Abstract
Objective
To determine the time to hematologic recovery and the incidence of secondary sepsis and mortality among neutropenic infants treated or not treated with granulocyte-colony stimulating factor (G-CSF).
Study Design
We identified all neutropenic infants discharged from 348 neonatal intensive care units in 1997–2012. Neutropenia was defined as an absolute neutrophil count ≤ 1500/μL for ≥1 day during the first 120 days of life. Incidence of secondary sepsis and mortality and number of days required to reach an absolute neutrophil count > 1500/μL for infants exposed to G-CSF were compared to those of unexposed infants.
Results
We identified 30,705 neutropenic infants, including 2142 infants (7%) treated with G-CSF. Treated infants had a shorter adjusted time to hematologic recovery (HR 1.36, 95% CI 1.30–1.44) and higher adjusted odds of secondary sepsis (OR 1.50, 95% CI 1.20–1.87), death (OR 1.33, 95% CI 1.05–1.68), and the combined outcome of sepsis or death (OR 1.41, 95% CI 1.19–1.67) at day 14 compared to untreated infants. These differences persisted at day 28.
Conclusions
G-CSF treatment decreased the time to hematologic recovery but was associated with increased odds of secondary sepsis and mortality in neutropenic infants. G-CSF should not routinely be used for infants with neutropenia.
Keywords: hematologic recovery, sepsis, prophylaxis
Neutropenia is common in infants, particularly infants who are small for gestational age (SGA) and in the setting of maternal pregnancy-induced hypertension (PIH) or neonatal sepsis.1–3 Granulocyte-colony stimulating factor (G-CSF) is a physiologic regulator of neutrophil function that increases neutrophil production and enhances neutrophil activity.4 Endogenous production of G-CSF in infants is relatively poor.5 Exogenous G-CSF can be used to increase the neutrophil count in neutropenic infants.6 This has been done in two settings: as prophylaxis for the prevention of infection in infants with neutropenia7 or at high risk for developing neutropenia,8 and as adjunctive therapy for septic infants with and without coexisting neutropenia.9,10 However, the use of G-CSF in infants for these indications is controversial due to conflicting data regarding its efficacy.
We sought to evaluate the effectiveness of G-CSF in neutropenic, hospitalized infants. The primary outcome was secondary sepsis or mortality at 14 days after the start of G-CSF therapy. The secondary outcomes were secondary sepsis or mortality at 28 days after G-CSF therapy, secondary sepsis at 14 and 28 days after G-CSF therapy, death at 14 and 28 days after G-CSF therapy, and the time to hematologic recovery.
METHODS
Study Population
We identified all infants discharged from 348 neonatal intensive care units (NICUs) managed by the Pediatrix Medical Group in 1997–2012 with ≥1 day of neutropenia during the first 120 days of life. The time frame of 1997–2012 was selected a priori to maximize the number of infants in our cohort who were treated with G-CSF. Infants with major congenital or chromosomal anomalies including congenital neutropenia syndromes were excluded from the analysis. Data were obtained from an electronic medical record that was generated prospectively and captured information from notes generated by clinicians on all infants cared for by the Pediatrix Medical Group. Information recorded included maternal history and demographics and, on a daily basis, medications, laboratory results, microbiology results, diagnoses, and procedures.11 Timing and method of drug administration and drug dosages were not included in the data available for analysis.
Definitions
Neutropenia was defined as an absolute neutrophil count ≤ 1500/μL.12 Because neutrophil counts were not obtained on a daily basis in most infants and some neutrophil counts prompting G-CSF therapy may have been obtained at other institutions, a neutropenic episode was considered to be treated with G-CSF if an absolute neutrophil count ≤ 1500/μL was documented within the window of 2 days before or 2 days after the first day of G-CSF exposure. For infants with no measurement obtained on the first day of G-CSF exposure, this window was used as a surrogate for the presence of neutropenia on the start day of G-CSF. Infants with more than one episode of neutropenia were included as follows: when all episodes were treated with G-CSF, the first episode of neutropenia was included; when none of the episodes were treated with G-CSF, the first episode of neutropenia was included; when an infant had both untreated and treated neutropenic episodes, the first episode with G-CSF exposure was included. The episode start day was defined as the first day of G-CSF exposure for episodes treated with G-CSF and the first day of neutropenia for episodes not treated with G-CSF. SGA was defined as previously described.13 PIH was considered to be present if there was a maternal diagnosis of eclampsia, preeclampsia, or HELLP (hemolysis, elevated liver enzymes, low platelet) syndrome. Concurrent bacteremia was considered to be present if a positive blood culture was obtained 2 days before or after the episode start day. Blood cultures positive for organisms generally considered to be contaminants (Bacillus species, diphtheroids, Corynebacterium species) were considered to be negative.14 Cultures positive for coagulase-negative Staphylococcus (CoNS) sepsis were considered positive if they met one of the following criteria: 2 positive blood cultures for CoNS within a 4-day period, 3 positive blood cultures for CoNS within a 7-day period, or 4 positive blood cultures for CONS within a 10-day period.15 Cultures growing CoNS that did not meet these criteria were considered to be negative.
Time to hematologic recovery was defined as the number of days from the start day to the first day with an absolute neutrophil count > 1500/μL. Secondary sepsis was defined as a positive blood culture from day 3 through days 14 and 28 after the start day. For neutropenic episodes with concurrent bacteremia, secondary sepsis was defined as the presence of a positive blood culture with an organism other than the original organism, or, in cases where a blood culture grew the same organism as the original organism, if there were ≥21 days between culture results. Mortality was defined as death occurring from day 3 through days 14 and 28. For infants who died within 2 days of starting G-CSF therapy, mortality was treated as missing.
Statistical Analysis
We compared demographic and baseline characteristics of G-CSF-treated and untreated neutropenic infants. Categorical variables were presented as counts (proportions), and continuous variables were presented as medians (25th and 75th percentiles). We performed chi-square or Fisher’s exact tests for comparison of categorical variables and two-sample Wilcoxon rank sum tests for comparison of continuous variables. The time to hematologic recovery between the two groups was compared using a log rank test and a Cox proportional hazards model stratified by gestational age group and adjusted for SGA status, inotropic support, mechanical ventilation, and concurrent bacteremia. Infants without hematologic recovery were censored at the time of discharge. We determined the odds of sepsis, mortality, and the combined outcome at both 14 and 28 days using a multivariable logistic regression analysis adjusted for SGA status, gestational age group, postnatal age at the start day, inotropic support, mechanical ventilation, and concurrent bacteremia. We used a fixed effects model to adjust for potential variation by NICU site. We also calculated the primary outcome using this same adjusted logistic regression model with neutropenia defined as an absolute neutrophil count <500/μL or with initial absolute neutrophil count as a covariate. We calculated the primary outcome with infants having concurrent bacteremia excluded; the adjustment variables were the same except that concurrent bacteremia was not included. Data were analyzed using STATA 13 (College Station, TX), and a p-value < 0.05 was considered statistically significant. This study was approved by the Duke University Institutional Review Board.
RESULTS
Patient Characteristics
We identified 30,705 infants with neutropenia during the first 120 days of life. Of these, 2142 infants (7.0%) were treated with G-CSF (Table 1). Treated infants had a lower median gestational age and birth weight than untreated infants: 28 weeks (25th and 75th percentile, 26, 31 weeks) vs. 29 weeks (27, 32), p<0.001, and 935 g (655, 1451) vs. 1173 g (820, 1720), p<0.001, respectively. Compared with untreated infants, more infants treated with G-CSF were SGA (33% vs. 21%, p<0.001), had concurrent bacteremia (6% vs. 4%, p<0.001), and received ventilator (47% vs. 45%, p<0.001) or inotropic support (30% vs. 12%, p<0.001). Treated infants were older than untreated infants (median postnatal age 3 days [0, 7] vs. 1 [0, 9], p<0.001) and had lower median absolute neutrophil counts, 540/uL (312, 752) vs. 720/uL (495, 874), p<0.001. The median platelet counts for both groups were within the normal range on the first day of the neutropenia episode (138,000 vs. 194,000 for treated and untreated infants, respectively). Infants treated with G-CSF were treated for a median of 3 days (1, 3).
Table 1.
Demographics
| G-CSF | No G-CSF | |
|---|---|---|
|
| ||
| N=2142 (%) | N=28,563 (%) | |
| Gestational age, weeks | ||
| <26 | 480 (22) | 4544 (16) |
| 26–28 | 751 (35) | 7942 (28) |
| 29–32 | 524 (24) | 9448 (33) |
| 33–36 | 230 (11) | 4230 (15) |
| ≥37 | 155 (7) | 2377 (8) |
| Birth weight, g | ||
| <1000 | 1170 (55) | 10,929 (38) |
| 1000–1499 | 463 (22) | 8169 (29) |
| 1500–2499 | 326 (15) | 6536 (23) |
| 2500–3499 | 137 (6) | 2150 (8) |
| ≥3500 | 45 (2) | 751 (3) |
| Postnatal age, days | ||
| ≤7 | 20,768 (73) | 1538 (72) |
| 8–30 | 3584 (13) | 358 (17) |
| >30 | 4211 (15) | 246 (11) |
| 5-min Apgar score | ||
| 0–3 | 110 (5) | 1255 (5) |
| 4–6 | 448 (21) | 4304 (15) |
| 7–10 | 1533 (73) | 22312 (80) |
| Race | ||
| White | 1037 (50) | 13,360 (48) |
| Black | 526 (25) | 7564 (27) |
| Hispanic | 401 (19) | 5305 (19) |
| Other | 108 (5) | 1410 (5) |
| Small for gestational age | 696 (33) | 6099 (21) |
| Male | 1311 (61) | 16948 (59) |
| Cesarean section | 1548 (73) | 20436 (72) |
| Maternal hypertension* | 356 (17) | 4296 (15) |
| Inotropic support† | 640 (30) | 3562 (12) |
| Ventilator support† | 1443 (47) | 12,745 (45) |
| Concurrent bacteremia | 134 (6) | 1007 (4) |
Maternal hypertension includes preeclampsia, eclampsia and HELLP (hemolysis, elevated liver enzymes, low platelet) syndrome.
On the first day of neutropenia (untreated group) or first day of G-CSF therapy (treated group).
Hematologic Recovery
The median hematological recovery times were similar: 2 days (2, 4) and 2 days (2, 3), respectively. However, on Cox proportional hazard analysis, we observed that G-CSF treatment was associated with a shorter time to hematologic recovery, hazard ratio = 1.36, 95% CI 1.30–1.44 (Figure).
Figure 1.
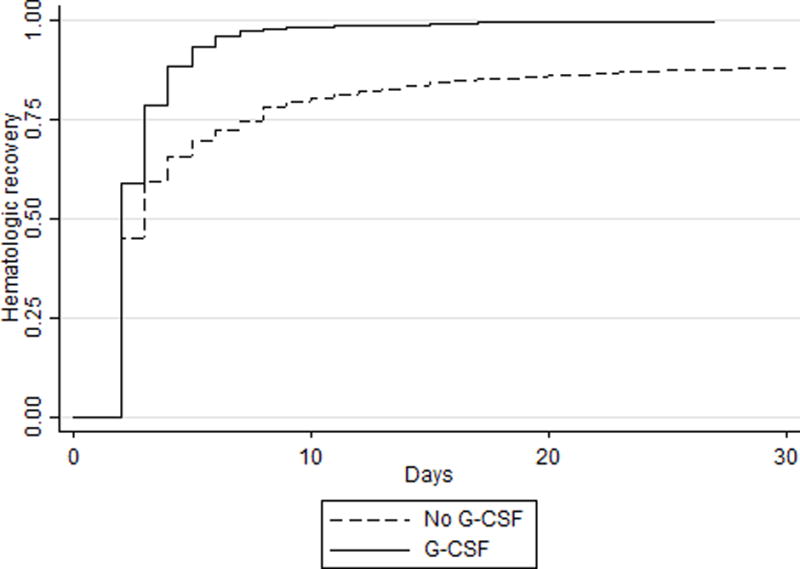
Time to hematologic recovery for neutropenic infants.
Sepsis and Mortality
Nine percent (192/2142) of treated infants and 6% (1772/28,563) untreated infants had a positive blood culture during the study period (Table 2). Sepsis occurred a median of 17 days (8, 35) after the start of the study period. On multivariable logistic regression, we observed an increase in sepsis, death, and the composite outcome within 14 days of the start day for G-CSF-treated infants compared with untreated infants. These differences persisted at 28 days. Sensitivity analyses that defined neutropenia as an absolute neutrophil count <500/μL or added the initial absolute neutrophil count as an adjustment covariate did not change the association of G-CSF exposure with an increased odds of death or sepsis at 14 days, odds ratio = 1.54, 95% CI 1.18, 2.01, and odds ratio = 1.34, 95% CI 1.13, 1.60, respectively. A second sensitivity analysis found that treatment with G-CSF exposure was still associated with an increased odds of death or sepsis at 14 days when infants with bacteremia at the start of the neutropenic period were excluded, odds ratio = 1.36, 95% CI 1.14, 1.62. Sepsis occurred while receiving G-CSF in 22 (0.1%) infants.
Table 2.
Outcomes
| G-CSF | No G-CSF | Adjusted Odds Ratio* (95% Confidence Interval) | |
|---|---|---|---|
|
| |||
| N=2142 (%) | N=28,563 (%) | ||
| Secondary sepsis | |||
| 3–14 days | 127 (6) | 1072 (4) | 1.50 (1.20–1.87) |
| 3–28 days | 192 (9) | 1772 (6) | 1.31 (1.09–1.57) |
| Death | |||
| 3–14 days | 124 (6) | 776 (3) | 1.33 (1.05–1.68) |
| 3–28 days | 157 (7) | 1073 (4) | 1.32 (1.07–1.62) |
| Death or secondary sepsis | |||
| 3–14 days | 235 (11) | 1779 (6) | 1.41 (1.19–1.67) |
| 3–28 days | 321 (15) | 2712 (9) | 1.29 (1.10–1.50) |
Odds of outcome for G-CSF group compared to non-G-CSF group adjusted for gestational age at birth, postnatal age, small for gestational age status, inotropic support, ventilator support, and concurrent bacteremia.
DISCUSSION
This study represents the largest evaluation of G-CSF use in neutropenic infants to date. In our study population, 7% of the neutropenic infants received G-CSF. In this cohort, G-CSF appeared to be used preferentially in infants with more severe illness because infants treated with G-CSF were younger, smaller, had lower Apgar scores, and more often required ventilator and inotropic support. Although the time to hematologic recovery was shorter in infants with G-CSF treatment, treated infants were more likely than untreated infants to develop secondary sepsis or die in the 14- and 28-day periods following their neutropenic episode even after adjustment for gestational age, SGA status, inotropic support, mechanical ventilation, and concurrent bacteremia.
Underlying causes of neonatal neutropenia are difficult to determine, and multiple factors may play a role. Blood dyscrasias did not appear to a prominent cause of neutropenia in our cohort; median platelet counts for both groups were >130,000. Prematurity and maternal PIH are important predisposing factors for neonatal neutropenia.16,17 This appears to be due to decreased neutrophil production during the first few days of life.3,18 On average, 40–80% of infants exposed to PIH develop neutropenia.1,19 For infants <1000 g birth weight, 68% of early neutropenia has been attributed to PIH.20 The majority of neutropenia related to PIH resolves within the first 3 days of life.20,21 Infants who are SGA are also more likely to develop neutropenia than those who are not SGA.22 In our cohort, more infants treated with G-CSF were SGA or were born to mothers with PIH than those not treated with G-CSF.
PIH-related neutropenia has been associated with an increased risk of secondary infection.3,23 This concern forms the basis for prophylactic use of G-CSF in neutropenic infants. A study of 28 infants with prolonged PIH-related neutropenia comparing the incidence of sepsis during the first 28 days of life found that treatment with G-CSF reduced the incidence of sepsis to 13% (2 of 15 infants) compared to 54% (7/13) in untreated infants, p<0.05.24 In infants <32 weeks gestation, use of a similar product, granulocyte-macrophage colony stimulating factor (GM-CSF), led to a slightly decreased incidence of sepsis in the 2 weeks following study enrollment compared to untreated infants, 11/36 vs. 18/39, OR 0.51, 95% CI 0.20–1.31.8 GM-CSF has been shown to increase the activity of neutrophils and macrophages, in addition to increasing neutrophil numbers, and for this reason has been theorized to be more effective than G-CSF as adjunctive therapy for sepsis.25 A study of non-neutropenic infants with presumed early onset sepsis found that that infants treated with G-CSF had increased ANCs and shorted hospital length of stay.26
Two other studies noted that the incidence of additional infections decreased when G-CSF was used during a sepsis episode occurring earlier in life, suggesting that G-CSF could be effective as prophylaxis for the prevention of infection.25,28 A multicenter randomized trial found that 102 neutropenic infants <32 weeks gestational age given prophylactic G-CSF had higher infection-free survival at 2 weeks compared to 98 untreated infants (84% vs. 71%, p=0.03). However, this difference was not present at 4 weeks (73% vs. 67%, p=0.42).7 Another multicenter, randomized trial of 280 infants <32 weeks gestational age found that although infants with neutropenia resolved their neutropenia more quickly when given GM-CSF compared with control infants, there was no difference in sepsis-free survival between treated and untreated infants, 67% vs. 74%.29 Paradoxically, there was a trend toward an increased incidence of sepsis in the group of infants treated with GM-CSF compared to untreated infants at both 14 (24% vs. 19%, respectively) and 28 days (30% vs. 24%, respectively).29 Similarly, we also observed an increase in sepsis in our cohort of infants treated with G-CSF.
Neutropenia may not only predispose infants to sepsis but may also arise as a result of sepsis. A study of 168 septic infants found that neutropenia was present in 38% of cases.2 Septic infants with neutropenia may have worse outcomes than their non-neutropenic septic peers.30 Several studies conducted to assess the usefulness of G-CSF as an adjunctive therapy for infants with sepsis-related neutropenia have demonstrated that G-CSF administration is effective at increasing the absolute neutrophil count in septic infants.10,21,26,28 Three small studies also suggested a reduction in mortality for septic infants with neutropenia following G-CSF administration. In one study, infants treated with G-CSF had a trend toward improved survival compared to historical controls, 12/14 (86%) vs. 15/24 (62%), p=0.1.31 In another study, a significant difference in survival was seen in the 28 days after a sepsis episode, with 13/14 (93%) of G-CSF-treated infants and 5/11 (45%) of conventionally treated infants surviving, p<0.03.21 A phase 1 study found that a greater proportion of septic infants treated with G-CSF survived to 6 months, 12/13 (92%), than those given placebo, 8/15 (53%), p=0.04.9 Conversely, another small study found that G-CSF administration to neutropenic infants with clinical sepsis did not improve survival compared to routine care, 8/10 vs. 7/10, respectively.32 A larger study found that neutropenic infants with suspected or confirmed sepsis who were treated with G-CSF had similar mortality compared to those given only antibiotics, 10/33 (30.3%) vs. 6/23 (26.6%).33 A study of 3644 SGA infants found that neutropenia was not independently associated with late onset sepsis, odds ratio =1.44, 95% CI 0.73, 2.61.22 With a much larger sample size, we also found that neutropenic infants had increased odds of death following treatment with G-CSF compared with untreated infants. However, the actual cause of death for infants who died is unknown, so we cannot hypothesize about the mechanism by which G-CSF exposure may be associated with increased odds of death. Additionally, it is possible that G-CSF exposure is a surrogate marker for other unmeasured confounders.
Although our study is the largest evaluation of G-CSF safety and outcomes in neutropenic infants to date, there are several important limitations to our findings. We included only one episode of neutropenia for each infant, and the role of G-CSF in recurrent neutropenia was not assessed. Because neutrophil counts were obtained at the discretion of the clinician, the median time to hematologic recovery could not be calculated on all infants and may be overestimated in some infants. Dosing information was not available; thus, we were not able to account for dose-dependent differences in outcomes. Finally, infants in our study were not randomized to receive G-CSF or not. G-CSF was prescribed at the discretion of the clinicians, and we were not able to determine what motivated clinicians to start G-CSF in some infants with neutropenia and not in others. Although we attempted to control for these important confounders and others in our analysis, it is likely that unmeasured confounders remain and may account for some of the differences noted between the treated and untreated infants.
In conclusion, we found that G-CSF treatment reduced the time to hematologic recovery but was associated with higher mortality and secondary sepsis in treated infants. Since prior studies have had conflicting efficacy findings and there is the possibility of harm, G-CSF should not routinely be used for infants with neutropenia.
Acknowledgments
This work was funded under National Institute for Child Health and Human Development (NICHD) contract HHSN275201000003I for the Pediatric Trials Network and under NICHD award number 1R25-HD076475-01. Research reported in this publication was also supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) under award number UL1TR001117. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Dr. Hornik receives salary support for research from the National Center for Advancing Translational Sciences of the NIH (UL1TR001117). Dr. Benjamin receives support from the NIH (award 2K24HD058735-06, National Center for Advancing Translational Sciences award UL1TR001117, NICHD contract HHSN275201000003I, and National Institute of Allergy and Infectious Diseases contract HHSN272201500006I). Dr. Smith receives salary support for research from the NIH and the National Center for Advancing Translational Sciences of the NIH (UL1TR001117), the NICHD (HHSN275201000003I and 1R01-HD081044-01), and the Food and Drug Administration (1R18-FD005292-01). Dr. Ericson receives support from the NICHD under award number 5T32HD060558.
Abbreviations
- G-CSF
granulocyte-colony stimulating factor
- GM-CSF
granulocyte-macrophage colony stimulating factor
- NICU
neonatal intensive care unit
- PIH
pregnancy-induced hypertension
- SGA
small for gestational age
APPENDIX
The Best Pharmaceuticals for Children Act – Pediatric Trials Network Steering Committee
Katherine Y. Berezny, BSMT, MPH, Michael Cohen-Wolkowiez, MD, PhD, Duke Clinical Research Institute, Durham, NC; Gregory L. Kearns, PharmD, PhD, Arkansas Children’s Hospital, Little Rock, AR; Matthew M. Laughon, MD, MPH, University of North Carolina, Chapel Hill, NC; Ian M. Paul, MD, MSc, Penn State College of Medicine, Hershey, PA; Michael J. Smith, MD, MSCE, University of Louisville, Louisville, KY; John van den Anker, MD, PhD, George Washington University School of Medicine and Health, Washington, DC; Kelly Wade, MD, Children’s Hospital of Philadelphia, Philadelphia, PA.
The Eunice Kennedy Shriver National Institute of Child Health and Human Development (Bethesda, MD): David Siegel, MD, Perdita Taylor-Zapata, MD, Anne Zajicek, PharmD, Zhaoxia Ren, MD, PhD, Ekaterini Tsilou, MD, Alice Pagan, BBA.
The EMMES Corporation (Data Coordinating Center; Rockville, MD): Ravinder Anand, PhD, Traci Clemons, PhD, Gina Simone, BS.
Footnotes
Conflicts of Interest
Drs. Benjamin and Smith receive research support from Cempra Pharmaceuticals (subaward to HHS0100201300009C) and industry for neonatal and pediatric drug development (www.dcri.duke.edu/research/coi.jsp). The other authors have no financial relationships to disclose.
The other authors have no financial disclosures relevant to this article.
References
- 1.Mouzinho A, Rosenfeld CR, Sanchez PJ, Risser R. Effect of maternal hypertension on neonatal neutropenia and risk of nosocomial infection. Pediatrics. 1992;90(3):430–435. [PubMed] [Google Scholar]
- 2.Funke A, Berner R, Traichel B, Schmeisser D, Leititis JU, Niemeyer CM. Frequency, natural course, and outcome of neonatal neutropenia. Pediatrics. 2000;106(1 Pt 1):45–51. doi: 10.1542/peds.106.1.45. [DOI] [PubMed] [Google Scholar]
- 3.Koenig JM, Christensen RD. Incidence, neutrophil kinetics, and natural history of neonatal neutropenia associated with maternal hypertension. N Engl J Med. 1989;321(9):557–562. doi: 10.1056/NEJM198908313210901. [DOI] [PubMed] [Google Scholar]
- 4.Lieschke GJ, Burgess AW. Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor (1) N Engl J Med. 1992;327(1):28–35. doi: 10.1056/NEJM199207023270106. [DOI] [PubMed] [Google Scholar]
- 5.Schibler KR, Liechty KW, White WL, Christensen RD. Production of granulocyte colony-stimulating factor in vitro by monocytes from preterm and term neonates. Blood. 1993;82(8):2478–2484. [PubMed] [Google Scholar]
- 6.La Gamma EF, Alpan O, Kocherlakota P. Effect of granulocyte colony-stimulating factor on preeclampsia-associated neonatal neutropenia. J Pediatr. 1995;126(3):457–459. doi: 10.1016/s0022-3476(95)70469-8. [DOI] [PubMed] [Google Scholar]
- 7.Kuhn P, Messer J, Paupe A, et al. A multicenter, randomized, placebo-controlled trial of prophylactic recombinant granulocyte-colony stimulating factor in preterm neonates with neutropenia. J Pediatr. 2009;155(3):324–330e321. doi: 10.1016/j.jpeds.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 8.Carr R, Modi N, Dore CJ, El-Rifai R, Lindo D. A randomized, controlled trial of prophylactic granulocyte-macrophage colony-stimulating factor in human newborns less than 32 weeks gestation. Pediatrics. 1999;103(4 Pt 1):796–802. doi: 10.1542/peds.103.4.796. [DOI] [PubMed] [Google Scholar]
- 9.Bedford Russell AR, Emmerson AJ, Wilkinson N, et al. A trial of recombinant human granulocyte colony stimulating factor for the treatment of very low birthweight infants with presumed sepsis and neutropenia. Arch Dis Child Fetal Neonatal Ed. 2001;84(3):F172–176. doi: 10.1136/fn.84.3.F172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gillan ER, Christensen RD, Suen Y, Ellis R, van de Ven C, Cairo MS. A randomized, placebo-controlled trial of recombinant human granulocyte colony-stimulating factor administration in newborn infants with presumed sepsis: significant induction of peripheral and bone marrow neutrophilia. Blood. 1994;84(5):1427–1433. [PubMed] [Google Scholar]
- 11.Clark RH, Gordon P, Walker WM, Laughon M, Smith PB, Spitzer AR. Characteristics of patients who die of necrotizing enterocolitis. J Perinatol. 2012;32(3):199–204. doi: 10.1038/jp.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mouzinho A, Rosenfeld CR, Sanchez PJ, Risser R. Revised reference ranges for circulating neutrophils in very-low-birth-weight neonates. Pediatrics. 1994;94(1):76–82. [PubMed] [Google Scholar]
- 13.Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics. 2010;125(2):e214–224. doi: 10.1542/peds.2009-0913. [DOI] [PubMed] [Google Scholar]
- 14.Boghossian NS, Page GP, Bell EF, et al. Late-onset sepsis in very low birth weight infants from singleton and multiple-gestation births. J Pediatr. 2013;162(6):1120–1124. doi: 10.1016/j.jpeds.2012.11.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hornik CP, Fort P, Clark RH, et al. Early and late onset sepsis in very-low-birth-weight infants from a large group of neonatal intensive care units. Early Hum Dev. 2012;88(Suppl 2):S69–74. doi: 10.1016/S0378-3782(12)70019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brazy JE, Grimm JK, Little VA. Neonatal manifestations of severe maternal hypertension occurring before the thirty-sixth week of pregnancy. J Pediatr. 1982;100(2):265–271. doi: 10.1016/s0022-3476(82)80653-7. [DOI] [PubMed] [Google Scholar]
- 17.Engle WD, Rosenfeld CR. Neutropenia in high-risk neonates. J Pediatr. 1984;105:982–986. doi: 10.1016/s0022-3476(84)80095-5. [DOI] [PubMed] [Google Scholar]
- 18.Zuppa AA, Girlando P, Florio MG, Cota F, Romagnoli C, Tortorolo G. Influence of maternal preeclampsia on recombinant human granulocyte colony-stimulating factor effect in neutropenic neonates with suspected sepsis. Eur J Obstet Gynecol Reprod Biol. 2002;102:131–136. doi: 10.1016/s0301-2115(01)00601-7. [DOI] [PubMed] [Google Scholar]
- 19.Juul SE, Haynes JW, McPherson RJ. Evaluation of neutropenia and neutrophilia in hospitalized preterm infants. J Perinatol. 2004;24(3):150–157. doi: 10.1038/sj.jp.7211057. [DOI] [PubMed] [Google Scholar]
- 20.Christensen RD, Henry E, Wiedmeier SE, Stoddard RA, Lambert DK. Low blood neutrophil concentrations among extremely low birth weight neonates: data from a multihospital health-care system. J Perinatol. 2006;26(11):682–687. doi: 10.1038/sj.jp.7211603. [DOI] [PubMed] [Google Scholar]
- 21.Kocherlakota P, La Gamma EF. Human granulocyte colony-stimulating factor may improve outcome attributable to neonatal sepsis complicated by neutropenia. Pediatrics. 1997;100(1):E6. doi: 10.1542/peds.100.1.e6. [DOI] [PubMed] [Google Scholar]
- 22.Christensen RD, Yoder BA, Baer VL, Snow GL, Butler A. Early-onset neutropenia in small for gestational age infants. Pediatrics. 2015;136(5):e1297–67. doi: 10.1542/peds.2015-1638. [DOI] [PubMed] [Google Scholar]
- 23.Doron MW, Makhlouf RA, Katz VL, Lawson EE, Stiles AD. Increased incidence of sepsis at birth in neutropenic infants of mothers with preeclampsia. J Pediatr. 1994;125(3):452–458. doi: 10.1016/s0022-3476(05)83294-9. [DOI] [PubMed] [Google Scholar]
- 24.Kocherlakota P, La Gamma EF. Preliminary report: rhG-CSF may reduce the incidence of neonatal sepsis in prolonged preeclampsia-associated neutropenia. Pediatrics. 1998;102(5):1107–1111. doi: 10.1542/peds.102.5.1107. [DOI] [PubMed] [Google Scholar]
- 25.Lejeune M, Cantinieaux B, Harag S, Ferster A, Devalck C, Sariban E. Defective functional activity and accelerated apoptosis in neutrophils from children with cancer are differentially corrected by granulocyte and granulocyte-macrophage colony stimulating factors in vitro. Br J Haematol. 1999;106(3):756–761. doi: 10.1046/j.1365-2141.1999.01589.x. [DOI] [PubMed] [Google Scholar]
- 26.Kucukoduk S, Sezer T, Yildiran A, Albayrak D. Randomized, double-blinded placebo-controlled trial of early administration of recombinant human granulocyte colony-stimulating factor to non-neutropenic preterm newborns between 33 and 36 weeks with presumed sepsis. Scand J Infect Dis. 2002;34(12):893–7. doi: 10.1080/0036554021000026966. [DOI] [PubMed] [Google Scholar]
- 27.Ahmad A, Laborada G, Bussel J, Nesin M. Comparison of recombinant granulocyte colony-stimulating factor, recombinant human granulocyte-macrophage colony-stimulating factor and placebo for treatment of septic preterm infants. Pediatr Infect Dis J. 2002;21(11):1061–1065. doi: 10.1097/00006454-200211000-00017. [DOI] [PubMed] [Google Scholar]
- 28.Miura E, Procianoy RS, Bittar C, et al. A randomized, double-masked, placebo-controlled trial of recombinant granulocyte colony-stimulating factor administration to preterm infants with the clinical diagnosis of early-onset sepsis. Pediatrics. 2001;107(1):30–35. doi: 10.1542/peds.107.1.30. [DOI] [PubMed] [Google Scholar]
- 29.Carr R, Brocklehurst P, Dore CJ, Modi N. Granulocyte-macrophage colony stimulating factor administered as prophylaxis for reduction of sepsis in extremely preterm, small for gestational age neonates (the PROGRAMS trial): a single-blind, multicentre, randomised controlled trial. Lancet. 2009;373(9659):226–233. doi: 10.1016/S0140-6736(09)60071-4. [DOI] [PubMed] [Google Scholar]
- 30.Christensen RD, Rothstein G, Anstall HB, Bybee B. Granulocyte transfusions in neonates with bacterial infection, neutropenia, and depletion of mature marrow neutrophils. Pediatrics. 1982;70(1):1–6. [PubMed] [Google Scholar]
- 31.Barak Y, Leibovitz E, Mogilner B, et al. The in vivo effect of recombinant human granulocyte-colony stimulating factor in neutropenic neonates with sepsis. Eur J Pediatr. 1997;156(8):643–646. doi: 10.1007/s004310050683. [DOI] [PubMed] [Google Scholar]
- 32.Schibler KR, Osborne KA, Leung LY, Le TV, Baker SI, Thompson DD. A randomized, placebo-controlled trial of granulocyte colony-stimulating factor administration to newborn infants with neutropenia and clinical signs of early-onset sepsis. Pediatrics. 1998;102(1 Pt 1):6–13. doi: 10.1542/peds.102.1.6. [DOI] [PubMed] [Google Scholar]
- 33.Aktas D, Demirel B, Gursoy T, Ovali F. A randomized case-controlled study of recombinant human granulocyte colony stimulating factor for the treatment of sepsis in preterm neutropenic infants. Pediatr Neonatol. 2015;56(3):171–5. doi: 10.1016/j.pedneo.2014.06.007. [DOI] [PubMed] [Google Scholar]


