Abstract
Previous research has shown that repeated testing with a stimulus male is required for ovariectomized, hormone-primed female mice to become sexually receptive (show maximal lordosis quotients; LQs) and that drug-induced, epigenetic enhancement of estradiol receptor function accelerated the improvement in LQs otherwise shown by estrous females with repeated testing. We asked whether pre-exposure to male pheromones (‘pheromone priming’) would also accelerate the improvement in LQs with repeated tests and whether optogenetic inhibition of accessory olfactory bulb (AOB) projection neurons could inhibit lordosis in sexually experienced estrous female mice. In Experiment 1, repeated priming with soiled male bedding failed to accelerate the progressive improvement in LQs shown by estrous female mice across 5 tests, although the duration of each lordosis response and females’ investigation of male body parts during the first test was augmented by such priming. In Experiment 2, acute optogenetic inhibition of AOB inputs to the forebrain during freely moving behavioral tests significantly reduced LQs, suggesting that continued AOB signaling to the forebrain during mating is required for maximal lordotic responsiveness even in sexually experienced females. Our results also suggest that pheromonal stimulation, by itself, cannot substitute for the full complement of sensory stimulation received by estrous females from mounting males that normally leads to the progressive improvement in their LQs with repeated testing.
Keywords: Lordosis, Pheromone, Optogenetics, Sexual Behavior, Odor Investigation
Introduction
Numerous studies have shown that sexually naïve ovariectomized female rats and hamsters show full-blown copulatory and paracopulatory behaviors when administered ovarian hormones and first tested with a sexually active male (Blaustein and Erskine, 2002). By contrast, sexually naïve female mice initially display very low levels of receptive sexual behavior following ovariectomy and the induction of estrus by administering estradiol followed two days later by progesterone. Repeated hormone priming and behavioral testing with a sexually experienced male is required for ovariectomized females to display maximal, high levels of sexual receptivity (Bakker et al., 2002; Kudwa and Rissman, 2003; Mani et al., 1997; Thompson and Edwards, 1971). Rissman and co-workers (Bonthuis et al., 2011) reported that systemic administration of a histone deacetylase inhibitor to ovariectomized female mice significantly enhanced the rate at which the incidence of lordosis (indexed by lordosis quotients; LQs) improved over the course of repeated hormone treatments and behavioral tests. These results point to an epigenetic mechanism whereby repeated testing with a stimulus male augments the actions of either estradiol or progesterone at respective cognate neural receptors for these two ovarian steroids. The expression of lordosis in female rodents is also sensitive to stress. Restraint stress reduces the expression of lordosis in ovariectomized female rats after treatment with ovarian hormones (White and Uphouse, 2004). Blaustein and co-workers (Laroche et al., 2009a, b) reported that the progressive improvement in LQs observed in estrous female mice over several tests in adulthood was significantly decreased by prior exposure to environmental or immune stressors around the age of puberty (specifically at 6 weeks of age). These observed behavioral defects induced by pubertal stress were correlated with a significant, long-lasting reduction in the expression of estradiol receptor type alpha (ERα) in the ventromedial hypothalamic nucleus (Ismail et al., 2011), raising the possibility that the stress-induced reduction in females’ receptivity resulted from an epigenetic down regulation of neural ERα expression.
In addition to the actions of ovarian hormones, male pheromones that are detected by the accessory olfactory system also play an essential role in the activation of courtship behaviors in estrous female mice (Haga et al., 2010; Keller et al., 2006b; Martel and Baum, 2009). High molecular weight pheromones are detected in the accessory olfactory system by sensory neurons in the murine vomeronasal organ (VNO), which send pheromonal information via projections to the accessory olfactory bulb (AOB) (Baum and Kelliher, 2009; Restrepo et al., 2004). The AOB in turn sends mitral cell projections to the medial amygdala, a major integration center for chemosensory cues (Samuelsen and Meredith, 2009), which then projects to hypothalamic regions that control reproductive behavior (Choi et al., 2005; Kevetter and Winans, 1981). Lesions placed at different points along the VNO-AOB pathway reduce the expression of lordosis as well as females’ interest in the investigation of opposite sex odor cues (DiBenedictis et al., 2012; Keller et al., 2006b; Martel and Baum, 2009).
Estradiol has been shown to significantly upregulate the ability of pheromones emitted from soiled male bedding to stimulate immediate-early gene expression in sensory neurons of the VNO, with a similar non-significant trend being seen in subnuclei of the medial amygdala of female mice (Halem et al., 1999). Thus ovarian steroids and pheromonal inputs appear to play a synergistic role in forebrain sites to facilitate the expression of female courtship behaviors in mice. However, it is not known whether repeated exposure (priming) to male pheromones can duplicate the effects of drug-induced, up regulation of hypothalamic ERα expression in augmenting lordotic responses in estrous female mice. It is also not known whether inputs from the accessory olfactory system continue to be required for the expression of lordosis in sexually experienced, hormone-primed females.
In a previous study (Haga et al., 2010), 30 minute pre-exposure of estrous female mice to the putative male tear pheromone, exocrine gland-secreting pepide-1 (ESP-1), significantly augmented LQs. In Experiment 1 we asked whether repeated pre-test exposure (30 minutes) to the pheromones emitted from soiled male bedding and urine would significantly accelerate the progressive improvement in the display of receptive (lordosis) as well as paracopulatory (investigative) behavior that occurs in ovariectomized female mice brought into estrus at 4-day intervals using estradiol followed by progesterone. In Experiment 2 we asked whether intermittent, optogenetic inhibition of activity in the AOB mitral cells would significantly inhibit the expression of lordosis otherwise seen in sexually experienced estrous females. We used a viral vector to insert the neuronal silencing opsin, ArchT (Han et al., 2011), into mitral cells of the AOB. This opsin, when activated by a narrow spectrum green laser, hyperpolarizes infected mitral cells thereby allowing us to create “reversible” lesions in the AOB to acutely block pheromone processing by the accessory olfactory system during freely moving behavioral tests.
Methods
Subjects
For Experiment 1, seventeen female C57BL/6J mice were purchased at 5–7 weeks of age (Charles River Laboratories, Wilmington, MA). Note that in previous studies by Blaustein et al. (reviewed in Blaustein and Ismail (2013)) mice shipped at 6 weeks of age and later tested after ovariectomy and priming with ovarian hormones showed average LQs of 30% on the last of 5 behavioral tests whereas mice shipped before 6 weeks or at 7 or more weeks of age showed average LQs of 60%. The mice used in Experiment 1 resembled this latter group from the Blaustein et al. work in so far as their maximal (test 5) LQs were 60% regardless of whether or not they were primed with male pheromones prior to each test. For Experiment 2, Protocadherin21-Cre (Pcdh21-Cre) mice were used in which expression of Cre-recombinase is restricted to mitral and tufted cells of both the main and accessory olfactory bulbs (Nagai et al., 2005). Subjects were generated in the Boston University vivarium by breeding heterozygous Pcdh21-Cre males with C57BL/6J females. Offspring were genotyped by PCR from genomic tail DNA and Cre-primers (Integrated DNA Technologies, Coralville, IA) constructed from sequences suggested by the Mutant Mouse Regional Resource Center (UC Davis, CA). Ten heterozygous female offspring and five female litter mate controls (age 8–14 weeks) were used in behavioral studies. Females were group housed (3–5 mice per cage) in both experiments. Stimulus C57BL/6J males for both experiments (n = 14 for Experiment 1 and n = 10 for Experiment 2 purchased at 5–7 weeks from Charles River Laboratories, Wilmington, MA) were individually housed after receiving sexual experience with an estrous female. Mice were maintained on a reversed 12:12 h light:dark cycle with food and water available ad libitum. All procedures were approved by the Boston University Charles River Campus Institutional Animal Care and Use Committee.
Surgery
Female mice from both experiments (sexually inexperienced, age 8–14 weeks) underwent bilateral ovariectomy under 2% isofluorane anesthesia and were allowed to recover for 1 week before behavioral testing (Experiment 1) or intra-cerebral virus injection (Experiment 2). Behavioral testing in Experiment 2 began three weeks after virus injection (a total of 4 weeks after bilateral ovariectomy). Subjects were given analgesic on the day of surgery and for two subsequent days (carprofen, 5 mg/kg, s.c.). During stereotaxic viral injections into the AOB (Experiment 2), mice were anaesthetized using 2% isofluorane whereupon the head was fixed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA). Injections were made using pulled glass micropipettes (Drummond Wiretrol-Precision Bores with plunger, Drummond Scientific Company, Broomall, PA,) with ~20 μm inner tip diameter using a Quintessential Stereotaxis Injector (Stoelting, Wood Dale, IL.) Adeno-associated viral (AAV) vectors were used to introduce the neuronal silencing opsin ArchT (Han et al., 2011) into the AOB. The ArchT vector, AAV8-CAG-Flex-ArchT-GFP (a gift from Dr. Ed Boyden, manufactured and distributed by the University of North Carolina Vector Core, Chapel Hill, NC), drives both ArchT and green fluorescent protein (GFP) reporter in cells that express Cre-recombinase. Pilot studies in our lab using Pcdh21-Cre mice that had previously been infected with a viral vector expressing Cre-dependent channelrhodopsin in AOB mitral cells showed that laser delivered via a single fiber implanted between the left and right AOBs induced bilateral c-fos expression in the medial amygdala (data not shown). These results indicate that laser from a single fiber is sufficient to activate opsins in infected mitral cells from both AOBs in our experimental set-up. Therefore, in the present study AOBs were injected bilaterally with 0.3 μl of the AAV-ArchT virus per site at a rate of 0.1–0.2 μl/min. Injections were made at a 40° angle off the horizontal plane at the following coordinates: 1.0 mm rostral to the inferior cerebral vein, 0.8 mm lateral to the midline and 1.8 mm below the dura. After each injection, the electrode remained in place for 10 minutes to allow for complete diffusion and stabilization of pressures before removal of the electrode. After virus injection, a single optical fiber (0.37 numerical aperture, 200 μm diameter, 2.0 mm in length; Doric Lenses, Quebec, Canada) was lowered through the inferior cerebral vein between the left and right AOBs. Bleeding was controlled by gentle pressure, and a ferrule attached to the optical fiber was secured to the skull using dental cement and two stainless steel screws. The surgical incision was closed with sutures, and post-operative analgesia was given as described previously. Females were group housed and behavioral experiments began three weeks after virus injection. Littermate control females were implanted with a single fiber optic ferrule similar to the experimental mice, but did not receive bilateral virus injections in the AOB.
Odor Stimuli
Urine and soiled bedding were collected from sexually inexperienced stimulus males (n=14) for use during olfactory priming (Experiment 1). Urine was collected using a metabolic chamber, pooled and stored in 1 ml aliquots at −80°C. To collect soiled bedding, 2–4 group-housed males were placed in a cage with clean bedding for 4 days, after which the soiled bedding was collected, pooled in plastic bags, and stored at −80°C. These stimulus males were given sexual experience after collection of odor stimuli and were also used to conduct the sexual behavior tests in Experiment 1. Males were considered sexually experienced after two overnight pairings with ovariectomized, hormone primed females (n=4; these females were not included in the behavioral studies). For Experiment 2, stimulus males were given sexual experience using the same procedure (n = 4 females for sexual experience).
Behavioral Tests
All tests were carried out under red light during the dark phase of the light:dark cycle. Tests were videotaped and coded prior to behavior scoring so that the investigator was blind to the treatment groups. To induce behavioral estrus, females were given a s.c. injection of estradiol benzoate (EB, 0.5 μg in 0.05 ml sesame oil, Sigma-Aldrich) 2 days before, and a s.c. injection of progesterone (P, 830 μg in 0.05 ml sesame oil, Sigma-Aldrich) 3–6 hours before testing (Bonthuis et al., 2011). At the end of each testing session, mice were returned to their home cage.
Experiment 1: Pheromone Priming Tests
Ovariectomized females were hormone primed prior to all 5 behavior tests, which were carried out every 4 days consecutively. Thirty minutes before the start of each test, stimulus males were placed into a plastic testing cage (29L x 18W x 13H cm) containing a mixture of bedding from their home cage and clean bedding. Estrous female subjects were placed into a different plastic exposure cage during that 30-minute period and exposed to either 20g clean bedding (Non-primed group, n = 8) or 20g male soiled bedding with an additional 1ml male urine applied (Primed group, n = 9). The type of bedding to which each female was exposed was kept consistent across all 5 tests. To begin a test, the female was placed in the testing cage containing the male, and courtship behavior was observed for either 20 minutes, 20 mount attempts by the stimulus male or until the male ejaculated. If a male ejaculated, it was not used for testing again on the same day. If a male did not mount during a test, it was replaced so that each female received mounting attempts on each testing day. Lordosis was scored when the female stood with all four limbs braced on the floor during a male mount and hold her snout parallel to the floor of the cage, without attempting to run away or sit (Bonthuis et al., 2011). Sexual receptivity of the females was determined during each of the 5 tests by calculating the lordosis quotient (LQ), which was the number of lordosis responses shown divided by the number of male mounts received multiplied by 100. Sexual receptivity was further assessed by determining the lordosis duration in each of the 5 tests. This was calculated by dividing the total time spent in lordosis by the total number of lordosis events per test. Close investigation of the male was scored and timed when the female subject made direct nasal contact with the body of the stimulus male. The body of the male was divided into three regions for analysis; the face (consisting of the snout, eyes or head), the back (consisting of both the dorsal and ventral portion of the male’s trunk and legs) and the anogenital region.
Experiment 2: Optogenetic Silencing of AOB Mitral Cells during Mating Tests
All ArchT infected and control females were hormone primed to induce behavioral estrus whereupon they received two sexual experience sessions (pairing overnight with a sexually experienced stimulus male) prior to the formal start of behavioral testing. This was done to ensure that the females would be maximally receptive during the study so that that any changes observed in receptivity during optogenetic treatment could be attributed to the acute inhibition of AOB mitral cell activity as opposed to lack of mating experience.
Females received a series of five mating tests (tests 1, 2 and 4 without any laser stimulation and tests 3 and 5 with continuous laser stimulation of the AOB), and they were also given one 20-minute habituation session in the absence of a stimulus male to familiarize them with the testing conditions. All tests were conducted in a plastic testing cage (26.5L x 20W x 30H cm). At the start of each test a fiber optic patch cord (0.37 N.A., 200 μm diameter, Doric Lenses, Quebec, Canada) was fastened to the ferrule secured to the head of the female, and the subject was transferred to a habituation cage (21L x 19W x 37H cm) for 30 minutes. The patch cord, which was protected from damage by a 7.6 cm metal sheath, was attached to a rotary joint that was suspended over the cage, and in turn connected to the laser source. The rotary joint prevented the cord from twisting due to the animal’s movement, thereby assuring unrestricted movement.
During the same 30-minute period, the stimulus male was placed into the testing cage containing a mixture of bedding from their home cage and clean bedding. After the habituation period the female was transferred into the testing cage containing the male. In tests 3 and 5, the optogenetic signal was generated for the duration of the test by a 532 nm DPSS laser (Fiber Coupled DPSS Green Laser, Shanghai Laser Corp, Shanghai, China). The fiber optic was set to deliver 10 mW at the tip (as measured with an optical power meter, Thor Labs, Inc., Newton, New Jersey). Similar, constant laser delivery parameters have been used previously to inhibit neuronal firing without damaging brain tissue or desensitizing the opsin (Stefanik and Kalivas, 2013; Tsunematsu et al., 2013). A 532 nm LED (Luxeon Star LEDs, Ontario, Canada) illuminated the cage during testing to mask light escaping the junction between the patch cord and the fiber optic ferrule when the laser was on. Each test lasted for either 20 minutes, until 20 mounts were received from the male or until the male ejaculated, as in Experiment 1. Females’ sexual receptivity was indexed by computing LQs and the lordosis duration across the 5 tests. The number of times each female crossed the midline of the cage during the 5 consecutive tests was also determined in order to index the locomotion behavior of both the ArchT infected and control females in the presence or absence of green laser stimulation of the AOB. Close investigation of the male was timed and scored as described in Experiment 1.
Brain histology
After the completion of behavioral testing in Experiment 2, mice were deeply anesthetized with sodium pentobarbital (150mg/kg i.p.) and perfused transcardially with 0.1M phosphate buffered saline (PBS, pH 7.4) and then with 4% paraformaldehyde (PFA). Brains were dissected from the skull, fixed for 2 hours in 4% PFA, and cryoprotected in 30% sucrose for 48 hours at 4°C. Olfactory bulbs and the forebrains were separated and stored in OCT at −80°C until sectioning. Sagittal olfactory bulb and coronal forebrain sections (30 μm) were made at 18°C using a cryostat (Microm HM 500M, Richard Allen Scientific, Kalamazoo, MI,). To visualize ArchT-GFP infections, olfactory bulb sections were mounted on gelatin-coated slides and coverslipped using Vectashield with DAPI counterstain (Vector Laboratories, Burlingame, CA).
To determine the infection rate of AOB mitral cells, images were collected on an Olympus Fluoview FV10i inverted confocal laser scanning microscope (Olympus Corporation) or a Nikon Eclipse Ni-E motorized microscope (Nikon Instruments, Inc.). Three representative sections (medial, middle and lateral) were taken from each AOB hemisphere approximately 150 μm apart, and the total number of mitral cells that expressed ArchT-GFP fluorescence was counted using ImageJ Software (NIH). The total number of DAPI-labeled neurons in the mitral cell layer of these sections was also counted in a subset of females receiving viral injections (n=4) to estimate the total number of cells per AOB in each hemisphere. These counts were then used to estimate the percentage of mitral cells that were infected with ArchT-GFP in each hemisphere. Of the ten females that received the bilateral viral injections, three were subsequently excluded from the analysis based on negligible levels of infection in one or both hemispheres.
Statistical Analysis
Data were expressed as mean ± SEM for all treatment groups across all five sexual behavior tests. Two-way repeated measures ANOVAs were used to determine the effect of treatment in Experiments 1 and 2 across the five tests, and Student Newman–Keuls post hoc tests were used to compare pairs of mean values. Effect sizes were determined by calculating the eta-squared (η2), which was defined as the sums of squares for the effect of interest divided by the total sums of squares for all the effects (Naule et al., 2016). All statistical analyses were carried out using SigmaPlot11 software.
Results
Experiment 1: Male Pheromone Priming Tests
Priming with male pheromones failed to accelerate the progressive increase in LQs that occurred in estrous female mice with repeated tests with a stimulus male (Fig. 1A). Overall there was a significant effect of repeated testing on females’ LQs (F4,60 = 9.845, p < 0.001, η2 = 0.29), with post hoc analysis showing that there was a significant increase in LQ in the non-primed group over baseline in tests 4 and 5, and in the primed group relative to baseline by test 5. There were no significant group differences in females’ LQs in any of the five tests. Priming with male pheromones significantly augmented the lordosis duration in estrous females across all 5 tests (Fig. 1B). This was revealed in a significant group effect in the overall ANOVA (F4,75 = 12.993, p < 0.001, η2 = 0.14), with post hoc analysis showing a significant increase in pheromone-primed females on test 4. There were no significant group differences in the number of mounts received or ejaculations received from stimulus males across the 5 tests (data not shown).
Fig. 1.
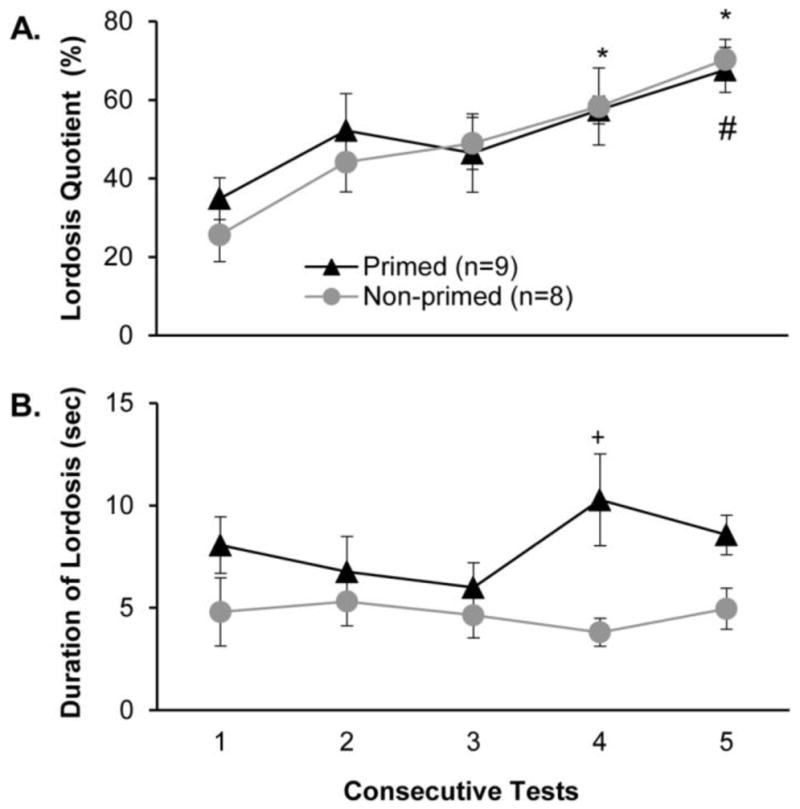
Effect of priming with male pheromones prior to each test on the display of lordosis shown by estrous female mice in response to mounts from a stimulus male. Females’ receptive behavior is indexed (panel A) as lordosis quotients and (panel B) as the duration of lordosis (seconds in lordosis posture/number of lordosis events). Each consecutive test was separated by 4 days. Data are expressed as mean +/− SEM; the number of subjects in each group is given in parentheses. * p< .05 post hoc comparisons within the non-primed group between tests 4 and 5 vs the baseline (test 1). # p < .05 post hoc comparisons within primed females between test 5 vs. test 1. + p < .05 post hoc between groups comparison on test 4.
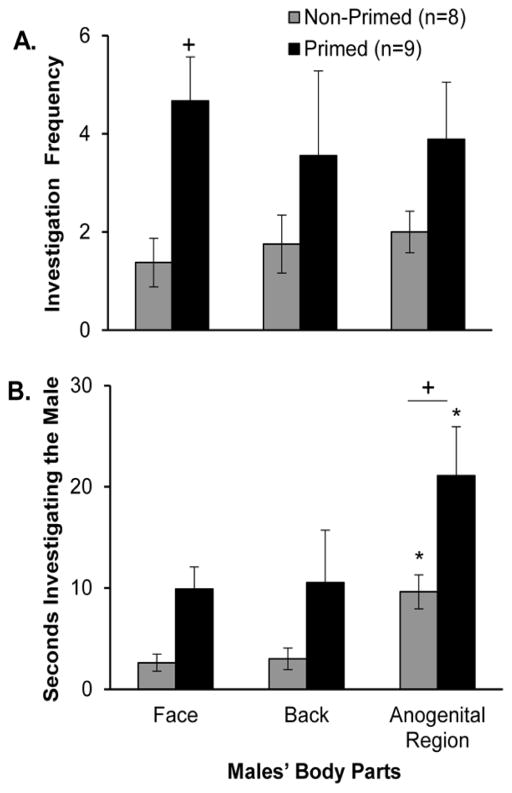
Exposing sexually naïve estrous female subjects to odor cues from stimulus males prior to mating tests increased the total time that that females spent in close nasal contact with the body (time spent investigating the face, back, and anogenital regions combined) of a stimulus male compared to controls during the first sexual behavior test (Fig. 2A). This was reflected in a significant treatment x test interaction effect (F4,60 = 2.694, p = 0.039, η2 = 0.10), and post hoc analysis revealed that pheromone-primed females showed significantly more investigation of the male than non-primed controls during test 1, but not during tests 2–5. Estrous females in the two treatment groups investigated the stimulus male for an equivalent, low percentage of the test time across the 5 tests (Fig. 2B). A detailed analysis of the time that females in the two treatment groups spent investigating different body parts of the stimulus male was made for test 1. Females that were exposed to male pheromones prior to test 1 showed a significantly higher frequency of investigation of the male body parts than non-primed controls (Fig. 3A) (F1,2 = 7.626, p = 0.008, η2 = 0.14), and post hoc analysis showed that this group difference was significant for investigation bouts directed towards the face region. Likewise, females given pre-test priming with male pheromones investigated male body parts for significantly longer than non-primed control females (Fig. 3B) (F1,2 = 9.072, p = 0.004, η2 = 0.14), with post hoc analysis showing that this group difference was significant for the duration of investigation of the male’s anogenital region. Finally, females in both treatment groups spent significantly more time investigating males’ anogenital region than either the face or back regions (F2,50 = 4.119, p = 0.023, η2 = 0.13), and post hoc analyses confirmed this result in both the control and pheromone-primed groups of estrous female subjects.
Fig. 2.
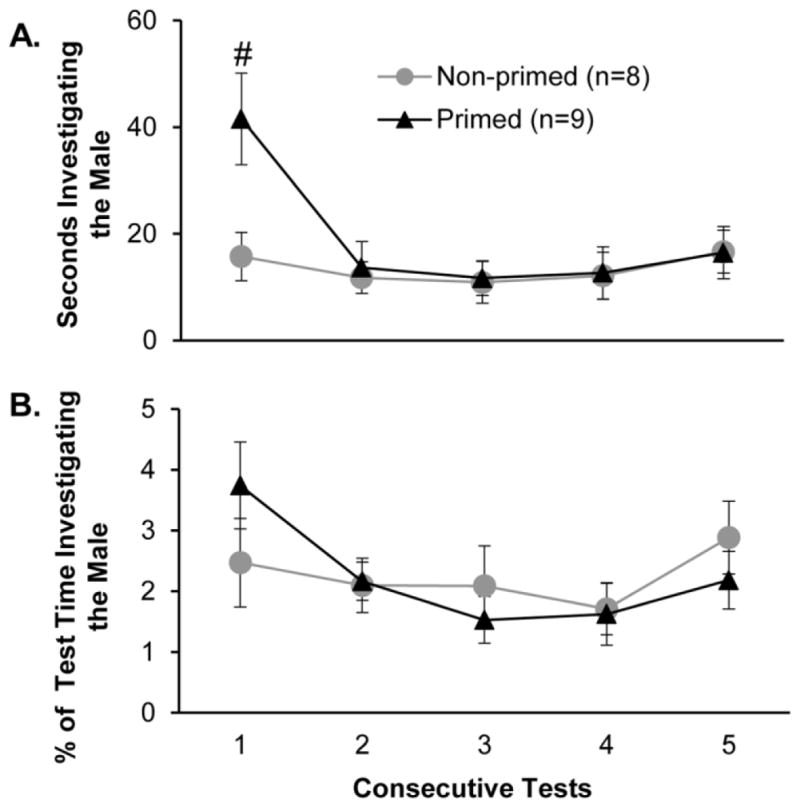
Effect of priming with male pheromones prior to each test on investigation of stimulus male conspecifics by estrous female mice. Females’ investigation is indexed (panel A) as the total time females investigated the male per test and (panel B) the percentage of the total test time during which the female investigated the stimulus male. Each consecutive test was separated by 4 days. Data are expressed as mean +/− SEM; the number of subjects in each group is given in parentheses. # p < .05 post hoc comparison of the 2 groups.
Fig. 3.
Effect of priming with male pheromones prior to test 1 on investigation of different parts of the stimulus males’ body by estrous female mice during that test. Females’ investigation is indexed as (panel A) the total frequency of investigation bouts and (panel B) the total duration of investigation of each body part. Data are expressed as mean +/− SEM; the number of subjects in each group is given in parentheses. + p < .01 post hoc between groups comparisons. *p < .05 post hoc within group comparisons with the time spent investigating either the face, back or the anogenital region.
Experiment 2: Optogenetic Silencing of AOB Mitral Cells during Mating Tests
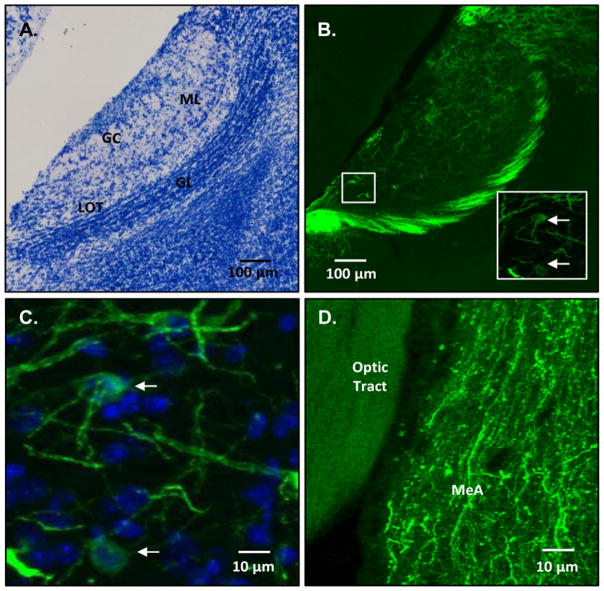
Bilateral infections of the AOB with ArchT were confirmed using fluorescent microscopy in all subjects whose data were used in the behavioral analyses (n=7) (Fig. 4; Table 1). GFP-fluorescent cell bodies indicative of ArchT infection were observed in the AOB mitral cells of the experimental subjects, and fluorescent axonal processes were also observed in the ipsilateral medial amygdala, which is a main target of AOB axonal inputs (Kang et al., 2011). Approximate infection rates for each hemisphere were estimated to be between 2 and 4% of all AOB mitral cells, based on counts of fluorescing mitral cells and DAPI counterstaining of all cells in the mitral cell layer in a subset of the experimental animals (Table 1). These values likely represent an underestimate of infection rates as DAPI stains glia in addition to neurons (Loesel et al., 2006), thereby inflating the denominator of the fraction used to calculate ArchT infection rates in the mitral cells. Inspection of sagittal brain sections revealed some fluorescent mitral cell bodies along with fluorescent axonal processes in the dorsal portion of the main olfactory bulb (MOB) abutting the AOB in both hemispheres of all 7 subjects.
Fig. 4.
Examples of the distribution of ArchT-GFP infected mitral cells in the accessory olfactory bulb (AOB) and ArchT-GFP fluorescent axons coursing to the medial amygdala. Panel A shows a photomicrograph from a sagittal section of Nissl-stained AOB. Panel B shows an epifluorescent photomicrograph of ArchT-GFP staining in the AOB; inset: confocal micrograph of ArchT-GFP staining in the AOB from the white boxed region. Panel C shows a high magnification of merged ArchT-GFP and DAPI staining in the AOB. Panel D shows ArchT-GFP staining in the axons of AOB mitral cells that project to the medial amygdala. Abbreviations: ML = AOB mitral cell layer; GC = AOB glomerular layer; LOT = lateral olfactory tract; GL = AOB granule cell layer. MeA = medial amygdala; White arrows in the panel B high magnification insert point to two ArchT-GFP expressing AOB mitral cells. White arrows in panel C point to two mitral cells expressing both ArchT-GFP and DAPI.
Table 1.
Average number of GFP-expressing ArchT-infected mitral cells and DAPI-positive cells in each hemisphere of the Accessory Olfactory Bulb of virus-injected mice.
| Treatment Group | Mouse | Hemisphere | Average Number Infected Mitral Cells | Average Number DAPI-positive Cells | Percent Infected Mitral Cells |
|---|---|---|---|---|---|
| ArchT Infected | 1 | Right | 8 ± 1 | 430 ± 58 | 2.1 % ± 0.2 |
| Left | 13 ± 3 | 335 ± 56 | 3.8 % ± 0.3 | ||
| 2 | Right | 11 ± 2 | 345 ± 34 | 3.2 % ± 0.6 | |
| Left | 9 ± 2 | 400 ± 73 | 2.2 % ± 0.6 | ||
| 3 | Right | 12 ± 2 | 364 ± 74 | 3.4 % ± 1.1 | |
| Left | 13 ± 4 | 299 ± 57 | 4.3 % ± 2.4 | ||
| 4 | Right | 12 ± 2 | 380 ± 37 | 3.0 % ± 0.3 | |
| Left | 13 ± 4 | 294 ± 76 | 4.2 % ± 0.8 | ||
| 5 | Right | 8 ± 2 | n/a | n/a | |
| Left | 9 ± 3 | n/a | n/a | ||
| 6 | Right | 8 ± 2 | n/a | n/a | |
| Left | 9 ± 3 | n/a | n/a | ||
| 7 | Right | 7 ± 2 | n/a | n/a | |
| Left | 8 ± 2 | n/a | n/a |
Data are expressed as the average number of ArchT-infected mitral cells and DAPI-positive cells in each of three sagittal AOB sections (30 μm) taken from the medial, middle and lateral segments of each hemisphere (mean + SEM). The percentage of infected mitral cells per hemisphere was also estimated (average # GFP expressing mitral cells/average number DAPI-positive cells x 100). n/a = not available
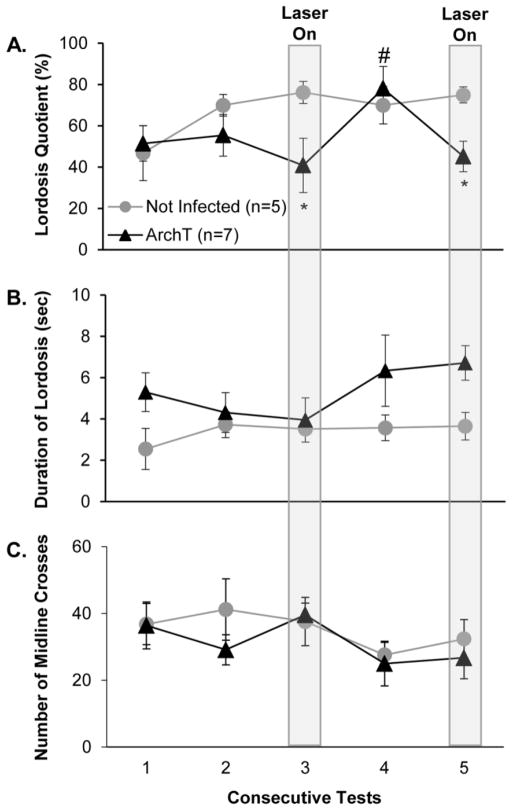
Optogenetic inhibition of activity in AOB mitral cells in females infected with ArchT resulted in significant reductions in LQs during tests 3 and 5 whereas application of the laser above the AOBs of non-infected control females failed to suppress LQs during these tests (Fig. 5A). Thus there was a significant group x test interaction effect (F4,56 = 3.904, p = 0.010, η2 = 0.13), and post hoc analysis showed that LQs of ArchT infected females were significantly lower than those of non-infected control females when the laser was applied above the AOBs during tests 3 and 5.
Fig. 5.
Effect of optogenetic inhibition (green laser on continuously above the AOB during tests 3 and 5) of activity in accessory olfactory bulb (AOB) mitral cells on the display of lordosis in response to mounts from a stimulus male and on locomotor activity in sexually experienced, ArchT-infected, estrous female mice. Data from non-infected, control females are shown for comparison. Females’ receptive behavior is indexed (panel A) as lordosis quotients, and (panel B) as the duration of lordosis (seconds in the lordosis posture/number of lordosis events). Females’ locomotor activity is indexed (panel C) by the total number of midline crosses in the cage during each test. Each consecutive test was separated by 4 days. Data are expressed as mean +/− SEM; the number of subjects in each group is given in parentheses, except that in the ArchT infected group the n for test 4 was 6 and for test 5 was 5. * p < .05 post hoc between groups comparisons; # p < .05 post hoc within groups comparisons for Arch T infected females between values for test 4 vs tests 3 and test 5.
Although there was a trend for lordosis duration values to be higher in ArchT-infected vs non-infected control females across the 5 tests (Fig. 5B), this difference was not significant. There was a significant difference between the total number of mounts received per group (data not shown), with the non-infected control group receiving more mounts (t = 2.689, p = 0.009, d = 0.74), which can be attributed to the ArchT-infected females showing less receptivity in later tests when the laser was applied. Within each treatment group, however, there was no significant difference in the number of mounts received between tests when the laser was switched on (tests 3 and 5) versus when the laser was not activated (tests 1,2 and 4) (data not shown). Application of the green laser over the AOB failed to affect the locomotor activity of either group of females (Fig. 5C) or the close nasal investigation behavior of either group of females (data not shown), suggesting that the significant reductions in LQ’s seen in Arch T infected females (Tests 3 and 5) did not reflect some non-specific action of this optogenetic stimulus.
Discussion
Priming with male pheromones prior to each test of mating behavior failed to accelerate the progressive increase in LQs that characterized estrous female mice used in Experiment 1 and in numerous previous studies (Bakker et al., 2002; Bonthuis et al., 2011; Kudwa and Rissman, 2003; Mani et al., 1997; Thompson and Edwards, 1971). This outcome suggests that the progressive improvement in females’ lordosis performance normally seen in mice does not depend solely on repeated exposure to male pheromonal cues. Likewise, an early study (Thompson and Edwards, 1971) found that simply giving ovariectomized mice estradiol and progesterone so as to induce estrus on 4 occasions, in the absence of any tests with a stimulus male, also failed to accelerate the progressive improvement in LQs later seen when the same females were further given ovarian hormones followed by tests with a male on three occasions. The results of that study show that repeated exposure to ovarian hormones, in the absence of repeated testing with a stimulus male, failed to accelerate the expression of lordosis. Taken together, these outcomes imply that the full range of sensory stimulation resulting from interaction with the male, including the receipt of mounts with pelvic thrusting/intromissions together with repeated exposure to ovarian hormones, is needed to augment females’ lordosis responses over a series of tests.
The absence of any effect of male pheromone priming in Experiment 1 contrasted with the striking ability of chronic treatment (via the drinking water) with the histone deacetylase blocking drug, sodium butyrate, to accelerate the progressive improvement in LQs among estrous female mice across a series of 5 behavioral tests with a stimulus male (Bonthuis et al., 2011). While treatment was systemic and there are many potential targets for sodium butyrate, the increased lordosis response observed after drug treatment in wild-type, hormone primed females was not observed in either ERα knockout females given ovarian hormones or in wild type mice that did not receive ovarian hormones. This outcome points to an epigenetic mechanism that normally enhances the efficacy of estradiol and/or progesterone signaling via cognate receptors as a result of the female’s receipt of mating stimulation over a series of tests. We found that both the pheromone primed and the non-primed control females used in Experiment 1 received an equivalent number of mounts across 5 tests, leading to an equivalent, progressive increase in LQs in these two groups of females. Thus pheromone-induced priming of the accessory olfactory system alone, in the absence of mounting that would provide additional, non-pheromonal physical cues from the male, failed to augment females’ progressive improvement in LQs. This outcome contrasts with the report (Haga et al., 2010) that 30 minutes of pre-exposure to the putative male tear pheromone, ESP-1, on a single occasion significantly augmented lordosis in ovariectomized, hormone-primed female mice.
It is possible that the lack of an effect of priming with male pheromones on the acceleration of lordosis could have reflected a lack of activation of the AOS by the soiled male bedding stimulus due to the absence of investigation of the soiled bedding by the female subjects. While we did not explicitly quantify the investigatory behavior of the females in Experiment 1 during the time they were placed onto either the clean or male soiled bedding stimuli, females were observed to explore the cage and to make nasal contact with the soiled bedding immediately after introduction into the test cage. This suggests that, at least initially, females were sampling the bedding stimuli during the exposure period. Additionally, this same method of exposing female mice to male soiled bedding has previously been shown to increase c-fos expression in the AOB and other forebrain regions that comprise the accessory olfactory system (Halem et al., 2001; Halem et al., 1999). For these reasons we are confident that the male pheromonal stimuli used in Experiment 1 successfully activated the accessory olfactory system in our estrous females.
The pheromone priming stimuli used in Experiment 1 were emitted from soiled bedding and urine pooled from the stimulus males that females were later tested with for lordosis responsiveness. Previous studies have shown that female mice are able to distinguish between individual conspecific males based on odor cues (Cheetham et al., 2007) and prefer to approach more dominant vs subordinate males (Mossman and Drickamer, 1996). It is possible that pre-exposure to the pheromones from a single dominant male, followed by testing with that specific male, might have accelerated the progressive improvement in LQs. Such an effect would have been lost in Experiment 1 by pooling pheromonal stimuli from a group of males with unknown social relationships.
Whereas repeated priming with male pheromones failed to augment LQs, this priming did significantly increase the average time estrous females subsequently displayed the lordosis posture across the 5 consecutive tests. This facilitation was especially evident on tests 4 and 5 in the sequence (see Fig. 1B). It is unknown whether the amount of time spent holding the lordosis posture affects females’ reproductive success. It is possible, however, that longer lordosis durations increase the likelihood of intromission and/or ejaculation thereby increasing the incidence of pregnancy. The total time investigating the stimulus male was increased by pheromone priming only in test 1 (Fig. 2A). There was no apparent relationship between tests showing altered investigation behavior (either the actual investigation time or the percentage of total test time spent with the stimulus male) and the priming-induced augmentation of the duration of lordosis responses. Thus, the priming effect on receptivity was greatest on tests 4 and 5 whereas the effect of priming on females’ subsequent nasal investigation of the stimulus male was statistically significant on test 1 but not during later tests. This observation raises the question of whether the female’s VNO receptors must always come into direct contact with putative non-volatile pheromones released from the male in order to facilitate interest in the male and lordosis responsiveness to the receipt of mounts from the male. Previous research in mice of both sexes (Keller et al., 2006b; Martel and Baum, 2009; Pankevich et al., 2004) suggests that a functional VNO/accessory olfactory system is needed to maximize subjects’ motivation to maintain nasal contact with opposite-sex urinary pheromones. Our observation that male pheromone priming (which involved direct nasal contact with putative chemosignals) failed to augment females’ direct investigation of the male across tests 2–5 while at the same time it augmented females’ lordosis duration in response to male mounts implies that VNO signaling in females may be initiated by unknown sources of non-volatile pheromones and/or by VNO responsiveness to volatile male pheromones (Sam et al., 2001; Trinh and Storm, 2003).
Pheromone-primed females showed a higher frequency of nasal investigation of the male face and spent significantly more time than non-primed controls investigating the stimulus male’s anogenital region during test 1. This outcome is interesting in so far as previous research has shown that two specific, chemically characterized putative male pheromones are secreted from these two sources in male mice. Thus, ESP-1, which is secreted in male mouse tears, is detected by the female’s VNO sensory neurons and facilitated lordosis (Haga et al., 2010; Kimoto et al., 2005). Contact with this putative male pheromone would be facilitated when females investigate the male’s face. Another compound, a major urinary protein (MUP) called darcin, has been isolated from male mouse urine and has been shown to attract females, again after its detection by VNO sensory neurons (Roberts et al., 2012; Roberts et al., 2010). Contact with darcin would likely be facilitated when females come into nasal contact with the male’s anogenital region. It was not obvious from our data set (tests 4–5 when lordosis durations of pheromone-primed females were augmented in the absence of increased nasal investigation of the stimulus males) how either of these putative male pheromones gained access to females’ VNO sensory neurons and thereby augmented lordosis duration.
Optogenetic inhibition of activity in AOB mitral cells reduced LQs in ArchT-infected females, but not in non-infected control females, when the green laser was turned on over the AOB continuously during tests 3 and 5 in Experiment 2. These reductions in females’ LQs could not be explained by differences in the number of mounts received by ArchT infected females when the laser was or was not activated across the 5 consecutive tests. Compared with tests 3 and 5, the LQs of ArchT infected females returned to a high value on the intervening test 4 when the laser was not applied. These results show that acute reductions of VNO inputs to the AOB/forebrain circuits controlling lordosis reduce the expression of this behavior, even in females with previous mating experience. They also imply that the repeated priming of estrous female mice with male pheromones over 5 consecutive tests given in Experiment 1 had the potential to augment VNO signaling in ways that might have increased lordotic responsiveness in estrous mice. The observed acute reductions in LQs by optogenetic inhibition of AOB mitral cell activity were statistically significant, but did not completely eliminate the lordosis response. This outcome may reflect that fact that only a small percentage of AOB mitral cells was successfully infected bilaterally with ArchT. It should be noted that the rate of neural ArchT infection in our study is comparable to that estimated in another study (Znamenskiy and Zador, 2013). In our study the optogenetic silencing of even a small percentage of AOB mitral cells, thereby preventing some aspects of VNO signaling in response to male pheromones, successfully reduced LQs. During the assessment of ArchT-infection of AOB mitral cells, no damage (either tissue lesions or scarring) was observed in the infected AOBs. This suggests that the reduction in LQs observed on test days 3 and 5 in the ArchT-infected animals compared to the controls was specifically due to optogenetic inhibition of AOB mitral cell activity as opposed to persistent damage to the AOB. Finally, our results complement those of several previous studies in which surgical removal of the VNO (Keller et al., 2006b; Martel and Baum, 2009), lesions of the AOB (Martel and Baum, 2009) as well as experimental mutation of the VNO receptor, V2Rp5, (Haga et al., 2010) reduced LQs in sexually naïve estrous female mice.
The main olfactory system also plays a role in females’ processing of male pheromones and the resultant expression of feminine courtship behavior (Baum and Cherry, 2015; Keller et al., 2006a; Ma et al., 2002). Previous research has shown that there is a population of mitral cells in the ventral region of the main olfactory bulb (MOB) that project to the medial amygdala (Bader et al., 2012; Thompson et al., 2012) and which are activated male pheromonal cues (Kang et al., 2009; Lin et al., 2004). The dorsal MOB, however, has been shown to specifically detect predator as opposed to sexually relevant pheromones (Kobayakawa et al., 2007). In Experiment 2, the Pcdh21-Cre subjects expressed Cre-recombinase in the mitral cells of both the AOB and the MOB, although the injections of the Cre-dependent AAV were centered in the AOB. While the ArchT infected females always showed some spread of the virus into the mitral cells located in the dorsal region of the MOB, no infected cell bodies were observed in the ventral region of the MOB. Also the positioning of the fiber optic ferrule directly above the center of the AOBs likely prevented the spread of laser light into the ventral MOB. As a result, it seems likely that we were optogenetically inhibiting AOB mitral cells as opposed to those MOB mitral cells that are potentially activated by male reproductive pheromones.
It was surprising that the duration of lordosis was not reduced by optogenetic stimulation of ArchT infected females during tests 3 and 5. Indeed, if anything the duration of lordosis on test 5 tended to be higher in ArchT infected than in non-infected control females when the laser was activated. This outcome finds a parallel with the results of Experiment 1 in which priming with male pheromones augmented lordosis duration, when it occurred, without affecting females’ LQs. It was possible that the green laser stimulation of the AOB could have caused ArchT-infected animals (and even control mice) to stop displaying all overt behaviors instead of simply the expression of lordosis. Alternately, green laser stimulation could have stimulated locomotion in infected females, making it more difficult for the male to initiate mating. An analysis of locomotion during the mating tests revealed, however, that there was no difference in this behavior (as measured by total number of times each female crossed the midline of the cage during each test) between the ArchT infected and control females on any of the test days regardless of whether or not the laser was switched on. Close nasal investigation directed towards the stimulus male both in ArchT-infected and in non-infected female subjects during the five consecutive tests was also observed. Again, there was no difference in close nasal investigation of the stimulus male between either treatment group in the presence or absence of green laser stimulation (data not shown). Taken together, these results indicate that the reduction in LQ observed in Arch T infected females when the green laser was applied to the AOB was specific to this receptive behavior and not the result of a more general alteration of females’ locomotor activity or investigation of the stimulus male.
In conclusion, priming sexually naïve female mice with male pheromones prior to mating tests did not accelerate the progressive increase in LQs normally observed with repeated testing, which indicates that activation of the olfactory system alone, in the absence of male mounting, is not sufficient to alter this behavior. Pheromone priming did increase the amount of time that females spent holding the lordosis posture; further research is needed to determine the role of the accessory olfactory system in this behavior. Acute optogenetic inhibition of AOB mitral cells during mating events in sexually experienced female subjects decreased their LQs, further highlighting the importance of the ongoing function of the accessory olfactory system in female rodent reproduction. Future studies looking at the timing of inhibition during specific parts of the mating sequence (either during close investigation behavior or the reception of a mount) will further pinpoint when during the mating sequence proper functioning of the accessory olfactory system is required for feminine courtship behavior.
Highlights.
Priming with male pheromones failed to augment lordosis quotients in estrous females.
Priming with male pheromones augmented females’ initial investigation of males.
Optogenetic inhibition of accessory olfactory bulb activity reduced lordosis.
Acknowledgments
We thank Drs. Xue Han, Jerome Mertz, Jason Ritt and Hua-an Tseng for help in setting up the optogenetic methods as well as Dr. Ian Davison for the gift of founder Pcdh21-Cre male mice. We also thank Matthew Bass, Sofia Georghiou, Curtis Hon, Vivian Lee and Siddhartha Vashi for help with behavioral testing and histology and Dr. Todd Blute for help with microscopy. This work was supported by NIDCD Grant DC008962 to JAC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bader A, Breer H, Strotmann J. Untypical connectivity from olfactory sensory neurons expressing OR37 into higher brain centers visualized by genetic tracing. Histochemistry and cell biology. 2012;137:615–628. doi: 10.1007/s00418-012-0919-2. [DOI] [PubMed] [Google Scholar]
- Bakker J, Honda S, Harada N, Balthazart J. The aromatase knock-out mouse provides new evidence that estradiol is required during development in the female for the expression of sociosexual behaviors in adulthood. J Neurosci. 2002;22:9104–9112. doi: 10.1523/JNEUROSCI.22-20-09104.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum MJ, Cherry JA. Processing by the main olfactory system of chemosignals that facilitate mammalian reproduction. Horm Behav. 2015;68:53–64. doi: 10.1016/j.yhbeh.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Baum MJ, Kelliher KR. Complementary roles of the main and accessory olfactory systems in mammalian mate recognition. Annu Rev Physiol. 2009;71:141–160. doi: 10.1146/annurev.physiol.010908.163137. [DOI] [PubMed] [Google Scholar]
- Blaustein JD, Erskine MS. Feminine Sexual Behavior: Cellular Integration of Hormonal and Afferent Information in the Rodent Brain. In: Pfaff DW, Arnold AP, Etgen AM, Farhrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Acadenic Press; San Diego: 2002. pp. 139–214. [Google Scholar]
- Blaustein JD, Ismail N. Enduring influence of pubertal stressors on behavioral response to hormones in female mice. Horm Behav. 2013;64:390–398. doi: 10.1016/j.yhbeh.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonthuis PJ, Patteson JK, Rissman EF. Acquisition of sexual receptivity: roles of chromatin acetylation, estrogen receptor-alpha, and ovarian hormones. Endocrinology. 2011;152:3172–3181. doi: 10.1210/en.2010-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham SA, Thom MD, Jury F, Ollier WE, Beynon RJ, Hurst JL. The genetic basis of individual-recognition signals in the mouse. Curr Biol. 2007;17:1771–1777. doi: 10.1016/j.cub.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Choi GB, Dong HW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Swanson LW, Anderson DJ. Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron. 2005;46:647–660. doi: 10.1016/j.neuron.2005.04.011. [DOI] [PubMed] [Google Scholar]
- DiBenedictis BT, Ingraham KL, Baum MJ, Cherry JA. Disruption of urinary odor preference and lordosis behavior in female mice given lesions of the medial amygdala. Physiol Behav. 2012;105:554–559. doi: 10.1016/j.physbeh.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga S, Hattori T, Sato T, Sato K, Matsuda S, Kobayakawa R, Sakano H, Yoshihara Y, Kikusui T, Touhara K. The male mouse pheromone ESP1 enhances female sexual receptive behaviour through a specific vomeronasal receptor. Nature. 2010;466:118–122. doi: 10.1038/nature09142. [DOI] [PubMed] [Google Scholar]
- Halem HA, Baum MJ, Cherry JA. Sex difference and steroid modulation of pheromone-induced immediate early genes in the two zones of the mouse accessory olfactory system. J Neurosci. 2001;21:2474–2480. doi: 10.1523/JNEUROSCI.21-07-02474.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halem HA, Cherry JA, Baum MJ. Vomeronasal neuroepithelium and forebrain Fos responses to male pheromones in male and female mice. J Neurobiol. 1999;39:249–263. [PubMed] [Google Scholar]
- Han X, Chow BY, Zhou H, Klapoetke NC, Chuong A, Rajimehr R, Yang A, Baratta MV, Winkle J, Desimone R, Boyden ES. A high-light sensitivity optical neural silencer: development and application to optogenetic control of non-human primate cortex. Front Syst Neurosci. 2011;5:18. doi: 10.3389/fnsys.2011.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail N, Garas P, Blaustein JD. Long-term effects of pubertal stressors on female sexual receptivity and estrogen receptor-alpha expression in CD-1 female mice. Horm Behav. 2011;59:565–571. doi: 10.1016/j.yhbeh.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang N, Baum MJ, Cherry JA. A direct main olfactory bulb projection to the ‘vomeronasal’ amygdala in female mice selectively responds to volatile pheromones from males. Eur J Neurosci. 2009;29:624–634. doi: 10.1111/j.1460-9568.2009.06638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang N, Baum MJ, Cherry JA. Different profiles of main and accessory olfactory bulb mitral/tufted cell projections revealed in mice using an anterograde tracer and a whole-mount, flattened cortex preparation. Chem Senses. 2011;36:251–260. doi: 10.1093/chemse/bjq120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M, Douhard Q, Baum MJ, Bakker J. Destruction of the main olfactory epithelium reduces female sexual behavior and olfactory investigation in female mice. Chem Senses. 2006a;31:315–323. doi: 10.1093/chemse/bjj035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M, Pierman S, Douhard Q, Baum MJ, Bakker J. The vomeronasal organ is required for the expression of lordosis behaviour, but not sex discrimination in female mice. Eur J Neurosci. 2006b;23:521–530. doi: 10.1111/j.1460-9568.2005.04589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevetter GA, Winans SS. Connections of the corticomedial amygdala in the golden hamster. I. Efferents of the “vomeronasal amygdala”. J Comp Neurol. 1981;197:81–98. doi: 10.1002/cne.901970107. [DOI] [PubMed] [Google Scholar]
- Kimoto H, Haga S, Sato K, Touhara K. Sex-specific peptides from exocrine glands stimulate mouse vomeronasal sensory neurons. Nature. 2005;437:898–901. doi: 10.1038/nature04033. [DOI] [PubMed] [Google Scholar]
- Kobayakawa K, Kobayakawa R, Matsumoto H, Oka Y, Imai T, Ikawa M, Okabe M, Ikeda T, Itohara S, Kikusui T, Mori K, Sakano H. Innate versus learned odour processing in the mouse olfactory bulb. Nature. 2007;450:503–508. doi: 10.1038/nature06281. [DOI] [PubMed] [Google Scholar]
- Kudwa AE, Rissman EF. Double oestrogen receptor alpha and beta knockout mice reveal differences in neural oestrogen-mediated progestin receptor induction and female sexual behaviour. J Neuroendocrinol. 2003;15:978–983. doi: 10.1046/j.1365-2826.2003.01089.x. [DOI] [PubMed] [Google Scholar]
- Laroche J, Gasbarro L, Herman JP, Blaustein JD. Enduring influences of peripubertal/adolescent stressors on behavioral response to estradiol and progesterone in adult female mice. Endocrinology. 2009a;150:3717–3725. doi: 10.1210/en.2009-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laroche J, Gasbarro L, Herman JP, Blaustein JD. Reduced behavioral response to gonadal hormones in mice shipped during the peripubertal/adolescent period. Endocrinology. 2009b;150:2351–2358. doi: 10.1210/en.2008-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Arellano J, Slotnick B, Restrepo D. Odors detected by mice deficient in cyclic nucleotide-gated channel subunit A2 stimulate the main olfactory system. J Neurosci. 2004;24:3703–3710. doi: 10.1523/JNEUROSCI.0188-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesel R, Weigel S, Braunig P. A simple fluorescent double staining method for distinguishing neuronal from non-neuronal cells in the insect central nervous system. J Neurosci Methods. 2006;155:202–206. doi: 10.1016/j.jneumeth.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Ma D, Allen ND, Van Bergen YC, Jones CM, Baum MJ, Keverne EB, Brennan PA. Selective ablation of olfactory receptor neurons without functional impairment of vomeronasal receptor neurons in OMP-ntr transgenic mice. Eur J Neurosci. 2002;16:2317–2323. doi: 10.1046/j.1460-9568.2002.02303.x. [DOI] [PubMed] [Google Scholar]
- Mani SK, Blaustein JD, O’Malley BW. Progesterone receptor function from a behavioral perspective. Horm Behav. 1997;31:244–255. doi: 10.1006/hbeh.1997.1393. [DOI] [PubMed] [Google Scholar]
- Martel KL, Baum MJ. Adult testosterone treatment but not surgical disruption of vomeronasal function augments male-typical sexual behavior in female mice. J Neurosci. 2009;29:7658–7666. doi: 10.1523/JNEUROSCI.1311-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossman CA, Drickamer LC. Odor preferences of female house mice (Mus domesticus) in seminatural enclosures. J Comp Psychol. 1996;110:131–138. doi: 10.1037/0735-7036.110.2.131. [DOI] [PubMed] [Google Scholar]
- Nagai Y, Sano H, Yokoi M. Transgenic expression of Cre recombinase in mitral/tufted cells of the olfactory bulb. Genesis. 2005;43:12–16. doi: 10.1002/gene.20146. [DOI] [PubMed] [Google Scholar]
- Naule L, Marie-Luce C, Parmentier C, Martini M, Albac C, Trouillet AC, Keller M, Hardin-Pouzet H, Mhaouty-Kodja S. Revisiting the neural role of estrogen receptor beta in male sexual behavior by conditional mutagenesis. Horm Behav. 2016;80:1–9. doi: 10.1016/j.yhbeh.2016.01.014. [DOI] [PubMed] [Google Scholar]
- Pankevich DE, Baum MJ, Cherry JA. Olfactory sex discrimination persists, whereas the preference for urinary odorants from estrous females disappears in male mice after vomeronasal organ removal. J Neurosci. 2004;24:9451–9457. doi: 10.1523/JNEUROSCI.2376-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restrepo D, Arellano J, Oliva AM, Schaefer ML, Lin W. Emerging views on the distinct but related roles of the main and accessory olfactory systems in responsiveness to chemosensory signals in mice. Horm Behav. 2004;46:247–256. doi: 10.1016/j.yhbeh.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Roberts SA, Davidson AJ, McLean L, Beynon RJ, Hurst JL. Pheromonal induction of spatial learning in mice. Science. 2012;338:1462–1465. doi: 10.1126/science.1225638. [DOI] [PubMed] [Google Scholar]
- Roberts SA, Simpson DM, Armstrong SD, Davidson AJ, Robertson DH, McLean L, Beynon RJ, Hurst JL. Darcin: a male pheromone that stimulates female memory and sexual attraction to an individual male’s odour. BMC Biol. 2010;8:75. doi: 10.1186/1741-7007-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sam M, Vora S, Malnic B, Ma W, Novotny MV, Buck LB. Neuropharmacology. Odorants may arouse instinctive behaviours. Nature. 2001;412:142. doi: 10.1038/35084137. [DOI] [PubMed] [Google Scholar]
- Samuelsen CL, Meredith M. Categorization of biologically relevant chemical signals in the medial amygdala. Brain Res. 2009;1263:33–42. doi: 10.1016/j.brainres.2009.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, Kalivas PW. Optogenetic dissection of basolateral amygdala projections during cue-induced reinstatement of cocaine seeking. Front Behav Neurosci. 2013:7. doi: 10.3389/fnbeh.2013.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JA, Salcedo E, Restrepo D, Finger TE. Second-order input to the medial amygdala from olfactory sensory neurons expressing the transduction channel TRPM5. J Comp Neurol. 2012;520:1819–1830. doi: 10.1002/cne.23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson ML, Edwards DA. Experiential and Strain Determinants of the Estrogen-Progesterone Induction of Sexual Receptivity in Spayed Female Mice. Hormones and Behavior. 1971;2:299–305. [Google Scholar]
- Trinh K, Storm DR. Vomeronasal organ detects odorants in absence of signaling through main olfactory epithelium. Nat Neurosci. 2003;6:519–525. doi: 10.1038/nn1039. [DOI] [PubMed] [Google Scholar]
- Tsunematsu T, Tabuchi S, Tanaka KF, Boyden ES, Tominaga M, Yamanaka A. Long-lasting silencing of orexin/hypocretin neurons using archaerhodopsin induces slow-wave sleep in mice. Behav Brain Res. 2013;255:64–74. doi: 10.1016/j.bbr.2013.05.021. [DOI] [PubMed] [Google Scholar]
- White S, Uphouse L. Estrogen and progesterone dose-dependently reduce disruptive effects of restraint on lordosis behavior. Horm Behav. 2004;45:201–208. doi: 10.1016/j.yhbeh.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Znamenskiy P, Zador AM. Corticostriatal neurons in auditory cortex drive decisions during auditory discrimination. Nature. 2013;497:482–485. doi: 10.1038/nature12077. [DOI] [PMC free article] [PubMed] [Google Scholar]