Abstract
Drug-induced liver injury (DILI) is a major public health problem. Intrinsic (dose-dependent) DILI associated with acetaminophen overdose is the number one cause of acute liver failure in the US. However the most problematic type of DILI impacting drug development is idiosyncratic, occurring only very rarely among treated patients and often only after several weeks or months of treatment with the offending drug. Recent advances in our understanding of the pathogenesis of DILI suggest that three mechanisms may underlie most hepatocyte effects in response to both intrinsic and idiosyncratic DILI drugs: mitochondrial dysfunction, oxidative stress, and alterations in bile acid homeostasis. However, in some cases, hepatocyte stress promotes an immune response that results in clinically important idiosyncratic DILI. This review discusses recent advances in our understanding of the pathogenesis of both intrinsic and idiosyncratic DILI as well as emerging tools and techniques that will likely improve DILI risk identification and management.
Keywords: Drug-induced liver injury, Idiosyncratic DILI, Intrinsic DILI, Mechanisms, Risk Management
INTRODUCTION
Drug-induced liver injury (DILI) is a major public health problem impacting patients, health care providers, drug developers, and drug regulators. DILI is the number one cause of acute liver failure (ALF) in the US 1. Although complete recovery is expected for patients experiencing less serious DILI, the associated symptoms (e.g. fatigue, itching, nausea) can be debilitating and recovery can be prolonged, with about 20% of patients having biochemical evidence of continuing liver injury 6 months after diagnosis 2.
Diagnosis of DILI remains a significant challenge for health care providers 3. When a patient develops DILI, there are currently no tests available to physicians that can confidently establish the diagnosis. The clinical presentation of DILI varies widely and is indistinguishable from other hepatic disorders such as viral hepatitis. The diagnosis of DILI can also be confounded by preexisting liver disease. Furthermore, in patients taking multiple drugs, it is often challenging to confidently identify the specific drug causing DILI. While an extensive diagnostic evaluation is underway, the physician may be forced to unnecessarily terminate or substitute treatments resulting in exposure to new adverse drug event risks and possible suboptimal treatment of underlying diseases. Finally, until DILI has resulted in organ dysfunction, current biomarkers cannot predict whether a patient with DILI will recover quickly, recover slowly, or progress to ALF so that all patients experiencing DILI must be monitored closely until recovery is underway.
DILI also remains a major adverse event that leads to termination of clinical drug development programs 4. In some cases, this results from discovery of dose-dependent or “intrinsic” hepatotoxicity in Phase 1 clinical trials which may not have been suspected based on preclinical studies. But the most problematic form of DILI in drug development is “idiosyncratic”, occurring only very rarely among treated patients and often only after several months of treatment with the offending drug. As a result, this liability is typically detected only late in clinical development. A recent example was termination of development of the promising diabetes drug fasiglifam due to DILI recognized only late in phase 3 clinical trials 5. Termination this late in development often equates to a more than $1B investment and in this case, over 5,000 patients in the clinical trials exposed to risk without public health benefit.
The prediction of idiosyncratic DILI is complicated by the apparent need for individual susceptibility factors that are not typically represented in preclinical models or even among subjects in small clinical trials. While drugs that cause idiosyncratic DILI typically also cause a more frequent mild and asymptomatic liver injury – and this can usually be detected early in clinical development as transient, asymptomatic elevations in serum alanine aminotransferase (ALT) occurring in some treated subjects, the frequency and magnitude of serum ALT elevations does not correlate well with the risk of idiosyncratic DILI. For example, there are drugs that cause frequent and significant (>3x ULN) elevations in serum ALT but yet have little or no liver safety risk (e.g. heparins, cholestyramine, statins). There are no tests available that have been shown to distinguish elevations in serum ALT that do or do not portend idiosyncratic DILI. As a result, the only current way to make this distinction is to continue to treat patients who develop asymptomatic elevations in serum ALT to determine whether or not they will develop elevations in serum bilirubin, indicating global liver dysfunction. However, continued treatment of patients after drug-induced ALT elevations to determine whether they will develop global liver dysfunction, even with careful monitoring, is placing clinical trial subjects at risk for ALF 4. Dr. Hy Zimmerman observed that hepatocellular injuries sufficient to impair bilirubin excretion are capable of causing potentially lethal consequences, i.e. at least a 10% rate of death or transplant, and clinical trial subjects with drug-induced hepatocellular injuries and elevations in total serum bilirubin > 2x ULN are referred to as “Hy’s Law Cases” 6.
Unfortunately, once a new drug candidate has been shown to cause elevations in serum ALT, regulators increasingly demand larger and longer clinical trials to better characterize the liver safety risk. This creates a bottleneck in the regulatory approval process, delaying patient access to important new medications and substantially driving up their cost 4. For example, an FDA Advisory Committee recommended approval of rivaroxaban for short term clot prophylaxis but the drug did not receive approval for marketing in the US until 2 years later and after an additional 14,000 subjects received long term treatment with this drug 7. In addition, when a drug with liver safety concerns is marketed, its use may be tied to onerous requirements for frequent liver chemistry testing and then may be available only to patients who have failed other treatments 8.
It is understandable then that companies may be quick to terminate drugs once suspicions of hepatotoxicity emerge at any point in preclinical or clinical development. But it is important to note that this practice is undoubtedly resulting in the abandonment of drugs that would be relatively safe for the liver. For example, acetaminophen causes severe liver injury in rodents and in man at much less than 10-fold exposure safety margins. In addition, therapeutic dosing of acetaminophen causes frequent serum ALT elevations in healthy adults 9. It is therefore unlikely that acetaminophen would enter Phase 2 clinical trials even if it were a new drug candidate addressing an unmet medical need. Likewise, ibuprofen, clearly among the safest drugs on the market, can cause kidney and liver failure at therapeutic doses in the dog. If ibuprofen were a new drug addressing an unmet medical need, and the dog was used in preclinical testing, it probably would never progress into phase 1 clinical studies.
Finally, it should also be noted that DILI due to herbal and dietary supplements (HDS) has also become a major public health problem, accounting for about 1/5th of the cases of acute DILI entering the registry of the US Drug Induced Liver Injury Network 10. This is likely due to a combination of the growing use of HDS by the American population and the limited regulation of HDS conferred by the Dietary Supplement Health and Education Act of 1994 – which does not require manufacturers to demonstrate a product’s safety or efficacy prior to marketing 11.
INTRINSIC DILI
Drugs that induce liver injury in a predictable, dose-dependent manner in both preclinical models and humans are said to cause “intrinsic DILI”. Acetaminophen is the most common cause of intrinsic DILI in the US, representing approximately half of all ALF cases 1. While most of these cases are the result of intentional overdose, many are the result of unintentional overdose caused by failure to adhere to dosing recommendations (particularly for opiate:acetaminophen combinations), unknowing consumption of multiple medications containing acetaminophen, or other factors that lower the threshold for acetaminophen hepatotoxicity 12. If provided shortly after exposure, N-acetyl cysteine (NAC) can very be effective in the treatment of acetaminophen-induced hepatotoxicity. However, successful treatment is more common in cases of intentional overdose where patients have acute exposure, are more likely to present to an emergency room, and acetaminophen overdose is immediately recognized 13. Few other drugs on the market cause life-threatening intrinsic DILI because this liability is generally identified during preclinical or early clinical studies. Such drugs are either abandoned from further development, may be progressed at doses well below those anticipated to cause liver injury, or administered in controlled situations (e.g. intravenous chemotherapy).
Mechanisms of Intrinsic DILI
Three mechanisms appear to account for the majority of intrinsic DILI: mitochondrial dysfunction, oxidative stress, and alterations in bile acid homeostasis. Support for the important role of these three mechanisms in DILI comes from studies that have shown the ability to retrospectively identify DILI liability for most drugs by their abilities to interfere with one or more of these mechanisms at clinically relevant plasma drug concentrations 14, 15, 16, 17. Mitochondria produce ATP that is required to maintain all vital cellular functions. DILI causing drugs can inhibit mitochondrial function, resulting in reduced levels of ATP, a decline in cell function, and eventually cell death 17. Oxidative stress is the result of reactive oxygen species (ROS) which are a byproduct of normal metabolism and have roles in cell signaling and homeostasis. However, some DILI causing drugs can increase ROS accumulation though a variety of mechanisms 15. When the processes that exist to regulate cellular levels of ROS are exceeded, oxidative stress can result in damage to key cellular components and eventually cell death. Finally, a major function of the liver is the transport of bile salts from blood into bile. DILI causing drugs can disrupt this process in many ways, most importantly through reducing hepatic bile acid efflux by inhibition of the bile salt export protein (BSEP) 14. This results in the intracellular accumulation of toxic bile acids, which can lead to hepatocyte death.
While mitochondrial dysfunction, oxidative stress, and alterations in bile acid homeostasis may each have a distinct contribution to intrinsic DILI, they are often interlinked and work together to promote toxicity 16. For example, mitochondrial dysfunction results in a decline in ATP production which can in turn reduce ATP-dependent bile acid transport by BSEP into the canaliculi. Similarly, bile acid accumulation can disrupt mitochondrial oxidative phosphorylation. Furthermore, mitochondrial dysfunction can result in increased production of ROS and ROS can target mitochondrial DNA and proteins leading to mitochondrial dysfunction. Acetaminophen toxicity for example is the result of a reactive intermediate, N-acetyl-p-benzoquinone imine (NAPQI), produced by cytochrome P450 (CYP) metabolism. In small quantities, NAPQI is readily detoxified by conjugation with glutathione (GSH). However, in excess, NAPQI production depletes mitochondrial GSH reducing the ability to neutralize ROS resulting in mitochondrial injury. Once GSH is depleted, the NAPQI concentration rises causing NAPQI to bind to mitochondrial proteins probably promoting mitochondrial injury18. The antidote, N-acetyl cysteine (NAC), acts primarily by facilitating the regeneration of GSH and promoting the detoxification of NAPQI.
Limitations of Preclinical Models to Detect Intrinsic DILI
While intrinsic DILI resulting from these mechanisms can often be accurately predicted in preclinical in vitro or animal studies, there are drugs eliciting toxicities in humans via these mechanisms that appear “clean” in all preclinical screens. This is likely explained by species differences in animal models and/or physiological differences in cultured human hepatocytes.
One major reason preclinical models fail to detect intrinsic DILI liability is due to differences in drug metabolism and disposition. In many cases, toxicity is not from the parent drug, but rather results from metabolites, often catalyzed by CYP metabolism. Some human CYPs differ from those in animals with regards to expression and catalytic activities 19. For example, substrate specificities for CYP2C are very different between human and animal isoforms, and some dogs do not express CYP2C. And while cultures of human hepatocytes may better replicate the processes of human drug metabolism, robust enzyme levels are only retained for about 4–6 h in suspension and drop dramatically once cells are plated for toxicity studies 20. Species differences can also exist in the handling of reactive metabolites. Rats, for example, are significantly less susceptible to the oxidative stress induced by acetaminophen compared to humans and mice. While the metabolism of acetaminophen is similar among these species, fewer mitochondrial protein adducts are observed in rats, suggesting that rats are more resistant to the mitochondrial toxicity induced by this compound 18.
Current preclinical safety testing in animals also appears to be particularly inadequate at predictions of DILI liability resulting from alterations in bile acid homeostasis. This species difference appears to relate in part to differences in transporter inhibition kinetics, but also to species differences in the toxicity profile of bile acids 21. Humans have a higher portion of the more toxic hydrophobic bile acids while rodents and dogs have a higher percentage of less toxic polar bile acids. And while cultured human hepatocytes may produce a physiologically relevant complement of endogenous bile acids 22, the majority (>95%) of bile acids present in the liver come from the extrahepatic pool (i.e. enterohepatic recirculation). This pool is absent in static 2D cultures, preventing any significant intracellular accumulation due to transport inhibition. However, recent efforts have shown promise for eliciting bile acid-mediated toxicity in vitro by adding physiologically relevant concentrations of human bile acids to the culture media 23. Furthermore, recognizing the inadequacies of current preclinical testing to detect bile acid-mediated DILI, experts in the pharmaceutical industry and academia are recommending that new drug candidates be screened for human BSEP inhibition potential 24. While both the EMA and FDA (draft) guidances on drug interaction studies suggest that BSEP inhibition should be considered, there are currently no specific guidelines for this practice. Key considerations in screening for BSEP inhibition include assaying for potential inhibition by major drug metabolites, determining the type of inhibition (i.e. competitive, non-competitive, or mixed), including assay standards to allow for comparisons across experiments and lab, and accounting for exposure and protein binding 24.
Cholestatic vs. Hepatocellular Injury
It should also be noted that DILI due to alterations in bile acid homeostasis is often referred to as “cholestatic injury” by toxicologists. To a clinician however, “cholestatic injury” refers to the patient’s pattern of serum liver chemistries characterized by elevations in serum alkaline phosphatase and bilirubin associated with selective interference with bile flow; initially, hepatocyte injury is minimal, resulting in only modest elevations in serum aminotransferases. This results in some confusion as DILI resulting from BSEP inhibition may present clinically as “hepatocellular injury”, resulting in a liver chemistry profile reflecting substantial hepatocyte death (i.e. prominent elevations in serum aminotransferases with minimal elevation in serum alkaline phosphatase and bilirubin). This is in part because DILI caused by certain types of BSEP inhibition (competitive vs. non-competitive or mixed) may not reduce bile flow. Rather, rising bile acid concentrations within the hepatocyte may “out compete” BSEP inhibition by the drug 24. In this case, bile acid-dependent bile flow is only minimally affected although the hepatocyte bile acid concentrations may rise to exceed the threshold of toxicity.
It now seems likely that cholestatic DILI, as defined clinically, involves selective damage to the canalicular membrane of hepatocytes, or to the cells lining the biliary tree (cholangiocytes). This can occur as a result of toxic species excreted into bile 25. It is also likely that biliary damage can result from drug-mediated inhibition of the phospholipid transporter multidrug resistance protein 3 (MDR3). MDR3, is a phospholipid filppase located on the canalicular membrane of hepatocytes. It mediates the biliary secretion of phosphatidylcholine, which is essential for the formation of phoslipid/bile acid/cholesterol micelles which encase bile acids and prevent their direct contact with cell membranes. Disruption of MDR3 activity results in bile acid-mediated damage to the biliary epithelium and is associated with liver injury in both mice and humans 26. Several DILI causing drugs including chlorpromazine, itraconazole, haloperidol, ketoconazole, and ritonavir have been shown to inhibit MDR3 activity 27. While some of these drugs also inhibit BSEP, chlorpromazine and itraconazole for example, are weak inhibitors of BSEP suggesting that MDR3 inhibition may play a more important role in the liver injury associated with these drugs.
IDIOSYNCRATIC DILI
Idiosyncratic DILI typically describes a serious liver injury occurring very rarely (typically 1 in greater than 10,000 treated patients). The term idiosyncrasy derives from the Greek “idiosynkrasia” which combines idio-, one’s own, and synkrasis, a mixing together. Accordingly, susceptibility to toxicity is thought to reflect an individual’s unique genetic and non-genetic influences on multiple risk factors that in a rare combination results in susceptibility to DILI. Although it is stated in text books that idiosyncratic DILI is not dose-related, recent studies have supported dose as a variable in the response, although not a straightforward one 28. A key concept is that drugs that display idiosyncratic DILI are believed to be completely safe for the vast majority of patients treated at therapeutic doses. The most problematic idiosyncratic DILI occurs suddenly and typically after 1–6 months of continuous treatment and may progress for weeks after discontinuing the offending drug 2. For example, tolvaptan-induced liver injury in subjects with autosomal dominant polycystic kidney disease (ADPKD) was typically observed after 3 months of receiving therapy and in some cases serum aminotransferases and/or bilirubin continued to rise even after stopping treatment with the drug 29. Because of the rarity and prolonged latency, idiosyncratic DILI liability may not be recognized until very late in clinical development or even post marketing approval.
Delayed-onset Idiosyncratic DILI Appears to be the Result of an Adaptive Immune Attack
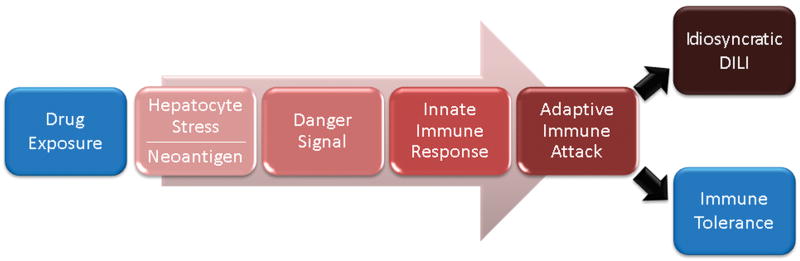
It is now widely believed that the idiosyncratic DILI with prolonged latency usually reflects an adaptive immune attack on the liver. This is consistent with the finding that it is typical for the liver injury to recur promptly if, after complete recovery, the DILI patient is rechallenged with the offending drug. The prolonged latency to initial onset can in part be attributed to the time required for antigen-specific lymphocytes to be activated and proliferate to sufficient numbers to mediate a DILI event. The rapid recurrence upon rechallenge is expected since the expanded antigen-specific population of lymphocytes remains in the body. A critical role for cell-mediated immunity in idiosyncratic DILI is also supported by observations that drug-reactive T-cells can sometimes be identified in the blood of patients who have experienced idiosyncratic DILI due to the particular drug, but the T-cells are generally not detected in patients who tolerate treatment with that same drug 30. For example, flucloxacillin-responsive CD4+ and CD8+ T-cells have been isolated from patients who experienced liver injury attributed to this drug 31. In addition, during an episode of idiosyncratic DILI, the liver is typically infiltrated by cytotoxic CD8 T-cells 32 and in one DILI case, the liver-infiltrating T-cells were shown to be activated 33. Finally, a role for adaptive immunity is supported by the observation that in human genome-wide association studies (GWAS) only HLA alleles have emerged as significant risk factors for DILI 34.The associations between HLA alleles and DILI risk have generally been drug specific (Table 1). A proposed sequence of events that lead to delayed idiosyncratic DILI are shown in Fig. 1.
TABLE 1.
HLA Risk Alleles and DILI Susceptibility
| Drug | HLA | Population | Odds Ratio |
|---|---|---|---|
| Anti TB drugs (isoniazid, rifampicin, pyrazinamide) | DQA1*01:02 | Indian | 0.2 |
| DQB1*02:01 | Indian | 1.9 | |
| Amoxicillin-clavulanate | A*02:01 | Caucasian (NW Europe) | 2.2 |
| A*30:02 | Caucasian (S Europe) | 6.7 | |
| B*18:01 | Caucasian (S Europe) | 2.8 | |
| DQB1*04:02 | Caucasian (NW Europe) | - | |
| DRB1*07 | Caucasian (NW Europe) | 0.18 | |
| DRB1*15:01-DQB1*06:02 a | Caucasian (Europe) | 2.3–10 | |
| Clometacin | B*08 | - | - |
| Flucloxacillin | B*57:01 | Caucasian (N Europe) | 80.6 |
| DRB*07:01-DQB1*03:03 | Caucasian (N Europe) | 7 | |
| DRB1*15 | Caucasian (N Europe) | - | |
| Flupirtine | DRB1*16:01-DQB1*05:02a | Caucasian (Europe) | 18.7 |
| Lapatinib | DQB1*02:02 | Caucasian | 6.9–8.6 |
| DRB1*07:01- DQA1*02:01 a | Caucasian | 2.6–9 | |
| Lumiracoxib | DRB1*15:01-DQA1*01:02-DQB1*06:02-DRB5*01:01a | Caucasian (N Europe) | 5 |
| Nevirapine | B*58:01 | South African | - |
| DRB1*01 | Caucasian (Europe) | 3 | |
| DRB1*01:02 | South African | - | |
| Ticlopidine | A*33:03 | Japanese | 13 |
| B*44:03 | Japanese | 6.7 | |
| Cw*14:03 | Japanese | 7.3 | |
| DRB1*13:02 | Japanese | 9.0 | |
| DQB1*06:04 | Japanese | 10.1 | |
| Tiopronine (mercaptopropionylglycine) | A*33 B*44 DR6 | Japanese | - |
| Ximelagatran | DRB1*07:01-DQA1*02:01a | Caucasian (N Europe) | 4.4 |
FIG. 1. Proposed pathogenesis for idiosyncratic DILI.
It is hypothesized that idiosyncratic DILI (IDILI) drugs produce neoantigen in the liver. IDILI drugs also result in hepatocyte stress (not necessarily cell death) and this appears to involve the same primary mechanisms of intrinsic DILI: oxidative stress, mitochondrial dysfunction, and altered bile acid homeostasis. In susceptible individuals, these events lead to the release of danger signals that result in activation of innate immune cells. Activation of macrophages and other antigen presenting cells is required to stimulate T-cells and promote an adaptive immune attack. An adaptive immune attack may result in asymptomatic elevations in serum liver chemistries that typically resolve despite continued drug treatment. This likely reflects activation of immune tolerance mechanisms. If immune tolerance does not promptly occur, progressive and symptomatic liver injury may result. The sequential steps of IDILI described here are consistent with the prolonged latency characteristic of the initial liver injury, the characteristic rapid recurrence with rechallenge after recovery from IDILI, as well as the identification of HLA risk alleles associated with transient and asymptomatic elevations in serum ALT in clinical trials of IDILI drugs.
The Creation of Neoantigens
In order to mount a cell-mediated adaptive immune attack, HLA molecules must present antigens to stimulate T-cell activation. Because the adaptive immune response in DILI is usually liver specific, it is assumed that the antigens that are the target of the T-cell response are present primarily on liver cells. For hepatocellular DILI involving adaptive immune mechanisms, the presumed target antigens are present primarily on hepatocytes, whereas in immune-mediated cholestatic DILI, the target antigens are present predominantly on cholangiocytes. Furthermore, the target antigens are presumably “neoantigens” that are only formed in response to drug treatment and were not considered as “self” during the immune inventory undertaken early in life.
There are a variety of mechanisms proposed for the creation of neoantigens as a result of drug exposure (Fig. 2). The longest standing model is commonly referred to as the “hapten hypothesis”. In this model, hepatocytes produce a reactive intermediate via metabolism of the parent drug. The covalent binding of this metabolite to liver proteins generates hapten-protein adducts that can be processed into a pool of chemically-modified peptides. These peptide neoantigens, when presented by HLA molecules, are recognized as “foreign” by T-cells and elicit an adaptive immune response. This hypothesis is supported by the observation that most drugs that cause idiosyncratic DILI are capable of generating reactive metabolites that covalently bind proteins 35. Furthermore, at least two studies have shown a requirement for antigen presenting cells to be pulsed with parent compound for at least 16 h in order to elicit a T-cell response 36, 37. This delay is consistent with hapten formation and antigen processing prior to neoantigen presentation.
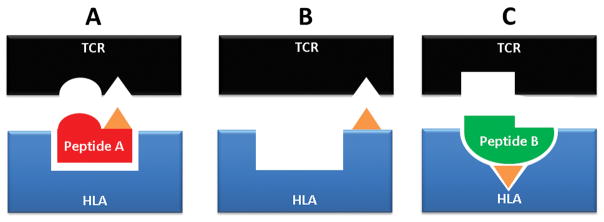
FIG. 2. Models of neoantigen presentation by HLA molecules.
Currently, three main hypotheses have been proposed to explain drug-induced T-cell activation in idiosyncractic DILI. (A) Hapten model: hepatocytes produce a reactive intermediate (represented in orange) via metabolism of the parent drug. The covalent binding of this metabolite to liver proteins generates hapten-protein adducts that can be processed into a pool of chemically-modified peptides. When presented by a HLA (human leukocyte antigen) molecules, these peptide neoantigens may be recognized as “foreign” and elicit an adaptive immune response. (B) Pharmacological interaction or p-i model: a drug (represented in orange) binds noncovalently to either the T-cell receptor (TCR) or HLA molecule and directly actives T-cells in a peptide-independent manner. (C) Altered peptide repertoire model: a drug (represented in orange) binds to the peptide-binding pocket of the HLA molecule, thereby changing the chemistry of the binding cleft and altering the repertoire of the endogenous (unmodified) peptides it presents. When new peptides are presented, they may be seen as neoantigens by T-cells; they are not “new” to the cell but have never been presented by HLA molecules before.
More recently, two hypotheses have emerged suggesting that T-cell mediated immune responses may result from a drug (or its metabolites) interacting directly with immune receptors 38. The “pharmacological interaction” or “p-i” model suggests that a drug binds noncovalently to either the T-cell receptor (TCR) or HLA molecule and directly actives T-cells in a peptide-independent manner. The second hypothesis is the “altered peptide repertoire” model where a drug or metabolite binds to the peptide-binding pocket of the HLA molecule, thereby changing the chemistry of the binding cleft and altering the repertoire of self peptides the HLA molecule presents. This is plausible because only a small percentage of the peptides generated within cells bind to HLA molecules on the surface of cells and hence are recognized as self. When new peptides are presented, they are seen as neoantigens by T-cells, even though they are not “new” to the cell. This has been shown to be the mechanism underlying abacavir hypersensitivity reactions, predominately impacting skin 38.
Regardless of the mechanism of neoantigen formation, the question remains, what makes these reactions liver specific? Liver specificity could reflect higher concentrations of parent drug or drug metabolites in the liver (especially after oral administration). Alternatively, the neoantigen could involve liver specific peptides (e.g. albumin) as either part of a hapten-peptide complex or presented only after the peptide binding pocket has been altered and thus considered “foreign”.
Finally, it is important to note that even the strongest association yet observed between an HLA risk allele and DILI (HLA-B*57:01 and flucloxacillin DILI) explains only a small fraction of individual patient risk, as fewer than 1:500 individuals with the risk allele will develop clinically evident DILI when treated with the drug 39. Furthermore, it should not take several months to mount an adaptive response, supporting preceding non-immune-mediated events as required for an adaptive immune attack on the liver. Finally, it is believed that a robust adaptive immune response requires local release of “danger signals” or “alarmins” to stimulate an adaptive immune attack 40. Presumably, this would result as a consequence of events occurring in response to the drug effects on the hepatocyte.
Hepatocyte Stress
It is widely assumed that the cascade of events culminating in idiosyncratic DILI begins with some level of direct drug-induced stress to the hepatocyte. The mechanisms of this initial drug stress may be very similar to those for intrinsic DILI compounds. This is supported by the recent development of several cell based assays that appear to have some sensitivity and specificity for predicting idiosyncratic DILI liability using hepatocytes or hepatocyte-like cell lines in the absence of immune cells 41, 42. Many of these approaches rely on endpoints based on the same three mechanisms that typically underlie intrinsic DILI: mitochondrial dysfunction, oxidative stress, and alterations in bile acid homeostasis. The ability to detect responses in hepatocytes obtained from random donors (i.e. those statistically very unlikely to be susceptible to idiosyncratic DILI), suggest that relevant stress responses are probably occurring to some extent in a large proportion of individuals exposed to the drug and might be elicited in all individuals when exposed at high enough concentration of the drugs. However, there may in fact be susceptibility factors involved in these early steps as well – both variation in susceptibility to the stress responses at pharmacologically relevant exposures and in mechanisms to adapt to the stress responses. Without prompt adaptation, the stress responses may result in the release of “danger signals” that are necessary to provoke a robust immune response (Fig. 1).
Danger Signals and Innate Immune Response
The role of the danger signals is to stimulate innate immune cells and create inflammation. Viruses contain pathogen-associated molecular patterns (PAMPs) that constitute the danger signal by binding to toll-like and other receptors on macrophages and other cells of the innate immune system, resulting in their activation. The activation of innate immune cells promotes the release of cytokines and chemokines that act through a variety of mechanisms to enhance the adaptive immune response and target the response to the infected tissues. Commonly used vaccine adjuvants mimic PAMPs without which, most vaccines would be ineffective. In the case of DILI, cytotoxicity results in the release of damage associated molecular patterns (DAMPs) 43. DAMPs act similarly to PAMPs, signaling to and activating innate immune cells. Previously identified DAMPS include, high mobility group box 1 protein (HMGB1), certain heat shock proteins (HSPs), and S100 proteins 44. These molecules upregulate costimulatory factors on professional antigen presenting cells and promote cytokine and chemokine release which help to stimulate the adaptive immune attack on the liver. In addition, some cytokines such as tumor necrosis factor-alpha (TNFα) and interferon-gamma (IFNγ) can make hepatocytes more susceptible to drug-induced stress by shifting cellular responses away from cell survival and towards cell death, and it has been proposed that some inflammatory conditions not caused directly by the drug may be a risk factor for idiosyncratic DILI 45.
It has been assumed that the release of danger signals is a consequence of cell death, resulting in the broad release of hepatocyte proteins including DAMPs into the circulation. However, it is not clear that hepatocyte necrosis is a prerequisite for the release of these danger signals. For example, strong HLA associations have been observed with relatively minor elevations in serum ALT in clinical trials of ximelagatran 46, lumiracoxib 47 and lapatinib 48. In addition, in the case of idiosyncratic DILI due the antibiotic isoniazid, drug reactive T-cells have been identified in a patient’s blood prior to the onset of elevations in serum ALT 49. These observations suggest that mild elevations in serum ALT do not necessarily reflect hepatocyte death that could initiate an adaptive immune attack, but rather that the mild ALT elevations may reflect an adaptive immune attack on the liver initiated without hepatocyte death. This may help explain why overt toxicity is usually not observed in animal and hepatocyte culture models treated with even high doses of drugs that cause idiosyncratic DILI. The potential for an adaptive immune attack on the liver to be triggered in the absence of hepatocyte death is consistent with infection of the hepatocyte by the hepatitis B virus. The hepatitis B virus is not cytolytic yet generates a liver-specific adaptive immune attack resulting in hepatocyte necrosis and the clinical disease 50. A common mechanism may account for why microscopic analysis of liver biopsies from idiosyncratic DILI with prolonged latency often appears similar to viral hepatitis. However, if the release of danger signals does not require cell death, how dose immune activation occur?
Recent work suggests that the danger signals may travel in hepatocyte-derived exosomes 51. Exosomes, the smallest class of extracellular vesicles (<150 nm), can be released from the liver and diffuse into circulation due to the porous fenestrations that are unique to the sinusoidal endothelium. Liver-derived exosomes have been detected under basal conditions in biofluids such as plasma, and more recent evidence has demonstrated that the abundance and cargo of exosomes released from hepatocytes changes in in response to drug-induced stress, prior to and in the absence of overt necrosis 51. It also appears that exosomes from hepatocytes treated with sub- toxic doses of drugs can stimulate the activation of monocots (personal communication, Natalie Holman). This may explain why the traditional histological and biochemical endpoints for liver toxicity have frequently failed to detect idiosyncratic DILI liability in new drug candidates. Interestingly, exosomes have been shown to mediate hepatitis B virus transmission and influence natural killer (NK) cell function in the liver 52.
The Adaptive Immune Response and Immune Tolerance
The culmination of the proposed early events is the targeting of cytotoxic lymphocytes to liver cells by HLA molecules presenting neoantigen. Cytotoxic T cells kill the target cells through the secretion of cytolytic molecules including Fas-L, perforin, and granzyme-B. As noted previously, drugs that can cause idiosyncratic DILI typically cause asymptomatic elevations in serum aminotransferases that resolve despite continuation of treatment with the offending drug. In cases where information is available, these transient liver injuries are associated with the same HLA risk alleles as the clinically important liver injuries. This suggests that the initiation of a drug-induced adaptive immune attack on the liver is usually reversible, presumably through immune tolerance mechanisms. An important development in the understanding of the role of immune tolerance comes from recent reports of a mouse model that recapitulates some of the clinical characteristics of idiosyncrasy including delayed onset and involvement of the innate and adaptive immune responses 53. This is achieved by inhibiting functions of programmed cell death 1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA4), negative regulators of T cell activation that are important for the induction of immune tolerance. It is also of note that inhibitors of immune tolerance that are very promising additions to cancer treatment regimens are also increasingly associated with DILI and other immune-mediated adverse reactions 54.
Finally, it is important to note that biologic agents and even monoclonal antibodies have been associated with liver injury that is indistinguishable from idiopathic autoimmune hepatitis but generally reverses when treatment is discontinued 55. Presumably, biologics are not generating reactive metabolites that participate in the formation of neoantigens. However, biologics themselves may interact directly with HLA molecules as in the “p-i” or “altered peptide repertoire” models. And they may activate hepatocyte stress response pathways by mediating changes at the gene transcript level 56.
Evidence Against the Proposed Model for Idiosyncratic DILI
To date, GWAS approaches have only found robust associations with HLA alleles and not with genes involved in other steps along the proposed pathogenesis of idiosyncratic DILI (Fig. 1). Furthermore, most reported non-HLA genetic associations that have evolved from hypothesis-directed interrogation (minimizing the requirement for multiple test correction), have not been confirmed in subsequent studies. This may reflect the drug-specific nature of non-HLA risk factors and/or the relatively small sample sizes generally available for DILI due to specific drugs (although the HLA risk factors are also largely drug specific and found in the available sample sizes). It is also possible that the events preceding the adaptive immune attack occur in all patients treated with idiosyncratic DILI, and that the missing risk factors reside in T-cell receptor variation resulting from genetic recombination that would not be detected in genomic DNA. The lack of non-HLA risk associations may also relate to the fact that the liver is a “plastic” organ where gene expression is particularly influenced by epigenetic change which may result from exposure to drugs and other chemicals 57. Epigenetic changes induced by drugs and environmental exposures do not cause direct changes to the nucleotide sequence of DNA, and would therefore not be discovered by the analytical techniques currently used in typical genetic studies. Moreover, it seems unlikely that epigenetic changes present in the liver would be mirrored by changes in DNA from blood or saliva that are most commonly used in genetic studies. Epigenetic analysis should be possible in tissue taken from liver biopsies, however most patients with DILI due not undergo biopsy as part of routine care. Furthermore, the risks associated with the procedure would make it hard to justify performing biopsies for research purposes only.
Quantitative systems pharmacology (QSP) modeling has also made a strong case for the ability of some idiosyncratic DILI causing drugs to have prolonged latency to DILI without involving an adaptive immune attack. Idiosyncratic DILI associated with troglitizone was the first example of this – where modeling based solely on alterations in bile acid homeostasis, accurately predicted not only the incidence of serum ALT elevations observed in the clinical trials, but the latency to peak serum ALT elevations observed in the clinical trials 58. The latency in the model resulted from several factors including the gradual accumulation of the sulfate metabolite within hepatocytes, the compensatory regulation of bile acid transporters through the farnesoid X receptor (FXR), and the fact that the mechanism of BSEP inhibition was competitive (as opposed to non-competitive). Because there is no genomic DNA available from troglitazone DILI patients, rechallenge experience is not well documented, and patients are no longer treated with this drug, it remains unclear whether the idiosyncratic DILI with this drug involved an adaptive immune response.
QSP modeling has also suggested that the idiosyncratic DILI observed in tolvaptan-treated patients with ADPKD might be explained by non-immune mediated mechanisms. Based only on the ability of the parent drug and major metabolite to inhibit BSEP and to interfere with mitochondrial respiration, QSP modeling was able to recapitulate the incidence of elevation in serum aminotransferases observed in the clinical trials 59. However, in the clinical trials, the observed onset of the enzyme elevations had latencies sometimes exceeding one year. This was not accurately captured in the modeling. It was postulated that the latency observed in the clinic was due to progression of the ADPKD, perhaps associated with kidney dysfunction and/or liver cysts that might lower the threshold for toxicity. However, patients who have recovered from tolvaptan DILI have been reported to have rapid recurrence upon rechallenge with the drug, supporting involvement of the adaptive immune system in the liver injury 29. It is possible that the QSP modeling does not contain all the relevant mechanisms of adaption to stress and, by accounting for only the initial processes in the cascade, overestimates the impact of the non-immunologic events (Fig. 1).
FUTURE DIRECTIONS: MANAGING THE RISK OF DILI
Looking ahead, continued advances in genetics, biomarkers, preclinical models, and QSP will further inform our understanding of the mechanisms underlying DILI reactions. These advances will lead to improved diagnosis and management of DILI risk and also allow for the ability to distinguish benign elevations in serum aminotransferases from those that may represent a serious health threat. Ultimately, the knowledge gained will lead to the confident assessment of DILI liability for new drug candidates thus allowing shorter and smaller clinical trials to confirm liver safety. Together, these advances will make new drugs available faster, more affordable, and safer.
Genetic Tests in Risk Management and Diagnosis of DILI
To date, even the most robust HLA risk allele associations have not led to clinically accepted risk management strategies. This is in part because the associations found-to-date identify a relatively large subpopulation at increased risk of idiosyncratic DILI, but the majority of those who will test positive for carrying the allele can in fact take the drug safely. Nonetheless, there has been one attempt to introduce genetic testing for the management of DILI risk. This involved Novartis’ lumiracoxib, a COX-2 inhibitor withdrawn from world-wide markets due to drug-induced ALF estimated to occur in less than 1:10,000 treated patients 47. As previously mentioned, a retrospective GWAS of DNA samples obtained in lumiracoxib clinical trials revealed a risk allele (HLA-DRB1*1501) that was highly sensitive for identifying patients at risk for developing DILI 47. However, over 25% of the subjects in the clinical trials carried this risk allele, the vast majority of whom could be treated safely with the drug. The 75% of subjects not carrying the risk allele had a DILI risk comparable to that of naproxen, a comparator treatment that is generally regarded as safe for the liver.
Novartis attempted reintroduction of lumiracoxib into the market by linking it to a genetic screen. This was practical because an assay for the risk allele was already part of routine transplant genotyping and widely available. However, this effort was not successful, largely because of the lack of a clear unmet medical need for lumiracoxib. Furthermore, there was no way to ensure that a drug useful for aches and pains would only be consumed by those who had undergone genotyping. Nonetheless, the effectiveness of genotyping in reducing the risk of DILI due to lumiracoxib was not challenged, and the FDA remains a strong advocate for the use of genotyping in precision medicine (see FDA guidance on Clinical Pharmacogenomics). With the right drug and indication it can be anticipated that genotyping as a means of managing DILI risk will make it into the clinic in the future.
As DILI gene banks increase in size, and next generation sequencing and associated analytical techniques continue to advance, many new risk alleles for DILI will likely be identified. In addition to improving DILI risk management, these findings should also identify novel stress response and adaptive pathways that underlie DILI susceptibility which may further inform DILI risk management strategies. HLA genotyping could also be very useful to identify the DILI causing drug in a patient receiving multiple potentially hepatotoxic medications. For example, if a patient carried a previously identified risk allele for a specific drug (Table 1), it would make it easier to confidently identify this drug as the culprit. Finally, it should be noted that in a clinical trial for a new drug candidate, the finding of a strong HLA allele association with asymptomatic and transient elevations in serum ALT may be a strong indication that that drug will be capable of idiosyncratic and clinically important DILI.
Role of Non-Genetic Tests in Risk Management and Diagnosis of DILI
Because non-genetic risk factors also contribute to DILI susceptibility, the optimal risk management strategies may involve assessment of a combination of genetic and non-genetic risk factors. The blood tests (biomarkers) used to detect and monitor the course of liver disease have not changed in over 50 years. However, there are currently two large consortia efforts aimed at identifying novel, more sensitive and mechanistic biomarkers of liver injury: The US Predictive Safety Testing Consortium (PSTC) managed by the Critical Path Institute and the European Safer and Faster Evidence-based Translation (SAFE-T) Consortium sponsored by the Innovative Medicines Initiative. These efforts are identifying new serum biomarkers that may represent a major advance in the detection and management of DILI. For example, there are now serum biomarkers that can potentially monitor the successive steps in the proposed model for idiosyncratic DILI (Table 2). It therefore seems likely that monitoring the right set of biomarkers during a clinical trial could identify drugs with idiosyncratic DILI potential—perhaps even in a relatively small patient cohort—greatly improving the efficiency of clinical development programs and making the “Hy’s Law Case” obsolete. Because DILI is usually an acute and then resolving injury, and because each biomarker may have distinct release and clearance kinetics, the optimal interpretation of biomarkers will require pharmacokinetic modeling of drug, relevant metabolites and biomarkers. Clinical pharmacologists are uniquely suited to take on this challenge.
TABLE 2.
Select Novel Biomarkers to Assess the Pathogenesis of Idiosyncratic DILI
| Step | Biomarker |
|---|---|
| Hepatocyte Stress | Possibly traditional biomarkers or miR-122 |
| Oxidative Stress | Various metabolites 68 |
| Mitochondrial Toxicity | Glutamate dehydrogenase and mitochondrial DNA 69 |
| Altered Bile Acid Homeostasis | Bile acids 21 |
| Danger Signals | HMGB1, HSPs, S100, exosomes 40, 51 |
| Apoptosis vs. Necrosisa | Ratio of the caspase-cleaved fragment of cytokeratin 18 (CK18) to the full length CK18 70 |
| Innate Immune Response | Acetylated HMGB1, various miRNAs and mRNAs, cytokines 71, 72, 73, 74 |
| Adaptive Immune Attack | Lymphocyte transformation test 36 |
This may be important since apoptosis is not thought to involve release of active DAMPs.
These new biomarkers may also help identify novel therapeutic strategies to treat DILI. For example, monoclonal antibodies against HMGB1 have been shown to ameliorate immune activation and reduce liver injury in a mouse model of acetaminophen-induced liver injury 60. Other areas of research such as metabolomics and the role of the gut microbiome also hold promise to identify predictive biomarkers and new therapeutic strategies 20, 61. However, regulatory acceptance of the new DILI biomarkers will probably require prospective collection and archiving of serial serum samples from large numbers of patients with diverse underlying diseases and taking diverse drugs with and without idiosyncratic DILI potential. Unfortunately, banking serum samples on this scale is not a common practice within the pharmaceutical industry. The industry should now be aware that once liver safety concerns are raised regarding a new drug candidate, collection and archiving of serial serum samples in clinical trials, particularly during elevations in serum aminotransferase, will make it possible to assay the new biomarkers that may in the future speed regulatory approval and even save otherwise failed development programs.
New Model Systems
Preclinical cell culture and animal models will also play an increasingly important role in predicting and understanding DILI. There has been continued advancement in the area of liver cell culture systems that permit sustained maintenance of differentiated hepatocytes and improved performance in toxicity assessment 62. The inclusion of nonparenchymal cell types, more complex 3-dimensional cytoarchitecture, and fluid flow helps to create a more organotypic model that better mimics the dynamic in vivo environment. Eventually the hope is that these liver models could be connected to other organ systems to create a “human on a chip”. The use of cells sourced from multiple human donors is introducing some, albeit limited, patient heterogeneity into these cultures, while the advancement of induced pluripotent stem cell-derived (iPSC) human hepatocytes offers the promise of a more reliable supply of hepatocytes that can be sourced from DILI patients with specific genetic susceptibility factors. However, the limited supply of high quality cells, fragility in handling, and high cost of primary and iPSC-derived cells still leaves a need for hepatocyte-like cell lines that can provide a robust, consistent, and convenient alternative. Old favorites such as HepG2 and HepaRG demonstrate some utility for this purpose and are even getting upgraded with transfected metabolic enzymes and more user-friendly versions. New commercially available models that are hepatocyte-derived, such as Upcyte Hepatocytes (http://www.upcyte.com/products/human-upcyte-hepatocytes.html) and HepatoCells (https://www.corning.com/worldwide/en/products/life-sciences/products/adme-tox-research/hepatocells.html), also show promise for combining the phenotypic relevance of hepatocytes with the ease and consistency of an immortalized cell line. However, these models have not been extensively studied.
There have also been recent advances in the development of preclinical animal models that recapitulate aspects of clinical idiosyncratic DILI, capture human population diversity, or have “humanized” features that may better replicate human physiology. PD1 knockout mice treated with an anti-CTLA4 antibody have been used to recapitulate aspects of idiosyncratic DILI produced by amodiaquine, isoniazid, and nevirapine – helping to uncover an important role for impaired immune tolerance in these toxicities 53. The genetically diverse Collaborative Cross mouse population model has recently been applied to study susceptibility factors and mechanisms for idiosyncratic DILI due to tolvaptan 63. And it is also now possible to repopulate a mouse liver with human hepatocytes; to date this has generally involved hepatocytes from random donors and required ablation of the mouse immune system which may limit the utility of this model in the study of idiosyncratic DILI. However, mice with humanized livers and human immune systems have also been reported but are not widely available 64. The advancement of induced pluripotent stem cell technology may ultimately permit creation of mice with hepatocytes and immune systems derived from patients who have actually experienced DILI.
Finally, new high-throughput and high-content analytical techniques hold promise for providing more comprehensive insights into mechanisms and risk factors even earlier in the drug development process. Although traditional histological and biochemical endpoints can be used for the identification of a toxic response, recent evidence suggests gene expression profiling may be a more sensitive and translational method to predict idiosyncratic DILI in humans 65. Furthermore, evaluation of molecular signaling pathways can provide insight into mechanisms of drug toxicity as well as phenotypes to help identify susceptibility factors. Combining transcriptomics with epigenetics has helped to identify new genomic perturbations resulting from drug exposure 57. Proteomics and metabolomics can be used to identify functional molecular changes and even new biomarkers for drug toxicity 20. And high-content imaging applied to cellular systems can survey a large number of mechanistic endpoints across multiple treatments in an automated fashion.
Quantitative Systems Pharmacology Modeling
Now in its 6th year, the DILI-sim Initiative (Dilisym.com) is one of the most promising areas of research poised to influence DILI risk management. The DILI-sim Initiative is a public-private partnership including scientists from academia, 12 major pharmaceutical companies, and the FDA. Grounded in QSP, the Initiative has developed the DILIsym software, a mechanistic, mathematical model that can be used to understand and predict DILI in humans and preclinical species. DILIsym combines PBPK compound distribution and metabolism with mechanisms of hepatotoxicity to describe the effect of drug exposure on the hepatocyte, liver, and whole organism. Recently, activation of innate immunity was added to DILIsym, and modeling of adaptive immune mechanisms is underway.
DILIsym is parameterized using available experimental data and is being optimized by successive modeling of “exemplar” drugs where liver safety liability has been established across multiple species 59. By varying model parameters, heterogeneous patient populations are simulated enabling the prediction of DILI occurrence in large populations of treated patients. Eventually, such predictions should reduce the size and duration of clinical trials required for FDA approval. Critical model variables that determine susceptible and resistant individuals can then be identified, and this may support the use of certain clinical characteristics or biomarkers in DILI risk management. Various dosing regimens can be simulated to help optimize design of clinical study protocols to reduce DILI events. Ultimately, modeling drug therapy in various specific simulated populations (e.g. active liver diseases and cirrhosis, nonalcoholic steatohepatitis, diabetes, etc) could reduce or obviate the need for clinical trials in these populations.
Using compound specific data, DILIsym has been able to correctly model the DILI liability of over 95% of the drugs/metabolites studied to date (Brett Howell, personal communication). This has included both intrinsic and idiosyncratic DILI drugs. The majority of these analyses have been retrospective, but several prospective modeling predictions are now being tested in clinical trials. However, a challenge here is the relatively low throughput of analysis resulting from both the time required for data gathering and running simulations. The DILI-sim Initiative is working to address both of these areas. Needed advances include liver cell culture systems that will take into account the effects of metabolites even before they have been identified, enable better PBPK estimates, and also identify the relevant toxicity pathways to focus the modeling effort. Cloud computing will help to increase computational power and speed the rate of patient population simulations to provide more rapid results that can inform drug development decisions. Because the predictive ability of QSP modeling improves as more data becomes available, modeling should in the future be an iterative process ongoing throughout the preclinical and clinical development of new drug candidate.
CONCLUSIONS
The increased use of medications in an aging and overweight population, and growing DILI risk from herbal and dietary supplements will ensure that DILI remains a significant public health challenge in the years to come. However much has been learned about the mechanisms of idiosyncratic DILI and a clear picture of the pathogenesis is beginning to emerge. This understanding combined with the identification of predictive genetic and non-genetic biomarkers should lead to improved management and prediction of DILI risk.
Acknowledgments
Funding for PBW was provided by the National Institute of Diabetes and Digestive and Kidney Diseases Project Number 5U01DK065201-13.
Footnotes
CONFLICT OF INTEREST/DISCLOSURE
PBW chairs the Scientific Advisory Committee of the DILI-sim Initiative and owns equity in DILIsym Service, Inc.
AUTHOR CONTRIBUTIONS
MM and PBW participated equally in writing this review.
References
- 1.Lee WM. Drug-induced acute liver failure. Clin Liver Dis. 2013;17(4):575–586. viii. doi: 10.1016/j.cld.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fontana RJ, Hayashi PH, Gu J, Reddy KR, Barnhart H, Watkins PB, et al. Idiosyncratic Drug-Induced Liver Injury Is Associated With Substantial Morbidity and Mortality Within 6 Months From Onset. Gastroenterology. 2014;147(1):96–108. e104. doi: 10.1053/j.gastro.2014.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watkins PBPB. How to Diagnose and Exclude Drug-Induced Liver Injury. Digestive diseases (Basel) 2015;33(4):472–476. doi: 10.1159/000374091. [DOI] [PubMed] [Google Scholar]
- 4.Watkins PB. Drug safety sciences and the bottleneck in drug development. Clin Pharmacol Ther. 2011;89(6):788–790. doi: 10.1038/clpt.2011.63. [DOI] [PubMed] [Google Scholar]
- 5.Takeda Shares Drop After Scrapping Development of Diabetes Drug. http://www.bloomberg.com/news/2013-12-27/takeda-scraps-development-of-diabetes-drug-on-risks-shares-drop.html. [cited December 27.2013]Available from.
- 6.Temple RR. Hy’s law: predicting serious hepatotoxicity. Pharmacoepidemiology and drug safety. 2006;15(4):241–243. doi: 10.1002/pds.1211. [DOI] [PubMed] [Google Scholar]
- 7.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus Warfarin in Nonvalvular Atrial Fibrillation. New England Journal of Medicine. 2011;365(10):883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 8.Watkins PB. Managing the risk of drug-induced liver injury. Clin Pharmacol Ther. 2013;94(6):629–631. doi: 10.1038/clpt.2013.182. [DOI] [PubMed] [Google Scholar]
- 9.Watkins PB, Kaplowitz N, Slattery JT, et al. Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily: A randomized controlled trial. JAMA. 2006;296(1):87–93. doi: 10.1001/jama.296.1.87. [DOI] [PubMed] [Google Scholar]
- 10.Navarro VJ, Barnhart H, Bonkovsky HL, Davern T, Fontana RJ, Grant L, et al. Liver injury from Herbals and Dietary Supplements in the US Drug Induced Liver Injury Network. Hepatology (Baltimore, Md) 2014;60(4):1399–1408. doi: 10.1002/hep.27317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Navarro VJ, Lucena MI. Hepatotoxicity Induced by Herbal and Dietary Supplements. Semin Liver Dis. 2014;34(02):172–193. doi: 10.1055/s-0034-1375958. [DOI] [PubMed] [Google Scholar]
- 12.Fontana RJ. Acute Liver Failure including Acetaminophen Overdose. The Medical clinics of North America. 2008;92(4):761–794. doi: 10.1016/j.mcna.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee WM. Drug-induced Acute Liver Failure. Clinics in liver disease. 2013;17(4) doi: 10.1016/j.cld.2013.1007.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morgan RE, van Staden CJ, Chen Y, Kalyanaraman N, Kalanzi J, Dunn RT, et al. A Multifactorial Approach to Hepatobiliary Transporter Assessment Enables Improved Therapeutic Compound Development. Toxicological Sciences. 2013;136(1):216–241. doi: 10.1093/toxsci/kft176. [DOI] [PubMed] [Google Scholar]
- 15.Gómez-Lechón MJ, Tolosa L, Donato MT. Metabolic activation and drug-induced liver injury: in vitro approaches for the safety risk assessment of new drugs. Journal of Applied Toxicology. 2016;36(6):752–768. doi: 10.1002/jat.3277. [DOI] [PubMed] [Google Scholar]
- 16.Aleo MD, Luo Y, Swiss R, Bonin PD, Potter DM, Will Y. Human drug-induced liver injury severity is highly associated with dual inhibition of liver mitochondrial function and bile salt export pump. Hepatology. 2014;60(3):1015–1022. doi: 10.1002/hep.27206. [DOI] [PubMed] [Google Scholar]
- 17.Will Y, Dykens J. Mitochondrial toxicity assessment in industry – a decade of technology development and insight. Expert Opinion on Drug Metabolism & Toxicology. 2014;10(8):1061–1067. doi: 10.1517/17425255.2014.939628. [DOI] [PubMed] [Google Scholar]
- 18.Jaeschke H, Xie Y, McGill MR. Acetaminophen-induced Liver Injury: from Animal Models to Humans. Journal of Clinical and Translational Hepatology. 2014;2(3):153–161. doi: 10.14218/JCTH.2014.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martignoni M, Groothuis GM, de Kanter R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin Drug Metab Toxicol. 2006;2(6):875–894. doi: 10.1517/17425255.2.6.875. [DOI] [PubMed] [Google Scholar]
- 20.Jiang J, Wolters JEJ, van Breda SG, Kleinjans JC, de Kok TM. Development of novel tools for the in vitro investigation of drug-induced liver injury. Expert Opinion on Drug Metabolism & Toxicology. 2015;11(10):1523–1537. doi: 10.1517/17425255.2015.1065814. [DOI] [PubMed] [Google Scholar]
- 21.Schadt HS, Wolf A, Pognan F, Chibout S-D, Merz M, Kullak-Ublick GA. Bile acids in drug induced liver injury: Key players and surrogate markers. Clinics and Research in Hepatology and Gastroenterology. 2016;40(3):257–266. doi: 10.1016/j.clinre.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 22.Marion TL, Perry CH, StClaire RL, Brouwer KLR. Endogenous Bile Acid Disposition in Rat and Human Sandwich-Cultured Hepatocytes. Toxicology and Applied Pharmacology. 2012;261(1):1–9. doi: 10.1016/j.taap.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Susukida T, Sekine S, Nozaki M, Tokizono M, Ito K. Prediction of the Clinical Risk of Drug-Induced Cholestatic Liver Injury Using an In Vitro Sandwich Cultured Hepatocyte Assay. Drug Metabolism and Disposition. 2015;43(11):1760–1768. doi: 10.1124/dmd.115.065425. [DOI] [PubMed] [Google Scholar]
- 24.PSTC. PSTC BSEP Webinar Series. Current trends in BSEP inhibition and perturbation to bile acid homeostasis as mechanisms of drug-induced liver injury. 2006;2006 http://www.cvent.com/events/pstc-bsep-webinar-series-current-trends-in-bsep-inhibition-and-perturbation-to-bile-acid-homeostasis/agenda-5e6e6654752947d6b1eb71341a9b09de.aspx. [Google Scholar]
- 25.Jean PA, Roth RA. Naphthylisothiocyanate disposition in bile and its relationship to liver glutathione and toxicity. Biochemical Pharmacology. 1995;50(9):1469–1474. doi: 10.1016/0006-2952(95)02051-9. [DOI] [PubMed] [Google Scholar]
- 26.Sundaram SS, Sokol RJ. The multiple facets of ABCB4 (MDR3) deficiency. Current treatment options in gastroenterology. 2007;10(6):495–503. doi: 10.1007/s11938-007-0049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He K, Cai L, Shi Q, Liu H, Woolf TF. Inhibition of MDR3 Activity in Human Hepatocytes by Drugs Associated with Liver Injury. Chemical Research in Toxicology. 2015;28(10):1987–1990. doi: 10.1021/acs.chemrestox.5b00201. [DOI] [PubMed] [Google Scholar]
- 28.Uetrecht J, Naisbitt DJ. Idiosyncratic Adverse Drug Reactions: Current Concepts. Pharmacological Reviews. 2013;65(2):779–808. doi: 10.1124/pr.113.007450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watkins P, Lewis J, Kaplowitz N, Alpers D, Blais J, Smotzer D, et al. Clinical Pattern of Tolvaptan-Associated Liver Injury in Subjects with Autosomal Dominant Polycystic Kidney Disease: Analysis of Clinical Trials Database. Drug Safety. 2015:1–11. doi: 10.1007/s40264-015-0327-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maria V, Victorino R. Diagnostic value of specific T cell reactivity to drugs in 95 cases of drug induced liver injury. Gut. 1997;41(4):534–540. doi: 10.1136/gut.41.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monshi MM, Faulkner L, Gibson A, Jenkins RE, Farrell J, Earnshaw CJ, et al. Human leukocyte antigen (HLA)-B*57:01-restricted activation of drug-specific T cells provides the immunological basis for flucloxacillin-induced liver injury. Hepatology. 2013;57(2):727–739. doi: 10.1002/hep.26077. [DOI] [PubMed] [Google Scholar]
- 32.Foureau DM, Walling TL, Maddukuri V, Anderson W, Culbreath K, Kleiner DE, et al. Comparative analysis of portal hepatic infiltrating leucocytes in acute drug-induced liver injury, idiopathic autoimmune and viral hepatitis. Clinical and Experimental Immunology. 2015;180(1):40–51. doi: 10.1111/cei.12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mennicke M, Zawodniak A, Keller M, Wilkens L, Yawalkar N, Stickel F, et al. Fulminant liver failure after vancomycin in a sulfasalazine-induced DRESS syndrome: fatal recurrence after liver transplantation. Am J Transplant. 2009;9(9):2197–2202. doi: 10.1111/j.1600-6143.2009.02788.x. [DOI] [PubMed] [Google Scholar]
- 34.Urban TJ, Daly AK, Aithal GP. Genetic Basis of Drug-Induced Liver Injury: Present and Future. Semin Liver Dis. 2014;34(02):123–133. doi: 10.1055/s-0034-1375954. [DOI] [PubMed] [Google Scholar]
- 35.Tailor A, Faulkner L, Naisbitt D, Park B. The chemical, genetic and immunological basis of idiosyncratic drug–induced liver injury. Human & Experimental Toxicology. 2015;34(12):1310–1317. doi: 10.1177/0960327115606529. [DOI] [PubMed] [Google Scholar]
- 36.Kim S-H, Saide K, Farrell J, Faulkner L, Tailor A, Ogese M, et al. Characterization of amoxicillin- and clavulanic acid-specific T cells in patients with amoxicillin-clavulanate–induced liver injury. Hepatology. 2015;62(3):887–899. doi: 10.1002/hep.27912. [DOI] [PubMed] [Google Scholar]
- 37.Nattrass R, Faulkner L, Vocanson M, Antoine DJ, Kipar A, Kenna G, et al. Activation of Flucloxacillin-Specific CD8+ T-Cells With the Potential to Promote Hepatocyte Cytotoxicity in a Mouse Model. Toxicological Sciences. 2015;146(1):146–156. doi: 10.1093/toxsci/kfv077. [DOI] [PubMed] [Google Scholar]
- 38.White KD, Chung W-H, Hung S-I, Mallal S, Phillips EJ. Evolving models of the immunopathogenesis of T cell–mediated drug allergy: The role of host, pathogens, and drug response. Journal of Allergy and Clinical Immunology. 2015;136(2):219–234. doi: 10.1016/j.jaci.2015.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daly AK, Donaldson PT, Bhatnagar P, Shen Y, Pe’er I, Floratos A, et al. HLA-B*5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nat Genet. 2009;41(7):816–819. doi: 10.1038/ng.379. [DOI] [PubMed] [Google Scholar]
- 40.Li J, Uetrecht JP. The danger hypothesis applied to idiosyncratic drug reactions. Handb Exp Pharmacol. 2010;(196):493–509. doi: 10.1007/978-3-642-00663-0_18. [DOI] [PubMed] [Google Scholar]
- 41.Garside H, Marcoe KF, Chesnut-Speelman J, Foster AJ, Muthas D, Gerry Kenna J, et al. Evaluation of the use of imaging parameters for the detection of compound-induced hepatotoxicity in 384-well cultures of HepG2 cells and cryopreserved primary human hepatocytes. Toxicology in Vitro. 2014;28(2):171–181. doi: 10.1016/j.tiv.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 42.Takemura A, Izaki A, Sekine S, Ito K. Inhibition of bile canalicular network formation in rat sandwich cultured hepatocytes by drugs associated with risk of severe liver injury. Toxicology in Vitro. 2016;35:121–130. doi: 10.1016/j.tiv.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 43.Martin-Murphy BV, Holt MP, Ju C. The Role of Damage Associated Molecular Pattern Molecules in Acetaminophen-Induced Liver Injury in Mice. Toxicology Letters. 2010;192(3):387. doi: 10.1016/j.toxlet.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Foell D, Wittkowski H, Roth J. Mechanisms of Disease: a ‘DAMP’ view of inflammatory arthritis. Nat Clin Pract Rheum. 2007;3(7):382–390. doi: 10.1038/ncprheum0531. [DOI] [PubMed] [Google Scholar]
- 45.Maiuri AR, Breier AB, Gora LFJ, Parkins RV, Ganey PE, Roth RA. Cytotoxic Synergy Between Cytokines and NSAIDs Associated With Idiosyncratic Hepatotoxicity Is Driven by Mitogen-Activated Protein Kinases. Toxicological Sciences. 2015;146(2):265–280. doi: 10.1093/toxsci/kfv091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kindmark A, Jawaid A, Harbron CG, Barratt BJ, Bengtsson OF, Andersson TB, et al. Genome-wide pharmacogenetic investigation of a hepatic adverse event without clinical signs of immunopathology suggests an underlying immune pathogenesis. Pharmacogenomics J. 2008;8(3):186–195. doi: 10.1038/sj.tpj.6500458. [DOI] [PubMed] [Google Scholar]
- 47.Singer JB, Lewitzky S, Leroy E, Yang F, Zhao X, Klickstein L, et al. A genome-wide study identifies HLA alleles associated with lumiracoxib-related liver injury. Nat Genet. 2010;42(8):711–714. doi: 10.1038/ng.632. [DOI] [PubMed] [Google Scholar]
- 48.Spraggs CF, Budde LR, Briley LP, Bing N, Cox CJ, King KS, et al. HLA-DQA1*02:01 is a major risk factor for lapatinib-induced hepatotoxicity in women with advanced breast cancer. J Clin Oncol. 2011;29(6):667–673. doi: 10.1200/JCO.2010.31.3197. [DOI] [PubMed] [Google Scholar]
- 49.Warrington RJ, McPhilips-Feener S, Rutherford WJ. The predictive value of the lymphocyte transformation test in isoniazid-associated hepatitis. Clin Allergy. 1982;12(3):217–222. doi: 10.1111/j.1365-2222.1982.tb02521.x. [DOI] [PubMed] [Google Scholar]
- 50.Chisari FV, Isogawa M, Wieland SF. Pathogenesis of Hepatitis B Virus Infection. Pathologie-biologie. 2010;58(4):258–266. doi: 10.1016/j.patbio.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holman NS, Mosedale M, Wolf KK, LeCluyse EL, Watkins PB. Subtoxic Alterations in Hepatocyte-Derived Exosomes: An Early Step in Drug-Induced Liver Injury? Toxicological Sciences. 2016;151(2):365–375. doi: 10.1093/toxsci/kfw047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang Y, Han Q, Hou Z, Zhang C, Tian Z, Zhang J. Exosomes mediate hepatitis B virus (HBV) transmission and NK-cell dysfunction. Cell Mol Immunol. 2016 doi: 10.1038/cmi.2016.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mak A, Uetrecht J. The Combination of Anti-CTLA-4 and PD1−/− Mice Unmasks the Potential of Isoniazid and Nevirapine To Cause Liver Injury. Chemical Research in Toxicology. 2015;28(12):2287–2291. doi: 10.1021/acs.chemrestox.5b00305. [DOI] [PubMed] [Google Scholar]
- 54.Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treatment Reviews. 2016;44:51–60. doi: 10.1016/j.ctrv.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 55.Rossi RE, Parisi I, Despott EJ, Burroughs AK, O’Beirne J, Conte D, et al. Anti-tumour necrosis factor agent and liver injury: Literature review, recommendations for management. World Journal of Gastroenterology : WJG. 2014;20(46):17352–17359. doi: 10.3748/wjg.v20.i46.17352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mosedale M, Jackson JP, Brouwer KR, Button D, Caggiano A, Eisen A, et al. Transient Changes in Hepatic Physiology Impacting Bilirubin and Bile Acid Transport May Help Explain the Elevations in Liver Chemistries Observed in Clinical Trials of GGF2 (Cimaglermin Alpha) Society of Toxicology Annual Meeting. 2016 doi: 10.1093/toxsci/kfx222. Submitted. [DOI] [PubMed] [Google Scholar]
- 57.Thomson JP, Moggs JG, Wolf CR, Meehan RR. Epigenetic profiles as defined signatures of xenobiotic exposure. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2014;764–765:3–9. doi: 10.1016/j.mrgentox.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 58.Yang K, Woodhead JL, Watkins PB, Howell BA, Brouwer KL. Systems pharmacology modeling predicts delayed presentation and species differences in bile Acid-mediated troglitazone hepatotoxicity. Clin Pharmacol Ther. 2014;96(5):589–598. doi: 10.1038/clpt.2014.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Woodhead JL, Brock WJ, Roth SE, Shoaf SE, Brouwer KLR, Church RJ, et al. Application of a Mechanistic Model to Evaluate Putative Mechanisms of Tolvaptan Drug-Induced Liver Injury and Identify Patient Susceptibility Factors. Toxicological Sciences. 2016 doi: 10.1093/toxsci/kfw193. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lundbäck P, Lea JD, Sowinska A, Ottosson L, Fürst CM, Steen J, et al. A novel high mobility group box 1 neutralizing chimeric antibody attenuates drug-induced liver injury and postinjury inflammation in mice. Hepatology. 2016 doi: 10.1002/hep.28736. n/a–n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fontana RJ. Pathogenesis of idiosyncratic drug-induced liver injury and clinical perspectives. Gastroenterology. 2014:146. doi: 10.1053/j.gastro.2013.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Godoy P, Hewitt NJ, Albrecht U, Andersen ME, Ansari N, Bhattacharya S, et al. Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Archives of Toxicology. 2013;87(8):1315–1530. doi: 10.1007/s00204-013-1078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mosedale M, Kim Y, Brock WJ, Roth SE, Wiltshire T, Eaddy JS, et al. Candidate Risk Factors and Mechanisms for Tolvaptan-Induced Liver Injury Are Identified Using a Collaborative Cross Approach. Toxicological Sciences. 2016 doi: 10.1093/toxsci/kfw269. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheng L, Li F, Bility MT, Murphy CM, Su L. Modeling hepatitis B virus infection, immunopathology and therapy in mice. Antiviral Research. 2015;121:1–8. doi: 10.1016/j.antiviral.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Laifenfeld D, Qiu L, Swiss R, Park J, Macoritto M, Will Y, et al. Utilization of Causal Reasoning of Hepatic Gene Expression in Rats to Identify Molecular Pathways of Idiosyncratic Drug-Induced Liver Injury. Toxicological Sciences. 2014;137(1):234–248. doi: 10.1093/toxsci/kft232. [DOI] [PubMed] [Google Scholar]
- 66.Grove JI, Aithal GP. Human leukocyte antigen genetic risk factors of drug-induced liver toxicology. Expert Opinion on Drug Metabolism & Toxicology. 2015;11(3):395–409. doi: 10.1517/17425255.2015.992414. [DOI] [PubMed] [Google Scholar]
- 67.Nicoletti P, Werk AN, Sawle A, Shen Y, Urban TJ, Coulthard SA, et al. HLA-DRB1*16: 01-DQB1*05: 02 is a novel genetic risk factor for flupirtine-induced liver injury. Pharmacogenet Genomics. 2016;26(5):218–224. doi: 10.1097/FPC.0000000000000209. doi:210.1097/FPC.0000000000000209. [DOI] [PubMed] [Google Scholar]
- 68.Beger RD, Bhattacharyya S, Yang X, Gill PS, Schnackenberg LK, Sun J, et al. Translational biomarkers of acetaminophen-induced acute liver injury. Archives of Toxicology. 2015;89:1497–1522. doi: 10.1007/s00204-015-1519-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. The Journal of Clinical Investigation. 2012;122(4):1574–1583. doi: 10.1172/JCI59755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Adebayo D, Morabito V, Andreola F, Pieri G, Luong T-V, Dhillon A, et al. Mechanism of cell death in acute-on-chronic liver failure: a clinico-pathologic-biomarker study. Liver International. 2015;35(12):2564–2574. doi: 10.1111/liv.12850. [DOI] [PubMed] [Google Scholar]
- 71.Steuerwald NM, Foureau DM, Norton HJ, Zhou J, Parsons JC, Chalasani N, et al. Profiles of Serum Cytokines in Acute Drug-Induced Liver Injury and Their Prognostic Significance. PLoS ONE. 2013;8(12):e81974. doi: 10.1371/journal.pone.0081974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fannin RD, Gerrish K, Sieber SO, Bushel PR, Watkins PB, Paules RS. Blood transcript immune signatures distinguish a subset of people with elevated serum ALT from others given acetaminophen. Clinical Pharmacology & Therapeutics. 2015 doi: 10.1002/cpt.328. n/a–n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Antoine DJ, Jenkins RE, Dear JW, Williams DP, McGill MR, Sharpe MR, et al. Molecular forms of HMGB1 and keratin-18 as mechanistic biomarkers for mode of cell death and prognosis during clinical acetaminophen hepatotoxicity. J Hepatol. 2012;56(5):1070–1079. doi: 10.1016/j.jhep.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 74.Arrese M, Eguchi A, Feldstein AE. Circulating microRNAs: Emerging Biomarkers of Liver Disease. Semin Liver Dis. 2015;35(01):043–054. doi: 10.1055/s-0034-1397348. [DOI] [PubMed] [Google Scholar]