Abstract
Animal models have shown that myopic defocus is a potent inhibitor of ocular growth: brief (1–2 hours) daily periods of defocus are sufficient to counter the effects of much longer periods of hyperopic defocus, or emmetropic vision. While the variables of duration and frequency have been well-documented with regard to effect, we ask whether the efficacy of the exposures might also depend on the time of day that they are given. We also ask whether there are differential effects on the rhythms in axial length or choroidal thickness.
2-week-old chickens were divided into 2 groups: (1) “2-hr lens-wear”. Chicks wore monocular +10D lenses for 2 hours per day for 5 days at one of 3 times of day: 5:30 am (n=11), 12 pm (n=8) or 7:30 pm (n=11). (2) “2-hr minus lens-removal”. Chicks wore monocular −10D lenses continually for 7 days, except for a 2-hr period when lenses were removed; the removal occurred at one of 2 times: 5:30 am (n=8) or 7:30 pm (n=8). Both paradigms exposed eyes to brief myopic defocus that differed in its magnitude, and in the visual experience for the rest of the day. High frequency A-scan ultrasonography was done at the start of the experiment; on the last day, it was done at 6-hr intervals, starting at noon, over 24-hr, to assess rhythm parameters. Refractive errors were measured using a Hartinger’s refractometer at the end.
In both paradigms, myopic defocus in the evening was significantly more effective at inhibiting eye growth than in the morning (“2-hr lens-wear”: X-C: −149 vs −83 μm/5d; “2-hr lens-removal: X-C: 91 vs 245 μm/7d; post-hoc Bonferroni test, p<0.01 for both). Data for “noon” was similar to that of “evening”. In general, the refractive errors were consistent with the eye growth. In both paradigms, a 2-way ANOVA showed that “time of day” accounted for the differences between the morning versus evening groups (“2-hr lens-wear”: p=0.0161; “2-hr lens-removal”: p=0.038). In the “plus-lens” morning exposure, the rhythm in axial length could not be fit to a sinusoid. In both paradigms, the rhythm in axial length for the evening group was phase-advanced relative to noon or morning (“2-hr lens-wear: evening vs noon; 1:24 pm vs 6:42 pm; “2-hr lens-removal: evening vs morning: 12:15 pm vs 6:18 pm; p<0.05 for both). Finally, the amplitude of the rhythm as assessed by the “day vs night” maximum and minimum respectively, was larger in the “evening” than in the “morning” group (“2-hr lens-wear: 88 vs 38 μm; “2-hr lens-removal: 104 vs 48 μm; p<0.05 for both). For the choroidal rhythm, there was no effect on phase, however, the amplitude was larger in most, but not all, experimental groups.
These findings have potential translational applications to myopia prevention in schoolchildren, who are exposed to extended periods of hyperopic defocus during reading sessions, due to the nearness of the page. We propose that bouts of such near-work might best be scheduled later in the day, along with frequent breaks for distance vision.
Keywords: defocus, myopia, hyperopia, choroid, axial length, diurnal rhythms, amplitude
Introduction
The development of myopia in children has long been associated with prolonged near work (reading), that is presumably due to the hyperopic defocus experienced because of the nearness of the page, and possibly contributed to by accommodative lag or insufficiency (Gwiazda et al. 1995). It has been proposed that taking brief breaks from reading to focus at distance, which may induce transient myopic defocus, might be a means of ameliorating the development of myopia (review: (Wallman and Winawer 2004). By the same token, perhaps transient myopic defocus induced by accommodative spasm that some eyes might experience after near work might also aid in preventing myopia development. This idea is supported by much work in animal models that demonstrate a robust non-linearity in the temporal integration characteristics found in response to spectacle lens-induced myopic and hyperopic blur. If myopic defocus is indeed an enduring “stop growth” signal that counters the tendency towards myopia development in response to the hyperopic defocus induced by near work, there are two variables that are known to determine its efficacy: the duration of exposure and its frequency. With regard to duration, it is known that very brief periods of myopic defocus, one or two hours, are sufficient to outbalance the other 12 hours of emmetropic vision (Schmid and Wildsoet 1996), or hyperopic defocus (Winawer and Wallman 2002; Zhu et al. 2003), to cause a hyperopic shift via an inhibition of eye growth. Furthermore, for lens-induced myopic defocus, the “fall” time (time it takes for the response to decay) is longer than that for lens-induced hyperopic defocus; that is, the response is more enduring (Zhu and Wallman 2009). Regarding frequency, it is known that multiple brief periods of myopic defocus are more potent than a single episode even if the total duration is the same (Winawer and Wallman 2002; Zhu et al. 2005; Zhu and Wallman 2009). We propose a third variable that might be important in the potency of the response: that of time of day.
It has been shown that eyes of chickens (Nickla et al. 1998; Papastergiou et al. 1998) and primates (Nickla et al. 2002), including humans (Stone et al. 2004; Brown et al. 2009; Chakraborty et al. 2011), exhibit rhythms in axial length and choroidal thickness, such that eyes elongate during the day and decrease growth (or shrink) at night, while the choroid thins during the day and thickens at night. The phases of these rhythms change in eyes receiving myopic defocus either by positive spectacle lenses or by removing a diffuser from a previously form-deprived eye, so that both rhythms shift into phase, and have an acrophase at around 8 pm (Nickla et al. 1998). These defocus-induced phase shifts have been corroborated in humans as well (Chakraborty et al. 2012). In chicks, the rhythms might contribute to a rhythm in refractive error; in fact, in chickens, eyes are more myopic in the morning than in the evening (personal observation). Putting a positive lens onto eyes in the morning, when they are more myopic, would add more myopia to the refraction than it would in the evening when eyes are more hyperopic. Therefore, one might suppose that the signal to stop growth might be more robust when given in the morning than when given in the evening. One might further ask, would the efficacy of brief periods of myopic defocus differ depending on whether the eye was otherwise emmetropic (image focussed on the retina) or hyperopic (image focused behind the retina by a negative lens)? Finally, would a greater efficacy at one time of day versus another be associated with alterations in the phase and/or amplitude of the rhythms in axial length and/or choroidal thickness?
In this series of experiments, we chose 2 paradigms to mimic the above two conditions. In the first, brief myopic defocus was induced by the wearing of positive spectacle lenses (+10 D) for 2 hours, at different times of day, in eyes that were otherwise emmetropic (no lenses). In the second, eyes wearing negative lenses (hyperopic defocus) had the lenses removed for 2 hours at different times of day. This latter paradigm might emulate the situation of a child reading for long periods and taking a break to look at distance. We asked whether the growth-inhibiting response was dependent on time of day in either condition, whether it was more robust in one or the other condition, and finally, whether the efficacy was associated with changes in the phase or amplitude of the rhythms in axial length or choroidal thickness. Parts of this manuscript have been published in abstract form (Nickla and Totonelly 2013; Nickla et al. 2014).
Methods
Subjects
White Leghorn chicks (Gallus gallus domesticus) were hatched in an incubator and raised from day one in temperature – controlled brooders. The light cycle was 14L/10D; the light level in the brooders at chick height was about 500 lux. Food and water were supplied ad libitum. Care and use of the animals conformed to the ARVO Resolution for the Care and Use of Animals in Research.
Paradigms
Brief myopic defocus induced by +10 D lenses (alternating myopic defocus and emmetropia): “Plus lens-wear”
Starting at 2 weeks of age, birds received daily 2 hours of myopic defocus induced by the wearing of a monocular +10 D lens at one of three different times of day for 5 days (3 groups): “morning”: 5:30 am – 7:30 am (n=11), “noon”: 12:00 pm – 2:00 pm (n=8), and “evening”: 7:30 pm – 9:30 pm (n=11). A separate group wore the lenses continually, as a “constant lens-wear” control (n=6). The L/D cycle was 14/10 in all groups: “morning”: 5:30 am to 7:30 pm; “noon” and “evening”: 7:30 am to 9:30 pm. Lenses were attached to Velcro rings the matching one of which was glued to the feathers around the eyes. The fellow eyes were untreated.
Ocular dimensions were measured using high-frequency A-scan ultrasonography at noon on the first day of the experiment, under inhalation anesthesia (for details see: (Nickla et al. 1998)). On day 5 of the experiment, eyes were re-measured with ultrasound at 12 pm, 6 pm, 12 am, 6 am and 12 pm to obtain the parameters of the diurnal rhythms in axial length and choroidal thickness (see below for details) for the groups getting the 2 hours of defocus. Data on the rhythms for continuous lens-wearing controls have been published (Nickla 2005), and so were not measured here. Measurements at night were done under a photographic safe light; measurements typically took about 5 minutes per eye, after which the birds were returned to the dark cage. Refractive errors were measured at the end of the experiment using a Hartinger’s refractometer.
Brief myopic defocus induced by removal of a −10 D lens (alternating myopic and hyperopic defocus): “Minus lens-removal”
Starting at 2 weeks of age, birds wore a monocular −10 D lens continuously for 7 days1, except for daily 2-hour intervals when the lenses were removed. Three groups had their lenses removed at 3 different times of day: “morning”: 5:30 am – 7:30 am (n=11), noon: 12 pm—2pm (n=10) or “evening”: 7:30 pm–9:30 pm (n=11). A separate group wore the lenses continually, as a “constant lens-wear” control (n=5). The L/D cycle was 14/10 in all groups. Ocular dimensions were measured using high-frequency A-scan ultrasonography at noon on the first day of the experiment and again on day 7 to determine growth rates, and refractions were measured using a Hartinger’s refractometer. Because the data for “noon” and “evening” did not differ, to determine rhythm parameters, we used a subset of only two groups, the “morning” (n=8) and “evening” (n=8), which were measured with ultrasound at 12 pm, 6 pm, 12 am, 6 am and 12 pm on the last day of the experiment (day 7). Data on the rhythms for continual lens-wearing controls have been published (Nickla 2005) and were not measured here. In both paradigms the fellow eyes were untreated, but the focus of our investigation was the time-of-day effects of the defocus, therefore for simplicity when presenting the data related to phase (figures 2 and 4; Table 1), we combined the fellow-eye data from both paradigms.
TABLE 1.
Choroid and axial rhythm parameters of amplitude (amp, in μm) and phase (peak) for all groups.
| Positive Lens-wear | Minus Lens-removal | FELLOW EYES Paradigms combined |
|||||
|---|---|---|---|---|---|---|---|
| MORN (n=11) | NOON (n=8) | EVE (n=11) | MORN (n=8) | EVE (n=8) | MORN +/− (n=19) | EVE +/− (n=19) | |
| CHOROID | |||||||
| Max-Min amp | 140 (18) | 123 (27) | 121 (15) | 77 (9) | 139 (14) | 83/81 # | 88/54 # |
| Night-Day amp | 138 (19) | 112 (30) | 109 (19) | 68 (13) | 139 (14) | 77/73 # | 88/46 # |
| Mean sine amp | 109 | 88 | 69 | 54 | 126 | 51 | |
| Mean sine peak | 2:30 | 22:45 | 0:30 | 4:12 | 3:12 | 2:05 | |
| Individual sine peak | 1:36 (7) | 1:00 (6) | 0:45 (8) | 3:45 (6) | 3:18 (8) | 1:50 (16) | |
| AXIAL | |||||||
| Max-Min amp | 73 (10) | 97 (19) | 104 (11) | 76 (10) | 165 (18) | 49/83 | 55/100 |
| Day-Night amp | 38 (14) | 62 (18) | 88 (13) | 48 (14) | 104 (11) | 35/58 | 46/52 |
| Mean sine amp | ---- | 31 | 98 | 20 | 50 | 10 | |
| Mean sine peak | ---- | 18:30 | 14:30 | 19:12 | 10:48 | 16:50 | |
| Individual sine peak | ---- | *18:42 (7) | *13:24 (8) | *18:18 (5) | *12:15 (5) | 16:15 (12) | |
Shaded data: Max-min and Night-day amplitude; analyses described in methods. SEMs in parentheses.
Two values represent the “plus” and “minus-removal” data respectively.
Un-shaded data derived from longitudinal data. “Mean sine amp” and “mean sine peak” are from the 24-hr sine function fit to the mean normalized data in figures 2 and 4 A, C and E. “Individual sine peak” is from individual eyes (n’s in parentheses) fit to sine waves with a 24-hr period; circular statistics were used to analyze group differences.
Noon vs Eve and Morn vs Eve differ using circular statistics (p<0.05).
---- Data are not sinusoidal.
Data Analysis
Ocular growth rate was defined as the change in axial length (from the front of the cornea to the front of the sclera) over the experimental interval, from noon to noon. The diurnal rhythms in eye length, as determined by the 6-hour interval measurements on the last day of the experiment (day 5–6 for “plus lens-wear”; day 7–8 for “minus lens-removal”), was assessed in 2 ways: first, we calculated the change over each of the four 6-hour intervals, which includes the “steady state” eye growth occurring over the 24 hour period (i.e the slope of the longitudinal data). These data are plotted as bar graphs in figures 2 and 4 B and D. One-way ANOVAs (StatPlus; AnaystSoft) were used to assess between group differences for each of the 4 intervals. Second, the “steady state” growth rate (regression) was subtracted from the data for each individual eye, as described in Nickla et al. (Nickla et al. 1998), in order to assess the sine function. To control for the large between-animal variability, these data were then normalized to their mean, and the means of these longitudinal data are plotted as line graphs in figures 2 and 4A, C and E, with the standard error bars. 2-way ANOVAs were used on these data to determine interactions. Sine functions with a fixed 24-hour period were fit to these data (Nickla et al. 1998), and were one way of determining phase and amplitude (“mean sine peak” and “mean sine amp” in Table 1). Only those eyes whose data could be fit to a sine function (numbers indicated in parenthesis in Table 1) were used in the determination of “individual sine peak”, and phase differences between groups were analyzed using circular statistics. “Goodness of fit” required that the mean standard deviation of the residuals of the sine wave fit was less than 85% of the standard deviation of the raw data (Nickla et al. 1998). The other two ways of assessing “amplitude” are described below.
For choroidal thickness, the longitudinal data did not require the subtraction of the regression because unlike eye length, that reflects normal growth over 24 hours, choroid thickness does not change in any discernable way (i.e. the slope is approximately zero). These data were normalized to the mean, for consistency. One- and two-way ANOVAs were used to analyze the effect of “time of day”, and to determine the interaction as indicated for both axial length and choroid thickness. Where appropriate, post-hoc t-tests were used to test between-group differences. We used two other methods to assess “amplitude” apart from the mean of the sine wave fit mentioned above: In one, we took the minimum vs maximum differences from the 5 diurnal measurements, regardless of the time of day; this is referred to as “max-min amp” in Table 1. In the second, we restricted the maximum to either the day, for axial length, or night, for choroid thickness, and the minimum to either night, for axial length, or day, for the choroid thickness; this is referred to as “night-day amp” in Table 1 (both in shaded cells). This second one is a more accurate means of assessing if there is a diurnal excursion (i.e. if there is a day vs night change). The degree of similarity between the two methods of assessing “amplitude” also serves as a measure of whether the excursion is a diurnal one (that is, has its peak and trough about 12 hours apart).
Results
Eye growth and refractive error
Plus lens-wear
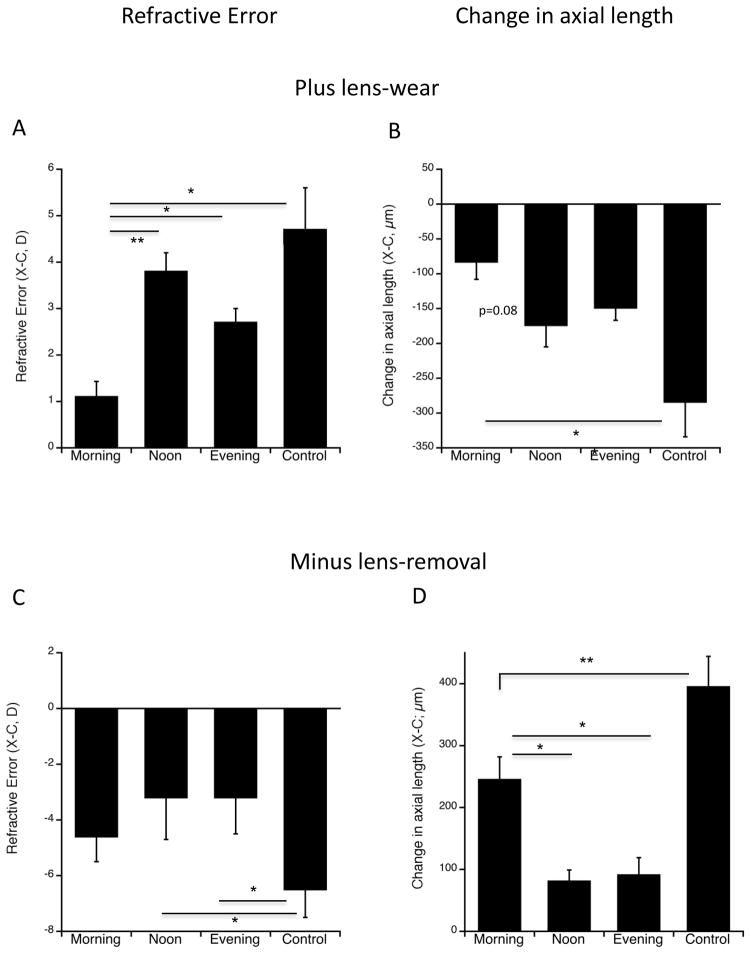
Continuous lens-wearing control eyes exposed to myopic defocus by the wearing of +10 D lenses for 5 days developed 4.7 D of hyperopia (Figure 1A). This did not differ significantly from the amount of hyperopia induced by a mere 2 daily hours of lens-induced myopic defocus when it was given at either noon or evening (figure 1A: 3.8 D and 2.9 D; ANOVA p<0.00001; post-hoc Bonferroni: p=0.99; p=0.084 respectively). By contrast, when the 2 hours of defocus was given in the morning, it was significantly less effective at inducing hyperopia than at any of the other times of day (morning vs noon and evening: 1.1 D vs 3.8 and 2.9 D respectively; p=0.0004 and p=0.0095 respectively). It was also significantly less effective than that induced by the continuous lens-wear (morning vs controls: p=0.00003). This refractive effect was the result of differences in axial elongation (Figure 1B): defocus in the morning produced less growth inhibition than that at noon or by continuous lens-wear (change in axial length over 5d; X-C: −83 μm vs −174 and −284 μm respectively; p=0.087, p=0.0004); it should be noted that if the full-lens controls are excluded, the ANOVA p=0.033, and the difference between morning vs noon becomes significant at the p=0.039 level. The difference between morning and evening was not significant (−83 μm vs −149 μm p=0.35). There were no significant differences in eye growth or refractive error between eyes given defocus at noon versus those given defocus in the evening.
Figure 1.
Refractive errors and changes in axial length for 2 hours of +10 D lens wear (A and B) and 2 hours of removal of −10 D lenses (C and D). A. End refractive error, (experimental minus fellow eyes; X-C) in diopters (D) for the three times of day of exposure to myopic defocus induced by 2 hours of +10 D lens-wear, and continuous lens-wear controls. The “morning” group developed significantly less hyperopia than did the noon or evening groups; all 2-hour lens groups were significantly less hyperopic than full-lens controls. * p<0.01; ** p<0.005. B. Change in axial length (X-C) for the three times of day of exposure to 2 hours of +10 D lens-wear, and continuous lens-wear controls. The morning group showed significantly less growth inhibition (relative to fellow controls) than did the evening group. All 2-hour lens groups showed significantly less growth inhibition relative to full-lens controls. * p<0.0005. C. End refractive error, (X-C) in diopters (D) for the three times of day of exposure to 2 hours of myopic defocus induced by the removal of a −10 D lens, and continuous lens-wear controls. There wasn’t a significant difference between experimental groups (ANOVA p=0.26), however, both noon and evening groups were significantly less myopic than full-lens controls, while the morning group did not differ. Hence lens-removal in the morning is not effective in inhibiting myopia. * p<0.05. D. Change in axial length (X-C) for the three times of day of 2 hours of negative lens-removal, and continuous lens-wear controls. The morning group showed significantly less growth inhibition relative to fellow eyes than did the noon and evening groups, and also than full-lens controls. All experimental groups showed more growth inhibition than full lens controls. *p<0.01; **p<0.005.
Minus lens-removal
Continuous lens-wearing control eyes exposed to hyperopic defocus by the wearing of −10 D lenses for 7 days developed −6.5 D of myopia (Figure 1C). As predicted (Schmid and Wildsoet 1996), 2 daily hours of myopic defocus induced by removal of the negative lens over the course of 7 days resulted in significantly less myopia, but only in eyes in which lenses were removed at noon or evening (−3.2 for both vs −6.5; ANOVA p=0.01; post-hoc Bonferroni: p=0.022 and 0.014 respectively). Surprisingly, eyes with lenses removed in the morning were as myopic as those exposed to continuous lens-wear (−4.6 vs −6.5; p=0.49). While exposure in the morning tended to be less effective than exposure at noon or evening, the refractive error differences between experimental groups did not reach statistical significance (ANOVA p=0.26; figure 1C).
The tendency of morning defocus being less effective at preventing myopia than noon or evening was borne out in the axial elongation data (Figure 1D). Eyes that had lenses removed at morning grew significantly faster than eyes with removal at noon or evening (ANOVA p<0.00001; 245 μm vs 81 and 91 μm/7d; p=0.006 and 0.010 respectively), in accordance with the more myopic refractive errors. All experimental groups grew significantly slower than continuous lens controls, indicating that the 2 hours of lens-removal had an inhibitory effect on growth rates at all times of day.
Evening defocus phase-advances the rhythm in axial length
Plus lens-wear
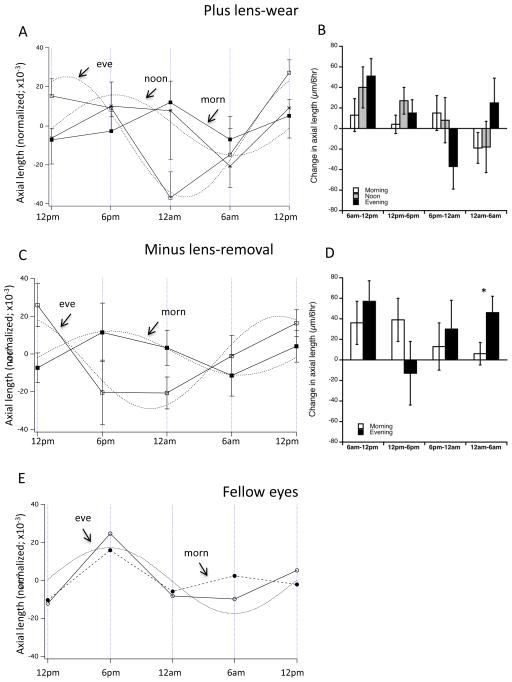
The mean normalized longitudinal data shows significant effects on rhythm “shape” and phase (figure 2A). First, the morning group (black squares) does not appear to oscillate in a normal sinusoidal 24-hour rhythm, while that for both other groups do. A 2-way ANOVA of morning vs evening data shows that time-of-day accounts for the between-group differences (F 4,76=3.25; p=0.0161). This difference is also apparent in the interval data (Figure 2B, white bars) in which the morning group (open bars) oscillates between the 6-hour intervals. However, these interval data showed no significant between-group differences at any intervals (1-way ANOVA, p >0.1 for all). The mean acrophases from the sine waves fit to the data for noon and evening are at 6:30 pm and 2:30 pm respectively (Table 1); this difference is significant using circular statistics (6:42 vs 1:24 pm for noon and evening; p<0.05; Table 1). (Because the morning data cannot be fit to sine waves, this analysis is not possible).
Figure 2.
Mean normalized longitudinal data for axial length (A, C and E) and changes over successive 6-hour intervals (B and D) for both paradigms and fellow controls. A and B: 2 hours of +10 D lens-wear. Note that the longitudinal data for the morning group (dark squares) cannot be fit to a 24-hr sinusoid, while that of both other groups can (evening: white squares; asterisks: noon) (A). A 2-way ANOVA shows that time of day accounts for the between group differences (p=0.016). There are no between-group differences in the interval data (B). C and D: 2 hours of −10 D lens-removal. There is a significant difference in phase between the morning (black squares) and evening (open squares) groups using circular statistics (p<0.05), and a 2-way ANOVA shows that time of day accounts for the between group difference (p=0.038). *p<0.05. E. Mean longitudinal data for all fellow eyes combining both paradigms for morning and evening groups.
Minus lens-removal
The mean normalized longitudinal data shows significant effects on rhythm phase (figure 2C). A 2-way ANOVA of the longitudinal data indicates that time-of-day accounted for the between-group differences (Figure 2D: F4,56=2.74; p=0.038). The mean acrophases for the sine waves fit to the data for morning and evening are 7:12 pm and 10:48 am respectively; this difference is significant using circular statistics (6:18 pm vs 12:15 pm; p<0.05; Table 1). Similar to the “plus lens-wear” group, the mean raw changes in axial length for three of the 6-hour intervals showed no significant between-group differences (first 3 intervals, figure 2D; p-values >0.1). However, between 12 am and 6 am, eyes exposed to morning defocus grew significantly less that those of eyes with evening exposure (6 vs 45 μm; p=0.046).
Fellow eyes
Figure 2E shows the fellow eye data combining the two paradigms for the two separate time groups; 2-way ANOVAs showed no significant between-paradigm differences. For “evening”, the rhythm is sinusoidal, and peaks at about 6 pm, while the morning data appears somewhat abnormal, possibly revealing a “contralateral” or “yoking” effect from the experimental eyes. Because potential yoking was not the purpose of these studies, and because we did not include an untreated control group, this will not be discussed.
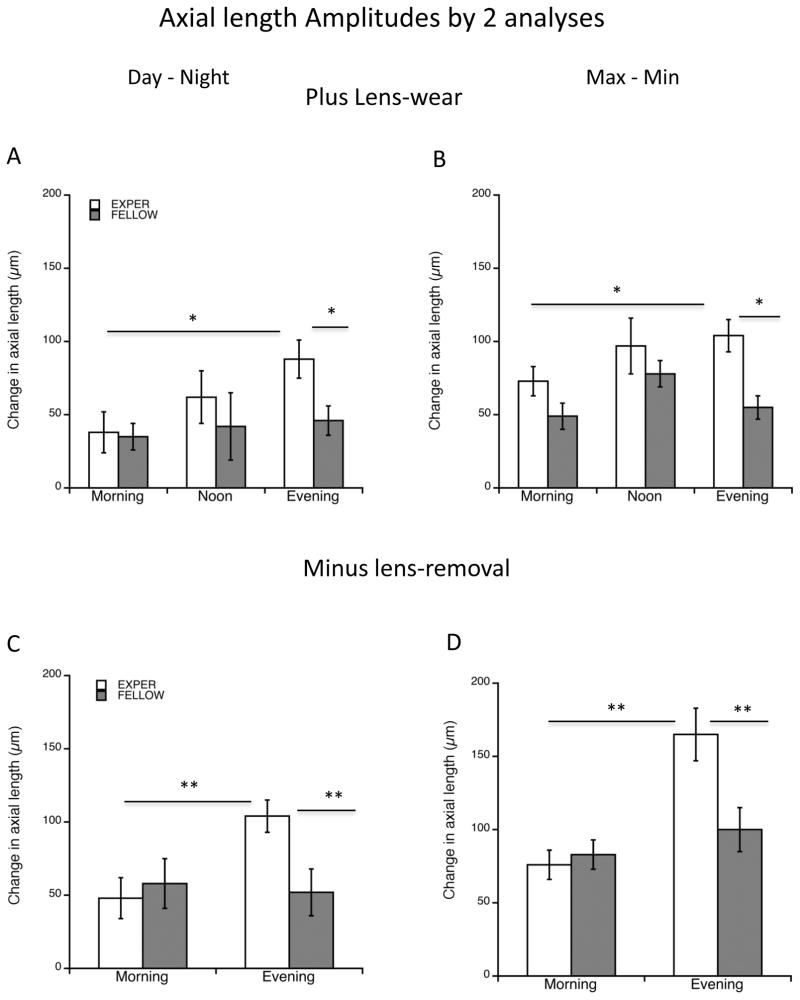
Evening defocus increases the amplitude of the rhythm in axial length
We calculated “amplitude” in two ways, as described in Methods. For the “plus lens-wear” group, the “day-night” derivation shows that the experimental amplitudes differ as a function of time of defocus (ANOVA, p=0.0375; Figure 3A; Table 1): the evening group amplitude was significantly larger than that of the morning group (88 vs 38 μm; p=0.033, post-hoc Bonferroni correction). For evening as well, the experimental eye amplitudes were significantly greater than that of fellow eyes (88 vs 46 μm; p=0.0186). Amplitudes of fellow eyes did not differ significantly between groups ANOVA p>0.5). For the “max-min” derivation, while an ANOVA showed no significant differences between experimental groups, a comparison between “evening” and “morning” using a Student’s t-test showed that evening was higher in this derivation as well (104 vs 73 μm; p=0.045; Figure 3B; Table 1). Here too, “evening” amplitude was significantly greater than that of fellow eyes (104 vs 55 μm; p=0.001). A similarity between these two ways of calculating amplitude (compare figures 3A and B) is a measure of the “normal” rhythm having an acrophase during the day (Nickla et al. 1998); this is seen for experimental eyes in noon and evening, and for fellow eyes. However, for experimental eyes in the morning group the two means are significantly different (38 vs 73 μm; p=0.04). Together, the results support the conclusion that the acrophase of the rhythms in the noon and evening groups are normal, and occur during the day, while the morning group is anomalous.
Figure 3.
“Amplitude” for axial length as assessed by 2 means (Methods). “Day-Night” is the largest excursion between day and night points (left graphs); Max-Min is the largest difference regardless of time of day. A and B: 2 hours of +10 D lens-wear. A. The “evening” amplitude is significantly larger than the “morning” group amplitude (p=0.033) and fellow controls (p=0.019). B. The same pattern is found here. The “evening” amplitude is significantly larger than the “morning” group amplitude (p=0.047) and fellow controls (p=0.001). C and D: 2 hours of −10 D lens-removal. C. The “evening” amplitude is significantly larger than the “morning” group amplitude (p=0.0047) and fellow controls (p=0.009). D. The same pattern is found here (p=0.0004; p=0.0087 respectively). *p<0.05; **p<0.01.
We find a similar result for the “minus lens-removal” paradigm (Figures 3C and D): the amplitude of the rhythms in “evening” are larger than those of “morning” in both means of derivation (C:104 vs 48 μm; p=0.0047; D:165 vs 76 μm; p=0.0004), similarly, the experimental amplitudes are larger than those of fellow controls (C: 104 vs 52 μm; p=0.009; D: 165 vs 100 μm; p=0.0087). These data are consistent with the amplitudes as derived by the “mean sine amplitude” showing the evening amplitude as being significantly greater than the morning one (50 vs 20 μm; p<0.01; Figure 2A; Table 1).
In summary, (1) there is a significant difference in the phases of the rhythms between the evening and mid-day group in the “plus lens-wear” paradigm, and between evening and morning groups in the “minus lens-removal” paradigm, with the “evening” being relatively phase-advanced in both paradigms, and (2) the rhythm amplitude is larger in the evening groups compared to morning and to fellow controls, in both paradigms.
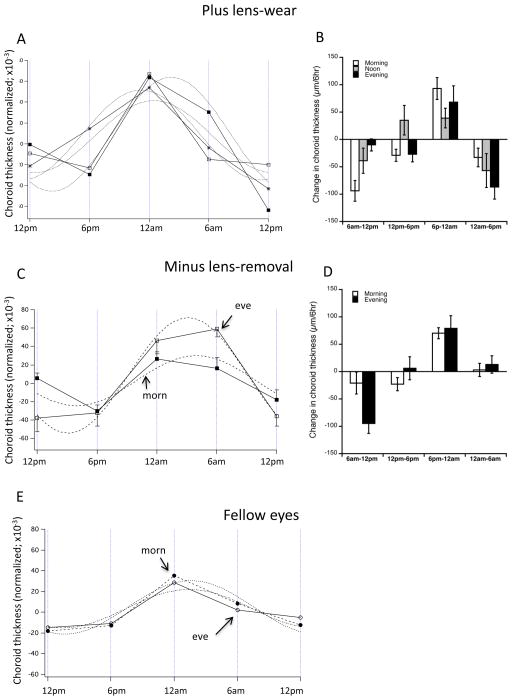
Choroidal thickness
We found no evidence for between-group differences in the sinusoidal rhythm in choroidal thickness by any analysis in either paradigm (Figure 4). Neither paradigm showed between-group differences in the longitudinal data (Figures 4A and C compared to E). For the “plus lens-wear”, the acrophase in all three groups occurred between 11:30 pm (“evening”) and 2:20 am (“morning”) (Figure 4A), and there were no significant differences between experimental groups when analyzed by circular statistics (Table 1). For the “minus lens-removal” the acrophases occurred at 2 am (“morning”) and 3 am (“evening”) (Figure 4C); these did not differ using circular statistics. The acrophases for the combined fellow eyes (morning vs evening) was 1:45 am for both sets (Figure 4E). There were no significant between-group differences in the interval data at any interval data (Figures 4B and D; ANOVAs >0.1).
Figure 4.
Mean normalized longitudinal data for choroidal thickness (A, C and E) and changes over successive 6-hour intervals (B and D) for both paradigms and fellow controls. A and B: 2 hours of +10 D lens-wear. Data for all three experimental groups are well-fit to a 24-hr sinusoid. Evening: white squares; Morning: black squares; Noon: asterisks. B. There are no significant between-group differences at any 6-hr time interval. C and D: 2 hours of −10 D lens-removal. Data for both morning (black squares) and evening (white squares) experimental groups are well-fit to a 24-hr sinusoid. D. There are no significant between-group differences at any 6-hr time interval. E. Mean longitudinal data for all fellow eyes combining both paradigms for morning (black symbols) and evening (white symbols) groups. Note the difference in amplitude between experimental eyes in both paradigms and the fellow controls (y axes range the same).
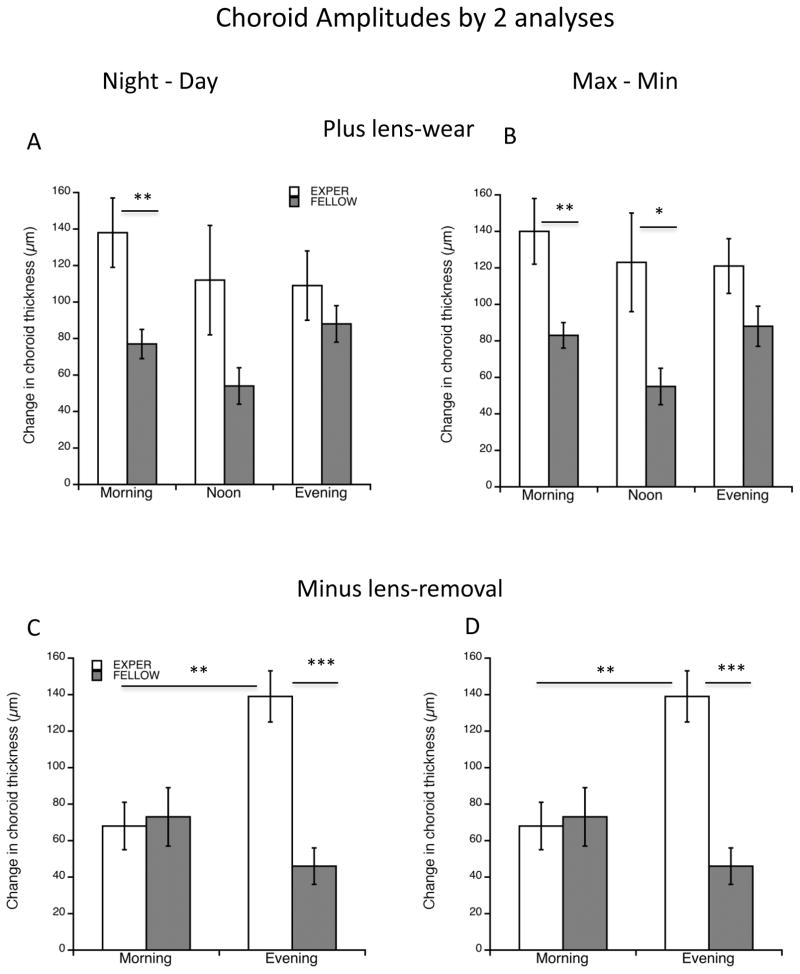
For the rhythm amplitude, an ANOVA of the plus-lens wear group showed no significant differences between experimental groups for either means of assessing amplitude (Figure 5A and B). However, there was a tendency for the experimental amplitudes to be larger than those of fellow eyes; this reached statistical significance in the morning group in both analyses (Figure 5A: 138 vs 77 μm; p=0.005; “Max-min”: 140 vs 83 μm; p=0.0057) as well as in the noon group in the “max-min” analysis (Figure 5B: 123 vs 55 μm; p=0.023). By contrast, for the “minus lens-removal” paradigm, the amplitudes for the experimental eyes in the evening group were larger than their fellows in both analyses (Figure 5C: 139 vs 46 μm; p=0.0008; Figure 5D: 139 vs 54 μm; p=0.00003) and larger than the “morning” experimental eye amplitudes (Figure 5C: 139 vs 68 μm; p=0.0014; Figure 5D: 139 vs 77 μm; p=0.0012).
Figure 5.
“Amplitude” for choroidal thickness as assessed by 2 means (Methods). “Day-Night” is the largest excursion between day and night points (left graphs); Max-Min is the largest difference regardless of time of day. A and B: 2 hours of +10 D lens-wear. There are no significant differences between experimental groups in either means of amplitude derivation. In both means of derivation, the amplitude for the morning group is larger than that of fellow controls (p<0.01 for both). For max-min (B) this is also true for the noon group (p<0.05). C and D: 2 hours of −10 D lens-removal. In both means of derivation the amplitude for the evening group is greater than that for the morning group (p<0.001), and the experimental eye amplitude is greater than that of fellow eyes (p<0.005). *p<0.05; **p<0.01; ***p<0.005.
In summary, in the “plus lens-wear” paradigm, the choroidal rhythm amplitude in experimental eyes tended to be larger than that of fellow eyes. In the “minus lens-removal” paradigm, however, the experimental “evening” amplitudes were larger than those of “morning”.
Discussion
We here show that brief myopic defocus is most effective at inhibiting eye growth when given later in the day as opposed to earlier; this is true for both paradigms of imposing myopic defocus. This “time of day” effect is associated with two effects on the axial length rhythm parameters: first, “time of day” accounts for the differences between longitudinal rhythm data for morning vs evening in both paradigms. In the “plus lens-wear” paradigm, the data for the “morning” group cannot be fit to a 24-hour period sine function, and the rhythm of the evening group is phase-advanced relative to the noon group; similarly, in the minus lens-removal paradigm the rhythm of the evening group is phase-advanced relative to morning. Second, there is an increase in the amplitude of the rhythm in the “evening” vs the “morning” groups in both paradigms. For the choroidal rhythm, there is no differential effect of “time of day” on rhythm parameters: defocus at any time of day tends to increase the amplitudes, but has no discernable effect on phase.
Alterations in “shape” or phase of the rhythm in axial length
Previous studies have shown that certain visual conditions that caused changes in ocular growth rates also resulted in alterations in either the phase of the rhythm in axial length or its “shape”. With regard to “shape”, 2 hours of exposure to light in the middle of the night caused an acute growth stimulation that altered the rhythm such that it no longer could be fit to a sinusoidal function (Nickla and Totonelly 2016). With regard to phase, continuous exposure to myopic defocus, induced either by a positive spectacle lens or the removal of a diffuser from a form-deprived eye resulted in a significant phase-delay (Nickla et al. 1998; Nickla 2005). In the current study, the imposition of the myopic defocus was a mere 2 hours, regardless, there was significant growth inhibition at all exposure times, in both paradigms, with growth being more inhibited in the evening exposure groups than in the morning ones. In both paradigms as well, the rhythm in axial length showed a phase-advance relative to that of the earlier exposure groups (evening vs noon for plus lens-wear, and evening vs morning for minus lens-removal). Because “evening” and “noon” were similarly effective at growth inhibition in the plus-lens wear paradigm, while their phases were significantly different, the phase advance in the evening group is not likely to play a role in the greater efficacy. Furthermore, this phase-advance is contrary to the phase-delay found in the earlier studies of growth inhibition, weakening, but not disproving, the hypothesis linking phase shifts to eye growth regulation. In both the earlier myopic defocus studies mentioned above, the defocus was continuous, and thus the growth inhibition much more robust and longer-lasting, while in the current study there were alternating signals, which could account for the different results regarding phase.
Our data indicate that myopic defocus at the end of the day causes a phase advance in the axial length rhythm, the shift being in the opposite direction to that found in response to light pulses: light exposures at the end of subjective day usually cause phase-delays. We know that the light/dark cycle entrains the rhythm in eye length in chicks: the rhythm free-runs in constant darkness with an acrophase during subjective day (Nickla et al. 2001). There is evidence in the circadian literature, however, that in certain situations, some external cues, such as temperature, can cause effects on phase that are opposite (i.e. in the other direction) than those traditionally seen to light pulses (Yoshikawa et al. 2013), supporting the possibility that defocus may act as an “external cue” that has opposite effects on rhythm phase than does that of light.
We found that the wearing of positive-lenses in the morning altered the rhythm to a greater extent than did the (morning) removal of a negative lens: in the former, the rhythm could no longer be fit to a 24-hour sinusoid. It is likely that this greater deleterious effect was due to the higher amount of blur induced by the positive lens, (between 8–10 D; chicks this age are about +1 to +2 D), as opposed to significantly less myopic blur in the “minus lens-removal” (the cumulative effect after 7 days is only about - 4 D; see figure 1C).
Alterations in rhythm amplitude
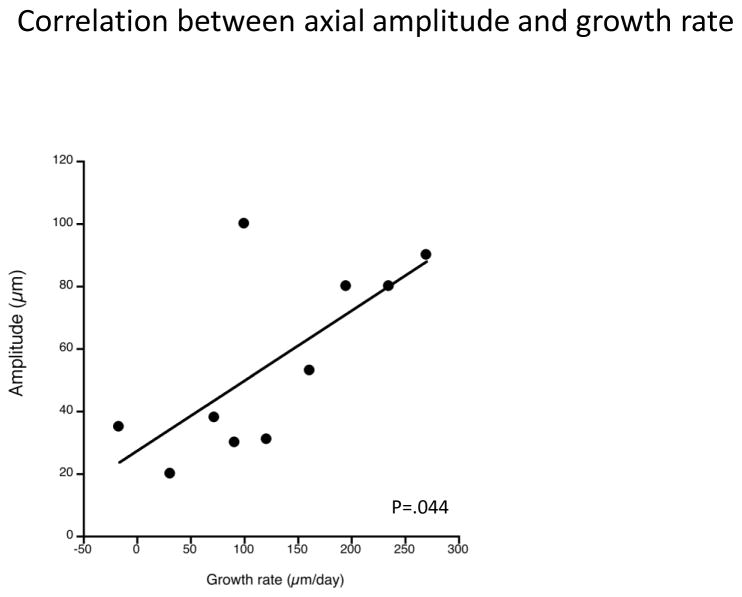
We found that the amplitude of the rhythm in axial length was significantly greater in the evening exposure groups relative to the morning exposure groups, and to fellow controls, in both paradigms, and by all means of derivation, hence a greater efficacy in ocular growth inhibition is associated with higher amplitude growth rhythms. Because the morning amplitudes do not differ from fellow controls indicates that the relevant variable is related to time-of-day (i.e. evening defocus). This is a curious finding, because larger amplitude growth rhythms might be expected to accompany higher rates of growth, not slower ones. In fact, a retrospective analysis of old data (Nickla et al. 1998; Nickla 2005) shows a significant correlation between amplitude of the growth rhythm and the rate of growth over a day (Figure 6 open circles; Pearson’s coefficient r=0.680; p=0.044). Because rhythm amplitude is normally a measure of the strength of the Zeitgeber, we speculate that the concurrence of myopic blur with another oscillating variable (melatonin release?) might effect this amplitude change. Finally, while there are no significant amplitude differences between paradigms (plus vs minus lenses) in the morning for either derivation, or in the evening in the day-night derivation, amplitudes are significantly greater in the minus-lens removal vs plus-wear for the max-min derivation. Because this difference is not apparent in either the day-night derivation nor the mean sine fit data in Figure 2 and Table 1, and, in fact, is in the opposite direction, with the plus lens amplitudes being larger, this is probably an anomaly.
Figure 6.
Scatterplot of mean amplitudes of the rhythms in axial length and the growth rates associated with that day, from published data (Nickla et al., 1998; Nickla 2005). There is a significant correlation between the two (Pearson’s coefficient r=0.680; p=0.044).
For the choroidal rhythm, the data were not consistent: in the minus lens-removal paradigm, the evening exposure group showed a greater amplitude than the morning exposure group, but for the plus lens-wear, the morning group showed a larger amplitude. We had previously shown that all visual conditions that altered ocular growth rates (form deprivation, diffuser removal and both plus and minus spectacle lenses) were associated with larger choroidal rhythm amplitudes than normal (Nickla et al. 1998). Together, these data show that retinal blur in either direction increases the choroidal rhythm amplitude, and that furthermore, that amplitude is unrelated to changes in eye growth rates. One reasonable explanation is that larger oscillations in either rhythm functions to search for the image plane, in order to signal to the sclera the correct direction and magnitude of growth. These results emphasize that rhythm amplitude is probably the result of a complex combination of oscillating variables. Its relationship to emmetropization (if any) awaits further study.
Temporal integration characteristics of hyperopic blur
Myopic defocus induced by lenses is a robust signal for ocular growth inhibition: only a few hours per day effects growth inhibition and hyperopia (Schmid and Wildsoet 1996). Our data on 2 daily hours of positive lens-wear corroborates that earlier study. However, the results of our negative lens-removal paradigm differs: we show that 12 daily hours of lens-wear with 2 hours of removal resulted in significant myopia (paired t-tests: morning, noon, evening: p=0.009, p=0.012, p=0.0005), whereas Schmid and Wildsoet reported that nearly constant wearing of the minus lens was required for myopia development (one hour of lens removal was sufficient to prevent myopia). The cumulative number of hours of lens-wear is not the relevant factor for this difference (our study: 12 hours on × 7 days = 84; S&W: 9 hours on × 10 days = 90). Perhaps the relevant factor is our higher ratio of duration of hyperopic defocus to myopic defocus (84 hrs “on” to 14 hours “off” = 6:1; S&W: 45 hrs “on” to 15 hrs “off” = 3:1). It is also possible that this difference can be accounted for by breed difference: 5 days of lens-wear in their White leghorn-New Hampshire cross caused only −1.7 D of myopia while we found −6.5 D in 7 days in our White leghorns. Nonetheless, these discrepancies do not alter the main conclusion, which is that brief periods of myopic defocus alternating with either “normal” emmetropic vision, or hyperopic defocus, is a strong inhibitory signal.
May time of day be important in emmetropization in humans?
If requiring children to take breaks from reading to expose their eyes to myopic defocus might be an effective means of preventing the development of myopia, as has been previously suggested, then it would be important to determine whether one time of day is more effective than another. Our study addressed this question. The paradigm of removing a negative spectacle lens for a brief daily period is more directly analogous to the visual experience of children taking a break from reading, than is the wearing of a positive lens, but in both conditions we found that the defocus was most effective at inhibiting eye growth in the evening. This suggests that imposing myopic defocus at intervals during bouts of reading would be more advantageous later than earlier in the day, although it remains to be seen whether there is a similar time-dependent mechanism at work for the deleterious (i.e. myopia-enhancing) effects of hyperopic defocus, that would suggest that teachers schedule reading activities for the afternoon. Current work in our lab supports this hypothesis (unpublished data).
To our knowledge this is the only such study to have systematically addressed the issue of time of day for defocus. In the study by Schmid and Wildsoet, time of day was controlled for by having equal numbers of birds given defocus at either the start or end of the day. They stated that “data for equivalent am and pm groups were pooled…”, inferring that the groups did not differ, however, that data were not shown, so a comparison with ours is not possible.
Another potential myopia preventative in school-aged children is increasing the amount of time spent out of doors (Rose et al. 2008; Guggenheim et al. 2012; Guo et al. 2013). This effect was shown to be unrelated to exercise (Rose et al. 2008) and so is probably visual. A lot of effort has been expended on the hypothesis that light intensity is the crucial variable, and animal studies have supported this: high intensity light ameliorates the development of form deprivation myopia (Ashby et al. 2009), and slows the response to negative lenses (Ashby and Schaeffel 2010). However, other potential variables, such as time of day of exposure, have not been sufficiently explored. Several recent studies hint that time of day might be important: 2 hours of bright light was more effective at myopia inhibition in form-deprived eyes when given mid-day versus evening (Backhouse et al. 2013). Another study, however, showed that 5 hours of bright light was more effective in the morning than in the evening at reducing negative lens-induced myopia (Feldkaemper et al. 2016). Although not at all conclusive, it remains likely that time of day might matter here as well.
In summary, brief myopic defocus is less effective at inhibiting eye growth when exposures are in the morning than later in the day. This weakening of the compensatory response in the morning is associated with alterations in the “shape” of the rhythm in axial length. The greater efficacy of the evening exposures for ocular growth inhibition is associated with an increased amplitude of the rhythm in axial length. How these alterations in rhythms may be mechanistically related to alterations in eye growth remains to be determined. We submit that these results may be translatable to school children, so that intensive bouts of near-work like reading should probably be scheduled later in the day, along with scheduled times for frequent breaks for exposure to myopic defocus.
Highlights.
Brief myopic defocus in the evening is most effective at eye growth inhibition.
Myopic defocus in the morning alters the rhythm in axial length.
Evening myopic defocus causes increased amplitude in the rhythm in axial length.
Acknowledgments
This work was funded by NIH-NEI-013636, NIH-NEI-025307 and T35-EY007149.
Footnotes
The experimental duration was extended from 5 days (used for plus lens-wear) to 7 to get a more robust defocus effect from the minus lenses: Measurements at 5 days showed borderline significant differences between “noon” and “evening” versus controls that reached significance using the longer duration.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashby R, Ohlendorf A, Schaeffel F. The effect of ambient illuminance on the development of deprivation myopia in chicks. Invest Ophthalmol Vis Sci. 2009;50:5348–5354. doi: 10.1167/iovs.09-3419. [DOI] [PubMed] [Google Scholar]
- Ashby R, Schaeffel F. The effect of bright light on lens-compensation in chicks. Invest Ophthalmol Vis Sci. 2010;51:5247–5253. doi: 10.1167/iovs.09-4689. [DOI] [PubMed] [Google Scholar]
- Backhouse S, Collins AV, Phillips JR. Influence of periodic vs continous daily bright light exposure on development of experimental myopia in the chick. Ophthal Physiol Opt. 2013;33:563–572. doi: 10.1111/opo.12069. [DOI] [PubMed] [Google Scholar]
- Brown JS, Flitcroft DI, Ying G, Francis EL, Schmid GF, Quinn GE, Stone RD. In vivo human choroidal thickness measurements: Evidence for diurnal fluctuations. Invest Ophthalmol Vis Sci. 2009;50:5–12. doi: 10.1167/iovs.08-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty R, Read SA, Collins MJ. Diurnal variations in axial length, choroidal thickness, intraocular pressure, and ocular biometrics. Invest Ophthalmol Vis Sci. 2011;52:5121–5129. doi: 10.1167/iovs.11-7364. [DOI] [PubMed] [Google Scholar]
- Chakraborty R, Read SA, Collins MJ. Monocular myopic defocus and daily changes in axial length and choroidal thickness of human eyes. Exp Eye Res. 2012;103:47–54. doi: 10.1016/j.exer.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Feldkaemper MP, Bernhard-Kurz S, Schaeffel F. Time of day influence of exposure to intense illuminance and inherent variability of intense illuminance on the development of experimentally induced myopia in chicks. 2016 ARVO E-Abstract #5528. [Google Scholar]
- Guggenheim JA, Northstone K, McMahon G, et al. Time outdoors and physical activity as predictors of indident myopia in childhood: a prospective cohort study. Invest Ophthalmol Vis Sci. 2012;53:2856–2865. doi: 10.1167/iovs.11-9091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Liu LJ, Xu L, et al. Outdoor activity and myopia among primary students in rural and urban regions of Beijing. Ophthamol. 2013;120:277–283. doi: 10.1016/j.ophtha.2012.07.086. [DOI] [PubMed] [Google Scholar]
- Gwiazda J, Bauer J, Thorn F, Held R. A dynamic relationship between myopia and blur-driven accommodation in school-aged children. Vision Res. 1995;35:1299–1304. doi: 10.1016/0042-6989(94)00238-h. [DOI] [PubMed] [Google Scholar]
- Nickla D. The phase relationships between the diurnal rhythm in axial length and choroidal thickness and the association with ocular growth rate in chicks. J Comp Physiol A. 2005;192:399–407. doi: 10.1007/s00359-005-0077-2. [DOI] [PubMed] [Google Scholar]
- Nickla D, Totonelly K. Brief light exposure at night disrupts the circadian rhythms in eye growth and choroidal thickness in chicks. Exp Eye Res. 2016;146:189–195. doi: 10.1016/j.exer.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickla D, Wildsoet C, Troilo D. Endogenous rhythms in axial length and choroidal thickness in chicks: Implications for ocular growth regulation. Invest Ophthalmol Vis Sci. 2001;42:584–588. [PubMed] [Google Scholar]
- Nickla D, Wildsoet C, Troilo D. Diurnal rhythms in intraocular pressure, axial length, and choroidal thickness in a primate model of eye growth, the common marmoset. Invest Ophthalmol Vis Sci. 2002;43:2519–2528. [PubMed] [Google Scholar]
- Nickla D, Zanzerkia R, Thai P, Totonelly K. Phase-dependent effects of brief periods of myopic defocus on the rhythms in axial length and choroid thickness in chicks. 2014 doi: 10.1016/j.exer.2016.11.012. ARVO E-Abstract #3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickla DL, Totonelly K. The efficacy of brief periods of “vision” in chick eyes wearing negative lenses is dependent on time of day. 2013 ARVO E-Abstract #5175. [Google Scholar]
- Nickla DL, Wildsoet C, Wallman J. Visual influences on diurnal rhythms in ocular length and choroidal thickness in chick eyes. Exp Eye Res. 1998;66:163–181. doi: 10.1006/exer.1997.0420. [DOI] [PubMed] [Google Scholar]
- Papastergiou GI, Schmid GF, Riva CE, Mendel MJ, Stone RA, Laties AM. Ocular axial length and choroidal thickness in newly hatched chicks and one-year-old chickens fluctuate in a diurnal pattern that is influenced by visual experience and intraocular pressure changes. Exp Eye Res. 1998;66:195–205. doi: 10.1006/exer.1997.0421. [DOI] [PubMed] [Google Scholar]
- Rose K, Morgan I, Ip J, Kifley A, Huynh S, Smith W, Mitchell P. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology. 2008;116:1229–1230. doi: 10.1016/j.ophtha.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Schmid K, Wildsoet CF. Effects of the compensatory responses to positive and negative lenses of intermittent lens wear and ciliary nerve section in chicks. Vision Res. 1996;36:1023–1036. doi: 10.1016/0042-6989(95)00191-3. [DOI] [PubMed] [Google Scholar]
- Stone RD, Quinn GE, Francis EL, Ying G, Flitcroft DI, Parekh P, Brown J, Orlow J, Schmid G. Diurnal axial length fluctuations in human eyes. Invest Ophthalmol Vis Sci. 2004;45:63–70. doi: 10.1167/iovs.03-0294. [DOI] [PubMed] [Google Scholar]
- Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43:447–468. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Winawer J, Wallman J. Temporal constraints on lens compensation in chicks. Vision Res. 2002;42:2651–2668. doi: 10.1016/s0042-6989(02)00300-0. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T, Matsuno A, Yamanaka Y, Nishide S, Honma S, Honma K. Daily exposure to cold phase-shifts the circadian clock of neonatal rats in vivo. Eur J Neurosci. 2013;37:491–497. doi: 10.1111/ejn.12052. [DOI] [PubMed] [Google Scholar]
- Zhu X, Park T, Winawer J, Wallman J. In a matter of minutes, the eye can know which way to grow. Invest Ophthalmol Vis Sci. 2005;46:2238–2241. doi: 10.1167/iovs.04-0956. [DOI] [PubMed] [Google Scholar]
- Zhu X, Wallman J. Temporal properties of compensation for positive and negative spectacle lenses in chicks. Invest Ophthalmol Vis Sci. 2009;50:37–46. doi: 10.1167/iovs.08-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Winawer J, Wallman J. Potency of myopic defocus in spectacle lens compensation. Invest Ophthalmol Vis Sci. 2003;44:2818–2827. doi: 10.1167/iovs.02-0606. [DOI] [PubMed] [Google Scholar]