Abstract
Background
Despite accumulating evidence of the important health benefits of bariatric surgery in morbidly obese patients in general, bariatric surgery outcomes are less clear in higher-risk, high-priority populations of patients with BMI ≥ 50 kg/m2. To help the Department of Veterans Affairs (VA) Health Services Research & Development Service (HSR&D) develop a research agenda, we conducted a rapid evidence review to better understand bariatric surgery outcomes in adults with BMI ≥ 50 kg/m2.
Methods
We searched MEDLINE®, the Cochrane Database of Systematic Reviews, the Cochrane Central Registry of Controlled Trials, and ClinicalTrials.gov through June 2016. We included trials and observational studies. We used pre-specified criteria to select studies, abstract data, and rate internal validity and strength of the evidence (PROSPERO registration number CRD42015025348). All decisions were completed by one reviewer and checked by another.
Results
Among 1892 citations, we included 23 studies in this rapid review. Compared with usual care, one large retrospective VA study provided limited evidence that bariatric surgery can lead to increased mortality in the first year, but decreased mortality long-term among super obese veterans. Studies that compared different bariatric surgical approaches suggested some differences in weight loss and complications. Laparoscopic gastric bypass generally resulted in greater short-term proportion of excess weight loss than did other procedures. Duodenal switch led to greater long-term weight loss than did gastric bypass, but with more complications.
Conclusions
The published literature that separates the super obese is insufficient for determining the precise balance of benefits and harms of bariatric surgery in this high-risk subgroup. Future studies should evaluate a more complete set of key outcomes with longer follow-up in larger samples of more broadly representative adults.
KEY WORDS: bariatric surgery, super obese, systematic review
INTRODUCTION
The growth rate of BMI ≥ 50 kg/m2, referred to as super obesity, rose more rapidly in the USA between 1986 and 2010 compared to prevalence of BMI categories < 50 kg/m2. This is important, because individuals with BMI ≥ 50 kg/m2 are more likely to have more complex health issues such as diabetes, hypertension, congestive heart failure, and chronic obstructive pulmonary disease that might increase surgical risk and challenges to the health care system.1 , 2
Although bariatric surgery is well-established as an effective treatment for morbid obesity, questions remain regarding the balance of benefits and harms of bariatric surgery in patients with the highest levels of obesity.3 – 11 Studies consistently show associations between increasing BMI and lower odds of successful weight loss and higher risk of morbidity and mortality after surgery.10 , 12 – 15 Potential surgical risk factors for poorer outcomes include the association between super obesity and greater prevalence of comorbid health conditions and greater technical and resource challenges.16 – 18 More research is needed to better understand the reasons for higher morbidity and mortality in the super obese and to identify specific clinical predictors that would inform optimization of bariatric surgery in this high-risk population.16
In the past few years, bariatric surgery research has increasingly focused on super obesity. However, no previous systematic review has examined evidence exclusively in super obesity across a wide range of bariatric surgery procedures. The purpose of our review is to synthesize the literature on the comparative effectiveness and safety of surgical and non-surgical treatments for obesity among super obese adults. This review was conducted by the Department of Veterans Affairs (VA) Evidence-based Synthesis Program (ESP)19 to help inform the VA Health Services Research and Development (HSR&D) “State of the Art” (SOTA) Conference on Weight Management in their efforts to develop a research agenda for super obesity, among a broad range of other obesity-related topics. This paper is a succinct version of the full evidence report,20 and includes an updated search and the addition of new studies published within the last year.
METHODS
Upon initiation of this review, we developed a protocol, with input from experts, and registered it in the public PROSPERO database (CRD42015025348). Additionally, our full-evidence report provides complete details of our methods and data for this review, including search strategy, full key questions and inclusion criteria, data abstraction, and risk of bias and strength of evidence ratings.20 We reported this review following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.21
Topic Development
The ESP Coordinating Center investigators and representatives of the SOTA committee worked together to identify the population, intervention, comparator, outcome, timing, setting, and study design characteristics of interest. These eligibility criteria are presented in Table 1.
Table 1.
Eligibility Criteria
| Population: Our primary focus is adult patients with BMI ≥50 kg/m2. To maximize applicability, we included only studies that focused exclusively on the super obese or that included a subgroup analysis of super obese. We did not include studies that had patients with mean or median BMI ≥ 50 kg/m2 but that encompassed a broader range of patients overall. |
| Intervention: Bariatric surgery interventions. |
| Comparator: Non-surgical weight loss interventions (including lifestyle, dietary changes, medications) and different bariatric surgical procedures, usual care |
| Outcomes: Primary outcomes of interest include long-term (defined as ≥ 5 years, based on recent NIH Funding Opportunity Announcement #PAR-14-262 for long-term outcomes of bariatric surgery using large data sets) weight loss (% excess weight lost, BMI change), mortality, remission/resolution of physical and mental health conditions, complications, and cost. Secondary outcomes include barriers to obtaining bariatric surgery (patient attitudes, provider attitudes, access, etc.) and short-term (<5 years) weight loss (% excess weight lost, BMI change), mortality, remission/resolution of physical and mental health conditions, complications, and cost. |
| Timing: No restrictions |
| Setting: Within and outside VA. We will prioritize VA studies, but will look outside of the VA to fill gaps in VA evidence, including international studies. |
| Study design: Using a best evidence approach, we will prioritize evidence from systematic reviews and multi-site comparative studies that adequately controlled for potential patient-, provider-, and system-level confounding factors. Inferior study designs (e.g., single-site, inadequate control for confounding, non-comparative) will be accepted only to fill gaps in higher-level evidence. |
Search Strategy
To identify articles relevant to the key questions, our research librarian searched MEDLINE®, the Cochrane Central Registry of Controlled Trials, PsycINFO, and ClinicalTrials.gov through June 2016 using various terms for bariatric surgery and obesity. Due to the large volume of well-conducted systematic reviews, we relied on reference lists for studies published through 2012 and conducted new searches for studies published from 2013 onward. Additional citations were identified from hand-searching reference lists and consultation with content experts. We limited the search to articles involving human subjects available in the English language.
Study Selection
Two reviewers selected studies based on the eligibility criteria described above. Titles and abstracts and full-text articles were first reviewed by one investigator and then checked by another. All disagreements were resolved by consensus.
Data Abstraction and Quality Assessment
We abstracted data on each of the eligibility criteria items (Table 1). We assessed the internal validity of all studies using predefined criteria. For randomized controlled trials, we used Cochrane’s Risk of Bias Tool.22 For observational studies we assessed selection, performance, attrition, detection, and reporting biases and assigned overall ratings of high, medium, and low risk of bias.23 For systematic reviews we used the AMSTAR tool.24 We used a standardized form to abstract data from all included studies on key study and patient characteristics and results. All data abstraction and internal validity ratings were completed by one reviewer and then checked by another. All disagreements were resolved by consensus.
Data Synthesis
We graded the overall strength of the evidence for each outcome as high, moderate, low, or insufficient based on study limitations, consistency, directness, precision, and reporting bias, according to the AHRQ Methods Guide for Comparative Effectiveness Reviews.25 We did not perform meta-analyses, due the small number of studies and heterogeneity in outcomes and comparison evaluated. Instead, we synthesized the evidence qualitatively by grouping studies by similarity in bariatric surgery comparison.
Among the wide variety of weight loss metrics reported, we preferred percent baseline weight loss per the 2013 analysis by Hatoum and Kaplan, which showed it was least influenced by variation in preoperative BMI.26 When percent baseline weight loss was not reported, we evaluated proportion excess body weight loss (%EWL). As Hatoum and Kaplan found that both %EWL and change in BMI were similarly sensitive to preoperative BMI (r = −0.52 and r = 0.56, respectively), we selected %EWL as the secondary weight loss metric, as it was most commonly reported and allowed for greater comparison across studies. We used Microsoft Excel 2010 (Microsoft Corp., Redmond, WA) to calculate descriptive statistics and generate a graph for observational study risk of bias indicators.
RESULTS
Literature Flow
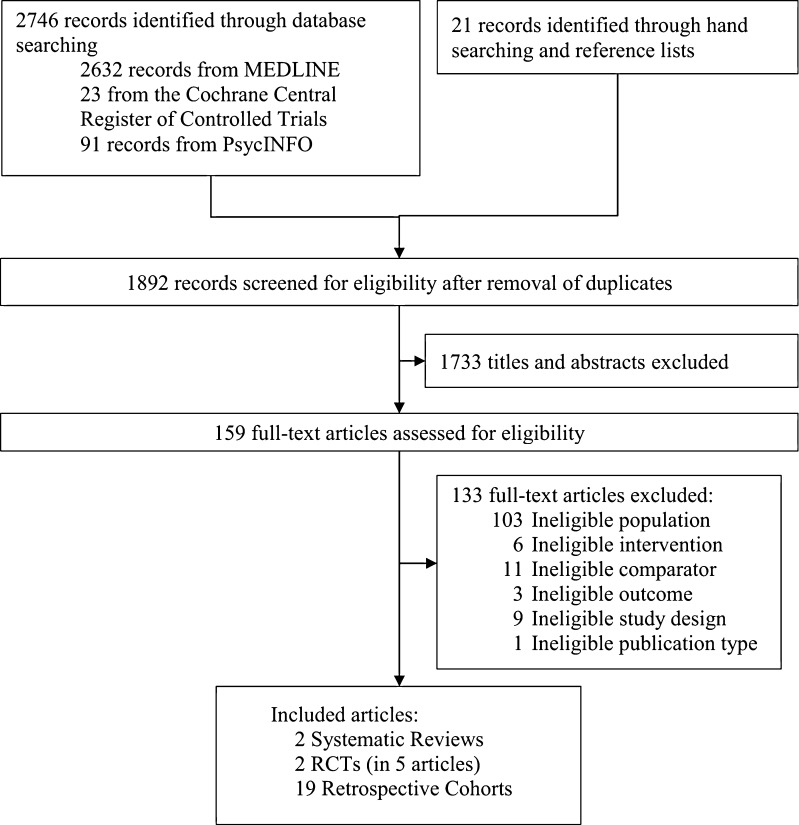
The literature flow diagram (Fig. 1) summarizes the results of search and study selection processes. Bibliographic database searches resulted in 1892 potentially relevant articles. Of these, we included two systematic reviews, two randomized controlled trials (RCTs; in five publications), and 19 retrospective cohort studies. The previous Cochrane review focused only on RCTs and found super obesity data insufficient for analysis.6 Searches of ClinicalTrials.gov identified one unpublished study in patients with BMI > 50 kg/m2 that compared long-limb to very-long-limb gastric bypass (NCT00868543).
Figure 1.
Literature Flow Chart
Overview of Study Characteristics
Table 2 displays the characteristics of the included primary studies. For the comparison of bariatric surgery to non-surgical treatment, we identified one subgroup analysis from a VA observational study.27 For the comparison of different bariatric surgery types, data were available from one systematic review,28 two RCTs,29 , 30 and 18 retrospective cohorts.31 – 48 The majority of studies involved mostly women aged 35 to 45 years.
Table 2.
Characteristics of Included Studies
| Author, year (N) | Location (time frame) | Study design | Interventions compared | Mean baseline BMI* (kg/m2) | Mean age* (years) | Gender* (% female) |
Type 2 diabetes* (% patients) | Risk of bias |
|---|---|---|---|---|---|---|---|---|
| Arterburn, 2015 (2860)27 | VA Medical Centers (2000–2006) | RC | Surgery (RYGB, sleeve gastrectomy, adjusted GB) vs. no surgery | NR† | NR † | NR † | NR † | Medium |
| Bowne, 2006 (106)31 | USA (2001–2004) | RC | LRYGB vs. LAGB | 55.96 | 42.29 | 80 | 17.9 | Medium |
| Daigle, 2015 (30)32 | USA (2006–2012) | RC | LRYGB vs. LSG vs. LAGB | 55.9 | 67.1 | 86.7 | 30 | High |
| Giordiano, 2015 (181)33 | Finland (2006–2009) | RC | LRYGB vs. LAGB | 55 | 41.9 | 64 | NR | High |
| Heneghan, 2014 (189)34 | USA (2007–2010) | RC | Banded vs. non-banded LRYGB | NR † | NR † | NR † | NR† | Medium |
| Khorgami, 2014 (3268)35 | USA (2002–2012) | RC | LRYGB vs. LAGB | NR † | NR † | NR † | NR | High |
| Laurenius, 2010 ‡
(32)36 |
Sweden (2001–2004) | RC | LRYGB vs. LDS | 56.6 | 37.8 | 62.6 | 37.5 | Medium |
| Mognol, 2005 (290)37 | France (1994–2004) | RC | LRYGB vs. LAGB | 55.9 | 40 | 77.6 | NR | High |
| Nelson, 2012 (26510)38 | USA (2007–2010) | RC | GBP vs. DS (laparoscopic or open) | NR † | NR † | NR † | NR † | High |
| O’Rourke, 2006 ‡
(452)39 |
USA (2000–2003) | RC | GBP vs. BPD-DS (open and laparoscopic) | 55 | 44 | 82 | NR | Medium |
| Parikh, 2005 (332)40 | USA (2000–2004) | RC | LAGB vs. LRYGB vs. BPD (with/without DS) | 55.7 | 42 | 75 | NR | High |
| Prachand, 2006 ‡ (350)41 | USA (2002–2005) | RC | DS vs. RYGB | 57.75 | 40.4 | 83 | NR | High |
| Risstad, 2015; Sovik 2010 ‡, 2011 ‡, 2013 (60)29 , 49 – 51 | Norway & Sweden (2006–2007) | RCT | RYGB vs. BPD-DS | 54.86 | 35.6 | 70 | NR | Low |
| Roland, 2011 (89)42 | USA (2003–2007) | RC | ORYGB vs. LRYGB | 78.9 | 42 | 65 | 41.7 | Medium |
| Sekhar, 2007 (967)43 | USA (2001–2005) | RC | ORYGB vs. LRYGB | 54.9 | 42.9 | 81.8 | NR | High |
| Serrano, 2015 (135)44 | USA (2008–2013) | RC | LRYGB vs. LSG | 66.95 | 38.82 | 65 | 26.75 | Medium |
| Stephens, 2008 (291)45 | USA (2002–2006) | RC | LAGB vs. VBG-RYGB vs. LRYGB | 67 § | 41 § | 63.23 | 17.87 | High |
| Svanevik, 2015 (113)30 | Norway (2011–2013) | RCT | Proximal vs. Distal RYGB | 53.4 | 40.6 | 64.4 | 29.4 | Unclear |
| Thereaux, 2015 (359)46 | France (2004–2013) | RC | LSG vs. LRYGB | 56.8 | 41.8 | 71.8 | 38.49 | Medium |
| Topart, 2013 ‡
(179)47 |
France (2002–2009) | RC | BPD-DS vs. RYGB | 55 | 39.76 | 68.6 | 17.8 | High |
| Zerrweck, 2014 (77)48 | Mexico (2010–2012) | RC | LGBP vs. LSG | 53.38 | 36.6 | 72 | 19.16 | Medium |
*ESP calculated averages/weighted averages based on information provided for subgroups of interest
†Study does not report data for the super obese subgroup
‡Article included in systematic review, Hedberg (2014)
§ Median value shown here; mean value not provided in study results
Abbreviations: BPD = biliopancreatic diversion, BPD-DS = biliopancreatic diversion with duodenal switch, DS = duodenal switch, GBP = gastric bypass, LAGB = laparoscopic adjustable gastric banding, LGBP = laparoscopic gastric bypass, LSG = laparoscopic sleeve gastrectomy, LDS = laparoscopic biliopancreatic diversion/duodenal switch, LRYGB = laparoscopic Roux-en-Y gastric bypass, NR = not reported, ORYGB = open Roux-en-Y gastric bypass, RC = retrospective cohort, RCT = randomized controlled trial, RYGB = Roux-en-Y gastric bypass, VBG-RYGB = vertical banded gastroplasty–Roux-en-Y gastric bypass
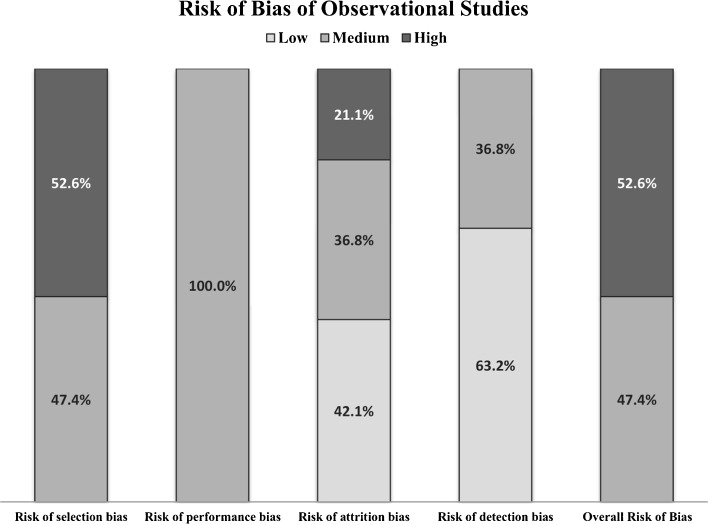
We rated the systematic review as fair quality,28 one RCT (in four studies) as low risk of bias,29 , 49 – 51 and one RCT as unclear risk of bias.30 Figure 2 summarizes the main risk of bias indicators of the 19 retrospective cohorts. Their overall risk of bias was medium in 47% and high in 53%. Frequent methodological limitations were unbalanced comparison groups at baseline and lack of control for confounders or co-interventions. Risk of reporting bias was generally low based on comparing the study reports to outcomes pre-specified in the methods, but protocols typically were not available.
Figure 2.
Risk of Bias of Included Observational Studies (N = 19)
Comparison of Bariatric Surgery to Non-Surgical Treatment
No studies evaluated the comparative effectiveness of bariatric surgery versus any specific, active non-surgical treatment in super obese adults. One retrospective study used administrative data to compare long-term survival in veterans who underwent bariatric surgery between 2000–2011 to veterans matched for sex, diabetes diagnosis, race, VA region, BMI, and age.27 The control group was described as representing usual care, but there was no information about what care was provided or their eligibility for bariatric surgery. This study conducted post hoc analyses to determine whether the relationship between surgery and mortality might differ in the super obese.27
Overall, 74% of the veterans in this study were men, and their mean age was 52 years. The surgical procedure types were primarily open (53%) or laparoscopic (21%) Roux-en-Y gastric bypass. Rates of diet, exercise, lifestyle interventions, or weight loss medication use were not reported for either group. However, the authors reported that participation in the VA’s national MOVE!® weight management program was mandatory for bariatric surgery candidates starting in 2006, and it is likely that many control group patients also participated, as it is VA policy to refer all severely obese patients to MOVE!®.27
Compared to usual care, post hoc analyses of the super obese subgroup provided low-strength evidence that risk of mortality associated with bariatric surgery was higher at 1 year (4.93% vs. 2.77%; aHR 1.57; 95% CI, 1.08–2.76; N = 2860), then lower from 1 year to 5 years (5.48% vs. 11.4%; aHR 0.46, 95% CI, 0.33–0.64; N = 2723) and from 5 years to 14 years (9.5% vs. 17.5%; aHR 0.45, 95% CI, 0.34–0.60; N = 2054). Compared to the overall study population (mean BMI = 47 kg/m2), mortality rates for the super obese subgroup were numerically higher across all time periods, and the increased risk in the first year reached statistical significance.
Although matching for some known confounders was done well, the main methodological limitations of the study overall were that (1) there was no information about the care provided to the controls, and (2) information from administrative data about many key covariates was either unavailable or missing, including severity of comorbid conditions and smoking. We cannot rule out the possibility that the greater mortality risk factors characteristic of surgical ineligibility were overrepresented in the non-surgery group. Additionally, this study does not fully address the balance of benefits and harms of surgery, as it did not evaluate other outcomes of great interest, including comorbid disease remission, complications, and quality of life.
Comparison of Different Bariatric Surgery Types
We identified 20 primary studies29 – 51 and one systematic review28 which compared various types of bariatric surgery among super obese patients. Duodenal switch (DS) versus laparoscopic Roux-en-Y gastric bypass (LRYGB) is the only comparison that has outcome data with over 5 years of follow-up. For follow-up over 5 years, an RCT (reported in four publications)29 , 49 – 51 provided low-strength evidence that, compared to LRYGB, DS achieves better weight control (% patients with BMI > 40 kg/m2: DS = 14% vs. LRYGB = 55.3%, P = 0.001) and has comparable diabetes remission (100% vs. 80%; P = 0.45) and mortality (3% vs. 0%; P = 0.48), but higher risk of hospital admissions (59% vs. 29%; P = 0.02) and surgeries related to the initial procedure (45% vs. 10%; P = 0.002). Table 3 summarizes best evidence for each surgical comparison for up to 5 years of follow-up. These studies provide low-strength evidence that laparoscopic gastric bypass generally resulted in greater short-term proportion of excess weight loss (%EWL) than did other procedures, particularly when banding versus non-banding was used and when the bypass was proximal versus distal.
Table 3.
Summary of Findings: Best Evidence on Comparative Effectiveness of Different Types of Bariatric Surgery in Super Obese (BMI ≥ 50 kg/m2) Patients at < 5 Years
| Comparison | Number and type of studies, sample sizes, endpoint | %EWL | Mortality | Complications | Diabetes remission |
|---|---|---|---|---|---|
| DS vs. LRYGB | Short-term: 6 primarily US retrospective cohorts31 , 36 , 38 – 40 , 48 N = 27,645 | ★ | ★ | ★ | ★ |
| LRYGB vs. LAGB | 1 US, single-center, retrospective cohort in Brooklyn31 N = 106, 16 months | 52% vs. 31%, P < 0.001 ★★ |
No deaths ★★ |
Early complications: 17% vs. 18%, P = 0.33 ★★ Late complications: 28% vs. 78%, P < 0.05 ★★ |
NR |
| LRYGB vs. LSG | 2 non-US, single-center retrospective cohorts in Mexico and France46 , 48 N = 436, 12 months | 55–63.9% vs. 40.2–43.9%, P < 0.05 ★★ |
30 days: 1% vs. 0%, P = 0.9946
12 months: No deaths48 ★★ |
<30 days: Complications: 9% vs. 22%, P > 0.248 Major adverse events*: ★★ 5.7% vs. 6.7%, P = 0.8546 |
Resolution: 70.7% vs. 47.5%, P = 0.01 ★★ |
| LRYGB vs. LSG in super super obese (BMI ≥ 60 kg/m2) | 1 US single-center retrospective cohort in New York44 N = 135, 37 months | ★ | NR | NR | NR |
| Banded vs. non-banded LRYGB | 1 US, single-center retrospective cohort in Ohio34 N = 189, 2 years | 57.5% vs. 47.6%, P = 0.003 ★★ |
NR | NR | NR |
| Laparoscopic vs. open gastric bypass | 1 US, subgroup analysis from a single-center retrospective cohort in Tennessee43 N = unknown for super obese subgroup | ★ | NR | NR | NR |
| Laparoscopic vs. open gastric bypass in mega obese (BMI > 70 kg/m2) | 1 US, single-center retrospective cohort in Ohio42 N = 89, 2 years | 3 months: 22.7% vs. 17.5, P = 0.016 6 months: 37.6% vs. 30.8%, P = 0.037 1 year: 48% vs. 48% 2 year: 60% vs. 60% ★★ |
0 vs. 2% ★★ |
Hernia: 19% vs. 3%; P = 0.02 ★★ No differences in other complications ★★ |
NR |
| Proximal vs. distal RYGB | 1 non-US, double-blind RCT with two participating centers in Norway30 N = 113, 2 years | NR | No deaths ★★ |
Severe complications: 0% vs. 10.7%; P = 0.01 ★★ Reoperations: 0% vs. 10.7% ★★ No significant differences in other complications ★★ |
NR |
Note: Strength of evidence: ★★★★ = High; ★★★ = Moderate; ★★ = Low; ★ = Insufficient
*Major adverse events: death, percutaneous or endoscopic interventions or repeat surgery, venous thromboembolism (VTE) and failure to be discharged from the hospital
Abbreviations: aHR = adjusted hazard ratio, DS = duodenal switch, LAGB = laparoscopic adjustable gastric banding, LRYGB = laparoscopic Roux-en-Y gastric bypass, LSG = laparoscopic sleeve gastrectomy, NR = not reported, RYGB Roux-en-Y gastric bypass, %EWL = % excess weight loss
DISCUSSION
To our knowledge, this is the first evidence review that has focused exclusively on adults with BMI ≥ 50 kg/m2 across a wide variety of bariatric surgery types. This rapid evidence review found the published literature that separately evaluates adults with BMI ≥ 50 kg/m2 to be insufficient for determining the precise balance of benefits and harms of bariatric surgery compared to non-surgical treatment (i.e., lifestyle, dietary changes, pharmacotherapy) in this subpopulation. This paucity of research in individuals with BMI ≥ 50 kg/m2 is important, because it may be contributing to under- or overuse of bariatric surgery in this unique population.
One large retrospective VA study provided limited evidence that, compared with usual care, bariatric surgery could lead to increased mortality in the first year but reduced mortality over the long term among super obese veterans. However, the care provided to the control group, whether non-surgical or no treatment, was not well-defined. Also, information about many key issues was missing (e.g., smoking, severity of comorbidities), and the study did not evaluate a complete set of key outcomes including weight loss, obesity-related disease remission, complications, and cost.
Studies comparing different bariatric surgical approaches in people with BMI ≥ 50 kg/m2 provide low-strength evidence of some differences in weight loss and complications. Laparoscopic gastric bypass generally resulted in a greater short-term proportion of excess weight loss (%EWL) than did other procedures, particularly when banding versus non-banding was used and when the bypass was proximal versus distal. The exception was that duodenal switch led to greater long-term weight loss than did gastric bypass, but this was at the expense of more complications for duodenal switch. However, the applicability of these findings may be limited primarily to women in their mid-30s to 40s, and information was missing on diabetes, mental illness, and other important comorbidities.
The potential limitations of this rapid evidence review are related to the modifications we made to standard systematic review methodology in order to meet a constrained timeline of 3 months. With 100 rapid reviews published between 1997 and 2013, they have become an increasingly common form of evidence synthesis used to inform more urgent needs of health care decision-makers.52 Surveys of rapid evidence review end-users found that they were willing to accept certain methodological shortcuts in order to increase reviewer efficiency,53 and that availability of rapid reviews increased their uptake of evidence to inform time-sensitive system-level decision-making.54 However, there is no consensus yet on what represents best practice for rapid reviews. A scoping review of rapid reviews found that shortcut approaches varied widely across all steps of the review process and were applied inconsistently.52 Concerns have been expressed about methodological shortcuts potentially increasing the risk of bias in rapid reviews, leading to suggestions for future research comparing findings of standard and rapid reviews.52 , 53 , 55 , 56 The two main methodological limitations of this evidence brief are its scope and our abbreviated search methods. Regarding scope, because of our abbreviated time frame, we limited our focus to the SOTA committee’s highest-priority outcomes of weight loss, mortality, obesity-related disease remission, complications, and cost. Therefore, this review does not address additional outcomes that can also have important clinical implications (e.g., surgical time, conversion to open procedure, quality of life, functional capacity, minor adverse effects). Also, given our time constraint, to obtain the most precise estimates of outcomes in the super obese, we focused on studies that included only super obese patients or that separated out the super obese subgroup. However, given more time, further assessment of the very large body of existing evidence of broader patient populations with BMI > 35 kg/m2 could provide additional information about patients with BMI ≥ 50. As many studies that enrolled patients with BMI > 35 kg/m2 included a subgroup of patients with BMI ≥ 50 kg/m2, individual patient data meta-analysis could be used to evaluate patients with BMI ≥ 50 kg/m2. Regarding our search methods, although we attempted to use an exhaustive list of search terms, the lack of a standard taxonomy for describing super obesity in the literature made searching for this topic somewhat difficult. Also, for studies published through 2012, we relied on the reference lists of previous well-conducted systematic reviews, and only conducted new searches for studies published from 2013 onward. For these two reasons, our search may have missed relevant studies. However, extensive peer review by multiple bariatric surgery experts did not uncover any additional studies.
This rapid evidence brief identified several key gaps in the evidence base in adults with BMI ≥ 50 kg/m2 that helped the VA HSR&D realign its obesity treatment future research priorities57 and which may be actionable for other research efforts. First, for studies comparing different bariatric surgery treatments in adults with BMI ≥ 50 kg/m2, half were from non-US settings. Differences in health care systems and standards of care in these studies may have low applicability to US health care systems (e.g., accreditation, level and type of multidisciplinary care, pre-procedure preparation/post-procedure support). Next, the majority of studies in adults with BMI ≥ 50 kg/m2 comprised primarily females in their mid-30s to 40s, and lacked information about other key patient characteristics (e.g., smoking, presence and severity of medical and mental health comorbidities), preoperative care and requirements, care in quaternary systems of care, and adherence to post-procedure recommendations, which could have influenced their outcome. These gaps greatly limit the applicability of their findings. To broaden the applicability of evidence in adults with BMI ≥ 50 kg/m2 and to help identify predictors of best outcomes, future research should better report and evaluate the role of a broader range of key covariates.
Lastly, although the majority of studies consistently reported short-term weight loss outcomes, reporting on complications was limited and inconsistent, and studies generally lacked long-term follow-up and neglected important obesity-related disease remission outcomes. To better determine the precise balance of benefits and harms of bariatric surgery in people with BMI ≥ 50 kg/m2, future research needs to measure a more complete set of long-term outcomes. For weight loss outcome assessment, in 2013 Hatoum and Kaplan recommended adoption of percentage of baseline weight loss as the preferred weight loss measure, because it was the least influenced by preoperative BMI.26 However, studies used a wide variety of methods, including BMI loss, weight loss, proportion of excess weight loss, proportion of patients with a BMI over a certain threshold, and proportion of patients that failed to lose 50% or more of their excess weight, and many studies did not report measures of variance. There is also a lack of standardized definitions for surgical complication outcomes. This heterogeneity makes it difficult to combine and compare findings across studies. The bariatric surgery field in general would benefit from work toward standardization of outcome definitions.11 , 58 Further, we found no defined goals for the magnitude of weight loss that is required for a meaningful benefit in longevity and resolution of obesity-related comorbidity. Philosophically, it may be ideal to strive to reduce BMI to a level that would eliminate eligibility for bariatric surgery.41 But as this is more difficult to achieve in super obese patients, it could be clinically useful to document what level of weight loss is really necessary to achieve the greater overall goals. As the current evidence is very limited in the super obese, we recommended that the HSR&D SOTA committee, in setting their research agenda, prioritize confirmation of the subgroup findings from Arterburn et al. about the comparison of bariatric surgery to non-surgical treatment in the super obese.27 Answering questions about the long-term comparative effectiveness of surgical and non-surgical weight loss interventions will help to determine the relevance of questions about choice of surgical approach.
In conclusion, our rapid evidence review found that, in adults with BMI ≥ 50 kg/m2, there is limited evidence that, compared to usual care, bariatric surgery can increase mortality in the first year, but decrease longer-term mortality. Despite their potentially greater health care challenges, existing evidence suggests that people with BMI ≥ 50 kg/m2 may benefit from bariatric surgery. However, to more precisely determine the balance of benefits and harms of bariatric surgery in this high-risk subgroup, future research should (1) evaluate and better characterize larger samples of more broadly representative adults and (2) better define and assess a more complete set of key outcomes with longer follow-up.
Acknowledgements
We would like to thank Linda Humphrey, MD, FACP, for providing clinical expertise; Julia Haskin, MA, for editorial support; and Robin Paynter, MLIS, for searching support.
Compliance with Ethical Standards
Funders
This material is based upon work supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Quality Enhancement Research Initiative (QUERI), Evidence-Based Synthesis Program (ESP).
Prior Presentations
None.
Conflict of Interest
The authors declare that they do not have a conflicts of interest.
References
- 1.Sturm R. Increases in morbid obesity in the USA: 2000–2005. Public Health. 2007;121(7):492–496. doi: 10.1016/j.puhe.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sturm R, Hattori A. Morbid obesity rates continue to rise rapidly in the United States. Int J Obes (Lond) 2013;37(6):889–891. doi: 10.1038/ijo.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindekilde N, Gladstone BP, Lubeck M, et al. The impact of bariatric surgery on quality of life: a systematic review and meta-analysis. Obes Rev. 2015;16(8):639–651. doi: 10.1111/obr.12294. [DOI] [PubMed] [Google Scholar]
- 4.Canadian Agency for Drugs and Technologies in Health. Bariatric Surgical Procedures for Obese and Morbidly Obese Patients: A Review of Comparative Clinical and Cost-Effectiveness, and Guidelines. CADTH Rapid Response Service. 2014. [PubMed]
- 5.Canadian Agency for Drugs and Technologies in Health. Bariatric Surgery for Obese Patients with Co-Morbidities: A Review of Clinical Effectiveness, Cost-Effectiveness, and Guidlines. CADTH Rapid Response Service. 2013.
- 6.Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. Cochrane Database Syst Rev. 2014;8:CD003641. doi: 10.1002/14651858.CD003641.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gloy VL, Briel M, Bhatt DL, et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:f5934. doi: 10.1136/bmj.f5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Heart Lung and Blood Institute. Managing overweight and obesity in adults: systematic evidence review for the Obesity Expert Panel: National Institutes of Health 2013.
- 9.Padwal R, Klarenbach S, Wiebe N, et al. Bariatric surgery: a systematic review of the clinical and economic evidence. J Gen Intern Med. 2011;26(10):1183–1194. doi: 10.1007/s11606-011-1721-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puzziferri N, Roshek TB, 3rd, Mayo HG, Gallagher R, Belle SH, Livingston EH. Long-term follow-up after bariatric surgery: a systematic review. JAMA. 2014;312(9):934–942. doi: 10.1001/jama.2014.10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Washington State Health Care Authority. Bariatric Surgery: Final Evidence Report. Institute for Clinical and Economic Review. 2015.
- 12.Livhits M, Mercado C, Yermilov I, et al. Preoperative predictors of weight loss following bariatric surgery: systematic review. Obes Surg. 2012;22(1):70–89. doi: 10.1007/s11695-011-0472-4. [DOI] [PubMed] [Google Scholar]
- 13.Petry NM, Barry D, Pietrzak RH, Wagner JA. Overweight and obesity are associated with psychiatric disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychosom Med. 2008;70(3):288–297. doi: 10.1097/PSY.0b013e3181651651. [DOI] [PubMed] [Google Scholar]
- 14.Livingston EH, Arterburn D, Schifftner TL, Henderson WG, DePalma RG. National Surgical Quality Improvement Program analysis of bariatric operations: modifiable risk factors contribute to bariatric surgical adverse outcomes. J Am Coll Surg. 2006;203(5):625–633. doi: 10.1016/j.jamcollsurg.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Schauer DP, Arterburn DE, Livingston EH, et al. Impact of bariatric surgery on life expectancy in severely obese patients with diabetes: a decision analysis. Ann Surg. 2015;261(5):914–919. doi: 10.1097/SLA.0000000000000907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arterburn D, Livingston EH, Schifftner T, Kahwati LC, Henderson WG, Maciejewski ML. Predictors of long-term mortality after bariatric surgery performed in Veterans Affairs medical centers. Arch Surg. 2009;144(10):914–920. doi: 10.1001/archsurg.2009.134. [DOI] [PubMed] [Google Scholar]
- 17.Galinsky T, Hudock S, Streit J. Addressing the Need for Research on Bariatric Patient Handling. Rehabil Nurs. 2010;35(6):242–247. doi: 10.1002/j.2048-7940.2010.tb00054.x. [DOI] [PubMed] [Google Scholar]
- 18.Sanford JA, Kadry B, Brodsky JB, Macario A. Bariatric surgery operating room time--size matters. Obes Surg. 2015;25(6):1078–1085. doi: 10.1007/s11695-015-1651-5. [DOI] [PubMed] [Google Scholar]
- 19.US Department of Veterans Affairs. Health Services Research & Development: Evidence-based Synthesis Program. 2016; http://www.hsrd.research.va.gov/publications/esp/. Accessed November 2, 2016.
- 20.Peterson K AJ, Ferguson L, Erickson K, Humphrey L. Evidence brief: the comparative effectiveness of bariatric surgery in super obesity (BMI >50 kg/m2). 2015. http://www.hsrd.research.va.gov/publications/esp/bariatricsurgery.cfm. Accessed November 2, 2016.
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Viswanathan M, Ansari M, Berkman ND, et al. Assessing the Risk of Bias of Individual Studies in Systematic Reviews of Health Care Interventions:Methods Guide for Comparative Effectiveness Reviews. Rockville, MD2012.
- 24.Shea BJ, Hamel C, Wells GA, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol. 2009;62(10):1013–1020. doi: 10.1016/j.jclinepi.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Berkman ND, Lohr KN, Ansari M, et al. Grading the Strength of a Body of Evidence When Assessing Health Care Interventions for the Effective Health Care Program of the Agency for Healthcare Research and Quality: An Update Methods Guide for Effectiveness and Comparative Effectiveness Reviews. 2013. [PubMed]
- 26.Hatoum IJ, Kaplan LM. Advantages of percent weight loss as a method of reporting weight loss after Roux-en-Y gastric bypass. Obesity (Silver Spring, Md) 2013;21(8):1519–1525. doi: 10.1002/oby.20186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arterburn DE, Olsen MK, Smith VA, et al. Association between bariatric surgery and long-term survival. JAMA. 2015;313(1):62–70. doi: 10.1001/jama.2014.16968. [DOI] [PubMed] [Google Scholar]
- 28.Hedberg J, Sundstrom J, Sundbom M. Duodenal switch versus Roux-en-Y gastric bypass for morbid obesity: systematic review and meta-analysis of weight results, diabetes resolution and early complications in single-centre comparisons. Obes Rev. 2014;15(7):555–563. doi: 10.1111/obr.12169. [DOI] [PubMed] [Google Scholar]
- 29.Risstad H, Sovik TT, Engstrom M, et al. Five-year outcomes after laparoscopic gastric bypass and laparoscopic duodenal switch in patients with body mass index of 50 to 60: a randomized clinical trial. JAMA Surg. 2015;150(4):352–361. doi: 10.1001/jamasurg.2014.3579. [DOI] [PubMed] [Google Scholar]
- 30.Svanevik M, Risstad H, Hofso D, et al. Perioperative Outcomes of Proximal and Distal Gastric Bypass in Patients with BMI Ranged 50–60 kg/m(2)--A Double-Blind, Randomized Controlled Trial. Obes Surg. 2015;25(10):1788–1795. doi: 10.1007/s11695-015-1621-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bowne WB, Julliard K, Castro AE, Shah P, Morgenthal CB, Ferzli GS. Laparoscopic gastric bypass is superior to adjustable gastric band in super morbidly obese patients: a prospective, comparative analysis. Arch Surg. 2006;141(7):683–689. doi: 10.1001/archsurg.141.7.683. [DOI] [PubMed] [Google Scholar]
- 32.Daigle CR, Andalib A, Corcelles R, Cetin D, Schauer PR, Brethauer SA. Bariatric and metabolic outcomes in the super-obese elderly. Surg Obes Relat Dis. 2015; doi:10.1016/j.soard.2015.04.006. [DOI] [PubMed]
- 33.Giordano S, Tolonen P, Victorzon M. Laparoscopic Roux-en-Y gastric bypass versus laparoscopic adjustable gastric banding in the super-obese: peri-operative and early outcomes. Scand J Surg. 2015;104(1):5–9. doi: 10.1177/1457496914553148. [DOI] [PubMed] [Google Scholar]
- 34.Heneghan HM, Annaberdyev S, Eldar S, Rogula T, Brethauer S, Schauer P. Banded Roux-en-Y gastric bypass for the treatment of morbid obesity. Surg. 2014;10(2):210–216. doi: 10.1016/j.soard.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 35.Khorgami Z, Arheart KL, Zhang C, Messiah SE, de la Cruz-Munoz N. Effect of ethnicity on weight loss after bariatric surgery. Obes Surg. 2015;25(5):769–776. doi: 10.1007/s11695-014-1474-9. [DOI] [PubMed] [Google Scholar]
- 36.Laurenius A, Taha O, Maleckas A, Lönroth H, Olbers T. Laparoscopic biliopancreatic diversion/duodenal switch or laparoscopic Roux-en-Y gastric bypass for super-obesity—weight loss versus side effects. Surg Obes Relat Dis. 2010;6(4):408–414. doi: 10.1016/j.soard.2010.03.293. [DOI] [PubMed] [Google Scholar]
- 37.Mognol P, Chosidow D, Marmuse JP. Laparoscopic gastric bypass versus laparoscopic adjustable gastric banding in the super-obese: a comparative study of 290 patients. Obes Surg. 2005;15(1):76–81. doi: 10.1381/0960892052993486. [DOI] [PubMed] [Google Scholar]
- 38.Nelson DW, Blair KS, Martin MJ. Analysis of obesity-related outcomes and bariatric failure rates with the duodenal switch vs gastric bypass for morbid obesity. Arch Surg. 2012;147(9):847–854. doi: 10.1001/archsurg.2012.1654. [DOI] [PubMed] [Google Scholar]
- 39.O’Rourke RW, Andrus J, Diggs BS, Scholz M, McConnell DB, Deveney CW. Perioperative morbidity associated with bariatric surgery: an academic center experience. Arch Surg. 2006;141(3):262–268. doi: 10.1001/archsurg.141.3.262. [DOI] [PubMed] [Google Scholar]
- 40.Parikh MS, Shen R, Weiner M, Siegel N, Ren CJ. Laparoscopic bariatric surgery in super-obese patients (BMI > 50) is safe and effective: a review of 332 patients. Obes Surg. 2005;15(6):858–863. doi: 10.1381/0960892054222632. [DOI] [PubMed] [Google Scholar]
- 41.Prachand VN, Davee RT, Alverdy JC. Duodenal switch provides superior weight loss in the super-obese (BMI > or =50 kg/m2) compared with gastric bypass. Ann Surg. 2006;244(4):611–619. doi: 10.1097/01.sla.0000239086.30518.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roland JC, Needleman BJ, Muscarella P, Cook CH, Narula VK, Mikami DJ. Laparoscopic Roux-en-Y gastric bypass in patients with body mass index >70 kg/m2. Surg Obesity Relat Dis: Off J Am Soc Bariatric Surg. 2011;7(5):587–591. doi: 10.1016/j.soard.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 43.Sekhar N, Torquati A, Youssef Y, Wright JK, Richards WO. A comparison of 399 open and 568 laparoscopic gastric bypasses performed during a 4-year period. Surg Endosc. 2007;21(4):665–668. doi: 10.1007/s00464-006-9151-2. [DOI] [PubMed] [Google Scholar]
- 44.Serrano OK, Tannebaum JE, Cumella L, et al. Weight loss outcomes and complications from bariatric surgery in the super super obese. Surg Endosc. 2015:1–7. [DOI] [PubMed]
- 45.Stephens DJ, Saunders JK, Belsley S, et al. Short-term outcomes for super-super obese (BMI ≥ 60 kg/m 2) patients undergoing weight loss surgery at a high-volume bariatric surgery center: laparoscopic adjustable gastric banding, laparoscopic gastric bypass, and open tubular gastric bypass. Surg Obes Relat Dis. 2008;4(3):408–415. doi: 10.1016/j.soard.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 46.Thereaux J, Corigliano N, Poitou C, Oppert J-M, Czernichow S, Bouillot J-L. Comparison of results after one year between sleeve gastrectomy and gastric bypass in patients with BMI > 50 kg/m2. Surg. 2015;11(4):785–790. doi: 10.1016/j.soard.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 47.Topart P, Becouarn G, Ritz P. Weight loss is more sustained after biliopancreatic diversion with duodenal switch than Roux-en-Y gastric bypass in superobese patients. Surg. 2013;9(4):526–530. doi: 10.1016/j.soard.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 48.Zerrweck C, Sepulveda EM, Maydon HG, et al. Laparoscopic gastric bypass vs sleeve gastrectomy in the super obese patient: early outcomes of an observational study. Obes Surg. 2014;24(5):712–717. doi: 10.1007/s11695-013-1157-y. [DOI] [PubMed] [Google Scholar]
- 49.Sovik TT, Aasheim ET, Taha O, et al. Weight loss, cardiovascular risk factors, and quality of life after gastric bypass and duodenal switch: a randomized trial. Ann Intern Med. 2011;155(5):281–291. doi: 10.7326/0003-4819-155-5-201109060-00005. [DOI] [PubMed] [Google Scholar]
- 50.Sovik TT, Karlsson J, Aasheim ET, et al. Gastrointestinal function and eating behavior after gastric bypass and duodenal switch. Surg. 2013;9(5):641–647. doi: 10.1016/j.soard.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 51.Sovik TT, Taha O, Aasheim ET, et al. Randomized clinical trial of laparoscopic gastric bypass versus laparoscopic duodenal switch for superobesity. Br J Surg. 2010;97(2):160–166. doi: 10.1002/bjs.6802. [DOI] [PubMed] [Google Scholar]
- 52.Tricco AC, Antony J, Zarin W, et al. A scoping review of rapid review methods. BMC Med. 2015;13(1):1. doi: 10.1186/s12916-014-0241-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hartling L, Guise J-M, Hempel S, et al. EPC Methods: AHRQ End-User Perspectives of Rapid Reviews: Agency for Healthcare Research and Quality;2016. [PubMed]
- 54.Peterson K FN, Ferguson L, Christensen V, Helfand M. User survey finds rapid evidence reviews increased uptake of evidence by Veterans Health Administration leadership to inform fast-paced health-system decision-making. Systematic Reviews. Forthcoming 2016. [DOI] [PMC free article] [PubMed]
- 55.Polisena J, Garritty C, Umscheid CA, et al. Rapid Review Summit: an overview and initiation of a research agenda. Sys Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hartling L, Guise J-M, Kato E, et al. EPC methods: an exploration of methods and context for the production of rapid reviews. 2015. [PubMed]
- 57.US Department of Veterans Affairs. Health Services Research & Development Research Topics: Obesity. 2016; http://www.hsrd.research.va.gov/research_topics/obesity.cfm. Accessed November 2, 2016.
- 58.Brethauer S, Kim J, el Chaar M, et al. Standardized Outcomes Reporting in Metabolic and Bariatric Surgery. Obes Surg. 2015;25(4):587–606. doi: 10.1007/s11695-015-1645-3. [DOI] [PubMed] [Google Scholar]