Abstract
After corneal epithelial injury, the ensuing inflammatory response is necessary for efficient wound healing. While beneficial healing effects are attributed to recruited neutrophils and platelets, dysregulated inflammation (too little or too much) is associated with impaired wound healing. The purpose of this study was to use an established C57BL/6J mouse model of corneal injury to evaluate the potential modulatory role of interleukin-20 (IL-20) on the inflammatory and healing responses to epithelial wounding. In the uninjured cornea, immunofluorescence staining for IL-20 and its receptor, IL-20RA, was observed on basal epithelial cells at the limbus. After a 2mm central epithelial abrasion, IL-20 staining was also observed in stromal keratocytes and ELISA studies showed a significant increase (nearly 3-fold) in IL-20 expression. Injured corneas healed more slowly when treated with a topical application of a neutralizing anti-IL-20 antibody. While corneal epithelial cell division and epithelial nerve recovery measured at 24 hours post-injury were reduced compared to controls, neutrophil influx into the cornea was increased. In contrast, topical application of recombinant IL-20 (rIL-20) decreased corneal inflammation as evidenced by reductions in limbal vessel dilatation, platelet extravasation, neutrophil recruitment and CXCL1 expression. In wild type mice, topical rIL-20 had a limited effect on corneal wound healing and resulted in only a slight increase in epithelial cell division and epithelial nerve recovery; the rate of wound closure was unaffected. To clarify the effect of IL-20 on corneal wound healing, rIL-20 was topically applied to neutropenic wild type (WT) mice and mutant mice (γδ T cell deficient mice and CD11a deficient mice), all of which have well characterized reductions in neutrophil recruitment and delayed wound healing after corneal injury. In each case, rIL-20 restored corneal wound healing to baseline levels while neutrophil recruitment remained low. Thus, it appears that IL-20 plays a beneficial and direct role in corneal wound healing while negatively regulating neutrophil and platelet infiltration.
Keywords: Interleukin-20, cornea, injury, healing, inflammation
Introduction
The stratified epithelium of the cornea contains leukocytes of the innate immune system 1–9 that reside mostly in the peripheral regions near and within the limbus 4, 5, 7. Animal models of epithelial injury reveal rapid accumulation of leukocytes within the avascular dense connective tissue stroma and the epithelium. Neutrophils migrate to corneal wounds mostly within the anterior stroma 3, 7, 10, lymphocytes, macrophages and dendritic cells migrate within both stroma and stratified epithelium 7, 11–13, and platelets after extravasation, accumulate in the stroma near limbal vessels 14, 15. In a mouse model, locally produced CCL20 attracts CCR6+ IL-17A+ IL-22+ IL-23R+ RORγt+ γδ T cells 7, 14–17 that become the most abundant CD3+ lymphocytes in the epithelium within 24 hours after corneal wounding 16. In mice deficient in γδ T cells, restoration of normal epithelial stratification and basal cell density is deficient even 2 weeks after a defined central epithelial lesion, though wildtype mice restore corneal epithelium to preinjury thickness and density within a few days 7, 16. IL-22 produced by the γδ T cells 15, 17 directly stimulates epithelial secretion of CXCL1, a chemokine that promotes recruitment of neutrophils 17, a source of important growth factors (e.g., VEGF-A 15). The importance of IL-22 to corneal wound healing is confirmed by experiments in which topical application of neutralizing anti-IL-22 antibody markedly diminishes neutrophil recruitment and delays epithelial wound closure. Topical application of recombinant IL-22 in mutant mice lacking γδ T cells, the major cellular source of IL-22 in the cornea, restores corneal wound healing 17. While the inflammatory response induced by epithelial injury directly benefits wound healing, there is potential for damage to corneal tissue, as demonstrated in mice 18 with dysregulated control mechanisms resulting in excessive neutrophil accumulation 19, 20. Modulatory influences within the inflammatory response are critical to effective wound healing. Haworth et al. 21 published evidence that NK cells are important for resolution of adaptive inflammation involving eosinophil and activated T cell infiltration into the lung. Liu et al. 22 demonstrated in a mouse model that γδ T cells are also responsible for significant accumulation of NKp46+ NK1.1+ NKG2D+ EOMES+ CD3− CD94− RORγt− IL-22− CD127− NK cells in the wounded cornea. These NK cells are important in limiting the tenure of neutrophil accumulation in the mouse cornea after epithelial wounding through an NKG2D-dependent mechanism. This function is apparently important, tipping the balance of inflammation in favor of wound healing. In the current study we analyzed another potential modulatory influence on the corneal inflammatory response to epithelial wounding. Recent evidence indicates that IL-22 can induce expression in epithelial cells of IL-20, a member of the IL-10 family of cytokines that is closely related to IL-22. IL-20 is reported to exhibit effects on acute inflammatory processes distinct from those of IL-22 23, 24. Here we report that corneal tissues express IL-20, and we present evidence that IL-20 promotes wound healing at two levels, modulation of neutrophil influx and stimulation of epithelial recovery.
Materials and Methods
Studies were carried out at Baylor College of Medicine (Houston, TX). 8–12 week old mice were used for all experiments and mice were treated following the guidelines in the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Corneal abrasion model
Female C57BL/6J wild type mice, as well as γδ T cell deficient mice (TCR−/−) mice and CD11a deficient mice (CD11a−/−) on the C57BL/6J background were purchased from Jackson Laboratory (Bar Harbor, ME). Neutropenic mice were prepared by intraperitoneally injecting anti-Ly6G antibody 25 (0.25mg/mouse, BD Pharmingen, San Jose, CA). A central corneal wound was made following an established protocol 16. Briefly, mice were anesthetized by intraperitoneal injection of pentobarbital sodium solution (50mg/kg) and a 2 mm diameter central corneal epithelial region was marked by a trephine and mechanically debrided with a golf club spud under a dissecting microscope while taking care not to penetrate the basement membrane.
Topical application of IL-20
Every 4 hours over a 24 hour period, wounded wild-type, mutant and neutrophil depleted mice were given topical applications of 200ng/ml recombinant mouse IL-20 or neutralizing anti-IL-20 antibody (R&D Systems, Minneapolis, MN) dissolved in phosphate buffered saline (PBS). Control mice received PBS or non-immune IgG.
Analysis of wound closure
The rate of corneal wound closure was determined using an established protocol 12. Briefly, sodium fluorescein solution was applied to the wounded cornea to mark the size of the open wound. Images were obtained from time 0, 4, 8, 12, 16, 24 and 36 hours using a digital camera and analyzed by Optimus 6.2 software (Media Cybernetics, Rockville, MD).
Immunohistology
Dissected corneas (including limbus) were fixed for 30 minutes in phosphate buffered saline (PBS, pH 7.2) containing 2% paraformaldehyde. Fixed corneas were washed in PBS 3 times, permeabilized with 0.1% Triton X-100 for 30 minutes, and then incubated for 30 min with PBS containg 1% bovine serum albumin followed by fluorescent-labeled antibodies raised against mouse antigens. Antibodies used were: anti-CD31 (BD Pharmingen, San Jose, CA) for detection of blood vessels; anti-β-III tubulin (R&D Systems, Minneapolis, MN) for detection of nerves, anti-α-tubulin (Sigma, St. Louis, MO) for detection of mitotic spindles, anti-Ly6G (BD Pharmingen, San Jose, CA) for detection of neutrophils and anti-CD41 (BD Pharmingen, San Jose, CA) for detection of platelets. For detection of IL-20 and its receptor, dissected corneas were incubated with labeled anti-IL-20 (Abcam, Cambridge, MA) or anti-IL-20RA (Millipore, Billerica, MA) antibodies. Labeled non-immune isotype matched antibodies served as controls for non-specific staining. To evaluate IL-20 and IL20RA expression in human corneal epithelial cells, we cultured human telomerase human corneal epithelial cells (HTCEpi) for 4 hours in KBM-2 medium (Fisher Scientific, Pittsburgh, PA) containing 10 units/ml IL-1β (R&D Systems, Minneapolis, MN) after which the cells were labeled with anti-IL-20 (Abcam, Cambridge, MA) or anti-IL-20RA (Millipore, Billerica, MA) antibodies. Again, labeled non-immune isotype matched antibodies served as controls for non-specific staining.
Microscopic imaging and morphologic evaluation
Immunostained corneas were imaged as whole mounts using a 40X oil immersion lens or a 20X dry lens mounted on a DeltaVision Core Spectris microscope system (Applied Precision, Issequah, WA). Captured images were deconvolved and analyzed using SoftWorx software. Platelets were imaged at the limbus using a 20X lens. Deconvolved projected images were then used for counting intra- and extra-vascular platelets. Nerves were imaged after staining with anti-β-III tubulin antibody and 40X serial epithelial images of the cornea at the paralimbus, parawound, wound and wound center were collected at 0.2 μm Z-steps through the entire thickness of the epithelium, deconvolved and saved as maximum projected images. The projected images were analyzed using a custom nerve tracing program (Matlab) that quantifies linear nerve density. Neutrophil counts were made at the parawound region using Z-stack images taken at 40X magnification. Dividing basal epithelial cells with positively stained mitotic spindles were imaged and counted at the limbus, paralimbus, parawound, wound and wound center. For imaging the limbal vasculature, overlapping images recorded at 20x were deconvolved, saved as maximum projections and stitched together to form multipanel images detailing the entire limbal vasculature. Arteriole and venule diameters were measured at 50 μm intervals along the entire length of each vessel type and the values obtained were used to compute the average arteriole and venule diameters within each cornea.
Neutralization of endogenous IL-20
IL-20 function was blocked by topical application of a neutralizing goat-anti-mouse IL-20 antibody (R&D Systems, Minneapolis, MN). Briefly, wild type mice received corneal epithelial abrasions and were divided into 3 groups. In one group, mice received topical application (10μl) of goat-anti-mouse IL-20 antibody dissolved in PBS (200μg/ml). This was repeated every 4 hours for 24 hours. Another group of mice received non-immune isotype matched IgG, and a third group received only PBS. The rate of corneal wound closure, epithelial nerve density, number of dividing epithelial cells and magnitude of the neutrophil influx were analyzed and comparisons were made between anti-IL-20 treated and control groups.
ELISA assays
IL-20 was analyzed in corneal extracts collected at 0, 24, 48, 72 hours and 7 days after epithelial injury using an ELISA kit (R&D Systems, Minneapolis, MN) and following the manufacturer’s instructions. Briefly, 10 corneas from each time point were dissected (including limbus) and pulverized in 500μL of radioimmunoprecipitation buffer containing protease inhibitors (Roche). All samples were stored at −80°C until needed. Mice received central corneal epithelial abrasions. The corneas were immediately excised, placed in RPMI 1640 (6 corneas per ml) and incubated for 6 hours. The culture supernatants were then collected and stored at −80°C until analyzed for CXCL1 expression (ELISA kit (R&D Systems, Minneapolis, MN) in the following experimental conditions.. The following groups were prepared: Unwounded corneas, abraded corneas, abraded corneas incubated with recombinant IL-20 (200ng/ml), and abraded corneas incubated with recombinant IL-22 (200ng/ml).
Statistical analyses
Data were analyzed using a Student’s t-test or, for multiple comparisons, a two-way analysis of variance (ANOVA) followed by a Tukey post-test. Data are expressed as means ± SEM and P values ≤0.05 were considered statistically significant.
Results
IL-20 and its receptor subunit, IL20RA, in corneal tissues
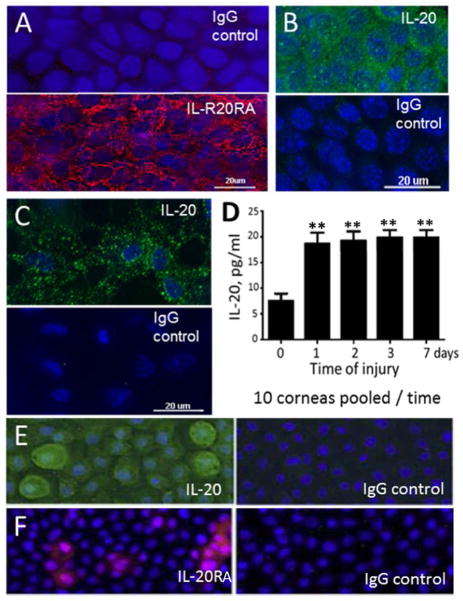
In the uninjured cornea, basal corneal epithelial cells at the limbus exhibited punctate fluorescence after labeling with anti-IL-20RA while no labeling was observed with non-immune IgG. Injured corneas gave essentially the same results consistent with the interpretation that after central corneal abrasion, IL-20RA was present in the regenerating epithelium (Figure 1A, 24 hours post-injury). The use of fluorescence microscopy also indicated that IL-20 was present in the basal cells throughout the epithelium after injury (Figure 1B, 18 hours post-injury). Additionally, viable keratocytes within the peripheral regions of the corneal stroma showed positive staining for IL-20 after epithelial injury (Figure 1C, 18 hours post-injury). This experimental approach failed to reveal labeled antibody binding in corneal macrophages, though macrophages in other tissues have been reported to contain IL-20 26.
Figure 1.
IL-20 and IL-20RA are detected in the mouse cornea. (A) Binding of TRITC-anti-IL-20RA or TRITC-IgG to normal mouse corneal epithelium. (B) Binding of FITC-anti-IL-20 or FITC-IgG to normal mouse corneal epithelium, and (C) to stromal keratocytes. (D) ELISA determinations of IL-20 from extracted corneas without wounding and at various times after central epithelial abrasion (n=10 corneas pooled at each timepoint; ** p<0.01). (E) Human epithelial cell line (HTCEpi cells) stained with FITC-anti-IL-20 or FITC-IgG and (F) with TRITC-anti-IL-20RA or TRITC-IgG.
ELISA studies indicating that IL-20 was present in the mouse cornea involved pooling corneas at different times after central epithelial abrasion. The values obtained in fluid extractions of these corneas were ~7 pg/ml from corneas injured only by removing them from the eye, and ~ 19 pg/ml for corneas receiving abrasions in vivo and collected for analysis out to 7 days post-injury (Figure 1D, 10 corneas pooled at each time point).
Using fluorescence microscopy to detect labeled antibodies against human IL-20 and human IL-20RA provided evidence for these antigens in a cultured human corneal epithelial cell line (HTCEpi cells) stimulated for 4 hours with an inflammatory cytokine (IL-1β, 10 units/ml; Figures 1E and 1F).
IL-20 and corneal wound healing
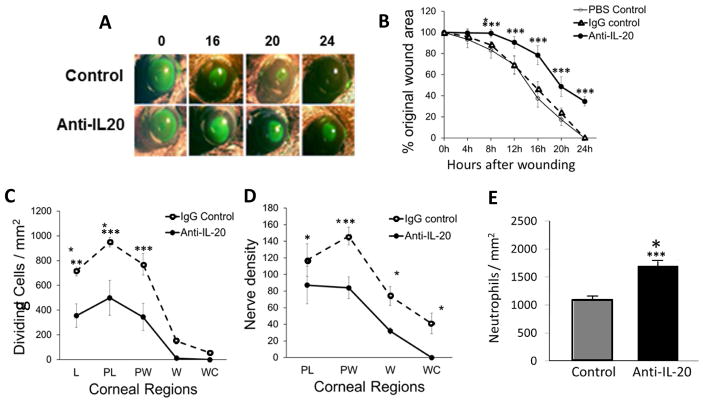
To investigate the role of IL-20 in corneal wound healing, abraded mouse corneas received topical applications of a neutralizing anti-IL-20 antibody, an isotype matched non-immune IgG or vehicle (PBS). The rate of epithelial wound closure was reduced compared to controls when anti-IL-20 was applied, and in contrast to controls, the wound remained open at 24 hours (Figures 2A, B, n=5, p<0.001). Corneal epithelial cell division measured at 24 hours after epithelial abrasion was reduced at the limbus, paralimbus and parawound compared to controls when anti-IL-20 antibody was applied topically (Figures 2C, n=5 p<0.01, p<0.001 and p<0.001, respectively). Similarly, anti-IL-20 impaired epithelial nerve recovery at the paralimbus and parawound (Figure 2D, n=5 p<0.05 and p<0.01, respectively). In contrast to these measures of wound healing, neutrophil influx into abraded corneas at 24 hours post-injury was increased with topical application of anti-IL-20 (Figure 2E, n=5 p<0.01).
Figure 2.
Corneal wound healing in wildtype mice. (A) Representative images of open wounds revealed by topical fluorescein solution. (B) Percentage of open wound area over time after wounding (n=5, *** p<0.001). (C) Dividing epithelial cells in five regions of the cornea at 24 hours after epithelial abrasion (n=5, ** p<0.01 and *** p<0.001). (D) Nerve density in the epithelium in four regions of the epithelium at 24 hours after abrasion (n=5, * p<0.05 and ** p<0.01). (E) Neutrophil counts in the paralimbal region of the corneal stroma at 24 hours after abrasion (n=5, *** p<0.001). L, limbus; PL, paralimbus; PW, parawound; W, wound margin; WC, wound center.
Topical rIL-20 and the inflammatory response to corneal epithelial abrasion
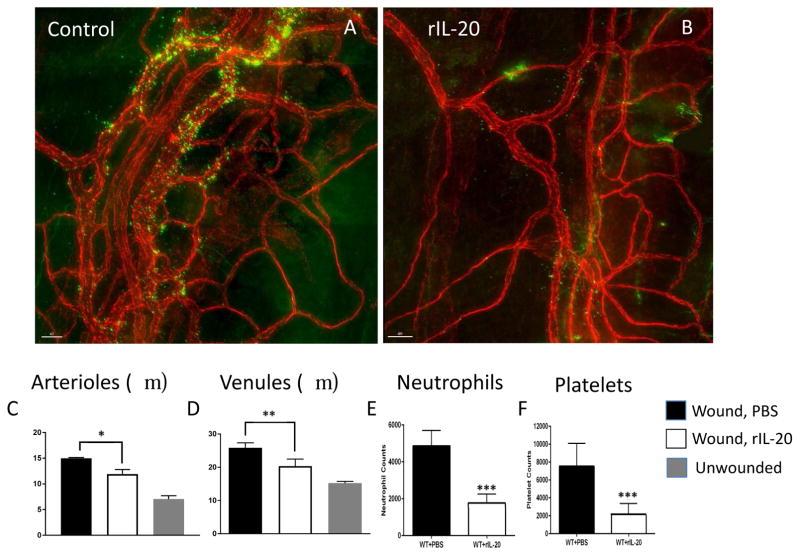
Topical rIL-20 (200 ng/ml) or vehicle (PBS, control) was applied to the cornea every 4 hours for 24 hours after epithelial abrasion before inflammatory parameters were analyzed. Uninjured corneas served as additional controls. Inflammatory parameters evaluated at 24 hours post-injury were: 1) limbal arteriole and venule dilatation, 2) platelet recruitment in and around the limbal vessels and 3) extravasation of neutrophils into the corneal stroma. All three measures of inflammation were reduced (in mice receiving topical rIL-20 as shown in Figure 3).
Figure 3.
Effects of topical rIL-20 on inflammatory changes induced by central corneal epithelial abrasion. Representative photos of limbal vessels at 24 hours after corneal epithelial abrasion in mice treated topically with PBS (A) or rIL-20 (B). Stained tissues showing vessels (red) with extravasating platelets (green) (A, B), and showing arterioles (▶) and venules (↓) with diameters marked (lines). Data on vessel diameters are plotted (C, D, n=4, * p<0.05 and ** p<0.05) and data on platelet and neutrophil extravasation are plotted. (E, F, n=4, *** p<0.001)
In a previous study, we showed that levels of CXCL1 (a potent PMN chemoattractant) in the cornea increased after epithelial abrasion and this increase mirrored the increase in PMN infiltration12. Most cell types can produce CXCL1, including PMNs. We wished to determine if the reduction in PMN infiltration observed after topical application of rIL-20 was associated with decreased corneal production of CXCL1. An in vivo experiment documenting reduced CXCL1 expression would not distinguish between decreased CXCL1 levels resulting from reduced PMN infiltration and decreased CXCL1 levels resulting from diminished corneal production. For this reason, an ex vivo model was chosen to evaluate CXCL1 levels in supernatants of unwounded and wounded mouse corneas incubated in culture medium containing rIL-20 without the potentially confounding effects of the inflammatory response. Supernatants analyzed by ELISA showed that in corneas cultured for 6 hours without injury other than the trauma of excising the cornea, the culture medium contained 606 pg/ml of CXCL1. Corneas with a 2 mm diameter epithelial abrasion yielded 1070 pg/ml CXCL1 in the medium. Abraded corneas yielded 1601 pg/ml of CXCL1 with rIL-22 added to the culture medium. In contrast, abraded corneas with rIL-20 added to the culture medium yielded 351 pg/ml.
Topical IL-20 and corneal wound healing
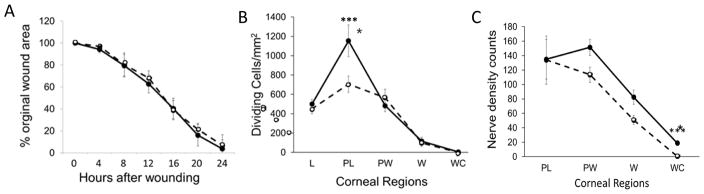
Wildtype mice received topical applications of recombinant IL-20 (rIL-20) or PBS after epithelial abrasion. At 24 hours post-injury, the rate of wound closure in corneas treated with rIL-20 was not significantly different from controls (Figure 4A, (n=4)) whereas there was a mean increase in dividing epithelial cells in the paralimbal region (Figure 4A, (n=4, p<0.001). There was a small influence on epithelial nerve density near the wound center at 24 hours after abrasion following application of rIL-20 (n=4, p<0.001).
Figure 4.
Effects of topical rIL-20 on corneal wound healing in wildtype mice. Corneas were treated topically with rIL-20 and compared to controls treated with PBS. (A) Wound closure was evaluated as shown in Figure 2A. (B) Dividing epithelial cells were evaluated in the regions indicated at 24 hours after epithelial abrasion (n=5, *** p<0.001). (C) Epithelial nerve density was determined in the corneal regions indicated at 24 hours after abrasion (n=5, *** p<0.001). L, limbus; PL, paralimbus; PW, parawound; W, wound margin; WC, wound center.
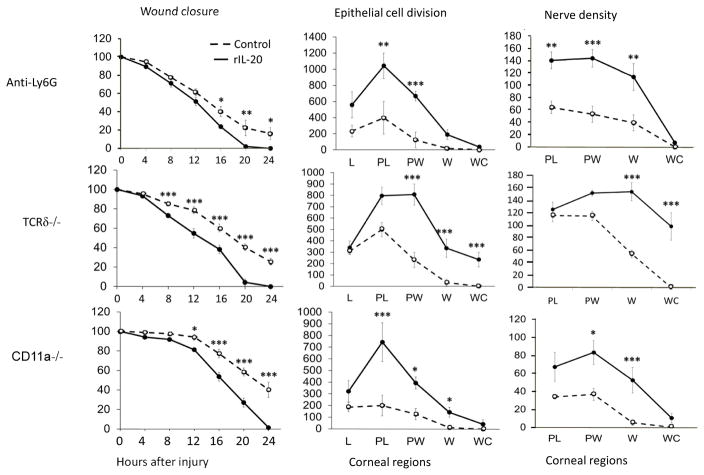
Following a corneal abrasion, the ensuing inflammatory response is necessary for efficient wound healing12, 14, 15. Given the limited effect of topical rIL-20 in wildtype mice, mice with reduced wound healing induced by specific interventions were analyzed for a possible influence of topical rIL-20 on healing. Three interventions that have been shown to significantly reduce healing of corneal abrasions were assessed: 1) systemic anti-Ly6G antibody administration, which results in neutropenia15, 2) TCRδ−/− mice, deficient in γδ T cells 7, 15, 17 and 3) CD11a−/− mice18. Each intervention shows a reduced inflammatory response to epithelial abrasion and a marked delay in wound healing. Topical application of rIL-20 compensated for the loss of beneficial effects provided by a normal inflammatory response and increased the rate of wound closure, numbers of dividing epithelial cells and extent of epithelial nerve recovery at 24 hours in these mice (Figure 5).
Figure 5.
Effects of topical rIL-20 on corneal wound healing. Mice with reduced wound healing resulting from deficiency of neutrophils (anti-Ly6G treatment), γδ T cells (TCRδ−/−), or integrin CD11a (CD11a−/−) were treated topically with rIL-20 or PBS. Three parameters of healing were evaluated: closure of a 2 mm central epithelial abrasion over a 24 hour observation period, numbers of dividing epithelial cells in the regions indicated at 24 hours after epithelial abrasion, and nerve density in the recovering epithelium at 24 hours after central epithelial abrasion. L, limbus; PL, paralimbus; PW, parawound; W, wound margin; WC, wound center. (n=4, * p<0.05, ** p<0.01 and *** p<0.001).
Discussion
The results of this study are consistent with the conclusion that the cytokine, IL-20, is present in corneal tissue during wound healing and influences the reaction of corneal tissues to epithelial injury. This conclusion is based on interpretation of the results in this report as follows: 1) In the abraded mouse cornea, epithelial cells and keratocytes stained positively for IL-20, IL-20 levels in the cornea increased after abrasion, and the corneal basal epithelium was positive for the IL-20 receptor subunit IL-20RA. 2) In wildtype mice, topical application of a neutralizing antibody against IL-20 delayed epithelial wound closure, epithelial cell division and epithelial nerve recovery, and it enhanced neutrophil and platelet infiltration. 3) Topical application of rIL-20 to the injured corneas of wildtype mice inhibited neutrophil and platelet recruitment, an effect possibly linked to an associated reduction in CXCL1 chemokine expression demonstrated in an experiment ex vivo. 4) Topical application of rIL-20 to the injured corneas of neutropenic, TCR−/−, CD11a−/− mice enhanced wound healing as evidenced by accelerated wound closure, increased epithelial cell division and enhanced nerve recovery. Mutant mice deficient in γδ T cells (TCRδ−/−) and CD11a (CD11a−/−), and neutrophil-depleted mice each have reduced neutrophil recruitment and an associated delay in corneal wound healing 7, 12, 18. Thus, it appears that IL-20 plays a beneficial and direct role in corneal wound healing while negatively regulating neutrophil and platelet infiltration.
IL-20 participates in tissue repair and wound healing by inducing genes regulating cell proliferation 26 and promoting host protection by releasing antimicrobial peptides 27. IL-20 induces keratinocyte proliferation and differentiation in normal skin, and activates a STAT3-dependent pathway, promoting cell proliferation as shown in human keratinocyte and glioblastoma cell lines 28, 29. IL-20 apparently plays a role in diseases like psoriasis 30–32 and rheumatoid arthritis 33, 34 through a chronically elevated expression that results in excessive stimulation of epithelial cell division. Our observations in the present study are consistent with functions of IL-20 in other tissues, promoting epithelial proliferation and wound healing. In that regard, IL-20 shares activity with IL-22, previously shown to promote epithelial proliferation and wound closure in the cornea 17 and skin 23. It appears that both IL-20 and IL-22 are involved in corneal epithelial wound healing since blocking antibodies to either inhibit healing 23, and topical recombinant IL-20 or IL-22 restores healing in murine models with deficient healing.
Beyond the direct effects of IL-20 and IL-22 on epithelial cells, they influence innate immune functions. In the present study, they exhibit distinctions that are potentially significant to wound healing of the cornea. Sa et al. 26 using gene expression analysis found that IL-20 and IL-22 induced correlated expression profiles from reconstituted human epidermis, but there were some important differences. IL-22 is a potent inducer of chemokines such as IL-8, CXCL7 and CXCL1. IL-20 exhibits very limited ability to induce expression of these chemokines, and in studies by Myles et al. 35, IL-20 was found to inhibit cutaneous production of IL-1β and IL-17A, two cytokines critical to development of an innate inflammatory response. It appears that these two cytokines may influence innate inflammation distinctly. In a dermal infection model with methicillin-resistant Staphylococcis aureus, IL-20 promotes infection 35. In contrast, IL-22 has been shown to serve a protective role in infection models 23. Thus, observations in the current model of corneal epithelial abrasion are apparently consistent with the effects of IL-20 and IL-22 in other tissues. Recombinant IL-20 reduced the production of CXCL1, a proinflammatory chemokine, by abraded mouse corneas while rIL-22 enhanced its production. Further, topical rIL-20 reduced limbal vessel dilatation, neutrophil influx and platelet extravasation in abraded corneas (Figure 5). In published studies we found that topical rIL-22 restored corneal healing and leukocyte influx in TCRδ−/− mice 17.
A general model for the contribution of IL-22 and IL-20 to wound healing in the cornea appears to be that these two cytokines, derived from different cellular sources within the cornea, directly promote epithelial proliferation with attendant recovery of the epithelium including regeneration of epithelial nerves. Injury-induced innate inflammatory responses contribute to corneal wound healing 3, 7, 10–15, but in excess can cause tissue injury 18. In epithelial abrasion injury to the cornea, IL-22 induces inflammation and IL-20 inhibits inflammation. Their presence within the cornea in the days after wounding, along with immigrating NKG2D+ IL-22− NK cells 22, 36, modulates the inflammatory response, maintaining a healing environment.
HIGHLIGHTS.
Interleukin-20 (IL-20) is present in the mouse cornea during wound healing
Antibody neutralization of IL-20 delays epithelial wound healing
Topically applied recombinant IL-20 inhibits leukocyte recruitment
IL-20 plays a beneficial and direct role in inflammation and corneal wound healing
Acknowledgments
FUNDING: This work was supported by the National Institutes of Health: EY18239, P30 EY007551, EY017120 and HL116524 and the National Natural Science Foundation of China: 39970250, 30772387 and 81070703.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Toulon A, Breton L, Taylor KR, et al. A role for human skin-resident T cells in wound healing. J Exp Med. 2009;206:743–750. doi: 10.1084/jem.20081787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Havran WL, Jameson JM. Epidermal T cells and wound healing. J Immunol. 2010;184:5423–5428. doi: 10.4049/jimmunol.0902733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamagami S, Hamrah P, Miyamoto K, et al. CCR5 chemokine receptor mediates recruitment of MHC class II-positive Langerhans cells in the mouse corneal epithelium. Invest Ophthalmol Vis Sci. 2005;46:1201–1207. doi: 10.1167/iovs.04-0658. [DOI] [PubMed] [Google Scholar]
- 4.Hamrah P, Huq SO, Liu Y, Zhang Q, Dana MR. Corneal immunity is mediated by heterogeneous population of antigen-presenting cells. J Leukoc Biol. 2003;74:172–178. doi: 10.1189/jlb.1102544. [DOI] [PubMed] [Google Scholar]
- 5.Knickelbein JE, Watkins SC, McMenamin PG, Hendricks RL. Stratification of Antigen-presenting Cells within the Normal Cornea. Ophthalmol Eye Dis. 2009;1:45–54. doi: 10.4137/oed.s2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chinnery HR, Ruitenberg MJ, Plant GW, Pearlman E, Jung S, McMenamin PG. The chemokine receptor CX3CR1 mediates homing of MHC class II-positive cells to the normal mouse corneal epithelium. Invest Ophthalmol Vis Sci. 2007;48:1568–1574. doi: 10.1167/iovs.06-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Z, Burns AR, Rumbaut RE, Smith CW. gamma delta T cells are necessary for platelet and neutrophil accumulation in limbal vessels and efficient epithelial repair after corneal abrasion. Am J Pathol. 2007;171:838–845. doi: 10.2353/ajpath.2007.070008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shirane J, Nakayama T, Nagakubo D, et al. Corneal epithelial cells and stromal keratocytes efficently produce CC chemokine-ligand 20 (CCL20) and attract cells expressing its receptor CCR6 in mouse herpetic stromal keratitis. Curr Eye Res. 2004;28:297–306. doi: 10.1076/ceyr.28.5.297.28682. [DOI] [PubMed] [Google Scholar]
- 9.Huang LC, Jean D, Proske RJ, Reins RY, McDermott AM. Ocular surface expression and in vitro activity of antimicrobial peptides. Curr Eye Res. 2007;32:595–609. doi: 10.1080/02713680701446653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrescu MS, Larry CL, Bowden RA, et al. Neutrophil interactions with keratocytes during corneal epithelial wound healing: a role for CD18 integrins. Invest Ophthalmol Vis Sci. 2007;48:5023–5029. doi: 10.1167/iovs.07-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin Y, Shen L, Chong EM, et al. The chemokine receptor CCR7 mediates corneal antigen-presenting cell trafficking. Mol Vis. 2007;13:626–634. [PMC free article] [PubMed] [Google Scholar]
- 12.Li Z, Burns AR, Smith CW. Two waves of neutrophil emigration in response to corneal epithelial abrasion: distinct adhesion molecule requirements. Invest Ophthalmol Vis Sci. 2006;47:1947–1955. doi: 10.1167/iovs.05-1193. [DOI] [PubMed] [Google Scholar]
- 13.Lee EJ, Rosenbaum JT, Planck SR. Epifluorescence intravital microscopy of murine corneal dendritic cells. Invest Ophthalmol Vis Sci. 2010;51:2101–2108. doi: 10.1167/iovs.08-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Z, Rumbaut RE, Burns AR, Smith CW. Platelet Response to Corneal Abrasion Is Necessary for Acute Inflammation and Efficient Re-epithelialization. Invest Ophthalmol Vis Sci. 2006;47:4794–4802. doi: 10.1167/iovs.06-0381. [DOI] [PubMed] [Google Scholar]
- 15.Li Z, Burns AR, Han L, Rumbaut RE, Smith CW. IL-17 and VEGF are necessary for efficient corneal nerve regeneration. Amer J Pathol. 2011;178:1106–1116. doi: 10.1016/j.ajpath.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Byeseda SE, Burns AR, Dieffenbaugher S, Rumbaut RE, Smith CW, Li Z. ICAM-1 is necessary for epithelial recruitment of gammadelta T cells and efficient corneal wound healing. Am J Pathol. 2009;175:571–579. doi: 10.2353/ajpath.2009.090112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Z, Burns AR, Byeseda MS, Smith CW. CCL20, {gamma}{delta} T cells, and IL-22 in corneal epithelial healing. FASEB J. 2011;25:2659–2668. doi: 10.1096/fj.11-184804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Z, Burns AR, Smith CW. Lymphocyte function-associated antigen-1-dependent inhibition of corneal wound healing. Am J Pathol. 2006;169:1590–1600. doi: 10.2353/ajpath.2006.060415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Z, Zhao M, Li N, Diaz LA, Mayadas TN. Differential roles for beta2 integrins in experimental autoimmune bullous pemphigoid. Blood. 2006;107:1063–1069. doi: 10.1182/blood-2005-08-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayadas TN, Johnson RC, Rayburn H, Hynes RO, Wagner DD. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell. 1993;74:541–554. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- 21.Haworth O, Cernadas M, Levy BD. NK cells are effectors for resolvin E1 in the timely resolution of allergic airway inflammation. J Immunol. 2011;186:6129–6135. doi: 10.4049/jimmunol.1004007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Q, Smith CW, Zhang W, Burns AR, Li Z. NK Cells Modulate the Inflammatory Response to Corneal Epithelial Abrasion and Thereby Support Wound Healing. Am J Pathol. 2012;181:452–462. doi: 10.1016/j.ajpath.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rutz S, Wang X, Ouyang W. The IL-20 subfamily of cytokines--from host defence to tissue homeostasis. Nat Rev Immunol. 2014;14:783–795. doi: 10.1038/nri3766. [DOI] [PubMed] [Google Scholar]
- 24.Wolk K, Witte E, Warszawska K, et al. The Th17 cytokine IL-22 induces IL-20 production in keratinocytes: a novel immunological cascade with potential relevance in psoriasis. Eur J Immunol. 2009;39:3570–3581. doi: 10.1002/eji.200939687. [DOI] [PubMed] [Google Scholar]
- 25.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 26.Sa SM, Valdez PA, Wu J, et al. The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. J Immunol. 2007;178:2229–2240. doi: 10.4049/jimmunol.178.4.2229. [DOI] [PubMed] [Google Scholar]
- 27.Wolk K, Witte K, Witte E, et al. Maturing dendritic cells are an important source of IL-29 and IL-20 that may cooperatively increase the innate immunity of keratinocytes. J Leukoc Biol. 2008;83:1181–1193. doi: 10.1189/jlb.0807525. [DOI] [PubMed] [Google Scholar]
- 28.Blumberg H, Conklin D, Xu WF, et al. Interleukin 20: discovery, receptor identification, and role in epidermal function. Cell. 2001;104:9–19. doi: 10.1016/s0092-8674(01)00187-8. [DOI] [PubMed] [Google Scholar]
- 29.Chen WY, Chang MS. IL-20 is regulated by hypoxia-inducible factor and up-regulated after experimental ischemic stroke. J Immunol. 2009;182:5003–5012. doi: 10.4049/jimmunol.0803653. [DOI] [PubMed] [Google Scholar]
- 30.Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866–873. doi: 10.1038/nature05663. [DOI] [PubMed] [Google Scholar]
- 31.Otkjaer K, Kragballe K, Funding AT, et al. The dynamics of gene expression of interleukin-19 and interleukin-20 and their receptors in psoriasis. Br J Dermatol. 2005;153:911–918. doi: 10.1111/j.1365-2133.2005.06800.x. [DOI] [PubMed] [Google Scholar]
- 32.Romer J, Hasselager E, Norby PL, Steiniche T, Thorn CJ, Kragballe K. Epidermal overexpression of interleukin-19 and -20 mRNA in psoriatic skin disappears after short-term treatment with cyclosporine a or calcipotriol. J Invest Dermatol. 2003;121:1306–1311. doi: 10.1111/j.1523-1747.2003.12626.x. [DOI] [PubMed] [Google Scholar]
- 33.Kragstrup TW, Greisen SR, Nielsen MA, et al. The interleukin-20 receptor axis in early rheumatoid arthritis: novel links between disease-associated autoantibodies and radiographic progression. Arthritis Res Ther. 2015;18:61. doi: 10.1186/s13075-016-0964-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kragstrup TW, Otkjaer K, Holm C, et al. The expression of IL-20 and IL-24 and their shared receptors are increased in rheumatoid arthritis and spondyloarthropathy. Cytokine. 2008;41:16–23. doi: 10.1016/j.cyto.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 35.Myles IA, Fontecilla NM, Valdez PA, et al. Signaling via the IL-20 receptor inhibits cutaneous production of IL-1beta and IL-17A to promote infection with methicillin-resistant Staphylococcus aureus. Nat Immunol. 2013;14:804–811. doi: 10.1038/ni.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao Y, Li Z, Hassan N, et al. NK cells are necessary for recovery of corneal CD11c+ dendritic cells after epithelial abrasion injury. J Leukoc Biol. 2013;94:343–351. doi: 10.1189/jlb.1212633. [DOI] [PMC free article] [PubMed] [Google Scholar]