Abstract
Objective
Temporomandibular joint (TMJ) diseases predominantly afflict women, suggesting a role of estrogen in the disease etiology. Previously, we determined that decreased occlusal loading (DOL) inhibited collagen type II (Col2) expression in the mandibular condylar cartilage (MCC) of female wild-type (WT) mice whereas no change was observed in males. This decrease in chondrogenesis was abolished by estrogen receptor beta (ERβ) deficiency in females. Therefore, the goal of this study was to examine the role of estradiol – ERβ signaling in mediating DOL effects in male mice to further decipher sex differences.
Methods
Male 21 day-old WT and ERβKO male mice were treated with either placebo or estradiol and exposed to normal or DOL for 4 weeks. Cartilage thickness and cell proliferation, gene expression and immunohistochemistry of chondrogenic markers and estrogen receptor alpha (ERα), and analysis of bone histomorphometry via microCT were completed to ascertain the effect of estradiol on DOL effects to the TMJ.
Results
ERβKO male mice lack a MCC phenotype. In both genotypes, estradiol treatment increased Col2 gene expression and trabecular thickness. DOL in combination with estradiol treatment caused a significant increase in Col2 gene expression in both genotypes.
Conclusions
The sex differences in DOL-induced inhibition of Col2 expression do not appear to be mediated by differences in estradiol levels between male and female mice. Greater understanding on the role of estrogen and altered loading are critical in order to decipher the sex dimorphism of TMJ disorders.
Keywords: Temporomandibular joint, mandibular condylar cartilage, estradiol, estrogen receptor β, sex differences, decreased occlusal loading
1. INTRODUCTION
Temporomandibular joint (TMJ) pain afflicts approximately 10% of the United States population with roughly half of this pain associated with joint degenerative disease (TMJ-DD)1, 2. It has been postulated that excessive TMJ remodeling in response to altered mechanical loading is a major factor in the development of TMJ-DD3, 4. Further, women who engage in increased oral parafunctional behavior are the population most subject to developing TMJ-DD5–8. These clinical statistics suggest the role of estrogen and mechanical loading-induced TMJ remodeling in the disease process.
Unlike most hyaline articular cartilages of the appendicular joints, the mandibular condylar cartilage (MCC) is derived from periosteal tissue and comprised of fibrocartilage9, 10. In response to altered loading, the MCC is known to regulate the size and properties of the tissue to adapt to the change in load11–17. This response is mediated by the osteochondral progenitor cells located within the proliferative zone of the cartilage18–20. These cells are typically quiescent and are activated to undergo proliferation. Afterwards, they either continue to proliferate or proceed through cell cycle arrest to then differentiate into chondrocytes which express collagen type II and aggrecan18, 21. This process is further regulated by estrogen signaling22–24 which predominantly occurs through two receptor isoforms, estrogen receptor alpha (ERα) and beta (ERβ). Ligand binding of estrogen to either receptor induces a conformational change, receptor dimerization, nuclear translocation, and target gene transcription. Elucidating the combined role of altered loading and estradiol to the TMJ and deciphering sex differences that mediate these responses is vital for developing treatments to combat accelerated alterations to the joint.
In the TMJ, we have optimized a decreased occlusal loading (DOL) mouse model that consists of incisor trimming and soft diet administration25. Utilizing this model, we have observed a decrease in MCC thickness in both male and female mice in response to this underloading treatment25–27. Surprisingly, DOL reduced collagen type II (Col2) expression only in females, suggesting sex differences in the role estradiol plays in load altered load-induced MCC chondrogenesis25, 26. Estrogen-mediated sex differences have been previously observed in periosteal-derived tissue. Male and female mice deficient in ERα express a characteristic skeletal phenotype28, 29. In contrast, only female mice deficient in ERβ display a unique skeletal phenotype29–32. In bone, female mice deficient in ERβ experience an increase in mechanical loading-induced periosteal bone formation suggesting this receptor may inhibit altered load-induced remodeling33. However, the role of estradiol on the MCC of male mice in decreased loading conditions is unknown.
In the female MCC, we have previously determined that DOL inhibition of Col2 expression was abolished by ERβ deficiency suggesting that estradiol inhibition of TMJ chondrogenesis in this altered loading state is mediated by ERβ signaling27. Therefore, the major goal of this study was to examine whether the sex differences in DOL-induced inhibition of Col2 expression are controlled by an estradiol-ERβ signaling pathway utilizing male mice. In order to examine this, 21 day-old male WT and ERβKO mice were treated with placebo or estradiol and exposed to either normal or DOL for 4 weeks. We hypothesized that estradiol treatment would result in a DOL-induced inhibition of Col2 expression in male WT mice but not ERβKO mice. As such, cartilage thickness, cell proliferation, gene expression of chondrogenic markers and estrogen receptors, and microCT analysis of bone architecture were completed. Determining a mechanism by which estradiol mediates altered loading-induced TMJ remodeling is critical in order to decipher the sex dimorphism of the disease.
2. MATERIALS AND METHODS
2.1 Mice
All experiments were performed in accordance with animal welfare based on an approved Institutional Animal Care and Use Committee (IACUC) protocol (#AAAH9166) from Columbia University. Breeding pairs of C57BL/6 WT (Cat# 000664) and ERβKO mice (homozygous male, heterozygous female, Cat# 004745) were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Twenty-one day-old WT and ERβKO male mice were divided into four groups (n = 12 for each group: n = 6 for histology/microCT and n = 6 for mRNA): placebo (Plb)/normal load (NL), placebo (Plb)/decreased occlusal loading (DOL), estradiol (Esd)/normal load (NL), and estradiol (Esd)/decreased occlusal loading (DOL) (Table 1). This age was chosen for two reasons: (1) eruption of molars and occlusion are complete at this age34 (2) majority of MCC growth in mice is completed by 60 days of age35. Mice were administered placebo or 17β-estradiol (60 day release, Innovative Research of America, FL) for 28 days at a daily dose of 10 ng/g body weight. The dose of estradiol utilized in this study matches the dose found effective to restore MCC thickness in female, ovariectomized WT mice of the same age36. Half of the mice were subjected to DOL involving mandibular incisor trimming and a soft-dough diet (Transgenic Dough Diet; Bioserv, Frenchtown, NJ) as detailed previously26. The normal load groups received a hard-dough diet. Following 4 weeks of treatment, mice were injected intraperitoneally with 0.13mg bromodeoxyuridine (BrdU) per gram body weight at 3 and 19 hours prior to euthanasia to track proliferating cells.
Table 1.
Groups and sample sizes investigated in this study.
| Group | Genotype | Estradiol | Loading Condition | Sample Size (n) |
|---|---|---|---|---|
| 1 | WT | Placebo | NL | 6 (Histology); 6 (PCR and μCT) |
| 2 | WT | Estradiol | NL | 6 (Histology); 6 (PCR and μCT) |
| 3 | WT | Placebo | DOL | 6 (Histology); 6 (PCR and μCT) |
| 4 | WT | Estradiol | DOL | 6 (Histology); 6 (PCR and μCT) |
| 5 | ERβKO | Placebo | NL | 6 (Histology); 6 (PCR and μCT) |
| 6 | ERβKO | Estradiol | NL | 6 (Histology); 6 (PCR and μCT) |
| 7 | ERβKO | Placebo | DOL | 6 (Histology); 6 (PCR and μCT) |
| 8 | ERβKO | Estradiol | DOL | 6 (Histology); 6 (PCR and μCT) |
2.2 Histomorphometry and Immunohistochemistry
Histomorphometry and immunohistochemistry techniques were employed to determine the effect of estradiol and DOL treatment on condylar cartilage cellular and matrix changes in male WT and ERβKO mice. Whole mouse heads were sectioned into halves, fixed in 10% formalin for 4 days at room temperature and decalcified in 14% ethylenediaminetetraacetic acid (EDTA) (pH 7.1) (Sigma, St. Louis, MO, USA) for 28 days. Subsequently, the samples were processed through progressive concentrations of ethanol, cleared in xylene, and embedded in paraffin. Sagittal serial sections of 5 μm thickness were made of the TMJ utilizing a Microm HM 355s microtome (Thermo Fisher Scientific, Waltham, MA, USA). Sections representing the mid-coronal portion of the mandibular head were stained with hematoxylin and eosin (H&E) and Safranin-O (SafO) and used as the representative central section for analysis.
Histomorphometry measurements were made in a blinded, nonbiased manner using the BioQuant computerized image analysis system (BioQuant, Nashville, TN, USA). MCC analysis was performed on H&E sagittal sections corresponding to the mid-coronal portion of the mandibular condylar head. Average thicknesses were determined for each region and summed for the total cartilage thickness. Condyles from six mice within each group were analyzed and the average of three-five sections was taken for each sample.
For immunohistochemistry, tissue sections were deparaffinized with xylene and rehydrated in progressive ethanol/water solutions with increasing concentrations of deionized water. Following rehydration, the sections were digested for 10 minutes with pepsin for unmasking (Lab Vision, Fremont, CA, USA), washed with PBS, and treated with a 3 vol% hydrogen peroxide in methanol solution to inhibit endogenous peroxidase activity. All sections were blocked with 10% normal goat serum (Life Technologies) to reduce non-specific binding of the antigen with the primary antibody. Immunohistochemical staining was performed using the SuperPicture™ Polymer HRP Broad Spectrum Detection Kit (Life Technologies) following the procedure recommended by the manufacturer. Collagen type II (Millipore; MAB8887, 1:100 dilution),ERα (Abcam; ab75635, 1:100 dilution) and androgen receptor (Abcam, ab74272, 1:100 dilution) primary antibodies were utilized in this study. After incubation with the primary antibodies (Col2: 60 minutes at RT; ERα and androgen receptor: overnight at 4°C), sections were washed twice in PBS. The secondary antibody – horseradish peroxidase (HRP) conjugate (SuperPicture™, Life Technologies) was added and incubated for 10 minutes at RT. Following 2X PBS wash, sections were stained with DAB chromogen (30 μL DAB in 1 mL diluent, ImmPACT DAB, Vector Laboratories) for 2 minutes. Sections were then counterstained with either hematoxylin (Col2 staining) or 0.2% Fast Green (ERα and androgen receptor staining) for 30 seconds, dehydrated, and mounted.
BrdU immunohistochemical analysis to determine proliferating cells was completed using a BrdU staining kit following the manufacturer's instructions (Zymed Laboratories-Invitrogen Corporation, Carlsbad, CA, USA). To quantify BrdU, the labeling index (number of BrdU positive cells divided by the total number of cells) was calculated. Three to six sections, corresponding to the same anatomical region utilized to determine total cell number (mid-coronal), were counted for each group and the average index of these sections was used for the labeling index.
2.3 mRNA Extraction and PCR Amplification
After duration of treatment, mRNA from the condylar cartilage of all groups was extracted to analyze the effects of estradiol and DOL on the expression of chondrocyte markers and ERα. For each mouse, the MCC (left and right) was carefully isolated from all other soft tissue and dissected under a dissecting microscope. mRNA was extracted with TRIzol Reagent (Ambion by Life Technologies) following the manufacturer’s protocol and treated with DNase treatment and removal kit (Ambion by Life Technologies). Reverse transcription was performed to convert mRNA to cDNA utilizing the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Real-time polymerase chain reaction (RT-PCR) was conducted to assess the relative levels of genes of interest using the ViiA™ 7 Real-Time PCR System (Applied Biosystems, Life Technologies) following the protocol detailed in Chen et al 37. Expression of each gene of interest was determined relative to the Gapdh housekeeping gene (Gapdh – MM99999915_g1) utilizing the ΔΔCT method. Gene expression was analyzed for the following chondrocyte markers: parathyroid hormone-related peptide (PTHrP – Mm00436057_m1), SRY-box containing gene 9 (Sox9 – MM00448840_m1), collagen type II (Col 2a1 – Mm00491889_m1), collagen type X (Col 10a1 – Mm00487041_m1), sclerostin (Sost – Mm00470479_m1), indian hedgehog (Ihh – Mm00439613_m1), estrogen receptor α (Esr1 – Mm00433149_m1), and estrogen receptor β (Esr2 – Mm00599819). All primers were purchased from Applied Biosystems.
2.4 Micro-CT Analysis
The effect of estradiol and DOL on WT and ERβKO male mice subchondral bone architecture was accessed utilizing cone-beam micro-focus x-ray computed tomography (μCT40, Scanco Medical AG, Bassersdorf, Switzerland) as previously described26. Briefly, serial tomographic images from 6 mice per group were acquired at 55 kV and 145 μA and 2000 projections per rotation were collected at 300 msec integration time. Three-dimensional 16-bit grayscale images were reconstructed with standard convolution back-projection algorithms with Shepp and Logan filtering. Bone was segmented from marrow and surrounding soft tissue including cartilage using a constrained Gaussian filter to reduce noise at a threshold of 480 mg/cm3. Volumetric regions were selected to include the mandibular condyle. Bone volume fraction, trabecular number, trabecular thickness, and trabecular spacing were determined.
2.5 Statistical Analysis
Data points, averages, and confidence intervals are presented in the scatter plots. For histomorphometry and BrdU analysis, each data point represents the average value for each mouse sample obtained from 3–6 histological sections. Confidence intervals were determined for the data sets using a t-distribution for sample sizes smaller than 30. T values were determined for a 95% confidence with degrees of freedom dependent on sample size (e.g.the value for 5 degrees of freedom for a sample size of 6 for a 95% confidence interval is 2.571). The standard deviation divided by the square root of the sample size was multiplied by the t value. This value was then added and subtracted from the sample means to determine the upper and lower bounds for the confidence interval. Normal distribution of the data was confirmed via the Shapiro-Wilk test using SPSS. Significant outliers were removed using Tukey’s outlier method which was only necessary for the BrdU data. Specifically, the interquartile range was multiplied by 2.2 and subtracted from the lower quartile and added to the upper quartile. Observations were removed if they fell below or above these determined values. Statistical significance of differences among means was determined by a Student’s t-test when comparing one factor (genotype, estradiol, or DOL alone) and two-way analysis of variance (ANOVA) with post hoc analysis by the Bonferonni method using SPSS when determining the combined effect of estradiol and DOL. The combined effects of estradiol and DOL were determined by significance between NL+Esd vs. DOL+Esd and DOL+Plb vs. DOL+Esd. Statistical significance was defined as p < 0.05 and the specific p values are denoted in the respective plots.
3. RESULTS
Experimental groups and sample sizes utilized for this study are detailed in Table 1. Estradiol treatment resulted in a significant decrease in body weight in both WT (WT Plb = 21.8 ± 1.3 vs. WT Esd = 19.7 ± 1.6) and ERβKO (KO Plb = 21.7 ± 1.4 vs. KO Esd = 19.2 ± 1.6) mice, an effect which has been shown previously38. However, DOL treatment had no significant effect on male mice body weight in either genotype.
ERβKO male mice do not exhibit a MCC or subchondral bone phenotype
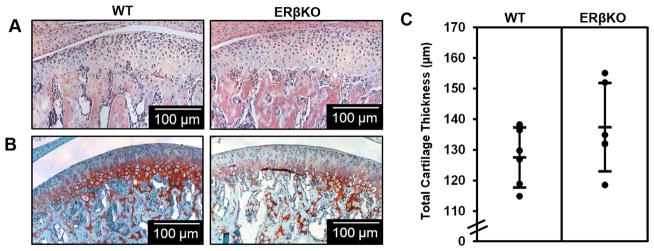
There was no significant difference in cartilage morphology (Figure 1A and 1B) or cartilage thicknesses as determined by histomorphometry (Figure 1C) between male WT and ERβKO mice. Gene expression and immunohistochemical staining of Col2 and bone morphology as determined by μCT also revealed no statistically significant differences comparing the WT and ERβKO mice (Supplementary Figure 1).
Figure 1. ERβ mandibular condylar cartilage phenotype in male mice.
Representative hematoxylin & eosin (H&E) images (A), Safranin-O images (B), and histomorphometric cartilage thicknesses (C) for 49–day WT and ERβKO male mice treated with placebo and subjected to normal load. For histomorphometric analysis, n=6 mice were utilized from each group and the average of 3–6 sections/mouse was analyzed. Statistical significance was determined by a Student’s t-test and p = 0.18.
Estradiol treatment results in anabolic changes in the MCC and subchondral bone
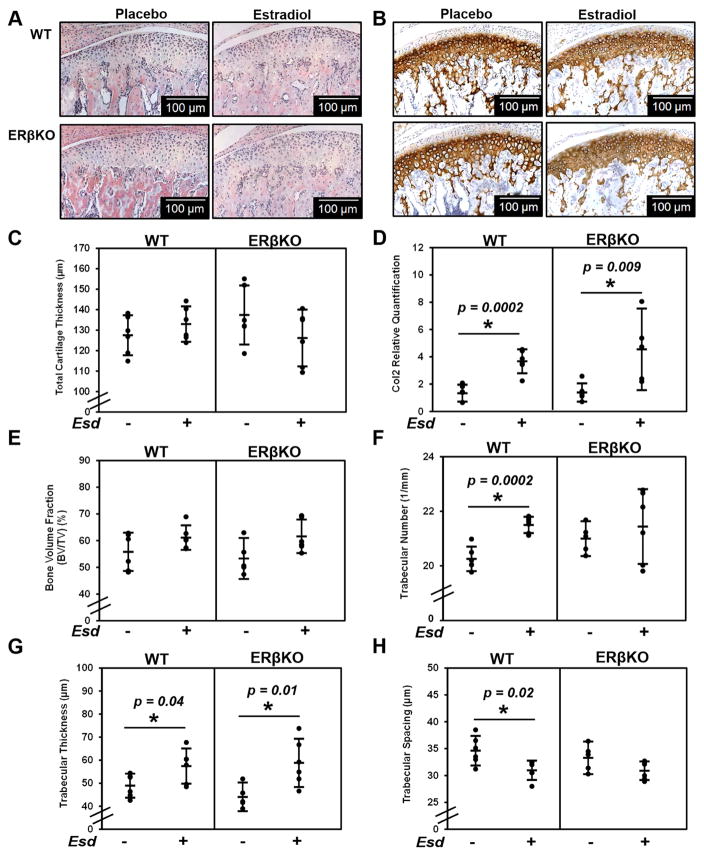
Estradiol treatment did not result in any significant change in cartilage thickness in 49 day-old male WT and ERβKO mice. (Figure 2A and 2C). However, estradiol treatment increased the production and localization of Col2 in both genotypes (Figure 2B and 2D). In the subchondral bone, estradiol treatment did not result in a significant change in bone volume fraction (Figure 2E) but did result in an increase in trabecular number and trabecular thickness and a decrease in trabecular spacing in WT mice (Figure 2F–2H). An increase in trabecular thickness was observed with estradiol treatment in ERβKO mice (Figure 2G). Overall, estradiol promotes remodeling of both the MCC and subchondral bone in WT and ERβKO male mice.
Figure 2. Effect of estradiol on cartilage thickness, Col2 expression and production, and bone architecture.
The data represent WT and ERβKO mice under normal load with either placebo or estradiol treatment. Representative hematoxylin & eosin (H&E) images (A), Col2 immunohistochemical staining (B), cartilage thickness as determined by histomorphometry (C), Col2 gene expression (D), bone volume fraction (E), trabecular number (F), trabecular thickness (G), and trabecular spacing (H) are shown. For histomorphometric analysis, n=6 mice were utilized for all groups and the average of 3–6 sections/mouse was analyzed. For gene expression, n=6 mice were utilized for all groups and mandibular condylar cartilage from left and right were pooled together. Statistical significance was determined by a Student’s t-test and p < 0.05.
Estradiol promotes chondrogenesis in response to DOL independent of ERβ
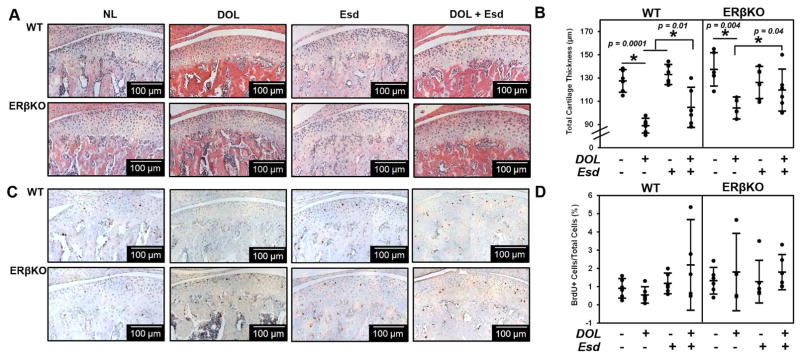
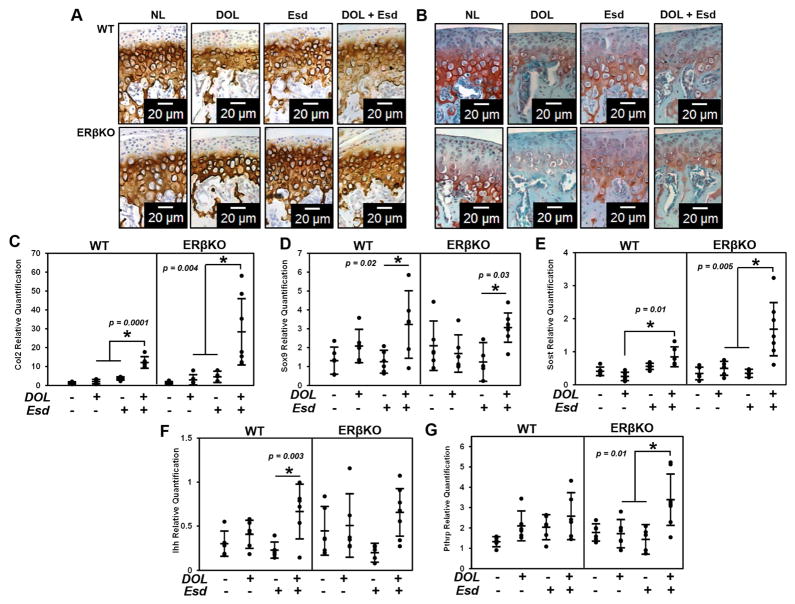
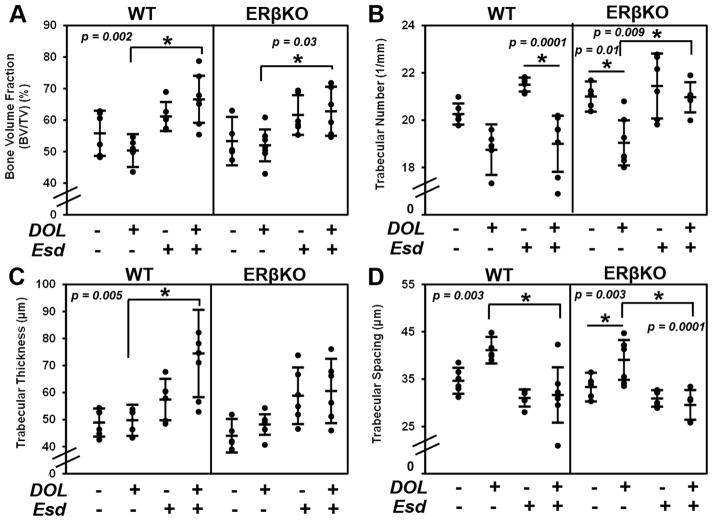
While DOL resulted in a decrease in cartilage thickness for both genotypes, estradiol administered during this treatment partly inhibited the DOL-induced reduction in cartilage thickness (Figure 3A and 3B). Figure 3C and 3D illustrate the negligible effect of estradiol and DOL on cell proliferation for both genotypes. Figure 4A illustrates an increase in Col2 production and localization when estradiol is administered during DOL compared to DOL alone. A similar effect was observed with glycosaminoglycan staining with safranin O in Figure 4B. Gene expression analysis revealed that estradiol and DOL caused a significant increase in Col2 (Figure 4C), Sox9 (Figure 4D), and Sost (Figure 4E) expression in both WT and ERβKO mice. In WT mice, Ihh expression (Figure 4F) was significantly increased whereas Pthrp expression (Figure 4G) was significantly increased in ERβKO. No significant change in Col10 expression was observed (data not shown). In the subchondral bone, estradiol and DOL resulted in an increase in bone volume fraction in both genotypes (Figure 5A). This effect is likely attributed to the increase in trabecular number (Figure 5B) and/or trabecular thickness (Figure 5C) with a resulting decrease in trabecular spacing (Figure 5D).
Figure 3. Effect of estradiol and decreased occlusal loading on cartilage thickness and cell proliferation.
The data represent WT and ERβKO mice under normal load (NL) or decreased occlusal loading (DOL) with either placebo (Plb) or estradiol (Esd) treatment. Specifically, the labels indicate the following: NL = normal load and placebo; DOL = decreased occlusal loading and placebo; Esd = normal load and estradiol; DOL + Esd = decreased occlusal loading and estradiol. Representative hematoxylin & eosin (H&E) images (A), cartilage thickness as determined by histomorphometry (B), BrdU proliferative immunohistochemical staining (C), and quantified percentage of BrdU+ cells (D) are shown. For histomorphometric and BrdU analysis, n=6 mice were utilized for all groups and the average of 3–6 sections/mouse was analyzed. BrdU+ cells were normalized to the total number of cells in the cartilage region as determined from the adjacent H&E section. Statistical significance was determined by a two-way ANOVA followed by posthoc analysis with the Bonferonni method with p < 0.05. Exact p values are listed above the bars that denote significance.
Figure 4. Effect of estradiol and decreased occlusal loading on chondrogenic markers.
The data represent WT and ERβKO mice under normal load or decreased occlusal loading with either placebo or estradiol treatment. Specifically, the labels indicate the following: NL = normal load and placebo; DOL = decreased occlusal loading and placebo; Esd = normal load and estradiol; DOL + Esd = decreased occlusal loading and estradiol. Representative Col2 immunohistochemical images (A), representative safranin O images (B) and gene expression of Col2 (C), Sox9 (D), Pthrp (E) and Ihh (F) and Sost (G) are shown. For gene expression, n=6 mice were utilized for all groups and mandibular condylar cartilage from left and right were pooled together. Statistical significance was determined by a two-way ANOVA followed by posthoc analysis with the Bonferonni method with p < 0.05. Exact p values are listed above the bars that denote significance.
Figure 5. Effect of estradiol and decreased occlusal loading on bone architecture.
The data represent WT and ERβKO mice under normal load or decreased occlusal loading with either placebo or estradiol treatment. Bone volume fraction (A), trabecular number (B), trabecular thickness (C), and trabecular spacing (D) as determined by microCT are shown. Statistical significance was determined by a two-way ANOVA followed by posthoc analysis with the Bonferonni method with p < 0.05. Exact p values are listed above the bars that denote significance.
Sex steroid levels in response to DOL and estradiol treatment
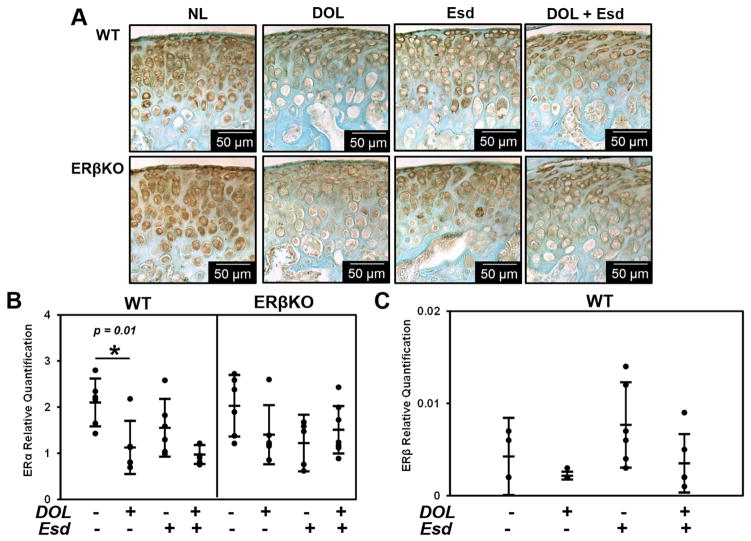
Estradiol treatment did not significantly affect ERα expression for either genotype. DOL resulted in a decrease in ERα expression in WT mice which was not changed when estradiol was administered as seen by immunohistochemistry (Figure 6A) and gene expression analysis (Figure 6B). Neither DOL nor estradiol treatment significantly affected the gene expression of ERβ in the WT groups as seen in Figure 6C. However, estradiol treatment did increase the localization of androgen receptor in the MCC in the WT mice but not the ERβKO mice as shown in Supplementary Figure 2.
Figure 6. Effect of estradiol and decreased occlusal loading on ER- gene expression and immunostaining.
The data represent WT and ERβKO mice under normal load or decreased occlusal loading with either placebo or estradiol treatment. Specifically, the labels indicate the following: NL = normal load and placebo; DOL = decreased occlusal loading and placebo; Esd = normal load and estradiol; DOL + Esd = decreased occlusal loading and estradiol. Representative ERα immunohistochemical images (A), and gene expression of ERα (B) and ERβ (C) are shown. For gene expression, n=6 mice were utilized for all groups and mandibular condylar cartilage from left and right were pooled together. Statistical significance was determined by a two-way ANOVA followed by posthoc analysis with the Bonferonni method with p < 0.05. Exact p values are listed above the bars that denote significance.
4. DISCUSSION
The major goal of this work was to examine whether the sex differences in DOL-induced inhibition of chondrocyte maturation were mediated by an estradiol-ERβ signaling pathway. Overall, two major findings resulted from this study. First, male mice lack an ERβ mandibular condylar phenotype indicating estrogen-induced TMJ growth and remodeling are not directly mediated by ERβ in male mice. Second, the sex differences in DOL-inhibition of chondrocyte maturation, specifically decreased Col2 gene expression and protein production seen in females but not males, are not mitigated by supraphysiological estradiol levels.
ERβ has no significant effect on mandibular condylar phenotype or remodeling in male mice
In 49 day-old male mice, no statistically significant differences were observed in any cartilage or subchondral bone measurementsof the WT mandibular condyle compared to ERβKO mice. In addition, we found no significant change in the response to DOL in placebo treated WT mice compared to placebo treated ERβKO mice. This is in contrast to 49 day-old female mice, in which we found that ERβKO mice exhibited increased cartilage thickness, increased chondrocyte maturation gene expression, and increased total volume in the subchondral bone compared to age-matched WT controls36. We also found that DOL-induced inhibition of Col2 expression that occurs in WT female mice did not occur in female ERβKO mice27. These combined results are consistent with previous findings that illustrate an ERβ phenotype solely in the cortical bone, trabecular bone, and growth plate cartilage of female mice29, 32, 39. These results highlight the fundamental differences in the role of ERβ in mediating TMJ growth and remodeling in males versus females.
Effects of estradiol on the mandibular condyle in male mice
Multiple studies have illustrated the effect of estradiol on MCC metabolism in females22, 23, 40–42. However, the role of estradiol supplementation in male mice is unclear. Estradiol treatment resulted in differential effects to the MCC thickness and proliferation in male mice compared to effects previously observed in female mice. In females, it has been shown that estrogen treatment causes a decrease in MCC thickness in ovariectomized WT mice but not in ERβKO mice 23, 37. Further, ovariectomy results in increased proliferation in female WT but not ERβKO mice 37, 42. In this study, we found that estrogen treatment did not significantly affect the MCC thickness or proliferation in male WT or ERβKO mice. One reason for the observed sex differences may be that estradiol treatment was conducted in gonad-intact mice in this study, whereas estradiol treatment was performed in ovariectomized female mice in our previous studies. However, Figueroba et al. showed that estradiol treatment in gonadectomized male rats did not drastically effect MCC thickness compared to the changes observed in female rats supporting the findings from this study 43. Therefore taken together, the results suggest that estrogen via ERβ inhibits proliferation and subsequent thinning of the MCC in female but not in male WT mice.
On the other hand, estradiol treatment promoted chondrogenesis in male mice. In this study, we found that similar to female mice, estradiol caused a significant increase in Col2 gene expression and protein production in both genotypes37. Estradiol is known to promote chondrogenesis in hyaline cartilage and delay experimental arthritis44, emphasizing the anabolic effects of estrogen observed in this study45. Specifically in males, estradiol has been shown to increase bone mineral density and exhibit a bone sparing effect46–49 and promote epiphyseal closure.50 These results strongly suggest that estrogen promotes TMJ chondrogenesis independent of ERβ in both sexes.
While a few studies have looked at the role of estrogen in mediating the microarchitecture of the mandibular condylar subchondral bone in females, not much is known in male mice. In this study, we found that estrogen caused an increase in trabecular thickness independent of ERβ. In females, estrogen deficiency induced bone loss as measured by trabecular thickness and bone volume fraction in the mandibular subchondral bone of female rats51. Further, studies investigating the role of genistein, a phytoestrogen that structurally resembles 17β-estradiol, illustrated an increase in trabecular thickness and bone volume fraction dependent on ERβ in female rats52. Thus, estradiol promotes anabolic changes in the mandibular condylar subchondral bone of both sexes; however, the increase in trabecular thickness may only depend on ERβ in females.
Sex differences in the effects of estradiol and DOL on the TMJ
We previously found that DOL caused inhibition of Sox9 and Col2 expression in female mice only 26 and these effects were abolished by ERβ deficiency 27. Thus, we hypothesized that estradiol treatment to DOL male mice would cause inhibition of Col2 expression in WT but not in ERβKO mice. However, we observed the opposite effect. Estradiol treatment with DOL resulted in an increase in Col2 and Sox9 expression and a decrease in Sost expression in both genotypes. These results suggest that the sex differences in response to DOL are not mediated by differences in estradiol levels between male and female mice.
Sex differences in the estrogen receptor expression levels and/or the role of these receptors in mediating mechanical loading-induced TMJ remodeling may exist. For example, in the rat condylar cartilage, the number of cells expressing ERα and ERβ were significantly larger in males compared to females from through 12 months 53. It has previously been shown that estrogen deficiency alone or in combination with decreased loading increases ERα expression in female rats 40. While we did observe a decrease in ERα expression with DOL in WT male mice in this study, no significant change was observed with estradiol or the combined treatments. Thus, the significant increase in Col2 expression in response to estradiol treatment and DOL is not directly dependent on ERα expression levels in male mice.
Further, it is probable that estradiol treatment may affect TMJ growth and remodeling differently in males and females resulting in differential responses to altered mechanical loading. Estradiol inhibits proliferation solely in female mice which may result in fewer cells available for chondrogenesis compared to male mice that are exposed to DOL. In support, it has previously been shown that ovariectomized female rats exposed to a soft diet exhibited an increased Col2 area compared to non-ovariectomized female rats exposed to a soft diet suggesting that estradiol and decreased loading reduce the number of cells undergoing chondrogenesis 41. Also, sex differences in cell proliferation in response to strain have been shown in murine osteoblasts 54. Collectively, these results suggest the male MCC exhibits an increased estrogen-mediated adaptive capacity to resist altered mechanical loading.
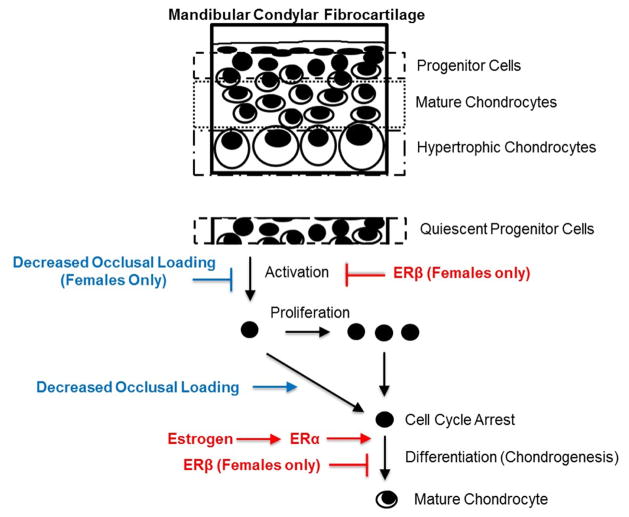
A working model illustrating the effects of estradiol and DOL in the MCC is provided in Figure 7. We posit that DOL causes cell cycle arrest in both sexes. However, we propose that DOL and/or estradiol via ERβ inhibits TMJ progenitor cell activation solely in female mice. Therefore, when estradiol and DOL treatment are combined in the male mice, we propose that DOL results in an increase in the number of cells undergoing cycle arrest and primed for chondrogenic differentiation, which is then promoted by estradiol. This results in the increase in Col2 expression and one possible mechanism for the partial increase in cartilage thickness compared to DOL alone. In contrast, ERβ causes a decrease in the number of activated progenitor cells in female mice, thereby causing a decrease in the number of cells available for chondrogenesis. These sex differences related to MCC chondrogenesis provide one mechanism to explain the discrepancies in TMJ disorders between males and females.
Figure 7.
Working model illustrating the sex-dependent role of estradiol via ERβ and decreased occlusal loading on the mandibular condylar cartilage growth and remodeling.
We recognize that our study has many limitations. First, the mice utilized in this study were young, growing 49-day old mice. Thus, any changes observed cannot be directly translated to effects that may occur in older mice. Also, the aim of this study was to determine if supraphysiological estradiol levels in male mice resulted in similar results compared to the female studies. Naturally, circulating estradiol levels in male mice are negligible 55. However, aromatization of testosterone is a common way in which males produce estrogen suggesting that estradiol levels may have been fluctuating throughout the experiment 39, 56. Also, the supraphysiological levels of estrogen administered to male mice in this study may be suppressing androgen levels and causing the observed effects 57. Supplementary Figure 2 illustrates an increase in androgen receptor staining solely in WT mice after estradiol treatment possibly indicating a change in androgen levels in these mice. Lastly, this study was conducted using global ERβKO mice that may express ERβ splice variants. Thus, future studies using conditional ERβKO mice with complete deletions of ERβ in specific cell populations will be advantageous in ascertaining the local effects of estradiol and DOL.
In conclusion, this study illustrates that estradiol treatment independent of ERβ results in an increase in chondrogenesis in the male MCC. Further, estradiol treatment administered with DOL causes enhanced chondrogenesis in male mice independent of ERβ. Taken together, these results suggest that there are sex differences in response to DOL and estradiol in the TMJ. Thus, additional investigation of the role of estradiol signaling in both males and females is necessary to gain greater understanding of the sexual dimorphism in TMJ disorders and develop sex-specific regeneration strategies.
Supplementary Material
The data represent WT and ERβKO mice under normal load with placebo treatment. Representative Col2 immunohistochemical images (A), gene expression of Col2 (B), bone volume fraction (C), trabecular number (D), trabecular thickness (E) and trabecular spacing (F) are shown. For gene expression, n=6 mice were utilized for all groups and mandibular condylar cartilage from left and right were pooled together. Statistical significance was determined by a Student’s t-test and p < 0.05.
The data represent WT and ERβKO mice under normal load or decreased occlusal loading with either placebo or estradiol treatment. Specifically, the labels indicate the following: NL = normal load and placebo; DOL = decreased occlusal loading and placebo; Esd = normal load and estradiol; DOL + Esd = decreased occlusal loading and estradiol. Representative androgen receptor immunohistochemical images are shown for all groups.
Highlights.
ERβKO male mice lack a mandibular condylar cartilage phenotype.
Estradiol and decreased occlusal loading promote Col2 expression independent of ERβ.
Sex differences exist in the role of estradiol and decreased occlusal loading on mandibular condylar cartilage remodeling.
Acknowledgments
The authors acknowledge Don McMahon for his statistical expertise and Alina O’Brien, Vikas Gupta, and Patricia Solarte for their help in imaging.
8. Role of Funding Source
Funding for this work was supported by the National Institute for Health (R56 DE020097). Additionally, this material is based upon work supported by the National Institute for Health K12 TMJ Training Grant No. 5K12DE023583-02.
Abbreviations
- TMJ
Temporomandibular joint
- ERβKO
Estrogen receptor beta knock-out
- ERα
Estrogen receptor alpha
- ERβ
Estrogen receptor beta
- MCC
Mandibular condylar cartilage
- DOL
Decreased occlusal loading
- NL
Normal Load
- Plb
Placebo
- Esd
Estradiol
- Col2
Collagen type II
- Col10
Collagen type X
- Ihh
Indian hedgehog
- PTHrP
Parathyroid hormone-related protein
- Sox9
SRY-box containing gene 9
- H&E
Hematoxylin and eosin
Footnotes
7. Author Contributions
J. Robinson was involved in study design, data collection, data analysis, drafting and approval of the manuscript.
K. Cass was involved in data collection, drafting and approval of the manuscript.
R. Aronson was involved in data collection, drafting and approval of the manuscript.
T. Choi was involved in data collection, drafting and approval of the manuscript.
M. Xu was involved in data collection, drafting and approval of the manuscript.
R. Buttenbaum was involved in data collection, drafting and approval of the manuscript.
H. Drissi was involved in study design, drafting and approval of the manuscript.
H. H. Lu was involved in data analysis, drafting, and approval of the manuscript.
J. Chen was involved in study design, data analysis, drafting and approval of the manuscript.
S. Wadhwa was involved in study design, data analysis, drafting and approval of the manuscript.
J. Robinson and S. Wadhwa take responsibility for the integrity of the work as a whole.
9. Conflict of Interest No competing financial interests exist for any of the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lipton JA, Ship JA, Larach-Robinson D. Estimated prevalence and distribution of reported orofacial pain in the United States. J Am Dent Assoc. 1993;124:115–121. doi: 10.14219/jada.archive.1993.0200. [DOI] [PubMed] [Google Scholar]
- 2.Manfredini D, Guarda-Nardini L, Winocur E, Piccotti F, Ahlberg J, Lobbezoo F. Research diagnostic criteria for temporomandibular disorders: a systematic review of axis I epidemiologic findings. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:453–462. doi: 10.1016/j.tripleo.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka E, Detamore MS, Mercuri LG. Degenerative disorders of the temporomandibular joint: etiology, diagnosis, and treatment. J Dent Res. 2008;87:296–307. doi: 10.1177/154405910808700406. [DOI] [PubMed] [Google Scholar]
- 4.Milam SB. Pathogenesis of degenerative temporomandibular joint arthritides. Odontology. 2005;93:7–15. doi: 10.1007/s10266-005-0056-7. [DOI] [PubMed] [Google Scholar]
- 5.Manfredini D, Arveda N, Guarda-Nardini L, Segù M, Collesano V. Distribution of diagnoses in a population of patients with temporomandibular disorders. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114:e35–e41. doi: 10.1016/j.oooo.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 6.Manfredini D, Piccotti F, Ferronato G, Guarda-Nardini L. Age peaks of different RDC/TMD diagnoses in a patient population. Journal of Dentistry. 2010;38:392–399. doi: 10.1016/j.jdent.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Ohrbach R, Fillingim RB, Mulkey F, Gonzalez Y, Gordon S, Gremillion H, et al. Clinical Findings and Pain Symptoms as Potential Risk Factors for Chronic TMD: Descriptive Data and Empirically Identified Domains from the OPPERA Case-Control Study. J Pain. 2011;12:T27–T45. doi: 10.1016/j.jpain.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rutkiewicz T, Kononen M, Suominen-Taipale L, Nordblad A, Alanen P. Occurrence of clinical signs of temporomandibular disorders in adult Finns. J Orofac Pain. 2006;20:208–217. [PubMed] [Google Scholar]
- 9.Hinton RJ. Genes that regulate morphogenesis and growth of the temporomandibular joint: a review. Dev Dynam. 2014;243:864–874. doi: 10.1002/dvdy.24130. [DOI] [PubMed] [Google Scholar]
- 10.Owtad P, Park JH, Shen G, Potres Z, Darendeliler MA. The biology of TMJ growth modification: a review. J Dent Res. 2013;92:315–321. doi: 10.1177/0022034513476302. [DOI] [PubMed] [Google Scholar]
- 11.Shen G, Darendeliler MA. The adaptive remodeling of condylar cartilage-a transition from chondrogenesis to osteogenesis. J Dent Res. 2005;84:691–699. doi: 10.1177/154405910508400802. [DOI] [PubMed] [Google Scholar]
- 12.Kantomaa T, Ronning O. In: Fundamentals of craniofacial growth. Dixon A, Hoyte D, Ronning O, editors. CRC Press; New York: 1997. pp. 189–204. [Google Scholar]
- 13.Nakano H, Watahiki J, Kubota M, Maki K, Shibasaki Y, Hatcher D, et al. Micro X-ray computed tomography analysis for the evaluation of asymmetrical condylar growth in the rat. Orthodontics & craniofacial research. 2003;6(Suppl 1):168–182. doi: 10.1034/j.1600-0544.2003.252.x. [DOI] [PubMed] [Google Scholar]
- 14.Uekita H, Takahashi S, Domon T, Yamaguchi T. Changes in collagens and chondrocytes in the temporomandibular joint cartilage in growing rats fed a liquid diet. Ann Anat. 2015;202:78–87. doi: 10.1016/j.aanat.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Sato I, Uneno R, Miwa Y, Sunohara M. Distribution of tenascin-C and tenascin-X, apoptotic and proliferating cells in postnatal soft-diet rat temporomandibular joint (TMJ) Ann Anat. 2006;188:127–136. doi: 10.1016/j.aanat.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Carlson D, McNamara J, Graber L, Hoffman D. In: Current advances in oral surgery. Irby W, editor. Vol. 3. Mosby; St. Louis: 1980. pp. 28–77. [Google Scholar]
- 17.Petrovic AG, Stutzmann JJ, Oudet CL. In: Determinants of mandibular form and growth. McNamara JA Jr, editor. University of Michigan; Ann Arbor, MI: 1975. pp. 101–154. [Google Scholar]
- 18.Luder HU, Leblond CP, von der Mark K. Cellular stages in cartilage formation as revealed by morphometry, radioautography and type II collagen immunostaining of the mandibular condyle from weanling rats. Am J Anat. 1988;182:197–214. doi: 10.1002/aja.1001820302. [DOI] [PubMed] [Google Scholar]
- 19.Glineburg RW, Laskin DM, Blaustein DI. The effects of immobilization on the primate temporomandibular joint: a histologic and histochemical study. J Oral Maxillofac Surg. 1982;40:3–8. doi: 10.1016/s0278-2391(82)80007-4. [DOI] [PubMed] [Google Scholar]
- 20.Lydiatt DD, Davis LF. The effects of immobilization on the rabbit temporomandibular joint. J Oral Maxillofac Surg. 1985;43:188–193. doi: 10.1016/0278-2391(85)90158-2. [DOI] [PubMed] [Google Scholar]
- 21.Hinton RJ, Carlson DS. Regulation of growth in mandibular condylar cartilage. Semin Orthod. 2005;11:209–218. [Google Scholar]
- 22.Ng MC, Harper RP, Le CT, Wong BS. Effects of estrogen on the condylar cartilage of the rat mandible in organ culture. J Oral Maxillofac Surg. 1999;57:818–823. doi: 10.1016/s0278-2391(99)90823-6. [DOI] [PubMed] [Google Scholar]
- 23.Talwar RM, Wong BS, Svoboda K, Harper RP. Effects of estrogen on chondrocyte proliferation and collagen synthesis in skeletally mature articular cartilage. J Oral Maxillofac Surg. 2006;64:600–609. doi: 10.1016/j.joms.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 24.Yasuoka T, Nakashima M, Okuda T, Tatematsu N. Effect of estrogen replacement on temporomandibular joint remodeling in ovariectomized rats. J Oral Maxillofac Surg. 2000;58:189–196. doi: 10.1016/s0278-2391(00)90337-9. [DOI] [PubMed] [Google Scholar]
- 25.Chen J, Sorensen KP, Gupta T, Kilts T, Young M, Wadhwa S. Altered functional loading causes differential effects in the subchondral bone and condylar cartilage in the temporomandibular joint from young mice. Osteoarthritis and Cartilage. 2009;17:354–361. doi: 10.1016/j.joca.2008.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J, Sobue T, Utreja A, Kalajzic Z, Xu M, Kilts T, et al. Sex differences in chondrocyte maturation in the mandibular condyle from a decreased occlusal loading model. Calcif Tissue Int. 2011;89:123–129. doi: 10.1007/s00223-011-9498-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polur I, Kamiya Y, Xu M, Cabri BS, Alshabeeb M, Wadhwa S, et al. Oestrogen receptor beta mediates decreased occlusal loading induced inhibition of chondrocyte maturation in female mice. Archives of Oral Biology. 2015;60:818–824. doi: 10.1016/j.archoralbio.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Börjesson AE, Lagerquist MK, Windahl SH, Ohlsson C. The role of estrogen receptor α in the regulation of bone and growth plate cartilage. Cell Mol Life Sci. 2013;70:4023–4037. doi: 10.1007/s00018-013-1317-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sims NA, Dupont S, Krust A, Clement-Lacroix P, Minet D, Resche-Rigon M, et al. Deletion of estrogen receptors reveals a regulatory role for estrogen receptors-β in bone remodeling in females but not in males. Bone. 2002;30:18–25. doi: 10.1016/s8756-3282(01)00643-3. [DOI] [PubMed] [Google Scholar]
- 30.Chagin AS, Lindberg MK, Andersson N, Moverare S, Gustafsson JÅ, Sävendahl L, et al. Estrogen receptor-β inhibits skeletal growth and has the capacity to mediate growth plate fusion in female mice. J Bone Miner Res. 2004;19:72–77. doi: 10.1359/JBMR.0301203. [DOI] [PubMed] [Google Scholar]
- 31.Lindberg M, Alatalo S, Halleen J, Mohan S, Gustafsson J, Ohlsson C. Estrogen receptor specificity in the regulation of the skeleton in female mice. J Endocrinol. 2001;171:229–236. doi: 10.1677/joe.0.1710229. [DOI] [PubMed] [Google Scholar]
- 32.Windahl SH, Vidal O, Andersson G, Gustafsson JA, Ohlsson C. Increased cortical bone mineral content but unchanged trabecular bone mineral density in female ERbeta(−/−) mice. The Journal of clinical investigation. 1999;104:895–901. doi: 10.1172/JCI6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saxon LK, Robling AG, Castillo AB, Mohan S, Turner CH. The skeletal responsiveness to mechanical loading is enhanced in mice with a null mutation in estrogen receptor-β. Am J Physiol Endocrinol Metab. 2007;293:E484–E491. doi: 10.1152/ajpendo.00189.2007. [DOI] [PubMed] [Google Scholar]
- 34.Shibata S, Suzuki S, Tengan T, Yamashita Y. A histochemical study of apoptosis in the reduced ameloblasts of erupting mouse molars. Arch Oral Biol. 1995;40:677–680. doi: 10.1016/0003-9969(95)00021-g. [DOI] [PubMed] [Google Scholar]
- 35.Festing M. A multivariate analysis of subline divergence in the shape of the mandible in C57BL/Gr mice. Genet Res. 1973;21:121–132. doi: 10.1017/s0016672300013306. [DOI] [PubMed] [Google Scholar]
- 36.Kamiya Y, Chen J, Xu M, Utreja A, Choi T, Drissi H, et al. Increased mandibular condylar growth in mice with estrogen receptor beta deficiency. J Bone Miner Res. 2013;28:1127–1134. doi: 10.1002/jbmr.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen J, Kamiya Y, Polur I, Xu M, Choi T, Kalajzic Z, et al. Estrogen via estrogen receptor beta partially inhibits mandibular condylar cartilage growth. Osteoarthr Cartilage. 2014;22:1861–1868. doi: 10.1016/j.joca.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dakin RS, Walker BR, Seckl JR, Hadoke PW, Drake AJ. Estrogens protect male mice from obesity complications and influence glucocorticoid metabolism. International journal of obesity (2005) 2015;39:1539–1547. doi: 10.1038/ijo.2015.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vidal O, Lindberg MK, Hollberg K, Baylink DJ, Andersson G, Lubahn DB, et al. Estrogen receptor specificity in the regulation of skeletal growth and maturation in male mice. Proc Natl Acad Sci U S A. 2000;97:5474–5479. doi: 10.1073/pnas.97.10.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orajarvi M, Hirvonen O, Yu SB, Liu X, Tiilikainen P, Wang M, et al. Effect of estrogen and altered diet hardness on the expression of estrogen receptor alpha and matrix metalloproteinase-8 in rat condylar cartilage. J Orofac Pain. 2011;25:261–268. [PubMed] [Google Scholar]
- 41.Orajarvi M, Puijola E, Yu SB, Liu X, Tiilikainen P, Wang M, et al. Effect of estrogen and dietary loading on condylar cartilage. J Orofac Pain. 2012;26:328–336. [PubMed] [Google Scholar]
- 42.Orajarvi M, Thesleff I, Hartikainen H, Raustia A, Pirttiniemi P. Effect of estrogen and food hardness on metabolism and turnover of condylar cartilage. J Oral Facial Pain Headache. 2015;29:297–307. doi: 10.11607/ofph.1287. [DOI] [PubMed] [Google Scholar]
- 43.Figueroba SR, Franco GCN, Omar NF, Groppo MF, Groppo FC. Dependence of cytokine levels on the sex of experimental animals: a pilot study on the effect of oestrogen in the temporomandibular joint synovial tissues. Int J Oral Maxillofac Surg. 2015 doi: 10.1016/j.ijom.2015.06.025. [DOI] [PubMed] [Google Scholar]
- 44.Engdahl C, Borjesson AE, Forsman HF, Andersson A, Stubelius A, Krust A, et al. The role of total and cartilage-specific estrogen receptor alpha expression for the ameliorating effect of estrogen treatment on arthritis. Arthritis research & therapy. 2014;16:R150. doi: 10.1186/ar4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kato M, Takaishi H, Yoda M, Tohmonda T, Takito J, Fujita N, et al. GRIP1 enhances estrogen receptor alpha-dependent extracellular matrix gene expression in chondrogenic cells. Osteoarthritis and cartilage /OARS, Osteoarthritis Research Society. 2010;18:934–941. doi: 10.1016/j.joca.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 46.Eriksson AL, Wilhelmson AS, Fagman JB, Ryberg H, Koskela A, Tuukkanen J, et al. The Bone Sparing Effects of 2-Methoxyestradiol Are Mediated via Estrogen Receptor-alpha in Male Mice. Endocrinology. 2016;157:4200–4205. doi: 10.1210/en.2016-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eriksson S, Eriksson A, Stege R, Carlstrom K. Bone mineral density in patients with prostatic cancer treated with orchidectomy and with estrogens. Calcif Tissue Int. 1995;57:97–99. doi: 10.1007/BF00298427. [DOI] [PubMed] [Google Scholar]
- 48.Moverare S, Venken K, Eriksson AL, Andersson N, Skrtic S, Wergedal J, et al. Differential effects on bone of estrogen receptor alpha and androgen receptor activation in orchidectomized adult male mice. Proc Natl Acad Sci U S A. 2003;100:13573–13578. doi: 10.1073/pnas.2233084100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Windahl SH, Börjesson AE, Farman HH, Engdahl C, Movérare-Skrtic S, Sjögren K, et al. Estrogen receptor-α in osteocytes is important for trabecular bone formation in male mice. Proceedings of the National Academy of Sciences. 2013;110:2294–2299. doi: 10.1073/pnas.1220811110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. The New England journal of medicine. 1994;331:1056–1061. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 51.Tanaka M, Ejiri S, Nakajima M, Kohno S, Ozawa H. Changes of cancellous bone mass in rat mandibular condyle following ovariectomy. Bone. 1999;25:339–347. doi: 10.1016/s8756-3282(99)00179-9. [DOI] [PubMed] [Google Scholar]
- 52.Li YQ, Xing XH, Wang H, Weng XL, Yu SB, Dong GY. Dose-dependent effects of genistein on bone homeostasis in rats' mandibular subchondral bone. Acta pharmacologica Sinica. 2012;33:66–74. doi: 10.1038/aps.2011.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu SB, Wang MQ, Li YQ, Lv X, Jiang Y, Dong GY, et al. The effects of age and sex on the expression of oestrogen and its receptors in rat mandibular condylar cartilages. Archives of oral biology. 2009;54:479–485. doi: 10.1016/j.archoralbio.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 54.Meakin LB, Galea GL, Sugiyama T, Lanyon LE, Price JS. Age-related impairment of bones' adaptive response to loading in mice is associated with sex-related deficiencies in osteoblasts but no change in osteocytes. J Bone Miner Res. 2014;29:1859–1871. doi: 10.1002/jbmr.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McNamara KM, Harwood DT, Simanainen U, Walters KA, Jimenez M, Handelsman DJ. Measurement of sex steroids in murine blood and reproductive tissues by liquid chromatography–tandem mass spectrometry. J Steroid Biochem Mol Biol. 2010;121:611–618. doi: 10.1016/j.jsbmb.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 56.Vanderschueren D, Venken K, Ophoff J, Bouillon R, Boonen S. Sex steroids and the periosteum - reconsidering the roles of androgens and estrogens in periosteal expansion. J Clin Endocrinol Metab. 2006;91:378–382. doi: 10.1210/jc.2005-1766. [DOI] [PubMed] [Google Scholar]
- 57.Laurent MR, Vanderschueren D. Reproductive endocrinology: functional effects of sex hormone-binding globulin variants. Nature reviews. Endocrinology. 2014;10:516–517. doi: 10.1038/nrendo.2014.120. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The data represent WT and ERβKO mice under normal load with placebo treatment. Representative Col2 immunohistochemical images (A), gene expression of Col2 (B), bone volume fraction (C), trabecular number (D), trabecular thickness (E) and trabecular spacing (F) are shown. For gene expression, n=6 mice were utilized for all groups and mandibular condylar cartilage from left and right were pooled together. Statistical significance was determined by a Student’s t-test and p < 0.05.
The data represent WT and ERβKO mice under normal load or decreased occlusal loading with either placebo or estradiol treatment. Specifically, the labels indicate the following: NL = normal load and placebo; DOL = decreased occlusal loading and placebo; Esd = normal load and estradiol; DOL + Esd = decreased occlusal loading and estradiol. Representative androgen receptor immunohistochemical images are shown for all groups.