Abstract
Objective
The objective of this study was to monitor the progression of joint damage in two animal models of knee joint trauma using two non-invasive, clinically available imaging modalities.
Methods
A 3-T clinical magnet and micro-computed tomography (mCT) was used to document changes immediately following injury (acute) and post-injury (chronic) at time points of 4, 8, or 12 weeks. Joint damage was recorded at dissection and compared to the chronic magnetic resonance imaging (MRI) record. Fifteen Flemish Giant rabbits were subjected to a single tibiofemoral compressive impact (ACLF), and 18 underwent a combination of anterior cruciate ligament (ACL) and meniscal transection (mACLT).
Results
All ACLF animals experienced ACL rupture, and 13 also experienced acute meniscal damage. All ACLF and mACLT animals showed meniscal and articular cartilage damages at dissection. Meniscal damage was documented as early as 4 weeks and worsened in 87% of the ACLF animals and 71% of the mACLT animals. Acute cartilage damage also developed further and increased in occurrence with time in both models. A progressive decrease in bone quantity and quality was documented in both models. The MRI data closely aligned with dissection notes suggesting this clinical tool may be a non-invasive method for documenting joint damage in lapine models of knee joint trauma.
Conclusions
The study investigates the acute to chronic progression of meniscal and cartilage damage at various time points, and chronic changes to the underlying bone in two models of posttraumatic osteoarthritis (PTOA), and highlights the dependency of the model on the location, type, and progression of damage over time.
Keywords: MRI, meniscus, cartilage, trauma, acute, chronic
1. Introduction
Posttraumatic osteoarthritis (PTOA) is a form of secondary osteoarthritis that can result from joint trauma. Tearing of the anterior cruciate ligament (ACL) due to jump landings is a common sports related knee injury1–3. The current literature indicates the occurrence of PTOA does not depend on whether or not the ACL is reconstructed following injury4. Magnetic resonance imaging (MRI) of the knee is a common method for diagnosing damage to knee joint structures. This noninvasive method is often used clinically by surgeons to determine the severity of damage5,6, In experimental studies, micro-computed tomography (μCT) is frequently used in addition to MR imaging to monitor OA development7,8. The use of MR imaging in lapine models is less studied9,10, but could be a useful tool for monitoring soft tissue damage throughout a traumatized joint.
Previous models have been used to recapitulate PTOA for the purpose of investigating the pathogenesis of OA. One of the most widely used models is an ACL transection (ACLT) model, where the ACL is transected and degradation of the joint is monitored over time. However, ACLT models so not take into account acute meniscal damage that is often documented in conjunction with ACL tears11,12 following large compressive tibiofemoral forces through the joint at the time of injury1–3,13–16. For this reason, two new lapine models have been developed: a modified ACLT (mACLT) model17 and a traumatic impact (ACLF) model18. Similar to the ACLT model, the mACLT model destabilizes the knee by transecting the ACL; however, in the mACLT model partial meniscal transections are also introduced. The ACLF model induces ACL rupture and damage to the surrounding structures, including the meniscus, via a single blunt force impact to the tibiofemoral joint.
The objective of the current study was to use conventional MR and μCT imaging to document joint damage immediately following trauma and longitudinally in both the mACLT and ACLF models. It was hypothesized that 1) untreated acute soft tissue damage will be progressive, 2) the MR images will provide a description of damage documented at dissection in the lapine model and 3) differences will be evident between the two experimental models. If successful, we could be assured that conventional MRI techniques can be used confidently in longitudinal studies of PTOA in these lapine models, and understand how differences in experimental models may translate to differences in soft tissue and bone damage.
2. Methods
2.1 Animal Care
All animals were housed in individual cages (60 × 60 × 14 in) for the duration of this study, which was approved by Michigan State University and Colorado State University All-University Committees on Animal Use and Care. Thirty-three skeletally mature (5–8 months of age) Flemish Giant rabbits (5.4 ± 0.6 kg, 13 females and 20 males) were used in the study. Animals were placed under anesthesia (2% isoflurane and oxygen) and the right limb was subjected to trauma with the contralateral limb unaffected to serve as an internal control. All animals received buprenorphine for post-trauma pain every 8 hours for 3 days. For the duration of the study animals were monitored by a licensed veterinary technician. Animals were randomly placed into two experimental groups and three time points as described below.
2.2 ACLF Model
Fifteen animals received a closed-joint impact to the tibiofemoral joint (ACLF), similar to previous studies19,20,18. In brief, the impacting force was applied using a gravity accelerated mass of 1.75 kg dropped from a height of 70 cm and attached to a pre-crushed aluminum honeycomb head (Hexweb Rigicell, Hexcel Corp. Stamford, Conn.). To ensure a single insult, the impact sled was arrested following impact. ACL rupture was confirmed with an anterior drawer test. ACL tearing and acute meniscal damages were documented with a post impact MRI. Three animals were euthanized at 4 weeks, six at 8 weeks, and six at 12 weeks.
2.3 mACLT model
The remaining 18 animals received an ACL transection as well as a radial transection in the white zone of the central region of the medial meniscus with a longitudinal transection extending though the main body and a radial transection of the lateral meniscus in the white zone of the central region with a longitudinal tear extending anteriorly (mACLT)17. Limited lateral joint access prevented the longitudinal tear from extending into the posterior lateral region. Six animals were euthanized at each time point (4, 8, 12 weeks).
2.4 Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) was used to document tissue damages in each joint following initial trauma (acute damage), as well as just prior to euthanasia (4, 8, or 12 weeks post trauma, chronic damage). Meniscal damage was identified as being in the anterior horn, anterior junction, body, posterior junction, or posterior horn. Cartilage damage on the tibial plateau was characterized as anterior, central, or posterior as well as sub-meniscal and peripheral when appropriate. Femoral cartilage damage was characterized in the weight bearing, non-weight bearing, or peripheral regions. Imaging was performed with a GE (Waukesha, WI) HDxt 3.0 T magnet using an 8 channel HD wrist coil. Sagittal and coronal proton density sequences were performed with 3000–5000 ms repetition time, 32–34 ms time to echo, ±62.5 kHz receiver bandwidth, 2 excitations, 1.5 mm slice thickness with 0 interslice gap, 512 × 384 matrix size, and an 8 cm field of view. Sagittal and coronal fat suppressed proton density sequences were also performed with 3000–5000 ms repetition time, 32–34 ms time to echo, ±50 kHz receiver bandwidth, 2 excitations, 1.5 mm slice thickness with 0 interslice gap, 416 × 256 matrix size, and an 8 cm field of view. The images were interpreted by a fellowship-trained musculoskeletal radiologist with 8 years of clinical experience (RF).
2.5 Micro-Computed Tomography
Bones from each animal were scanned via micro-computed tomography (Scanco μCT 80, Scanco Medical AG, Brüttisellen, Switzerland) with an isotropic voxel size of 25 µm. Four spatially distributed cylindrical volumes of interest (VOI) were identified for each tibia and femur based on anatomical markers21. The following measurements were obtained: volumetric trabecular material bone mineral density (mgHA/cm3) (Tb.BMD), trabecular bone volume fraction (bone volume/total volume, Tb.BV/TV), trabecular number (Tb.N), trabecular thickness (mm) (Tb.Th), trabecular separation (mm) (Tb.Sp), cortical material bone mineral density (mgHA/cm3) (Ct.BMD), cortical bone porosity (Ct.Po), and volume of osteophytes (mm3)21.
2.6 Cartilage Morphology
Following euthanasia, India ink was lightly applied to the articular cartilage surfaces to highlight surface fissures, cartilage degradation, and other irregularities. The surfaces were digitally photographed (Polaroid DMC2, Polaroid Corp., Waltham, MA) under a dissecting microscope at 12× and 25× (Wild TYP 374590, Heerbrugg, Switzerland). Two blinded graders assessed the tissues for morphological damage using a semi-quantitative grading scheme, as previously described22. Grades ranged from 1 indicating normal appearing cartilage to 4 representing full thickness erosion and exposed underlying bone. Grades were averaged between the two blinded graders with an ICC value of 0.84.
2.7 Statistical Analysis
For trabecular and cortical bone parameters obtained from μCT, a mixed model analysis of variance (ANOVAs) with Tukey post-hoc tests were performed using Minitab software (Minitab, Inc., State College, PA) to compare injured limbs to uninjured limbs with significance corresponding to a p-value less than 0.05.
3. Results
Rabbits in the ACLF groups favored the contralateral limb for the first 3–5 days, while mACLT animals favored the contralateral limb for 1–3 days post-surgery. All ACLF animals experienced ACL tears, and all but two impacted joints showed MRI evidence of edema acutely and joint effusion with synovitis chronically. The average impact failure load across all animals was 943.7 ± 209.7 N with no statistically significant differences between time points (983.7 ± 255.5 N, 829.95 ±177.0 N, 1037.3 ± 195.9 N for 4, 8, and 12 weeks respectively).
3.1 Meniscal Results
Based on the MRIs, acute (immediately after impact) damage was present in 13/15 ACLF animals, while all experienced some form of chronic damage (i.e. damages noted at either 4, 8 or 12 weeks prior to euthanasia) (Tables 1–3). Representative MR images of a complex meniscal tear from both the sagittal and coronal planes are shown in Figure 1. Acute damage was common in both hemijoints with 9 instances in the medial meniscus and 11 in the lateral (Tables 1–3). All acute medial damage, and all but one instance in the lateral meniscus, was characterized as damage to the posterior horn or posterior junction. Chronically tears propagated or new tears developed, in both hemijoints as early as 4 weeks with 2 of 3 medial menisci and all 3 lateral menisci showing progressive damage. A trend of progressive posterior damage continued with time. Combining time points, 14/15 medial menisci and 12/15 lateral menisci presented with chronic damage at the time of euthanasia (Tables 1–3). There was one case, animal 3 at 12 weeks, where an initial tear was detected in the acute MRI in the lateral meniscus (italicized in Table 3) that was not noted in the chronic images.
Table 1.
Meniscal damage from 4 week samples with T identifying mACLT animals and F identifying ACLF animals (PH= posterior horn, AH = anterior horn, PJ = posterior junction, AJ = anterior junction, CXT= complex tear, FTR = full thickness radial tear, FEF = free edge fraying, LVT = longitudinal vertical tear). Damage identified as new or worsened chronically is in bold text in the chronic column. Italicized text in the dissection or acute notes represents damage not seen in the chronic MRI. Italicized text in the chronic notes identifies damage not seen at dissection.
| Rabbit | Acute MRI Notes | Chronic 4 Week MRI Notes | Dissection Notes | |||
|---|---|---|---|---|---|---|
| Medial | Lateral | Medial | Lateral | Medial | Lateral | |
| F1 | Intact | FTR in PJ |
LVT in PH →
body, and in AH |
FTR in PJ, free edge tearing in PH |
CXT in PH → body | FTR in body, PH maceration |
| F2 | Horizontal tear in PJ → body, LVT in PJ |
LVT in PH |
Bucket handle tear in PH → body |
CXT in PH | Bucket handle tear in PH → body |
CXT in PH → body |
| F3 | Intact | AH entrapped |
Intact | AH entrapped
AH maceration |
Parrot beak tear in body | Intact |
| T1 | FTR in body | FTR in body |
FTR in PH | CXT in PH |
FTR in body, radial tear
in PH |
CXT in PH |
| T2 | Radial tear in body | FTR in PJ | FTR in body | FTR in body | FTR in body, radial
tear in AH |
FTR in body |
| T3 | FTR in AJ, flap tear in AH |
FTR in body |
Complex tear in AJ
→ body, PH maceration |
FTR in body, FEF in PH | FTR in body, Radial tear to AH, PH maceration |
FTR in body, LVT
in AH, PH maceration |
| T4 | FTR in PH, radial tear in AJ, FEF in AH |
FTR in body |
FTR in AJ and PH | FTR in body | FTR in AJ and PH,
PH maceration |
FTR in body |
| T5 | FTR in PJ | FTR in body |
FTR in PJ | FTR in PJ, horizontal
tear from AJ → body, FEF in PH |
Radial tear in AH and PH, PH and body maceration |
FTR in body, radial tear in AJ |
Table 2.
Meniscal damage from 8 week samples with T identifying mACLT animals and F identifying ACLF animals (PH= posterior horn, AH = anterior horn, PJ = posterior junction, AJ = anterior junction, CXT= complex tear, FTR = full thickness radial tear, FEF = free edge fraying, LVT = longitudinal vertical tear). Damage identified as new or worsened chronically is in bold text in the chronic column. Italicized text in the dissection or acute notes represents damage not seen in the chronic MRI. Italicized text in the chronic notes identifies damage not seen at dissection.
| Rabbit | Acute MRI Notes | Chronic 8 Week MRI Notes | Dissection Notes | |||
|---|---|---|---|---|---|---|
| Medial | Lateral | Medial | Lateral | Medial | Lateral | |
| F1 | Intact | CXT in PH, LVT in AH |
Flap tear in PH, PH
→ body maceration |
CXT in PH, AH maceration |
CXT in PH → body | CXT in PH → AH |
| F2 | Vertical tear in PJ | Horizontal tear in PJ |
PH maceration, FEF
in body |
Horizontal tear in body and PJ |
CXT in PH → body | Horizontal tear in PH and body |
| F3 | Intact | Horizontal tear in PJ |
CXT in PJ, PH
maceration, bucket handle component |
CXT in PJ,
PH maceration |
CXT in PJ | CXT in PJ → body |
| F4 | Intact | Intact | CXT in PH |
PH maceration, FEF in AH |
CXT of PH → body | PH maceration, surface damage in AH |
| F5 | CXT to PH, PH maceration |
LVT in PJ | CXT in PH →body, PH maceration, bucket handle component |
CXT in PH | CXT in PH → body, PH maceration |
Horizontal tear in body, FEF in AH |
| F6 | LVT in PH → body, PH maceration |
Radial tear in PH |
CXT in PH → AH | CXT in PH → body | FTR in body and AH, maceration |
Maceration (prox/distal) in body |
| T1 | FTR in body | FTR in body | Radial tear in PJ,
PH maceration |
FTR in PJ, FEF in AH, PH maceration |
FTR in body | FTR in body, FEF in AH, PH maceration |
| T2 | FTR in AJ | FTR in body, LVT in PH. |
Radial tear in the AJ, LVT in AH |
FTR in PJ, FEF in PH | FTR in AJ, LVT in AH | FTR in body, PH maceration |
| T3 | FTR in PJ | FTR in body. |
Radial tear in PJ
and PH maceration |
FTR in PJ, FEF
in AH, PH maceration |
Maceration (proximal/distal in body), radial tear to AH |
FTR in body, PH maceration |
| T4 | Radial tear in AJ | FTR in PJ |
PH and body maceration, LVT in AJ, FEF in AH |
FTR in PJ, FEF
in AH |
FTR in body, LVT in AH, PH maceration |
FTR in body, LVT
in AH |
| T5 | Intact | Radial tear in body, LVT in AJ |
FEF in AH | Radial tear in body, LVT in AH, FEF in PH |
CXT in AH | FTR in body, maceration in all |
| T6 | Radial tear in PJ | FTR in body |
CXT in PH → body,
PH maceration |
FTR in body, LVT
in AH. |
Radial tear in AJ, CXT in PH |
FTR in AJ, surface damage in AH |
Table 3.
Meniscal damage from 12 week samples with T identifying mACLT animals and F identifying ACLF animals (PH= posterior horn, AH = anterior horn, PJ = posterior junction, AJ = anterior junction, CXT= complex tear, FTR = full thickness radial tear, FEF = free edge fraying, LVT = longitudinal vertical tear). Damage identified as new or worsened chronically is in bold text in the chronic column. Italicized text in the dissection or acute notes represents damage not seen in the chronic MRI. Italicized text in the chronic notes identifies damage not seen at dissection.
| Rabbit | Acute MRI Notes | Chronic 12 Week MRI Notes | Dissection Notes | |||
|---|---|---|---|---|---|---|
| Medial | Lateral | Medial | Lateral | Medial | Lateral | |
| F1 | LVT in PH | Intact | CXT in PH → body | Horizontal tear in PH | CXT in PH → body | Horizontal tear in PH |
| F2 | CXT in PH → body |
Intact | CXT in PH →
body, maceration |
undersurface tear in
PH → PJ |
CXT in PH → body, PH maceration |
Surface damage in PH |
| F3 | Radial tear in PH |
Horizontal tear
in PH |
FTR in PH | Indeterminate for tear |
Tissue maceration
in body |
Intact |
| F4 | Intact | Intact |
Bucket handle tear in PH → body |
Horizontal tear in
AJ, CXT in PH → body |
Bucket handle tear PH → body |
CXT in PH → body |
| F5 | Peripheral vertical tear in PJ |
CXT in PJ → body |
CXT with
PH macerated |
CXT in body,
PH maceration |
CXT in body, PH maceration |
CXT in body, PH maceration |
| F6 | LVT PH → body | FTR in PJ, PH maceration |
FTR in PJ, FEF
in body, PH maceration |
FTR in PJ, FEF in PH. | CXT in PH → body, PH maceration |
Radial tears in AH, FTR in PJ, FEF in PH |
| T1 | FTR in PH | FTR in PJ | FTR in PH |
CXT in PH → body,
PH macerated |
FTR in PH | CXT in PH → body, PH macerated |
| T2 | FTR in PH | FTR in PH | Radial tear in PJ,
PH maceration |
FTR tear in PJ, FEF
in PH |
CXT in body, PH maceration |
CXT in PJ, FEF in PH |
| T3 | FTR in PH, FEF in PJ → body |
FTR in PJ, FEF in PH |
FTR in body, FEF
in AH |
PH maceration, FEF in PH |
FTR in body, FEF in AH |
CXT in PH → body, FEF in PH |
| T4 | FTR in PH | CXT in AH → PH | FTR in PJ | CXT in AH → PH | FTR tear in PJ | CXT in body,
surface damage in AH |
| T5 | FTR in AJ | FTR in body, horizontal tear in PJ |
Radial tear in body,
PH maceration |
FTR in PJ, FEF in PH | Radial tear in body, PH maceration |
CXT in body, FEF in PH |
| T6 | Radial tear in PH | FTR in body | CXT in AJ | CXT in body | CXT in AJ | CXT in body |
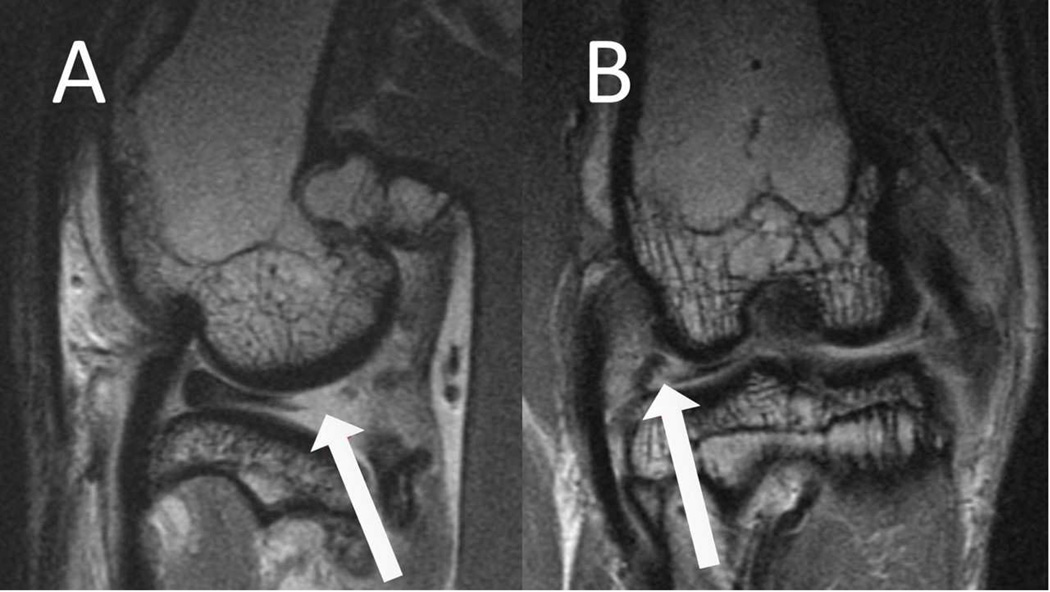
Figure 1.
MRI images of meniscal damage. Arrows on images A, B, and C demonstrate a complex tear of the posterior junction to posterior horn of the lateral meniscus as seen in the sagittal and coronal planes.
Since meniscal transections were introduced during surgery, all animals in the mACLT group had similar acute damage. Only 1/5 medial menisci and 3/5 lateral menisci showed signs of progressive damage at 4 weeks. However at the 8 week time point, 6/6 medial and 5/6 lateral menisci showed progressive damage. Across all time points in the mACLT model, 11/17 medial and 13/17 lateral menisci were characterized as showing damage chronically. There were two cases where damage was noted in the acute MRI and not noted in the chronic MRI.
3.2 Cartilage Results
Based on MRIs, the impact created acute damage to articular cartilage in all but 3 joints of the ACLF animals, with all joints showing some chronic damage at the time of euthanasia. Acute damage most frequently affected the posterior aspect of the medial tibia, occurring in 11 of 15 joints. Thinning of the posterior aspect of the lateral plateau was the second most common acute injury affecting 4 of the joints. Chronically, the location of damage was similar in all 4, 8, and 12 week animals (Figure 2). Full or high grade defects (10/15) or other partial damage such as fibrillation (3/15) was identified in the medial posterior tibial plateau. Chronic MR images also identified some lateral tibial plateau damage (10/15), typically as thinning of the posterior or peripheral aspect. Chronic femoral defects were also noted in both the medial (7/15) and lateral (4/15) femoral condyles.
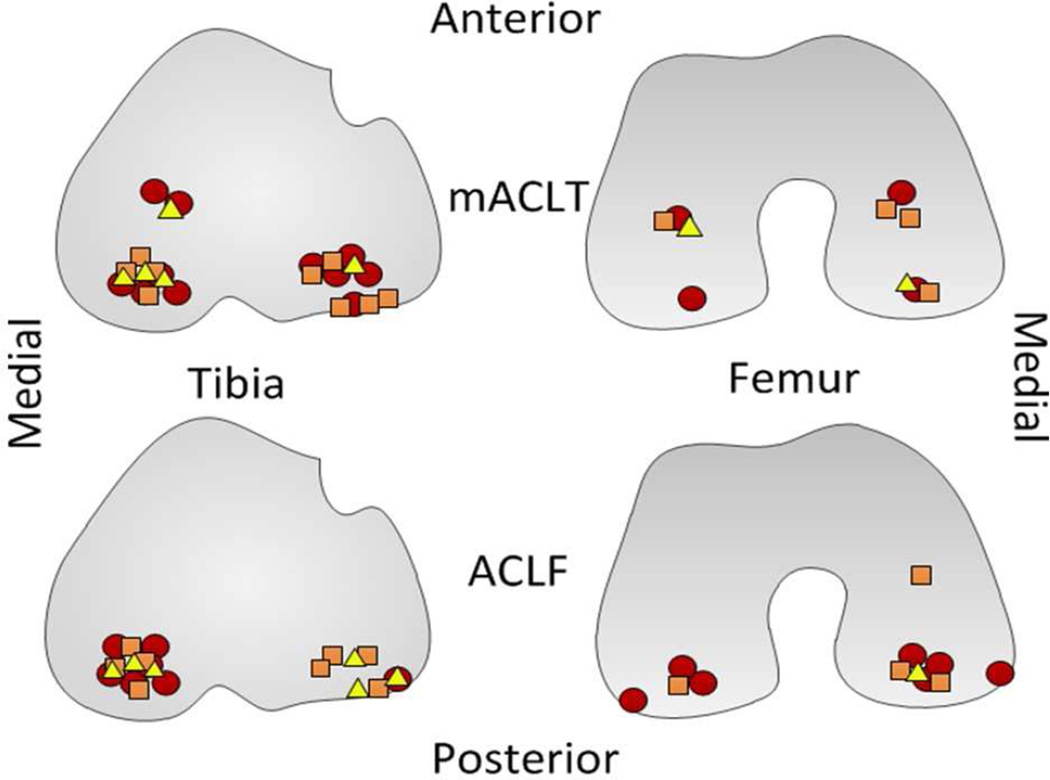
Figure 2.
Visual representation of regional location of damage when noted in chronic MRI results (damage reported within a hemijoint without a specific region has been excluded) at 4 weeks, yellow triangle, 8 weeks, orange square, and 12 weeks, red circle. Top images correspond to mACLT and bottom images ACLF. Moving left to right is the medial tibia, lateral tibia, lateral femur, medial femur.
At the 4 week time point there was high grade or full thickness defects to the posterior medial tibial plateau (3/6), as well as lateral posterior tibial cartilage thinning or defects (2/6) in the mACLT animals. The mACLT animals euthanized at 8 weeks began to show more condylar damage in the posterior aspect of the medial condyle (4/6) and lateral condyle (1/6) in addition to medial (4/6) and lateral (5/6) tibial plateau damage. By 12 weeks, damage was more evident across the whole joint affecting the medial condyle (2/6), lateral condyle (3/6), medial plateau (6/6), and lateral plateau (5/6). However, similar to earlier time points most of the damage at 12 weeks occurred in the posterior aspect of the joint (Figure 2).
3.3 Bone Summary
Following injury, femurs and tibias showed decreases in cortical and trabecular bone quantity and quality. Diminished bone quality was indicated by decreases in bone mineral density. Loss of bone quantity in the trabecular bone presented as decreases in bone volume fraction, number of trabecular struts and thickness of trabecular struts, as well as increases in spacing between trabecular struts. For cortical bone, loss of bone quantity presented as a higher porosity of the bone.
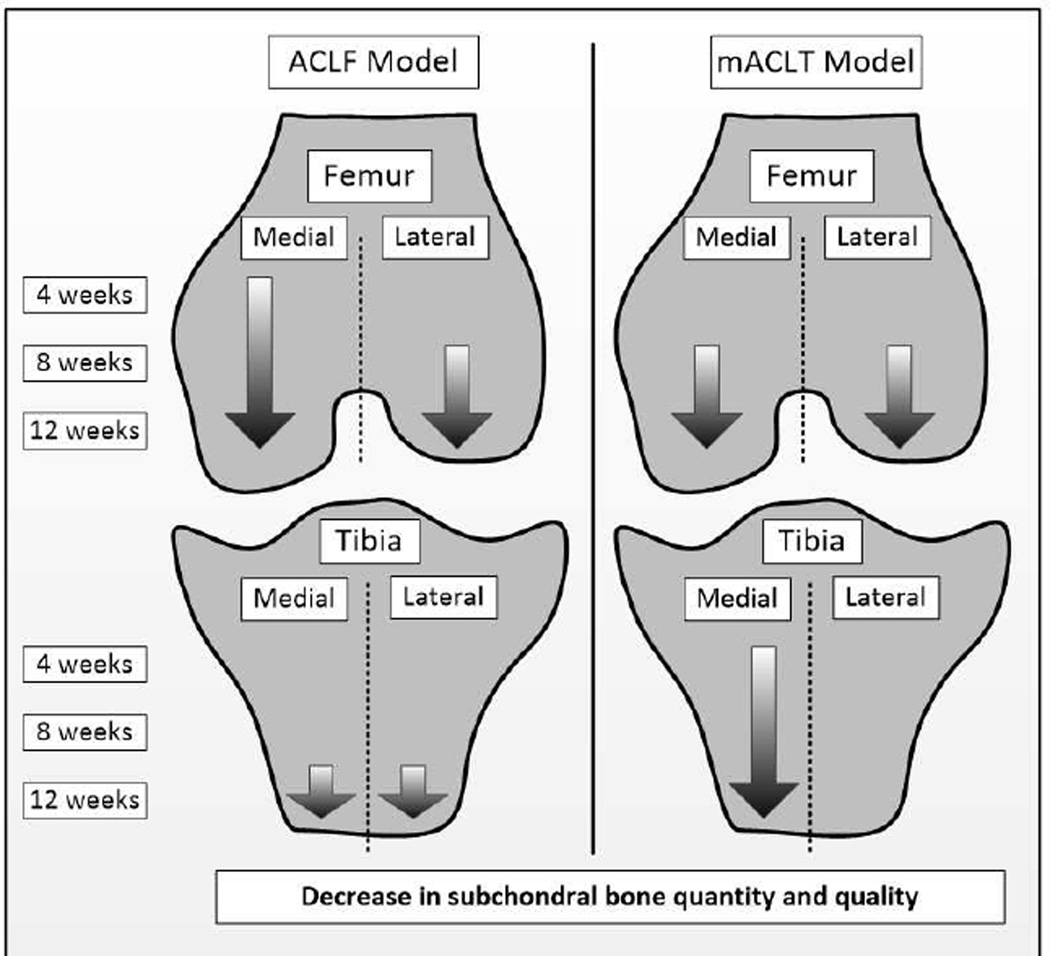
In the ACLF model statistically significant changes to cortical bone porosity in the medial hemijoint of the femur presented 4 weeks after injury. At 8 weeks bone loss in the medial hemijoint continued, as evidenced by a decrease in trabecular strut thickness in both the femur (13%) and tibia (9%). By 12 weeks statistically significant decreases in bone mineral density and bone volume were observed in the cortical and trabecular bone of the femur and tibia in both hemijoints. Thus in the ACLF model bone changes in the medial hemijoint were initially evident at 4 weeks and got progressively worse, however bone changes in the lateral hemijoint presented primarily at the 12 week time point (Figure 3).
Figure 3.
Trends in injured femurs and tibias from progressive decreases in subchondral bone quantity and quality.
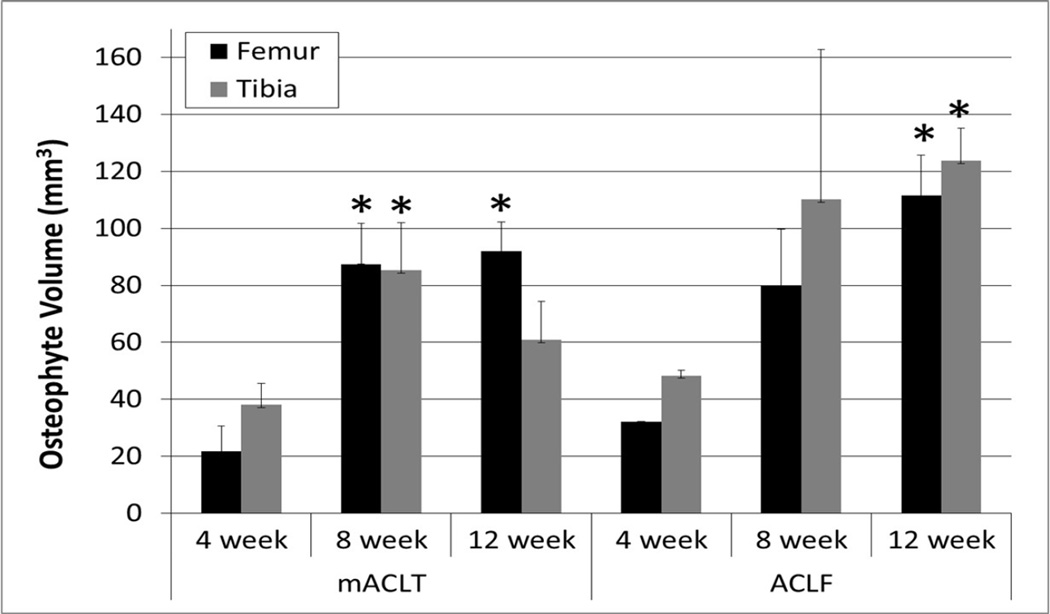
In the mACLT model statistically significant trabecular bone changes were evident after 4 weeks in the medial tibia. By 8 weeks in both hemijoints of injured femurs there were statistically significant losses of bone quality and quantity: less thick trabecular struts in both hemijoints (12% medially and 17% laterally), 6% higher cortical bone porosity in the medial hemijoint, and 5% lower cortical bone mineral density. Twelve weeks after injury major changes were documented in the medial hemijoint of both bones, and some change in the lateral femur. Notably in the medial hemijoint of both bones of injured limbs, there were statistically significant decreases in trabecular bone volume (13% in the femur, and 16% in the tibia) and Tb.BMD (4% in the femur, and 3% in the tibia). In the lateral injured femur there was 2% lower bone mineral density compared to the control limb. Thus in the mACLT model bone damage progressively worsened in both hemijoints of the femur and the medial tibia, but were generally not evident in the lateral tibia (Figure 3). No osteophytes were identified on control limbs; however osteophytes were present on injured femurs (250% increase) and tibias (150% increase) of animals in both models (Figure 4).
Figure 4.
Volume of osteophytes on injured femurs and tibias. * indicates statistically significant difference from volume of osteophytes at 4 weeks.
3.4 MRI vs. meniscus dissection
Comparing the ACLF dissection notes to chronic MRI notes, there were few instances in which the MRI-indicated damage which was not consistent with the dissection notes. Amongst these, there were only 4 instances where damage was noted at dissection that did not appear in the chronic MRI. All other inconsistencies involved the severity of damage rather than its location (Tables 1–3). The majority of discrepancies between the chronic MRI and dissection notes for the mACLT were in the 4 week animals. Radial tears were missed by the MRI in three medial menisci (animals 1, 2, and 5) and a longitudinal tear was missed in a lateral meniscus (animal 3). As was the case in the ACLF group the damage noted at dissection was frequently more severe than initially suspected from the chronic MRI, but only occasionally was damage identified at dissection in locations where no damage was noted in the MRI.
3.5 MRI vs. cartilage morphology
Generally speaking, MRI readings for articular cartilage matched well with cartilage morphology scores (Table 4). However, since control limbs were not imaged, only relative medial and lateral comparisons can be made in the injured limbs. Both MRI and morphological scores showed the most damage in the medial tibial hemijoint for the ACLF animals. While the MRI readings suggested femoral articular cartilage damage in mostly the medial hemijoint at 4 and 8 weeks, which progressed to both the lateral and medial hemijoints at 12 weeks, the morphological scores for the lateral and medial femur suggested equal damage at all time points.
Table 4.
Morphological scores for cartilage of ACLF and mACLT animals
| ACLF Average ± standard deviation | |||||||||
| Tibia | Femur | ||||||||
| Medial Covered |
Medial Uncovered |
Lateral Covered |
Lateral Uncovered |
Medial Top |
Medial Bottom |
Lateral Top |
Lateral Bottom |
||
| 12 W | Control | 2.2 ± 1.1 | 2.4 ± 0.5 | 1.1 ± 0.2 | 1.2 ± 0.3 | 1.8 ± 0.5 | 1.3 ± 0.5 | 1.4 ± 0.5 | 1 ± 0 |
| Injured | 2.8 ± 1.3 | 1.8 ± 0.7 | 1.5 ± 0.8 | 1.7 ± 0.8 | 3.3 ± 1 | 3.1 ± 1.1 | 1.8 ± 0.5 | 1.3 ± 0.4 | |
| 8W | Control | 2.7 ± 0.4 | 2.7 ± 0.5 | 1.8 ± 0.6 | 1.5 ± 0.4 | 1.8 ± 0.8 | 1.5 ± 0.6 | 1.6 ± 0.5 | 1.9 ± 0.5 |
| Injured | 3.1 ± 0.8 | 2.6 ± 0.4 | 2.0 ± 0.5 | 1.6± 0.5 | 3.5 ± 1 | 3.7 ± 0.4 | 2.4 ± 1 | 1.9 ± 0.5 | |
| 4W | Control | 2.8 ± 0.4 | 2.5 ± 0.7 | 1.8 ± 0.4 | 1.3 ± 0.4 | 1.5 ± 0 | 2.3 ± 0.4 | 2 ± 0 | 2.3 ± 0.4 |
| Injured | 2 ± 0.7 | 2.3 ± 0.4 | 2.8 ± 1.8 | 2 ± 0 | 3.5 ± 0.7 | 3 ± 1.4 | 3.3 ± 1.1 | 2 ± 0 | |
| mACLT Average ± standard deviation | |||||||||
| Tibia | Femur | ||||||||
| Medial Covered |
Medial Uncovered |
Lateral Covered |
Lateral Uncovered |
Medial Top |
Medial Bottom |
Lateral Top |
Lateral Bottom |
||
| 12 W | Control | 1.8 ± 0.5 | 2.4 ± 0.4 | 1.3 ± 0.4 | 1.3 ± 0.4 | 1.3 ± 0.8 | 1.3 ± 0.4 | 1.3 ± 0.4 | 1.3 ± 0.6 |
| Injured | 2.5 ± 1.2 | 1.9 ± 0.4 | 1.5 ± 0.8 | 1.6 ± 0.7 | 2.8 ± 1 | 2.7 ± 1.2 | 2.9 ± 1.2 | 2.3 ± 1.4 | |
| 8W | Control | 2.0 ± 0.5 | 2.5 ± 0.4 | 1.6 ± 0.5 | 1.3 ± 0.3 | 1.5 ± 0.4 | 1.5 ± 0.6 | 1.4 ± 0.5 | 1.4 ± 0.4 |
| Injured | 2.0 ± 0.8 | 2.4 ± 0.5 | 2.3 ± 1.2 | 2.4 ± 1.1 | 2.8 ± 0.8 | 2.4 ± 0.9 | 3.3 ± 0.8 | 1.8 ± 2.0 | |
| 4W | Control | 0.8 ± 0.9 | 0.5 ± 0.6 | 0.3 ± 0.4 | 0.3 ± 0.4 | 0.3 ± 0.4 | 0.1 ± 0.2 | 0.3 ± 0.4 | 0.4 ± 0.5 |
| Injured | 0.4 ± 0.6 | 0.3 ± 0.4 | 0.4 ± 0.6 | 0.4 ± 0.5 | 0.6 ± 0.7 | 0.5 ± 0.6 | 0.3 ± 0.5 | 0.4 ± 0.5 | |
For the mACLT model, MRI readings indicated similar damage to both hemijoints at all time points, with damage progressively worsening with time. Morphological scores for the medial and lateral tibia generally agree with this (Table 4). Comparing the two models, fewer high grade lesions were seen in the mACLT model MR images compared to the ACLF model, which is also seen with the morphological scores of the mACLT being lower than the ACLF scores (Table 4).
4. Discussion
The study showed traumatic and surgical ACL rupture and meniscal tear models resulted in progressive degeneration of meniscus and articular cartilage as early as 4 weeks post-injury, if left untreated. Chronic damage was noted in 87% of impacted menisci and 71% of surgically transected menisci across all time points. It is interesting to note that in the ACLF model, menisci had more acute damage to the lateral hemijoint, however chronically the medial hemijoint showed more meniscal damage. The opposite was true in the mACLT model; acute damage was more prevalent in the medial meniscus, and chronically the lateral hemijoint showed more damage. It is unclear how acute meniscal damage affects the progression of OA in bone and cartilage, however the mACLT starts to shed light on this since acutely there was no traumatic impaction, only ACL and meniscal damage.
The distribution of damage across hemijoints was model dependent. Chronic damage was more common in the medial meniscus (93% medial vs. 73% lateral menisci), and the medial cartilage (93%, vs. 80% for lateral cartilage) of the ACLF model. Similarly, progressive bone damage was primarily present in the medial hemijoint of injured femurs and tibiae, and damage to the lateral hemijoint was less prevalent and only manifested significantly at 12 weeks. Chronic damage was more evenly dispersed between hemijoints in the mACLT model for the menisci (medial 65%, lateral 76%) and cartilage (medial 82%, Lateral 76%). In femurs and tibias bone quantity and quality progressively decreased from 4 to 12 weeks in both hemijoints of the femur as well as the lateral tibia. Interestingly, in the ACLF model acute tears of the lateral meniscus were documented in 73% of the specimens, while medial tears were present in only 60% of the animals. Hence, the traumatic model closely mimicked what is seen clinically, with regards to meniscal damage. Both clinically and in the traumatic injury model, acute damage was primarily to the lateral meniscus (Bellabarba et al.23 reported acute tears occurring more often in the lateral (56%) than medial meniscus (44%)), and chronic damage was primarily to the medial meniscus (70% of chronic meniscal injuries are reported to occur to the medial meniscus23). Chronic damage is clinically most commonly documented in the posterior regions24–26, which was consistent with the current MRI findings in both models. Having an animal model such as the ACLF model that not only simulates a traumatic loading event through the joint, but also results in both acute and chronic damage similar to clinical cases would seem to be an extremely valuable tool in experimental studies of PTOA.
The MR imaging used in this study and the dissection notes for the meniscal tissue and articular cartilage matched well. Identifying specific regions, for example anterior junction vs. anterior horn, is difficult given the small size of the tissue and the slice thickness. Defining AC “surface damage” was difficult with MRI.
Hough et al. suggests longitudinal and radial tears are the most common tear orientations when excessive force is applied to the meniscus, while horizontal tears are more common in degenerative cases27. In the ACLF model the most common meniscal tear type was longitudinal vertical tears. This, in addition to the joint experiencing a loading scenario, may make it a more mimetic model of ACL and meniscal injury. Likewise the mACLT model is advantageous compared to the traditional ACLT model as it recreates a combination of radial and longitudinal meniscal tears. Longitudinal, bucket-handle, and complex tears have been shown to strongly correlate with articular cartilage damage, as opposed to radial, flap, and horizontal cleavage tears28,29. This is supported by the findings in these two models. The ACLF model had more occurrences of complex and longitudinal tearing and, as such, had a higher percentage of observed articular cartilage damage compared to the mACLT model. MRI data from the current study suggested acute damage may serve as a location for damage to propagate and worsen over time. Acute longitudinal meniscal tears in the ACLF model typically became complex tears chronically and partial surgical radial tears in mACLT animals turned into segmented menisci. The articular cartilage in the medial posterior aspect of the tibial plateau was the site of greatest damage both acutely and chronicly in both models. Animals in the mACLT model did, however, have appreciable damage to the lateral plateau as well.
It is relevant to compare the location and progression of damage of the menisci and articular cartilage to the location and progression of subchondral bone damage. In both the ACLF and mACLT models the location of chronic meniscus and cartilage damage primarily corresponded with the location of bone damage. For ACLF animals the primary location of progressive bone damage was in the medial hemijoint, and similarly the menisci and cartilage were chronically more commonly damaged in the medial hemijoint. For mACLT animals progressive subchondral bone damage was present in both hemijoints of the femur, as well as the medial tibia. This generally corresponds with the locations of damage to menisci and cartilage; however the subchondral bone in the lateral tibia of injured limbs remained largely unaffected, despite demonstrated damage to the lateral menisci and cartilage. The similar locations of damage could suggest a relationship between the progressive damage in soft tissue and the loss of subchondral bone volume and bone mineral density; however it is challenging to discern a cause and effect relationship between the degeneration of joint structures in the current study. In general, both the mACLT and ACLF models resulted in bone damage 12 weeks after injury, however the longitudinal progression of damage in the two models was significantly different. This suggests the method of induction of soft tissue injury significantly affects progression of bone damage. Both models tended towards a similar amount of osteophytic formation, which was surprising given the large compressive load in the ACLF model. Thus, there continues to be an intricate relationship between soft tissue and bone degradation.
Although the mACLT model of the current study is the first to inflict surgical damage to both the ACL and menisci in a rabbit knee, other groups have assessed the longitudinal progression of bone damage after soft tissue transection8,30,31. Batiste et al. 2004 identified losses of bone mineral density in a rabbit ACLT model 4 and 8 weeks post-surgery, with bone mineral density values returning to normal levels at 12 weeks7. This biphasic response may be suggestive of an active bone remodeling process taking place after injury. In contrast, a surgical ACL transection model in canines identified a decrease in bone mineral density only 3 weeks after surgery with subsequent decreases to 12 weeks post-surgery32.
The study is not without limitations. First, all samples sizes were not equal, with the 4 week ACLF group only having 3 samples. Despite this limitation, statistical analyses were completed on the data and meaningful conclusions were drawn. Additionally, only one blinded grader read the MRI slices. In the future, both a veterinary and human radiologist should be included for comparison between readers. Discrete time points were chosen for the analysis, out to 12 weeks. Former studies with the ACLT model have shown significant degradation of the joint in 8 weeks, including fibrillations, local erosions, and eburnation of bone7,33, during the so-called “period of joint degradation34. Given the amount of degeneration that was seen, we believe these conditions represent chronic OA. Lastly, it is important to note that statistical significance is not equivalent to scientific, human, or economic significance. Statistical significance for this study was taken as p<0.05. Since smaller p-values do not necessarily imply the presence of larger or more important effects, and larger p-values do not imply a lack of importance or even lack of effect35, it is important to note that both practical relevance and inferential uncertainly are important issues to address.
It was evident that both of these models resulted in osteoarthritic-type changes, as evidenced by osteophyte formation, progressive degeneration of articular cartilage, menisci and bone. Collectively looking at the articular cartilage and menisci it was noted that the mACLT model provided a slightly different pattern of degeneration than the ACLF model, which could be due to the slightly different acute damages or lack of an initial traumatic impact onto the joint. Nevertheless, damage identified using the chronic MR images did closely match damage seen at dissection, thus MR imaging could be used as a means for non-invasively assessing progressive damage in future studies with these models reducing the total number of animals needed.
Acknowledgments
This research was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R21 AR060464. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors would also like to thank Jean Atkinson, licensed Veterinarian Technician for animal care and handling, and Cliff Beckett for the design and fabrication of the test fixture and help in conduction of the impact experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions:
All authors have contributed to the analysis and interpretation of data, provided a critical review of the article for important intellectual content and have approved the final submitted version of the manuscript. Dr. Tammy Haut Donahue (Tammy.Donahue@colostate.edu) and Dr. Roger Haut (Haut@msu.edu) take responsibility for the integrity of the work as a whole, from inception to finished article.
Conflict of Interest/ Competing Interest:
There are no competing interests to report.
References
- 1.Meyer EG, Haut RC. Excessive compression of the human tibio-femoral joint causes ACL rupture. J Biomech. 2005;38(11):2311–2316. doi: 10.1016/j.jbiomech.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Speer KP, Warren RF, Wickiewicz TL, Horowitz L, Henderson L. Observations on the injury mechanism of anterior cruciate ligament tears in skiers. Am J Sports Med. 1995;23(1):77–81. doi: 10.1177/036354659502300113. [DOI] [PubMed] [Google Scholar]
- 3.Boden BP, Dean GS, Feagin JA, Garrett WE. Mechanisms of anterior cruciate ligament injury. Orthopedics. 2000;23(6):573–578. doi: 10.3928/0147-7447-20000601-15. [DOI] [PubMed] [Google Scholar]
- 4.Myklebust G, Bahr R. Return to play guidelines after anterior cruciate ligament surgery. Br J Sports Med. 2005;39(3):127–131. doi: 10.1136/bjsm.2004.010900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atkinson PJ, Cooper TG, Anseth S, Walter NE, Kargus R, Haut RC. Association of knee bone bruise frequency with time postinjury and type of soft tissue injury. Orthopedics. 2008;31(5):440. doi: 10.3928/01477447-20080501-01. [DOI] [PubMed] [Google Scholar]
- 6.Guenoun D, Le Corroller T, Amous Z, Pauly V, Sbihi a, Champsaur P. The contribution of MRI to the diagnosis of traumatic tears of the anterior cruciate ligament. Diagn Interv Imaging. 2012;93(5):331–341. doi: 10.1016/j.diii.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Batiste DL, Kirkley A, Laverty S, Thain LMF, Spouge AR, Holdsworth DW. Ex vivo characterization of articular cartilage and bone lesions in a rabbit ACL transection model of osteoarthritis using MRI and micro-CT. Osteoarthr Cartil. 2004;12(12):986–996. doi: 10.1016/j.joca.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Bouchgua M, Alexander K, Carmel EN, et al. Use of routine clinical multimodality imaging in a rabbit model of osteoarthritis--part II: bone mineral density assessment. Osteoarthritis Cartilage. 2009;17(2):197–204. doi: 10.1016/j.joca.2008.06.023. [DOI] [PubMed] [Google Scholar]
- 9.Lozano J, Saadat E, Li X, Majumdar S, Ma CB. Magnetic resonance T(1 rho) imaging of osteoarthritis: a rabbit ACL transection model. Magn Reson Imaging. 2009;27(5):611–616. doi: 10.1016/j.mri.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Anastasiou A, Hall LD. A novel RF coil configuration for in-vivo and ex-vivo imaging of arthritic rabbit knee joints. Magn Reson Imaging. 2003;21(1):61–68. doi: 10.1016/s0730-725x(02)00636-7. [DOI] [PubMed] [Google Scholar]
- 11.McDaniel WJ, Dameron TB. Untreated ruptures of the anterior cruciate ligament. A follow-up study. J Bone Joint Surg Am. 1980;62(5):696–705. [PubMed] [Google Scholar]
- 12.Felson DT, Neogi T. Osteoarthritis: is it a disease of cartilage or of bone? Arthritis Rheum. 2004;50(2):341–344. doi: 10.1002/art.20051. [DOI] [PubMed] [Google Scholar]
- 13.Felson DT. An update on the pathogenesis and epidemiology of osteoarthritis. Radiol Clin North Am. 2004;42(1):1–9. v. doi: 10.1016/S0033-8389(03)00161-1. [DOI] [PubMed] [Google Scholar]
- 14.Yeow CH, Cheong CH, Ng KS, Lee PVS, Goh JCH. Anterior cruciate ligament failure and cartilage damage during knee joint compression: a preliminary study based on the porcine model. Am J Sports Med. 2008;36(5):934–942. doi: 10.1177/0363546507312645. [DOI] [PubMed] [Google Scholar]
- 15.Hewett TE, Stroupe AL, Nance TA, Noyes FR. Plyometric training in female athletes. Decreased impact forces and increased hamstring torques. Am J Sports Med. 1996;24(6):765–773. doi: 10.1177/036354659602400611. [DOI] [PubMed] [Google Scholar]
- 16.Ettlinger CF, Johnson RJ, Shealy JE. A method to help reduce the risk of serious knee sprains incurred in alpine skiing. Am J Sport Med. 1995;23(5):531–537. doi: 10.1177/036354659502300503. [DOI] [PubMed] [Google Scholar]
- 17.Fischenich KM, Coatney Ga, Haverkamp JH, et al. Evaluation of meniscal mechanics and proteoglycan content in a modified anterior cruciate ligament transection model. J Biomech Eng. 2014;136(7):1–8. doi: 10.1115/1.4027468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isaac DI, Meyer EG, Haut RC. Development of a traumatic anterior cruciate ligament and meniscal rupture model with a pilot in vivo study. J Biomech Eng. 2010;132(6):064501. doi: 10.1115/1.4001111. [DOI] [PubMed] [Google Scholar]
- 19.Isaac DI, Meyer EG, Haut RC. Chondrocyte damage and contact pressures following impact on the rabbit tibiofemoral joint. J Biomech Eng. 2008;130(4):041018. doi: 10.1115/1.2948403. [DOI] [PubMed] [Google Scholar]
- 20.Killian ML, Isaac DI, Haut RC, Déjardin LM, Leetun D, Donahue TLH. Traumatic anterior cruciate ligament tear and its implications on meniscal degradation: a preliminary novel lapine osteoarthritis model. J Surg Res. 2010;164(2):234–241. doi: 10.1016/j.jss.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Pauly HM, Larson BE, Coatney GA, et al. Assessment of Cortical and Trabecular Bone Changes in Two Models of Post-Traumatic Osteoarthritis. J Orthop Res. 2015 Dec;:1835–1845. doi: 10.1002/jor.22975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshioka M, Coutts RD, Amiel D, Hacker SA. Characterization of a model of osteoarthritis in the rabbit knee. Osteoarthritis Cartilage. 1996;4(2):87–98. doi: 10.1016/s1063-4584(05)80318-8. [DOI] [PubMed] [Google Scholar]
- 23.Bellabarba C, Bush-Joseph CA, Bach BR. Patterns of meniscal injury in the anterior cruciate-deficient knee: a review of the literature. Am J Orthop (Belle Mead NJ) 1997;26(1):18–23. [PubMed] [Google Scholar]
- 24.Smith JP, Barrett GR. Medial and lateral meniscal tear patterns in anterior cruciate ligament-deficient knees. A prospective analysis of 575 tears. Am J Sports Med. 2001;29(4):415–419. doi: 10.1177/03635465010290040501. [DOI] [PubMed] [Google Scholar]
- 25.Chan WP, Lang P, Stevens MP, et al. Osteoarthritis of the knee: comparison of radiography, CT, and MR imaging to assess extent and severity. AJR Am J Roentgenol. 1991;157(4):799–806. doi: 10.2214/ajr.157.4.1892040. [DOI] [PubMed] [Google Scholar]
- 26.Greis PE, Bardana DD, Holmstrom MC, Burks RT. Meniscal injury: I. Basic science and evaluation. J Am Acad Orthop Surg. 2002;10(3):168–176. doi: 10.5435/00124635-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Hough AJ, Webber RJ. Pathology of the Meniscus. Clin Orthop Relat {…} 1990;252(252):32–40. [PubMed] [Google Scholar]
- 28.Lewandrowski KU, Müller J, Schollmeier G. Concomitant meniscal and articular cartilage lesions in the femorotibial joint. Am J Sports Med. 1997;25(4):486–494. doi: 10.1177/036354659702500411. [DOI] [PubMed] [Google Scholar]
- 29.Tandogan RN, Taşer O, Kayaalp A, et al. Analysis of meniscal and chondral lesions accompanying anterior cruciate ligament tears: relationship with age, time from injury, and level of sport. Knee Surg Sports Traumatol Arthrosc. 2004;12(4):262–270. doi: 10.1007/s00167-003-0398-z. [DOI] [PubMed] [Google Scholar]
- 30.Dedrick DK, Goldstein Sa, Brandt KD, O’Connor BL, Goulet RW, Albrecht M. A longitudinal study of subchondral plate and trabecular bone in cruciate-deficient dogs with osteoarthritis followed up for 54 months. Arthritis Rheum. 1993;36(10):1460–1467. doi: 10.1002/art.1780361019. [DOI] [PubMed] [Google Scholar]
- 31.Boyd SK, Müller R, Leonard T, Herzog W. Long-term periarticular bone adaptation in a feline knee injury model for post-traumatic experimental osteoarthritis. Osteoarthritis Cartilage. 2005;13(3):235–242. doi: 10.1016/j.joca.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Messner K, Fahlgren a, Ross I, Andersson B. Simultaneous changes in bone mineral density and articular cartilage in a rabbit meniscectomy model of knee osteoarthrosis. Osteoarthritis Cartilage. 2000;8(3):197–206. doi: 10.1053/joca.1999.0290. [DOI] [PubMed] [Google Scholar]
- 33.Sah RL, Yang aS, Chen aC, et al. Physical properties of rabbit articular cartilage after transection of the anterior cruciate ligament. J Orthop Res. 1997;15(2):197–203. doi: 10.1002/jor.1100150207. [DOI] [PubMed] [Google Scholar]
- 34.Papaioannou N, Krallis N, Triantafillopoulos I, Khaldi L, Dontas I, Lyritis G. Optimal timing of research after anterior cruciate ligament resection in rabbits. Contemp Top Lab Anim Sci. 2004;43(6):22–27. quiz 58. [PubMed] [Google Scholar]
- 35.Wasserstain RL, Lazar NA. The ASA’s statement on p-values: context, process, and purpose. The American Statistician. 2016 [Google Scholar]