Abstract
The goal of the study was to examine secreted protein response and withdrawal profiles from cultured human trabecular meshwork (HTM) cells following short- and long-term glucocorticoid treatment. Primary cultures of five human HTM cell strains isolated from 5 different individual donor eyes were tested. Confluent HTM cells were differentiated in culture media containing 1% FBS for at least one week, and then treated with Dexamethasone (Dex, 100 nM) 3 times/week for 1 or 4 weeks. Cell culture supernatant was collected 3 times per week for 8 weeks. Secretion profiles of myocilin (MYOC), matrix metalloproteinase-2 (MMP2) and fibronectin (FN) were determined by Western blot analysis and MMP2 activity by zymography. Dex treatment reduced MMP2 expression and activity, returning to normal levels shortly after Dex withdrawal in 5 HTM cell strains. All five cell strains significantly upregulated MYOC in response to Dex treatment by an average of 17-fold, but recovery to basal levels after Dex withdrawal took vastly different periods of time depending on cell strain and treatment duration. Dex treatment significantly increased FN secretion in all strains but one, which decreased FN secretion in the presence of Dex. Interestingly, secretion of FN and MYOC negatively correlated during a 4 week recovery period following 4 weeks of Dex treatment. Taken together, the time course and magnitude of response and recovery for three different secreted, extracellular matrix-associated proteins varied greatly between HTM cell strains, which may underlie susceptibility to glucocorticoid-induced ocular hypertension.
Keywords: glaucoma, dexamethasone, extracellular matrix, conventional outflow, ocular hypertension
1. Introduction
Glucocorticoids (GC) have been a mainstay of therapy in reducing systemic and ocular inflammation since the 1950s (Gordon, 1956). However, GC use often induces ocular hypertension, optic nerve head damage, and visual field defects if left untreated. Elevation of intraocular pressure (IOP) usually occurs weeks to months after GC administration, and happens in ~40% of patients without glaucoma, called “steroid responder”, and interestingly in up to 90% of open angle glaucoma (OAG) patients (Becker and Mills, 1963; Bernstein et al., 1963; Jones and Rhee, 2006; Kersey and Broadway, 2006).
The exact mechanism for the GC-induced IOP elevation is uncertain, but due to its time course likely involves at least two cellular processes in the resistance-generation region of the conventional outflow pathway: increased barrier function at the inner wall of Schlemm’s canal (SC) and alterations in cell contractility and extracellular matrix (ECM) turnover in the trabecular meshwork (TM) (Clark and Wordinger, 2009). Morphological examination of the TM in patients having GC-induced glaucoma shows increased deposition of extracellular materials, specifically, increased ECM content in the juxtacanalicular tissue (cribriform region) (Johnson et al., 1997; Overby et al., 2014; Ueda et al., 2002). Moreover, GC treatment results in decreased intra-trabecular spaces (Fautsch et al., 2000) as a result of increased collagen, fibronectin and elastin deposition and an unbalance in ECM enzymes (Theocharis et al., 2016). It has been proposed that reduction in matrix metalloproteinase (MMP) activity might lead to enhanced deposition of ECM material in the trabecular meshwork and thus induce an elevated juxtacanalicular outflow resistance (Johnson et al., 1997; Rohen et al., 1973). Furthermore, an imbalance between MMPs and the tissue inhibitors of MMPs (TIMPs) within the trabecular meshwork is thought to contribute to POAG (Ashworth Briggs et al., 2015). Other common features of GC treated eyes are myocilin induction, cytoskeletal rearrangement (Clark et al., 2005; Hoare et al., 2009; Read et al., 2007) and inhibition of phagocytosis (Matsumoto and Johnson, 1997), which all may contribute to increased outflow resistance. Recently, it was noted that both humans and mice on prolonged corticosteroid treatment display increased deposition of basement membrane materials below the inner wall of Schlemm’s canal (SC), possibly contributing to increased barrier function (Johnson et al., 1997; Overby et al., 2014; Tektas et al., 2010). This was modeled in vitro by Alvarado showing that Dex increased junction complexes in both SC and TM cells, which resulted in increased transendothelial fluid flow resistance (Underwood et al., 1999).
The trabecular meshwork is a porous connective tissue with complex three-dimensional structure. The resident TM cells are responsible for maintenance of its unique architecture and their ECM constituents. The ECM is a dynamic structure composed of a number of different matrix proteins (Acott and Kelley, 2008) that is constantly remodeled by new deposition and proteolysis. In the current study, we focused on three secreted ECM-related proteins: matrix metalloproteinase-2 (MMP-2); myocillin (MYOC); and fibronectin (FN) whose expression is altered by Dex and which have also been shown to play important roles in ECM formation and remodeling (Stamer et al., 2013). MMP2, also called type IV collagenase is involved in the breakdown of ECM in normal physiological processes. The function of MYOC is unknown, however mutations in MYOC is a cause of hereditary open-angle glaucoma with ocular hypertension (Gharahkhani et al., 2015; Stone et al., 1997). FN is a high-molecular weight ECM glycoprotein whose expression is increased in TM tissues and the aqueous humor of glaucoma patients (Acott and Kelley, 2008; Wordinger et al., 2007).
Cultured human TM cells share many properties with human TM cells in situ (Porter et al., 2015) (Stamer and Clark, 2016) and are commonly used as a model to study the biological effects of corticosteroids (Raghunathan et al., 2015). Our previous study showed that TM cells modified the secretion of ECM proteins in response to three different types of GC treatment (Stamer et al., 2013). However, the variability in secretory responses by different TM cell strains to prolonged GC treatment and after withdrawal is unknown. In the current study, we examined primary cultures of HTM cells isolated from five different donor eyes of different ages at time of death. Cells were treated with dexamethasone (Dex) for short (1 week) or long (4 weeks) periods and assessed for individual responses to Dex treatment and the temporal expression patterns of the ECM-related proteins after withdrawal.
2. Material and Methods
2.1 HTM cell culture
Five strains of human trabecular meshwork cells (HTM120, 122, 123, 124, and 133) were isolated from donors aged 11 months, 54 years, 39 years, 81 years and 79 years, respectively at time of death, with no eye disease documented. Cells were isolated and characterized as previously described (Stamer et al., 2000; Stamer et al., 1995). Human eye tissues were sourced ethically from accredited US eye banks and their research use was in accordance with the terms of the informed consents of the donors and/or donor family.
HTM cells in passages 3–6 were seeded into 6 well plates in DMEM containing 10% fetal bovine serum (FBS) (Atlanta Biologicals, Atlanta, GA) until the cells reached confluency. The cells were then switched to DMEM medium containing 1% FBS for at least 7 days prior to experimentation.
2.2 Dexamethasone (Dex) treatment
HTM cells were treated with Dex (100 nM) or vehicle in fresh 1.8 ml DMEM containing 1% FBS. The cell culture supernatant was collected 3 times/week and replaced with fresh medium containing Dex. After 1 week or 4 weeks of Dex treatment, HTM cells were then incubated with fresh media without Dex and the culture media was collected/changed every 3 days for a total of 8 weeks.
2.3 Western Blot analysis
Media was collected from wells of culture plates 3 times/week for 8 weeks. At end of 8 weeks, cells were harvested and rinsed twice with cold PBS. Cells were scraped into 0.2 ml of lysis buffer (25% glycerol, 0.0625M Tris.HCl, 2% SDS) containing 5% beta mercaptoethanol. Cell culture media at each time point was mixed with 4 × loading buffer (50% glycerol, 0.125M Tris.HCl, 4% SDS) containing 5% beta mercaptoethanol and boiled for 10 min before storing at −20°C. For Western blotting, 24 μl of solubilized proteins containing 4 × loading buffer were loaded into 8% polyacrylamide gel slabs and separated via SDS-PAGE. Fractionated proteins were then transferred electrophoretically to nitrocellulose membranes. Non-specific binding of antibodies to membranes containing transferred proteins was reduced by incubating with Tris-buffered saline with 0.1% Tween 20 (TBS-T) containing 5% nonfat dry milk (blocking buffer). Monoclonal antibodies against MMP2 (1:1000, Calbiochem, Gibbstown, NJ) in blocking buffer were incubated overnight with membranes at 4°C. The next day, membranes were washed in TBS-T (3 times for 10 minutes) and were incubated again in blocking buffer containing horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) for 1 hour. After incubation, membranes were washed with TBS-T as before. Protein-antibody complexes were visualized using a chemiluminescent HRP antibody detection reagent spray (HyGLO; Denville Scientific, Inc., Metuchen, NJ) and exposure to x-ray film (Phenix Research Company, Candler, NC). Antibody complexes were removed from membranes using stripping buffer (Thermo Scientific, Rockford, IL), and re-blotted using affinity purified polyclonal antibodies raised in rabbits against myocilin (dilution of 300 ng/ml stock, 1:5000). Detection proceeded as described above. This process was repeated using monoclonal antibody recognizing fibronectin (1:1000, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and the same detection process as above. The protein abundance in each band was quantified by densitometry using ImageJ image analysis software (GeneSnap/GeneTools; Syngene, Frederick, MD).
In order to compare the three treatments (con, dex 1w and dex 4w), samples were controlled in three ways: 1) the same cell number of cells were seeded into 6-well plates, treated with same amount of media with identical volumes of media loaded onto gels to run Western blot (loading control). 2) The three samples at each time point were run on the same blot and the protein levels were normalized by the total proteins in the membrane using Pierce™ Reversible Protein Stain Kit for Nitrocellulose Membranes (Thermal Scientific, Waltham, MA) (internal control). 3) At end of the 8 weeks, total cell lysates from each well were collected, and identical volumes of each sample were tested using Western blot analysis for beta-actin content with monoclonal antibodies (1:5000, Sigma, St. Louis, MO) to verify that the same number of the cells were present in each well (Cell number control). After the normalizations, differences of three protein levels represent the different protein secretion profiles following short- and long-term Dex treatment.
2.4 Assessment of gelatinase activity by zymography
Zymography was performed as described previously (Frankowski et al., 2012). Briefly, conditioned media from HTM cells was mixed with 4× Laemmli buffer (50% glycerol, 0.125M Tris.HCl, 4% SDS) and incubated for 10 minutes at room temperature. Samples were loaded (10 μl/well) into substrate incorporated polyacrylamide gels (10 % v:v Tris–HCl acrylamide gels containing 0.1 % (w/v) gelatin, BioRad, Hercules, CA.) and proteins separated at a constant current of 60mA. Gels were then washed 3 times with washing buffer (2.5% Triton X-100 in ddH2O), 15 minutes per wash and then incubated with zymographic development buffer (50mM Tris-HCl, pH 7.4; 200mM NaCl; 50mM CaCl2) for 24 hours at 37°C. Gels were stained with 0.2% Coomassie blue for 2 hours, then de-stained for 1–2 hours and areas void of blue stain were indicative of areas of enzyme activity. Semi-quantitative densitometric analysis was performed on all gels to determine the extent of enzymatic digestion using ImageJ software (GeneSnap/GeneTools; Syngene, Frederick, MD) and expressed as the intensity of total-MMP2 bands of interest (72 kDa pro-form and 68 kDa active-form).
2.5 Statistics
Normality assumption of data (MYOC and FN in 4 weeks Dex treatment and recovery groups) was tested. Non-parametric correlation tests (Spearman and Kendall Tau) were used to measure total and between-group correlation between MYOC and FN in 4 weeks Dex treatment and 4 weeks recovery groups. The between-group correlation was obtained by averaging the FN and MYOC values for each cell strain across 4 weeks treatment and 4 weeks recovery time points, and then computing correlation coefficient across the 5 cell strains. Mann–Whitney U test was used to analyze statistical significant difference between groups. A P value ≤ 0.05 was considered statistically significant.
3. Results
In order to better understand the spectrum of responses to GCs, we monitored the secretion of three different ECM-related proteins (MYOC, MMP2 and FN) from five different HTM cell strains, exposed to either 1 week or 4 weeks of dexamethasone (100 nM) treatment. We then followed the secretion of these three proteins after treatment was stopped, terminating the experiments after 8 weeks for a total of 21 time points for each cell strain. Thus, cells treated for one week were followed for an additional seven weeks, while cells treated for 4 weeks were observed for an additional 4 weeks after Dex withdrawal. Shown in figure 1 are representative western blot data from these five HTM cell strains at weekly intervals for total 8 weeks. Treatments did not appear to alter cell morphology over the eight weeks of study (supplemental figure 1).
Figure 1. Dexamethasone (Dex)-induced changes in the secretion of MYOC, MMP2 and FN from five different HTM cell strains.
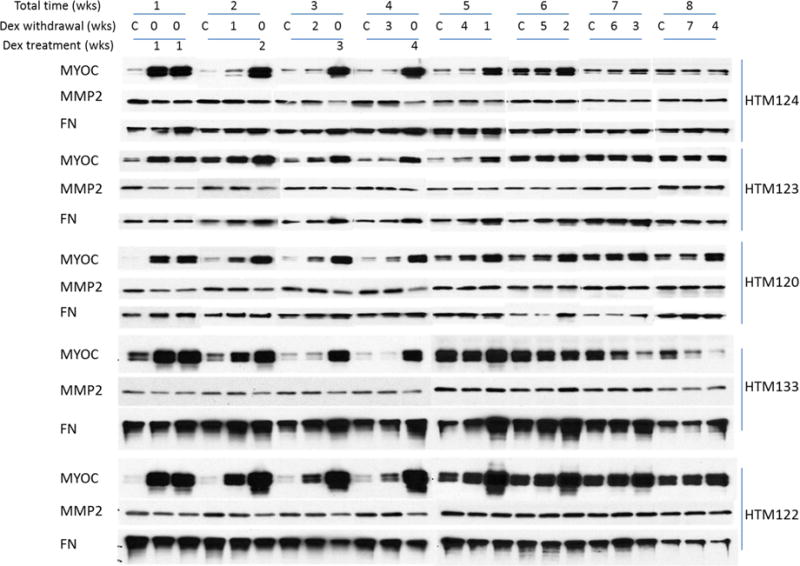
Five HTM cell strains were cultured in 1% DMEM medium for at least one week and then treated with Dex (100 nM, three times/week) for 1 or 4 weeks. In the 1 week treatment group, the secretion profile after cessation of Dex treatment was monitored for an additional 7 weeks, while the 4 week treatment group was monitored for an additional 4 weeks after Dex withdrawal. Protein levels of MYOC, MMP2 and FN in cell culture supernatant was monitored by Western blot: MYOC (size: 55/57kDa), MMP2 (size: 72kDa), and FN (size: 263kDa). Secretion levels by all five HTM cell strains (HTM124, 123, 120, 133 and 122) are displayed.
3.1 Myocilin
To better compare cellular responses to Dex treatment and their recovery, we specifically focused on individual protein secretion by different cell strains; the secretion profiles for MYOC are shown in figure 1. As reported previously, we observed that MYOC was highly induced in all five HTM cell strains treated with Dex for one or four weeks (Stamer et al., 1998). In the one week Dex treatment group, induction of MYOC ranged from 3.5-fold to 26.3-fold in presence of Dex, depending upon the cell strain (figure 2A). Recovery was also variable, with some cell strains returning back to normal levels within a few days, whereas others took weeks. Interestingly, one cell strain (HTM133) responded by continuing to elevate MYOC levels even after treatment withdrawal, and then having reduced levels compared to control weeks later. While the magnitude increase in secretion varied (3.9-fold to 12.5-fold), the secretion pattern for MYOC was more consistent amongst the different cell strains after 4 weeks of Dex treatment (figure 2B). In all strains, recovery from Dex withdrawal took much longer, with MYOC secretion remaining elevated for two weeks; gradually decreasing to normal levels. HTM133 was unique in that during recovery MYOC descended below starting levels two weeks after withdrawal. Combined data from all five cell strains is shown in figure 2C. On average, we observed about a 17-fold increase in MYOC secretion in both the one week and four weeks Dex treatment groups. The one week treatment group recovered to baseline levels at mean of 7 days after withdrawal, while the four week treatment group took over two weeks to recover on average.
Figure 2. Quantitative analysis of MYOC protein secretion by five HTM cell strains over time during and after Dexamethasone (Dex) treatment.
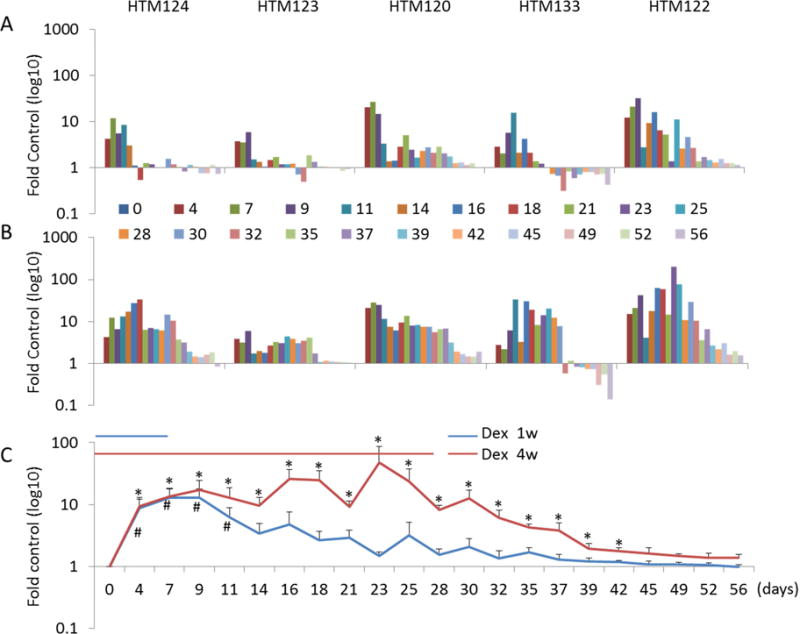
Densitometry was performed on Western blot bands (55/57 doublet) that correspond to MYOC using ImageJ software. Panel A shows results from cells treated with Dex for 1 week and were followed for 7 weeks after withdrawal; Panel B shows protein profile of cells treated with Dex for 4 weeks and were followed for an additional 4 weeks after cessation of treatment. Panel C shows combined data (mean ± SE) from all five cell strains in each treatment group over the 8 week evaluation period. *, P < 0.5 by Mann-Whitney U Test.
3.2 Matrix Metalloproteinase-2
Next we analyzed MMP2 secretion patterns by the same 5 HTM strains (figure 3). Densitometry of MMP2-specific bands on Western blots that correspond to cells treated with Dex (or after withdrawal) were normalized to untreated control at each time point. Shown in figure 3A are normalized data showing individual responses of five HTM cell strains treated for 1 week with Dex and were monitored for 7 weeks after removal of Dex. Figure 3B shows normalized data from cells treated with Dex for 4 weeks and were observed for an additional 4 weeks without Dex. Secretion of MMP2 was significantly lower after 1 week of Dex treatment; however individual cell strain responses ranged from 28% to 48%. MMP2 levels returned to normal in all cell strains 11 days after Dex removal. Similarly, data in figure 3B show that individual cell strains responded differently to 4 weeks of Dex treatment, with MMP2 secretion reduced by 28% to 58%. Interestingly, recovery profiles from Dex removal was also quite different between cell strains, with some strains returning MMP2 secretion level to normal within 9 days and others (HTM123 and HTM133) actually increasing MMP2 secretion weeks later. Combined data from all five cell strains are shown in figure 3C. Average decrease in MMP2 secretion after 1 week and 4 weeks treatment was about the same at 41% and 40%, respectively. The mean recovery time back to normal levels took about 7 days for the 1 week treated group and 4 days in 4 week treatment group, apparently due to disparity in recovery between individual cell strains.
Figure 3. Quantitative analysis of MMP2 protein levels in conditioned media from five HTM cell strains.
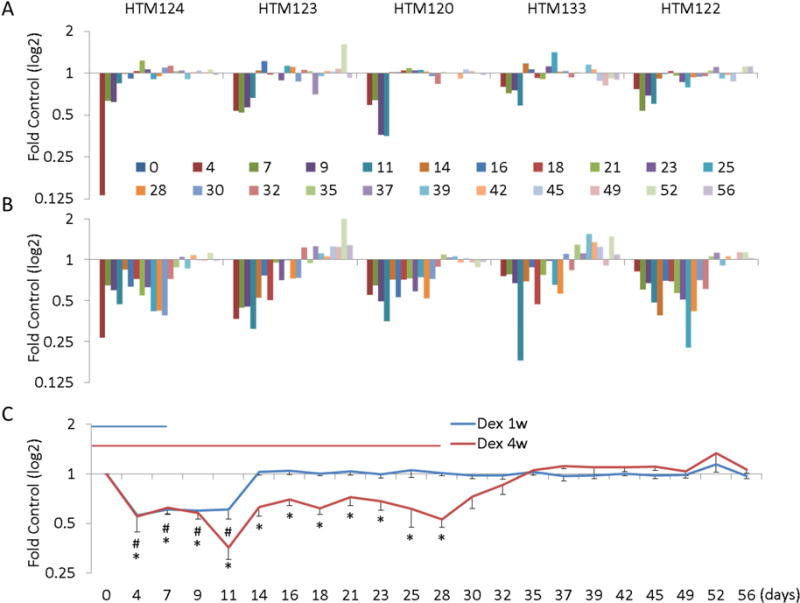
Western blots were immunostained with mouse monoclonal antibody against MMP2 and the bands intensities were analyzed by densitometry. Panel A shows results following 1 week of Dex treatment and 7 weeks of withdrawal. Panel B show data obtained after 4 weeks of Dex treatment and 4 weeks of withdrawal. Summarized data from all five cell strains show mean expression (± SE) levels at each time point are shown in panel C. *, P < 0.5 by Mann-Whitney U Test.
Effects of dex on MMP2 activity were also examined at select treatment and recovery time points as shown in figure 4. Both active- (68 kDa) and pro-forms (72 kDa) of MMP2 are visible on gels. However, the majority of MMP2 in HTM cell culture supernatant appears its pro-form (72 kDa), which is consistent to previous reports (Li et al., 2011). Summarized data from both forms of MMP2 show that MMP2 activity was significantly reduced but at lower levels at 1 week and 4 weeks treatment compared to protein measured by western blot (26% and 23% respectively). MMP2 activity returned to baseline levels after 3 weeks of recovery from either 1 week or 4 weeks of dex treatment.
Figure 4. Reduction of MMP2 activity in HTM cell supernatant by dexamethasone treatment.
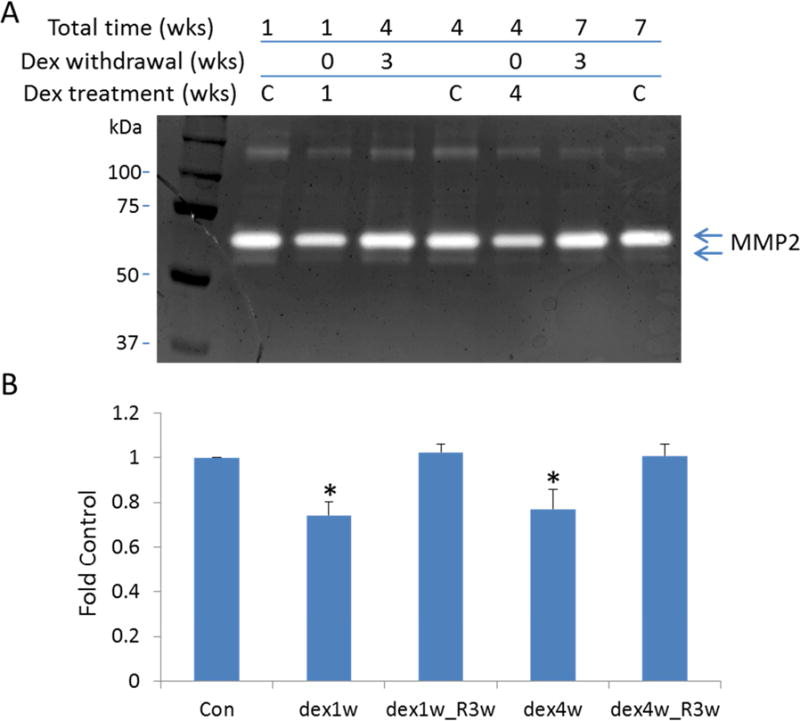
Zymography was performed to assess gelatinase activity in media conditioned by HTM cells treated with dexamethasone (1 week and 4 weeks), and followed up 3 weeks recovery for both treatment time points. Panel A shows a representative zymogram with prominent MMP2 bands of digestion in the presence/absence of Dex for 1 week or 4 weeks, plus following 3 weeks Dex withdrawal. Panel B displays quantitative densitometry of MMP2 activity results from all five cell strains. *, p < 0.05 compared to vehicle treated group at each time point. Labels: Dex1w or dex4w: Dex treatment for 1 week or 4 weeks; dex1w_R3w or dex4w_R3w: after 1 week or 3 weeks Dex treatment, cells were incubated in fresh medium for additional 3 more weeks.
3.3 Fibronectin
The last secreted protein that we examined was FN. Shown in figure 5A are results from experiments testing effects of Dex treatment for 1 week and recovery over 7 weeks. Interestingly, we found that FN secretion was elevated in 4 strains of HTM cells, but was decreased in 1 cell strain (HTM122). The increase in FN was relatively small for four cell strains compared to MYOC (range: 1.14–1.46-fold). Conversely, the secretion of FN decreased by 26% in HTM122. Recovery was rather slow in all but HTM122, taking nearly two weeks to reach starting levels. The induction of FN by four cell strains was variable, but more robust (1.4–2.5-fold) after 4 weeks of Dex treatment (figure 5B). Similar to before, FN secretion from HTM122 was reduced as a result of prolonged Dex treatment. The decrease in FN secretion stayed down for one additional week after Dex withdrawal. Figure 5C shows combined data of FN secretion in the presence and absence of Dex treatment from all 5 cell strains. Despite the fact that HTM122 cell secretion of FN was more than two standard deviations outside of range established by other four cell strains, the summary from all five stains still showed clear increase of FN at both 1 week and 4 weeks of Dex treatment. Mean increase in FN secretion was 1.24-fold after one week of Dex treatment (average of day 11 to day 21) and 1.52-fold after four weeks of treatment (average of day 11 to day 52 or day 28 to day 52). Recovery was slow in both groups, with the one week treatment group taking two weeks to return to baseline and the four week group on average taking almost four weeks.
Figure 5. Quantitative analysis of FN protein levels in conditioned supernatant from five HTM cell strains.
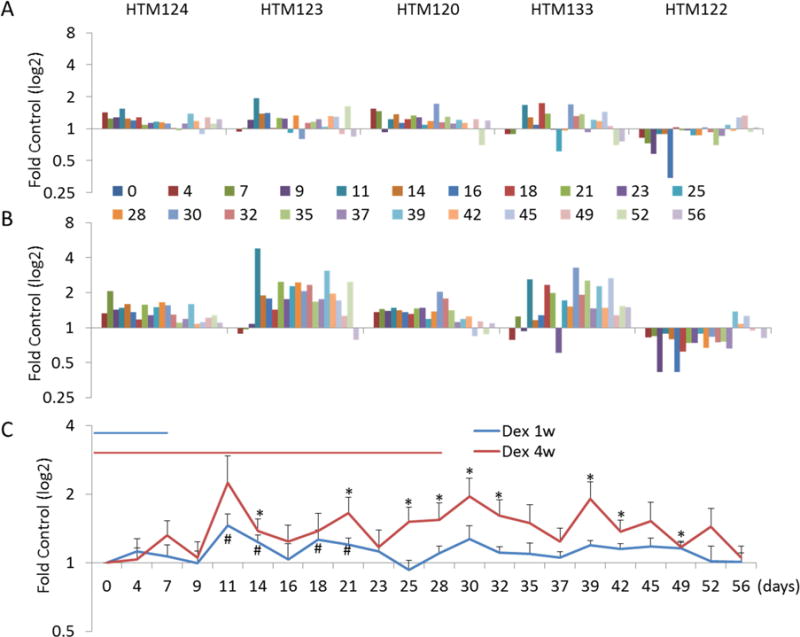
Western blots were immunostained with mouse monoclonal antibody against FN and the band intensities from each time point in each cell strain was analyzed. Panel A shows results following 1 week of Dex treatment and 7 weeks of withdrawal. Panel B displays findings of 4 weeks of Dex treatment and 4 weeks of withdrawal. Summarized expression levels (mean ± SE) of all five cell lines are shown in panel C. *, P < 0.5 by Mann-Whitney U Test.
3.4 Correlation between Secretion of FN and MYOC by Cells Treated with Dex
The level of FN secretion after Dex treatment appeared to negatively correlate changes in secretion of MYOC when comparing individual cell strains (compare figures 2A/B with 5A/B). In order to assess the degree of correlation between secretion of FN and MYOC, Spearman and Kendaul Tau rank correlation tests were conducted with magnitude of response in secretion of FN to the change of MYOC induction based on Western blot data of FN and MYOC from 4 weeks Dex treatment and recovery of all five HTM cell strains. Analysis of the correlation between FN and MYOC from all time points in 4 weeks treatment plus recovery groups was as follows: Spearman correlation coefficient = −0.15 (P = 0.14) and Kendall Tau correlation coefficient = −0.10 (P = 0.15), which were suggestive of relationship but not significantly. Analysis of only the 4 weeks Dex treatment times, averaging magnitude of effect for each cell strain the results were as follows: Spearman correlation coefficient improved = −0.7 (P = 0.19) and Kendall Tau correlation coefficient = −0.6 (P =0.14) (Table 1). Lastly, examination of the 4 week recovery period, comparing mean effect of each cell strain, produced the following results: Spearman correlation coefficient = − 0.9 (P = 0.04) and Kendall Tau correlation coefficient = −0.8 (P = 0.05), indicating a significant negative correlation between FN and MYOC using both correlation analysis methods (table 1). In contrast, we observed no correlation between the age of donors from which HTM cell strains were obtained and response to Dex (table 1).
Table 1.
Response of FN and MYOC to 4 weeks Dex treatment and recovery after cessation of treatment in individual cell strains and correlation analysis between FN and MYOC
| HTM124 (81 yrs) | HTM123 (39 yrs) | HTM120 (11 mo) | HTM133 (79 yrs) | HTM122 (54 yrs) | Spearman | Kendall Tau | |
|---|---|---|---|---|---|---|---|
| T (FN) | 1.49 ± 0.07 | 1.98 ± 0.33 | 1.39 ± 0.03 | 1.5 ± 0.2 | 0.68 ± 0.05 | Coefficient −0.7 P = 0.19 |
Coefficient −0.6 P = 0.14 |
| T (MYOC) | 12.8 ± 2.93 | 3.26 ± 0.39 | 13.3 ± 2.33 | 14.5 ± 3.21 | 57.6 ± 17.25 | ||
| R (FN) | 1.25 ± 0.06 | 1.91 ± 0.2 | 1.27 ± 0.12 | 1.85 ± 0.22 | 0.97 ± 0.07 | Coefficient −0.9 P = 0.04 |
Coefficient −0.8 P = 0.05 |
| R (MYOC) | 4.1 ± 1.47 | 1.91 ± 0.38 | 3.81 ± 0.8 | 1.4 ± 0.7 | 6.87 ± 2.6 |
Note: data represent mean ± S.E.M.; T (treatment); R (recovery).
4. Discussion
In this study, we document the secretion response and recovery profile of five different HTM cell strains exposed to short and long-term Dex. We monitored three proteins involved in ECM formation and remodeling, MMP2, MYOC and FN, over an eight weeks examination period. Our major finding was that responses to treatment and recovery was highly variable, depending upon the cell strain. In 14 out of 15 cases, cells responded to treatment in the same direction; however, the magnitude of response, duration and recovery times were unpredictable. Interestingly, we observed that MYOC and FN secretion levels were inversely related when comparing responses and recovery from different cell strains. To our knowledge, this is the most comprehensive study of differential response profiles of 5 different cell strains following Dex treatment and withdrawal that models treatment times in vivo.
The treatment regimen and drug concentrations were selected based on patient responses in the clinic and in vivo models. Data show GC-induced ocular hypertension onset is 1–4 weeks after treatment, with IOP returning to normal between 1–3 weeks after treatment stoppage. (Armaly, 1963; Clark et al., 1995; Overby et al., 2014). The 100 nM Dex concentration was chosen for treatments based on previous data showing measureable responses to long term treatment in outflow cells without inducing cytotoxicity (Jain et al., 2013; Sharma et al., 2013; Shepard et al., 2001; Stamer et al., 2013; Steely et al., 1992; Underwood et al., 1999; Yun et al., 1989) and aqueous humor concentration after topical application in the range of 50–1000 nM (Awan et al., 2009).
MMP2, MYOC, and FN were selected for study because they are constitutively secreted, and thus easily monitored over time in culture. Moreover, all three have been shown to be responsive to Dex treatment in multiple studies and are thought to be critical proteins involved ECM homeostasis in the trabecular meshwork (Acott and Kelley, 2008; Chuang et al., 2015; Nguyen et al., 1998; Polansky et al., 2000; Stamer et al., 2013; Steely et al., 1992; Wordinger et al., 2007). Although, we could not account for their proportional incorporation into the TM cell monolayer ECM, we were able to monitor their steady-state levels in conditioned media. MMP2 is involved in the proteolysis of type IV collagen, usually associated with basement membranes (De Groef et al., 2013). Reduction of MMP2 by Dex (Chuang et al., 2015; Stamer et al., 2013), as we observed in all five cell strains in the present study, may contribute to increased basement membrane accumulation below the SC inner wall. Previous studies found that GC treatment resulted in a doubling of SC inner wall covered by basement membrane in both living mice and humans (Johnson et al., 1997; Overby et al., 2014; Tektas et al., 2010).
MYOC was first discovered as a protein that is uniquely induced upon glucocorticoid treatment of TM cells (Nguyen et al., 1998; Polansky et al., 1997; Stone et al., 1997). In fact, the increased expression of MYOC caused by Dex is currently used to characterize cultured TM cells (Lin et al., 2007; Mao et al., 2012; Stamer et al., 1998). In the present study and others, the profile of MYOC up-regulation by DEX was dose- and time-dependent, very similar to the course of development of steroid-induced glaucoma (Nguyen et al., 1998) (Shepard et al., 2001; Tamm et al., 1999); suggesting that MYOC plays a role in GC-induced glaucoma (Shepard et al., 2001). While it was found that MYOC was dramatically induced in all five HTM cell strains, differences between cell strains were noticeable in their recovery profiles. For example, we observed that MYOC expression was elevated for much longer after Dex withdrawal in HTM120 (11 month-old) and 122 (54 year-old) than other cell strains. Similarly, others have observed previously that a higher concentration of Dex (500 nM) treatment for 6 days resulted in a two week recovery period for MYOC (Faralli et al., 2015). The significance of this delay in terms of GC-induced glaucoma is uncertain and needs to be explored further. Clearly, induction of myocilin by GCs alone does not impede outflow and cause GC-induced glaucoma (Gould et al., 2004). It is likely that MYOC participates in a pathway that impacts outflow function, which is evident when mutated, causing ocular hypertension and glaucoma (Stone et al., 1997).
In contrast, a clear association between enhanced deposition of the ECM glycoprotein FN with POAG and GC-induced glaucoma has been documented (Babizhayev and Brodskaya, 1989; Rodrigues et al., 1980; Steely et al., 1992). Increases in FN expression have been found in glaucomatous TM tissues and aqueous humor of patients (Acott and Kelley, 2008; Wordinger et al., 2007). FN is a multifunctional and ubiquitous ECM glycoprotein that is one of the major ECM proteins in the TM (Hann et al., 2001). FN plays a number of important roles in the ECM of outflow tissues, likely providing mechanical support for cell attachment and regulating many of the biological processes including matrix production, ECM turnover, gene expression, growth factor signaling, and cytoskeletal organization (Calderwood, 2004; Ivaska and Heino, 2000; Lee and Juliano, 2004; Morgan et al., 2007; Schwartz and Assoian, 2001). FN and its receptors also are one of the modulators of cellular response to physical forces such as tissue stretching (Katsumi et al., 2005), which may underlie a role for FN in outflow resistance changes in response to changes in IOP (Faralli et al., 2009). In our study, we found that FN secretion increased by ~47 % in two cell strains and ~2-fold in two other cell strains. Unexpectedly, we observed that FN secretion by one cell strain (HTM122) actually decreased in response to both one and four week Dex treatments, which to our knowledge has not been previously reported. Our results can be compared to that of Clark’s group who observed a ~2-fold elevation of FN in glaucomatous TM cells exposed to Dex (100 nM), ~50–60% increase in two normal TM cell strains and no increase in one normal strain (Steely et al., 1992). Taken together, our results and that of Clark emphasize the importance of using more than one primary TM cell strain for studies.
We found that there is an apparent inverse relationship between recovery of MYOC and FN in the five HTM cell lines. For example, after 4 weeks Dex treatment, MYOC levels in HTM133 returned to normal within 2 weeks, however, FN stayed up 4 more weeks. In another instance, the dramatic upregulation of MYOC in HTM122 corresponded to the reduction of FN. By comparison, a relatively lower induction of MYOC by Dex in HTM123 was correlated to the consistent higher levels of FN (table 1). In HTM120 and 124, the small increase of FN corresponded to higher induction of MYOC by Dex. When comparing all data points (four weeks of treatment and response) or mean change due to treatment for each cell strain, correlation analysis of MYOC and FN showed a trending inverse relationship that did not reach statistical significance. However, when comparing just the recovery phase after four weeks of Dex, we found a significant negative correlation between FN and MYOC secretion (table 1). This apparent relationship needs to be explored further.
In summary, this in vitro study examined the effects of two different Dex treatment regimens, mimicking the 1 and 4 week topical treatment times practiced in vivo. Using 5 different TM cell strains, we monitored the secretion of three proteins that are important regulators of ECM homeostasis in the TM, during and 4–7 weeks after stoppage of Dex treatment. We observed that each cell strain produced a unique response and recovery profile, emphasizing the need to develop personalized treatments for those with ocular hypertension in GC-induced and other types of glaucoma.
Supplementary Material
HTM cells were treated with Dex 100 nM for 1 and 4 weeks and then maintained in DMEM medium containing 1% FBS for additional 7 and 4 weeks, respectively. Images were captured at end of 8 weeks experiment under light microscope. Figures show images from three representative HTM cell strains: HTM120 (top panel), HTM123 (middle panel) and HTM124 (lower panel) under 10 × magnification.
Highlights.
Secretory responses to dexamethasone and recovery after cessation of treatment was highly variable, depending upon the human TM cell strain tested.
In 14 out of 15 cases, human TM cell strains responded to dexamethasone treatment in the same direction, however the magnitude of response, duration and recovery times were unpredictable.
MYOC and FN secretion levels were inversely related when comparing dexamethasone responses and recovery from different human TM cell strains.
Acknowledgments
This work was supported in part by Allergan Inc., Bright Focus Foundation (G2015100), Research to Prevent Blindness Foundation and NIH (EY019696).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acott TS, Kelley MJ. Extracellular matrix in the trabecular meshwork. Experimental eye research. 2008;86:543–561. doi: 10.1016/j.exer.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armaly MF. Effect of Corticosteroids on Intraocular Pressure and Fluid Dynamics. I. The Effect of Dexamethasone in the Normal Eye. Archives of ophthalmology. 1963;70:482–491. doi: 10.1001/archopht.1963.00960050484010. [DOI] [PubMed] [Google Scholar]
- Ashworth Briggs EL, Toh T, Eri R, Hewitt AW, Cook AL. TIMP1, TIMP2, and TIMP4 are increased in aqueous humor from primary open angle glaucoma patients. Molecular vision. 2015;21:1162–1172. [PMC free article] [PubMed] [Google Scholar]
- Awan MA, Agarwal PK, Watson DG, McGhee CN, Dutton GN. Penetration of topical and subconjunctival corticosteroids into human aqueous humour and its therapeutic significance. The British journal of ophthalmology. 2009;93:708–713. doi: 10.1136/bjo.2008.154906. [DOI] [PubMed] [Google Scholar]
- Babizhayev MA, Brodskaya MW. Fibronectin detection in drainage outflow system of human eyes in ageing and progression of open-angle glaucoma. Mechanisms of ageing and development. 1989;47:145–157. doi: 10.1016/0047-6374(89)90017-1. [DOI] [PubMed] [Google Scholar]
- Becker B, Mills DW. Corticosteroids and Intraocular Pressure. Archives of ophthalmology. 1963;70:500–507. doi: 10.1001/archopht.1963.00960050502012. [DOI] [PubMed] [Google Scholar]
- Bernstein HN, Mills DW, Becker B. Steroid-induced elevation of intraocular pressure. Archives of ophthalmology. 1963;70:15–18. doi: 10.1001/archopht.1963.00960050017005. [DOI] [PubMed] [Google Scholar]
- Calderwood DA. Integrin activation. Journal of cell science. 2004;117:657–666. doi: 10.1242/jcs.01014. [DOI] [PubMed] [Google Scholar]
- Chuang TD, Pearce WJ, Khorram O. miR-29c induction contributes to downregulation of vascular extracellular matrix proteins by glucocorticoids. American journal of physiology Cell physiology. 2015;309:C117–125. doi: 10.1152/ajpcell.00254.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AF, Brotchie D, Read AT, Hellberg P, English-Wright S, Pang IH, Ethier CR, Grierson I. Dexamethasone alters F-actin architecture and promotes cross-linked actin network formation in human trabecular meshwork tissue. Cell motility and the cytoskeleton. 2005;60:83–95. doi: 10.1002/cm.20049. [DOI] [PubMed] [Google Scholar]
- Clark AF, Wilson K, de Kater AW, Allingham RR, McCartney MD. Dexamethasone-induced ocular hypertension in perfusion-cultured human eyes. Investigative ophthalmology & visual science. 1995;36:478–489. [PubMed] [Google Scholar]
- Clark AF, Wordinger RJ. The role of steroids in outflow resistance. Experimental eye research. 2009;88:752–759. doi: 10.1016/j.exer.2008.10.004. [DOI] [PubMed] [Google Scholar]
- De Groef L, Van Hove I, Dekeyster E, Stalmans I, Moons L. MMPs in the trabecular meshwork: promising targets for future glaucoma therapies? Investigative ophthalmology & visual science. 2013;54:7756–7763. doi: 10.1167/iovs.13-13088. [DOI] [PubMed] [Google Scholar]
- Faralli JA, Clark RW, Filla MS, Peters DM. NFATc1 activity regulates the expression of myocilin induced by dexamethasone. Experimental eye research. 2015;130:9–16. doi: 10.1016/j.exer.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faralli JA, Schwinn MK, Gonzalez JM, Jr, Filla MS, Peters DM. Functional properties of fibronectin in the trabecular meshwork. Experimental eye research. 2009;88:689–693. doi: 10.1016/j.exer.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fautsch MP, Bahler CK, Jewison DJ, Johnson DH. Recombinant TIGR/MYOC increases outflow resistance in the human anterior segment. Investigative ophthalmology & visual science. 2000;41:4163–4168. [PubMed] [Google Scholar]
- Frankowski H, Gu YH, Heo JH, Milner R, Del Zoppo GJ. Use of gel zymography to examine matrix metalloproteinase (gelatinase) expression in brain tissue or in primary glial cultures. Methods in molecular biology. 2012;814:221–233. doi: 10.1007/978-1-61779-452-0_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharahkhani P, Burdon KP, Hewitt AW, Law MH, Souzeau E, Montgomery GW, Radford-Smith G, Mackey DA, Craig JE, MacGregor S. Accurate Imputation-Based Screening of Gln368Ter Myocilin Variant in Primary Open-Angle Glaucoma. Investigative ophthalmology & visual science. 2015;56:5087–5093. doi: 10.1167/iovs.15-17305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon DM. Prednisone and prednisolone in ocular disease. American journal of ophthalmology. 1956;41:593–600. [PubMed] [Google Scholar]
- Gould DB, Miceli-Libby L, Savinova OV, Torrado M, Tomarev SI, Smith RS, John SW. Genetically increasing Myoc expression supports a necessary pathologic role of abnormal proteins in glaucoma. Molecular and cellular biology. 2004;24:9019–9025. doi: 10.1128/MCB.24.20.9019-9025.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hann CR, Springett MJ, Wang X, Johnson DH. Ultrastructural localization of collagen IV, fibronectin, and laminin in the trabecular meshwork of normal and glaucomatous eyes. Ophthalmic research. 2001;33:314–324. doi: 10.1159/000055687. [DOI] [PubMed] [Google Scholar]
- Hoare MJ, Grierson I, Brotchie D, Pollock N, Cracknell K, Clark AF. Cross-linked actin networks (CLANs) in the trabecular meshwork of the normal and glaucomatous human eye in situ. Investigative ophthalmology & visual science. 2009;50:1255–1263. doi: 10.1167/iovs.08-2706. [DOI] [PubMed] [Google Scholar]
- Ivaska J, Heino J. Adhesion receptors and cell invasion: mechanisms of integrin-guided degradation of extracellular matrix. Cellular and molecular life sciences: CMLS. 2000;57:16–24. doi: 10.1007/s000180050496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A, Liu X, Wordinger RJ, Yorio T, Cheng YQ, Clark AF. Effects of thailanstatins on glucocorticoid response in trabecular meshwork and steroid-induced glaucoma. Investigative ophthalmology & visual science. 2013;54:3137–3142. doi: 10.1167/iovs.12-11480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D, Gottanka J, Flugel C, Hoffmann F, Futa R, Lutjen-Drecoll E. Ultrastructural changes in the trabecular meshwork of human eyes treated with corticosteroids. Archives of ophthalmology. 1997;115:375–383. doi: 10.1001/archopht.1997.01100150377011. [DOI] [PubMed] [Google Scholar]
- Jones R, 3rd, Rhee DJ. Corticosteroid-induced ocular hypertension and glaucoma: a brief review and update of the literature. Current opinion in ophthalmology. 2006;17:163–167. doi: 10.1097/01.icu.0000193079.55240.18. [DOI] [PubMed] [Google Scholar]
- Katsumi A, Naoe T, Matsushita T, Kaibuchi K, Schwartz MA. Integrin activation and matrix binding mediate cellular responses to mechanical stretch. The Journal of biological chemistry. 2005;280:16546–16549. doi: 10.1074/jbc.C400455200. [DOI] [PubMed] [Google Scholar]
- Kersey JP, Broadway DC. Corticosteroid-induced glaucoma: a review of the literature. Eye. 2006;20:407–416. doi: 10.1038/sj.eye.6701895. [DOI] [PubMed] [Google Scholar]
- Lee JW, Juliano R. Mitogenic signal transduction by integrin- and growth factor receptor-mediated pathways. Molecules and cells. 2004;17:188–202. [PubMed] [Google Scholar]
- Li A, Leung CT, Peterson-Yantorno K, Stamer WD, Civan MM. Cytoskeletal dependence of adenosine triphosphate release by human trabecular meshwork cells. Investigative ophthalmology & visual science. 2011;52:7996–8005. doi: 10.1167/iovs.11-8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Lee OT, Minasi P, Wong J. Isolation, culture, and characterization of human fetal trabecular meshwork cells. Current eye research. 2007;32:43–50. doi: 10.1080/02713680601107058. [DOI] [PubMed] [Google Scholar]
- Mao W, Liu Y, Mody A, Montecchi-Palmer M, Wordinger RJ, Clark AF. Characterization of a spontaneously immortalized bovine trabecular meshwork cell line. Experimental eye research. 2012;105:53–59. doi: 10.1016/j.exer.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Johnson DH. Dexamethasone decreases phagocytosis by human trabecular meshwork cells in situ. Investigative ophthalmology & visual science. 1997;38:1902–1907. [PubMed] [Google Scholar]
- Morgan MR, Humphries MJ, Bass MD. Synergistic control of cell adhesion by integrins and syndecans. Nature reviews Molecular cell biology. 2007;8:957–969. doi: 10.1038/nrm2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TD, Chen P, Huang WD, Chen H, Johnson D, Polansky JR. Gene structure and properties of TIGR, an olfactomedin-related glycoprotein cloned from glucocorticoid-induced trabecular meshwork cells. The Journal of biological chemistry. 1998;273:6341–6350. doi: 10.1074/jbc.273.11.6341. [DOI] [PubMed] [Google Scholar]
- Overby DR, Bertrand J, Tektas OY, Boussommier-Calleja A, Schicht M, Ethier CR, Woodward DF, Stamer WD, Lutjen-Drecoll E. Ultrastructural changes associated with dexamethasone-induced ocular hypertension in mice. Investigative ophthalmology & visual science. 2014;55:4922–4933. doi: 10.1167/iovs.14-14429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polansky JR, Fauss DJ, Chen P, Chen H, Lutjen-Drecoll E, Johnson D, Kurtz RM, Ma ZD, Bloom E, Nguyen TD. Cellular pharmacology and molecular biology of the trabecular meshwork inducible glucocorticoid response gene product. Ophthalmologica. Journal international d’ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. 1997;211:126–139. doi: 10.1159/000310780. [DOI] [PubMed] [Google Scholar]
- Polansky JR, Fauss DJ, Zimmerman CC. Regulation of TIGR/MYOC gene expression in human trabecular meshwork cells. Eye. 2000;14(Pt 3B):503–514. doi: 10.1038/eye.2000.137. [DOI] [PubMed] [Google Scholar]
- Porter K, Hirt J, Stamer WD, Liton PB. Autophagic dysregulation in glaucomatous trabecular meshwork cells. Biochimica et biophysica acta. 2015;1852:379–385. doi: 10.1016/j.bbadis.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghunathan VK, Morgan JT, Park SA, Weber D, Phinney BS, Murphy CJ, Russell P. Dexamethasone Stiffens Trabecular Meshwork, Trabecular Meshwork Cells, and Matrix. Investigative ophthalmology & visual science. 2015;56:4447–4459. doi: 10.1167/iovs.15-16739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read AT, Chan DW, Ethier CR. Actin structure in the outflow tract of normal and glaucomatous eyes. Experimental eye research. 2007;84:214–226. doi: 10.1016/j.exer.2005.10.035. [DOI] [PubMed] [Google Scholar]
- Rodrigues MM, Katz SI, Foidart JM, Spaeth GL. Collagen, factor VIII antigen, and immunoglobulins in the human aqueous drainage channels. Ophthalmology. 1980;87:337–345. doi: 10.1016/s0161-6420(80)35242-1. [DOI] [PubMed] [Google Scholar]
- Rohen JW, Linner E, Witmer R. Electron microscopic studies on the trabecular meshwork in two cases of corticosteroid-glaucoma. Experimental eye research. 1973;17:19–31. doi: 10.1016/0014-4835(73)90164-4. [DOI] [PubMed] [Google Scholar]
- Schwartz MA, Assoian RK. Integrins and cell proliferation: regulation of cyclin-dependent kinases via cytoplasmic signaling pathways. Journal of cell science. 2001;114:2553–2560. doi: 10.1242/jcs.114.14.2553. [DOI] [PubMed] [Google Scholar]
- Sharma A, Patil AJ, Mansoor S, Estrago-Franco MF, Raymond V, Kenney MC, Kuppermann BD. Effects of dexamethasone on human trabecular meshwork cells in vitro. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2013;251:1741–1746. doi: 10.1007/s00417-013-2343-2. [DOI] [PubMed] [Google Scholar]
- Shepard AR, Jacobson N, Fingert JH, Stone EM, Sheffield VC, Clark AF. Delayed secondary glucocorticoid responsiveness of MYOC in human trabecular meshwork cells. Investigative ophthalmology & visual science. 2001;42:3173–3181. [PubMed] [Google Scholar]
- Stamer DW, Roberts BC, Epstein DL, Allingham RR. Isolation of primary open-angle glaucomatous trabecular meshwork cells from whole eye tissue. Current eye research. 2000;20:347–350. [PubMed] [Google Scholar]
- Stamer WD, Clark AF. The many faces of the trabecular meshwork cell. Experimental eye research. 2016 doi: 10.1016/j.exer.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamer WD, Hoffman EA, Kurali E, Krauss AH. Unique response profile of trabecular meshwork cells to the novel selective glucocorticoid receptor agonist, GW870086X. Investigative ophthalmology & visual science. 2013;54:2100–2107. doi: 10.1167/iovs.12-11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamer WD, Roberts BC, Howell DN, Epstein DL. Isolation, culture, and characterization of endothelial cells from Schlemm’s canal. Investigative ophthalmology & visual science. 1998;39:1804–1812. [PubMed] [Google Scholar]
- Stamer WD, Seftor RE, Williams SK, Samaha HA, Snyder RW. Isolation and culture of human trabecular meshwork cells by extracellular matrix digestion. Current eye research. 1995;14:611–617. doi: 10.3109/02713689508998409. [DOI] [PubMed] [Google Scholar]
- Steely HT, Browder SL, Julian MB, Miggans ST, Wilson KL, Clark AF. The effects of dexamethasone on fibronectin expression in cultured human trabecular meshwork cells. Investigative ophthalmology & visual science. 1992;33:2242–2250. [PubMed] [Google Scholar]
- Stone EM, Fingert JH, Alward WL, Nguyen TD, Polansky JR, Sunden SL, Nishimura D, Clark AF, Nystuen A, Nichols BE, Mackey DA, Ritch R, Kalenak JW, Craven ER, Sheffield VC. Identification of a gene that causes primary open angle glaucoma. Science. 1997;275:668–670. doi: 10.1126/science.275.5300.668. [DOI] [PubMed] [Google Scholar]
- Tamm ER, Russell P, Epstein DL, Johnson DH, Piatigorsky J. Modulation of myocilin/TIGR expression in human trabecular meshwork. Investigative ophthalmology & visual science. 1999;40:2577–2582. [PubMed] [Google Scholar]
- Tektas OY, Hammer CM, Danias J, Candia O, Gerometta R, Podos SM, Lutjen-Drecoll E. Morphologic changes in the outflow pathways of bovine eyes treated with corticosteroids. Investigative ophthalmology & visual science. 2010;51:4060–4066. doi: 10.1167/iovs.09-4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK. Extracellular matrix structure. Advanced drug delivery reviews. 2016;97:4–27. doi: 10.1016/j.addr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Ueda J, Wentz-Hunter K, Yue BY. Distribution of myocilin and extracellular matrix components in the juxtacanalicular tissue of human eyes. Investigative ophthalmology & visual science. 2002;43:1068–1076. [PubMed] [Google Scholar]
- Underwood JL, Murphy CG, Chen J, Franse-Carman L, Wood I, Epstein DL, Alvarado JA. Glucocorticoids regulate transendothelial fluid flow resistance and formation of intercellular junctions. The American journal of physiology. 1999;277:C330–342. doi: 10.1152/ajpcell.1999.277.2.C330. [DOI] [PubMed] [Google Scholar]
- Wordinger RJ, Fleenor DL, Hellberg PE, Pang IH, Tovar TO, Zode GS, Fuller JA, Clark AF. Effects of TGF-beta2, BMP-4, and gremlin in the trabecular meshwork: implications for glaucoma. Investigative ophthalmology & visual science. 2007;48:1191–1200. doi: 10.1167/iovs.06-0296. [DOI] [PubMed] [Google Scholar]
- Yun AJ, Murphy CG, Polansky JR, Newsome DA, Alvarado JA. Proteins secreted by human trabecular cells. Glucocorticoid and other effects. Investigative ophthalmology & visual science. 1989;30:2012–2022. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
HTM cells were treated with Dex 100 nM for 1 and 4 weeks and then maintained in DMEM medium containing 1% FBS for additional 7 and 4 weeks, respectively. Images were captured at end of 8 weeks experiment under light microscope. Figures show images from three representative HTM cell strains: HTM120 (top panel), HTM123 (middle panel) and HTM124 (lower panel) under 10 × magnification.


