Abstract
Objective
Enhanced tissue factor (TF) expression in epithelial ovarian cancer (EOC) is associated with aggressive disease. Our objective was to evaluate the role of the TF-factor VIIa-protease-activated receptor-2 (PAR-2) pathway in human EOC.
Methods
TCGA RNAseq data from EOC databases were analyzed for PAR expression. Cell and microparticle (MP) associated TF protein expression (Western blot) and MP-associated coagulant activity were determined in human EOC (SKOV-3, OVCAR-3 and CaOV-3) and control cell lines. PAR-1 and PAR-2 protein expression were similarly examined. The PAR dependence of VEGF-A release (ELISA) and chemotactic migration in response to FVIIa and cellular proliferation in response to thrombin was evaluated with small molecule antagonists.
Results
Relative mRNA expression consistently demonstrated PAR-2>PAR-1≫PAR-3/4 in multiple EOC datasets. Human EOC cell line lysates confirmed expression of TF, PAR-1 and PAR-2 proteins. MPs isolated from EOC cell lines demonstrated markedly enhanced (4–10 fold) TF coagulant activity relative to control cell lines. FVIIa induced a dose-dependent increase in VEGF-A release (2.5-3 fold) from EOC cell lines that was abrogated by the PAR-2 antagonist ENMD-1068. FVIIa treatment of CaOV-3 and OVCAR-3 cells resulted in increased chemotactic migration that was abolished by ENMD-1068. Thrombin induced dose-dependent EOC cell line proliferation was completely reversed by the PAR-1 antagonist vorapaxar. Small molecule antagonists had no effect on these phenotypes without protease present.
Conclusions
Enhanced activity of the TF-FVIIa-PAR-2 axis may contribute to the EOC progression via PAR-2 dependent signaling that supports an angiogenic and invasive phenotype and local thrombin generation supporting PAR-1 dependent proliferation.
Keywords: Protease activated receptor 2, tissue factor-factor VIIa complex, ovarian cancer, microparticles, vascular endothelial growth factor
Introduction
Epithelial ovarian cancer (EOC) is the fifth leading cause of cancer deaths among women in the United States and the most fatal of all the gynecologic cancers [1]. The increased number of venous thromboembolic (VTE) events at the time of diagnosis, association of VTE with clinically aggressive variants, and adverse impact of VTE on overall survival suggests that systemic hypercoagulability is intrinsic to ovarian cancer biology [2–4]. Despite the importance of the procoagulant phenotype to ovarian cancer biology, the contribution of the hemostatic system to EOC progression is not well understood. The contribution of specific hemostatic factors (including tissue factor, prothrombin, fibrinogen, and platelets) have been established in animal models of hematogenous metastasis [5], but these models are not representative of the natural history of EOC, which primarily spreads along the mesothelium-lined surface of the peritoneal cavity.
Progression of malignancy is often associated with a procoagulant phenotype, characterized by increased tissue factor (TF) expression and enhanced metastasis [5, 6]. Activated factor VII (FVIIa) is the physiologic ligand for TF and formation of the tissue factor-factor VIIa (TF-FVIIa) complex is associated with both activation of blood coagulation and cellular signaling via protease activated receptor-2 (PAR-2) [7]. TF expression is almost uniformly increased in EOC relative to normal ovarian tissues [8]. FVII may be ectopically expressed in ovarian cancer (especially clear cell carcinoma) and further induced by hypoxia [9]. Transfection of human FVII cDNA into an EOC cell line enhances coagulant activity, migration, and Matrigel invasion [10]. Likewise, PAR-2 signaling is associated with a promigratory, invasive, and proangiogenic phenotype in experimental models of breast cancer [11–13]. PAR-2 activation and induction of growth factor/chemokine expression, including prominently vascular endothelial growth factor (VEGF), has been described in renal cell carcinoma and colon cancer cell lines [14, 15]. Thus, the TF/FVIIa/PAR-2 axis may directly contribute to the progression of malignancy.
The protease activated receptor (PAR) family represents a potential mechanistic link between blood coagulation and cancer. Invasive subtypes of ovarian cancer demonstrate broadly enhanced expression of PAR-1 compared to normal ovarian epithelium by immunohistochemistry [16] and matrix metalloproteinase-1 (MMP-1) activation of PAR-1 enhances angiogenesis, ascites formation and metastasis in mouse models of ovarian cancer [17]. In contrast, relatively little is known about PAR-2 in ovarian cancer. Increased PAR-2 expression in primary human ovarian cancer specimens relative to stromal cells has been detected by immunohistochemistry; with higher intensity staining correlating with advanced stage, microvessel density, proliferation, and decreased survival [18]. However, the cellular consequences of PAR-2 activation in EOC have not been described. We hypothesized that TF-FVIIa dependent PAR-2 activation on EOC cells up-regulates expression of inflammatory/angiogenic mediators that enhance vascular leakage, ascites formation, angiogenesis and disease progression. Elevated VEGF concentrations in preoperative serum, ascites and tissue are associated with advanced stage and decreased survival in ovarian cancer patients [19–21]. The goals of this study were to evaluate the global expression of PAR receptors in ovarian cancer and characterize the impact of the TF-FVIIa-PAR-2 pathway on coagulant activity, VEGF release, and migration in human EOC cell lines. The results suggest that the TF/FVIIa/PAR-2 axis may modify the peritoneal microenvironment to enhance progression of ovarian cancer.
Materials and Methods
Materials
Refer to Table S1 for information on Materials and Reagents.
Methods
Refer to Supplemental Methods for a more detailed description of the methods.
PAR-1–4, TF and VEGF expression analysis
The R2: Genomics Analysis and Visualization Platform (R2, http://r2.amc.nl) created by Dr. Jan Koster at the Academic Medical Center Amsterdam, the Netherlands, was used to examine publicly available expression data from human patient samples (serous carcinoma and normal control samples). Transcript expression levels were obtained from Mas5.0 analysis and derived from the R2 datasets. The Affymetrix probe-sets were selected with the R2 software using HUGO. Shown are the absolute expression levels on a log2 scale. Statistical significance for differences in expression was determined using the Student’s t-test. See Tables S2 and S3 (supplementary information) for a list and description of the human patient datasets that were analyzed, along with corresponding sample information, statistics and fold change about each set.
Cell culture
EOC cell-line (SKOV-3, OVCAR-3 and CaOV-3) identification was verified through analysis of short tandem repeat (STR) DNA markers using polymerase chain reaction (PCR) by Dr. Manish Patankar’s laboratory (University of Wisconsin-Madison). Cells were grown in the following media: SKOV-3 in RPMI1640; OVCAR-3 in RPMI1640 containing 10 µg/ml insulin; and CaOV-3, EA.hy926 and HEK 293T in DMEM, with all containing 10% FBS and 0.1 mg/mL P/S. Cell lines were grown in a humidified atmosphere containing 5% CO2 at 37°C. For sub-culturing, treatment with trypsin/EDTA was used. Since trypsin itself activates PAR-type receptors, care was taken to remove all traces of trypsin upon sub-cultivation and prior to treatment, i.e. washing twice with appropriate growth media.
Western Blot
Lysates and appropriate controls were extracted at the indicated times using standard protocols. Lysates were separated by 10% SDS-PAGE, transferred to Immobilon®-FL membrane (Millipore), blocked in milk and membranes incubated with antibody according to manufacturer’s instructions. Horseradish peroxidase-conjugated antibodies were used for detection with Pierce® ECL 2 HRP Western Blot Substrate via film.
Microparticle (MP) isolation and TF activity
EOCs were cultured until confluent, washed with Dulbecco’s Phosphate-Buffered Saline (DPBS) and serum free media added. After 24 hrs, media was centrifuged at 1500×g for 10 min at 4°C. Supernatant was centrifuged at 20,800×g for 10 min to isolate the MP fraction. Supernatant was removed and MPs were washed twice with HBS buffer (10 mM HEPES, 140 mM NaCl), restored to the original volume, aliquoted and snap frozen. TF activity of the MP fraction was measured in a 2-stage factor Xa (FXa) generation assay as described previously [22] in buffer containing 2 mM CaCl2, 5 nM human FVIIa and 100 nM FX for 20 min. After the reaction was stopped by addition of EDTA, S-2765 substrate was added and FXa generation determined by the rate of hydrolysis detected at 405nm on a Spectra Max 340PC plate reader (Molecular Devices) for 5 min. TF-dependence was confirmed by pre-incubation with the inhibitory anti-TF (α-HTF-1) antibody (0.025mg/ml).
Quantification of VEGF-A
VEGF-A concentration in conditioned media from EOC cell lines was determined using a human VEGF-A ELISA kit (Peprotech, Burlington, NC) per the manufacturer’s instructions. EOC cells (5×103 cells) were grown in a 48-well plate for 24 hrs in complete media with serum, followed by serum starvation for at least 6 hrs and then treatment with FVIIa (0–50nM final concentrations). Cell culture supernatants (100 µl) were harvested at indicated times and centrifuged 10 min at 1500×g to remove cellular debris.
Cell proliferation assay
EOC cells were seeded in 6-well plates, SKOV-3 (1×104 cells), OVCAR-3 (3 ×104 cells) and CaOV-3 (3 ×104 cells) in complete media overnight before being serum starved for at least 6 hrs prior to treatment. Specificity of the proliferative response was determined by preincubating serum starved cells with or without 5–50 nM vorapaxar (PAR-1 antagonist) for at least 1 hr prior to thrombin (0.1–1 U/ml) exposure for 48 hrs. Cells were counted with a hemocytometer using trypan blue exclusion both before and after 48 hr treatment.
Migration assay
EOC cells were serum starved overnight, trypsinized, washed, and counted. EOC cells (1–30 × 104 cells) were preincubated with FVIIa (0 or 50 nM) in serum free media within the upper migration chamber (8 µm Membrane transwell insert). After 2 hrs, the serum free media in the lower chamber was replaced with appropriate growth media containing 10% (all cells) or 20% (positive control) FBS and incubated for an additional 6–18 hrs at 37°C in humidified air with 5% CO2. Migration of cells into the lower surface of the membrane was assessed by fixing the cells with ice-cold methanol at room temperature for 30 min and staining with 0.2% crystal violet (Sigma Aldrich, St Louis, MO) for ~5 min. The number of migratory cells was counted in > 4 different fields/well for n ≥ 3 wells. Images of the membrane inserts were obtained using an unmounted Canon Powershot A470 camera on a Hund Wetzlar microscope at × 10 objective (Aph 10/0.25).
Statistical Analysis
Graphs, tables and associated statistics were generated using Microsoft Excel™ 2010 (version 14) (Microsoft Corporation, Redmond, WA), KaleideGraph (version 4.5.) (Synergy Software, Reading, PA) or MSTAT (version 6.1) (McArdle Laboratory for Cancer Research-School of Medicine and Public Health, University of Wisconsin, Madison, WI). Mean values ± SD were calculated (n≥3) and compared for significant differences (p < 0.05) using the unpaired, heteroscedastic Student t-test.
Results
PAR expression in ovarian cancer
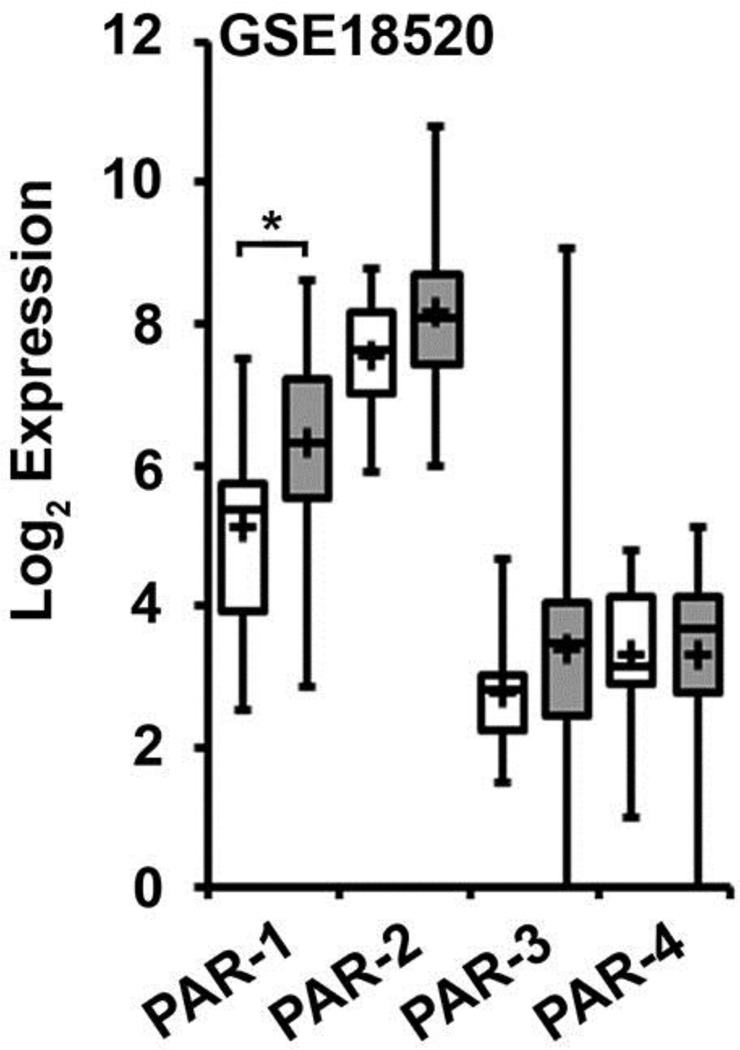
Relative mRNA expression levels for PAR-1–4 in human ovarian cancer tissue were analyzed from available gene expression datasets (Table S2) that employed the Affymetrix Human Genome U133 or U133 Plus 2.0 Array. Three datasets (Mixed Ovarian-Birrer-63-MAS5.0-u133p2 [GSE18520], Tumor Ovarian Serous Cystadenocarcinoma-TCGA-527-MAS5.0-htu133a [GSE68661], and Mixed Ovarian Cancer (CAFs)-Wong-77-MAS5.0-u133p2 [GSE40595]) contained normal ovarian tissue for comparison, while four additional datasets contained only ovarian cancer tissue (Tumor Ovary-EXPO-256-MAS5.0-u133p2 [GSE2109], Tumor Ovarian-Anglesio-90-MAS5.0-u133p2 [GSE12172], Tumor Ovarian-Bowtell-285-MAS5.0-u133p2 [GSE9891], and Tumor Ovarian-Pamula-Pilat-101-MAS5.0-u133p2 [GSE63885]). The Birrer [GSE18520] data set, containing 53 advanced stage high-grade primary ovarian tumors and 10 normal human ovarian surface epithelium (HOSE) samples, is representative of the findings (Fig 1). Additional serous carcinoma datasets are included in supplementary data (Fig S1) as well as a malignant fallopian tube dataset (Fig S3). Elevated levels of PAR-2 and PAR-1 expression were noted in all ovarian cancer tissues with a consistent pattern of PAR mRNA expression across all the datasets: PAR-2 > PAR-1 ≫ PAR-3/PAR-4. Additionally, while PAR-2 expression was similar in ovarian cancer and normal tissues (NT), PAR-1 expression was relatively enhanced in ovarian cancer relative to normal tissue (GSE18520: 2.3-fold increase, p=0.048; GSE40595: 3.51, p=0.002; GSE68661: 4.08, p=0.004). PAR-3 and PAR-4 mRNA expression were undetectable or barely detectable in the majority of the datasets. Thus, PAR-2 and PAR-1 were the predominant PAR subtypes expressed in ovarian cancer tissues.
Figure 1. Gene array analysis of PAR expression in ovarian cancer and human ovarian surface epithelial tissue.
Gene expression data for PAR-1–4 was downloaded from the dataset Mixed Ovarian-Birrer-63-MAS5.0-u133p2[GSE18520], which contains a panel of 53 ovarian cancer tissue and 10 normal ovarian tissue samples evaluated by expression micro-array analysis. All analyses were performed using R2 Genomics Analysis and Visualization Platform, an Affymetrix analysis and visualization platform developed at the Academic Medical Center Amsterdam, the Netherlands. Transcript expression levels were obtained from Mas5.0 analysis and derived from the R2 dataset. The Affymetrix probe-sets were selected using the R2 bio-informatic platform. The unpaired, heteroscedastic Student’s t-test was used to determine significant differences. Shown are the absolute expression levels on a log2 scale. PAR-1–4 expression in normal ovarian tissue (white bars) and ovarian cancer tissue (gray bars) were compared by student’s t-test (* p<0.05).
Cellular and MP-associated TF expression in human EOC cell lines
Genomic comparisons demonstrate pronounced differences between common ovarian cancer cell lines and tumor samples from high grade serous ovarian cancer, the most common ovarian cancer subtype [23]. OVCAR-3 and CaOV-3 cell lines ranked as “possibly” to “likely” high-grade serous subtype, suggesting these cell lines are representative disease models. In contrast, SKOV-3 was ranked “unlikely” related to the high-grade serous subtype and contains wild type TP53 [24].
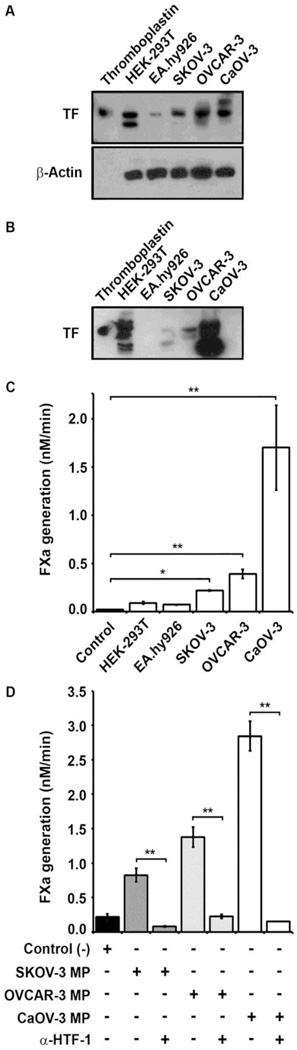
TF antigen expression was evaluated by Western blot analysis of whole cell lysates and MP fractions obtained from control and EOC cell lines. As expected, only modest TF antigen expression was noted in the EA.hy926 endothelial cell line lysate compared to substantial expression in the HEK-293T epithelial cell line with both the 50 kD band consistent with fully glycosylated TF and a lower MW band (Fig 2A).[25] The human EOC cell lines all demonstrated substantial TF antigen expression similar to the control epithelial cell line, with relatively higher levels in OVCAR-3 and CaOV-3 relative to the SKOV-3 line. An additional higher MW band was noted in the CaOV-3 line. MP fractions isolated from control and EOC cell lines demonstrated no detectable TF antigen from the EA.hy926 endothelial cell line, substantial TF antigen from the HEK-293T and CaOV-3 cell lines and modest TF antigen from SKOV-3 and OVCAR-3 cell lines (Fig 2B). TF antigen levels in the MP fractions from the EOC cell lines were roughly similar to the cell lysates (CaOV-3 ≫ OVCAR-3 > SKOV-3). An increased number of lower MW bands were observed in the MP fractions relative to the cell lysates for HEK-293T and the EOC cell lines, suggesting partial proteolytic degradation of TF during MP isolation.
Figure 2. TF expression and MP-associated coagulant activity in epithelial ovarian cancer cell lines.
For Western blots whole cell lysates or MPs were prepared in lysis buffer containing 1% Triton-X 100, clarified by centrifugation and separated by 10% SDS-PAGE. (A–B) TF antigen was detected by immunoblotting (upper panel). (A) A placental-derived TF (Thromboplastin) standard (20ng) was loaded in lane 1 and cell lysates equivalent to 8×104 cells cells/lane were loaded in the remaining 5 lanes for HEK-293T (epithelial) and EA.hy926 (endothelial) cell lines; and human EOC cell lines SKOV-3, OVCAR-3 and CaOV-3. β-actin loading control is shown in the lower panel control. (B) MP fractions derived from each cell line were immunoblotted. Placental-derived TF (Thromboplastin) standard (20ng) was loaded in lane 1. TF coagulant activity of the MP fractions was determined by FX activation assay. (C) The rate of FXa generation (nM/min) by MP isolated from HEK-293T, EA.hy926, SKOV-3, OVCAR-3 and CaOV-3 cell lines is shown as the mean ± SD (n=3). Negative (buffer) control is shown on the left. (D) TF coagulant activity was inhibited by inhibitory anti-human TF antibody (α-HTF-1) versus IgG isotype control. The rate of FXa generation triggered by MP isolated from the human EOC cell lines in the absence or presence of α-HTF-1 (0.025mg/ml) is shown as the mean ± SD (n=3). Addition of α-HTF-1 reduced the rate of FXa generation to background levels for all three EOC cell lines, while the IgG isotype control (0.025mg/mL) had no significant effect. (C and D) Student’s t-test: * p<0.05, ** p<0.001).
MP-associated TF coagulant activity derived from human EOC cell lines
TF coagulant activity was detected by FXa generation in the presence of excess FVIIa for MP fractions derived from control and human EOC cell lines. TF activity levels were determined by comparison to a standard curve for FXa generation established with known TF (human thromboplastin) concentrations. Control MP fractions derived from the EA.hy926 and HEK-293T cell lines demonstrated minimal TF activity over background (FXa generation <0.075 nM/min), despite the substantial TF antigen present in the epithelial cell line (Fig 2C). In contrast, all three human EOC cell lines demonstrated significant TF activity, with rates of FXa generation trending similarly to antigen expression in the MP fractions: CaOV-3 (1.70nM/min) ≫ OVCAR-3 (0.5nM/min) > SKOV-3 (0.25nM/min) (Fig 2C). The TF-dependence of FXa generation was confirmed by preincubation of MP fractions from EOC cell lines with inhibitory anti-TF (α-HTF-1) antibody, which reduced coagulant activity to background levels (Fig 2D). Thus, TF activity roughly correlated with TF antigen present in the human EOC cell lines but was not representative of TF antigen levels in the HEK-293T epithelial cell line control.
Factor VIIa stimulates VEGF-A release from EOC cell lines in a PAR-2 dependent manner
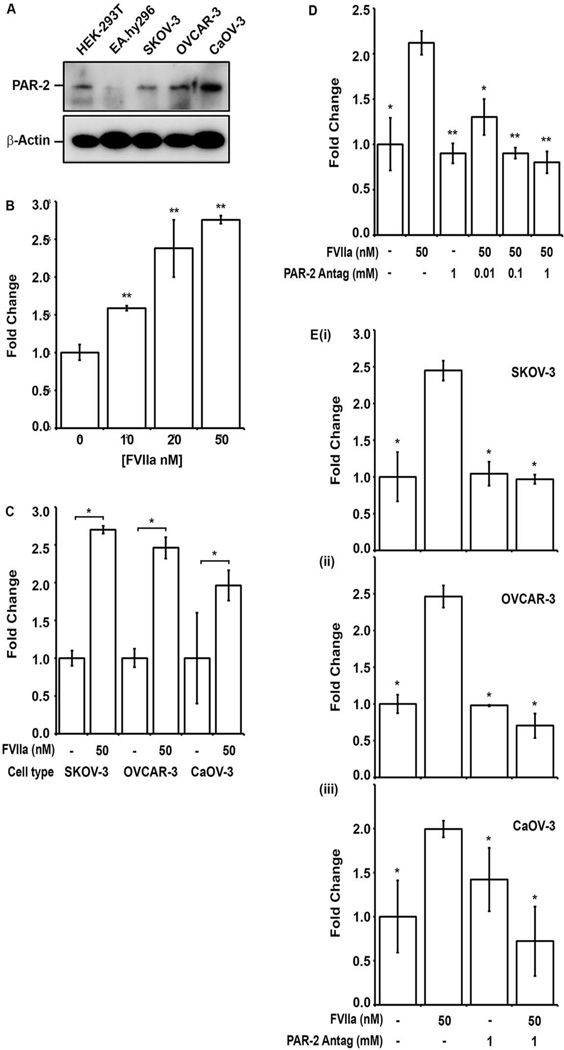
Western blot analysis of whole cell lysates demonstrated PAR-2 expression in the control HEK-293T epithelial cell line and all EOC cell lines, with moderately enhanced antigen levels in the CaOV-3 cell line (Fig 3A). The EA.hy926 endothelial cell line showed negligible PAR-2 expression. Given the co-expression of TF and PAR-2 in the human EOC cell lines, the effect of PAR-2 activation by FVIIa on VEGF-A release was examined. Incubation of serum starved SKOV-3 cells with FVIIa (0–50 nM) demonstrated dose-dependent induction in VEGF-A secretion (Fig 3B), which reached maximal levels after 48–72 hrs. Exposure of OVCAR-3 and CaOV-3 cells to 50 nM FVIIa resulted in a similar 23-fold induction of VEGF-A levels in the cultured media (p<0.05) (Fig 3C) relative to untreated controls at 48 hrs. The PAR-2 dependence of FVIIa induced VEGF release by EOC cell lines was examined using a small molecule PAR-2 antagonist (ENMD-1068) [26]. Preincubation of SKOV-3 cells with 0.01–1 mM ENMD-1068 resulted in dose-dependent reduction of FVIIa-induced VEGF-A release back to control levels (no FVIIa) (Fig 3D). The presence of 1.0 mM ENMD-1068 antagonized the FVIIa-induced increase in VEGF levels back to control levels (p<0.05) similarly for SKOV-3, OVCAR-3 and CaOV-3 cells (Fig 3Ei–iii). In contrast, preincubation with 1.0 mM ENMD-1068 did not significantly reduce VEGF levels for any of the EOC cell lines in the absence of FVIIa (Fig 3Ei–iii) and MTT-based cytotoxicity assays demonstrated the absence of direct toxicity. These results suggest that the FVIIa induced VEGF release by the EOC cell lines was PAR-2 dependent.
Figure 3. FVIIa stimulates VEGF-A release from human EOC cell lines in a PAR-2 dependent manner.
(A) Whole cell lysates prepared from control HEK-293T (epithelial) and EA.hy926 (endothelial) cell lines; and EOC cell lines SKOV-3, OVCAR-3 and CaOV-3 were separated by 10% SDA-PAGE and immunoblotted with anti-PAR-2 (upper panel) and β-actin loading controls (lower panel). (B) Relative VEGF-A concentration in serum free media 48 hours after exposure of SKOV-3 cell line to FVIIa (0–50 nM) was quantified. VEGF-A was detected by ELISA and expressed as fold increase over baseline. (C) Comparison of fold increase in VEGF-A concentration in response to FVIIa (50nM) for EOC cell lines SKOV-3, OVCAR-3 and CaOV-3 at 48 hr (first bar- basal levels). (D) Dose dependent inhibition of FVIIa-induced VEGF-A release at 48 hr in SKOV-3 cells preincubated with the small molecule PAR-2 antagonist ENMD-1068 (0.01–1 mM). (E) Inhibition of FVIIa induced VEGF-A release in (i) SKOV-3, (ii) OVCAR-3 and (iii) CaOV-3 cells at 48 hrs by preincubation with 1 mM PAR-2 antagonist (ENMD-1608). All results are expressed as the mean fold increase in VEGF-A concentration (n=3) with error bars representing ± S.D. (* p<0.05, ** p<0.001).
Thrombin induces proliferation of EOC cell lines in a PAR-1 dependent manner
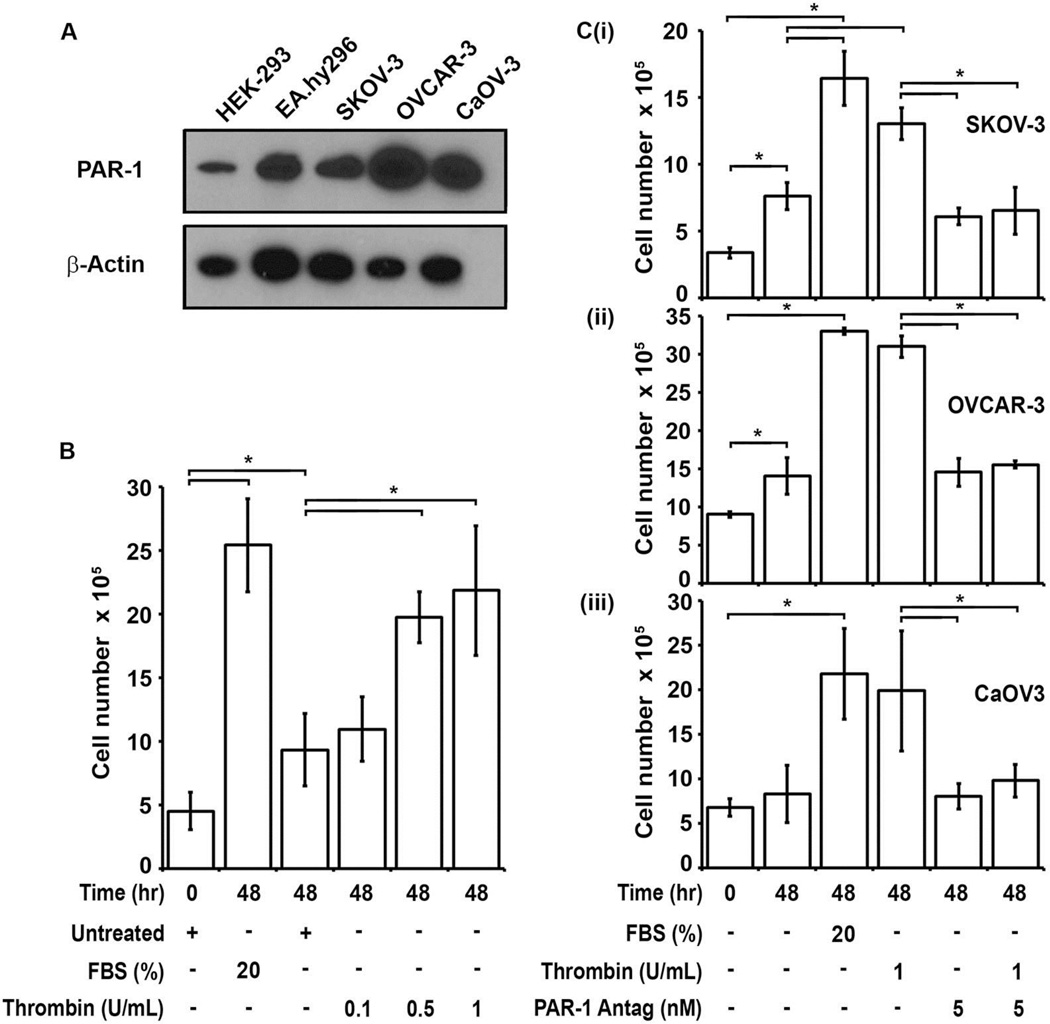
In addition to signaling through PAR-2 activation, the TF-FVIIa complex also activates FX, ultimately resulting in thrombin generation in vivo. Thrombin, the penultimate product of the coagulation pathway, may also induce cellular proliferation via PAR-1 activation. Western blot analysis of whole cell lysates showed ubiquitous PAR-1 expression in control and EOC cell lines (Fig 4A). Exposure of serum-starved SKOV-3 cells to thrombin (0.1–1 U/ml) or 20% FBS (positive control) induced dose-dependent cellular proliferation relative to untreated cells (Fig 4B). Likewise, thrombin (1 U/ml) induced proliferation of OVCAR-3 and CaOV-3 cell lines to levels comparable with the 20% FBS control (Fig 4Cii–iii). The magnitude of the thrombin response relative to untreated cells was modestly dampened for SKOV-3 due to continued growth of this cell line under serum free conditions. To assess the PAR-1 dependence of the thrombin-induced proliferation in these cell lines, the small molecule PAR-1 antagonist vorapaxar (5 nM) was employed. Preincubation of all three EOC cell lines with vorapaxar (5 nM) reduced thrombin induced cellular proliferation back to basal levels (Fig 4Ci–iii). In the absence of thrombin, vorapaxar had no effect on baseline levels of cellular proliferation. These results suggest that thrombin induced proliferation of the EOC cell lines was PAR-1- dependent.
Figure 4. Thrombin induces proliferation of EOC cell lines in a PAR-1 dependent manner.
(A) PAR-1 was detected by western blot analysis of whole cell lysates prepared from control HEK-293T epithelial and EA.hy926 endothelial cell lines; and the human EOC cell lines SKOV-3, OVCAR-3 and CaOV-3. Cell lysates equivalent to 8×104 cells / lane were separated by 10% SDS-PAGE and immunoblotted for PAR-1. β-actin loading controls are shown in the lower panel. (B) Dose-dependent proliferation of serum starved SKOV-3 cells in response to thrombin. Mean cell numbers at 0 hr (left column) and 48 hr are shown for 20% FBS (positive control), no treatment (negative control), and increasing doses of thrombin (0.1–1.0 U/ml). (C) Inhibition of thrombin-induced proliferation in (i) SKOV-3, (ii) OVCAR-3 and (iii) CaOV-3 cells at 48 hrs by preincubation with the small molecule PAR-1 antagonist vorapaxar (5 nM). All results are expressed as mean cell number (n=3) with error bars representing ± SD (* p<0.05).
Factor VIIa induces EOC migration in a PAR-2 dependent manner
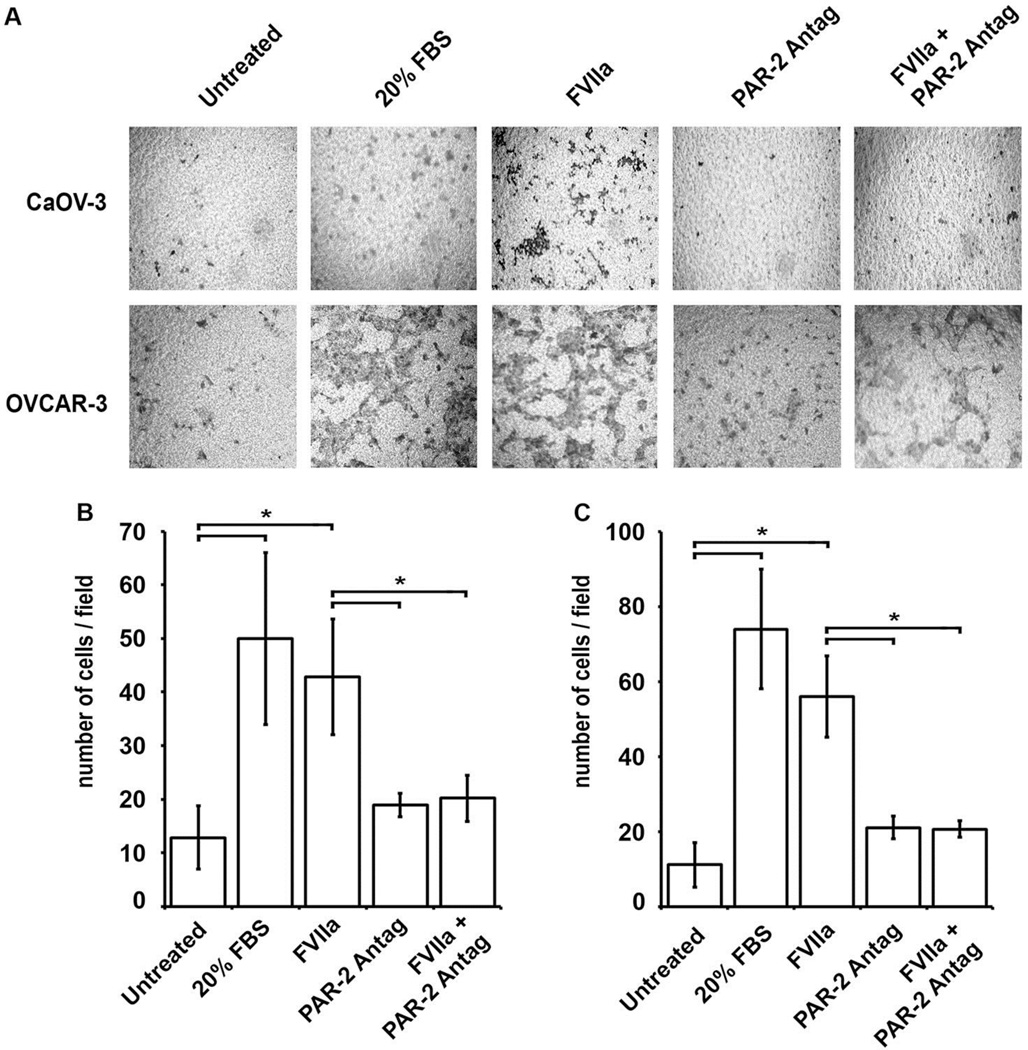
The ability of FVIIa to enhance the migratory properties of the high grade serous subtype related EOC cell lines, CaOV-3 and OVCAR-3, was assessed in a trans-well assay. Serum starved cells were pretreated with 50 nM FVIIa for 2 hrs in the upper chamber of the transwell with serum free media in the bottom chamber. At the end of the incubation, the bottom chamber was replaced with 10% or 20% (positive control) FBS containing media and migration toward the serum-containing chamber was assessed. Preincubation of CaOV-3 (Fig 5A & B) and OvCAR-3 (Fig 5A & C) cell lines with 50 nM FVIIa significantly enhanced migration toward the 10% FBS-containing lower chamber relative to untreated cells. In the presence of the PAR-2 antagonist ENMD-1068 (1 mM), the FVIIa-induced increase in cellular migration was reduced to basal levels for both CaOV-3 and OvCAR-3 cell lines. In contrast, without FVIIa present, ENMD-1068 had no effect on EOC cell migration. These results suggest that FVIIa-induced migration in these EOC cell lines was PAR-2 dependent.
Figure 5. FVIIa induction of EOC migration is PAR-2 dependent.
The effect of pretreatment of serum starved CaOV-3 and OVCAR-3 cells with FVIIa (50 nM) on cellular migration towards 10% serum was analyzed in a trans-well migration assay. (A) Representative images of CaOV-3 and OvCAR-3 cell migration across the insert membrane after 6 hr or 18 hr incubation, respectively, are shown: untreated (negative control), 20% FBS (positive control), 50 nM FVIIa, 1.0 mM PAR-2 antagonist, and 50 nM FVIIa plus 1.0 mM PAR-2 antagonist. Mean migrated cell counts for (B) CaOV-3 and (C) OvCAR-3, corresponding to upper and lower panels in (A), respectively: untreated, 20% FBS, and FVIIa (50 nM) treated cells and inhibition of cell migration after 2 hr pretreatment with 1.0 mM PAR-2 antagonist, and a 6hr or 18 hr incubation, respectively. All results are expressed as mean cell number (n=3) with error bars representing ± SD (*p<0.05).
Discussion
EOC is highly sensitive to initial platinum-based chemotherapy; however, re-growth of the cancer occurs in the vast majority of patients (up to 80%), often with development of platinum-resistant disease with no widely accepted salvage therapy [27]. While enhanced TF expression has been associated with the “angiogenic switch” and proclivity for hematogenous metastasis [28], the contribution(s) of a procoagulant phenotype to intra-peritoneal progression of ovarian cancer is not well understood. The protease activated receptor (PAR) family represents a potential mechanistic link between the hypercoagulable phenotype and disease progression. Increased PAR-2 expression levels in advanced EOC are associated with reduced survival, but the contribution of the TF-FVIIa-PAR-2 axis to cellular mechanisms for EOC progression has not been examined [18]. We have undertaken: 1) a global analysis of PAR family mRNA expression in ovarian cancer databases, and 2) characterization of the TF-FVIIa-PAR-2 and PAR-1 effects on cellular mechanisms of growth factor release, cellular migration and proliferation in human EOC cell lines. These results demonstrate that human epithelial ovarian cancer samples predominantly express PAR-2 and PAR-1 mRNA, and human EOC cell lines exhibit enhanced TF coagulant activity, PAR-2 dependent VEGF release and chemotactic migration and PAR-1 dependent cellular proliferation.
Evaluation of seven human ovarian cancer gene expression databases consistently demonstrated the highest expression levels for PAR-2, followed closely by PAR-1, with minimal or undetectable expression of PAR-3 and PAR-4 (Figs 1 and S1). In databases that contained normal human ovarian surface epithelium for comparison, PAR-2 mRNA expression levels appeared similar but PAR-1 expression was relatively enhanced in the EOC tissue. Consistent with this observation, abundant PAR-1 protein expression has been reported in both borderline (low malignant potential) tumors and invasive ovarian cancer, while normal ovarian epithelium does not express detectable PAR-1 [16]. Similarly, passage of the human ovarian cancer cell line OVCAR-4 in a mouse xenograft model resulted in a 2.5-fold increase in surface PAR-1 expression [17]. Examination of cell lysates demonstrated ubiquitous expression of PAR-1 protein in control and EOC cell lines (Fig 4A) and significant PAR-2 protein in both the epithelial cell line (HEK-293T) and human EOC cell lines, modestly enhanced in CaOV-3 (Fig 3A). Semi-quantitative immunohistochemical detection of PAR-2 expression levels in ovarian cancer specimens has likewise been associated with advanced stage, microvessel density, proliferation, and decreased survival in ovarian cancer [18]. Thus, both mRNA and protein expression data are consistent with roles for PAR-1 and PAR-2 in ovarian cancer biology.
As noted for other solid tumors, increased TF expression (relative to normal ovarian tissue) and increased serum TF antigen are associated with reduced survival in ovarian cancer [8, 29]. Further, ectopic expression of FVII enhances the migratory/invasive and procoagulant properties of ovarian cancer cells, suggesting a role for the TF-FVIIa complex [9, 10]. TF antigen expression in cell lysates and cell-derived MP fractions demonstrated 4–5 fold higher expression in the epithelial cell lines (both control HEK-293T and EOC lines) relative to the endothelial cell line (EA.hy926) (Fig 2A–B). However, only the human EOC cell lines demonstrated significantly enhanced TF-dependent coagulant activity in their MP fractions (Fig 2C–D). TF undergoes post-translational N-glycosylation at 3 sites in the extracellular domain, however, none of these sites are essential for coagulant activity, PAR-2 signaling or surface expression [25]. The discrepancy between TF antigen expression and activity suggests that MP-associated TF from HEK-293T cells is partially encrypted relative to MP-TF from the EOC cell lines. Reduced phosphatidylserine exposure, inhibition of TF-FVIIa by membrane bound TFPI, or conformational regulation of TF via labile disulfide “switching” may contribute to encryption of TF activity [30, 31]. Disruption of TF regulatory mechanisms in EOC may contribute to an enhanced procoagulant phenotype relative to normal epithelium.
The procoagulant activity of the TF-FVIIa complex on EOC cells and their respective MP likely contributes to local thrombin generation. Thrombin-like activity has been reported in ovarian cancer ascites [32], suggesting the potential relevance of thrombin-dependent responses in the peritoneal microenvironment. Thrombin induced dose-dependent cellular proliferation of all three human EOC cells lines was abrogated by preincubation with vorapaxar, suggesting these responses were dependent on PAR-1 (Fig 4B–C). In addition to procoagulant activity, TF-FVIIa can also activate PAR-2 dependent intracellular signaling pathways [7]. FVIIa induced dose-dependent VEGF-A release from all three human EOC cell lines at 48 hrs (Fig 3B–C) that was completely inhibited by preincubation with ENMD-1068, suggesting growth factor release was dependent on PAR-2 (Fig 3D–E) [26]. VEGF-A is an important angiogenic factor in a broad array of malignancies that plays a critical role in ascites formation in EOC [33]. Consistent with these findings, VEGF-A mRNA levels were significantly elevated in EOC tissue samples compared to normal ovarian tissue in 2 of 3 datasets (Fig S2). Enhanced migration/invasion is another important characteristic of invasive cancer cells. FVIIa triggered chemotactic migration of the OVCAR-3 (Fig 5A–B) and CaOV-3 (Fig 5A,C) cell lines towards 10% serum that was similarly inhibited by ENMD-1068. Thus, FVIIa induced both PAR-2 dependent VEGF-A release and chemotactic migration in the OVCAR-3 and CaOV-3 cell lines.
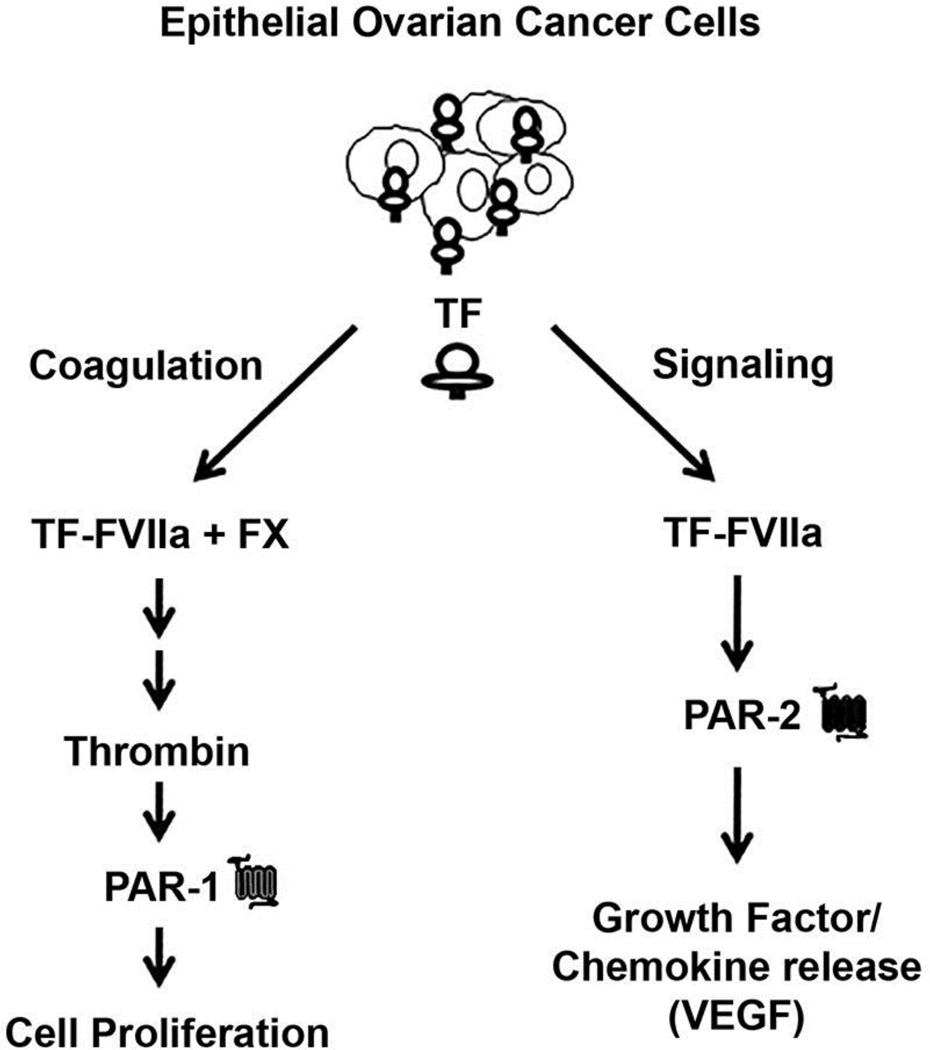
The potential impact of the TF-FVIIa-PAR-2 pathway on the tumor microenvironment has been increasingly recognized [7]. PAR-2 expression/activation has been described in a number of human epithelial malignancies [14, 15, 34] and triggers growth factor/chemokine release from malignant cells, including IL-6 [14], IL-8 [12], and VEGF [13]. PAR-2 activation in EOC may similarly impact the peritoneal microenvironment [35]. The potential role of coagulation proteases in the intra-peritoneal EOC progression is depicted (Fig 6). Increased TF expression enhances TF-FVIIa complex formation on the EOC cell, which triggers: 1) local thrombin generation (coagulation arm) and 2) PAR-2 activation (signaling arm). Thrombin generation in the peritoneal space triggers PAR-1 dependent cellular proliferation while TF-FVIIa directly triggers PAR-2 dependent growth factor/chemokine release that facilitates tumor migration/invasion, angiogenesis and ascites formation. Local thrombin generation in the peritoneal cavity is likely given the substantial presence of coagulation zymogens (10–60% plasma activity) in malignant effusions and the thrombin-like activity reported in ovarian cancer ascites [32, 36]. Likewise, increased IL-8 expression [20, 37] and elevated levels of IL-6 or VEGF in serum and ascites are associated with decreased survival in EOC patients [38–40]. Disruption of these PAR-dependent pathways represents an attractive adjunctive approach for preventing EOC relapse/regrowth in the peritoneal cavity that may exhibit limited toxicity relative to chemotherapy. Upstream targeting of PAR-2 activation may concomitantly down-regulate the release of multiple growth factors with adverse prognostic implications in EOC, which may prove more effective in prolonging progression-free survival than current anti-VEGF therapy.
Figure 6. Proposed model of the TF-FVIIa-PAR-2 axis.
Formation of TF-FVIIa complex (on TF-containing MP) triggers thrombin generation via the extrinsic coagulation pathway and PAR-2 signaling in EOC cells. Local thrombin generation activates PAR-1 on EOC, triggering cellular proliferation. Direct PAR-2 activation by TF-FVIIa results in VEGF-A (and other growth factor) release by EOC cells, promoting ascites formation, angiogenesis and tumor migration/invasion.
Supplementary Material
Gene array analysis of PAR-1–4 expression in ovarian cancer and human ovarian surface epithelial tissue was performed using the following mRNA expression datasets: (A) Mixed Ovarian Cancer (CAFs)-Wong-77-MAS5.0-u133p2 [GSE40595], which contains a panel of 32 ovarian cancer tissue and 6 normal ovarian tissue samples (B) Tumor Ovarian Serous Cystadenocarcinoma-TCGA-527-MAS5.0-htu133a [GSE68661], which contains a panel of 509 ovarian cancer tissue and 10 normal ovarian tissue samples, (C) Tumor Ovary-EXPO-256-MAS5.0-u133p2 [GSE2109],which contains a panel of 91 ovarian cancer tissue, (D) Tumor Ovarian-Bowtell-285-MAS5.0-u133p2 [GSE9891], which contains a panel of 243 ovarian cancer tissue (E) Tumor Ovarian-Anglesio-90-MAS5.0-u133p2 [GSE12172], which contains a panel of 74 ovaruian cancer tissue and (F) Tumor Ovarian-Pamuła-Piłat-101-MAS5.0-u133p2 [GSE63885], which contains a panel of 73 ovarian cancer tissues. All analyses were performed using R2: Genomics Analysis and Visualiztion Platform, an Affymetrix analysis and visualization platform developed in the Department of Oncogenomics at the Academic Medical Center at the University of Amsterdam. Transcript expression levels were obtained from Mas5.0 analysis and derived from the R2 dataset. The Affymetrix probe-sets were selected using the R2 bio-informatic platform. The unpaired, heteroscedastic, Student’s t-test was used to determine significant differences. Shown are the absolute expression levels on a log2 scale. PAR-1–4 expression in normal ovarian tissue (white bars) and ovarian cancer tissue (gray bars) were compared (* p<0.009).
Gene array analysis of VEGFA expression in ovarian cancer and human ovarian surface epithelial tissue was performed using the following mRNA expression datasets: (A) Mixed Ovarian-Birrer-63-MAS5.0–u133p2 [GSE18520], which contains a panel of 53 ovarian cancer tissue and 10 normal ovarian tissue samples, (B) Mixed Ovarian Cancer (CAFs)-Wong-77-MAS5.0-u133p2 [GSE40595], which contains a panel of 32 ovarian cancer tissue and 6 normal ovarian tissue samples, and (C) Tumor Ovarian Serous Cystadenocarcinoma-TCGA-527-MAS5.0-htu133a [GSE68661], which contains a panel of 509 ovarian cancer tissue and 10 normal ovarian tissue samples. All analyses were performed using R2: Genomics Analysis and Visualiztion Platform, an Affymetrix analysis and visualization platform developed at the Department of Oncogenomics at the Academic Medical Center at the University of Amsterdam, the Netherlands. Transcript expression levels were obtained from Mas5.0 analysis and derived from the R2 dataset. The Affymetrix probe-sets were selected using the R2 bioinformatic platform. The unpaired, heteroscedastic Student’s t-test was used to determine significant differences. Shown are the absolute expression levels on a log2 scale. VEGFA expression in normal ovarian tissue (white bars) and ovarian cancer tissue (gray bars) were compared (* p≤0.01 ).
Highlights.
PAR-1 and PAR-2 expression predominate in human epithelial ovarian cancer (EOC)
PAR-2 activation enhances angiogenic and migratory properties of EOC cells
The TF-FVIIa-PAR-2 axis promotes EOC spread by modification of the microenvironment
Acknowledgments
The authors would like to thank Dr. Manish Pantakar at the University of Wisconsin for providing EOC cell lines (SKOV-3, OVCAR-3 and CaOV-3). This work was in part supported by research grants: NIH T32 HL007899 to AC; UL1TR000427 from the Clinical and Translational Science Award (CTSA) program of the National Center for Advancing Translational Science (NCATS) and the University of Wisconsin Carbone Cancer Center Cancer Center Support Grant P30 CA014520 to IMO; and a University of Wisconsin Carbone Cancer Center Investigator-initiated Pilot Project Grant to JPS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Conflict of Interests
The authors declare that there are no conflicts of interest.
References
- 1.Group, U.S.C.S.W. United States Cancer Statistics: 1999–2012 Incidence and Mortality Web-based Report. 2015 Available from: http://www.cdc.gov/uscs.
- 2.Satoh T, Oki A, Uno K, Sakurai M, Ochi H, Okada S, et al. High incidence of silent venous thromboembolism before treatment in ovarian cancer. British journal of cancer. 2007;97:1053–1057. doi: 10.1038/sj.bjc.6603989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsuura Y, Robertson G, Marsden DE, Kim SN, Gebski V, Hacker NF. Thromboembolic complications in patients with clear cell carcinoma of the ovary. Gynecologic oncology. 2007;104:406–410. doi: 10.1016/j.ygyno.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez AO, Wun T, Chew H, Zhou H, Harvey D, White RH. Venous thromboembolism in ovarian cancer. Gynecologic oncology. 2007;105:784–790. doi: 10.1016/j.ygyno.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 5.Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, et al. Tumor cell-associated tissue factor and circulating hemostatic factors cooperate to increase metastatic potential through natural killer cell-dependent and-independent mechanisms. Blood. 2007;110:133–141. doi: 10.1182/blood-2007-01-065995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu JL, May L, Lhotak V, Shahrzad S, Shirasawa S, Weitz JI, et al. Oncogenic events regulate tissue factor expression in colorectal cancer cells: implications for tumor progression and angiogenesis. Blood. 2005;105:1734–1741. doi: 10.1182/blood-2004-05-2042. [DOI] [PubMed] [Google Scholar]
- 7.Schaffner F, Ruf W. Tissue factor and PAR2 signaling in the tumor microenvironment. Arterioscler Thromb Vasc Biol. 2009;29:1999–2004. doi: 10.1161/ATVBAHA.108.177428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cocco E, Varughese J, Buza N, Bellone S, Lin KY, Bellone M, et al. Tissue factor expression in ovarian cancer: implications for immunotherapy with hI-con1, a factor VII-IgGF(c) chimeric protein targeting tissue factor. Clin Exp Metastasis. 2011;28:689–700. doi: 10.1007/s10585-011-9401-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yokota N, Koizume S, Miyagi E, Hirahara F, Nakamura Y, Kikuchi K, et al. Self-production of tissue factor-coagulation factor VII complex by ovarian cancer cells. British journal of cancer. 2009;101:2023–2029. doi: 10.1038/sj.bjc.6605406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koizume S, Jin MS, Miyagi E, Hirahara F, Nakamura Y, Piao JH, et al. Activation of cancer cell migration and invasion by ectopic synthesis of coagulation factor VII. Cancer research. 2006;66:9453–9460. doi: 10.1158/0008-5472.CAN-06-1803. [DOI] [PubMed] [Google Scholar]
- 11.Versteeg HH, Schaffner F, Kerver M, Ellies LG, Andrade-Gordon P, Mueller BM, et al. Protease-activated receptor (PAR) 2, but not PAR1, signaling promotes the development of mammary adenocarcinoma in polyoma middle T mice. Cancer research. 2008;68:7219–7227. doi: 10.1158/0008-5472.CAN-08-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hjortoe GM, Petersen LC, Albrektsen T, Sorensen BB, Norby PL, Mandal SK, et al. Tissue factor-factor VIIa-specific up-regulation of IL-8 expression in MDA-MB-231 cells is mediated by PAR-2 and results in increased cell migration. Blood. 2004;103:3029–3037. doi: 10.1182/blood-2003-10-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, Mueller BM. Protease-activated receptor-2 regulates vascular endothelial growth factor expression in MDA-MB-231 cells via MAPK pathways. Biochem Biophys Res Commun. 2006;344:1263–1270. doi: 10.1016/j.bbrc.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, Wang W, Mize GJ, Takayama TK, True LD, Vessella RL. Protease-activated receptor 2 signaling upregulates angiogenic growth factors in renal cell carcinoma. Experimental and molecular pathology. 2013;94:91–97. doi: 10.1016/j.yexmp.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Zhang C, Gao GR, Lv CG, Zhang BL, Zhang ZL, Zhang XF. Protease-activated receptor-2 induces expression of vascular endothelial growth factor and cyclooxygenase-2 via the mitogen-activated protein kinase pathway in gastric cancer cells. Oncol Rep. 2012;28:1917–1923. doi: 10.3892/or.2012.1998. [DOI] [PubMed] [Google Scholar]
- 16.Grisaru-Granovsky S, Salah Z, Maoz M, Pruss D, Beller U, Bar-Shavit R. Differential expression of protease activated receptor 1 (Par1) and pY397FAK in benign and malignant human ovarian tissue samples. Int J Cancer. 2005;113:372–378. doi: 10.1002/ijc.20607. [DOI] [PubMed] [Google Scholar]
- 17.Agarwal A, Covic L, Sevigny LM, Kaneider NC, Lazarides K, Azabdaftari G, et al. Targeting a metalloprotease-PAR1 signaling system with cell-penetrating pepducins inhibits angiogenesis, ascites, and progression of ovarian cancer. Mol Cancer Ther. 2008;7:2746–2757. doi: 10.1158/1535-7163.MCT-08-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jahan I, Fujimoto J, Alam SM, Sato E, Sakaguchi H, Tamaya T. Role of protease activated receptor-2 in tumor advancement of ovarian cancers. Ann Oncol. 2007;18:1506–1512. doi: 10.1093/annonc/mdm190. [DOI] [PubMed] [Google Scholar]
- 19.Hefler LA, Zeillinger R, Grimm C, Sood AK, Cheng WF, Gadducci A, et al. Preoperative serum vascular endothelial growth factor as a prognostic parameter in ovarian cancer. Gynecologic oncology. 2006;103:512–517. doi: 10.1016/j.ygyno.2006.03.058. [DOI] [PubMed] [Google Scholar]
- 20.Kassim SK, El-Salahy EM, Fayed ST, Helal SA, Helal T, Azzam Eel D, et al. Vascular endothelial growth factor and interleukin-8 are associated with poor prognosis in epithelial ovarian cancer patients. Clin Biochem. 2004;37:363–369. doi: 10.1016/j.clinbiochem.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 21.Liang B, Guo Z, Li Y, Liu C. Elevated VEGF concentrations in ascites and serum predict adverse prognosis in ovarian cancer. Scand J Clin Lab Invest. 2013 doi: 10.3109/00365513.2013.773593. [DOI] [PubMed] [Google Scholar]
- 22.Wang JG, Manly D, Kirchhofer D, Pawlinski R, Mackman N. Levels of microparticle tissue factor activity correlate with coagulation activation in endotoxemic mice. J Thromb Haemost. 2009;7:1092–1098. doi: 10.1111/j.1538-7836.2009.03448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Domcke S, Sinha R, Levine DA, Sander C, Schultz N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nature communications. 2013;4:2126. doi: 10.1038/ncomms3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaw TJ, Senterman MK, Dawson K, Crane CA, Vanderhyden BC. Characterization of intraperitoneal, orthotopic, and metastatic xenograft models of human ovarian cancer. Mol Ther. 2004;10:1032–1042. doi: 10.1016/j.ymthe.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 25.Kothari H, Rao LV, Pendurthi UR. Glycosylation of tissue factor is not essential for its transport or functions. J Thromb Haemost. 2011;9:1511–1520. doi: 10.1111/j.1538-7836.2011.04332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelso EB, Lockhart JC, Hembrough T, Dunning L, Plevin R, Hollenberg MD, et al. Therapeutic promise of proteinase-activated receptor-2 antagonism in joint inflammation. J Pharmacol Exp Ther. 2006;316:1017–1024. doi: 10.1124/jpet.105.093807. [DOI] [PubMed] [Google Scholar]
- 27.Vergote IB, Garcia A, Micha J, Pippitt C, Bendell J, Spitz D, et al. Randomized multicenter phase II trial comparing two schedules of etirinotecan pegol (NKTR-102) in women with recurrent platinum-resistant/refractory epithelial ovarian cancer. J Clin Oncol. 2013;31:4060–4066. doi: 10.1200/JCO.2012.45.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mueller BM, Reisfeld RA, Edgington TS, Ruf W. Expression of tissue factor by melanoma cells promotes efficient hematogenous metastasis. Proc Natl Acad Sci U S A. 1992;89:11832–11836. doi: 10.1073/pnas.89.24.11832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han LY, Landen CN, Jr, Kamat AA, Lopez A, Bender DP, Mueller P, et al. Preoperative serum tissue factor levels are an independent prognostic factor in patients with ovarian carcinoma. J Clin Oncol. 2006;24:755–761. doi: 10.1200/JCO.2005.02.9181. [DOI] [PubMed] [Google Scholar]
- 30.Chen VM, Hogg PJ. Encryption and decryption of tissue factor. J Thromb Haemost. 2013;11(Suppl 1):277–284. doi: 10.1111/jth.12228. [DOI] [PubMed] [Google Scholar]
- 31.Rao LV, Kothari H, Pendurthi UR. Tissue factor encryption and decryption: facts and controversies. Thromb Res. 2012;129(Suppl 2):S13–S17. doi: 10.1016/j.thromres.2012.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naldini A, Morena E, Belotti D, Carraro F, Allavena P, Giavazzi R. Identification of thrombin-like activity in ovarian cancer associated ascites and modulation of multiple cytokine networks. Thromb Haemost. 2011;106:705–711. doi: 10.1160/TH11-05-0311. [DOI] [PubMed] [Google Scholar]
- 33.Mesiano S, Ferrara N, Jaffe RB. Role of vascular endothelial growth factor in ovarian cancer: inhibition of ascites formation by immunoneutralization. The American journal of pathology. 1998;153:1249–1256. doi: 10.1016/S0002-9440(10)65669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang LH, Pan SL, Lai CY, Tsai AC, Teng CM. Activated PAR-2 regulates pancreatic cancer progression through ILK/HIF-alpha-induced TGF-alpha expression and MEK/VEGF-A-mediated angiogenesis. The American journal of pathology. 2013;183:566–575. doi: 10.1016/j.ajpath.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 35.Matte I, Lane D, Laplante C, Rancourt C, Piche A. Profiling of cytokines in human epithelial ovarian cancer ascites. American journal of cancer research. 2012;2:566–580. [PMC free article] [PubMed] [Google Scholar]
- 36.Gieseler F, Luhr I, Kunze T, Mundhenke C, Maass N, Erhart T, et al. Activated coagulation factors in human malignant effusions and their contribution to cancer cell metastasis and therapy. Thromb Haemost. 2007;97:1023–1030. [PubMed] [Google Scholar]
- 37.Uslu R, Sanli UA, Dikmen Y, Karabulut B, Ozsaran A, Sezgin C, et al. Predictive value of serum interleukin-8 levels in ovarian cancer patients treated with paclitaxel-containing regimens. Int J Gynecol Cancer. 2005;15:240–245. doi: 10.1111/j.1525-1438.2005.15210.x. [DOI] [PubMed] [Google Scholar]
- 38.Stone RL, Nick AM, McNeish IA, Balkwill F, Han HD, Bottsford-Miller J, et al. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med. 2012;366:610–618. doi: 10.1056/NEJMoa1110352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li L, Wang L, Zhang W, Tang B, Zhang J, Song H, et al. Correlation of serum VEGF levels with clinical stage, therapy efficacy, tumor metastasis and patient survival in ovarian cancer. Anticancer Res. 2004;24:1973–1979. [PubMed] [Google Scholar]
- 40.Rudlowski C, Pickart AK, Fuhljahn C, Friepoertner T, Schlehe B, Biesterfeld S, et al. Prognostic significance of vascular endothelial growth factor expression in ovarian cancer patients: a long-term follow-up. Int J Gynecol Cancer. 2006;16(Suppl 1):183–189. doi: 10.1111/j.1525-1438.2006.00307.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gene array analysis of PAR-1–4 expression in ovarian cancer and human ovarian surface epithelial tissue was performed using the following mRNA expression datasets: (A) Mixed Ovarian Cancer (CAFs)-Wong-77-MAS5.0-u133p2 [GSE40595], which contains a panel of 32 ovarian cancer tissue and 6 normal ovarian tissue samples (B) Tumor Ovarian Serous Cystadenocarcinoma-TCGA-527-MAS5.0-htu133a [GSE68661], which contains a panel of 509 ovarian cancer tissue and 10 normal ovarian tissue samples, (C) Tumor Ovary-EXPO-256-MAS5.0-u133p2 [GSE2109],which contains a panel of 91 ovarian cancer tissue, (D) Tumor Ovarian-Bowtell-285-MAS5.0-u133p2 [GSE9891], which contains a panel of 243 ovarian cancer tissue (E) Tumor Ovarian-Anglesio-90-MAS5.0-u133p2 [GSE12172], which contains a panel of 74 ovaruian cancer tissue and (F) Tumor Ovarian-Pamuła-Piłat-101-MAS5.0-u133p2 [GSE63885], which contains a panel of 73 ovarian cancer tissues. All analyses were performed using R2: Genomics Analysis and Visualiztion Platform, an Affymetrix analysis and visualization platform developed in the Department of Oncogenomics at the Academic Medical Center at the University of Amsterdam. Transcript expression levels were obtained from Mas5.0 analysis and derived from the R2 dataset. The Affymetrix probe-sets were selected using the R2 bio-informatic platform. The unpaired, heteroscedastic, Student’s t-test was used to determine significant differences. Shown are the absolute expression levels on a log2 scale. PAR-1–4 expression in normal ovarian tissue (white bars) and ovarian cancer tissue (gray bars) were compared (* p<0.009).
Gene array analysis of VEGFA expression in ovarian cancer and human ovarian surface epithelial tissue was performed using the following mRNA expression datasets: (A) Mixed Ovarian-Birrer-63-MAS5.0–u133p2 [GSE18520], which contains a panel of 53 ovarian cancer tissue and 10 normal ovarian tissue samples, (B) Mixed Ovarian Cancer (CAFs)-Wong-77-MAS5.0-u133p2 [GSE40595], which contains a panel of 32 ovarian cancer tissue and 6 normal ovarian tissue samples, and (C) Tumor Ovarian Serous Cystadenocarcinoma-TCGA-527-MAS5.0-htu133a [GSE68661], which contains a panel of 509 ovarian cancer tissue and 10 normal ovarian tissue samples. All analyses were performed using R2: Genomics Analysis and Visualiztion Platform, an Affymetrix analysis and visualization platform developed at the Department of Oncogenomics at the Academic Medical Center at the University of Amsterdam, the Netherlands. Transcript expression levels were obtained from Mas5.0 analysis and derived from the R2 dataset. The Affymetrix probe-sets were selected using the R2 bioinformatic platform. The unpaired, heteroscedastic Student’s t-test was used to determine significant differences. Shown are the absolute expression levels on a log2 scale. VEGFA expression in normal ovarian tissue (white bars) and ovarian cancer tissue (gray bars) were compared (* p≤0.01 ).