599399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slipczuk L, et al. BDNF activates mTOR to regulate GluR1 expression required for memory formation. PLoS One. 2009;4(6):e6007. doi: 10.1371/journal.pone.0006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santini E, Huynh TN, Klann E. Mechanisms of translation control underlying long-lasting synaptic plasticity and the consolidation of long-term memory. Prog Mol Biol Transl Sci. 2014;122:131–167. doi: 10.1016/B978-0-12-420170-5.00005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jobim PF, et al. Inhibition of mTOR by rapamycin in the amygdala or hippocampus impairs formation and reconsolidation of inhibitory avoidance memory. Neurobiol Learn Mem. 2012;97(1):105–112. doi: 10.1016/j.nlm.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Speisman RB, et al. Daily exercise improves memory, stimulates hippocampal neurogenesis and modulates immune and neuroimmune cytokines in aging rats. Brain Behav Immun. 2013;28:25–43. doi: 10.1016/j.bbi.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernandes J, et al. Aerobic exercise attenuates inhibitory avoidance memory deficit induced by paradoxical sleep deprivation in rats. Brain Res. 2013;1529:66–73. doi: 10.1016/j.brainres.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 33.Lovatel GA, et al. Treadmill exercise induces age-related changes in aversive memory, neuroinflammatory and epigenetic processes in the rat hippocampus. Neurobiol Learn Mem. 2013;101:94–102. doi: 10.1016/j.nlm.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Liu L, et al. Rapamycin inhibits cell motility by suppression of mTOR-mediated S6K1 and 4E–BP1 pathways. Oncogene. 2006;25(53):7029–7040. doi: 10.1038/sj.onc.1209691. [DOI] [PubMed] [Google Scholar]
- 35.McAuliffe PF, et al. Deciphering the role of PI3K/Akt/mTOR pathway in breast cancer biology and pathogenesis. Clin Breast Cancer. 2010;10(Suppl 3):S59–S65. doi: 10.3816/CBC.2010.s.013. [DOI] [PubMed] [Google Scholar]
- 36.Dwyer JM, et al. Ribosomal protein S6 kinase 1 signaling in prefrontal cortex controls depressive behavior. Proc Natl Acad Sci U S A. 2015;112(19):6188–6193. doi: 10.1073/pnas.1505289112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hullinger R, O’Riordan K, Burger C. Environmental enrichment improves learning and memory and long-term potentiation in young adult rats through a mechanism requiring mGluR5 signaling and sustained activation of p70s6k. Neurobiol Learn Mem. 2015;125:126–134. doi: 10.1016/j.nlm.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60(2):236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- 39.Radley JJ, et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125(1):1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 40.Li N, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sen S, et al. Glucose regulation of load-induced mTOR signaling and ER stress in mammalian heart. J Am Heart Assoc. 2013;2(3):e004796. doi: 10.1161/JAHA.113.004796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nave BT, et al. Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem J. 1999;344(Pt 2):427–431. [PMC free article] [PubMed] [Google Scholar]
- 43.Hara K, et al. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J Biol Chem. 1998;273(23):14484–14494. doi: 10.1074/jbc.273.23.14484. [DOI] [PubMed] [Google Scholar]
- 44.Oshiro N, Rapley J, Avruch J. Amino Acids Activate Mammalian Target of Rapamycin (mTOR) Complex 1 without Changing Rag GTPase Guanyl Nucleotide Charging. Journal of Biological Chemistry. 2014;289(5):2658–2674. doi: 10.1074/jbc.M113.528505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bergstrom J, Furst P, Hultman E. Free amino acids in muscle tissue and plasma during exercise in man. Clin Physiol. 1985;5(2):155–160. doi: 10.1111/j.1475-097x.1985.tb00591.x. [DOI] [PubMed] [Google Scholar]
- 46.Peake JM, et al. Metabolic and hormonal responses to isoenergetic high-intensity interval exercise and continuous moderate-intensity exercise. Am J Physiol Endocrinol Metab. 2014;307(7):E539–E552. doi: 10.1152/ajpendo.00276.2014. [DOI] [PubMed] [Google Scholar]
- 47.Melancon MO, Lorrain D, Dionne IJ. Exercise increases tryptophan availability to the brain in older men age 57–70 years. Med Sci Sports Exerc. 2012;44(5):881–887. doi: 10.1249/MSS.0b013e31823ede8e. [DOI] [PubMed] [Google Scholar]
- 48.Takei N, et al. Brain-derived neurotrophic factor induces mammalian target of rapamycin-dependent local activation of translation machinery and protein synthesis in neuronal dendrites. Journal of Neuroscience. 2004;24(44):9760–9769. doi: 10.1523/JNEUROSCI.1427-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou JX, et al. TNFalpha signaling regulates cystic epithelial cell proliferation through Akt/mTOR and ERK/MAPK/Cdk2 mediated Id2 signaling. PLoS One. 2015;10(6):e0131043. doi: 10.1371/journal.pone.0131043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ying Z, et al. BDNF-exercise interactions in the recovery of symmetrical stepping after a cervical hemisection in rats. Neuroscience. 2008;155(4):1070–1078. doi: 10.1016/j.neuroscience.2008.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vary TC, Lang CH. IGF-I activates the eIF4F system in cardiac muscle in vivo. Mol Cell Biochem. 2005;272(1–2):209–220. doi: 10.1007/s11010-005-7551-6. [DOI] [PubMed] [Google Scholar]
- 52.Starkman BG, et al. IGF-I stimulation of proteoglycan synthesis by chondrocytes requires activation of the PI 3-kinase pathway but not ERK MAPK. Biochem J. 2005;389(Pt 3):723–729. doi: 10.1042/BJ20041636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Intlekofer KA, et al. Exercise and sodium butyrate transform a subthreshold learning event into long-term memory via a brain-derived neurotrophic factor-dependent mechanism. Neuropsychopharmacology. 2013;38(10):2027–2034. doi: 10.1038/npp.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Church DD, et al. l-Leucine Increases Skeletal Muscle IGF-1 but Does Not Differentially Increase Akt/mTORC1 Signaling and Serum IGF-1 Compared to Ursolic Acid in Response to Resistance Exercise in Resistance-Trained Men. J Am Coll Nutr. 2016:1–12. doi: 10.1080/07315724.2015.1132019. [DOI] [PubMed] [Google Scholar]
- 55.Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20(10):2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- 56.Duman CH, et al. Peripheral insulin-like growth factor-I produces antidepressant-like behavior and contributes to the effect of exercise. Behav Brain Res. 2009;198(2):366–371. doi: 10.1016/j.bbr.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Minichiello L, et al. Mechanism of TrkB-mediated hippocampal long-term potentiation. Neuron. 2002;36(1):121–137. doi: 10.1016/s0896-6273(02)00942-x. [DOI] [PubMed] [Google Scholar]
- 58.Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288(5469):1254–1257. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- 59.Jourdi H, et al. Positive AMPA receptor modulation rapidly stimulates BDNF release and increases dendritic mRNA translation. J Neurosci. 2009;29(27):8688–8697. doi: 10.1523/JNEUROSCI.6078-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Watson K, Baar K. mTOR and the health benefits of exercise. Semin Cell Dev Biol. 2014;36:130–139. doi: 10.1016/j.semcdb.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 61.Elfving B, et al. Transient activation of mTOR following forced treadmill exercise in rats. Synapse. 2013;67(9):620–625. doi: 10.1002/syn.21668. [DOI] [PubMed] [Google Scholar]
- 62.Fang ZH, et al. Effect of treadmill exercise on the BDNF-mediated pathway in the hippocampus of stressed rats. Neurosci Res. 2013;76(4):187–194. doi: 10.1016/j.neures.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 63.E L, Burns JM, Swerdlow RH. Effect of high-intensity exercise on aged mouse brain mitochondria, neurogenesis, and inflammation. Neurobiol Aging. 2014;35(11):2574–2583. doi: 10.1016/j.neurobiolaging.2014.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alaei H, Moloudi R, Sarkaki AR. Effects of treadmill running on mid-term memory and swim speed in the rat with Morris water maze test. J Bodyw Mov Ther. 2008;12(1):72–75. doi: 10.1016/j.jbmt.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 65.Wang XQ, Wang GW. Effects of treadmill exercise intensity on spatial working memory and long-term memory in rats. Life Sci. 2016;149:96–103. doi: 10.1016/j.lfs.2016.02.070. [DOI] [PubMed] [Google Scholar]
- 66.Greenwood BN, Fleshner M. Exercise, stress resistance, and central serotonergic systems. Exerc Sport Sci Rev. 2011;39(3):140–149. doi: 10.1097/JES.0b013e31821f7e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen C, et al. The role of medial prefrontal corticosterone and dopamine in the antidepressant-like effect of exercise. Psychoneuroendocrinology. 2016;69:1–9. doi: 10.1016/j.psyneuen.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 68.Cunha MP, et al. The antidepressant-like effect of physical activity on a voluntary running wheel. Med Sci Sports Exerc. 2013;45(5):851–859. doi: 10.1249/MSS.0b013e31827b23e6. [DOI] [PubMed] [Google Scholar]
- 69.Duman CH, et al. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Res. 2008;1199:148–158. doi: 10.1016/j.brainres.2007.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Herrera JJ, et al. Neurochemical and behavioural indices of exercise reward are independent of exercise controllability. Eur J Neurosci. 2016;43(9):1190–1202. doi: 10.1111/ejn.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Greenwood BN, et al. Voluntary freewheel running selectively modulates catecholamine content in peripheral tissue and c-Fos expression in the central sympathetic circuit following exposure to uncontrollable stress in rats. Neuroscience. 2003;120(1):269–281. doi: 10.1016/s0306-4522(03)00047-2. [DOI] [PubMed] [Google Scholar]
- 72.Greenwood BN, et al. Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behav Brain Res. 2011;217(2):354–362. doi: 10.1016/j.bbr.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Duman RS, et al. Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology. 2012;62(1):35–41. doi: 10.1016/j.neuropharm.2011.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chong ZZ, et al. Mammalian target of rapamycin: hitting the bull’s-eye for neurological disorders. Oxid Med Cell Longev. 2010;3(6):374–391. doi: 10.4161/oxim.3.6.14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shi D, et al. Caveolin-1 contributes to realgar nanoparticle therapy in human chronic myelogenous leukemia K562 cells. Internation Journal of Nanomedicine. 2016:115823–115835. doi: 10.2147/IJN.S115158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sarfstein R, et al. The Mechanism of Action of the Histone Deacetylase Inhibitor Vorinostat Involves Interaction with the Insulin-Like Growth Factor Signaling Pathway. PLoS One. 2011 doi: 10.1371/journal.pone.0024468. Online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shang J, et al. Antiapoptotic and antiautophagic effects of glial cell line-derived neurotrophic factor and hepatocyte growth factor after transient middle cerebral artery occlusion in rats. J Neurosci Res. 2010;88(10):2197–2206. doi: 10.1002/jnr.22373. [DOI] [PubMed] [Google Scholar]
- 78.Fu L, et al. Inhibition of AMP-activated protein kinase alleviates focal cerebral ischemia injury in mice: Interference with mTOR and autophagy. Brain Res. 2016;1650:103–111. doi: 10.1016/j.brainres.2016.08.035. [DOI] [PubMed] [Google Scholar]
- 79.Nonoda Y, et al. Activation of microglia/macrophages expressing phosphorylated S6 ribosomal protein in a case of hemimegalencephaly with progressive calcification and atrophy. J Neurol Sci. 2009;287(1–2):52–59. doi: 10.1016/j.jns.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 80.Dreyer HC, et al. Resistance exercise increases leg muscle protein synthesis and mTOR signalling independent of sex. Acta Physiol (Oxf) 2010;199(1):71–81. doi: 10.1111/j.1748-1716.2010.02074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mascher H, et al. Enhanced rates of muscle protein synthesis and elevated mTOR signalling following endurance exercise in human subjects. Acta Physiol (Oxf) 2011;202(2):175–184. doi: 10.1111/j.1748-1716.2011.02274.x. [DOI] [PubMed] [Google Scholar]
- 82.Garza-Lombo C, Gonsebatt ME. Mammalian Target of Rapamycin: Its Role in Early Neural Development and in Adult and Aged Brain Function. Front Cell Neurosci. 2016;10:157. doi: 10.3389/fncel.2016.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Noble EE, et al. Exercise reduces diet-induced cognitive decline and increases hippocampal brain-derived neurotrophic factor in CA3 neurons. Neurobiol Learn Mem. 2014;114:40–50. doi: 10.1016/j.nlm.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Soya H, et al. BDNF induction with mild exercise in the rat hippocampus. Biochem Biophys Res Commun. 2007;358(4):961–967. doi: 10.1016/j.bbrc.2007.04.173. [DOI] [PubMed] [Google Scholar]
- 85.Hong YP, Lee HC, Kim HT. Treadmill exercise after social isolation increases the levels of NGF, BDNF, and synapsin I to induce survival of neurons in the hippocampus, and improves depression-like behavior. J Exerc Nutrition Biochem. 2015;19(1):11–18. doi: 10.5717/jenb.2015.19.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lambert KG. The activity-stress paradigm: possible mechanisms and applications. J Gen Psychol. 1993;120(1):21–32. doi: 10.1080/00221309.1993.9917859. [DOI] [PubMed] [Google Scholar]
- 87.Lambert KG, et al. Activity-stress induces atrophy of apical dendrites of hippocampal pyramidal neurons in male rats. Physiol Behav. 1998;65(1):43–49. doi: 10.1016/s0031-9384(98)00114-0. [DOI] [PubMed] [Google Scholar]
- 88.Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010;40(2):310–322. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sun P, et al. Anger Emotional Stress Influences VEGF/VEGFR2 and Its Induced PI3K/AKT/mTOR Signaling Pathway. Neural Plast. 2016;2016:4129015. doi: 10.1155/2016/4129015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ochi E, Ishii N, Nakazato K. Time Course Change of IGF1/Akt/mTOR/p70s6k Pathway Activation in Rat Gastrocnemius Muscle During Repeated Bouts of Eccentric Exercise. J Sports Sci Med. 2010;9(2):170–175. [PMC free article] [PubMed] [Google Scholar]
- 91.Ross CE, Hayes D. Exercise and psychologic well-being in the community. Am J Epidemiol. 1988;127(4):762–771. doi: 10.1093/oxfordjournals.aje.a114857. [DOI] [PubMed] [Google Scholar]
- 92.McCann IL, Holmes DS. Influence of aerobic exercise on depression. J Pers Soc Psychol. 1984;46(5):1142–1147. doi: 10.1037//0022-3514.46.5.1142. [DOI] [PubMed] [Google Scholar]
- 93.Babyak M, et al. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom Med. 2000;62(5):633–638. doi: 10.1097/00006842-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 94.Tuon T, et al. Physical training prevents depressive symptoms and a decrease in brain-derived neurotrophic factor in Parkinson’s disease. Brain Res Bull. 2014;108:106–112. doi: 10.1016/j.brainresbull.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 95.Han LN, et al. Activation of serotonin(2C) receptors in the lateral habenular nucleus increases the expression of depression-related behaviors in the hemiparkinsonian rat. Neuropharmacology. 2015;93:68–79. doi: 10.1016/j.neuropharm.2015.01.024. [DOI] [PubMed] [Google Scholar]
- 96.Martinsen EW. Physical activity and depression: clinical experience. Acta Psychiatr Scand Suppl. 1994;377:23–27. doi: 10.1111/j.1600-0447.1994.tb05797.x. [DOI] [PubMed] [Google Scholar]
- 97.Stenman E, Lilja A. Increased monoaminergic neurotransmission improves compliance with physical activity recommendations in depressed patients with fatigue. Med Hypotheses. 2013;80(1):47–49. doi: 10.1016/j.mehy.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 98.Christianson JP, Greenwood BN. Stress-protective neural circuits: not all roads lead through the prefrontal cortex. Stress. 2014;17(1):1–12. doi: 10.3109/10253890.2013.794450. [DOI] [PubMed] [Google Scholar]
- 99.Dadalko OI, et al. mTORC2/rictor signaling disrupts dopamine-dependent behaviors via defects in striatal dopamine neurotransmission. J Neurosci. 2015;35(23):8843–8854. doi: 10.1523/JNEUROSCI.0887-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mustroph ML, et al. Wheel running can accelerate or delay extinction of conditioned place preference for cocaine in male C57BL/6J mice, depending on timing of wheel access. Eur J Neurosci. 2011;34(7):1161–1169. doi: 10.1111/j.1460-9568.2011.07828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ehringer MA, Hoft NR, Zunhammer M. Reduced alcohol consumption in mice with access to a running wheel. Alcohol. 2009;43(6):443–452. doi: 10.1016/j.alcohol.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 102.Smith MA, et al. Acute bouts of wheel running decrease cocaine self-administration: Influence of exercise output. Pharmacol Biochem Behav. 2016;150–151:94–99. doi: 10.1016/j.pbb.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Foley TE, Fleshner M. Neuroplasticity of dopamine circuits after exercise: implications for central fatigue. Neuromolecular Med. 2008;10(2):67–80. doi: 10.1007/s12017-008-8032-3. [DOI] [PubMed] [Google Scholar]
- 104.Greenwood BN, et al. 5-HT2C receptors in the basolateral amygdala and dorsal striatum are a novel target for the anxiolytic and antidepressant effects of exercise. PLoS One. 2012;7(9):e46118. doi: 10.1371/journal.pone.0046118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Neeper SA, et al. Exercise and brain neurotrophins. Nature. 1995;373(6510):109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- 106.Hopkins ME, Bucci DJ. BDNF expression in perirhinal cortex is associated with exercise-induced improvement in object recognition memory. Neurobiology of Learning and Memory. 2010;94(2):278–284. doi: 10.1016/j.nlm.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fahey B, et al. Interferon-alpha-induced deficits in novel object recognition are rescued by chronic exercise. Physiol Behav. 2008;95(1–2):125–129. doi: 10.1016/j.physbeh.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 108.Morrison SF. Central control of body temperature. F1000Res. 2016:5. doi: 10.12688/f1000research.7958.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Morrison SF, Nakamura K. Central neural pathways for thermoregulation. Front Biosci (Landmark Ed) 2011;16:74–104. doi: 10.2741/3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hu F, Xu Y, Liu F. Hypothalamic roles of mTOR complex I: integration of nutrient and hormone signals to regulate energy homeostasis. Am J Physiol Endocrinol Metab. 2016;310(11):E994–E1002. doi: 10.1152/ajpendo.00121.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rowsey PJ, Metzger BL, Gordon CJ. Effects of exercise conditioning on thermoregulatory response to anticholinesterase insecticide toxicity. Biol Res Nurs. 2001;2(4):267–276. doi: 10.1177/109980040100200406. [DOI] [PubMed] [Google Scholar]
- 112.Rowsey PJ, et al. Effects of exercise conditioning on thermoregulatory responses to repeated administration of chlorpyrifos. Environ Res. 2003;92(1):27–34. doi: 10.1016/s0013-9351(02)00096-8. [DOI] [PubMed] [Google Scholar]
- 113.Thompson RS, et al. Wheel running improves REM sleep and attenuates stress-induced flattening of diurnal rhythms in F344 rats. Stress. 2016;19(3):312–324. doi: 10.1080/10253890.2016.1174852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lalo U, Rasooli-Nejad S, Pankratov Y. Exocytosis of gliotransmitters from cortical astrocytes: implications for synaptic plasticity and aging. Biochem Soc Trans. 2014;42(5):1275–1281. doi: 10.1042/BST20140163. [DOI] [PubMed] [Google Scholar]
- 115.Takemoto T, et al. Neuroprotection elicited by nerve growth factor and brain-derived neurotrophic factor released from astrocytes in response to methylmercury. Environ Toxicol Pharmacol. 2015;40(1):199–205. doi: 10.1016/j.etap.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 116.Ji YF, et al. Upregulation of glutamate transporter GLT-1 by mTOR-Akt-NF-small ka, CyrillicB cascade in astrocytic oxygen-glucose deprivation. Glia. 2013;61(12):1959–1975. doi: 10.1002/glia.22566. [DOI] [PubMed] [Google Scholar]
- 117.Lisi L, et al. The mTOR kinase inhibitor rapamycin decreases iNOS mRNA stability in astrocytes. J Neuroinflammation. 2011;8(1):1. doi: 10.1186/1742-2094-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Song Q, et al. Rapamycin protects neurons from brain contusion induced inflammatory reaction via modulation of microglial activation. Mol Med Rep. 2015;12(5):7203–7210. doi: 10.3892/mmr.2015.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tateda S, et al. Rapamycin suppresses microglial activation and reduces the development of neuropathic pain after spinal cord injury. J Orthop Res. 2016 doi: 10.1002/jor.23328. [DOI] [PubMed] [Google Scholar]