Over the past few decades, numerous approaches have been proposed to achieve site-specific and time-controlled delivery of therapeutic agents, thus alleviating undesired side effects and enhancing the efficacy of a treatment.1 Meanwhile, rapid advances in the biomedical field pose new challenges to analytical chemistry in the field of chemical sensors: real-time, noninvasive analysis of chemical processes within tissues, inside live cells, and even subcellular subcompartments.2 One of the common challenges confronting the fields of therapeutics and bioanalytics is the need to deliver hydrophobic materials into a biological environment which, in most cases, requires a biocompatible coating/interface to minimize the “enhanced permeability and retention (EPR) effect”.3
Many drugs and molecular probes are hydrophobic and therefore require an appropriate vehicle to deliver them to a given target cell or tissue. Micelles based on block copolymer,4 ceramic5 and sapeptides,6 vesicles,7 liposomes,8 gel encapsulation,9 and prodrug/ sensor10 are examples of delivery systems for hydrophobic materials. Dinsmore et al.11 recently introduced a swelling method to adsorb rhodamine perchlorate from an organic medium into PMMA particles, enabling confocal microscopic study of colloidal dispersions. However, these systems have limitations: (1) they may require synthesis of new materials (copolymer or drug–polymer conjugate); (2) they may be stable only under wet conditions; (3) there may be difficulties in achieving uniform micro/nanocrystals.
Here, we describe a straightforward method for loading hydrophobic materials into commercially available polymer micro/ nanoparticles while, at the same time, retaining the native particle surface charge. This is achieved by using an ionic surfactant to stabilize swollen polymer particles. It is further demonstrated that subsequent surface modification of those particles based on layer-by-layer (LbL) self-assembly12 of oppositely charged polyelectrolytes is possible.
A schematic diagram of the process is illustrated in Scheme 1. The first step of the particle modification method is to adsorb surfactants onto the surfaces of the polymer particles in aqueous solution. A small amount of organic solvent is then added to swell the polymer matrix with gentle stirring. After this, a hydrophobic material is added and transferred into the hydrophobic polymer matrix. The organic solvent was partially removed by vaporizing. Finally, the particles were centrifuged and rinsed with DI water to remove unloaded substances.
Scheme 1. Loading of Hydrophobic Material into Polymer Particles.

A detailed description of the process is given in Supporting Information. Briefly, 25 μL of 5 mg/mL SDS was mixed into a microcentrifuge tube containing 100 μL of 0.5% (w/w) 1 μm PMMA particles in DI water. Then, 25 μL of CH2Cl2 was added into the dispersion for another 30 min to allow the organic solvent to swell the particles. The surfactant prevented swollen particles from aggregating and fusing. Next, 25 μL of 1 mg/mL Ru(dpp)3Cl2 in ethanol was added into the dispersion, followed by addition of 50 μL of acetone. The dispersion was stirred for 30 min and then kept open to the atmosphere at room temperature for 30 min. Finally, the particles were triple-rinsed with DI water and centrifuged at 10 000 rpm at 10 °C to remove unloaded dyes. The same procedure was confirmed to apply for FITC/ethanol, allowing FITC doping of PMMA particles.
Figure 1 contains confocal micrographs of the Ru(dpp) and FITC-loaded PMMA particles prepared by this procedure. It was shown that the loaded particles, with strong fluorescence, remained monodispersed. In hydrophilic matrices, Ru(dpp) exists as tiny crystals.9c However, the fluorescent dye is more miscible with the hydrophobic PMMA matrix at a molecular level; therefore, it is uniformly distributed inside the particles, which could be reasonably explained based on the principle of “like dissolves like” to lower the system free energy. It was observed that FITC had a loading result similar to that of Ru(dpp). This low molecular weight dye is used as a model drug or indicator in our study. The loading efficiency of the dye into PMMA particles can be easily adjusted by adding different concentrations of dye solution.
Figure 1.

Confocal microscope images of 1 μm PMMA particles with loaded (A) Ru(dpp)3Cl2 and (B) FITC.
Fluorescence spectroscopy was used to analyze the emission spectrum of Ru(dpp)-loaded PMMA particles and was compared to the emission observed in ethanol and DI water (Figure 2). It can be seen that the emission peak of Ru(dpp) in ethanol is at 608 nm, but this shifts to 623 nm due to polarity of the solvent when the medium is changed to DI water. The spectrum of Ru(dpp) in PMMA particles (610 nm) is clearly more similar to that of Ru(dpp) in ethanol solution, which indicates PMMA matrix may serve as a good solvent to Ru(dpp). It can also be observed that the Ru(dpp) remained sensitive and accessible to O2. Preliminary tests showed an oxygen sensitivity of ~42%, which is comparable with results from Ru(dpp) in ormosil and silica particles.2
Figure 2.
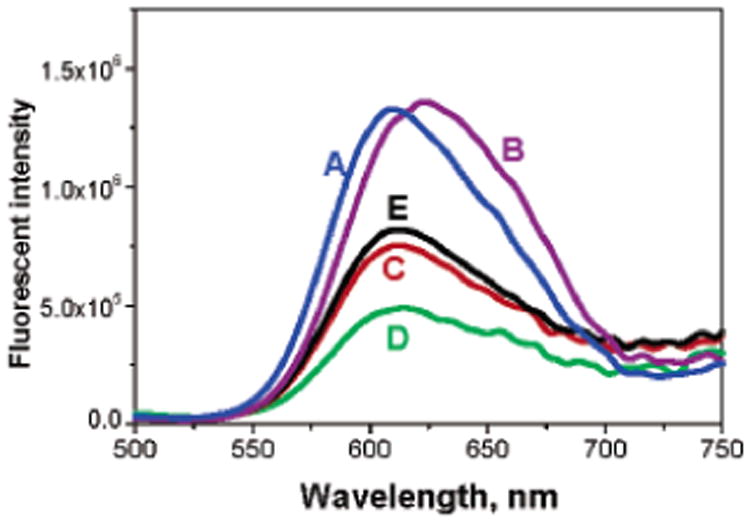
Emission spectrum of Ru(dpp) in different media: (A) EtOH, (B) DI water, (C) within PMMA particles in DI water, (D) within PMMA particles purged with O2, and (E) within PMMA particles, purged with N2.
The loaded particles are hardened again because the organic solvents are easily removed. The particles can be dried without aggregation and stored without loss of dye and can be further resuspended in DI water with sonication upon use. As a result, this approach may have advantages over other techniques for long-term storage.
Following production of Ru(dpp)-loaded particles, a polyelectrolyte (PE) pair {PAH/PSS} was used to deposit multilayers on the colloids. The LbL assembly of PE layers was monitored by electrophoretic mobility measurements. The surface potential of the PMMA particles was strongly negative (−57.52 ± 2.25 mV), only slightly changed after Ru(dpp) loading (−50.70 ± 3.23 mV), and was observed to alternate regularly from +50 mV for PAH to −50 mV for PSS, indicating the formation of {PAH/PSS}3 multilayer wall architecture. We note that it is also possible to use LbL to endow a stealth property by PEG or other hydrophilic biocompatible materials conjugated to PE as the outermost coating.13-15 Furthermore, targeting of selected cells can be achieved by decorating the particle surface- with cell-specific ligands.16
Figure 3 contains confocal images of Ru(dpp)-loaded particles deposited with/without PAH/PSS coating, then cultured with 3T3 fibroblasts. It was surprising to find that the 1 μm coated particles were apparently endocytosed, while the uncoated particles were not. It should be noted that all of the uncoated or coated particles are negatively charged, like the cell surface. This finding suggests that the surface property of the fluorescent dye-loaded particles has been tuned by surface modification via LbL assembly, and that cellular uptake can be accomplished without microinjection technique. Our further investigation is now focusing on other more biocompatible PE materials and O2 sensing properties of these particles.
Figure 3.

Confocal microscope images of 3T3 fibroblasts with Ru(dpp)3-loaded PMMA particles (a) with and (b) without {PAH/PSS}3 coating.
In additional experiments, the same loading result was also achieved for smaller PS particles (0.132–0.520 μm, Seradyn). The negatively charged surface also facilitates further assembly of LbL nanofilm coating onto dye-loaded particles. This simple and straightforward technique should be scalable to large batch or continuous processes and extendable to a variety of hydrophobic molecules into different polymer colloids for micro/nanosensor and drug-delivery applications.
In conclusion, we have demonstrated a simple method for loading hydrophobic material into stabilized polymer colloids. Although the experimental work presented here demonstrated only two different fluorescent dyes into PMMA and PS particles, the method should be applicable to a wide variety of hydrophobic materials and polymer colloids. It offers better physicochemical stability than other reported techniques and flexibility in processing and storage, and the post-treatment of loaded particles via LbL technique allows further surface modification of those particles according to requirements of specific application.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (Grant #R01 EB00739-01).
Footnotes
Supporting Information Available: Details of preparation and characterization of dye-loaded particles, LbL assembly of PEs on colloids, and culture of NIH-3T3 fibroblasts with particles. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Allen TA, Cullis PC. Science. 2004;303:1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]; (b) Langer R. Science. 2001;293:58–59. doi: 10.1126/science.1063273. [DOI] [PubMed] [Google Scholar]
- 2.(a) Brasuel M, Kopelman R, Miller TJ, Tjalkens R, Philbert MA. Anal Chem. 2001;73:2221–2228. doi: 10.1021/ac0012041. [DOI] [PubMed] [Google Scholar]; (b) Clark HA, Kopelman R, Tjalkens R, Philbert MA. Anal Chem. 1999;71:4837–4843. doi: 10.1021/ac990630n. [DOI] [PubMed] [Google Scholar]; (c) Koo YEL, Cao Y, Kopelman R, Koo SM, Brasuel M, Philbert MA. Anal Chem. 2004;76:2498–2505. doi: 10.1021/ac035493f. [DOI] [PubMed] [Google Scholar]
- 3.(a) Matsumura Y, Maeda H. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]; (b) Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. J Controlled Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 4.(a) Savic R, Luo L, Eisenberg A, Maysinger D. Science. 2003;300:615–618. doi: 10.1126/science.1078192. [DOI] [PubMed] [Google Scholar]; (b) Gao Z, Lukyanov AN, Singhal A, Torchilin VP. Nano Lett. 2002;2:979–982. [Google Scholar]; (c) Zhang L, Yu K, Eisenberg A. Science. 1996;272:1777–1779. doi: 10.1126/science.272.5269.1777. [DOI] [PubMed] [Google Scholar]; (d) Cao T, Munk P, Ramireddy C, Tuzar Z, Webber SE. Macromolecules. 1991;24:6300–6305. [Google Scholar]
- 5.Roy I, Ohulchanskyy TY, Pudavar HE, Bergey EJ, Oseroff AR, Morgan J, Dougherty TJ, Prasad PN. J Am Chem Soc. 2003;125:7860–7865. doi: 10.1021/ja0343095. [DOI] [PubMed] [Google Scholar]
- 6.Keyes-Baig C, Duhamel J, Fung SY, Bezaire J, Chen P. J Am Chem Soc. 2004;126:7522–7532. doi: 10.1021/ja0381297. [DOI] [PubMed] [Google Scholar]
- 7.Discher DE, Eisenberg A. Science. 2002;297:967–973. doi: 10.1126/science.1074972. [DOI] [PubMed] [Google Scholar]
- 8.(a) Emmelius M, Hörpel G, Ringsdorf H, Schmidt B. Polym Sci Technol. 1986;34:313–331. [Google Scholar]; (b) Paphadjopoulos D, Allen TM, Gabizon A, Mayhew E, Matthay K, Huang SK, Lee KD, Woodle MC, Lasic DD, Redemann C, Martin FJ. Proc Natl Acad Sci USA. 1991;88:11460–11464. doi: 10.1073/pnas.88.24.11460. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Lasic DD, Papahadjopoulos D. Science. 1995;267:1275–1276. doi: 10.1126/science.7871422. [DOI] [PubMed] [Google Scholar]
- 9.(a) Akiyoshi K, Kobayashi S, Shichibe S, Mix D, Baudys M, Kim SW, Sunamoto J. J Controlled Release. 1998;54:313–320. doi: 10.1016/s0168-3659(98)00017-0. [DOI] [PubMed] [Google Scholar]; (b) Rilling P, Walter T, Pommershein R, Vogt W. J Membrane Sci. 1997;129:283–287. [Google Scholar]; (c) Quincy Brown J, Srivastava R, Mc Shane MJ. Biosens Bioelectron. 2005;21:212–216. doi: 10.1016/j.bios.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 10.(a) Duncan R. Nat Rev Drug Discovery. 2003;2:347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]; (b) Thierry B, Kujawa P, Tkaczyk C, Winnik FM, Bilodeau L, Tabrizian M. J Am Chem Soc. 2005;127:1626–1627. doi: 10.1021/ja045077s. [DOI] [PubMed] [Google Scholar]; (c) Sanetra J, Bogdal D, Warzala M, Boron A. Chem Mater. 2002;14:89–95. [Google Scholar]
- 11.(a) Weeks ER, Crocker JC, Levitt AC, Schofield A, Weitz DA. Science. 1999;287:627–630. doi: 10.1126/science.287.5453.627. [DOI] [PubMed] [Google Scholar]; (b) Dinsmore AD, Weeks ER, Prasad V, Levitt AC, Weitz DA. Appl Opt. 2001;40:4152–4159. doi: 10.1364/ao.40.004152. [DOI] [PubMed] [Google Scholar]
- 12.Sukhorukov GB, Donath E, Davis S, Lichtenfeld H, Caruso F, Popov VI, Möhwald H. Polym Adv Technol. 1998;9:759. [Google Scholar]
- 13.Haque T, Chen H, Ouyang W, Martoni C, Lawuyi B, Urbanska AM, Prakash S. Mol Pharm. 2005;2:29–36. doi: 10.1021/mp049901v. [DOI] [PubMed] [Google Scholar]
- 14.Illum L, Davis SS. FEBS Lett. 1984;167:79–82. doi: 10.1016/0014-5793(84)80836-4. [DOI] [PubMed] [Google Scholar]
- 15.Gbadamosi JK, Hunter AC, Moghimi SM. FEBS Lett. 2002;532:338–344. doi: 10.1016/s0014-5793(02)03710-9. [DOI] [PubMed] [Google Scholar]
- 16.(a) Moghimi SM, Hunter AC, Murray J. Pharmacol Rev. 2001;53:283–318. [PubMed] [Google Scholar]; (b) Berry CC, Rudershausen S, Teller J, Curtis ASG. IEEE Trans Nanobio. 2002;1:105–109. doi: 10.1109/tnb.2003.809467. [DOI] [PubMed] [Google Scholar]; (c) Webster A, Compton SJ, Aylott JW. Analyst. 2005;130:163–170. doi: 10.1039/b413725f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


