Abstract
BACKGROUND:
The inflammatory biomarker α1-acid glycoprotein (AGP) was found to have the strongest association with 5-year mortality in a recent study of 106 biomarkers. We examined whether AGP is a better biomarker of mortality risk than the more widely used inflammatory biomarkers interleukin-6 (IL-6) and C-reactive protein (CRP).
METHODS:
We analyzed data for 6545 men and women aged 45–69 (mean 55.7) years from the Whitehall II cohort study. We assayed AGP, IL-6 and CRP levels from fasting serum samples collected in 1997–1999. Mortality followup was until June 2015. Cox regression analysis was used to model associations of inflammatory biomarkers with all-cause, cardiovascular and cancer-related mortality.
RESULTS:
Over the mean follow-up of 16.7 years, 736 deaths occurred, of which 181 were from cardiovascular disease and 347 from cancer. In the model adjusted for all covariates (age, sex, socioeconomic status, body mass index, health behaviours and chronic disease), AGP did not predict mortality beyond the first 5 years of follow-up; over this period, IL-6 and CRP had stronger associations with mortality. When we considered all covariates and biomarkers simultaneously, AGP no longer predicted all-cause mortality over the entire follow-up period (adjusted hazard ratio [HR] 0.99, 95% confidence interval [CI] 0.90–1.08). Only IL-6 predicted all-cause mortality (adjusted HR 1.22, 95% CI 1.12–1.33) and cancer-related mortality (adjusted HR 1.13, 95% CI 1.00–1.29) over the entire follow-up period, whereas CRP predicted only cardiovascular mortality (adjusted HR 1.30, 95% CI 1.06–1.61).
INTERPRETATION:
Our findings suggest that AGP is not a better marker of short-or long-term mortality risk than the more commonly used biomarkers IL-6 and CRP.
Inflammatory biomarkers are useful indicators of infection in care settings1 and have great value for monitoring chronic disease activity2 and overall health status in the wider population.3 Considerable research has shown that inflammatory biomarkers can predict mortality in adults with chronic conditions such as type 2 diabetes4 and cardiovascular disease,5 and interleukin-6 (IL-6) and C-reactive protein (CRP) have been shown to predict mortality and cardiovascular outcomes in general population settings.6 In older adults, IL-6 appears to have a stronger association than CRP with all-cause mortality.3 For cardiovascular outcomes, studies using Mendelian randomization have suggested causal effects for IL-6 but not for CRP.7,8
A recent metabolomics study examined 106 biomarkers and found α1-acid glycoprotein (AGP), an acute-phase protein, to be the strongest predictor of 5-year mortality.9 The importance of this finding has not been established for mortality follow-up beyond 5 years. It is also unknown how well AGP compares with other sensitive, dynamic and commonly measured markers of systemic inflammation, such as IL-6 and CRP, as a predictor of mortality. To address this question, we compared associations of these 3 inflammatory markers with short- and long-term risk of all-cause, cardiovascular and cancer-related mortality in a large cohort study. We also examined absolute differences in remaining life expectancy at age 50 in men and women with high versus low inflammation based on each marker.
Methods
Study design
The Whitehall II study is an ongoing cohort study of men and women originally employed by the British civil service in London-based offices.10 A total of 10 308 people (6895 men and 3413 women) aged 35–55 years were recruited during 1985–1988, with a response rate of 73%.9 Since the baseline medical examination, follow-up examinations have taken place about every 5 years.
The biomarkers were assayed from fasting serum samples collected in 1997–1999. For CRP measurement, a high-sensitivity immunonephelometric assay was used in a BN ProSpec nephelometer (Dade Behring); IL-6 levels were measured with a high-sensitivity enzyme-linked immunosorbent assay (R&D Systems); and AGP levels were measured with nuclear magnetic resonance spectroscopy as part of more complete biomarker profiling.11
Mortality
Mortality data until June 2015 were drawn from the British national mortality register (National Health Service Central Register). The tracing exercise was carried out using the National Health Service identification number of each participant. We used the 9th and 10th revisions of the International Classification of Diseases to examine deaths due to cardiovascular (ICD-9 codes 390–459 and ICD-10 codes I00-I99) and cancer-related (ICD-9 codes 140–208 and ICD-10 codes C00-C97) causes.
Covariates
Covariates used in the analyses were drawn from the 1997–1999 assessment, concurrent with the measurement of inflammatory markers.
Demographic covariates included age, sex and socioeconomic status (defined by employment grade, a 3-level marker of socioeconomic status used in the Whitehall II study10).
Health behaviour covariates included smoking status (categorized as current, former and never smoker), alcohol consumption (assessed via questions on the number of alcoholic drinks consumed in the last week and converted to units of alcohol), physical activity level (defined as active for ≥ 2.5 h/wk of moderate physical activity or ≥ 1 h/wk of vigorous physical activity, inactive for < 1 h/wk of moderate and vigorous activity, and intermediate for all other levels) and dietary behaviour (assessed using a question on frequency of fruit and vegetable consumption in a typical week). Body mass index (BMI) was used as a continuous variable.
Chronic disease burden was assessed by prevalence of cardiovascular disease (coronary artery disease or stroke), chronic obstructive pulmonary disease, cancer and type 2 diabetes mellitus. Cases of coronary artery disease included definite nonfatal myocardial infarction and definite angina determined on the basis of questionnaires, study electrocardiograms and cardiac enzyme levels; stroke was self-reported. As far as possible, all identified cases of cardiovascular disease were corroborated via linkage to the UK Hospital Episode Statistics database, which contains data on all inpatient and outpatient treatment. Chronic obstructive pulmonary disease was self-reported on a UK Medical Research Council respiratory questionnaire.12 Cancer was assessed using the National Health Service cancer registry. Type 2 diabetes was determined on the basis of glucose level (fasting level ≥ 7.0 mmol/L or 2-h postload level ≥ 11.1 mmol/L, or both), self-report of doctor diagnosis or use of diabetes medication.13
Statistical analysis
We examined participants’ baseline characteristics as a function of vital status at the end of follow-up and summarized the associations using standardized differences. Inflammatory markers were log-transformed owing to skewed distributions and standardized to z scores to allow comparison of effect sizes. For the 590 participants (9.0%) who had missing values for 1 or more variables, we imputed missing data using multiple imputations to generate 10 data sets, each analyzed separately, with the results combined using Rubin rules.14
For survival analyses, participants were followed until death or until the date of censoring for those who were alive (date of emigration or June 30, 2015), whichever occurred first. We used Cox proportional hazards models with years of follow-up as the underlying time variable to examine hazard ratios (HRs) and their 95% confidence intervals (CIs) for associations with mortality. Schoenfeld residuals suggested violation of the proportionality of hazards assumption, which justified our use of 3 follow-up periods for mortality outcomes (< 5 yr, 5–10 yr, ≥ 10 yr). There were no sex differences in associations between inflammatory markers and mortality (p values for interaction ranged from 0.2 to 0.4), which led us to combine men and women in analyses. In the first model, we adjusted for age and sex. In the second model, we also adjusted for socioeconomic status, BMI, health behaviours and prevalent chronic disease. In the final model, we included all of the covariates as well as all 3 inflammatory markers.
To take into account possible threshold effects, we repeated analyses with mortality using tertiles for each inflammatory marker (comparing highest v. lowest tertile). Using these same tertiles, we estimated remaining life expectancy at age 50 for men and women with high versus low inflammation by multiplying age-specific mortality in England and Wales for 2011–2013 with the hazard ratios for the inflammatory marker–mortality association in the 2 groups compared with the total sample. These analyses were undertaken separately for men and women because of known sex differences in life expectancy.
We performed all analyses using SAS 9.2 (SAS Institute Inc.). Statistical tests were 2-sided, with a p value of less than 0.05 considered to be statistically significant.
Ethics approval
Ethics approval was obtained from the Research Ethics Committee of the University College London Medical School. All participants provided written informed consent.
Results
A total of 6551 participants underwent clinical assessment in 1997–1999, 6 of whom were not linked to mortality records and were excluded from all analyses. The mean age of participants at the 1997–1999 clinical assessment was 55.7 (range 45–69) years. A total of 736 deaths occurred over a mean follow-up of 16.7 years; 14.5% occurred in the first 5 years, and 61.7% more than 10 years after assessment of biomarkers. Those who died were older than surviving participants (59.6 v. 55.2 years) but did not differ by sex (Table 1). They had an adverse socioeconomic and disease profile, including higher levels of all 3 inflammatory markers (Table 1). We found that AGP was correlated with IL-6 (r = 0.29, p < 0.001) and CRP (r = 0.33, p < 0.001); IL-6 and CRP were also correlated with each other (r = 0.46, p < 0.001).
Table 1:
Select baseline characteristics of participants in the Whitehall II study cohort aged 45–69 at start of follow-up (1997–1999)
| Characteristic | Status at end of follow-up (June 2015); % of patients or mean ± SD at baseline | Standardized difference* | |
|---|---|---|---|
| Alive n = 5809 |
Deceased n = 736 |
||
| Age, yr | 55.2 ± 5.9 | 59.6 ± 5.8 | 0.75 |
| Male | 70.9 | 71.1 | 0.00 |
| Low socioeconomic status | 13.2 | 17.9 | 0.13 |
| Current smoker | 9.0 | 16.1 | 0.22 |
| Units of alcohol consumed weekly | 13.6 ± 15.0 | 14.8 ± 18.9 | 0.07 |
| Inactive† | 8.1 | 8.5 | 0.02 |
| Daily consumption of fruits and vegetables | 73.8 | 69.7 | −0.09 |
| BMI | 26.1 ± 3.9 | 26.7 ± 4.6 | 0.16 |
| History of CVD (CAD or stroke) | 5.4 | 13.2 | 0.27 |
| History of diabetes | 5.6 | 12.5 | 0.24 |
| History of cancer | 2.3 | 5.0 | 0.14 |
| History of COPD | 7.5 | 11.9 | 0.15 |
| Inflammatory biomarker (standardized values)‡ | |||
| AGP | −0.02 (0.99) | 0.20 (1.05) | 0.22 |
| IL-6 | −0.05 (0.98) | 0.42 (1.04) | 0.47 |
| CRP | −0.03 (0.99) | 0.27 (1.03) | 0.30 |
Note: AGP = α1-acid glycoprotein, BMI = body mass index, CAD = coronary artery disease, COPD = chronic obstructive pulmonary disease, CRP = C-reactive protein, CVD = cardiovascular disease, IL-6 = interleukin-6, SD = standard deviation.
A standardized difference greater than 0.1 is considered meaningful.
Inactive for < 1 h/wk of moderate and vigorous activity.
The median (interquartile range) of each biomarker before standardization in the alive and deceased groups are as follows: AGP: 1.42 (1.29–1.57) and 1.47 (1.33–1.62); IL-6: 1.38 (0.98–2.04) and 1.84 (1.26–2.82); CRP: 0.99 (0.50–2.06) and 1.36 (0.64–6.87).
In the first Cox regression model, adjusted for age and sex, all 3 inflammatory biomarkers were associated with all-cause mortality (Table 2). All associations weakened over time and were stronger for deaths occurring in the first 5 years than for those occurring 10 or more years after enrolment. In the second model, adjusted for all covariates, AGP did not predict mortality beyond the first 5 years of follow-up. In the third model, which considered all covariates and biomarkers simultaneously, only IL-6 predicted short- and long-term mortality from all causes (adjusted HR over entire follow-up period 1.22, 95% CI 1.12–1.33). Table 3 shows the associations of all covariates with all-cause mortality before and after adjustment for all covariates and inflammatory markers.
Table 2:
Association of inflammatory biomarkers with all-cause, cardiovascular and cancer-related mortality
| Outcome; follow-up period, yr | Deaths/N | Biomarker; HR* (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Model 1† | Model 2‡ | Model 3§ | ||||||||
|
|
|
|
||||||||
| AGP | IL-6 | CRP | AGP | IL-6 | CRP | AGP | IL-6 | CRP | ||
| All-cause mortality | ||||||||||
|
| ||||||||||
| Overall | 736/6545 | 1.18 (1.09–1.27) | 1.35 (1.26–1.44) | 1.27 (1.17–1.37) | 1.06 (0.97–1.15) | 1.25 (1.16–1.35) | 1.17 (1.07–1.27) | 0.99 (0.90–1.08) | 1.22 (1.12–1.33) | 1.07 (0.97–1.18) |
|
| ||||||||||
| < 5 | 107/6545 | 1.43 (1.19–1.72) | 1.75 (1.49–2.06) | 1.65 (1.36–2.00) | 1.30 (1.06–1.59) | 1.61 (1.36–1.92) | 1.54 (1.25–1.90) | 1.09 (0.87–1.37) | 1.47 (1.20–1.81) | 1.18 (0.93–1.51) |
|
| ||||||||||
| 5–10 | 175/6438 | 1.24 (1.08–1.44) | 1.36 (1.18–1.56) | 1.28 (1.10–1.50) | 1.15 (0.98–1.34) | 1.28 (1.10–1.49) | 1.21 (1.02–1.43) | 1.06 (0.89–1.27) | 1.23 (1.04–1.46) | 1.06 (0.87–1.30) |
|
| ||||||||||
| ≥ 10 | 454/6263 | 1.09 (0.99–1.20) | 1.25 (1.14–1.37) | 1.18 (1.08–1.31) | 0.98 (0.88–1.09) | 1.15 (1.04–1.27) | 1.07 (0.96–1.20) | 0.93 (0.83–1.05) | 1.15 (1.03–1.28) | 1.04 (0.91–1.18) |
|
| ||||||||||
| Cardiovascular mortality¶ | ||||||||||
|
| ||||||||||
| Overall | 181/6541 | 1.27 (1.10–1.46) | 1.37 (1.19–1.57) | 1.51 (1.30–1.76) | 1.06 (0.90–1.24) | 1.19 (1.02–1.39) | 1.32 (1.12–1.57) | 0.94 (0.79–1.13) | 1.08 (0.90–1.29) | 1.30 (1.06–1.61) |
|
| ||||||||||
| < 5 | 31/6543 | 1.44 (1.02–2.05) | 1.57 (1.14–2.16) | 1.56 (1.08–2.24) | 1.28 (0.87–1.87) | 1.34 (0.96–1.88) | 1.49 (1.00–2.22) | 1.11 (0.72–1.71) | 1.17 (0.78–1.76) | 1.28 (0.78–2.11) |
|
| ||||||||||
| 5–10 | 42/6436 | 1.32 (0.98–1.77) | 1.51 (1.15–1.99) | 1.63 (1.19–2.21) | 1.06 (0.76–1.48) | 1.36 (1.01–1.85) | 1.39 (0.99–1.97) | 0.92 (0.64–1.31) | 1.25 (0.87–1.79) | 1.28 (0.84–1.95) |
|
| ||||||||||
| ≥ 10 | 108/6263 | 1.20 (1.00–1.45) | 1.26 (1.04–1.51) | 1.45 (1.19–1.77) | 0.99 (0.81–1.22) | 1.07 (0.86–1.33) | 1.25 (1.00–1.57) | 0.91 (0.72–1.14) | 0.97 (0.76–1.24) | 1.32 (1.00–1.75) |
|
| ||||||||||
| Cancer-related mortality¶ | ||||||||||
|
| ||||||||||
| Overall | 347/6541 | 1.09 (0.98–1.22) | 1.25 (1.13–1.39) | 1.23 (1.11–1.38) | 0.99 (0.88–1.12) | 1.17 (1.04–1.30) | 1.15 (1.02–1.30) | 0.92 (0.81–1.06) | 1.13 (1.00–1.29) | 1.12 (0.97–1.29) |
|
| ||||||||||
| < 5 | 54/6543 | 1.50 (1.17–1.91) | 1.66 (1.32–2.09) | 1.78 (1.37–2.33) | 1.35 (1.02–1.78) | 1.52 (1.18–1.97) | 1.64 (1.23–2.19) | 1.13 (0.83–1.53) | 1.30 (0.96–1.76) | 1.34 (0.95–1.90) |
|
| ||||||||||
| 5–10 | 87/6436 | 1.11 (0.90–1.38) | 1.21 (0.98–1.48) | 1.17 (0.94–1.46) | 1.01 (0.79–1.28) | 1.11 (0.88–1.40) | 1.07 (0.84–1.38) | 0.97 (0.75–1.27) | 1.09 (0.85–1.41) | 1.04 (0.78–1.39) |
|
| ||||||||||
| ≥ 10 | 206/6263 | 0.99 (0.85–1.15) | 1.17 (1.02–1.34) | 1.14 (0.99–1.32) | 0.90 (0.76–1.07) | 1.10 (0.95–1.28) | 1.08 (0.92–1.27) | 0.85 (0.71–1.02) | 1.10 (0.93–1.30) | 1.10 (0.91–1.34) |
Note: AGP = α1-acid glycoprotein, CI = confidence interval, CRP = C-reactive protein, HR = hazard ratio, IL-6 = interleukin-6.
Hazard ratios are per 1 standard deviation increase in the inflammatory biomarker.
Adjusted for age and sex.
Adjusted for all covariates (age, sex, socioeconomic status, body mass index, health behaviours and chronic disease).
Adjusted for all covariates and other inflammatory biomarkers.
Four participants with unknown cause of death were excluded from these analyses.
Table 3:
Association of baseline covariates with all-cause mortality*
| Covariate | Unadjusted HR (95% CI) | Adjusted HR† (95% CI) |
|---|---|---|
| Age (per 1-yr increase) | 1.12 (1.11–1.14) | 1.11 (1.10–1.13) |
| Female sex (v. male) | 0.98 (0.84–1.15) | 0.87 (0.72–1.04) |
| Socioeconomic status | ||
| Intermediate (v. high) | 1.04 (0.89–1.22) | 0.98 (0.83–1.16) |
| Low (v. high) | 1.41 (1.15–1.73) | 0.96 (0.75–1.24) |
| Smoking status | ||
| Former smoker (v. never) | 1.20 (1.03–1.41) | 1.07 (0.93–1.30) |
| Current smoker (v. never) | 2.08 (1.68–2.56) | 1.86 (1.49–2.38) |
| Alcohol consumption | ||
| None (v. moderate) | 1.29 (1.06–1.57) | 1.12 (0.91–1.38) |
| Heavy (v. moderate) | 1.04 (0.88–1.22) | 1.10 (0.93–1.30) |
| Physical activity | ||
| Inactive (v. active) | 1.08 (0.84–1.41) | 0.94 (0.72–1.24) |
| Moderately active (v. active) | 1.24 (0.97–1.59) | 1.18 (0.91–1.51) |
| Consumption of fruits and vegetables (≥ daily v. < daily) | 0.82 (0.70–0.96) | 0.81 (0.69–0.97) |
| BMI (per 1-kg/m2 increase) | 1.04 (1.02–1.05) | 1.01 (0.99–1.03) |
| History of CVD (CAD or stroke) | 2.45 (1.98–3.04) | 1.56 (1.25–1.94) |
| History of diabetes | 2.26 (1.82–2.81) | 1.61 (1.28–2.01) |
| History of cancer | 2.14 (1.54–2.98) | 1.86 (1.33–2.60) |
| History of COPD | 1.59 (1.26–2.00) | 1.20 (0.95–1.52) |
| Inflammatory biomarker (per 1-SD increase) | ||
| AGP | 1.23 (1.15–1.32) | 0.99 (0.90–1.08) |
| IL-6 | 1.48 (1.39–1.58) | 1.22 (1.12–1.33) |
| CRP | 1.33 (1.24–1.43) | 1.07 (0.97–1.18) |
Note: AGP = α1-acid glycoprotein, BMI = body mass index, CAD = coronary artery disease, CI = confidence interval, COPD = chronic obstructive pulmonary disease, CRP = C-reactive protein, CVD = cardiovascular disease, HR = hazard ratio, IL-6 = interleukin-6, SD = standard deviation.
All analyses were based on 736 deaths among 6545 individuals.
Adjusted for all covariates (age, sex, socioeconomic status, body mass index, health behaviours and chronic disease) and other inflammatory biomarkers.
A total of 181 deaths (24.6%) were attributed to cardiovascular disease, for which all 3 markers were significant predictors in the models adjusted for age and sex (model 1, Table 2). In analyses adjusted for all covariates and mutually adjusted for the 3 inflammatory markers, only CRP remained associated with cardiovascular death (adjusted HR 1.30, 95% CI 1.06–1.61). This pattern was also observed in analyses stratified by duration of follow-up.
A total of 347 deaths (47.1%) were due to cancer. All 3 biomarkers were associated with cancer-related mortality in models adjusted for age and sex over a 5-year follow-up period (model 1, Table 2). In the fully adjusted model including all 3 inflammatory markers, only IL-6 was associated with cancer-related mortality over the entire follow-up period (adjusted HR 1.13, 95% CI 1.00–1.29).
When we compared mortality risk in the top versus the bottom tertile of each inflammatory marker, we did not observe associations between AGP or CRP and mortality in the models adjusted for all covariates (models 2 and 3, Table 4). On the other hand, IL-6 was associated with all-cause mortality in all models, irrespective of the covariates included.
Table 4:
Association of highest versus lowest tertile of inflammatory biomarkers with all-cause, cardiovascular and cancer-related mortality over mean follow-up of 16.7 years
| Model; outcome | No. of participants* | No. of deaths* | Biomarker; HR (95% CI) | ||
|---|---|---|---|---|---|
| AGP | IL-6 | CRP | |||
| Model 1† | |||||
| All-cause mortality | 6545 | 736 | 1.43 (1.20–1.65) | 1.93 (1.58–2.37) | 1.52 (1.27–1.83) |
| Cardiovascular mortality | 6541¶ | 181 | 1.64 (1.11–2.43) | 1.96 (1.29–2.99) | 2.00 (1.35–2.97) |
| Cancer-related mortality | 6541¶ | 347 | 1.22 (0.94–1.59) | 1.56 (1.18–2.08) | 1.54 (1.18–2.02) |
| Model 2‡ | |||||
| All-cause mortality | 6545 | 736 | 1.12 (0.92–1.37) | 1.58 (1.27–1.96) | 1.22 (0.99–1.49) |
| Cardiovascular mortality | 6541¶ | 181 | 1.08 (0.71–1.63) | 1.36 (0.86–2.14) | 1.36 (0.88–2.10) |
| Cancer-related mortality | 6541¶ | 347 | 0.96 (0.72–1.27) | 1.28 (0.94–1.73) | 1.29 (0.96–1.73) |
| Model 3§ | |||||
| All-cause mortality | 6545 | 736 | 1.01 (0.82–1.26) | 1.53 (1.21–1.93) | 1.04 (0.83–1.32) |
| Cardiovascular mortality | 6541¶ | 181 | 0.94 (0.60–1.47) | 1.23 (0.76–2.02) | 1.27 (0.78–2.08) |
| Cancer-related mortality | 6541¶ | 347 | 0.85 (0.62–1.15) | 1.19 (0.86–1.65) | 1.29 (0.92–1.81) |
Note: AGP = α-1-acid glycoprotein, CI = confidence interval, CRP = C-reactive protein, HR = hazard ratio, IL-6 = interleukin-6.
Numbers of participants and deaths included in the analysis, including those in the middle tertile.
Adjusted for age and sex.
Adjusted for all covariates (age, sex, socioeconomic status, body mass index, health behaviours and chronic disease).
Adjusted for all covariates and other inflammatory biomarkers.
Four participants with unknown cause of death were excluded from these analyses.
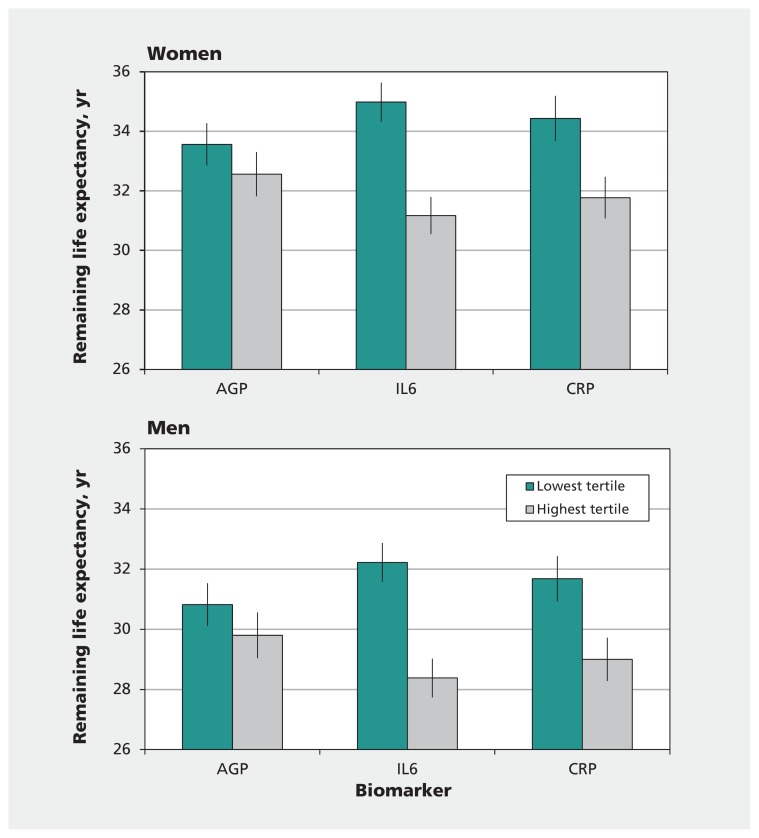
Figure 1 shows the remaining life expectancy at age 50 among participants with high versus low inflammation, defined using the highest and lowest tertiles for each biomarker. For AGP, there was a nonsignificant difference in life expectancy between those in the highest tertile and those in the lowest tertile (1.0 yr, 95% CI 0.0–2.0, for men and women; p = 0.054 and 0.06, respectively). The differences were significant between the highest and lowest tertiles of IL-6 (3.8 yr, 95% CI 2.9–4.7) and CRP (2.7 yr, 95% CI 1.6–3.7).
Figure 1:
Remaining life expectancy at age 50 among men and women with the lowest and highest tertiles of inflammatory biomarkers. We calculated the remaining years of life expectancy using the mortality hazard ratio for each tertile compared with the total sample and applied to the age-specific mortality in England and Wales for 2011–2013. Error bars represent 95% confidence limits. AGP = α1-acid glycoprotein, CRP = C-reactive protein, IL-6 = interleukin-6.
Interpretation
We examined the association of 3 inflammatory biomarkers with short- and long-term mortality in a large sample of middle-aged adults followed for 17 years. Analyses were motivated by a recent molecular profiling study that assessed the association of 106 metabolites with mortality and found AGP to have the strongest association with all-cause mortality.9 Because AGP is an acute-phase protein that is not widely measured,15 we compared its ability to predict mortality with the more commonly measured inflammatory biomarkers IL-6 and CRP. Our results showed that AGP was indeed associated with all-cause and cancer-related mortality in the short term; however, it was not associated with mortality beyond 5 years. Furthermore, when examining all 3 inflammatory markers in mutually adjusted models, AGP was not associated with mortality even in the short term. Thus, we found no evidence that AGP would be a better biomarker of mortality risk than the more commonly used inflammatory indicators IL-6 and CRP.
In analyses adjusted for all covariates where each marker was examined separately, both CRP and IL-6 were associated with all-cause and cancer-related mortality over the 17-year follow-up, with CRP also associated with cardiovascular mortality. However, when all inflammatory markers were considered together, only IL-6 was associated with all-cause and cancer-related mortality and CRP with cardiovascular mortality.
Identification of inexpensive prognostic markers for ill health in vulnerable populations, older patients or patients with chronic conditions is important for the management of care. In addition, a better understanding of the role of inflammatory markers in general population settings may help clarify their utility for guiding screening and prevention. Interactions between environmental, stochastic, genetic and epigenetic factors shape mortality risk. There is considerable interest in identifying biological markers of such risk, in particular those that can be measured noninvasively. Plasma biomarkers are ideal for this purpose, although observed associations do not necessarily reflect causality. In our study, CRP outweighed IL-6 as a predictor of cardiovascular mortality, although evidence from Mendelian randomization studies suggests a causal role for IL-616 but not for CRP17 in cardiovascular disease. Reanalysis of our data using tertiles of inflammatory markers rather than standardized z scores suggested a robust association only between IL-6 and all-cause mortality.
Plasma concentrations of acute-phase proteins fluctuate in response to inflammation. Interleukin-6 is a major proinflammatory cytokine, produced in a variety of tissues, and CRP and AGP are downstream products of the acute-phase response, derived via cytokine-dependent hepatic biosynthesis and secretion into the systemic circulation.18 Thus, IL-6 may be a more appropriate marker of long-term health status. There is considerable interest in the importance of inflammatory pathways for aging outcomes.19–21 It is possible that chronic low-grade inflammation plays a role in neurodegenerative diseases. This is supported by genome-wide association studies showing a number of genetic variants that influence inflammatory pathways associated with the development of Alzheimer disease.22, 23
In a study involving pregnant women with HIV infection, elevated AGP levels were associated with an increased risk of maternal death, postnatal transmission, and infant infection or death, possibly because of the impact of AGP on modulating immunity and binding or carrying drugs.1 There is little research on AGP and health status in general population samples, the exceptions being the study of metabolites and 5-year mortality, in which AGP was 1 of 106 biomarkers (no other inflammatory markers were examined in that study),9 and studies of mortality risk among healthy older people and older patients in hospital.24,25
Previous studies comparing AGP with other inflammatory markers for their associations with short- and long-term mortality are lacking. Our results showed a robust association of AGP with 5-year risk of all-cause, cardiovascular and cancer-related mortality in analyses adjusted for age and sex, with the magnitude of effect being similar to that in a previous study.9 However, by considering a longer follow-up period and other inflammatory markers simultaneously, we showed that IL-6 and CRP are more important predictors of mortality and that AGP does not provide additional information regarding mortality.
Strengths and limitations
A key strength of our study is the extended follow-up for mortality in a large cohort of men and women. We were also able to examine cancer-related and cardiovascular mortality.
Limitations of our study include the lack of ethnic diversity in the population. However, generalizability is unlikely to be compromised because the study was designed to include a wide socioeconomic spectrum, with more than a 10-fold salary difference across the socioeconomic hierarchy. Associations between common risk factors and incidence of cardiovascular disease in our study are comparable to those in previous studies based on general population samples.26 A further limitation is the use of only 3 serum markers of systemic inflammation. Although IL-6 and CRP are widely used in clinical practice and CRP is particularly inexpensive to measure, it remains unclear whether more relevant biomarkers of inflammation exist.
Conclusion
We found no evidence that AGP is a stronger prognostic marker of mortality than the widely used inflammatory markers IL-6 and CRP. As in previous studies, elevated AGP was associated with 5-year mortality. However, even with this length of follow-up, it did not do better than IL-6 in predicting mortality. Our analyses of all-cause, cardiovascular and cancer-related mortality suggest that IL-6 may be a better prognostic marker for all of these outcomes, in both the short and the long term.
Acknowledgements
The authors thank all of the participating civil service departments and their welfare, personnel and establishment officers; the British Occupational Health and Safety Agency; the British Council of Civil Service Unions; all of the civil servants who participated in the Whitehall II study; and all of the members of the Whitehall II study team, which comprises research scientists, statisticians, study coordinators, nurses, data managers, administrative assistants and data entry staff, who make the study possible.
Footnotes
See related article at www.cmaj.ca/lookup/doi/10.1503/cmaj.161033
Competing interests: None declared.
This article has been peer reviewed.
Contributors: Archana Singh-Manoux, Martin Shipley and Mika Kivimäki developed the hypothesis and study design. Martin Shipley performed the statistical analysis. Archana Singh-Manoux wrote the first and successive drafts of the manuscript. All of the authors contributed to the study concept and design, the analysis and interpretation of data, and the critical revision of the manuscript for important intellectual content. All of the authors approved the final version of the manuscript to be published and agreed to act as guarantors of the work.
Funding: The Whitehall II study is supported by grants from the US National Institute on Aging (grant nos. R01AG013196 and R01AG034454), the UK Medical Research Council (MRC grant no. K013351) and the British Heart Foundation. Mika Kivimäki is supported by the MRC and Nord-Forsk, the Nordic Programme on Health and Welfare.
References
- 1.Rawat R, Humphrey JH, Mutasa K, et al. Short communication: predicting adverse HIV-related outcomes in a resource-limited setting: use of the inflammation marker alpha(1)-acid glycoprotein. AIDS Res Hum Retroviruses 2010;26:1171–4. [DOI] [PubMed] [Google Scholar]
- 2.Wells G, Becker JC, Teng J, et al. Validation of the 28-joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann Rheum Dis 2009;68:954–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volpato S, Guralnik JM, Ferrucci L, et al. Cardiovascular disease, interleukin-6, and risk of mortality in older women: the women’s health and aging study. Circulation 2001;103: 947–53. [DOI] [PubMed] [Google Scholar]
- 4.Lowe G, Woodward M, Hillis G, et al. Circulating inflammatory markers and the risk of vascular complications and mortality in people with type 2 diabetes and cardiovascular disease or risk factors: the ADVANCE study. Diabetes 2014;63:1115–23. [DOI] [PubMed] [Google Scholar]
- 5.Lindahl B, Toss H, Siegbahn A, et al. Markers of myocardial damage and inflammation in relation to long-term mortality in unstable coronary artery disease. FRISC Study Group. Fragmin during Instability in Coronary Artery Disease. N Engl J Med 2000; 343: 1139–47. [DOI] [PubMed] [Google Scholar]
- 6.Kaptoge S, Di Angelantonio E, Lowe G, et al. ; Emerging Risk Factors Collaborators. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet 2010; 375:132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zacho J, Tybjaerg-Hansen A, Jensen JS, et al. Genetically elevated C-reactive protein and ischemic vascular disease. N Engl J Med 2008;359:1897–908. [DOI] [PubMed] [Google Scholar]
- 8.Sarwar N, Butterworth AS, Freitag DF, et al. ; IL6R Genetics Consortium Emerging Risk Factors Collaboration. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet 2012; 379:1205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischer K, Kettunen J, Wurtz P, et al. Biomarker profiling by nuclear magnetic resonance spectroscopy for the prediction of all-cause mortality: an observational study of 17 345 persons. PLoS Med 2014;11:e1001606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marmot MG, Smith GD, Stansfeld S, et al. Health inequalities among British civil servants: the Whitehall II study. Lancet 1991; 337:1387–93. [DOI] [PubMed] [Google Scholar]
- 11.Wurtz P, Havulinna AS, Soininen P, et al. Metabolite profiling and cardiovascular event risk: a prospective study of 3 population-based cohorts. Circulation 2015;131:774–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Council MR. Definition and classification of chronic bronchitis for epidemiological purposes. Lancet 1965;i:775–9. [PubMed] [Google Scholar]
- 13.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998;15:539–53. [DOI] [PubMed] [Google Scholar]
- 14.Rubin DB. Multiple imputation for nonresponse in surveys. New York: Wiley; 1987. [Google Scholar]
- 15.Luo Z, Lei H, Sun Y, et al. Orosomucoid, an acute response protein with multiple modulating activities. J Physiol Biochem 2015;71:329–40. [DOI] [PubMed] [Google Scholar]
- 16.Interleukin-6 Receptor Mendelian Randomisation Analysis Consortium. The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet 2012;379:1214–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wensley F, Gao P, Burgess S, et al. ; C Reactive Protein Coronary Heart Disease Genetics Consortium. Association between C reactive protein and coronary heart disease: mendelian randomisation analysis based on individual participant data. BMJ 2011;342:d548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rattazzi M, Puato M, Faggin E, et al. C-reactive protein and interleukin-6 in vascular disease: Culprits or passive bystanders? J Hypertens 2003;21:1787–803. [DOI] [PubMed] [Google Scholar]
- 19.Jurk D, Wilson C, Passos JF, et al. Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat Commun 2014;2:4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franceschi C, Capri M, Monti D, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev 2007;128: 92–105. [DOI] [PubMed] [Google Scholar]
- 21.Chung HY, Cesari M, Anton S, et al. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev 2009;8:18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tosto G, Reitz C. Genome-wide association studies in Alzheimer’s disease: a review. Curr Neurol Neurosci Rep 2013;13:381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lambert JC, Ibrahim-Verbaas CA, Harold D, et al. Meta-analysis of 74 046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet 2013;45:1452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carriere I, Dupuy AM, Lacroux A, et al. Biomarkers of inflammation and malnutrition associated with early death in healthy elderly people. J Am Geriatr Soc 2008;56:840–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henry OF, Blacher J, Verdavaine J, et al. Alpha 1-acid glycoprotein is an independent predictor of in-hospital death in the elderly. Age Ageing 2003;32:37–42. [DOI] [PubMed] [Google Scholar]
- 26.Batty GD, Shipley M, Tabak A, et al. Generalizability of occupational cohort study findings. Epidemiology 2014;25:932–3. [DOI] [PubMed] [Google Scholar]