Abstract
Recent studies have reported several genetic, health, and aging interaction effects in predicting cognitive performance and change. We used an accelerated longitudinal design to examine interactions among genetic, lifestyle, and aging for executive function (EF) in non-demented older adults (n=634; age range=53–95 years). The polymorphisms were Apolipoprotein E (APOE), Catechol-O-methyl transferase (COMT), and Brain-derived neurotrophic factor (BDNF). We tested (a) independent and additive effects of APOE, COMT, and BDNF and (b) APOE effect modification for COMT+ BDNF, on EF performance and 9-year change as separated by age and lifestyle activities. First, APOE ε4+ carriers had poorer EF performance and steeper 9-year decline. Second, APOE ε4+ carriers with (a) BDNF Met/Met genotype and (b) increasing allelic risk in the COMT+ BDNF risk panel had poorer EF performance; these effects were moderated by lifestyle activities (composite of everyday social, physical, cognitive activities). Examining APOE effect modification for COMT+ BDNF risk panel effects with other moderating factors may help identify complex neurobiological and genetic underpinnings of polygenic phenotypes such as EF in aging.
Keywords: Aging, Executive Function, Apolipoprotein E, Catechol-O-methyl transferase, Brain-derived neurotrophic factor, Victoria Longitudinal Study
1. Introduction
Research on biological and genetic markers of non-demented cognitive aging is in a transitional phase. Single candidate biomarker or gene association studies have produced encouraging but inconsistent associations with such prominent cognitive aging phenotypes as memory and executive functions (EF) (Harris & Deary, 2011; Laukka et al., 2013). Several recent observations provide promising and converging research directions for genetic marker research in non-demented aging. These observations include: (a) single gene-related individual differences in cognitive ability may increase in aging (Das et al., 2014; Deary et al., 2010); (b) genetic associations with cognitive phenotypes may be magnified as neurological resources decline (Belsky et al., 2009; Lindenberger et al, 2008); (c) cognitive phenotypes may be profitably measured with multiple indicators as they change over time (McFall et al., 2015a; Raz et al., 2009), and (d) biomarker predictors may be measured in terms of neurobiologically reasonable interactions, panels, or composites (Sapkota et al., 2015). Accordingly, some recent research has focused on identifying sets of genetic and other factors that may tap into underlying neurobiological mechanisms and thereby complement, modify, or intensify effects on cognitive changes with aging (McFall et al., 2015b). These changes may be differential, including patterns consistent with maintenance (McFall et al., 2015a; Nyberg et al., 2012), non-demented decline (Harris & Deary, 2011; Raz et al., 2009), and impairment (Dixon et al., 2014). Although the relevant research designs are sometimes complex, they afford the opportunity for examining interactions among concordant genetic variants and non-genetic biological and environmental risk factors as they predict longitudinal variations in cognitive trajectories and clinical outcomes (Thibeau et al., 2016). The present study contributes to this effort by including two typical cognitive aging polymorphisms, one prominent Alzheimer’s disease (AD) genetic risk variant, age, and environmental (lifestyle) factors— all in the context of longitudinal change in cognitive performance.
We focus on the cognitive domain of EF, which represents everyday goal-oriented performance (de Frias et al; 2006; de Frias et al., 2009; Luszcz, 2011). Some recent genetic studies of EF in non-demented aging have concentrated on two commonly examined dopaminergic- and neurotrophic-related variants (Das et al., 2014; Harris et al., 2006; Nagel et al., 2008; Sapkota et al., 2015) that may interact through basal ganglia-thalamocortical loops (Alexander et al., 1986). The single nucleotide polymorphisms (SNPs) identified for dopaminergic and neurotrophic-related factors include catechol-O-methyltransferase (COMT; rs4680) (Papenberg et al., 2014; Papenberg et al., 2015b; Wishart et al., 2011) and brain-derived neurotrophic factor (BDNF; rs6265) (Ghisletta et al., 2014; Nagel et al., 2008), respectively. In an earlier cross-sectional study, we observed synergistic associations between these two polymorphisms as they predicted concurrent EF performance (Sapkota et al., 2015). COMT and BDNF were selected because of the neurobiological and cognitive relationship of both genes (Nagel et al., 2008). COMT and BDNF have shown to influence the prefrontal and medial temporal lobe regions (Bertolini et al., 2006), which are both activated during EF tasks (Cabeza et al., 2003). Perhaps the most commonly considered polymorphism in cognitive and neurodegenerative aging is Apolipoprotein E (APOE; rs7412; rs429358). The APOE ε4 allele has been consistently linked to normal cognitive decline (Caselli et al., 2001; Laukka et al., 2013; Luciano et al., 2009; Wisdom et al., 2011), Mild Cognitive Impairment (MCI) (Brainerd et al., 2011; Dixon et al., 2014), and dementia (Barral et al., 2012). The role of APOE in cognitive aging may be pivotal in that it interacts with other genetic variants, as well as with health, lifestyle (e.g., physical activity), and neurobiological risk factors (McFall et al., 2016). In the present study, we extend our earlier cross-sectional report by assembling new 3-wave longitudinal data to test specific dynamic synergies among genetic markers (APOE plus COMT and BDNF) as moderated by age and lifestyle behaviors. Interactively, these factors are expected to shed light on mechanisms associated with EF change in brain aging. Specifically, we examine independent and additive effects of APOE, COMT, and BDNF. In addition, we test for an APOE moderation effect for COMT and BDNF, and APOE effect modification for COMT + BDNF. An APOE moderation would be observed when the APOE genotype interacts or influences the effect of COMT or BDNF on EF performance. An APOE effect modification would be observed if there is, as expected, a differing relationship of (COMT + BDNF) on executive functioning in the context of APOE stratification (i.e., ε4+ versus ε4- groups). According to the brain-resource modulation hypothesis (Lindenberger et al., 2008; Papenberg et al., 2015a), genetic effects may be magnified in late adulthood, as compared with earlier adulthood. Therefore, our dynamic synergistic analyses involves both the overall sample (n = 634; age range = 53–95 years) and as stratified by age group. Furthermore, some research has shown that lifestyle activity engagement (e.g., physical, cognitive, and social activities) can affect EF performance (Erickson, et al., 2008). For this reason, we test the moderating effects of a lifestyle activity composite on the synergistic associations of the genetic variants and potential magnification by chronological age on longitudinal trajectories of EF performance in a 40-year band of non-demented aging.
Our approach to predicting EF performance and change includes examining independent and additive associations for APOE, COMT, and BDNF genetic risk as moderated by age and lifestyle risk factors. The additive (gene + gene) model tests panels of risk, whereby an additional allelic risk may amplify the vulnerability already present with one risk allele (Sapkota et al., 2015; Verhaaren et al., 2013). The four main steps are as follows. First, we examine independent effects of APOE, COMT, and BDNF as moderated by age group and lifestyle activities. Second, we test APOE moderation for COMT and BDNF on EF performance and 9-year change. Third, we test whether a set of additive effects (i.e., APOE + COMT, APOE + BDNF, COMT + BDNF) separately and as moderated by age group and lifestyle activities influence EF performance and decline. Fourth, we test whether an additive effect for COMT + BDNF is modified by APOE. We now summarize the three polymorphisms as related to cognitive functioning in aging.
APOE is the most commonly studied genetic risk factor for AD and MCI (Brainerd et al., 2011; Dixon et al., 2014; Verghese et al., 2011). It is differentiated by three isoforms: ε2, ε3, and ε4. Carriers of the ε4 allele have a higher risk of AD development (Wisdom et al., 2011). In contrast, the ε2 allele has been found to be protective in numerous studies (Corder et al., 1994; de-Almada et al., 2012; McFall et al., 2015a; Panza et al., 2000). APOE is involved in transporting cholesterol to neurons, which is crucial for synaptic formation and axonal growth important in learning, memory, and neuronal injury repair. In addition, the APOE genotype presents an allelic dosage effect whereby the ε4/ε4 allele is associated with the highest risk followed by ε3/ε3 and ε2/ε2 (Liu et al., 2013). APOE ε4 allelic risk has been linked to lower dendritic spine density in the hippocampus and increased neuro-inflammation (Fotuhi et al., 2009; Liu et al., 2013). Current reports focus on synergistic associations of APOE with other biological (Das et al., 2014; Sapkota et al., 2015) and vascular-health (i.e., pulse pressure (McFall et al., 2015a)) risk factors.
The COMT enzyme regulates dopamine (DA) levels primarily in prefrontal cortex (Bilder et al., 2004; Chen et al., 2004; Papenberg et al., 2014). Multiple dopaminergic pathways in the prefrontal cortex (Raz et al., 2009) have been associated with EF processes (Bäckman et al., 2010). Because of lower enzymatic activity, the COMT polymorphism at codon 158 on chromosome 22q11 results in greater DA levels for COMT homozygotes for the Met allele as compared to Val allele homozygotes (Das et al., 2014; Egan et al., 2003). Carriers of the Val allele may thus be at higher risk for brain and cognitive deficits, including executive functioning (Das et al., 2014; Nagel et al., 2008; Sapkota et al., 2015; Wishart et al., 2011) and reduced white matter integrity (Papenberg et al., 2015b).
The BDNF (rs6265) Val66Met polymorphism located at 11p13 (Houlihan et al., 2009) is involved in BDNF secretion. BDNF is mostly present in hippocampus and prefrontal cortex, and may play an important role in memory (Miyajima et al., 2008), EF (Egan et al., 2003; Nagel et al., 2008; Sapkota et al., 2015), and cognitive plasticity (Poo, 2001). The BDNF Met allele is considered to be the higher risk allele as it is linked to lower levels of BDNF in the hippocampus and prefrontal cortex. However, BDNF-cognition association studies have reported an inconsistent pattern of results. For example, a meta-analysis examined 23 publications with a combined total of 7095 individuals and did not observe significant associations with any of the five most commonly studied phenotypes: general cognition, memory, EF, visual processing, and verbal fluency (Mandelman & Grigorenko, 2012). This meta-analysis was not focused on aging, and several known moderators of brain aging may affect observed BDNF-cognition associations in older adults. These include younger age and an active lifestyle. In aging, the latter may increase BDNF expression in the brain resulting in greater synaptic plasticity (Cotman & Berchtold, 2002) and reduced cognitive impairment (Erickson et al., 2012). This augmented effect in younger and higher lifestyle activities older adult groups may then lead to large detectable differences in cognitive performance and change, particularly for those with higher genetic risk combinations (Ward et al., 2014).
As applied to this study, a genetic magnification perspective suggests that more than one “copy” of a neurobiological aging risk factor may exacerbate the deleterious effects on phenotypes such as cognitive performance and change in aging. The sources of risk may be multimodal, including additional genetic risk, advanced biological aging, and low lifestyle activity. This study is a major longitudinal and predictor-related extension of an earlier cross-sectional report, which focused on determining the optimal operations for combining these variants (additive or multiplicative) in terms of examining synergistic effects of COMT and BDNF on EF in non-demented older adults (Sapkota et al., 2015). We adopt the additive operation combined with tests of moderation and effect modification by APOE and potential magnification by chronological age and lifestyle activities. We test magnification effects on longitudinal data across a 40-year band of aging. In addition, using a procedure established earlier (McFall et al., 2014), we measure EF as a single latent and invariant variable indicated by four standardized neuropsychological tests.
1.1. Research Questions
We examined two general research questions. For both, we predicted EF performance and 9-year change. Both general research questions were divided into two parts to represent the fact that two different ways of testing gene-cognition associations were stratified by age group, lifestyle activities, and APOE genotype. In general research question 1, we tested independent associations of APOE, COMT, and BDNF. In general research question 2, we tested additive associations of all possible dual-gene additive panels (i.e., APOE + COMT, APOE + BDNF, COMT + BDNF) as separated by age and lifestyle activities. Both research questions were divided into two corresponding parts. In parts 1a and 2a, we examined all three genotypes. In part 1b, we tested APOE moderation effect of COMT and BDNF (research question 1b) as separated by age and lifestyle activities. In part 2b, we tested APOE effect modification for COMT + BDNF (research question 2b) as separated by age and lifestyle activities. In part a (1a and 2a) the three genes were tested as separated by age group and lifestyle. In part b (1b and 2b) we added a test of further moderation and effect modification by APOE genotype. Based on our previous cross-sectional study, we expected to observe APOE moderation and effect modification for COMT and BDNF genotypes on EF performance and change.
1.1.1. Research question 1a (RQ1a)
Do higher allelic risk carriers for APOE (ε4+), COMT (Val/Val; Val/Met), and BDNF (Met/Met; Met/Val) show poorer performance and steeper decline in EF than their lower-risk counterparts? We test this question independently, by age group (younger versus older), and by lifestyle activities (higher versus lower activities)? We expected higher allelic risk carriers to have poorer EF performance and steeper decline overall. We also expected worse performance and exacerbated decline in the older group or the lower lifestyle activities group than in the younger or the higher lifestyle activities groups.
1.1.2. Research question 1b (RQ1b)
Does APOE status (ε4+ versus ε4-) moderate EF performance for COMT and BDNF higher allelic risk carriers such that COMT and BDNF higher allelic risk carriers in the APOE ε4+ group have poorer EF performance and steeper decline than those in the APOE ε4- group? We also examined whether this effect was magnified in the older age group or lower lifestyle activities groups than in the younger age group or higher lifestyle activities groups?
1.1.3. Research question 2a (RQ2a)
Is the additive (gene + gene) risk effect for each combination (i.e., APOE + COMT, APOE + BDNF, COMT + BDNF) associated with exacerbated EF deficits or decline? Is this exacerbation overall, by age group, or by lifestyle activities? We expected that the cumulative effect of higher allelic risk would produce poorer EF performance and steeper decline than would the lower-risk combinations, especially in the older age and lower lifestyle activities group.
1.1.4. Research question 2b (RQ2b)
Do APOE ε4+ carriers have poorer EF performance and steeper decline with increasing allelic risk in the COMT + BDNF risk panel compared to the APOE ε4-group? Is this effect larger in the older than in the younger age group or in the lower than in the higher lifestyle activities group? We expected APOE ε4+ carriers in the older group and in the lower lifestyle activities group to have poorer EF performance and steeper decline with increasing risk in the COMT + BDNF risk panel compared to those in the APOE ε4- group.
2. Method
2.1. Participants
We used data from the Victoria Longitudinal Study (VLS), a large scale, longitudinal sequential study examining biomedical, health, genetic, lifestyle, cognitive and other aspects of aging. We use the term longitudinal sequential to describe a complex design that includes the following characteristics: (a) more than one age-based sample is followed over time, and (b) these similar age-based samples are staggered in historical time, reflecting the fact that they represent different but overlapping birth cohorts (Baltes et al., 1977). In the VLS, three such samples (from the 1980s, 1990s, and 2000s) are included (Dixon & de Frias, 2004). General information on recruitment, methodological, and VLS characteristics are available elsewhere (Dixon & de Frias, 2004; Dolcos et al., 2012). All volunteers in the VLS were initially healthy, enrolled through advertisements, and received a small honorarium for their participation. The VLS and all present data collection procedures are in full and certified compliance with prevailing human/institutional research ethics guidelines. Written informed consent was obtained from all participants. Approximately 99.2% of participants were White, not of Hispanic Origin. All had complete access to Canadian national health care. The present sample reflects the implementation of exclusionary criteria affecting individuals with (a) diagnosis of dementia, (b) anti-psychotic medication, (c) Mini Mental State Exam (MMSE) scores less than 24, (d) uncontrolled hypertension, (e) insulin-controlled diabetes, and (f) history of serious head injury (e.g., hospitalized). Participants were screened for dementia and MMSE at each wave. Accordingly, 634 participants (age range = 53–95 years, mean age = 70.58, SD = 8.65), including 423 females and 211 males with genetic data were included at baseline (Table 1; Supplementary Table 1). We followed an accelerated longitudinal design by assembling three partial samples (S; S1, S2, S3) from the VLS. We note that the term “acceleration” refers not to a quickening in the rate of change but to a methodological adjustment whereby change trajectories are presented and analyzed according to an age (rather than a wave) metric and thus includes a broader band of aging (Galbraith et al., 2014; McArdle & Hamagami, 1991). The present Wave 1 (W1) and Wave 2 (W2) included participants from all three samples and Wave 3 (W3) included participants from S3. Specifically, throughout this report (a) W1 (n = 634) refers to S1W6, S2W4, and S3W1, (b) W2 (n = 518) refers to S1W7, S2W5, S3W2, and (c) W3 (n = 294) refers to S3W3 (see Table 1). As noted, with these data, we link a series of shorter individual longitudinal trajectories across the full available 40-year band of aging. The average interval was 4.4 years between W1 and W2, and 4.5 years between W2 and W3. The retention rates for each wave interval for: (a) S1: W1-W2 was 83%, (b) S2: W1-W2 was 77%, (c) S3: W1-W2 was 84%, (d) S3: W2-W3 was 88%, and (e) S3: W1-W3 was 74%.
Table 1.
Participant characteristics by age group and genotype.
| Characteristics | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Young-Old | COMT | BDNF | APOE | Total | ||||||||
| Met/Met | Met/Val | Val/Val |
p- value |
Met/Met | Met/Val | Val/Val |
p- value |
ε4- | ε4+ |
p- value |
||
| n | 70 | 152 | 74 | -- | 15 | 79 | 202 | -- | 201 | 79 | -- | 296 |
| Age (years) | 62.32 (4.47) |
62.70 (4.58) |
63.37 (4.39) |
0.356 | 64.09 (4.63) |
63.10 (4.24) |
62.55 (4.60) | 0.333 | 62.50 (4.52) |
63.28 (4.44) |
0.191 | 62.77 (4.51) |
| Education (years) |
15.21 (3.01) |
15.73 (2.75) |
15.38 (3.25) |
0.418 | 15.77 (2.97) |
15.15 (3.09) |
15.65 (2.88) | 0.425 | 15.44 (2.97) |
15.81 (3.02) |
0.345 | 15.52 (2.95) |
| Sex (F/M) | 54/16 | 105/47 | 52/22 | 0.458 | 12/3 | 58/21 | 141/61 | 0.625 | 149/52 | 49/30 | 0.045 | |
| MMSE | 29.04 (0.92) |
28.99 (1.05) |
28.76 (1.29) |
0.215 | 29.07 (0.80) |
29.05 (0.95) |
28.90 (1.16) | 0.513 | 28.87 (1.16) |
29.09 (0.95) |
0.129 | 28.95 (1.09) |
| Old-Old | COMT | BDNF | APOE | |||||||||
| Met/Met | Met/Val | Val/Val |
p- value |
Met/Met | Met/Val | Val/Val |
p- value |
ε4- | ε4+ |
p- value |
||
| n | 76 | 186 | 76 | -- | 12 | 110 | 214 | -- | 252 | 70 | -- | 338 |
| Age (years) | 77.37 (4.82) |
77.51 (4.55) |
77.23 (5.01) |
0.904 | 74.09 (2.59) |
77.44 (5.05) |
77.59 (4.56) | 0.043 | 77.55 (4.81) |
77.28 (4.35) |
0.665 | 77.42 (4.71) |
| Education (years) |
14.66 (3.20) |
15.03 (2.81) |
15.34 (3.06) |
0.361 | 15.67 (2.46) |
15.12 (2.93) |
14.94 (3.00) | 0.658 | 14.99 (2.96) |
15.25 (3.13) |
0.518 | 15.02 (2.96) |
| Sex (F/M) | 47/29 | 120/64 | 44/32 | 0.547 | 6/6 | 70/40 | 135/79 | 0.648 | 155/97 | 44/26 | 0.827 | |
| MMSE | 28.42 (1.35) |
28.48 (1.27) |
28.36 (1.32) |
0.799 | 29.25 (0.75) |
28.51 (1.23) |
28.36 (1.34) | 0.053 | 28.50 (1.27) |
28.19 (1.39) |
0.082 | 28.44 (1.30) |
Note. n = total number; COMT = Catechol-O-methyl transferase; BDNF = Brain-derived neurotrophic factor; APOE = Apolipoprotein E; p < .05. MMSE = Mini-Mental State Exam. Standard deviations are in parentheses. For the analyses involving the APOE genotypes, ε2/ε4 carriers (n = 30) were deleted from the sample.
2.2 DNA Extraction and Genotyping
Saliva was collected according to standard procedures from Oragene DNA Genotek and stored at room temperature in Oragene® disks until DNA extraction. DNA was manually extracted from 0.8 ml of saliva sample mix using the manufacturer’s protocol with adjusted reagent volumes. Genotyping was carried out by using a PCR-RFLP strategy to analyze the allele status for BDNF (rs6265), COMT (rs4680), and APOE (rs7412, rs429358). Genotyping was successful for the targeted SNPs for all present participants. Supplementary Table 1 shows participant characteristics by genotype for BDNF, COMT, and APOE. The genotype frequencies did not differ significantly from Hardy-Weinberg equilibrium: BDNF rs6265 (χ2 = 0.837, p = 0.36), COMT rs4680 (χ2 = 2.786, p = 0.10), and APOE rs7412, rs429358 (χ2 = 0.545, p = 0.909). We included all three allelic combinations for COMT and BDNF (Met/Met, Met/Val, and Val/Val). Both SNPs were coded from 1 to 3 (3 = highest risk). For evaluating moderation and effect modification by APOE, we deleted all ε2/ε4 carriers (n = 30) and then compared patterns between ε4+ carriers and ε4- group. The APOE ε4- group was coded as 1 (lower risk) and APOE ε4+ group as 2 (higher risk).
2.3. Executive Function Measures
Two dimensions of EF (inhibition, shifting) were each measured by two standard and frequently used tests for cognitive, clinical, and neurobiological studies in older adults (de Frias et al., 2006; McFall et al., 2014; Sapkota et al., 2015).
2.3.1 Hayling Sentence Completion (Inhibition)
This test (Burgess & Shallice, 1997) consists of two sections, each comprising 15 sentences. The standardized scores are based on errors from the second of two sections and the speed of each response from both sections, which are then combined to obtain the final score (1 = very low to 10 = very high).
2.3.2. Stroop (Inhibition)
This test (Taylor et al., 1997) consists of the standard three parts (Parts A, B and C), with the measures based on latencies. The score is the standardized Stroop interference index ([Part C- Part A]/ Part A), with a lower index reflecting better performance.
2.3.3. Brixton Spatial Anticipation (Shifting)
This test (Burgess & Shallice, 1997) consists of 10 different circles, one being blue, whereas the rest are colorless. Participants are asked to guess where the blue colored circle will appear on subsequent pages. The total number of incorrect guesses are measured and the final scores are calculated (1 = very low to 10 = very high).
2.3.4. Color Trails (Shifting)
This test (D’Elia et al., 1996) comprises two different sections in which participants connect different attributes, such as numbered and colored circles. Latency scores in the second of two sections were computed and used in the final analyses. Lower scores reflected better performance.
2.4. Lifestyle Activities Composite
The 67-item version of the VLS Activity Lifestyle Questionnaire (VLS-ALQ) was used to determine the level or frequency of participation in everyday activities. Based on previous research (e.g., Small et al., 2012; Thibeau et al., 2016) the following four activity domains were selected for this study: (a) social, such as visiting friends (7 items); (b) physical activity, such as gardening (4 items); (c) integrative information processing, such playing a musical instrument (12 items); and (d) novel information processing, such as completing jigsaw puzzles (27 items). The frequency of participation is rated on a 9-point scale (never, less than once a year to two or three times a week, and daily). The lifestyle activities composite was calculated by summing the scores across all four domains.
2.5. Statistical Analysis
Structural equation modeling (SEM) was used to analyze both parts of the two research questions with Mplus Version 7 (Muthén & Muthén, 1998–2015). All missing values for cognitive measures were assumed to be missing at random and handled using maximum likelihood. Missing predictor variables were handled using list-wise deletion in Mplus. Only two participants with missing measures on all four EF tasks were lost. Although we used the three waves to organize the demographic information (Supplementary Table 1), it is important to note that age rather than wave was used as the metric of longitudinal change in the analyses. Statistically, using age in this manner permitted us to account for variability associated with age as well or better than if it is used as a covariate in the statistical models.
2.5.1. Analyses for research questions
Older adults who were 70 years and older were in the old-old (OO) group and those below 70 years were in the young-old (YO) group. In the YO group, age was centered at 63 years and in the OO group, age was centered at 77 years, based on the mean age in each group. The lifestyle activities composite was split into low and high clusters of activity participation at the overall group mean (M = 133). As noted, age (as a continuous variable) was incorporated as the metric of change. Sex and education (continuous) were used as covariates in all analyses. For model fit statistics and significant results, we examined the regression estimate and p < .05, and −2 log likelihood (−2LL), Akaike information criteria (AIC), and Bayesian information criteria (BIC) values, with lower values indicating better model fit. We now turn to analyses for each research question.
For RQ1a, EF intercept and slope regression pathways were examined for APOE, COMT, and BDNF independently, and as separated by age group (YO and OO) and lifestyle activities composite (low and high).
For RQ1b, EF intercept and slope regression pathways were examined for COMT and BDNF as separated by APOE status (ε4+ versus ε4-). Next, we tested this regression model as further separated by age group (YO and OO) and lifestyle activities (low and high).
For RQ2a, EF intercept and slope regression pathways were examined separately for all additive genetic combinations. Specifically, for the additive models we tested (a) APOE + COMT, (b) APOE + BDNF, and (c) COMT + BDNF. We tested all three models independently, and as separated by age group (YO and OO) and lifestyle activities (low and high).
For RQ2b, EF intercept and slope regression pathways were examined for COMT + BDNF additive model as separated by APOE status (ε4+ versus ε4-). Next, we tested this regression model as further separated by age group (YO and OO) and lifestyle activities (low and high).
3. Results
First, we established several foundational results through preliminary analyses. The one-factor parsimonious model of EF provided the best fit to the data and was used as the final confirmatory factor analysis model (see supplementary material (text) and Supplementary Table 2). Unstandardized regression coefficients for the EF latent variable were examined to determine differences and changes in performance. Demonstrating longitudinal invariance of the latent variable, we obtained partial scalar longitudinal invariance across all three waves (χ2 (df) = 84.60 (49), p = .001; RMSEA (90% CI) = .034 (.021−.044); CFI = .977; and SRMR = .084) (Supplementary Table 2). We computed EF factor scores, which were used in all succeeding models for testing RQ1 and RQ2. The best latent-growth model was obtained with the random intercept and random slope model (Supplementary Table 3).
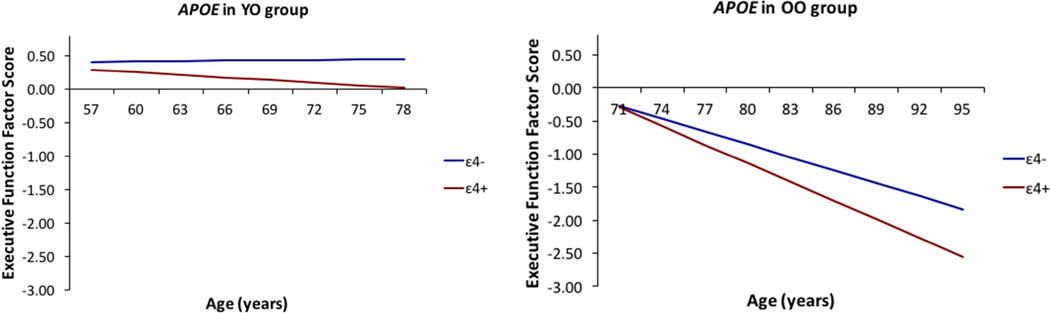
For RQ1a, we observed four significant independent effects of APOE on EF performance and change. First, overall, APOE higher risk carriers (ε4+) performed worse than their lower-risk (ε4-) counterparts at age 75 (β = −0.206; SE = 0.098; p = .036) (Supplementary Figure 1a). We have prepared a reference table as a guide to supplement all the research models tested in Supplementary Table 4. We did not observe significant differential decline between the APOE ε4+ and ε4- group. Second, in the YO group, APOE ε4+ carriers performed worse on EF than their ε4- counterparts at age 63 (β = −0.210; SE = 0.100; p = .036) and had steeper decline over the 9-year period (β = −0.015; SE = 0.007; p = .020). Third, in the OO group, APOE ε4+ carriers had steeper decline on EF with age than their lower-risk (ε4-) counterparts (β = −0.029; SE = 0.011; p = .007) (Figure 1). Level of lifestyle activities did not significantly moderate APOE genotype on EF performance or change. We did not observe significant independent effects for COMT or BDNF allelic risk on EF performance or change, either overall (Supplementary Figure 1b and 1c) or as separated by age or lifestyle activities.
Figure 1.
In the young-old (YO) group, APOE ε4+ carriers performed worse at age 63 and had steeper 9-year decline in EF than their non-risk (ε4-) counterparts. In the old-old (OO) group APOE ε4+ carriers showed steeper 9-year decline on EF than their non-risk counterparts.
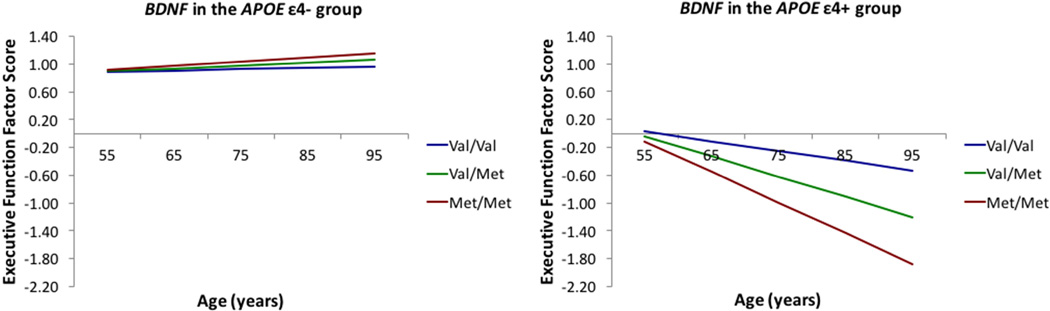
For RQ1b, we observed three significant associations. First, in the overall sample, there was a significant moderation effect for BDNF genotype by APOE status (ε4- versus ε4+). Specially, BDNF Met homozygotes in the APOE ε4+ group had the lowest EF performance at age 75 years compared to the BDNF Val/Met or Val/Val genotype (β = −0.373; SE = 0.179; p = .037). BDNF allelic higher risk carriers in the APOE ε4- group performed relatively well, as compared to the APOE ε4+ group (Figure 2). Second, in the YO group, BDNF Met homozygotes in the APOE ε4+ group had the lowest EF performance at age 63 (β = −0.330; SE = 0.145; p = .023) and a steeper slope than the BDNF Val homozygotes (β = −0.032; SE = 0.010; p = .023; Supplementary Figure 2). Third, in the higher lifestyle activities group, BDNF Met/Met homozygotes in the APOE ε4+ group had the lowest EF performance at 75 years (β = −0.525; SE = 0.252; p = .037) (Supplementary Figure 3), but did not differ from the other genotype groups in rate of change.
Figure 2.
In the APOE ε4+ group, BDNF Met/Met homozygotes had the worst EF performance compared to their non-risk counterparts (Val homozygotes) at 75 years. In contrast, in the APOE ε4- group, BDNF genotype did not affect EF performance.
For RQ2a, we did not observe any significant effects for (a) APOE + COMT, (b) APOE + BDNF, and (c) COMT + BDNF risk overall or as separated by age or lifestyle activities.
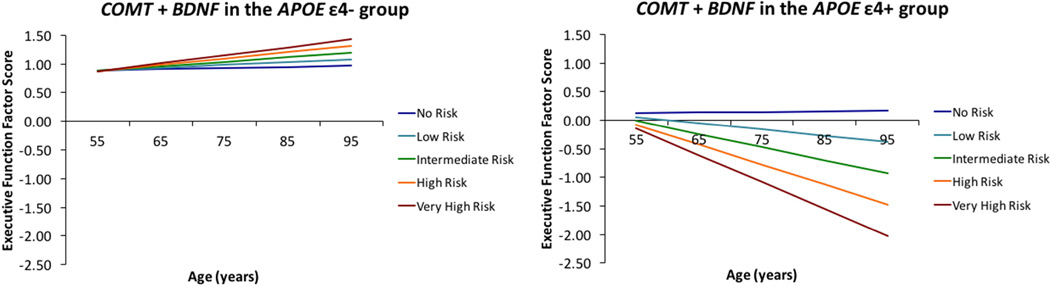
For RQ2b, we observed two significant synergistic effects for the COMT + BDNF combination. First, APOE effect modification was observed for the COMT + BDNF additive effect on EF performance. COMT + BDNF allelic risk carriers showed an additive risk effect at age 75 and borderline decline in the APOE ε4+ group. Specifically, participants displayed poorer EF performance with increasing allelic risk in the COMT + BDNF risk panel at age 75 (β = −0.307; SE = 0.123; p = .013), and borderline 9-year decline (β = −0.012; SE = 0.006; p = .054) (Figure 3; Supplementary Table 4). Second, greater COMT + BDNF allelic risk was associated with less steep decline in EF performance for the APOE ε4- group with higher lifestyle activities (β = 0.008; SE = 0.004; p = .046) (Supplementary Figure 4). We did not observe any significant effects for COMT and BDNF cumulative risk as separated by APOE ε4 status and age group.
Figure 3.
APOE effect modification was observed for COMT + BDNF additive effect on EF performance. APOE ε4+ carriers had poorer EF performance with increasing allelic risk in the COMT + BDNF risk panel at age 75 years and borderline 9-year decline. In contrast, the APOE ε4- group was protected from the deleterious effect on EF performance and decline with increasing allelic risk in the COMT + BDNF risk panel.
4. Discussion
In a previous cross-sectional study, we reported COMT + BDNF additive effects and APOE effect modification on EF performance (Sapkota et al., 2015). In the present and expanded longitudinal study, we first tested independent and additive associations of APOE, COMT, and BDNF allelic risk on EF performance and 9-year change in non-demented older adults. We then examined (a) APOE moderation effects separately for COMT and BDNF and (b) APOE effect modification for COMT + BDNF. Although this interactive and multimodal approach is growing (e.g., McFall et al., 2015b; Nagel et al., 2008; Papenberg et al., 2014; Sapkota et al., 2015), to our knowledge this is the first study to examine synergistic associations with EF performance and longitudinal change as separated by age group and lifestyle activities for these three aging-related genetic variants. Key results include the following. First, we observed a single-gene effect for APOE. The ε4+ carriers (and not COMT or BDNF risk carriers) were at higher risk for poor EF performance and steeper decline. Second, we observed that APOE ε4+ moderated the effects of the BDNF genotype such that the combined genetic risk was enough to negatively affect cognition even in the YO and higher lifestyle activity groups. Third, we observed APOE ε4 effect modification for COMT + BDNF: APOE ε4+ carriers had poorer EF performance with increasing allelic risk in the COMT + BDNF risk panel at age 75 and borderline 9-year decline. In contrast, adults in the APOE ε4- group with higher lifestyle activities were protected from the COMT + BDNF risk panel effect on EF. Specific comments for each research question follow.
For RQ1a, the first main result was the observation that APOE ε4 carriers performed worse than their ε4- counterparts at age 75 in the overall group, at age 63 years in the YO group, and had steeper EF decline in the YO and OO groups. Although some previous research and meta-analyses on APOE and cognitive associations have reported similar findings in non-demented older adults, observers have also concluded that the genetic associations may be selective to specific cognitive domains (Marioni et al., 2015; Raz et al., 2009; Small et al., 2004). In contrast, we found that COMT and BDNF allelic risk did not predict differences in EF. Second, we observed an overall age and lifestyle activities effect on EF performance and change. We found an age effect whereby adults in the OO group were declining more in EF performance than their YO counterparts. Notably, for lower versus higher lifestyle activities, we observed a similar pattern of results. There were no independent effects of APOE, COMT, and BDNF in either of two lifestyle groups, but participants with higher lifestyle activities showed shallower decline in EF performance overall compared to those with lower lifestyle activities.
For RQ1b, we observed an APOE moderation effect for BDNF genotype on EF performance in the overall sample, in the YO group, and in the higher lifestyle activities group. BDNF Met homozygotes showed the worst EF performance in the presence of APOE ε4+ genetic risk, and this effect was present even among YO and those with higher lifestyle activities. A recent study reported an APOE and BDNF interactive effect for episodic memory performance (Ward et al., 2014). This study found that BDNF Met+ carriers with APOE ε4 allele had poorer performance compared to BDNF Met+ carriers with the APOE ε2 allele, but no further interactions were tested. Another recent study examined amyloid beta deposition in cognitively normal older adults (Adamczuk et al., 2013), suggesting a possible biological interaction between APOE ε4 status and BDNF Met status. Specifically, adults who were carriers of both APOE ε4+ BDNF Met+ genotypes had a higher amyloid load in multiple brain regions than did those with a BDNF Met- genotype. Although no further interactions were tested, the authors suggested that the lipid-metabolism pathway influenced by APOE genotype and the role of BDNF in neuronal survival may be linked in a way that modifies amyloid deposition. In the present study, we observed that this moderation effect was present in YO adults and those with higher lifestyle activities. As noted earlier, both younger age and greater physical engagement (a common combination according to Evenson et al., 2012) may be “protective” for cognitive performance and change, possibly related to amplified BDNF expression in these favorable conditions (Erickson et al., 2012). Further, the APOE moderation effect on BDNF in our study implies that the (a) BDNF Met/Met risk may only be detrimental for EF in the presence of APOE ε4+ risk, and (b) younger age and higher lifestyle activities may not be protective for those with the highest genetic risk combination (BDNF Met/Met and APOE ε4+).
We briefly note that for RQ2a, we did not observe additive effects for all three pairwise combinations (APOE + COMT, APOE + BDNF, COMT + BDNF) overall or as separated by age or lifestyle activities. Absence of additive effect supports our findings that (a) the COMT + BDNF risk panel is only detectable in the presence of APOE ε4+ risk and (b) APOE has a moderating or effect modification (and not additive) role with COMT and BDNF in non-demented older adults.
For RQ2b, we observed an APOE effect modification for the COMT + BDNF additive association on EF performance. APOE ε4+ carriers displayed poorer EF performance with increasing allelic risk in the COMT + BDNF risk panel at age 75 and accentuated 9-year decline. An additional allelic risk for either COMT or BDNF among APOE ε4+ carriers resulted in poorer EF performance, whereas APOE non-risk carriers (ε4-) were protected from the deleterious effect of COMT + BDNF allelic risk. Previous studies have reported that aging exacerbates the association between lower prefrontal DA levels (i.e., COMT Val homozygotes) and poorer cognitive performance in (Bäckman et al., 2010; Lindenberger et al., 2008; Papenberg et al., 2014). Although we did not observe differential patterns in our YO versus OO age groups, we informally note a borderline aging magnification of COMT + BDNF genetic effects across the 40-year age range of this sample. This trend, which suggests magnification of genetic effects in older adults, deserves further research attention (Papenberg et al., 2015a). As for BDNF Val homozygotes, they have higher levels of neurotrophic factors (Marosi & Mattson, 2014), which has been associated with better cognitive performance (Nagel et al., 2008). In our additive association, we observed that an absence of COMT Val+ or BDNF Met + allelic risk did not eliminate the risk present with either COMT or BDNF genotype for APOE ε4+ carriers on EF performance.
We also observed that the groups carrying risk alleles for COMT and BDNF in the APOE ε4-group with the protective component of higher lifestyle activities showed least decline in EF performance over 9 years (Supplementary Figure 4). Some past studies show that higher levels of certain lifestyle activities may be protective against dementia (Scarmeas et al., 2001; Valenzuela et al., 2011) and support cognitive maintenance in non-demented older persons (Erickson et al., 2008). Among the proposed mechanisms are activity- or exercise-related increases in synaptic density and cognitive reserve, which may delay clinical symptoms (Scarmeas et al., 2001) and promote brain maintenance in old age (Wang et al., 2002). Higher lifestyle activities may counteract the negative effects and be most beneficial to persons with the highest combination of genetic risk (COMT + BDNF) for APOE ε4 non-carriers. Our results support this “differential susceptibility” model (Belsky et al., 2009; Ferencz et al., 2014), which suggests that adults with the highest allelic risk show the greatest amount of plasticity. Specifically, older adults with the highest genetic allelic risk combination in the COMT + BDNF risk panel and higher lifestyle activities had the least decline in EF performance compared to their lower genetic risk counterparts. This finding also extends our previous cross-sectional results by pointing to the moderating effect of lifestyle engagement on APOE, COMT, and BDNF genetic effects on EF.
We now note several strengths and limitations of the present study. A first strength is the sample of older adults (n = 634) tested at three waves and across a 40-year band of aging (age range = 53–95 years) from an ongoing longitudinal study. Although a larger sample size and additional waves would have been preferable, this design allowed us to compare age differences and changes across two older adult age groups and to examine and detect age magnification over a 40-year span. Second, we used an accelerated longitudinal design with age as the metric of change, thereby incorporating chronological age directly into our analyses (McFall et al., 2016). We note that although most longitudinal studies involving older adults are usually unstructured and do not address cohort effects, they do provide direct estimates of change (unlike cross-sectional designs) (Thompson et al., 2011). A latent growth modeling approach in Mplus by default accounts for missing data. The default method uses maximum likelihood estimation to generate factor scores for the dependent EF factor and maximizes the use of longitudinal data. Third, we used four standard cognitive tests contributing to a confirmed one-factor EF latent variable. The present latent variable approach represents the broader construct domain and it attenuates measurement error associated with single EF or other cognitive tests. Fourth, in extending an earlier cross-sectional study through 9 years with longitudinal data, we determined that the previous additive approach was effective and applied to change, but notably modified by the effects of APOE and lifestyle factors. Regarding limitations, first, we examined only one cognitive domain. Future studies should consider examining other domains, perhaps especially cognitive speed and episodic memory with APOE, COMT, and BDNF or other risk polymorphisms (Laukka et al., 2013). Second, the measurement of lifestyle activities was based on frequency and did not take into account the extent or intensity of participation. In addition, although we used multiple indicators and tapped four integrated aspects of lifestyle activities, the current approach was not designed to delineate separable contributions, if any, of these aspects. Third, because of ongoing data collection schedules, the longitudinal design did not include a third-wave opportunity for all participants. However, our results seem not to have been compromised because we used all data points available for each participant and confirmed that the latent EF variable was measurement invariant across all waves. By achieving partial scalar invariance, we accounted for any potential EF practice effects in our analyses (Kline, 2011). Fourth, we note that our participants were predominantly White, not of Hispanic origin, and that genotype allelic frequencies may differ in other racial populations. Our findings should be replicated in future studies with diverse or other racial backgrounds. Fifth, although we include sex and education as covariates, future studies should consider other relevant biomarkers including global cognition.
In conclusion, the APOE genotype presents a systematic array of potential associations with cognitive performance and change. When appropriate data are available, researchers may observe APOE associations that take the following forms: (a) overall independent effects on level and change, (b) moderation effects on BDNF genotype, and (c) effect modification of COMT + BDNF combined panel effects. In addition, as has been implicated in other contexts and with other cognitive phenotypes, both chronological age and lifestyle activities may moderate some or all of these forms of APOE associations. It is important to note that even in the absence of initial single-gene (independent) effects on EF, both COMT and BDNF allelic risk may play a role in predicting cognitive change but primarily in the context of interactive or magnified risk. At present, these synergistic neurobiological partners include Alzheimer’s genetic risk (APOE ε4+ carriers) and lifestyle engagement (lower lifestyle activities). Future research should be directed at detecting the roles played by the protective aspects of these risk factors: These include potential sources of protection ranging from the genetic (APOE ε2 carriers) to profiles of lifestyle and health activities that (independently or interactively) may protect against the neurobiological mechanisms underlying the various combinations of magnified risk examined in this study. Finally, we emphasize (a) the integrative role that APOE may play in all such complex and dynamic interactions, (b) the fact that the often-noted inconsistencies in single-gene COMT and BDNF association studies can be clarified in interactive and longitudinal contexts, and (c) the importance of multifactorial and dynamic approaches to understanding neurobiological aging and its influences on cognitive functioning.
Supplementary Material
Highlights.
APOE genotype affects cognitive aging both directly and synergistically
Both BDNF and COMT may affect EF aging through interactive risk combinations
APOE ε4+ carriers had poorer EF performance with increasing COMT+BDNF risk
COMT+BDNF risk in the APOE ε4- and high lifestyle group showed protected EF change
Acknowledgments
We thank the volunteer participants and the VLS staff for their many contributions. More information about the VLS may be found at: https://sites.ualberta.ca/~vlslab/index.html.
Role of the funding source
The present research is supported by grants to Roger A. Dixon from (a) the National Institutes of Health (National Institute on Aging; R01 AG008235), (b) Alberta Health Services (University Hospital Foundation) (to David Westaway, Jack Jhamandas, and Roger Dixon), and (c) the Canadian Consortium on Neurodegeneration in Aging (with funding from the Canadian Institutes of Health Research and partners, including SANOFI-AVENTIS R&D). RAD is also supported by the Canada Research Chairs program. Lars Bäckman acknowledges support from the Swedish Research Council, the Swedish Council for Health, Working Life, and Welfare, a donation from the af Jochnick Foundation, and an Alexander von Humboldt Research Award. The funding sources did not have a role in the study design, data collection, statistical analysis, results interpretation, report writing, or submission decisions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
All authors confirm that there is no actual or potential conflict of interest. All research has been approved continuously by relevant institutional review boards. Certificates are available and on file in the University of Alberta Research Services Office and the US National Institutes of Health. All participants have completed and signed informed consent forms.
References
- Adamczuk K, De Weer AS, Nelissen N, Chen K, Sleegers K, Bettens K, Van Broeckhoven C, Vandenbulcke M, Thiyyagura P, Dupont P, Van Laere K, Reiman EM, Vandenberghe R. Polymorphism of brain derived neurotrophic factor influences β amyloid load in cognitively intact apolipoprotein E ε4 carriers. Neuroimage Clini. 2013;2:512–520. doi: 10.1016/j.nicl.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9(1):357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Lindenberger U, Li SC, Nyberg L. Linking cognitive aging to alterations in dopamine neurotransmitter functioning: Recent data and future avenues. Neurosci Biobehav Rev. 2010;34(5):670–677. doi: 10.1016/j.neubiorev.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Baltes PB, Reese HW, Nesselroade JR. Life-span developmental psychology: Introduction to research methods. Monterey, CA: Brooks/Cole; 1977. [Google Scholar]
- Barral S, Bird T, Goate A, Farlow M, Diaz-Arrastia R, Bennett DA, Graff-Radford N, Boeve BF, Sweet RA, Stern Y, Wilson RS, Foroud T, Ott J, Mayeux R, et al. National Institute on Aging Late-Onszheimer’s Disease Genetics Study. Genotype patterns at PICALM, CR1, BIN1, CLU, and APOE genes are associated with episodic memory. Neurology. 2012;78(19):1464–1471. doi: 10.1212/WNL.0b013e3182553c48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R. Vulnerability genes or plasticity genes? Mol Psychiatry. 2009;14(8):746–754. doi: 10.1038/mp.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolino A, Rubino V, Sambataro F, Blasi G, Latorre V, Fazio L, Caforio G, Petruzzella V, Kolachana B, Hariri A, Meyer-Lindenberg A, Nardini M, Weinberger DR, Scarabino T. Prefrontal-hippocampal coupling during memory processing is modulated by COMT val158met genotype. Biol. Psychiatry. 2006;60(11):1250–1258. doi: 10.1016/j.biopsych.2006.03.078. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Volavka J, Lachman HM, Grace AA. The catechol-O-methyltransferase polymorphism: Relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology. 2004;29(11):1943–1961. doi: 10.1038/sj.npp.1300542. [DOI] [PubMed] [Google Scholar]
- Brainerd CJ, Reyna VF, Petersen RC, Smith GE, Taub ES. Is the apolipoprotein e genotype a biomarker for mild cognitive impairment? Findings from a nationally representative study. Neuropsychology. 2011;25(6):679–689. doi: 10.1037/a0024483. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Shallice T. The Hayling and Brixton Tests. Thurston, Suffolk, England: Thames Valley Test Company; 1997. [Google Scholar]
- Cabeza R, Dolcos F, Prince SE, Rice HJ, Weissman DH, Nyberg L. Attention-related activity during episodic memory retrieval: A cross-function fMRI study. Neuropsychologia. 2003;41(3):390–399. doi: 10.1016/s0028-3932(02)00170-7. [DOI] [PubMed] [Google Scholar]
- Caselli RJ, Osborne D, Reiman EM, Hentz JG, Barbieri CJ, Saunders AM, Hardy J, Graff-Radford NR, Alexander GE. Preclinical cognitive decline in late middle-aged asymptomatic apolipoprotein E-e4/4 homozygotes: A replication study. J Neurol Sci. 2001;189(1):93–98. doi: 10.1016/s0022-510x(01)00577-9. [DOI] [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J, Egan MF, Kleinman JE, Weinberger DR. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): Effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75(5):807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder E, Saunders AM, Risch N, Strittmatter W, Schmechel D, Gaskell PC, Rimmier JB, Locke PA, Conneally PM, Schmader KE, Small GW, Roses AD, Hainer JL, Pericak-Vance MA. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet. 1994;7(2):180–184. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: A behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25(6):295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Das D, Tan X, Bielak AA, Cherbuin N, Easteal S, Anstey KJ. Cognitive ability, intraindividual variability, and common genetic variants of catechol-O-methyltransferase and brain-derived neurotrophic factor: A longitudinal study in a population-based sample of older adults. Psychol Aging. 2014;29(2):393–403. doi: 10.1037/a0035702. [DOI] [PubMed] [Google Scholar]
- de Frias CM, Dixon RA, Strauss E. Structure of four executive functioning tests in healthy older adults. Neuropsychology. 2006;20(2):206–214. doi: 10.1037/0894-4105.20.2.206. [DOI] [PubMed] [Google Scholar]
- de Frias CM, Dixon RA, Strauss E. Characterizing executive functioning in older special populations: From cognitively elite to cognitively impaired. Neuropsychology. 2009;23:778–791. doi: 10.1037/a0016743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Elia LF, Satz P, Uchiyama CL, White T. Color trails test: Professional manual. Odessa, FL: Psychological Assessment Resources; 1996. [Google Scholar]
- de-Almada B, de-Almeida L, Camporez D, de-Moraes M, Morelato R, Perrone A, Belcavello L, Louro ID, de-Paula F. Protective effect of the APOE-e3 allele in Alzheimer’s disease. Braz J Med Biol Res. 2012;45(1):8–12. doi: 10.1590/S0100-879X2011007500151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Penke L, Johnson W. The neuroscience of human intelligence differences. Nat Rev Neurosci. 2010;11(3):201–211. doi: 10.1038/nrn2793. [DOI] [PubMed] [Google Scholar]
- Dixon RA, de Frias CM. The Victoria Longitudinal Study: From characterizing cognitive aging to illustrating changes in memory compensation. Aging Neuropsych C. 2004;11(2–3):346–376. [Google Scholar]
- Dixon RA, DeCarlo CA, MacDonald SW, Vergote D, Jhamandas J, Westaway D. APOE and COMT polymorphisms are complementary biomarkers of status, stability, and transitions in normal aging and early mild cognitive impairment. Front Aging Neurosci. 2014;6:236. doi: 10.3389/fnagi.2014.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos S, MacDonald SW, Braslavsky A, Camicioli R, Dixon RA. Mild cognitive impairment is associated with selected functional markers: Integrating concurrent, longitudinal, and stability effects. Neuropsychology. 2012;26(2):209–223. doi: 10.1037/a0026760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112(2):257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Kim JS, Suever BL, Voss MW, Francis BM, Kramer AF. Genetic contributions to age-related decline in executive function: A 10-year longitudinal study of COMT and BDNF polymorphisms. Front Hum Neurosci. 2008;2 doi: 10.3389/neuro.09.011.2008. Article 11, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Miller DL, Roecklein KA. The aging hippocampus: Interactions between exercise, depression, and BDNF. Neuroscientist. 2012;18(1):82–97. doi: 10.1177/1073858410397054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenson KR, Buchner DM, Morland KB. Objective measurement of physical activity and sedentary behavior among US adults aged 60 years or older. Prev Chronic Dis. 2012:9. [PMC free article] [PubMed] [Google Scholar]
- Ferencz B, Laukka EJ, Welmer AK, Kalpouzos G, Angleman S, Keller L, Graff C, Lövdén M, Bäckman L. The benefits of staying active in old age: Physical activity counteracts the negative influence of PICALM, BIN1, and CLU risk alleles on episodic memory functioning. Psychol Aging. 2014;29(2):440–449. doi: 10.1037/a0035465. [DOI] [PubMed] [Google Scholar]
- Fotuhi M, Hachinski V, Whitehouse PJ. Changing perspectives regarding late-life dementia. Nat Rev Neurol. 2009;5(12):649–658. doi: 10.1038/nrneurol.2009.175. [DOI] [PubMed] [Google Scholar]
- Galbraith S, Bowden J, Mander A. Accelerated longitudinal designs: An overview of modelling, power, costs and handling missing data. Stat Methods Med Res. 2014:1–25. doi: 10.1177/0962280214547150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghisletta P, Bäckman L, Bertram L, Brandmaier AM, Gerstorf D, Liu T, Lindenberger U. The Val/Met polymorphism of the brain-derived neurotrophic factor (BDNF) gene predicts decline in perceptual speed in older adults. Psychol Aging. 2014;29(2):384–392. doi: 10.1037/a0035201. [DOI] [PubMed] [Google Scholar]
- Harris SE, Deary IJ. The genetics of cognitive ability and cognitive ageing in healthy older people. Trends Cogn Sci. 2011;15(9):388–394. doi: 10.1016/j.tics.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Harris SE, Fox H, Wright AF, Hayward C, Starr JM, Whalley LJ, Deary IJ. The brain-derived neurotrophic factor Val66Met polymorphism is associated with age-related change in reasoning skills. Mol Psychiatry. 2006;11(5):505–513. doi: 10.1038/sj.mp.4001799. [DOI] [PubMed] [Google Scholar]
- Houlihan L, Harris S, Luciano M, Gow A, Starr J, Visscher P, Deary I. Replication study of candidate genes for cognitive abilities: The Lothian Birth Cohort 1936. Genes Brain Behav. 2009;8(2):238–247. doi: 10.1111/j.1601-183X.2008.00470.x. [DOI] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. 3rd. New York, NY: Guilford; 2011. [Google Scholar]
- Laukka EJ, Lövdén M, Herlitz A, Karlsson S, Ferencz B, Pantzar A, Keller L, Graff C, Fratiglioni L, Bäckman L. Genetic effects on old-age cognitive functioning: A population-based study. Psychol Aging. 2013;28(1):262–274. doi: 10.1037/a0030829. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Nagel IE, Chicherio C, Li SC, Heekeren HR, Bäckman L. Age-related decline in brain resources modulates genetic effects on cognitive functioning. Fronti Neurosci. 2008;2(2):234–244. doi: 10.3389/neuro.01.039.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: Risk, mechanisms and therapy. Nat Rev Neurol. 2013;9(2):106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano M, Gow AJ, Harris SE, Hayward C, Allerhand M, Starr JM, Visscher PM, Deary IJ. Cognitive ability at age 11 and 70 years, information processing speed, and APOE variation: The Lothian Birth Cohort 1936 study. Psychol Aging. 2009;24(1):129. doi: 10.1037/a0014780. [DOI] [PubMed] [Google Scholar]
- Luszcz M. Executive function and cognitive aging. In: Schaie KW, Willis SL, editors. The handbook of the psychology of aging. San Diego, CA: Academic Press; 2011. pp. 59–72. [Google Scholar]
- Mandelman SD, Grigorenko EL. BDNF Val66Met and cognition: All, none, or some? A meta-analysis of the genetic association. Genes Brain Behav. 2012;11(2):127–136. doi: 10.1111/j.1601-183X.2011.00738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marioni RE, Campbell A, Scotland G, Hayward C, Porteous DJ, Deary IJ. Differential effects of the APOE ε4 allele on different domains of cognitive ability across the life-course. Eur J Hum Genet. 2015 doi: 10.1038/ejhg.2015.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marosi K, Mattson MP. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol Metab. 2014;25(2):89–98. doi: 10.1016/j.tem.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle JJ, Hamagami F. Modeling in complete longitudinal and cross-sectional data using latent growth structural models. In: Collins LM, Horn JL, editors. Best methods for the analysis of change. Washington, DC: American Psychological Association; 1991. pp. 276–309. [Google Scholar]
- McFall GP, Sapkota S, McDermott KL, Dixon RA. Risk-reducing apolipoprotein E and clusterin genotypes protect against the consequences of poor vascular health on executive function performance and change in non-demented older adults. Neurobiol Aging. 2016;42:91–100. doi: 10.1016/j.neurobiolaging.2016.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall GP, Wiebe SA, Vergote D, Anstey KJ, Dixon RA. Alzheimer’s genetic risk intensifies neurocognitive slowing associated with diabetes in non-demented older adults. Alzheimers Dement (Amst) 2015b;1(4):395–402. doi: 10.1016/j.dadm.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall GP, Wiebe SA, Vergote D, Jhamandas J, Westaway D, Dixon RA. IDE (rs6583817) polymorphism and pulse pressure are independently and interactively associated with level and change in executive function in older adults. Psychol Aging. 2014;29(2):418–430. doi: 10.1037/a0034656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall GP, Wiebe SA, Vergote D, Westaway D, Jhamandas J, Bäckman L, Dixon RA. ApoE and pulse pressure interactively influence level and change in the aging of episodic memory: Protective effects among ε2 carriers. Neuropsychology. 2015a;29:388–401. doi: 10.1037/neu0000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyajima F, Ollier W, Mayes A, Jackson A, Thacker N, Rabbitt P, Pendleton N, Horan M, Payton A. Brain-derived neurotrophic factor polymorphism Val66Met influences cognitive abilities of the elderly. Genes Brain Behav. 2008;7(4):411–417. doi: 10.1111/j.1601-183X.2007.00363.x. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. 7th. Los Angeles, CA: Muthén & Muthén; 1998–2015. [Google Scholar]
- Nagel IE, Chicherio C, Li S-C, Von Oertzen T, Sander T, Villringer A, Heekeren HR, Bäckman L, Lindenberger U. Human aging magnifies genetic effects on executive functioning and working memory. Front Hum Neurosci. 2008;2:1. doi: 10.3389/neuro.09.001.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L, Lövden M, Riklund K, Lindenberger U, Bäckman L. Memory aging and brain maintenance. Trends Cogn Sci. 2012;16(5):292–305. doi: 10.1016/j.tics.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Panza F, Solfrizzi V, Torres F, Mastroianni F, Colacicco AM, Basile AM, Capurso C, D’Introno A, Del Parigi A, Capurso A. Apolipoprotein E in southern Italy: Protective effect of ε2 allele in early-and late-onset sporadic Alzheimer’s disease. Neurosci Lett. 2000;292(2):79–82. doi: 10.1016/s0304-3940(00)01447-6. [DOI] [PubMed] [Google Scholar]
- Papenberg G, Bäckman L, Nagel IE, Nietfeld W, Schroder J, Bertram L, Heekeren HR, Lindenberger U, Li SC. COMT polymorphism and memory dedifferentiation in old age. Psychol Aging. 2014;29(2):374–383. doi: 10.1037/a0033225. [DOI] [PubMed] [Google Scholar]
- Papenberg G, Lindenberger U, Bäckman L. Aging-related magnification of genetic effects on cognitive and brain integrity. Trends Cogn Sci. 2015a;19(9):506–514. doi: 10.1016/j.tics.2015.06.008. [DOI] [PubMed] [Google Scholar]
- Papenberg G, Lövden M, Laukka EJ, Kalpouzos G, Keller L, Graff C, Köhncke Y, Li TQ, Fratiglioni L, Bäckman L. Magnified effects of the COMT gene on white-matter microstructure in very old age. Brain Struct Funct. 2015b;220(5):2927–2938. doi: 10.1007/s00429-014-0835-4. [DOI] [PubMed] [Google Scholar]
- Poo M. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2(1):24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Land S. Genetic and vascular modifiers of age-sensitive cognitive skills: Effects of COMT, BDNF, ApoE, and hypertension. Neuropsychology. 2009;23(1):105–116. doi: 10.1037/a0013487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapkota S, Vergote D, Westaway D, Jhamandas J, Dixon RA. Synergistic associations of catechol-O-methyltransferase and brain-derived neurotrophic factor with executive function in aging are selective and modified by apolipoprotein E. Neurobiol Aging. 2015;36(1):249–256. doi: 10.1016/j.neurobiolaging.2014.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N, Levy G, Tang MX, Manly J, Stern Y. Influence of leisure activity on the incidence of Alzheimer’s disease. Neurology. 2001;57(12):2236–2242. doi: 10.1212/wnl.57.12.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small BJ, Dixon RA, McArdle JJ, Grimm KJ. Do changes in lifestyle engagement moderate cognitive decline in normal aging? Evidence from the Victoria Longitudinal Study. Neuropsychology. 2012;26(2):144–155. doi: 10.1037/a0026579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small BJ, Rosnick CB, Fratiglioni L, Bäckman L. Apolipoprotein E and cognitive performance: A meta-analysis. Psychol Aging. 2004;19(4):592. doi: 10.1037/0882-7974.19.4.592. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Kornblum S, Lauber EJ, Minoshima S, Koeppe RA. Isolation of specific interference processing in the Stroop task: PET activation studies. Neuroimage. 1997;6(2):81–92. doi: 10.1006/nimg.1997.0285. [DOI] [PubMed] [Google Scholar]
- Thibeau S, McFall GP, Wiebe SA, Anstey KJ, Dixon RA. Genetic factors moderate everyday physical activity effects on executive function in aging: Evidence from the Victoria Longitudinal Study. Neuropsychology. 2016;30(1):6–17. doi: 10.1037/neu0000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson WK, Hallmayer J, O’Hara R. Design considerations for characterizing psychiatric trajectories across the lifespan: Application to effects of APOE-ε4 on cerebral cortical thickness in Alzheimer’s disease. American Journal of Psychiatry. 2011;168:894–903. doi: 10.1176/appi.ajp.2011.10111690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela M, Brayne C, Sachdev P, Wilcock G. Cognitive lifestyle and long-term risk of dementia and survival after diagnosis in a multicenter population-based cohort. Am J Epidemiol. 2011;173(9):1004–1012. doi: 10.1093/aje/kwq476. [DOI] [PubMed] [Google Scholar]
- Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol. 2011;10(3):241–252. doi: 10.1016/S1474-4422(10)70325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaaren BF, Vernooij MW, Koudstaal PJ, Uitterlinden AG, van Duijn CM, Hofman A, Breteler MM, Ikram MA. Alzheimer’s disease genes and cognition in the nondemented general population. Biol Psychiatry. 2013;73(5):429–434. doi: 10.1016/j.biopsych.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Wang HX, Karp A, Winblad B, Fratiglioni L. Late-life engagement in social and leisure activities is associated with a decreased risk of dementia: A longitudinal study from the Kungsholmen project. Am J Epidemiol. 2002;155(12):1081–1087. doi: 10.1093/aje/155.12.1081. [DOI] [PubMed] [Google Scholar]
- Ward DD, Summers MJ, Saunders NL, Janssen P, Stuart KE, Vickers JC. APOE and BDNF Val66Met polymorphisms combine to influence episodic memory function in older adults. Behav Brain Res. 2014;271:309–315. doi: 10.1016/j.bbr.2014.06.022. [DOI] [PubMed] [Google Scholar]
- Wisdom NM, Callahan JL, Hawkins KA. The effects of apolipoprotein E on non-impaired cognitive functioning: A meta-analysis. Neurobiol Aging. 2011;32(1):63–74. doi: 10.1016/j.neurobiolaging.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Wishart HA, Roth RM, Saykin AJ, Rhodes CH, Tsongalis GJ, Pattin KA, Moore JH, McAllister TW. COMT Val158Met genotype and individual differences in executive function in healthy adults. J Int Neuropsychol Soc. 2011;17(1):174–180. doi: 10.1017/S1355617710001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.