Abstract
While essential tremor has been considered the most common movement disorder, it has largely remained a diagnosis of exclusion: many tremor and non-tremor features must be absent for the clinical diagnosis to stand. The clinical features of “essential tremor” overlap with or may be part of other tremor disorders and, not surprisingly, this prevalent familial disorder has remained without a gene identified, without a consistent natural history, and without an acceptable pathology or pathophysiologic underpinning. The collective evidence suggests that under the rubric of essential tremor there exists multiple unique diseases, some of which represent cerebellar dysfunction, but for which there is no intrinsic “essence” other than a common oscillatory behavior on posture and action. One approach may be to use the term “essential tremor” only as a transitional node in the deep phenotyping of tremor disorders based on historical, phenomenological, and neurophysiological features, to facilitate its etiologic diagnosis or serve for future gene- and biomarker-discovery efforts. This approach deemphasizes essential tremor as a diagnostic entity and facilitates the understanding of the underlying disorders in order to develop biologically tailored diagnostic and therapeutic strategies.
Keywords: Essential tremor, tremor, biomarkers, nomenclature
Essential tremor (ET) has been defined clinically as a disorder manifested by bilateral, largely symmetric, postural or kinetic tremor involving the hands, with with variable combination of midline tremors (head, vocal cords and face), in the absence of abnormal posturing, task specificity, or position dependence.1 In the seminal description by Critchley, ET variations involve the legs, in which there may be “dyskinetic movements”, ataxia, “foot clubbing” or “evidence of peroneal atrophy”.2 ET has remained conceptualized as a single disorder rather than a syndrome comprised of several distinct disorders in part because we, as clinicians, have accepted its unclear boundaries: it affects both hands, but can be asymmetric or even (if rarely) unilateral;3 it can occur over a wide, non-specific frequency range (4–12 Hz);4 it may exceed its postural amplitude at rest (as in parkinsonian tremor) or on action (as in cerebellar outflow tremor);5 and it may affect handwriting but not exclusively at onset (as in primary writing tremor).6 The diagnosis of ET is, thus, based on excluding distinctive tremor types rather than asserting what the tremor of ET is. It is now clear that no existing definition of ET converges into a single disease. As a result, ET has not been associated with a key neurophysiologic marker or a defined genetic etiology despite its apparent autosomal dominant pattern of inheritability. The diagnosis of ET has been largely one of exclusion.
High rate of misdiagnosis
The misdiagnosis rate of ET in two studies has been reported to range from 37%7 to 50%.8 While Parkinson’s disease (PD) and dystonia may be under-recognized when tremor is prominent, neuropathic tremor, unilateral leg tremor, drug-induced tremor, and functional tremor may also be misreported as ET.7, 8 In patients with orthostatic tremor (OT), “postural upper extremity tremor while seated”, documented in 22.8% of 184 patients with OT, has been suggested to represent coexistent ET rather than a harmonic of the original high frequency tremor characteristic of OT.9
Other important entities that may be commonly misattributed to ET include enhanced physiological tremor,10 spinocerebellar ataxia type 12,11 Fragile X tremor-ataxia syndrome,12 dystonic tremor,13 cortical tremor (truly a form of rhythmic cortical myoclonus), and benign tremulous parkinsonism (most of which represent PD).14 The latter may actually represent either dystonic tremor or mono- or oligo-symptomatic PD, poorly responsive to levodopa but with very slow progression and restricted pathology.15
The pitfalls in the diagnosis of ET
Lack of consensus on clinical boundaries
The clinical separation is initially impeded by the problem with the very nomenclature of essential, which is shrouded in as much obscurity as the terms primary, idiopathic, and senile (Table 1). The adjective essential does not truly appear to uncover the diagnostic essence of any disorder and may instead provide a false sense of security that obscures critical knowledge gaps. More detailed clinical characterization of patients and large families, has served to replace such reductive terminology with more appropriate syndromic or etiologically appropriate labels, in some cases with genetic and molecular ascertainment, amenable to inclusionary rather than exclusionary diagnosis.
Table 1.
Essential, Primary, Senile, and Idiopathic: Covering gaps in knowledge
| Early nomenclature | Revised nomenclature | Implication |
|---|---|---|
| Primary dystonia | Isolated dystonia | “Primary”: not reliable for etiology |
| Essential myoclonus | Myoclonus dystonia54 Benign hereditary chorea55 |
“Essential myoclonus” encompassed a syndrome |
|
Essential palatal tremor |
Isolated palatal tremor including special skills, functional palatal tremor and tics56, 57 |
“Essential” included other recognizable palatal tremors |
| Senile gait | Highest-level gait disorder Parkinsonian gait |
“Senile”58 wrongly implied changes due to normal aging |
| Senile chorea | Most often late-onset Huntington disease, antiphospholipid antibody syndrome, hypocalcemia, or tardive dyskinesia59 |
“Senile” chorea wrongly suggests it may be due to normal aging |
|
Idiopathic Parkinson’s disease |
PARK1, 2, 4, 7, 8… Ongoing genetic-molecular phenotyping |
“Idiopathic” becomes an ever narrowing slice of PD, as genetic/molecular etiologies are unveiled |
There also remains an undefined boundary between senile tremor (ranging from Critchley’s “senile variety of essential tremor”16 to Deuschl’s “age-related tremor” 17) and the reported distinct second epidemiological age peak of ET. Tremor onset after the age of 65 years is associated with an increased risk of dementia, a different natural history than those with tremor onset earlier in life.18 On the other hand, family history and alcohol responsiveness may be more common in early-onset “ET”,19 and may be associated with other tremor syndromes to a similar or greater extent. Yet they continue to be used as supportive of the diagnosis of ET.20, 21 Another controversial entity is isolated head tremor, which represents in most instances tremulous cervical dystonia. In fact, of 50 patients diagnosed as “essential tremor” randomly reviewed at the National Hospital for Neurology and Neurosurgery in London, only 25 (50%) were confirmed to have ET on subsequent analysis.8 Patients received alternative diagnoses (dystonia in 4, neuropathic tremor in 2, drug-induced tremor, functional tremor and myoclonus in 3 other cases) or atypical features suggested an alternative diagnosis of dystonic or parkinsonian tremor (7 cases with rest tremor, 2 with reduced arm swing), functional tremor (2 with sudden onset), and tremor associated with dystonia (1 case with a family member with an ‘isolated head tremor’); others had tremor in other body sides, including unilateral leg tremor in 2 cases.8 The authors concluded that the diagnosis of ET was overused and other types of tremor were overlooked when making this diagnosis.
Although the current diagnostic criteria of ET imply the lack of other overt neurological signs, many publications have assumed that ET and dystonia or PD may actually co-exist instead of representing ET mimics such as ‘tremor associated with dystonia’ and ‘Type II PD tremor’, respectively.1 For instance, in a large study on 463 patients from 97 kindreds with autosomal dominant mode of inheritance, "pure" ET was present in 365 individuals whereas remaining cases were felt to have a distinct ET subtype, “ET associated with dystonia” .22 Similar nosological confusion has affected PD research, resulting in the pursuit of a common genetic underpinning with ET.23, 24
A retrospective review of 350 patients with a diagnosis of ET found that 47% of patients had associated dystonia (spasmodic torticollis in 27%, writer’s cramp in 14%, blepharospasm in 7%, and spasmodic dysphonia in 4%) and 20% had parkinsonism.25 Nevertheless, the authors concluded that there was “no support for differentiation of ET subtypes” and that “although heterogeneous in its clinical presentation, [ET] is a single disease entity.”25 However, head or hand tremor can be the sole initial manifestation in patients with mutations in the anoctamin 3 (ANO3) gene, which results in autosomal dominant craniocervical dystonia (DYT24), often misdiagnosed as ET.26 Indeed, epidemiologic, clinical, and neurophysiologic data support non-task-specific dystonia as the appropriate nomenclature for a large proportion of patients currently classified as ET.27
Because postural and action tremor is a nonspecific feature of many diseases, there has been a large diversity of manifestations reported to be associated with ET (including the increased risk of PD, see also Long-term prognosis section below) based on the assumption of shared common biological mechanisms. For instance, some non-motor features have been recently suggested to represent “core non-motor symptoms” of ET: hyposmia,28 hearing impairment,29 cognitive impairment,30 and depression.31 Impairments in verbal fluency, naming, mental set-shifting, verbal memory, and working memory compared to a normative sample were reported in 18 ET surgical candidates, in a pattern similar to PD (not adjusting for depression, other comorbidities, or medication exposure).32 In a population based study, after adjusting for age, stroke, and educational level, patients with tremor onset after the age 65 were 70% more likely to be demented than controls.18 Hearing, olfactory, and cognitive impairments in each case appeared to be non-specific for a single entity, such as ET. In fact, the reported hyposmia of ET becomes indistinguishable from that of controls when the effects of age, age of onset, gender, and smoking are taken into account.33
Lack of consensus on prevalence
Because age-dependent tremors continue to be classified as ET there is wide variability in the reporting ET prevalence. Indeed, prevalence estimates vary 2,750 fold (8 to 22,000 cases per 100,000 population) among 20 studies.34 Similarly, “mildly abnormal tremor resembling mild ET”35 or any “signs of tremor”36 are documented in unselected ostensibly normal individuals with a mean of 7436 to 76 years.35 Thus, the cutoff between “senile” and ET tremor is poorly defined, suggesting either a substantial phenotypic heterogeneity in ET or a variety of disorders that exhibit tremor as one of their features.
Lack of consensus on electrophysiology
The diagnosis of ET cannot be confirmed with electrophysiology given the wide range of allowable frequency, 4 to 12 Hz,1 and the many allowable clinical variations, including the presence of both synchronous and alternating contraction of agonist and antagonist muscles.37 Surface electromyography and accelerometry can be used to support the presence of other tremor disorders rather than to confirm ET. In a population study from South Tyrol, tremor analyses of clinically suspected ET patients forced a substantial number to be reclassified as enhanced physiological tremor (because of reduction of the tremor frequency with weight loading in the latter), restricting the diagnosis of ET to the identification of a central tremor component on accelerometry.38 Electrophysiology has been used to separate ET from dystonic (evidence of overflow, effect of sensory tricks) and functional tremor (variability in frequency, entraintment),39, 40 but not from some forms of cerebellar tremor (the tremor generator likely differs in cerebellar neurodegenerative disorders compared to cerebellar lesions, such as in multiple sclerosis).5 In fact, there is no neurophysiological marker or single brain tremor-generating region common to what has been believed to represent ET.41 A synchronous rather than an alternating EMG burst pattern has been considered a hallmark of dystonia (assumed to represent co-contraction) but its diagnostic accuracy is debatable. More recently, temporal discrimination has been found to distinguish “tremor associated with dystonia” from ET42 and, interestingly, the same technique served to place isolated head and voice tremors fall into the category of dystonic tremors.43
Lack of consensus on pathology
Pathology studies have not yielded consistent findings, which may be due to the brain banking of heterogeneous “ET” populations. On one end of the spectrum, Lewy bodies in the locus ceruleus and torpedos in Purkinje cells have been reported by one group in a study of 33 ET brains.44, 45 A separate postmortem study of 24 brains of patients diagnosed with ET, however, found cerebellar gliosis and locus coeruleus depletion but no torpedos or Lewy bodies.46 The loss of Purkinje cells remains also confounded by the unclear long-term effect of medications and alcohol used to abate tremor and the extent to which it may be associated with disease duration. Nevertheless, a study comparing Purkinje cells in 7 ET and 6 PD brains found no difference across them, or with age-matched healthy controls.47
Lack of consensus on long-term prognosis (and the ET-PD conundrum)
While some epidemiologic studies have suggested that ET increases the risk of PD four- to five-fold,48 possibly reflecting the “Lewy body variant of ET”,44 other studies have suggested no such shared risk or pathology.46, 49 Nevertheless, this potential association raises the possibility that in some patients diagnosed with ET, this tremor represents a ‘transitional’ manifestation of a disorder yet to be characterized. This is supported by the fact that fewer than 10% of community dwellers with a diagnosis of ET had been diagnosed by their primary physician as having ET and only 5% were taking an anti-tremor medication,50 which suggests that those who come to medical attention have an unusually severe form of the disorder, a different disorder altogether, or a separate neurological abnormality beyond tremor. Further compounding the assessment of long-term outlook is the reported bimodal distribution of the age at onset of ET, a first peak in late teens and a second in late adulthood, which suggests the existence of at least two different pathophysiologic entities –and therefore two different courses. In particular, the late-onset ET group may be associated with dystonic features on exam (e.g., isolated rest tremor or the jaw and/or head and spasmodic dysphonia, which have a gender predilection for women) 34 or with non-motor symptoms possibly representing the aforementioned ‘transitional’ manifestations to developing PD, dementia51 and postural impairment.52
In sum, as a syndrome whose only “essence” is the abnormal oscillation in motor pathways resulting in rhythmic modulation of motor unit activity, ET lacks an electrophysiologic signature to distinguish it from most other tremor disorders, an identified gene or genes despite its prevalence, a consistent natural history, and an acceptable underlying pathological substrate.
Approach to postural and action tremor
Postural and action tremor can be seen in enhanced physiologic tremor due to many drugs or metabolic changes (mechanical-reflex oscillations), PD and other degenerative parkinsonisms, dopa-responsive dystonia, Wilson's disease, cerebellar diseases, and many other (central neurogenic oscillations) disorders. When a diagnostic work up fails to uncover an etiology, the term “ET” is generally applied and has become further enriched with a variety of features (Figure 1A) and associated movement disorders (Figure 1B). The reduction of postural and action tremor into the single essential construct of ET (exclusionary diagnosis if the features and any associated movements are “soft” or “non specific”) deserves reappraisal to facilitate its decomposition into what is most likely a number of distinct diagnostic etiologies (inclusionary diagnosis) and to assist future genomic and biomarker research.
Figure 1. Models of tremor.
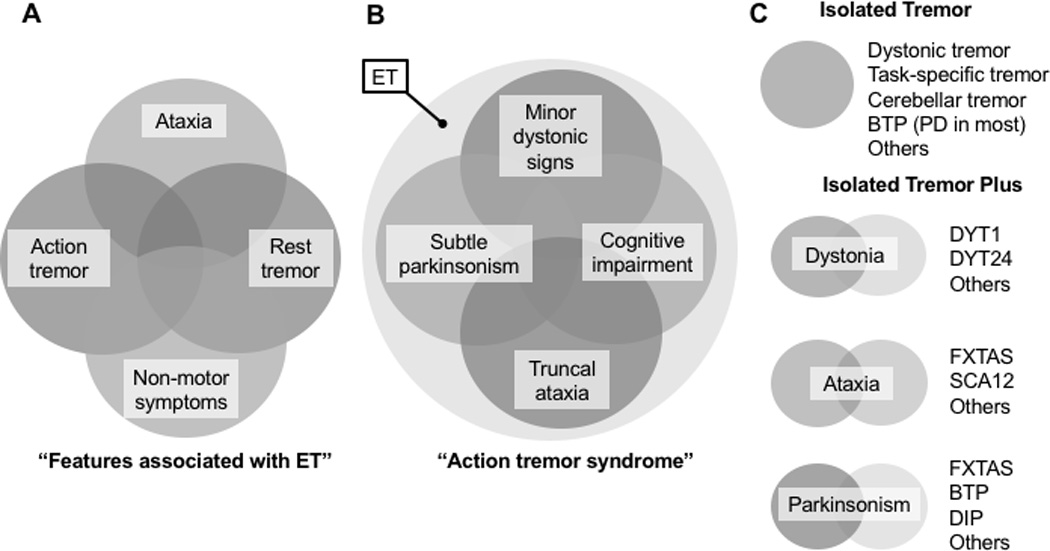
ET has been viewed as a single but heterogeneous disorder (A, model adapted from Louis et al60) and as an “isolated action tremor syndrome” encompassing other movement disorders (B; accounting for cases where “classic ET is too narrowly defined”61). An alternative model (C, brought to the fore in the reclassification effort for dystonia53) relies on the phenomenological characterization of tremor as a movement presented in isolation or in combination with other movement disorders. ET: essential tremor, BTP: benign tremulous parkinsonism; DIP: drug-induced parkinsonism; DYT: designation for genes associated with dystonia; PD; Parkinson disease; FXTAS: Fragile X tremor ataxia syndrome; SCA: spinocerebellar ataxia
An approach to tremor, as recently applied to a reclassification effort for dystonia (Figure 1C),53 avoids accepting “associated signs” as permissible for “ET” and relies instead on a two-step approach to postural and action tremor as presenting in isolation or in combination with other movement disorders, permitting their separation into “isolated” and “combined” tremor (Figure 2). In addition to the determination of age of onset (childhood, adolescence to early adulthood, and older than 45 years)19 and family history (of tremor, dystonia, parkinsonism, ataxia, and/or dementia), the following clinical characteristics of the tremor can facilitate the search for an underlying etiology: distribution (arms, legs, head, vocal cords, or a combination thereof), activating condition (rest, postural, action, intention, position/task specificity), and frequency (low [2–3], moderate [4–7], high [8 and above]). In the case of tremor presenting in combination with other features, key abnormalities to identify are dystonia (in the affected limb or distant body part), bradykinesia, and cerebellar features, with or without cognitive and/or gait impairment (Figure 2).
Figure 2. Approach to tremor.
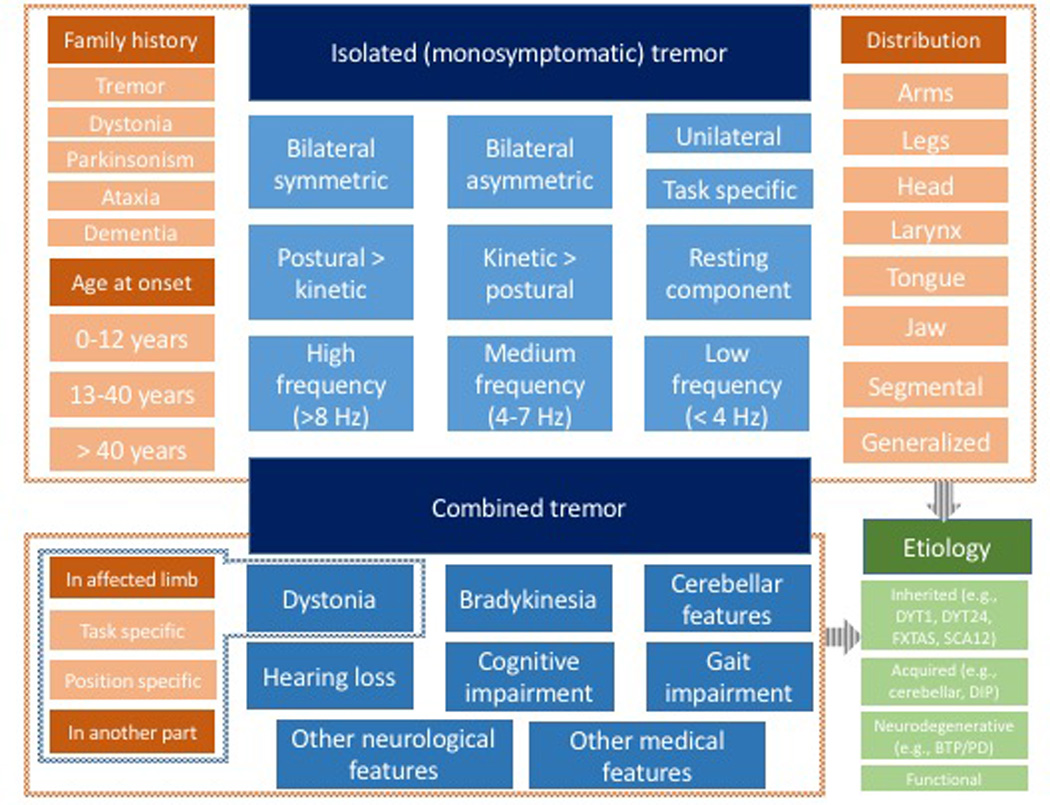
Historical (shades of red) and clinical features of isolated (upper box) or combined tremor (lower box) serve to provide the foundations on which the etiologic diagnosis (shades of green) can be formulated. The phenomenological clues and etiologic considerations stemming from them are similar to those used in the recent redefinition of dystonia.53
Subdividing cases in this fashion does not preclude combining categories when there is a clear justification for this. For example, the separation may permit the recognition of a genetic cause, which in turn may segregate with a broader clinical spectrum suggesting phenotypic heterogeneity with a single genetic cause. However, splitting into descriptive forms of isolated or isolated-plus tremors will reduce the “noise” that may prevent us from finding specific underlying causes.
Conclusions and future steps
Holding ET hostage to a single heteregenous construct may continue to prevent the identification of the constituent disorders that have been subsumed under this umbrella. While a change in nomenclature from ET to any other designation may not be critical in and of itself, the understanding that it represents a starting point rather than the end itself is important to facilitate etiologic diagnoses and a molecular approach to future therapeutic endeavors. To facilitate further studies, a better characterization of the historic and semiologic features of tremor are to represent the foundation for the ascertainment of unique etiologies. As a consequence of this approach, the waste basket of “ET” will continue to shrink from the absence of essential elements in its natural history, electrophysiology, genetic underpinnings, and pathology. Assisted by efforts in genomics and biomarker development applied to large tremor populations, the field should evolve into an era of etiologically-defined tremor disorders. Only then will biologically tailored interventions be selectively developed for specific tremor disorders.
Acknowledgments
Full financial disclosure for the previous 12 months
Dr. Espay has received grant support from the NIH, CleveMed/Great Lakes Neurotechnologies, Davis Phinney Foundation, and Michael J Fox Foundation; personal compensation as a consultant/scientific advisory board member for Solvay, Abbott, Chelsea Therapeutics, TEVA, Impax, Merz, Lundbeck, and Eli Lilly; honoraria from TEVA, UCB, the American Academy of Neurology, and the Movement Disorders Society; and publishing royalties from Lippincott Williams & Wilkins, Cambridge University Press, and Springer.
Prof. Lang has served as an advisor for AbbVie, Allon Therapeutics, Avanir Pharmaceuticals, Biogen Idec, Boehringer Ingelheim, Bristol-Myers Squibb, Ceregene, Cipla, InteKrin, Lilly, Medtronic, Merck, Novartis, NeuroPhage Pharmaceuticals, Teva, and UCB; received honoraria from Medtronic, Teva, UCB, and AbbVie; received grants from Brain Canada, the Canadian Institutes of Health Research, the Edmond J. Safra Philanthropic Foundation, The Michael J. Fox Foundation, the Ontario Brain Institute, National Parkinson Foundation, Parkinson Society Canada, Physicians Services Incorporated (PSI), Tourette Syndrome Association, W. Garfield Weston Foundation; received publishing royalties from Saunders, Wiley-Blackwell, Johns Hopkins Press, and Cambridge University Press; and has served as an expert witness in cases related to the welding industry.
Dr. Erro has received consultancies from Zambon.
Dr Merola has received grant support from UCB Pharma and speaker honoraria from CSL Behring, UCB Pharma, and Teva Pharmaceuticals. He has received personal compensation from Edge Consulting S.r.l., MediK S.r.l., and Sthetos S.r.l.
Dr. Fasano has received grant support from the McLaughlin Centre and the Michael J. Fox Foundation; he received speaking honoraria from UCB pharma, Medtronic, Boston Scientific, Abbvie, Novartis, and Chiesi pharmaceutical; he is in an advisory board for Abbvie and Ipsen; he provided consultancies for UCB pharma, Medtronic, Boston Scientific, and Abbvie.
Prof. Berardelli has received funds from the Benign Essential Blepharospasm Research Foundation for research on blepharospasm. He has received national grants from from Italian Ministry of University and Sapienza University of Rome, Chiesi, Lundbeck, Merz, Allergan, Ipsen. He has received honoraria for lecturing from Boehringer Ingelheim, GSK Pharmaceutical, Novartis Pharmaceuticals, Lundbeck, Chiesi.
Prof. Bhatia has received grant support from Welcome/MRC, NIHR, Parkinsons’s UK and EU Horizon 2020. He has received royalties from publication of the Oxford Specialist Handbook Parkinson's Disease and Other Movement Disorders (Oxford University Press, 2008, 2016) and of Marsden's Book of Movement Disorders (Oxford University Press, 2013). He has received honoraria/personal compensation for participating as consultant/scientific board member from Ipsen, Allergan, Merz and honoraria for speaking at meetings and from Allergan, Ipsen, Merz, Sun Pharma, Teva, UCBPharmaceuticals and from the American Academy of Neurology and Movement Disorders Society.
Footnotes
Financial disclosure related to research covered in this article: None
Authors’ roles
1. Research project: A. Conception, B. Organization, C. Execution
2. Manuscript Preparation: A. Writing of the first draft, B. Review and Critique
AJE: 1A, 1B, 1C, 2A, 2B
AEL, KPB: 1B, 1C, 2B
RE, AM, AF, AB: 1C, 2B
References
- 1.Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on Tremor. Ad Hoc Scientific Committee. Mov Disord. 1998;13(Suppl 3):2–23. doi: 10.1002/mds.870131303. [DOI] [PubMed] [Google Scholar]
- 2.Critchley E. Clinical manifestations of essential tremor. J Neurol Neurosurg Psychiatry. 1972;35:365–372. doi: 10.1136/jnnp.35.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phibbs F, Fang JY, Cooper MK, Charles DP, Davis TL, Hedera P. Prevalence of unilateral tremor in autosomal dominant essential tremor. Mov Disord. 2009;24:108–111. doi: 10.1002/mds.22113. [DOI] [PubMed] [Google Scholar]
- 4.Calzetti S, Baratti M, Gresty M, Findley L. Frequency/amplitude characteristics of postural tremor of the hands in a population of patients with bilateral essential tremor: implications for the classification and mechanism of essential tremor. J Neurol Neurosurg Psychiatry. 1987;50:561–567. doi: 10.1136/jnnp.50.5.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deuschl G, Wenzelburger R, Loffler K, Raethjen J, Stolze H. Essential tremor and cerebellar dysfunction clinical and kinematic analysis of intention tremor. Brain. 2000;123(Pt 8):1568–1580. doi: 10.1093/brain/123.8.1568. [DOI] [PubMed] [Google Scholar]
- 6.Soland VL, Bhatia KP, Volonte MA, Marsden CD. Focal task-specific tremors. Mov Disord. 1996;11:665–670. doi: 10.1002/mds.870110611. [DOI] [PubMed] [Google Scholar]
- 7.Jain S, Lo SE, Louis ED. Common misdiagnosis of a common neurological disorder: how are we misdiagnosing essential tremor? Arch Neurol. 2006;63:1100–1104. doi: 10.1001/archneur.63.8.1100. [DOI] [PubMed] [Google Scholar]
- 8.Schrag A, Munchau A, Bhatia KP, Quinn NP, Marsden CD. Essential tremor: an overdiagnosed condition? J Neurol. 2000;247:955–959. doi: 10.1007/s004150070053. [DOI] [PubMed] [Google Scholar]
- 9.Hassan A, Ahlskog JE, Matsumoto JY, Milber JM, Bower JH, Wilkinson JR. Orthostatic tremor: Clinical, electrophysiologic, and treatment findings in 184 patients. Neurology. 2016;86:458–464. doi: 10.1212/WNL.0000000000002328. [DOI] [PubMed] [Google Scholar]
- 10.Elble RJ. Physiologic and essential tremor. Neurology. 1986;36:225–231. doi: 10.1212/wnl.36.2.225. [DOI] [PubMed] [Google Scholar]
- 11.O'Hearn E, Holmes SE, Calvert PC, Ross CA, Margolis RL. SCA-12: Tremor with cerebellar and cortical atrophy is associated with a CAG repeat expansion. Neurology. 2001;56:299–303. doi: 10.1212/wnl.56.3.299. [DOI] [PubMed] [Google Scholar]
- 12.Leehey MA, Munhoz RP, Lang AE, et al. The fragile X premutation presenting as essential tremor. Arch Neurol. 2003;60:117–121. doi: 10.1001/archneur.60.1.117. [DOI] [PubMed] [Google Scholar]
- 13.Lalli S, Albanese A. The diagnostic challenge of primary dystonia: evidence from misdiagnosis. Mov Disord. 2010;25:1619–1626. doi: 10.1002/mds.23137. [DOI] [PubMed] [Google Scholar]
- 14.Josephs KA, Matsumoto JY, Ahlskog JE. Benign tremulous parkinsonism. Arch Neurol. 2006;63:354–357. doi: 10.1001/archneur.63.3.354. [DOI] [PubMed] [Google Scholar]
- 15.Selikhova M, Kempster PA, Revesz T, Holton JL, Lees AJ. Neuropathological findings in benign tremulous parkinsonism. Mov Disord. 2013;28:145–152. doi: 10.1002/mds.25220. [DOI] [PubMed] [Google Scholar]
- 16.Critchley M. Observations on essential (heredofamilial) tremor. Brain. 1949;72:113–139. doi: 10.1093/brain/72.2.113. [DOI] [PubMed] [Google Scholar]
- 17.Deuschl G, Petersen I, Lorenz D, Christensen K. Tremor in the elderly: Essential and aging-related tremor. Mov Disord. 2015;30:1327–1334. doi: 10.1002/mds.26265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benito-Leon J, Louis ED, Bermejo-Pareja F. Elderly-onset essential tremor is associated with dementia. Neurology. 2006;66:1500–1505. doi: 10.1212/01.wnl.0000216134.88617.de. [DOI] [PubMed] [Google Scholar]
- 19.Hopfner F, Ahlf A, Lorenz D, et al. Early- and late-onset essential tremor patients represent clinically distinct subgroups. Mov Disord. 2016 doi: 10.1002/mds.26708. [DOI] [PubMed] [Google Scholar]
- 20.Knudsen K, Lorenz D, Deuschl G. A clinical test for the alcohol sensitivity of essential tremor. Mov Disord. 2011;26:2291–2295. doi: 10.1002/mds.23846. [DOI] [PubMed] [Google Scholar]
- 21.Louis ED, Dogu O, Ottman R. Subclinical tremor in normal controls with versus without a family history of essential tremor: data from the United States and Turkey. Eur J Neurol. 2010;17:607–611. doi: 10.1111/j.1468-1331.2009.02875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hedera P, Phibbs FT, Fang JY, Cooper MK, Charles PD, Davis TL. Clustering of dystonia in some pedigrees with autosomal dominant essential tremor suggests the existence of a distinct subtype of essential tremor. BMC Neurol. 2010;10:66. doi: 10.1186/1471-2377-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Unal Gulsuner H, Gulsuner S, Mercan FN, et al. Mitochondrial serine protease HTRA2 p.G399S in a kindred with essential tremor and Parkinson disease. Proc Natl Acad Sci U S A. 2014;111:18285–18290. doi: 10.1073/pnas.1419581111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng H, Le WD, Hunter CB, Mejia N, Xie WJ, Jankovic J. A family with Parkinson disease, essential tremor, bell palsy, and parkin mutations. Arch Neurol. 2007;64:421–424. doi: 10.1001/archneur.64.3.421. [DOI] [PubMed] [Google Scholar]
- 25.Lou JS, Jankovic J. Essential tremor: clinical correlates in 350 patients. Neurology. 1991;41:234–238. doi: 10.1212/wnl.41.2_part_1.234. [DOI] [PubMed] [Google Scholar]
- 26.Stamelou M, Charlesworth G, Cordivari C, et al. The phenotypic spectrum of DYT24 due to ANO3 mutations. Mov Disord. 2014;29:928–934. doi: 10.1002/mds.25802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Defazio G, Conte A, Gigante AF, Fabbrini G, Berardelli A. Is tremor in dystonia a phenotypic feature of dystonia? Neurology. 2015;84:1053–1059. doi: 10.1212/WNL.0000000000001341. [DOI] [PubMed] [Google Scholar]
- 28.Giorelli M, Bagnoli J, Consiglio L, et al. Do non-motor symptoms in Parkinson's disease differ from essential tremor before initial diagnosis? A clinical and scintigraphic study. Parkinsonism Relat Disord. 2014;20:17–21. doi: 10.1016/j.parkreldis.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Benito-Leon J, Louis ED, Bermejo-Pareja F. Reported hearing impairment in essential tremor: a population-based case-control study. Neuroepidemiology. 2007;29:213–217. doi: 10.1159/000112463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benito-Leon J, Louis ED, Mitchell AJ, Bermejo-Pareja F. Elderly-onset essential tremor and mild cognitive impairment: a population-based study (NEDICES) J Alzheimers Dis. 2011;23:727–735. doi: 10.3233/JAD-2011-101572. [DOI] [PubMed] [Google Scholar]
- 31.Fabbrini G, Berardelli I, Falla M, et al. Psychiatric disorders in patients with essential tremor. Parkinsonism Relat Disord. 2012;18:971–973. doi: 10.1016/j.parkreldis.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 32.Lombardi WJ, Woolston DJ, Roberts JW, Gross RE. Cognitive deficits in patients with essential tremor. Neurology. 2001;57:785–790. doi: 10.1212/wnl.57.5.785. [DOI] [PubMed] [Google Scholar]
- 33.Shah M, Muhammed N, Findley LJ, Hawkes CH. Olfactory tests in the diagnosis of essential tremor. Parkinsonism Relat Disord. 2008;14:563–568. doi: 10.1016/j.parkreldis.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 34.Quinn NP, Schneider SA, Schwingenschuh P, Bhatia KP. Tremor--some controversial aspects. Mov Disord. 2011;26:18–23. doi: 10.1002/mds.23289. [DOI] [PubMed] [Google Scholar]
- 35.Elble RJ. Tremor in ostensibly normal elderly people. Mov Disord. 1998;13:457–464. doi: 10.1002/mds.870130314. [DOI] [PubMed] [Google Scholar]
- 36.Louis ED, Wendt KJ, Ford B. Senile tremor. What is the prevalence and severity of tremor in older adults? Gerontology. 2000;46:12–16. doi: 10.1159/000022127. [DOI] [PubMed] [Google Scholar]
- 37.Milanov I. Clinical and electromyographic examinations of patients with essential tremor. Can J Neurol Sci. 2000;27:65–70. doi: 10.1017/s0317167100052008. [DOI] [PubMed] [Google Scholar]
- 38.Wenning GK, Kiechl S, Seppi K, et al. Prevalence of movement disorders in men and women aged 50–89 years (Bruneck Study cohort): a population-based study. Lancet Neurol. 2005;4:815–820. doi: 10.1016/S1474-4422(05)70226-X. [DOI] [PubMed] [Google Scholar]
- 39.McAuley J, Rothwell J. Identification of psychogenic, dystonic, and other organic tremors by a coherence entrainment test. Mov Disord. 2004;19:253–267. doi: 10.1002/mds.10707. [DOI] [PubMed] [Google Scholar]
- 40.Schwingenschuh P, Katschnig P, Seiler S, et al. Moving toward "laboratory-supported" criteria for psychogenic tremor. Mov Disord. 2011;26:2509–2515. doi: 10.1002/mds.23922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muthuraman M, Heute U, Arning K, et al. Oscillating central motor networks in pathological tremors and voluntary movements. What makes the difference? Neuroimage. 2012;60:1331–1339. doi: 10.1016/j.neuroimage.2012.01.088. [DOI] [PubMed] [Google Scholar]
- 42.Tinazzi M, Fasano A, Di Matteo A, et al. Temporal discrimination in patients with dystonia and tremor and patients with essential tremor. Neurology. 2013;80:76–84. doi: 10.1212/WNL.0b013e31827b1a54. [DOI] [PubMed] [Google Scholar]
- 43.Conte A, Ferrazzano G, Manzo N, et al. Somatosensory temporal discrimination in essential tremor and isolated head and voice tremors. Mov Disord. 2015;30:822–827. doi: 10.1002/mds.26163. [DOI] [PubMed] [Google Scholar]
- 44.Louis ED, Faust PL, Vonsattel JP, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007;130:3297–3307. doi: 10.1093/brain/awm266. [DOI] [PubMed] [Google Scholar]
- 45.Axelrad JE, Louis ED, Honig LS, et al. Reduced Purkinje cell number in essential tremor: a postmortem study. Arch Neurol. 2008;65:101–107. doi: 10.1001/archneurol.2007.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shill HA, Adler CH, Sabbagh MN, et al. Pathologic findings in prospectively ascertained essential tremor subjects. Neurology. 2008;70:1452–1455. doi: 10.1212/01.wnl.0000310425.76205.02. [DOI] [PubMed] [Google Scholar]
- 47.Rajput AH, Robinson CA, Rajput ML, Rajput A. Cerebellar Purkinje cell loss is not pathognomonic of essential tremor. Parkinsonism Relat Disord. 2011;17:16–21. doi: 10.1016/j.parkreldis.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 48.Benito-Leon J, Louis ED, Bermejo-Pareja F. Risk of incident Parkinson's disease and parkinsonism in essential tremor: a population based study. J Neurol Neurosurg Psychiatry. 2009;80:423–425. doi: 10.1136/jnnp.2008.147223. [DOI] [PubMed] [Google Scholar]
- 49.Adler CH, Shill HA, Beach TG. Essential tremor and Parkinson's disease: lack of a link. Mov Disord. 2011;26:372–377. doi: 10.1002/mds.23509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Louis ED, Ford B, Wendt KJ, Cameron G. Clinical characteristics of essential tremor: data from a community-based study. Mov Disord. 1998;13:803–808. doi: 10.1002/mds.870130508. [DOI] [PubMed] [Google Scholar]
- 51.Bermejo-Pareja F, Louis ED, Benito-Leon J. Risk of incident dementia in essential tremor: a population-based study. Mov Disord. 2007;22:1573–1580. doi: 10.1002/mds.21553. [DOI] [PubMed] [Google Scholar]
- 52.Kronenbuerger M, Konczak J, Ziegler W, et al. Balance and motor speech impairment in essential tremor. Cerebellum. 2009;8:389–398. doi: 10.1007/s12311-009-0111-y. [DOI] [PubMed] [Google Scholar]
- 53.Albanese A, Bhatia K, Bressman SB, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord. 2013;28:863–873. doi: 10.1002/mds.25475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Valente EM, Edwards MJ, Mir P, et al. The epsilon-sarcoglycan gene in myoclonic syndromes. Neurology. 2005;64:737–739. doi: 10.1212/01.WNL.0000151979.68010.9B. [DOI] [PubMed] [Google Scholar]
- 55.Breedveld GJ, van Dongen JW, Danesino C, et al. Mutations in TITF-1 are associated with benign hereditary chorea. Hum Mol Genet. 2002;11:971–979. doi: 10.1093/hmg/11.8.971. [DOI] [PubMed] [Google Scholar]
- 56.Zadikoff C, Lang AE, Klein C. The 'essentials' of essential palatal tremor: a reappraisal of the nosology. Brain. 2006;129:832–840. doi: 10.1093/brain/awh684. [DOI] [PubMed] [Google Scholar]
- 57.Biller J, Espay AJ. Nosography of the "essential": volitional palatal tremor. Neurology. 2013;81:772–773. doi: 10.1212/WNL.0b013e3182a1aab3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elble RJ, Hughes L, Higgins C. The syndrome of senile gait. J Neurol. 1992;239:71–75. doi: 10.1007/BF00862975. [DOI] [PubMed] [Google Scholar]
- 59.Warren JD, Firgaira F, Thompson EM, Kneebone CS, Blumbergs PC, Thompson PD. The causes of sporadic and 'senile' chorea. Aust N Z J Med. 1998;28:429–431. doi: 10.1111/j.1445-5994.1998.tb02075.x. [DOI] [PubMed] [Google Scholar]
- 60.Louis ED. Essential tremors: a family of neurodegenerative disorders? Arch Neurol. 2009;66:1202–1208. doi: 10.1001/archneurol.2009.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Elble RJ. What is essential tremor? Curr Neurol Neurosci Rep. 2013;13:353. doi: 10.1007/s11910-013-0353-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


