Abstract
Identifying the cells that can be infected with HIV in vivo, and thus potentially persist as latent reservoirs is of high priority. Here, we report the major infected cells in a chronically-SIV infected macaque that developed AIDS and encephalitis. Majority of the infected cells were detected as non-proliferating resting cells. SIV infected non-proliferating resting cells were found to be playing an important role in viral pathogenesis, persistence or reservoir formation.
Keywords: Infection, Macrophage, Reservoir, Resting cells, SIV, SIV-RNA, T-cells
CASE REPORT
Eradication of human immunodeficiency virus (HIV) in infected patients remains a major obstacle due to the establishment of a pool of either long-lived, productively-infected cells, or latently infected cells that harbor proviral DNA. It is currently believed that we may be able to cure HIV-1 infection if viral reservoirs are eliminated. The simian immunodeficiency virus (SIV) infected rhesus macaque (RM) model is a well-accepted model of AIDS and has been extremely useful in exploring viral reservoir where SIV infection causes escalating immune dysfunction by rapid and persistent depletion of both central and effector memory CD4+ T-cells, and perpetual immune activation and inflammation [1–7]. Here, we report an interesting case where SIV infected cells were in majority are nonproliferating cells that include T-cells, macrophages, and dendritic cells. These nonproliferating SIV+ cells may play a key role in the formation of viral persistence or establishment of reservoirs.
An 11.8-year-old, male Chinese rhesus macaque (RM, Macaca mulatta) was intravenously inoculated with 1 ml of plasma (194,981 viral RNA copies) from a SIVMAC239 infected macaque. The animal was euthanized on day 316 after inoculation due to the end of the study. The macaque maintained a high plasma viral load (log10 6.6 RNA copies/ml of plasma using bDNA assay from Siemens with detection limit of 125 copies/ml of plasma) and low peripheral blood CD4 counts (below 200 cells/µl of blood) at the end of the study. Fresh and formalin-fixed tissues were collected at necropsy. All experiments were approved by the Tulane Institutional Animal Care and Use Committee.
Grossly and microscopically, this macaque had typical SIV lesions, like emaciation, lymphoid depletion in peripheral lymph nodes (LN), lymphoid follicular hyperplasia, lymphoplasmic and multinucleated giant cell enterocolitis. Consistent with neurological symptoms, this animal also had SIV giant cell meningoencephalitis characterized by lymphoplasmacytic perivascular cuffing mixed with many foamy histiocytes and multinucleate giant cells. Cytomegaly, karyomegaly and occasionally intranuclear inclusion bodies were noted in multiple organs that indicated this animal had opportunistic CMV infection due to SIV induced immunodeficiency. It is one of the most common opportunistic viral infections in both SIV infected macaques and HIV infected patients [8].
Quantification of SIV RNA positive cells was performed by in situ hybridization using antisense SIV riboprobes (Lofstrand Labs, Gaithersburg, MD) comprising essentially the entire SIV genome as described previously [9–13]. Labeled cells were visualized using horseradish alkaline phosphatase-conjugated sheep antidigoxigenin antibodies. For objective quantification of the number of infected cells/mm2 of tissue, computer assisted image analysis was used. Briefly, the number of infected cells/mm2 was determined by using a Leica DMLB microscope with SPOT insight digital camera (Digital Instrument Inc, Sterling Heights, MI) interfaced to Image-Pro Plus (Media Cybernetics, Inc.) image analysis software. In situ hybridization demonstrated that midbrain had the highest number of SIV-RNA+ cells, followed by ileum, jejunum, inguinal LN, mesenteric LN, axillary LN, spleen and colon (Supplemental Fig. 1A). SIV RNA+ cells diffusely distributed throughout these organs (Supplemental Figs. 1B–E). However, SIV-RNA+ cells were undetectable in bone marrow and thymus suggesting that these organs play a minor role in viral replication or as a source of reservoir.
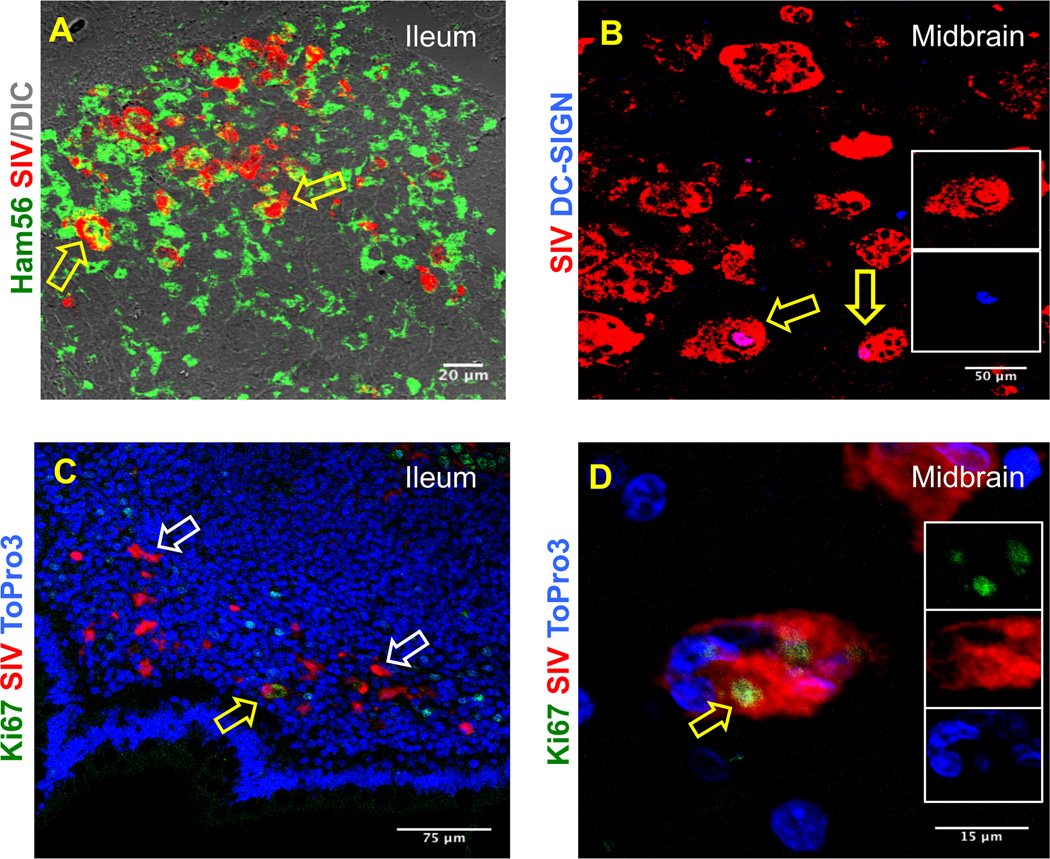
To further phenotype these SIV infected cells, tissues were processed for in situ hybridization for SIV mRNA and immunohistochemistry for CD3 (Rabbit anti-human polyclonal, Dako), Ham56 (clone Ham56, IgM kappa, Dako), dendritic cell specific ICAM-3 grabbing nonintegrin (DC-SIGN, clone DCN46, IgG2b kappa, BD Biosciences), Mac387 (clone MAC387, IgG1 kappa, Dako), and Ki67 (clone MIB-1, IgG1 kappa, Dako) by multilabel confocal microscopy. In brief, formalin-fixed, paraffin-embedded tissue sections were stained first with anti-sense SIV riboprobes. SIV mRNA positive cells were detected by a 2-hydroxy-3-naphthoic acid-2 phenylanilide phosphate (HNPP) fluorescence detection kit (Roche Diagnostic Corporation, USA). Sections were then co-labeled with any one of the unconjugated primary antibodies (CD3, Mac387, Ham56, DC-SIGN and Ki67) and then with secondary antibodies conjugated to either Alexa 488 or Alexa 633 (Life Technologies, USA). Nuclear staining was performed with anti-nuclear ToPro3 antibodies (Life Technologies). After staining, slides were washed, and labeled tissue sections were mounted using Prolong Gold antifade medium (Life Technologies) and imaged using a TCS SP2 confocal laser scanning microscope (Leica, Germany) [14–17]. Negative control slides were incorporated in each experiment either by omitting the primary antibody or using isotype IgG1 and IgG (H+L) controls [14–16]. The midbrain contained multiple multi-nucleated giant cells (MNGC) infected with SIV with occasional CD3+ T-cells around these MNGC (Supplemental Fig. 2A). In both jejunum and ileum, SIV infected CD3+ T-cells were also detected (Supplemental Fig. 3B–C). Consistent with previous findings [18, 19] SIV infected monocytes/histiocytes (Mac387+) and/or Ham56+ macrophages were also detected in midbrain, jejunum and ileum demonstrating these cells may act as reservoirs for the virus (Fig. 1 and Supplemental Figs. 2D–F). Finally, DC-SIGN (a marker of dendritic cells) positive cells were also found in midbrain, ileum and mesenteric LN tissues (Fig. 1 and Supplemental Figs. 2G–H). A few DC-SIGN+ cells distributed in the lamina propria of the ileum were positive for SIV. Similarly, DC-SIGN+ cells distributed along the barrier of the germinal center and T-cell zone of the follicle were also positive for SIV indicating that dendritic cells may also play an important role as a viral reservoir. The data consistent with previous findings [10, 18, 20] suggest that CD3+, Mac387+, Ham56+, and DC-SIGN+ cells were all contributing to the marked viral infection and replication in these tissues.
Figure 1.
SIV RNA positive cells were detected by multilabel confocal microscopy in ileum and midbrain. Ham56+ macrophages (A) and DC-SIGN positive dendritic cells (B) are shown in ileum and midbrain tissues respectively. Colocalization of Ham56 and SIV positive cells were shown in yellow. Distribution of SIV infected cells in proliferating (Ki67pos) and resting (Ki67neg) cells were shown in ileum (C) and midbrain (D) tissues by multilabel confocal microscopy. SIV positive cells in resting cells are indicated by white arrows. Yellow arrows represent the double positive cells for the respective markers in each panel. Inserts in figures B and D show DC-SIGN positive and Ki67 positive SIV infected cells respectively.
Total Ki67pos proliferating cells in various tissues were quantified (Table 1). Interestingly the colon (491 ± 54 cells/mm2) had the highest number of proliferating cells compared to other tissues examined. However, interestingly, a significant higher numbers of SIV positive cells were nonproliferating, resting (Ki67neg) cells in midbrain, mesenteric LN, and jejunum (Fig. 1, Table 1). Representative confocal images of midbrain, ileum, mesenteric LN and jejunum tissues (Fig. 1 and Supplemental Figs. 2I–J) suggested that majority of the SIV infected cells in this particular animal were nonproliferating (Ki67neg) cells.
Table 1.
Quantification of total Ki67posand SIV RNA positive proliferating (Ki67pos) and resting (Ki67neg) cells in different tissues.
| Tissue | Ki67pos (mean ± SEM)# |
SIVposKi67pos (mean ± SEM)# |
SIVposKi67neg (mean ± SEM)# |
Significance between SIVposKi67pos and SIVposKi67neg cells/mm2 |
|---|---|---|---|---|
| Midbrain | 11 ± 4 | 5 ± 2 | 58 ± 8 | P<0.0001 |
| Mesenteric LN | 265 ± 58 | 3 ± 2 | 13 ± 3 | P<0.001 |
| Jejunum | 191 ± 44 | 2 ± 1 | 28 ± 6 | P<0.0001 |
| Ileum | 459 ± 93 | 15 ± 4 | 36 ± 14 | NS |
| Colon | 491 ± 54 | 0.7 ± 0.5 | 1.4 ± 0.8 | NS |
cells/mm2; Twenty fields (40× magnification) in each slide were counted by two different individuals
SEM: standard error of mean
“pos” and “neg” denote positive and negative respectively
NS: not significant (using two-tailed paired t test, α=0.05)
Majority of the SIV infected cells were documented as resting memory CD4+ T-cells during acute stage of SIV infection [21, 22]. The presence of increased number of SIV-infected resting-cells suggested these cells may serve as a potential source of viral reservoirs and replications. It may be possible that these cells were initially infected while they were activated (expressed reservoir), yet later became quiescent or “resting” cells that had persistent virus (latent reservoir). Overall, we presented an interesting case where the nonproliferating resting cells could be the reservoir of SIV or HIV in AIDS patients.
Supplementary Material
Acknowledgments
The authors thank Andrew Lackner, Arpita Das, Carol Coyne, Cecily Conerly Midkiff, Maury Duplantis, Ronald S. Veazey, and Xavier Alvarez for their technical support and advice. The study was supported by NIH grants P20 GM103458-09, R21 AI080395 (B.P.), amfAR grant-106719-40-RGRL (B.P.) and Tulane Office of Research Bridge Funding (B.P.).
Footnotes
Declaration of conflict interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Pahar B, Amedee AM, Thomas J, Dufour JP, Zhang P, Nelson S, Veazey RS, Bagby GJ. Effects of alcohol consumption on antigen-specific cellular and humoral immune responses to SIV in rhesus macaques. J Acquir Immune Defic Syndr. 2013;64:332–341. doi: 10.1097/QAI.0b013e31829f6dca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kenway-Lynch CS, Das A, Lackner AA, Pahar B. Cytokine/Chemokine responses in activated CD4+ and CD8+ T cells isolated from peripheral blood, bone marrow, and axillary lymph nodes during acute simian immunodeficiency virus infection. J Virol. 2014;88:9442–9457. doi: 10.1128/JVI.00774-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kenway-Lynch CS, Das A, Pan D, Lackner AA, Pahar B. Dynamics of cytokine/chemokine responses in intestinal CD4+ and CD8+ T Cells during Acute Simian Immunodeficiency Virus Infection. J Virol. 2013;87:11916–11923. doi: 10.1128/JVI.01750-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pahar B, Gray WL, Phelps K, Didier ES, deHaro E, Marx PA, Traina-Dorge VL. Increased cellular immune responses and CD4+ T-cell proliferation correlate with reduced plasma viral load in SIV challenged recombinant simian varicella virus - simian immunodeficiency virus (rSVV-SIV) vaccinated rhesus macaques. Virol J. 2012;9:160. doi: 10.1186/1743-422X-9-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faul EJ, Aye PP, Papaneri AB, Pahar B, McGettigan JP, Schiro F, Chervoneva I, Montefiori DC, Lackner AA, Schnell MJ. Rabies virus-based vaccines elicit neutralizing antibodies, poly-functional CD8+ T cell, and protect rhesus macaques from AIDS-like disease after SIV(mac251) challenge. Vaccine. 2009;28:299–308. doi: 10.1016/j.vaccine.2009.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kimata JT. HIV-1 fitness and disease progression: insights from the SIV-macaque model. Curr HIV Res. 2006;4:65–77. doi: 10.2174/157016206775197628. [DOI] [PubMed] [Google Scholar]
- 7.Clements JE, Gama L, Graham DR, Mankowski JL, Zink MC. A simian immunodeficiency virus macaque model of highly active antiretroviral treatment: viral latency in the periphery and the central nervous system. Curr Opin HIV AIDS. 2011;6:37–42. doi: 10.1097/COH.0b013e3283412413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuhn EM, Stolte N, Matz-Rensing K, Mach M, Stahl-Henning C, Hunsmann G, Kaup FJ. Immunohistochemical studies of productive rhesus cytomegalovirus infection in rhesus monkeys (Macaca mulatta) infected with simian immunodeficiency virus. Vet Pathol. 1999;36:51–56. doi: 10.1354/vp.36-1-51. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Xu H, Pahar B, Lackner AA, Veazey RS. Divergent kinetics of proliferating T cell subsets in simian immunodeficiency virus (SIV) infection: SIV eliminates the "first responder" CD4+ T cells in primary infection. J Virol. 2013;87:7032–7038. doi: 10.1128/JVI.00027-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X, Xu H, Pahar B, Alvarez X, Green LC, Dufour J, Moroney-Rasmussen T, Lackner AA, Veazey RS. Simian immunodeficiency virus selectively infects proliferating CD4+ T cells in neonatal rhesus macaques. Blood. 2010;116:4168–4174. doi: 10.1182/blood-2010-03-273482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breed MW, Jordan AP, Aye PP, Lichtveld CF, Midkiff CC, Schiro FR, Haggarty BS, Sugimoto C, Alvarez X, Sandler NG, Douek DC, Kuroda MJ, Pahar B, Piatak M, Jr, Lifson JD, Keele BF, Hoxie JA, Lackner AA. Loss of a tyrosine-dependent trafficking motif in the simian immunodeficiency virus envelope cytoplasmic tail spares mucosal CD4 cells but does not prevent disease progression. J Virol. 2013;87:1528–1543. doi: 10.1128/JVI.01928-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahsan MH, Gill AF, Alvarez X, Lackner AA, Veazey RS. Kinetics of liver macrophages (Kupffer cells) in SIV-infected macaques. Virology. 2013;446:77–85. doi: 10.1016/j.virol.2013.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Rasmussen T, Pahar B, Poonia B, Alvarez X, Lackner AA, Veazey RS. Massive infection and loss of CD4+ T cells occurs in the intestinal tract of neonatal rhesus macaques in acute SIV infection. Blood. 2007;109:1174–1181. doi: 10.1182/blood-2006-04-015172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pahar B, Pan D, Lala W, Kenway-Lynch CS, Das A. Transforming growth factor-beta1 regulated phosphorylated AKT and interferon gamma expressions are associated with epithelial cell survival in rhesus macaque colon explants. Clin Immunol. 2015;158:8–18. doi: 10.1016/j.clim.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan D, Kenway-Lynch CS, Lala W, Veazey RS, Lackner AA, Das A, Pahar B. Lack of interleukin-10-mediated anti-inflammatory signals and upregulated interferon gamma production are linked to increased intestinal epithelial cell apoptosis in pathogenic simian immunodeficiency virus infection. J Virol. 2014;88:13015–13028. doi: 10.1128/JVI.01757-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan D, Das A, Lala W, Kenway-Lynch CS, Liu DX, Veazey RS, Pahar B. Interleukin-10 prevents epithelial cell apoptosis by regulating IFNgamma and TNFalpha expression in rhesus macaque colon explants. Cytokine. 2013;64:30–34. doi: 10.1016/j.cyto.2013.06.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan D, Das A, Liu D, Veazey RS, Pahar B. Isolation and characterization of intestinal epithelial cells from normal and SIV-infected rhesus macaques. PLoS One. 2012;7:e30247. doi: 10.1371/journal.pone.0030247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soulas C, Conerly C, Kim WK, Burdo TH, Alvarez X, Lackner AA, Williams KC. Recently infiltrating MAC387(+) monocytes/macrophages a third macrophage population involved in SIV and HIV encephalitic lesion formation. Am J Pathol. 2011;178:2121–2135. doi: 10.1016/j.ajpath.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nowlin BT, Burdo TH, Midkiff CC, Salemi M, Alvarez X, Williams KC. SIV encephalitis lesions are composed of CD163(+) macrophages present in the central nervous system during early SIV infection and SIV-positive macrophages recruited terminally with AIDS. Am J Pathol. 2015;185:1649–1665. doi: 10.1016/j.ajpath.2015.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ribeiro Dos Santos P, Rancez M, Pretet JL, Michel-Salzat A, Messent V, Bogdanova A, Couedel-Courteille A, Souil E, Cheynier R, Butor C. Rapid dissemination of SIV follows multisite entry after rectal inoculation. PLoS One. 2011;6:e19493. doi: 10.1371/journal.pone.0019493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Q, Duan L, Estes JD, Ma ZM, Rourke T, Wang Y, Reilly C, Carlis J, Miller CJ, Haase AT. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 22.Zhang ZQ, Wietgrefe SW, Li Q, Shore MD, Duan L, Reilly C, Lifson JD, Haase AT. Roles of substrate availability and infection of resting and activated CD4+ T cells in transmission and acute simian immunodeficiency virus infection. Proc Natl Acad Sci U S A. 2004;101:5640–5645. doi: 10.1073/pnas.0308425101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.