Abstract
Speakers respond automatically and rapidly to compensate for brief perturbations of pitch in their auditory feedback. The specific adjustments in vocal output require integration of brain regions involved in speech-motor-control in order to detect the sensory-feedback-error and implement the motor-correction. Cortical regions involved in the pitch-reflex phenomenon are highly vulnerable targets of network disruption in Alzheimer's disease (AD). We examined the pitch-reflex in AD patients (n=19) compared to an age-matched control group (n=16). We measured the degree of behavioral compensation (peak-compensation) and the extent of the adaptive response (pitch-response-persistence). Healthy-controls reached a peak-compensation of 18.7±0.8 cents, and demonstrated a sustained compensation at 8.9±0.69 cents. AD patients, in contrast, demonstrated a significantly elevated peak-compensation (22.4±1.2 cents, P<0.05), and a reduced sustained response (pitch-response-persistence, 4.5±0.88 cents, P<0.001). The degree of increased peak-compensation predicted executive dysfunction, while the degree of impaired pitch-response-persistence predicted memory dysfunction, in AD patients. The current study demonstrates pitch-reflex as a sensitive behavioral index of impaired prefrontal modulation of sensorimotor integration, and compromised plasticity mechanisms of memory, in AD.
Keywords: Alzheimer's disease, pitch perturbation, network disruption, executive dysfunction, prefrontal modulation, sensorimotor integration
1. INTRODUCTION
Alzheimer's disease (AD) is the most common neurodegenerative disease affecting more than 35 million people worldwide (World Health Organization., 2012). AD is characterized by progressive loss of specific cognitive functions including memory, executive skills, speech and language abilities, and visuospatial skills (Dubois, et al., 2007,McKhann, et al., 2011). Neuropsychological assessments in AD patients document the degree of deficits in specific cognitive constructs. Each cognitive test primarily targets a specific skill, which involves a series of mental processes ranging from the perception of sensory stimuli to an optimized behavioral output. For example, the Trail Making test, which is a widely used measure of executive ability (Delis, et al., 2004) involves serial alternating between numbers and letters, hence requires, visual scanning, attention, working memory, competence with numbers and letters, and motor performance. A growing body of evidence points out that AD is characterized by impaired connectivity between different cortical regions interacting with each other to produce a particular cognitive function (Delbeuck, et al., 2003,Golob, et al., 2001,Greicius, et al., 2004,Seeley, et al., 2009,Zhang, et al., 2009). There are however surprisingly no good measures that directly examines the deficits of integration between different brain areas.
Voice production involves both sensory inputs and motor commands and provides a unique opportunity to understand integration of neural processes involved in sensory perception and motor actions. In particular, auditory feedback constantly modulates the motor control of speech. For example, in a noisy background speakers automatically increase their voice amplitude (Bauer, et al., 2006,Heinks-Maldonaldo and Houde, 2005). Current models of speech motor control posit that ongoing execution of speech engages a widely distributed network of frontal, parietal, and temporal cortical areas where auditory feedback signals are compared with predictions derived from motor efference copies (Figure 1) (Behroozmand, et al., 2015b,Guenther and Hickok, 2015,Houde and Nagarajan, 2011,Indefrey and Levelt, 2008). Under normal conditions, feedback matches predictions, causing little response in sensory areas. However, if incoming feedback is perturbed to mismatch predictions, feedback prediction errors are generated. Sensory areas then respond by conveying these errors to motor regions, and ongoing speech output is modulated to compensate for the perturbation. This control loop is likely modulated by frontally mediated inhibitory mechanisms. A well-studied experimental paradigm that examines motor speech responses to auditory feedback distortions is pitch-reflex (Burnett, et al., 1998). In such experiments the participants listen to their own voice while the pitch of self-voice feedback is unexpectedly perturbed up or down. Participants respond to the pitch shift by compensating against the direction of the shift: e.g., up shift of audio feedback causes speakers to lower their pitch. Under physiological conditions this behavioral compensation partially corrects the applied pitch shift (Behroozmand, et al., 2014,Chang, et al., 2013).
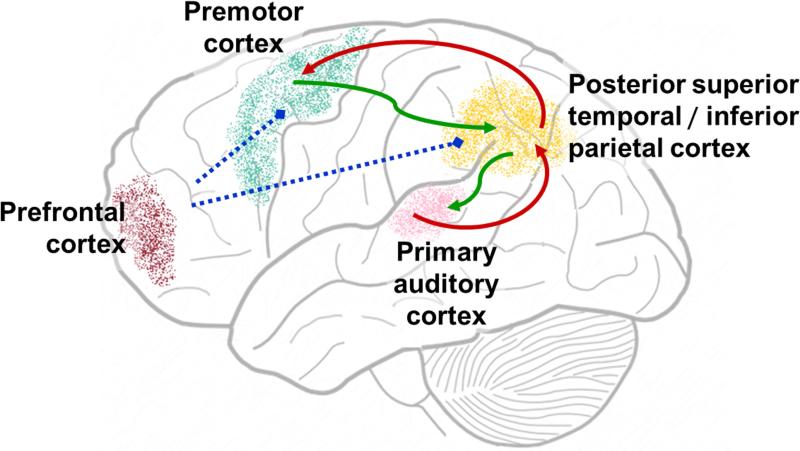
Figure 1. Cortical circuits of speech motor control.
Anatomical locations of candidate cortical areas are depicted on a schematic brain diagram. The arrows indicate auditory feedback control pathways where feedback predictions (green arrows) are compared with incoming feedback to generate feedback corrections (red arrows), whose key processing nodes (premotor cortex and posterior superior temporal/inferior parietal cortex) are modulated by prefrontal cortex (blue lines).
The network architecture of speech motor control circuit with its anatomic distribution involving the superior temporal, posterior parietal, premotor, and prefrontal regions is indeed a highly vulnerable target in Alzheimer's disease. Loss of neurons and synapses in AD characteristically involve the temporal and parietal cortices of the brain, and most predominantly the posterior superior temporal and inferior parietal regions (Rabinovici, et al., 2007). In addition to structural deficits of AD, functional neuroimaging studies have revealed a wealth of evidence that AD is also associated with disrupted functional connectivity of distributed networks involving temporal, parietal and prefrontal cortices (Greicius, et al., 2004). It is likely that the mechanisms of network disruptions are shared across networks involving higher-level cognitive behaviors and primary level processing circuits such as sensorimotor integration of speech. As such, abnormalities of sensorimotor integration may serve as sensitive indices of overall network integration deficits in AD. Only a few human psychophysical and functional imaging experiments have examined the patterns of sensorimotor integration in patients with AD. During the McGurk experiment, AD patients show significantly less influence of lip reading (Delbeuck, et al., 2007). The auditory evoked potentials of AD patients are significantly less affected by visual stimuli (Golob, et al., 2001). Here, we sought to examine the degree of sensorimotor integration of pitch-reflex in patients with AD, and examine the associations of abnormal pitch-reflex behavior and higher-level cognitive deficits.We characterized vocal responses to pitch feedback perturbations in AD patients compared to age-matched controls while producing a sustained vowel sound and listening to the real-time audio feedback of their own speech. We predicted that, AD patients, as a consequence of neural integration deficits of perisylvian— frontal-parietal-temporal and prefrontal brain areas that overlap with speech-motor-control network, would produce an altered behavioral response to pitch feedback perturbations. Next, we tested the hypothesis that the degree of altered pitch-reflex in AD patients would be associated with deficits of cognitive functions mediated by perisylvian and prefrontal regions including executive, fluency, memory, and language abilities.
2. METHODS
2.1. Participants
Patients were recruited from research cohorts at the University of California San Francisco (UCSF) Memory and Aging Center and consisted of 19 patients meeting the diagnostic criteria for AD (McKhann, et al., 2011). All patients underwent a complete clinical evaluation and structural brain imaging (Supplementary Table 1, AD-biomarker data). The diagnosis was made at a multidisciplinary consensus meeting for each patient individually. To make our cohort more uniform and more representative of typical AD we excluded atypical AD patients (Gorno-Tempini, et al., 2011,Mendez, et al., 2002). Eligibility criteria for age-matched healthy participants (n=16) included normal cognitive performance, normal structural brain imaging, and absence of neurologic, psychiatric, or other major illnesses. Informed consent was obtained from all participants or their assigned surrogate decision makers. The study was approved by UCSF institutional review board for human research.
2.2. Neuropsychological assessment
Both, patients and controls underwent Mini Mental State Examination (MMSE). In a structured caregiver interview, Clinical Dementia Rating scale (CDR), and CDR Sum of Boxes (CDR-SOB) (Morris, 1993) were documented for each patient. All AD patients underwent a battery of neuropsychological tests designed to assess major domains of cognition. The full battery of tests was detailed in previous reports (Kramer, et al., 2003,Ranasinghe, et al., 2016). We selected the cognitive domains that are primarily dependent on brain areas in close proximity to speech motor control network, specifically the frontal-temporal-parietal lobes, and prefrontal cortex, to examine their associations with the sensorimotor integration deficits detected from the pitch-perturbation experiment. These included executive, fluency, memory, and language functions. The specific neuropsychological tests used are described in the Supplementary methods.
2.3. Hearing status
All participants self-reported normal hearing and were assessed clinically for any hearing loss. Each participant underwent a bilateral tone hearing test to verify the hearing status, and to confirm the proper earphone placement during experiment.
2.4. Apparatus and procedure
The pitch-perturbation experiment consisted of two successive 74-trial sessions. In each trial, participants phonated the vowel /a/ to a microphone while listening to the real-time audio feedback via headphones (Figure 2). In each trial, onset of speech triggered a brief perturbation of the pitch of the auditory feedback. Perturbations shifted the pitch of auditory feedback upwards or downwards by 100 cents (1/12th of an octave) for 400ms, and occurred with a randomly jittered delay (200–500ms) from the vocalization onset. Further details of experimental setup are given in Supplementary methods.
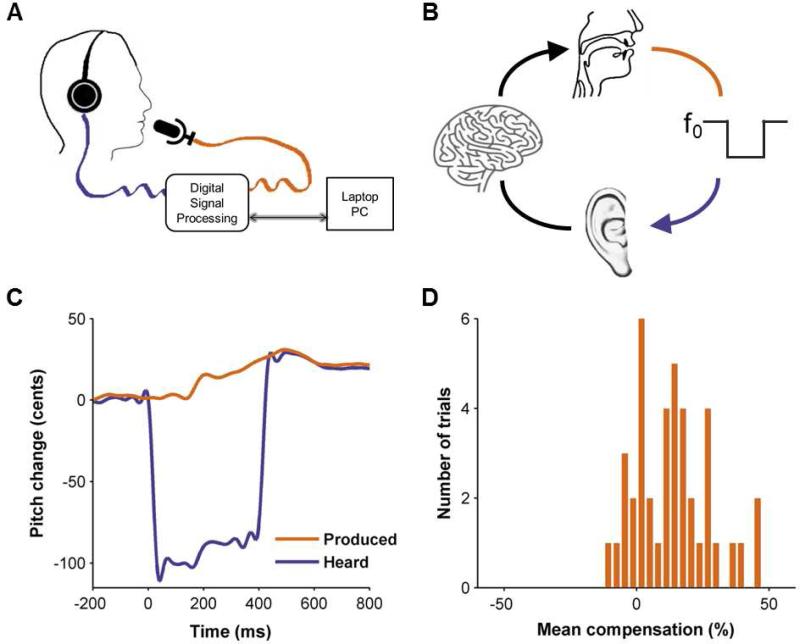
Figure 2. Apparatus and behavior.
(A) Diagram of the pitch-perturbation apparatus. A digital signal processing method was used to shift the pitch of participants’ vocalizations (orange line) and delivered this auditory feedback (purple line) to participants’ earphones. (B) A schematic of the mechanisms involved in pitch-reflex. Auditory feedback of the participant's voice (orange) is briefly perturbed in pitch and heard by the participant (purple). This perturbed auditory feedback is conveyed to auditory areas in the central nervous system, where it is mismatched with the motor-derived predictions. This mismatch gives rise to a feedback prediction error which then modulates the ongoing speech output to compensate for the perturbation. (C) An example of pitch-perturbation response from a single control participant. The purple line denotes the mean audio feedback of the auditory input across all down perturbation trials. Perturbations start at 0ms on the x-axis and last for 400ms. The orange line denotes the vocal output (mean response across all the down perturbation trials) demonstrating the compensatory response entailing an increase in pitch. (D) Histogram of peak compensatory responses as a percentage of pitch shift for the down perturbation trials for the same response shown in C.
2.5. Data processing and analysis
2.5.1. Audio data analysis
The raw audio data for each trial was first analyzed into time-course of pitch using an autocorrelation-based pitch tracking method (Parsons, 1987). Trials with pitch tracking errors or incomplete utterances within the analysis interval were excluded. An analysis interval of 1200ms (−200-1000ms from the perturbation onset) was extracted, and converted from hertz to cents (see Supplementary methods). For each participant, the pitch track for each trial was processed and expressed as deviations from the mean pitch track, averaged across all trials (i.e., up and down pitch-perturbations). Next, for each participant, responses to both upward and downward perturbations are combined into a single dataset. The number of trials in the combined data set for each participant was equal to the number of trials in upward perturbations plus the number of trials in downward perturbations. To account for the trial-by-trial variability within participants (Figure 2D) and the variable number of trials between participants, we analyzed the data by combining all trials per each group (total number of trials: AD=1245; controls=2029). Step-by-step detail of audio analysis is given in Supplementary methods.
2.5.2. Statistical analyses
We examined the statistical differences between patients and controls in their responses to altered auditory feedback of pitch, time locked to perturbation onset for 1000ms following perturbation. We identified two segments in the response including an immediate pitch-compensatory-response to perturbation followed by a period of pitch-response-persistence in which the participants retained a residual effect of compensation which slowed their return to baseline (Figure 3B).
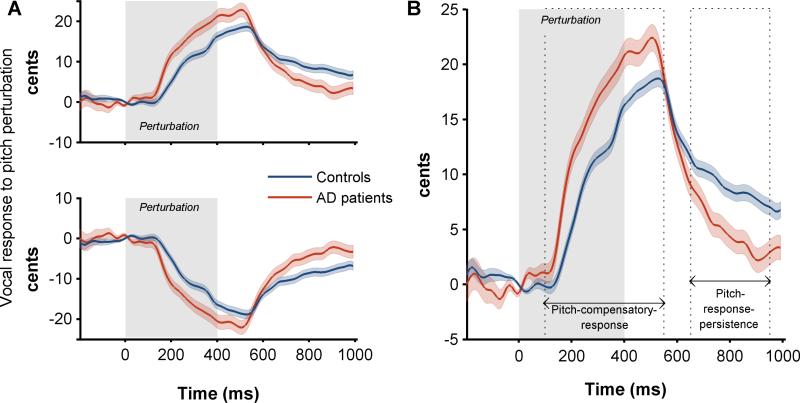
Figure 3. AD patients showed an altered behavioral response to auditory feedback perturbation.
(A) Vocal response to down (top panel) and up (bottom panel) perturbations of 100 cents, for controls and patients. (B) Single combined vocal response to pitch-perturbations including both up and down perturbations. Combined responses were generated by flipping the deviations from the mean time-course in response to up perturbations and adding these flipped trials to the data from down perturbations. The combined data were generated for each participant separately. Dark lines indicate the mean responses of each group and the shaded areas indicate standard error across the trials per each group. The time axis is time locked to perturbation onset (0ms). Grey shaded area indicates the duration of perturbation. The dotted lines in B indicate the two segments of the behavioral response—pitch-compensatory-response and pitch-response-persistence.
2.5.2.1. Pitch-compensatory-response and pitch-response-persistence
To define the onset and offset of these two response windows we used the time points where the two groups crossed over (the group responses not significantly different between AD and controls, Figure 3B). Specifically, we used 50 ms time bins across the time-course dataset and compared the group differences within each time bin using one-way-ANOVA in Matlab statistical toolbox (MATLAB 2012b, MathWorks, Inc.). The onset of first time bin where the groups differed significantly were after the onset of perturbation marked the inset of pitch-compensatory-response (100 ms after perturbation). The offset of the most distal time bin around the peak within which the groups remained significantly different marked the offset of pitch-compensatory-response (550 ms after perturbation) The onset and offset of pitch-response-persistence were marked by the onset of the most proximal bin and the offset of the most distal bin with significant differences, after the peak, respectively (650 ms, 950 ms after perturbation, onset and offset respectively). The time bins within which the groups were not statistically different were excluded.
2.5.2.2. Group comparisons
After defining the time windows, we ran mixed-model analyses using SAS Proc Mixed (SAS 9.4, SAS Institute Inc.) to compare the behavioral responses of AD and control groups. Mixed models are robust for analysis of data with variable numbers of observations and account for both within- and between- subject factors to provide a more accurate estimate of error (Littell, et al., 2006). To compare the average behavioral response we ran a mixed model with two fixed factors including group category (i.e. AD or Controls) and response-type (i.e. pitch-compensatory-response or pitch-response-persistence). The trials were included as a repeated factor nested within each subject by the response-type thus including the latter as a within subject factor, in this analysis. Post-hoc comparisons were adjusted with Tukey-Kramer. Next, to compare the peak compensation between the two groups, we ran a mixed model with the group category as a fixed factor.
2.5.3. Correlation analysis
We examined the correlations between: (1) peak-compensation (2) mean pitch-response-persistence and standard tests of cognitive function. The mean pitch-response-persistence for each participant was estimated as the degree of deviation from a grand mean response (including all patients and controls), at each time frame within the pitch-response-persistence window. As the two pitch response measures were correlated with each other (r=0.502, P=0.028), we examined the Pearson partial correlation coefficients of the residuals. We also regressed out the effect of MMSE, age and sex (using SAS 9.4, SAS Institute Inc.). We next computed composite cognitive scores for each domain (i.e., executive, fluency, memory and language) and examined the partial correlation coefficients between these and the pitch response measures. The composite scores were derived from averaging the standardized scores (z scores, derived based on age, education and sex matched normative data) across the individual tests categorized within a given domain. The tests that comprised composite scores included: executive composite—cognitive control and set-shifting; fluency composite—lexical, category and design fluency; memory composite—short delay verbal recall and delayed verbal recall; language composite—naming, repetition, and Peabody Picture Vocabulary Test.
3. RESULTS
3.1. Participant characteristics
AD patients were mildly impaired (median MMSE=22, Table 1). Control participants were matched with the AD patients in age, sex, handedness and race, yet showed a significantly higher number of years in education than patients (Table 1). Neuropsychological bedside performances of AD patients are given in Supplementary Table 2.
Table 1.
Participant Demographics
| Controls (n=16) | AD Patients (n=19) | P Value* | |
|---|---|---|---|
| Age – yr | 63.99 ± 5.25 | 60.09 ± 8.94 | 0.134 |
| Female sex – no. (%) | 11 (68.75) | 13 (68.42) | 1.000 |
| White – no. (%)† | 16 (100.00) | 18 (94.74) | 1.000 |
| Education – yr | 18.0 (17.0 – 18.5) | 16.0 (14.0 – 18.0) | 0.004 |
| Right handedness – no. (%) | 16 (100.00) | 15 (78.95) | 0.109 |
| MMSE‡ | 30.0 (29.5 – 30.0) | 22.0 (19.0 – 24.0) | <0.0001 |
Abbreviations: AD=Alzheimer's disease; MMSE=Mini-Mental State examination;
Values for age are means ±SD. Age ranges are 48.99 – 84.32 and 56.22 – 75.56, for AD patients and control participants respectively.
Values for education, MMSE are medians and interquartile ranges.
Statistical tests were unpaired t-test for age; Fisher Exact test for sex, race, and handedness; Wilcoxon-Mann-Whitney test for education and MMSE.
Race was self-reported.
Scores on the MMSE range from 0 to 30, with higher scores denoting better cognitive function.
3.2. Acoustic pitch-perturbations induced compensatory responses
The behavioral responses to brief pitch-perturbations in auditory feedback were compensatory. In the example shown in Figure 2C, the digital signal processing perturbed the participant's vocal feedback by abruptly lowering the pitch by 100 cents for 400ms. In response, the participant raised his/her pitch to partly cancel the effects of the perturbation. ~170ms after the perturbation onset, the pitch of the participant's vocalization began to elevate from baseline, and reached a peak response ~ 500ms (Figure 2C, perturbation onset=0ms). The behavioral response to pitch shift was variable from trial-to-trial in each subject (Figure 2D), and we combined all trials per group in our subsequent analyses to account for trial-by-trial variability (see methods).
3.3. AD patients showed an enhanced compensatory response compared to age-matched controls
Both patients and controls generated compensatory responses to pitch-perturbations in auditory feedback (Figure 3A). When time locked to the perturbation onset both groups reached a compensatory peak response after roughly 500ms (peak latency: controls=491 ± 27ms; patients=495 ± 29ms; unpaired t-test, t=0.34, P=0.74). The average peak-compensation of control participants was 18.7 ± 0.8 cents. AD patients in contrast showed a significantly elevated peak-compensation averaging at 22.4 ± 1.2 (Mixed-model analysis, F=18.30; P<0.0001, Figure 3B). The elevated compensatory response in AD patients was not only restricted to the peak amplitude but also was evident from the beginning of the pitch-compensatory-response and persisted throughout the duration (Figure 3B). For example, the average pitch-compensatory-response of controls was 11.3 ± 0.67 whereas the mean pitch-compensatory-response in AD patients was 15.6 ± 0.85 (Figure 3B; Mixed-model analysis, t=−4.03; P=0.0003 after Tukey-Kramer adjustment).
3.4. AD patients showed a poor pitch-response-persistence compared to age-matched controls
During the post compensatory period (~ 500 – 1000ms from perturbation onset) the behavioral response was returning towards baseline (Figure 3 A, B). However, even at 1000ms from perturbation onset (600ms after the end of the perturbation) control participants showed a significantly sustained compensatory response. We estimated the mean pitch-response-persistence (Figure 3B) for each participant. Pitch-response-persistence of control participants was 8.9 ± 0.67. In contrast, AD patients showed only a minimal pitch-response-persistence (4.7 ± 0.86), which was significantly reduced compared to that of controls (Figure 3B; Mixed-model analysis, t=3.84; P=0.0006 after Tukey-Kramer adjustment).
3.5. Altered auditory feedback response was correlated with cognitive deficits in AD patients
The abnormal behavioral response to pitch perturbations in AD patients is evidence for disrupted functional circuitry of sensorimotor integration and adaptive response mechanisms in AD. It is likely that similar mechanisms of functional disruptions are shared across cortical networks underlying other cognitive functions. We hypothesized that abnormally increased peak-compensation and reduced pitch-response-persistence in AD patients will be correlated with distinct cognitive deficits. To test this hypothesis we examined the correlations between abnormal pitch reflex measures and the cognitive performance on tasks primarily mediated by brain regions that are anatomically closely related to the speech-motor-control circuit including frontal executive functions (i.e. cognitive control and set-shifting), fluency, language, and verbal memory tasks, in AD patients (see experimental procedures for details of tests).
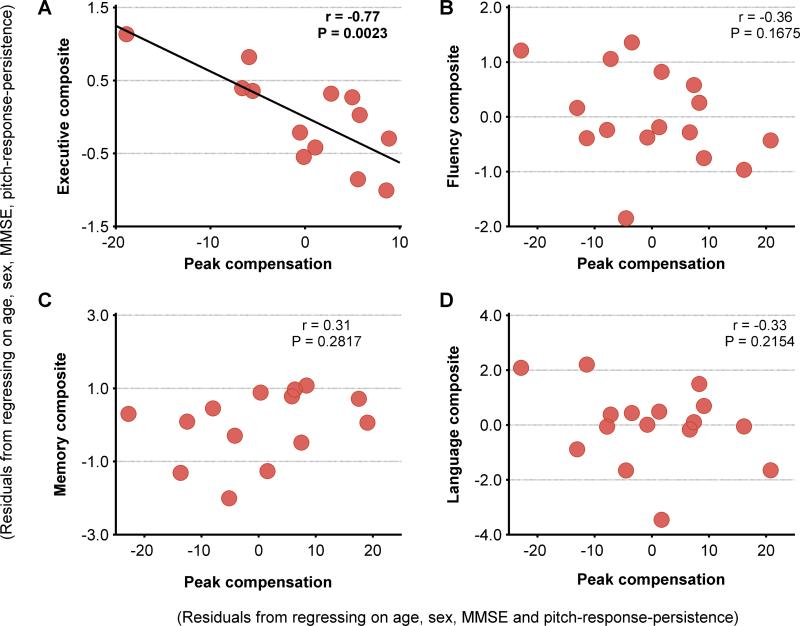
The peak-compensation was significantly negatively correlated with both cognitive control and set-shifting abilities of AD patients (Table 2). Executive composite derived from cognitive control and set-shifting scores showed a significant negative correlation with the enhanced peak-compensation of AD patients (Figure 4A), while none of the other cognitive tasks were significantly correlated (Table 2, Figure 4 B-D). Enhanced peak-compensation hence specifically predicted the executive deficits in AD, and did not predict fluency, memory and language dysfunctions.
Table 2.
Partial correlation coefficients between cognitive performance, peak-compensation and pitch-response-persistence
| Neuropsychological test | Partial correlation coefficient with peak-compensation r (P) |
Partial correlation coefficient with pitch-response-persistence r (P) |
|---|---|---|
| Executive | ||
| Cognitive control (Stroop test) | −0.770 (0.0008) | −0.490 (0.0635) |
| Set-shifting (Trail making test B) | −0.598 (0.0185) | 0.152 (0.5887) |
| Fluency | ||
| Lexical fluency (D words) | −0.464 (0.0606) | −0.382 (0.1303) |
| Semantic fluency (Animals) | −0.189 (0.4666) | 0.059 (0.8225) |
| Design fluency | −0.315 (0.2344) | −0.105 (0.6985) |
| Memory | ||
| Short delay verbal memory (CVLT 30 s recall) | 0.103 (0.7255) | 0.622 (0.0175) |
| Verbal free recall (CVLT 10 m recall) | 0.496 (0.0715) | 0.522 (0.0553) |
| Language | ||
| Naming (BNT) | −0.382 (0.1441) | 0.277 (0.2981) |
| Sentence repetition | −0.073 (0.7878) | −0.338 (0.2002) |
| PPVT | −0.217 (0.4026) | −0.390 (0.1220) |
Abbreviations: AD=Alzheimer's disease; BNT=Boston Naming Test; CVLT=California Verbal Learning Test (short form); PPVT=Peabody Picture Vocabulary Test.
Figure 4. Increased peak-compensation predicted poor executive function in AD patients.
Pearson partial correlations between the residuals of peak-compensation after regressing on pitch-response-persistence, age, sex and MMSE, and the composite scores of: (A) Executive; (B) Fluency (generation tasks); (C) Memory; (D) Language. Each composite score was estimated as the average of z scores derived for each of its component tasks. The component tasks included: executive – cognitive control (Stroop) and set-shifting (modified Trail Making); fluency (generation tasks) – lexical fluency (D words), category fluency (animals) and design fluency; memory – short delay verbal recall (CVLT 30 second recall) and delayed verbal recall (CVLT 10 minute recall); language – naming (BNT), repetition, and PPVT. Abbreviations: AD=Alzheimer's disease; BNT=Boston Naming Test; CVLT=California Verbal Learning Test (short form); MMSE=Mini-Mental State examination; PPVT=Peabody Picture Vocabulary Test.
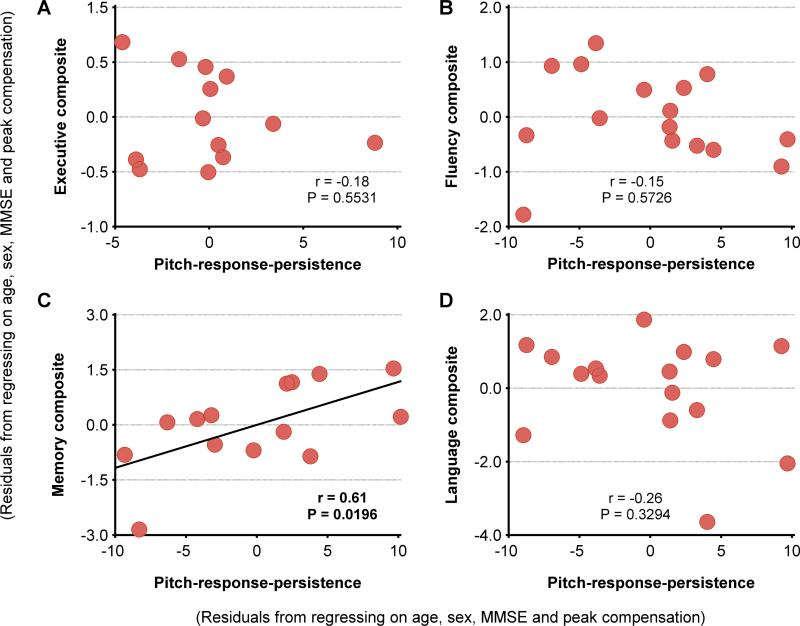
The pitch-response-persistence showed a significant positive correlation with short-delay-verbal recall (i.e., CVLT 30-seconds-recall; Table 2). The delayed-verbal recall also showed a positive correlation with a non-significant trend (Table 2). The pitch-response-persistence thus showed a significant positive correlation with the memory composite scores in AD patients (Figure 5C). Further, No other significant correlations were found between the pitch-response-persistence and cognitive scores in AD patients (Table 2; Figure 5 A,B,D). Higher degree of pitch-response-persistence hence specifically predicted better memory function in AD.
Figure 5. Decreased pitch-response-persistence predicted better memory function in AD patients.
Pearson partial correlations between the residuals of pitch-response-persistence after regressing on peak-compensation, age, sex and MMSE, and the composite scores of: (A) Executive; (B) Fluency; (C) Memory; (D) Language. Each composite score was estimated as the average of z scores derived for each of its component tasks (same as for Figure 4). Abbreviations: AD=Alzheimer's disease; MMSE=Mini-Mental State examination.
4. DISCUSSION
In this study we demonstrated a significantly abnormal pitch reflex in AD patients characterized by an enhanced compensatory response followed by a poorly sustained response-persistence, in response to brief perturbations of pitch in their auditory feedback. We further demonstrated that the degree of abnormally enhanced compensatory response is correlated with executive dysfunction, whereas the impaired pitch-response-persistence is correlated with memory dysfunction, in AD patients.
The elevated peak-compensation in AD patients showed a nearly 20% increase in error-correction compared to the more typical incomplete compensation seen in control participants. This increased corrective behavior indicates altered mechanisms of sensorimotor integration of speech-motor-control circuitry in AD. Likewise, the impaired pitch-response-persistence in Alzheimer's patients may indicate compromised mechanisms of neural plasticity enabling adaptive behavioral responses of learning and memory. In the subsequent sections we discuss each of these mechanistic phenomena and their potential causal relationships to AD.
4.1. Mechanisms of altered speech motor control responses in AD
Our current understanding suggests that motor control is based on statistically optimal integration of forward predictions and sensory feedback (Guenther and Hickok, 2015,Houde and Nagarajan, 2011,Shadmehr, et al., 2010,Todorov and Jordan, 2002). As shown in previous perturbation experiments using different sensory modalities, motor behavior is consistently adjusted to compensate for the feedback-error compared to an internal prediction (Burnett, et al., 1998,Choe and Welch, 1974,Houde and Jordan, 1998,Jones and Munhall, 2000,Purcell and Munhall, 2006). An important observation is that such compensation is incomplete, or that the error correction falls well short of cancelling out 100% of the effects of perturbation. From a theoretical perspective control systems based on larger feedback responses are inherently unstable (Franklin, et al., 1991). A partial correction of the sensory feedback perturbations hence may provide the optimal stability in biological control systems including the speech motor control circuit. Consistent with this phenomenon an incomplete auditory compensation is observed in humans as well as in animals (Chang, et al., 2013,Choe and Welch, 1974,Sober and Brainard, 2009,Troyer and Doupe, 2000). Based on studies conducted in humans and non-human primates and also the mechanisms proposed in current models of speech motor control three potential mechanisms facilitating an incomplete compensation can be identified. Below, we discuss each of these in relation to abnormally enhanced compensatory response observed in AD patients.
4.1.1. Allowances for feedback delays
Given the inherent delays in conveying and processing of sensory feedback from periphery, a complete correction based on sensory information will make the motor control unstable. The Kalman gain function incorporates this measure of uncertainty into the speech motor control models and attenuates the influence of feedback-errors on corrective behavior (Kalman, 1960). Thus, an impaired inhibitory Kalman gain, which depends on intrinsic function of higher auditory cortex, is one potential cause leading to increased compensation. Given the distribution of grey matter loss in AD patients, which predominantly involves the posterior parietal and posterior superior temporal regions, higher auditory areas are likely targets of AD related neurodegeneration (Thompson, et al., 2003). Thus, a disinhibited Kalman gain is a probable contributory mechanism for elevated compensation in AD patients.
4.1.2. Non-auditory sources of information
During speaking, sensory feedback consists not only of auditory but also of non-auditory sources such as somatosensory signals. In the pitch-perturbation experiment, at the same time the auditory feedback is detecting an off target error, somatosensory feedback from oropharyngeal structures is fully on target. Under physiological conditions, multiple modes of sensory information may integrate to generate an overall suppression of the corrective behavioral response. It is reasonable to think that impaired long-range connectivity of AD disrupts such neural integration. This effect is consistent with psychophysical experiments showing impaired McGurk effect in AD, where normal subjects fuse auditory and visual percepts of different speech sounds into an integrated percept (Delbeuck, et al., 2007).
4.1.3. Inhibitory control from prefrontal cortices
Physiological recordings during speech processing in non-human-primates as well as human functional magnetic resonance imaging studies have demonstrated that prefrontal cortices are tightly integrated at several levels of hierarchical auditory processing (Rauschecker, 1998,Rauschecker and Scott, 2009,Riecker, et al., 2005,Romanski, et al., 1999,Scott, 2012,Tourville, et al., 2008). Dual stream processing models consistently recruits prefrontal connections with both ventral and dorsal auditory pathways (Hickok and Poeppel, 2007,Rauschecker, 2011,Scott, 2012). As mapped in both lesion and neuroimaging studies, prefrontal cortex is characterized by strong reciprocal connections to sensory neocortical as well as motor systems, and thus plays a central role in establishing mapping between inputs, internal states, and outputs. (Fuster, 2015,Miller, 2000,Tanji and Hoshi, 2008). In this context, it is reasonable to assume that prefrontal cortex may serve as an ideal hub to modulate the compensatory response to auditory feedback discrepancies detected during perturbation. The top-down coordination of such frontal modulations may well be generating a net inhibitory influence leading to an incomplete compensatory response. Disruption of the rich functional connections of dorsolateral prefrontal cortex, a constituent brain region of prefrontal cortex, is a highly likely target in AD (Greicius, et al., 2004,Ranasinghe, et al., 2014). The current results suggest that lack of prefrontal mediated inhibition as a highly potential contributory factor, as we found that the degree of peak-compensation was significantly negatively correlated with executive control abilities, which specifically involves prefrontal connections (Dosenbach, et al., 2008,Tanji and Hoshi, 2008). Absence of significant correlations with other cognitive tasks (i.e. memory, language and fluency) adds further to the evidence implicating impaired prefrontal control during pitch-perturbation response in AD.
4.2. Pitch-response-persistence as an indicator of verbal memory deficits
Our results demonstrated a strong pitch-response-persistence in healthy older adults where the compensatory response to pitch-perturbation persisted several hundred milliseconds after the offset of perturbation. This response-persistence has been shown in several previous studies using a similar experimental paradigm of pitch-perturbation (Behroozmand, et al., 2014,Behroozmand, et al., 2015a). Similar persistent adaptation effects have been reported with other forms of auditory feedback manipulations, in humans as well as in animals (Houde and Jordan, 1998,Jones and Munhall, 2000,Sober and Brainard, 2009). Moreover, the response-persistence seems to be the norm across other domains of sensorimotor control. For example, after learning to reach a visual target while wearing prism goggles, the participants continue to miss targets in the direction opposite to the displacement created by the prisms even after the goggles have been removed (Held, 1965). It has been suggested that these effects represent the primary role of learning and memory as subserved by underlying mechanisms of neural plasticity (Della-Maggiore, et al., 2015). The current results not only demonstrated significantly impaired pitch-response-persistence in AD, but also that the degree of such impairment being significantly positively correlated with verbal memory deficits in AD. These findings are also consistent with previous reports suggesting that impaired consolidation rather than ineffective retrieval of new information, as the underlying mechanism of memory deficits in AD patients (Gallagher and Koh, 2011). The strong and significant correlations between impaired pitch-response-persistence and verbal memory deficits yet with no significant correlations with other cognitive tasks further attest to the specific associations with learning.
4.3. Nature of cognitive dysfunctions and their relation to pitch-perturbation response in AD
The composite cognitive scores clearly demonstrated the negative relationship of executive dysfunction to the degree of abnormally enhanced compensatory response, and the positive relationship of memory function to pitch-response-persistence. Although there was no significant correlation with the fluency composite score, lexical fluency ability also showed a non-significant negative trend with the peak-compensation of AD patients. Out of the three individual fluency tasks, the lexical fluency is relatively strongly mediated by frontal cortex, and hence it is likely that this effect is marginally reflected in the associations between peak-compensation. Similarly, the short delay verbal memory task showed a non-significant positive trend with the peak-compensation and cognitive control showed a non-significant negative trend with pitch-response-persistence in AD patients. It is likely that these effects represent the residual associations between the correlations of peak-compensation and pitch-response-persistence, although we have minimized such effects in our analyses by running partial correlations.
4.4. Pitch reflex in patients with Parkinson's disease
Abnormal vocal behavior towards altered auditory feedback was also reported in patients with Parkinson's disease in several previous experiments (Chen, et al., 2013,Huang, et al., 2016,Liu, et al., 2012,Mollaei, et al., 2013). Like the AD patients in the current study Patients with Parkinson's disease also showed an elevated pitch-compensatory response (Chen, et al., 2013,Huang, et al., 2016). Further, the Parkinson's disease patients also showed an elevated amplitude-compensatory response in experiments where amplitude was perturbed in the feedback signal (Liu, et al., 2012). The larger compensation error in Parkinson's disease patient was associated with an enhanced P2 signal of scalp EEG in the brain areas involved in speech motor control. It has been suggested that the elevated compensatory response results from an increased gain function due to abnormal basal ganglia circuit. However, the neural signatures of these potential mechanisms are yet to be demonstrated in neurophysiological experiments. Given that Parkinson's disease and AD have distinct underlying pathologies and affect spatially distinct brain regions, it is likely that diverse mechanisms target the speech motor control network in each condition, despite producing the same behavioral change.
4.5. Limitations and future directions
A distinct advantage of the current study is our access to uniformly characterized cohort of patients who were assessed via a consistent battery of neuropsychological tests. Nonetheless, we did not have complete bedside testing available for our age-matched controls. Future studies examining the same correlations in elderly controls will further validate the associations of executive abilities of human brain, in general, to behavioral pitch reflex measures. As the first study to examine the behavioral response within two separate time windows, we used a data-driven approach to define the onset and off set of each time window based on the time points where the two groups crossed over. Although the cross-over points were clearly identifiable in our response curves, the ideal analysis approach with minimal confounds from between and within subject variability would be to identify these time windows across several studies and multiple behavioral paradigms. Further, the current study used the methodological approach of analyzing the trial-by-trial variability in a pooled dataset from all the participants in respective groups of patients and controls. This approach allowed us to address the confounding effects of unequal number of trials per subject and trial-by-trial variability, though with the caveat that it obviated the between subject variability within our respective groups. Finally, the neural correlates of the key behavioral findings uncovered in this study remains to be explored in future research experiments.
4.6. Summary and conclusions
In conclusion, this study demonstrated and quantified the abnormal behavioral response of pitch reflex in AD patients. The results further revealed that the abnormally elevated compensation and the degree to which patients demonstrate a loss of response persistence are sensitive as well as specific indicators of executive dysfunction and memory dysfunction, respectively, in AD. This study also exemplified the associations between advanced cognitive functions and primary processing behaviors like pitch reflex, and showed that abnormal pitch reflex reliably reflects dysfunctions in higher cognitive domains in AD.
Supplementary Material
HIGHLIGHTS.
Abnormal primary processing behaviors may serve as biomarkers of higher-level cognitive dysfunctions.
Alzheimer's disease (AD) patients show an aberrant vocal response to altered auditory feedback of speech.
Enhanced compensation to perturbed pitch during speaking predicts executive deficits in AD.
Poor response-persistence to pitch perturbations predicts memory deficits in AD.
ACKNOWLEDGEMENTS
We would like to thank all of the study participants and their families for their generous support to our research. This study was supported by the National Institutes of Health grants: F32AG050434-01A1 (KGR), K23 AG038357 (KAV), P50 AG023501, P01 AG19724 (BLM), R21 NS76171 (SSN), R01 DC010145 (JFH), DC013979 (SSN), NS066654 (SSN), NS64060 (SSN), R01NS050915 (MLG), National Science Foundation Grant BCS-1262297 (SSN); a grant from John Douglas French Alzheimer's Foundation (KAV); a grant from Larry L. Hillblom Foundation, 2015-A-034-FEL (KGR); University of California San Francisco Alzheimer's Disease Research Center pilot project grant (KAV); grants from the Alzheimer's Association: (PCTRB-13-288476) (KAV), and made possible by Part the Cloud™, (ETAC-09-133596) (JFH); and a gift from the S. D. Bechtel Jr. Foundation.
KGR, JSG, HK, AJB, SH, DM, MLG, KAV, SSN and JFH declare no competing financial interests relevant for this work. BLM has the following disclosures: serves as Medical Director for the John Douglas French Foundation; Scientific Director for the Tau Consortium; Director/Medical Advisory Board of the Larry L. Hillblom Foundation; and Scientific Advisory Board Member for the National Institute for Health Research Cambridge Biomedical Research Centre and its subunit, the Biomedical Research Unit in Dementia, UK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bauer JJ, Mittal J, Larson CR, Hain TC. Vocal responses to unanticipated perturbations in voice loudness feedback: an automatic mechanism for stabilizing voice amplitude. J Acoust Soc Am. 2006;119(4):2363–71. doi: 10.1121/1.2173513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behroozmand R, Ibrahim N, Korzyukov O, Robin DA, Larson CR. Left-hemisphere activation is associated with enhanced vocal pitch error detection in musicians with absolute pitch. Brain Cogn. 2014;84(1):97–108. doi: 10.1016/j.bandc.2013.11.007. doi:10.1016/j.bandc.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behroozmand R, Ibrahim N, Korzyukov O, Robin DA, Larson CR. Functional role of delta and theta band oscillations for auditory feedback processing during vocal pitch motor control. Front Neurosci. 2015a;9:109. doi: 10.3389/fnins.2015.00109. doi:10.3389/fnins.2015.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behroozmand R, Shebek R, Hansen DR, Oya H, Robin DA, Howard MA, 3rd, Greenlee JD. Sensory-motor networks involved in speech production and motor control: an fMRI study. Neuroimage. 2015b;109:418–28. doi: 10.1016/j.neuroimage.2015.01.040. doi:10.1016/j.neuroimage.2015.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett TA, Freedland MB, Larson CR, Hain TC. Voice F0 responses to manipulations in pitch feedback. J Acoust Soc Am. 1998;103(6):3153–61. doi: 10.1121/1.423073. [DOI] [PubMed] [Google Scholar]
- Chang EF, Niziolek CA, Knight RT, Nagarajan SS, Houde JF. Human cortical sensorimotor network underlying feedback control of vocal pitch. Proc Natl Acad Sci U S A. 2013;110(7):2653–8. doi: 10.1073/pnas.1216827110. doi:10.1073/pnas.1216827110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Zhu X, Wang EQ, Chen L, Li W, Chen Z, Liu H. Sensorimotor control of vocal pitch production in Parkinson's disease. Brain Res. 2013;1527:99–107. doi: 10.1016/j.brainres.2013.06.030. doi:10.1016/j.brainres.2013.06.030. [DOI] [PubMed] [Google Scholar]
- Choe CS, Welch RB. Variables affecting the intermanual transfer and decay of prism adaptation. J Exp Psychol. 1974;102(6):1076–84. doi: 10.1037/h0036325. [DOI] [PubMed] [Google Scholar]
- Delbeuck X, Collette F, Van der Linden M. Is Alzheimer's disease a disconnection syndrome? Evidence from a crossmodal audio-visual illusory experiment. Neuropsychologia. 2007;45(14):3315–23. doi: 10.1016/j.neuropsychologia.2007.05.001. doi:10.1016/j.neuropsychologia.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Delbeuck X, Van der Linden M, Collette F. Alzheimer's disease as a disconnection syndrome? Neuropsychol Rev. 2003;13(2):79–92. doi: 10.1023/a:1023832305702. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Holdnack J. Reliability and validity of the Delis-Kaplan Executive Function System: an update. J Int Neuropsychol Soc. 2004;10(2):301–3. doi: 10.1017/S1355617704102191. doi:10.1017/S1355617704102191. [DOI] [PubMed] [Google Scholar]
- Della-Maggiore V, Landi SM, Villalta JI. Sensorimotor adaptation: multiple forms of plasticity in motor circuits. Neuroscientist. 2015;21(2):109–25. doi: 10.1177/1073858414545228. doi:10.1177/1073858414545228. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12(3):99–105. doi: 10.1016/j.tics.2008.01.001. doi:10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, Delacourte A, Galasko D, Gauthier S, Jicha G, Meguro K, O'Brien J, Pasquier F, Robert P, Rossor M, Salloway S, Stern Y, Visser PJ, Scheltens P. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6(8):734–46. doi: 10.1016/S1474-4422(07)70178-3. doi:10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- Franklin GF, Powell JD, Emami-Naeini A. Feedback control of dynamic systems. 2nd ed. Addison-Wesley; Reading, Mass: 1991. [Google Scholar]
- Fuster JM. The prefrontal cortex. 5 ed. Academic Press; London, UK: 2015. [Google Scholar]
- Gallagher M, Koh MT. Episodic memory on the path to Alzheimer's disease. Curr Opin Neurobiol. 2011;21(6):929–34. doi: 10.1016/j.conb.2011.10.021. doi:10.1016/j.conb.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golob EJ, Miranda GG, Johnson JK, Starr A. Sensory cortical interactions in aging, mild cognitive impairment, and Alzheimer's disease. Neurobiol Aging. 2001;22(5):755–63. doi: 10.1016/s0197-4580(01)00244-5. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Ogar JM, Rohrer JD, Black S, Boeve BF, Manes F, Dronkers NF, Vandenberghe R, Rascovsky K, Patterson K, Miller BL, Knopman DS, Hodges JR, Mesulam MM, Grossman M. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–14. doi: 10.1212/WNL.0b013e31821103e6. doi:10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101(13):4637–42. doi: 10.1073/pnas.0308627101. doi:10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther FH, Hickok G. Role of the auditory system in speech production. Handb Clin Neurol. 2015;129:161–75. doi: 10.1016/B978-0-444-62630-1.00009-3. doi:10.1016/B978-0-444-62630-1.00009-3. [DOI] [PubMed] [Google Scholar]
- Heinks-Maldonaldo TH, Houde JF. Compensatory responses to brief perturbations of speech amplitude. Acoust Res Lett Onl. 2005;6(3):131–7. doi:10.1121/1.1931747. [Google Scholar]
- Held R. Plasticity in sensory-motor systems. Sci Am. 1965;213(5):84–94. doi: 10.1038/scientificamerican1165-84. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8(5):393–402. doi: 10.1038/nrn2113. doi:10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Houde JF, Jordan MI. Sensorimotor adaptation in speech production. Science. 1998;279(5354):1213–6. doi: 10.1126/science.279.5354.1213. [DOI] [PubMed] [Google Scholar]
- Houde JF, Nagarajan SS. Speech production as state feedback control. Front Hum Neurosci. 2011;5:82. doi: 10.3389/fnhum.2011.00082. doi:10.3389/fnhum.2011.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Chen X, Yan N, Jones JA, Wang EQ, Chen L, Guo Z, Li W, Liu P, Liu H. The impact of parkinson's disease on the cortical mechanisms that support auditory-motor integration for voice control. Hum Brain Mapp. 2016 doi: 10.1002/hbm.23306. doi:10.1002/hbm.23306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indefrey P, Levelt W. The neural correlates of language production. In: Gazzaniga MS, editor. The new cognitive neurosciences. MIT Press; Cambridge, MA: 2008. pp. 845–65. [Google Scholar]
- Jones JA, Munhall KG. Perceptual calibration of F0 production: evidence from feedback perturbation. J Acoust Soc Am. 2000;108(3 Pt 1):1246–51. doi: 10.1121/1.1288414. [DOI] [PubMed] [Google Scholar]
- Kalman RE. A new approach to linear filtering and prediction problems. Journal of basic Engineering. 1960;82(1):35–45. [Google Scholar]
- Kramer JH, Jurik J, Sha SJ, Rankin KP, Rosen HJ, Johnson JK, Miller BL. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cognitive and behavioral neurology : official journal of the Society for Behavioral and Cognitive Neurology. 2003;16(4):211–8. doi: 10.1097/00146965-200312000-00002. [DOI] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS for mixed models. 2nd edition SAS Institute; 2006. [Google Scholar]
- Liu H, Wang EQ, Metman LV, Larson CR. Vocal responses to perturbations in voice auditory feedback in individuals with Parkinson's disease. PLoS One. 2012;7(3):e33629. doi: 10.1371/journal.pone.0033629. doi:10.1371/journal.pone.0033629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr., Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263–9. doi: 10.1016/j.jalz.2011.03.005. doi:10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez MF, Ghajarania M, Perryman KM. Posterior cortical atrophy: clinical characteristics and differences compared to Alzheimer's disease. Dement Geriatr Cogn Disord. 2002;14(1):33–40. doi: 10.1159/000058331. doi:58331. [DOI] [PubMed] [Google Scholar]
- Miller EK. The prefrontal cortex and cognitive control. Nat Rev Neurosci. 2000;1(1):59–65. doi: 10.1038/35036228. doi:10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- Mollaei F, Shiller DM, Gracco VL. Sensorimotor adaptation of speech in Parkinson's disease. Mov Disord. 2013;28(12):1668–74. doi: 10.1002/mds.25588. doi:10.1002/mds.25588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–4. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Parsons TW. Voice and Speech Processing. Mcgraw-Hill College; Blacklick, OH: 1987. [Google Scholar]
- Purcell DW, Munhall KG. Adaptive control of vowel formant frequency: evidence from real-time formant manipulation. J Acoust Soc Am. 2006;120(2):966–77. doi: 10.1121/1.2217714. [DOI] [PubMed] [Google Scholar]
- Rabinovici GD, Seeley WW, Kim EJ, Gorno-Tempini ML, Rascovsky K, Pagliaro TA, Allison SC, Halabi C, Kramer JH, Johnson JK, Weiner MW, Forman MS, Trojanowski JQ, Dearmond SJ, Miller BL, Rosen HJ. Distinct MRI atrophy patterns in autopsy-proven Alzheimer's disease and frontotemporal lobar degeneration. Am J Alzheimers Dis Other Demen. 2007;22(6):474–88. doi: 10.1177/1533317507308779. doi:10.1177/1533317507308779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranasinghe KG, Hinkley LB, Beagle AJ, Mizuiri D, Dowling AF, Honma SM, Finucane MM, Scherling C, Miller BL, Nagarajan SS, Vossel KA. Regional functional connectivity predicts distinct cognitive impairments in Alzheimer's disease spectrum. Neuroimage Clin. 2014;5:385–95. doi: 10.1016/j.nicl.2014.07.006. doi:10.1016/j.nicl.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranasinghe KG, Rankin KP, Lobach IV, Kramer JH, Sturm VE, Bettcher BM, Possin K, Christine You S, Lamarre AK, Shany-Ur T, Stephens ML, Perry DC, Lee SE, Miller ZA, Gorno-Tempini ML, Rosen HJ, Boxer A, Seeley WW, Rabinovici GD, Vossel KA, Miller BL. Cognition and neuropsychiatry in behavioral variant frontotemporal dementia by disease stage. Neurology. 2016;86(7):600–10. doi: 10.1212/WNL.0000000000002373. doi:10.1212/WNL.0000000000002373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker JP. Cortical processing of complex sounds. Curr Opin Neurobiol. 1998;8(4):516–21. doi: 10.1016/s0959-4388(98)80040-8. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP. An expanded role for the dorsal auditory pathway in sensorimotor control and integration. Hear Res. 2011;271(1-2):16–25. doi: 10.1016/j.heares.2010.09.001. doi:10.1016/j.heares.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker JP, Scott SK. Maps and streams in the auditory cortex: nonhuman primates illuminate human speech processing. Nat Neurosci. 2009;12(6):718–24. doi: 10.1038/nn.2331. doi:10.1038/nn.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riecker A, Mathiak K, Wildgruber D, Erb M, Hertrich I, Grodd W, Ackermann H. fMRI reveals two distinct cerebral networks subserving speech motor control. Neurology. 2005;64(4):700–6. doi: 10.1212/01.WNL.0000152156.90779.89. doi:10.1212/01.WNL.0000152156.90779.89. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Tian B, Fritz J, Mishkin M, Goldman-Rakic PS, Rauschecker JP. Dual streams of auditory afferents target multiple domains in the primate prefrontal cortex. Nat Neurosci. 1999;2(12):1131–6. doi: 10.1038/16056. doi:10.1038/16056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SK. The neurobiology of speech perception and production--can functional imaging tell us anything we did not already know? J Commun Disord. 2012;45(6):419–25. doi: 10.1016/j.jcomdis.2012.06.007. doi:10.1016/j.jcomdis.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62(1):42–52. doi: 10.1016/j.neuron.2009.03.024. doi:10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Smith MA, Krakauer JW. Error correction, sensory prediction, and adaptation in motor control. Annu Rev Neurosci. 2010;33:89–108. doi: 10.1146/annurev-neuro-060909-153135. doi:10.1146/annurev-neuro-060909-153135. [DOI] [PubMed] [Google Scholar]
- Sober SJ, Brainard MS. Adult birdsong is actively maintained by error correction. Nat Neurosci. 2009;12(7):927–U144. doi: 10.1038/nn.2336. doi:10.1038/nn.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji J, Hoshi E. Role of the lateral prefrontal cortex in executive behavioral control. Physiol Rev. 2008;88(1):37–57. doi: 10.1152/physrev.00014.2007. doi:10.1152/physrev.00014.2007. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, de Zubicaray G, Janke AL, Rose SE, Semple J, Herman D, Hong MS, Dittmer SS, Doddrell DM, Toga AW. Dynamics of gray matter loss in Alzheimer's disease. J Neurosci. 2003;23(3):994–1005. doi: 10.1523/JNEUROSCI.23-03-00994.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov E, Jordan MI. Optimal feedback control as a theory of motor coordination. Nat Neurosci. 2002;5(11):1226–35. doi: 10.1038/nn963. doi:10.1038/nn963. [DOI] [PubMed] [Google Scholar]
- Tourville JA, Reilly KJ, Guenther FH. Neural mechanisms underlying auditory feedback control of speech. Neuroimage. 2008;39(3):1429–43. doi: 10.1016/j.neuroimage.2007.09.054. doi:10.1016/j.neuroimage.2007.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyer TW, Doupe AJ. An associational model of birdsong sensorimotor learning I. Efference copy and the learning of song syllables. J Neurophysiol. 2000;84(3):1204–23. doi: 10.1152/jn.2000.84.3.1204. [DOI] [PubMed] [Google Scholar]
- World Health Organization . Dementia : a public health priority. World Health Organization; Geneva: 2012. [Google Scholar]
- Zhang Y, Schuff N, Du AT, Rosen HJ, Kramer JH, Gorno-Tempini ML, Miller BL, Weiner MW. White matter damage in frontotemporal dementia and Alzheimer's disease measured by diffusion MRI. Brain. 2009;132(Pt 9):2579–92. doi: 10.1093/brain/awp071. doi:10.1093/brain/awp071. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.