Abstract
Food insecurity is associated with negative chronic health outcomes, yet few studies have examined how providing medically appropriate food assistance to food-insecure individuals may improve health outcomes in resource-rich settings. We evaluated a community-based food support intervention in the San Francisco Bay Area for people living with HIV and/or type 2 diabetes mellitus (T2DM) to determine the feasibility, acceptability, and potential impact of the intervention on nutritional, mental health, disease management, healthcare utilization, and physical health outcomes. The 6-month intervention provided meals and snacks designed to comprise 100% of daily energy requirements and meet nutritional guidelines for a healthy diet. We assessed paired outcomes at baseline and 6 months using validated measures. Paired t tests and McNemar exact tests were used with continuous and dichotomous outcomes, respectively, to compare pre-post changes. Fifty-two participants (out of 72 initiators) had both baseline and follow-up assessments, including 23 with HIV, 24 with T2DM, and 7 with both HIV and T2DM. Median food pick-up adherence was 93%. Comparing baseline to follow-up, very low food security decreased from 59.6% to 11.5% (p < 0.0001). Frequency of consumption of fats (p = 0.003) decreased, while frequency increased for fruits and vegetables (p = 0.011). Among people with diabetes, frequency of sugar consumption decreased (p = 0.006). We also observed decreased depressive symptoms (p = 0.028) and binge drinking (p = 0.008). At follow-up, fewer participants sacrificed food for healthcare (p = 0.007) or prescriptions (p = 0.046), or sacrificed healthcare for food (p = 0.029). Among people with HIV, 95% adherence to antiretroviral therapy increased from 47 to 70% (p = 0.046). Among people with T2DM, diabetes distress (p < 0.001), and perceived diabetes self-management (p = 0.007) improved. Comprehensive, medically appropriate food support is feasible and may improve multiple health outcomes for food-insecure individuals living with chronic health conditions. Future studies should formally test the impact of medically appropriate food support interventions for food-insecure populations through rigorous, randomized controlled designs.
Keywords: Food, Nutrition, HIV, Diabetes, Community-based, Food security, Food support, Food assistance, Intervention, Medically tailored
Introduction
Food insecurity is a barrier to health and well-being [1, 2]. Thirteen percent of US households, or 42 million people, are food-insecure [3], with low-income households and households headed by racial/ethnic minorities disproportionately affected [3]. Food insecurity increases the risk of acquisition of and poor outcomes associated with both infectious and non-communicable diseases [4, 5]. In HIV, food insecurity is associated with increased risk of HIV acquisition [6] and transmission [7,8], poor medication adherence [9, 10], worse immunologic [10, 11] and virologic outcomes [10, 12, 13], morbidity [14], and mortality [15]. In diabetes, food insecurity is associated with increased risk of type 2 diabetes mellitus (T2DM) [4], poorer diabetes self-management [16, 17], poorer glycemic (i.e., blood sugar) control [4], and increased hypoglycemic events [18]. Our previously published conceptual framework posits that food insecurity negatively impacts health through nutritional (e.g., weight, diet quality), mental health (e.g., depression, stress), and behavioral pathways (e.g., medication adherence and disease self-management) [19, 20]. Intervention research is needed to understand not only whether medically appropriate food assistance may improve health but also to test whether food insecurity may be causally related to negative health outcomes.
There is a critical need for effective programs to improve the health of food-insecure populations with chronic illness or risk factors for chronic illness [19, 20]. Sixty percent of food-insecure US households have participated in at least one federal food and nutrition assistance program, the largest being the Supplemental Nutrition Assistance Program (SNAP, called Cal-Fresh in California) [21]. SNAP benefits may be insufficient for people with chronic illness to access healthy foods, which tend to cost more than less healthy foods [22–24]. In addition, many food-insecure individuals are excluded from participation in the federal food safety-net: in 2012, almost 30% of food-insecure households in the US had incomes above the eligibility thresholds for federal nutrition assistance [25]; SNAP also excludes undocumented immigrants. In California, the state with the second largest number of HIV diagnoses [26], individuals who receive Supplemental Security Income (SSI), a common source of disability income, are excluded from participating in SNAP [27].
Community-based food support programs, including food banks, food pantries, soup kitchens, and meal delivery organizations, fill important gaps in the federal and state food safety-nets, particularly in urban settings [25]. Some longstanding organizations—primarily serving chronically ill populations in large urban areas—have promoted that “food is medicine,” i.e., providing medically appropriate food to chronically ill populations, can improve health and reduce healthcare costs [28]. Yet few studies—none for HIV and few for diabetes [29] —have prospectively tested the role of community-based food assistance programs in improving the health of chronically ill individuals [30]. Little is known about whether, and through what mechanisms, nutritionally comprehensive, medically appropriate food assistance (i.e., designed to meet medical recommendations for specific health populations) can improve health outcomes for low-income, chronically ill populations.
To address these gaps, we conducted a study to evaluate the feasibility, acceptability, and potential impact [31] of Food = Medicine, a novel, medically appropriate 6-month food assistance intervention. We hypothesized that the intervention would improve nutrition, mental health, and health behaviors [19, 20].
Methods
Study Design
We assessed changes in nutritional and health outcomes among chronically ill individuals before and after the 6-month Food = Medicine intervention. Our study included people with HIV and/or T2DM because our previously published conceptual framework suggested similar mechanisms may govern the relationship between food insecurity and health in these groups [20].
Population and Recruitment
The study was conducted in partnership with Project Open Hand (POH), a San Francisco Bay Area-based non-profit organization that provides food assistance to individuals living with life-threatening and chronic illnesses, and to seniors. POH provides free meals and groceries to over 8000 clients either with HIV, other critical illnesses, or who are seniors. POH began serving people with T2DM around the same time as Food = Medicine was implemented, having served people with HIV for over 20 years.
POH eligibility criteria for the Food = Medicine intervention were: being (or in the process of becoming) a current POH client, certified by a physician as living with HIV and/or T2DM, English- or Spanish-speaking, age 18 or older, and low-income under ∼300% federal poverty line. For clients who had accessed regular services for at least 6 months, POH selected clients with service adherence >75% to maximize intervention fidelity. Clients requiring home-delivered meals or a special diet such as a renal, full vegetarian, or vegan diet were excluded from the intervention to simplify procedures, although plans are underway to expand to these groups in the future. Clients meeting the inclusion criteria were identified from a master list of POH clients, and recruited into the intervention until capacity was reached. After POH had recruited participants, but before the start of the intervention, the UCSF study team invited all participants to take part in the evaluation of Food = Medicine. All Food = Medicine clients were eligible to participate in the evaluation, but participation in the evaluation was not mandatory to receive the intervention.
Food = Medicine Intervention Description
The Food = Medicine intervention was developed in consultation with POH nutritionists and the study investigators, and implemented by POH from April 2014 to June 2015. The intervention provided meals and snacks fulfilling 100% of daily caloric requirements, tailored to meet nutritional guidelines for a healthy diet. Average energy requirements used to design daily meals were 1800–2000 kcal for people living with HIV and 1800 kcal for people with T2DM. This threshold evolved to account for varied energy requirements experienced by individuals of different size and metabolic needs. Meal plans varied each week but were the same across HIV and T2DM groups (see examples in Table 1). Meal plans were based on the Mediterranean diet featuring fresh fruits and vegetables, lean proteins, healthy fats (e.g., olive oil), and whole grains, and were low in refined sugars and saturated fats. The carbohydrate and saturated fat levels were set based on current recommendations from the American Diabetes Association and American Heart Association, respectively. All food was fresh, with limited pre-packaged food offered as snacks or grocery items (e.g., yogurt, sliced bread). Participants (or a surrogate) picked up their food twice per week at designated times from POH facilities.
Table 1.
Examples of Food = Medicine intervention meals and snacks
| Examples | |
|---|---|
| Breakfast | Bagel, 2 tbsp. low fat cream cheese, and 1 piece of fresh fruit 1 cup instant oatmeal, 1 cup 1% low fat milk, ½ banana, and 1 hard cooked egg 1 slice whole wheat bread, 2 tbsp. peanut butter, 1 orange, and 1 cup 1% low fat milk 1 cup plain yogurt, 2 tbsp. sliced almonds, and 1.5 cups frozen berries |
| Lunch | Mushroom-zucchini quiche Oven roasted cod w/ yogurt sauce Cajun style pork with red beans and rice Herb baked salmon w/ mushroom sauce w/ whole wheat penne |
| Dinner (meat) |
Pork loin chop w/ marinara sauce, wild rice and peas Chicken thigh w/ mushroom sauce, polenta, peas, and carrots Peruvian beef stew w/ tomato, pepper, onion, and quinoa Chicken and sausage gumbo w/ brown rice and corn |
| Dinner (vegetarian) | Black bean and corn chili Roasted tofu caponata and brown rice Stuffed bell peppers with tomato Provencal Veggie burger w/ tomato glaze and egg noodles |
| Snacks | 8 pieces of baby carrots, ¼ cup hummus, and 1 piece of fresh fruit ½ cup Tuscan bean salad and 1 piece of fresh fruit 1 apple, 2 tbsp. peanut butter, and 1 piece of fresh fruit ½ cup low fat 1% cottage cheese, 3 pieces Melba toast crackers, and 1 piece of fresh fruit |
Data Collection
Trained, master’s level research staff independent of POH conducted in-person survey interviews and anthropometric assessments, and coordinated blood draws (for participants with diabetes only). Survey topics broadly covered food security and nutrition, mental health and psychosocial outcomes, substance use, healthcare behaviors, and health status. Height and weight were measured. Phlebotomy was performed by certified phlebotomists using universal precautions. Participants were reimbursed $20 cash after each interview (including anthropometry) and $10 for completing the blood draw.
Measures
Nutritional Measures
We measured food security using the USDA Household Food Security Survey Module (HFSSM) [3, 32], which categorizes individuals as having high, marginal, low, or very low food security over the previous 6 months (Cronbach’s alpha 0.906). Diet quality was assessed using the 18-item Multifactor Screener [33], tailored to be relevant to low-income populations with HIV and/or diabetes. The screener assesses frequency of consumption of different types of foods in the previous 30 days; values were then converted to “times per day” participants consumed vegetables and fruits, protein, grains, starches, and dairy. We assessed height using a wall-mounted Seca stadiometer and weight using a Health O Meter scale (402KL). Body mass index (BMI) was then calculated as kg/m2.
Mental Health and Substance Use Measures
We assessed depressive symptoms using the Patient Health Questionnaire (PHQ-9) [34], with higher scores indicating higher depressive symptoms (probable depression ≥10). We assessed hazardous drinking using the Alcohol Use Disorders Identification Test (AUDIT-C) [35] (score >4 for men and >3 for women), and measured binge drinking as having five or more drinks at one occasion in the past 30 days. We also assessed current smoking (currently smoked every day, some days, or not at all) and illicit drug use (used crack/cocaine, methamphetamines, and/or heroin over the past 90 days (yes/no), and how many days in the past 90 days used any of these drugs).
Healthcare Behaviors
Competing demands were captured by asking how often the participant had to go without food because they needed the money for healthcare, or vice versa. We assessed acute-care utilization in the previous 90 days as the number of emergency department (ED) visits and hospitalizations as the number of overnight stays in a hospital bed.
HIV-Specific Measures
We assessed internalized HIV stigma using the negative self-image subscale of the HIV Stigma Scale [36, 37]; higher scores indicated higher levels of stigma (Cronbach’s alpha 0.875). Self-reported antiretroviral therapy (ART) adherence in the previous 7 days was assessed using the visual analog scale (VAS). Non-adherence was defined as <95% adherence [38, 39].
Diabetes-Specific Measures
We assessed diabetes-specific distress using the Diabetes Distress Scale (DDS) [40], with higher scores indicating higher levels of distress (Cronbach’s alpha 0.902). Diabetes self-efficacy was assessed using the Perceived Diabetes Self-Management Scale (PDSMS) [41]; higher scores indicated higher self-efficacy (Cronbach’s alpha 0.893). Fasting blood sugar and HbA1c were assessed via blood draw and analyzed by Quest Diagnostics using spectrophotometry and immunoturbidimetry, respectively. Higher levels of fasting glucose and HbA1c indicate worse current and longer-term glycemic control, respectively.
Demographics and Socioeconomic Status
We collected information on age, gender [42], race/ethnicity, non-US nativity, educational attainment, partnership status, location of receiving POH services, employment status, annual household income, savings, and receipt of government benefits.
Process Measures
We assessed program adherence (percentage of pick-ups attended by the participant or surrogate) using POH administrative records. Self-reported process outcomes included (1) reasons for missing pick-ups, (2) quantity of intervention food eaten, (3) prevalence and frequency of throwing away intervention food, (4) prevalence and frequency of sharing intervention food, and persons with whom food was shared, and (5) frequency and sources of non-intervention food eaten.
Analysis
We computed means and standard deviations for continuous variables, and proportions for categorical variables, separately for study completers and non-completers. We tested differences in baseline characteristics between these two groups using two sample t tests and χ 2 tests.
To compare pre-post changes in outcomes, we used paired t tests and McNemar exact tests with continuous and dichotomous outcomes, respectively. We compared pre-post outcomes for the study group as a whole, as well as by HIV and T2DM status. We considered a pre-post difference to be statistically significant at α = 0.05 for a one-tailed test. Stata 14 (Stata Corp., College Station, TX, USA) was used.
Ethics Statement
The Human Research Protection Program (HRPP) of the University of California, San Francisco (UCSF) approved the study. Participation in the study was voluntary and had no impact on the receipt of services from POH or participation in the intervention. Informed written consent was obtained from all participants.
Results
Sample Characteristics
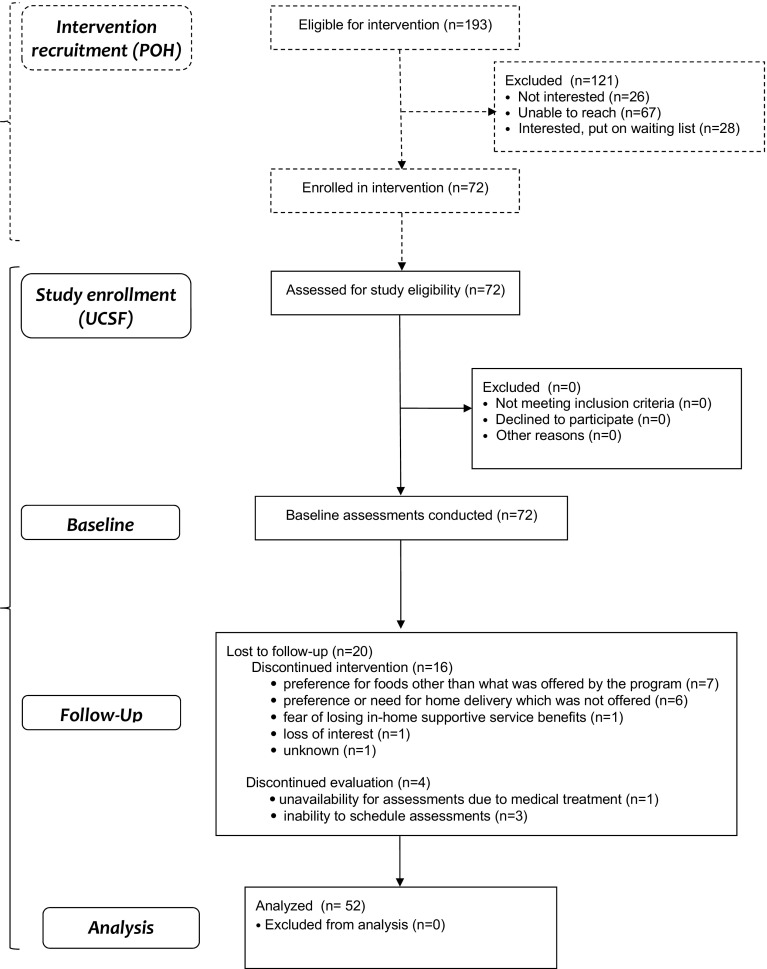
We completed baseline assessments on all 72 participants who initiated the Food = Medicine intervention. Of these, 56 (77.8%) completed the intervention, and 52 (72.2%) completed both the intervention and follow-up study assessments (Fig. 1). Compared to study completers, non-completers were significantly more likely to be based in Alameda County compared to San Francisco County, be of younger age, have lower educational attainment, and lower average income (Table 2).
Fig. 1.
Participant flow diagram
Table 2.
Characteristics of study participants at baseline by study completion status
| Characteristic | Completers (n = 52) | Non-completers (n = 20) | p value |
|---|---|---|---|
| HIV and T2DM diagnosisa, % | |||
| Has HIV (no T2DM) | 44.2 | 55.0 | 0.15 |
| Has T2DM (no HIV) | 42.3 | 25.0 | 0.38 |
| Has both HIV and T2DM | 13.5 | 20.0 | 0.45 |
| POH service location, % | |||
| Client in San Francisco County | 76.9 | 45.0 | 0.004 |
| Client in Alameda County (i.e., Oakland) | 23.1 | 55.0 | |
| Length of time POH client (months), median (IQR) | 212 (42.6, 560) | 153 (0.917, 475) | 0.734 |
| Age, median (IQR) | 57.2 (50.9, 60.7) | 52.9 (49.3, 56.6) | 0.016 |
| Gender, % | |||
| Male | 65.1 | 75.0 | 0.46 |
| Female | 26.9 | 15.0 | |
| TransFemale/Transwoman | 1.92 | 0 | |
| Other | 5.77 | 10.0 | |
| Race/ethnicityb, % | |||
| Native American | 9.62 | 5.00 | 0.40 |
| Asian/Pacific Islander | 1.92 | 5.00 | |
| Black/African American | 28.9 | 45.0 | |
| Hispanic/Latino | 21.2 | 30.0 | |
| White/Caucasian | 28.9 | 10.0 | |
| Other /Mixed | 9.62 | 5.00 | |
| Born outside the US, % | 21.2 | 30.0 | 0.51 |
| Education, % | |||
| Less than high school/GED | 13.5 | 25.0 | 0.014 |
| High school/GED | 17.3 | 40.0 | |
| More than high school/GED | 69.2 | 35.0 | |
| Housing, % | |||
| Apartment or house | 76.0 | 65.0 | 0.11 |
| SRO or nightly hotel | 20.0 | 25.0 | |
| Staying with friends or relatives | 2.00 | 10.0 | |
| Other | 2.00 | 0 | |
| Partnered (married, or committed relationship), % | 15.4 | 25.0 | 0.25 |
| Employed, % | 17.3 | 10.0 | 0.37 |
| Annual income, median (IQR) | $13,588 ($10,764, $20,000) | $10,500 ($9800, $11,556) | 0.021 |
| Less than $500 in savings, % | 73.1 | 95.0 | 0.09 |
| Receives SSI and/or SSDI, % | 65.4 | 70.0 | 0.89 |
| Receives SNAP/Cal-Fresh (i.e., food stamps), % | 21.6 | 35.0 | 0.1 |
T2DM type 2 diabetes mellitus, POH project open hand, GED general educational development, SRO single room occupancy, SSI supplemental security income, SSDI social security disability income, SNAP supplemental nutritional assistance program. P-values presented in italics are p<0.05.
aSample sizes by condition were n = 30 (HIV) and n = 29 (T2DM), including HIV/T2DM dually diagnosed individuals
bRace/ethnicity categories are not mutually exclusive
Among study completers, 23 individuals were living with HIV alone, 22 were living with T2DM alone, and 7 had a dual HIV/T2DM diagnosis (Table 2). Most participants identified as men (65.1%) and were between 50 and 64 years of age (71.2%). Less than one third of participants identified as white or Caucasian (28.9%). The majority had attained a high school degree/GED or higher (86.5%). Only 17.3% were employed; the majority were receiving either SSI and/or SSDI (65.4%), and 21.6% were receiving SNAP/CalFresh (i.e., “food stamps”). On average, the median time participants were POH clients before starting the intervention was 212 months, or about 17 years [interquartile range (IQR) 42.6, 560], but differed by condition. By condition, median months as a POH client were 435 [IQR 213, 569] (HIV) and 1.08 [IQR 0, 60.4] (T2DM).
Compared to participants living with HIV, those with T2DM were more likely to be older, female, African American, employed, and receiving SNAP benefits, and less likely to have a high school education and to be receiving SSI or SSDI (table not shown).
Changes during the Food = Medicine Intervention
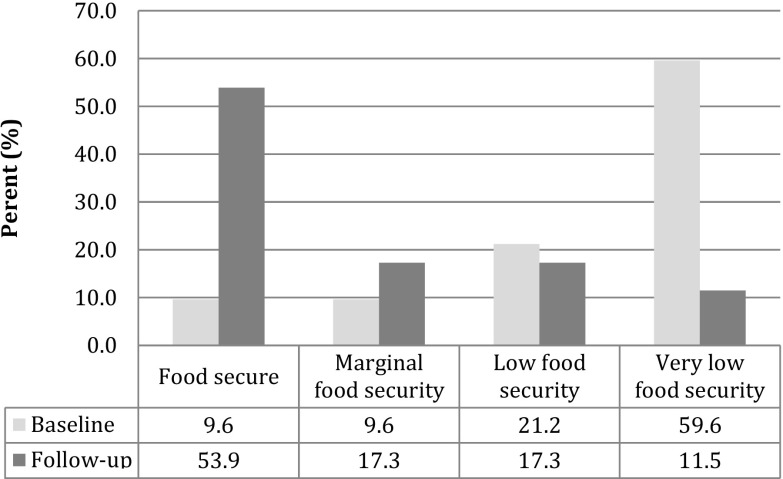
Over the course of the study, we observed a significant decrease in the severity of food insecurity experienced by participants (Fig. 2). Very low food security affected 59.6% of participants at baseline and only 11.5% at follow-up. Likewise, high food security was infrequent at baseline (9.62%) and experienced by the majority at follow-up (53.9%). Differences in food insecurity over time were statistically significant at p < 0.0001. The HIV and T2DM groups experienced similar changes in food insecurity.
Fig. 2.
Severity of food insecurity at baseline and follow-up (n = 52). Values are percents. Differences in food security categorization at baseline and follow-up statistically significant at p < 0.0001 (McNemar exact test)
Diet quality changed across several domains over the course of the study in the overall sample (Table 3). The frequency of consuming fatty foods decreased from 3.19 times per day to 2.21 times (p = 0.003), while the frequency of consuming fruits and vegetables increased from 1.85 to 2.34 times per day (p = 0.011). We observed a trend in decreased frequency of consumption of sugary foods (p = 0.07); however, this was only statistically significant in the T2DM group among whom consumption of sugary foods or drinks decreased from 0.994 to 0.650 times per day (p = 0.006) (diabetes-specific results not shown in table). In addition, average BMI decreased from 31.2 at baseline to 30.1 at follow-up (p = 0.08) in the overall group. Among participants with T2DM, BMI decreased from 36.1 at baseline to 34.8 at follow-up (p = 0.035); among participants with HIV, BMI did not change meaningfully from the baseline of 25.3 (disease-specific results not shown in table). While only one person in the overall sample was underweight at baseline, this individual was no longer underweight at follow-up.
Table 3.
Diet, mental health, and health behavior outcomes at baseline and follow-up (n = 52)
| Baseline | Follow-up | p valuea | |
|---|---|---|---|
| Diet quality (times consumed per day), mean (SD) | |||
| Fats | 3.19 (2.17) | 2.21 (1.5) | 0.003 |
| Proteins | 4.49 (2.38) | 4.89 (1.78) | 0.1 |
| Dairy | 1.63 (1.18) | 2.02 (1.13) | 0.009 |
| Grains and starches | 2.21 (1.36) | 3.02 (6.11) | 0.17 |
| Fruits and vegetables | 1.85 (1.54) | 2.34 (1.34) | 0.011 |
| Sugars | 1.95 (2.81) | 1.30 (1.69) | 0.07 |
| Body mass index | 31.2 (8.53) | 30.6 (8.39) | 0.08 |
| Mental health and substance use | |||
| Depressive symptoms (range 0–27), mean (SD) | 7.58 (6.92) | 5.84 (5.79) | 0.028 |
| Current smoking, % | 44.2 | 38.5 | 0.19 |
| Hazardous drinking, % | 17.3 | 13.5 | 0.31 |
| Binge drinking (≥5 drinks at same occasion in the past 30 days), % | 26.9 | 13.5 | 0.008 |
| Illicit drug use (in the last 3 months), % | 17.3 | 23.1 | 0.19 |
| Days of illicit drug use (in the last 3 months), mean (SD) | 4.96 (16.7) | 3.50 (11.8) | 0.24 |
| Competing demands | |||
| In previous 6 months, participant gave up… | |||
| Healthcare to spend time or money getting food, % | 34.6 | 19.2 | 0.029 |
| Food to spend money on healthcare (incl. transportation), % | 38.5 | 19.2 | 0.007 |
| Food to spend money on prescriptions, % | 28.9 | 15.4 | 0.046 |
| Acute-care utilization | |||
| Emergency department visits (≥1 in last 3 months), % | 26.9 | 17.3 | 0.15 |
| Hospitalizations (≥1 in past 3 months), % | 15.7 | 5.77 | 0.11 |
Values are means and standard deviations or percentages. T2DM type 2 diabetes mellitus
aData reported are p values based on paired data, i.e., data for individuals present at both baseline and follow-up. Paired t tests (for continuous variables) or McNemar test (for dichotomous variables) were used to compare outcomes across study assessments. This table does not include baseline data for 20 individuals who did not have follow-up data. Pvalues presented in italics are p<0.05.
Mental health and substance use also changed over the course of the study for the overall sample (Table 3). Compared to baseline, participants at follow-up had significantly fewer depressive symptoms (7.58 to 5.84; p = 0.028). Furthermore, participants reporting binge drinking decreased from 26.0 to 13.5% (p = 0.008). In addition, we observed decreases related to substance use that were not statistically significant, including decreased prevalence of hazardous drinking (17.3 to 13.5%, p = 0.31) and current smoking (44.2 to 38.5%, p = 0.19).
We investigated trends in competing needs and acute-care utilization between baseline and follow-up for the overall sample (Table 3). Over the previous 6 months, fewer participants reported giving up healthcare for food (decreased from 34.6 to 19.2%, p = 0.029), or giving up food to spend money on healthcare (decreased from 38.5 to 19.2%, p = 0.007) or spend money on prescriptions (decreased from 28.9 to 15.4%, p = 0.046). Although not statistically significant, there were decreased tendencies of having at least one hospitalization in the previous 3 months (15.7 to 5.77%, p = 0.11) and reporting at least one ED visit (26.9 to 17.3%, p = 0.15). In the DM group, hospitalizations fell from 25.0 to 6.90% (p = 0.09) and ED visits from 31.0 to 13.8% (p = 0.09). In the HIV group, hospitalizations fell from 10.0 to 3.33% (p = 0.31), while the number of ED visits did not change (disease-specific results not shown in Table 3).
Finally, we examined HIV- and T2DM-specific health outcomes (Table 4). Among participants with HIV, ARV medication adherence of 95% or greater increased from 46.7% of participants at baseline to 70.0% of participants at follow-up (p = 0.046). In addition, there was a decrease in internalized HIV stigma scores 12.2 to 11.5 (p = 0.21) which was not statistically significant.
Table 4.
HIV- and T2DM-specific study outcomes at baseline and follow-up
| Overall | |||
|---|---|---|---|
| Baseline | Follow-up | p valuea | |
| HIV-specific outcomes (n = 30) | |||
| Internalized HIV stigma (range 7–28]), mean (SD) | 12.2 (4.28) | 11.5 (4.10) | 0.21 |
| ART adherence ≥95%, % | 46.7 | 70.0 | 0.046 |
| T2DM-specific outcomes (n = 29) | |||
| Diabetes distress (range 1–6), mean (SD) | 2.64 (0.905) | 2.02 (0.777) | <0.001 |
| Perceived diabetes self-management score (range 8–40), mean (SD) | 24.8 (6.35) | 27.3 (6.73) | 0.007 |
| HbA1c %, mean (SD) | 9.23 (2.61) | 8.75 (1.95) | 0.41 |
| HbA1c <7% (optimal control), % | 10.3 | 19.2 | 0.08 |
| Fasting glucose, mean (SD) | 164 (86.1) | 151 (80.5) | 0.48 |
Values are means and standard deviations or percentages. ART antiretroviral, HbA1c glycated hemoglobin, HIV human immunodeficiency virus
aData reported are p values based on paired data, i.e., data for individuals present at both baseline and follow-up. Paired t tests (for continuous variables) or McNemar test (for dichotomous variables) were used to compare outcomes across study assessments. This table does not include baseline data for 15 individuals with HIV and 9 individuals with T2DM who did not have follow-up data.P-values presented in italics are p<0.05.
Among participants with T2DM, diabetes distress scores decreased from 2.64 to 2.02 (p < 0.001) and perceived diabetes self-management scores increased from 24.8 to 27.3 (p = 0.007). Finally, mean HbA1c was 9.23% at baseline and 8.75% at follow-up (p = 0.41), but this difference was not statistically significant. We also saw a higher prevalence of optimal glycemic control (defined as HbA1c <7%) [10.3 to 19.2%; p = 0.08].
Process Outcomes
The median adherence to food pick-ups was 93% based on administrative records (IQR 83, 100) (Table 5). The most common reasons participants reported for missing a pick-up were being too sick or injured to pick up food (38.5%), arrived too late (28.8%), having a healthcare appointment (25.0%), having a surrogate that did not follow through on the pick-up (21.2%), and having a logistical (not financial) transportation problem (19.2%). While almost 78.9% of participants reported eating all or most of the intervention food in a usual week, 90.4% reported throwing away intervention food at some point, 84.6% reported ever sharing intervention food with others, and 57.8% reported sharing intervention food at least once per week. Among participants who shared intervention food, they most commonly shared with friends (56.8%), neighbors (52.3%), and a spouse or partner (25.0%). Finally, almost 78.9% reported eating non-intervention foods at least once per week; only 3.9% reported never eating non-intervention food. Among those who consumed non-intervention foods, the most commonly consumed non-intervention foods were sweet snacks or desserts (76.9%), fast food (75.0%), vegetables (75.0%), and fruits (71.2%).
Table 5.
Process outcomes at follow-up
| Intervention food pick-up adherence and access | |
| Pick-up adherence (% of food pick-ups attended)a, median (IQR) | 93 (83, 100) |
| When missed a pick-up, the reason wasb: | |
| I was injured, or too sick to pick up food, % | 38.5 |
| I was running late and arrived after pick-up had ended, % | 28.8 |
| I had a healthcare appointment, % | 25.0 |
| My surrogate did not follow through in picking up food, % | 21.2 |
| I had a transportation problem (e.g., bus didn’t come), % | 19.2 |
| Intervention food utilization | |
| Ate “all” or “most” intervention food in a usual week, % | 78.9 |
| Ever threw away food, % | 90.4 |
| % of food thrown away in an average week, median (IQR) | 10 (5, 18) |
| Ever shared food with others, % | 84.6 |
| Shared food at least once per week, % | 57.8 |
| Among those sharing intervention food, the food was shared withc, %: | |
| Friend | 56.8 |
| Neighbor | 52.3 |
| Spouse or partner | 25.0 |
| Household member other than partner, including roommate | 15.9 |
| Family member that lives outside of your household | 13.6 |
| Non-intervention food eaten during the study | |
| Ate non-intervention foods at least once per week, % | 78.9 |
| Types of non- intervention food eaten during the study, %: | |
| Sweet snacks or desserts | 76.9 |
| Fast food, including hotdogs, pizza, etc. | 75.0 |
| Vegetables | 75.0 |
| Fruits | 71.2 |
| Sodas | 65.4 |
| Salty snacks | 57.7 |
| Foods from my culture | 48.1 |
Values are medians and interquartile ranges or percents
aBased on administrative records
bTop five reasons for missing a pick-up. Less common reasons included: I had enough food already (15%), coming twice a week was hard for me (13%), I was too busy to pick up food (12%), and I forgot (8%), among others
cFive most common types of people participants reported sharing intervention food with. Other less common answers included homeless individuals in their neighborhood (8%), a sexual partner other than a spouse (8%), and dependent children (4%)
Discussion
Provision of comprehensive, medically appropriate food assistance was feasible for both HIV and diabetes. Our results show that providing this assistance may improve outcomes for both conditions in food-insecure populations. Further rigorous testing of the intervention is needed to provide evidence as to whether policies to promote medically appropriate food assistance in chronically ill, economically distressed populations are merited.
The Food = Medicine intervention is part of a broader, nationwide “Food is Medicine” coalition [43] to mobilize food and nutrition safety-net programs and policies to mitigate the negative health, quality of life, and economic impacts of chronic illness. Nutrition-focused HIV service organizations are central in this coalition, supported by dedicated federal healthcare funding for nutritional wrap-around services via the Ryan White Care Act. The coalition includes organizations serving individuals with many life-threatening and/or chronic illnesses, particularly conditions for which access to a healthy diet is paramount, such as diabetes and heart disease. Rigorous evaluation of medically tailored meal services that may have concrete health impacts across diverse conditions is needed, especially given expanding opportunities to provide or reimburse nutrition services (including food) via Medicaid, Medicare, and private insurance under the Affordable Care Act [28].
Over 6 months, we observed significant improvements in food security and in outcomes related to all three mechanisms through which we posited food insecurity may impact HIV and diabetes health (nutritional, mental health, and behavioral). We observed dramatic improvements in depression, diabetes distress, diabetes self-management, trading-off between food and healthcare, and HIV medication adherence. Despite insufficient power to detect improvements in HbA1c or acute-care utilization, our results suggested possible improvements in glycemic control and reduced hospitalizations and ED visits among participants with T2DM.
While a growing literature has documented the mostly positive impacts of food assistance on medication adherence and other outcomes for people with HIV in low-income countries [44, 45], the USA and other high-income countries lack parallel studies. Among people with diabetes, a recent prospective study in the USA suggests that medically appropriate “food boxes” tailored for a diabetic diet, provided together with diabetes self-management and linkage to care, may improve glycemic control and other markers of diabetes health, including fruit and vegetable intake, self-management, and medication adherence [29]. The study observed improvements in diabetes distress and HbA1c of similar magnitude as our study. Two observational studies showed that medically appropriate food support may be associated with fewer missed appointments [46] as well as decreased acute-care utilization and healthcare costs [47]. Our study is one of the first to prospectively evaluate comprehensive, medically appropriate food assistance for people with chronic illness in high-income countries.
The focus on medically appropriate food assistance embodied by Food = Medicine and other similar interventions is especially salient given the need to address concurrent food insecurity and obesity in chronically ill populations. HIV-specific food assistance in resource-poor settings is generally tailored for underweight populations, although treated populations with HIV increasingly have higher BMIs [48, 49]. We previously showed that providing energy-dense forms of food assistance to overweight or obese, food-insecure individuals with treated HIV infection leads to weight gain [50], increasing the risk for chronic comorbidities. In contrast, our study suggests that providing three meals a day plus snacks that meet 100% of daily energy requirements while meeting guidelines for a heart-healthy, diabetes diet (for all participants) may result in weight loss or no change in weight among individuals with T2DM.
Society incurs high costs from uncontrolled chronic disease such as T2DM [51], particularly among low-income, food-insecure, and/or underinsured populations who are more likely to delay care, use the emergency room, and require hospital inpatient services [52]. Identifying less resource-intensive ways to help individuals manage their illnesses in the context of competing subsistence needs is therefore critical for the optimal use of public funds. A retrospective study conducted by the Metropolitan Area Neighborhood Nutrition Alliance (MANNA) compared chronically ill clients receiving three meals a day for 6 months with a similar group of Medicaid patients. They found that monthly healthcare costs were 28% lower and average inpatient costs were 30% lower among MANNA clients receiving the meal intervention compared to Medicaid patients not receiving meals [47]. In our study, food and packaging costs for the Food = Medicine intervention were $6.58 a day per participant ($1184 for 6 month intervention) in contrast to $2774, the cost per inpatient day in a California hospital [53]. While these preliminary studies are promising, formal, rigorously designed cost-effectiveness studies are needed to assess the economic value of medically appropriate food assistance for chronically ill populations.
As with any pre-post study without a comparison group, observed improvements in our study may be due to external factors or represent preexisting trends, rather than represent a change due to the intervention. Nevertheless, we believe it is plausible that improvements are attributable at least in part to the intervention. The external environment for low-income individuals in the San Francisco Bay Area during the time of our study was one of economic crisis characterized by an overwhelmingly high cost of living and severe housing shortage, compromising access to basic needs [54]. Thus, it is unlikely that the external environment was fully responsible for the observed improvements in health and well-being during the study. In addition, plausibility is enhanced by our observation of improvements related to each of the mechanisms (nutritional, mental health, and behavioral) linking food security to health posited by our conceptual framework. To obtain more conclusive data, however, it is critical to formally test the impact of comprehensive, medically appropriate food assistance using a rigorous, randomized controlled design.
In addition to the pre-post design, our study has additional limitations. Most HIV participants in our study were long-standing clients of POH while most T2DM participants were new clients. Thus, the transition from no services directly to a comprehensive nutrition intervention for participants with T2DM is likely to explain the greater improvements in several outcomes in this group. In addition, POH selected intervention participants with relatively high adherence to POH regular services to maximize likely exposure to the intervention. This selection process applied primarily to clients with HIV who had a longer history at POH to assess adherence. This group may have had greater housing stability, lower life chaos, less mental illness, and less drug addiction than less adherent clients. This may have either biased our results toward the null if excluded individuals had greater need for the intervention or away from the null because intervention adherence was high. Social desirability could have affected participants’ responses. Due to the small size of our study, we were underpowered to detect changes in a number of study outcomes, including diabetes control (HbA1c and fasting glucose), and hospitalizations. Furthermore, we did not collect laboratory measures of CD4 cell count or HIV viral load, and therefore could not directly assess the change in HIV clinical outcomes. Finally, our use of a brief diet quality instrument did not allow us to collect detailed information on diet composition or measure precise dietary intakes.
Our study provides initial support to the proposition that “Food is Medicine” may be an effective, low-cost strategy to improve health in vulnerable populations. By preventing worsened illness and acute illness episodes, medically appropriate food support may reduce societal healthcare costs [47] as well as prevent further impoverishment of critically ill individuals. The ultimate goal is to move toward greater health equity by disrupting the cycle of food insecurity and poor health created by the syndemics of poverty and chronic illness.
Acknowledgements
We deeply thank our research participants for sharing their experiences and time with this study. We also thank the Project Open Hand staff and Food = Medicine leadership team for their hard work, dedication, and collaboration. Finally, we thank student interns Irene Ching and Ajikarunia Palar for assisting with data collection, preliminary analyses, and data entry.
Abbreviations
- ARV
Antiretroviral
- ED
Emergency department
- GED
General Educational Development
- HbA1c
Glycated hemoglobin
- HFSSM
Household Food Security Survey Module
- PDSMS
Perceived Diabetes Self-Management Scale
- POH
Project Open Hand
- SNAP
Supplemental Nutrition Assistance Program
- SSDI
Social Security Disability Income
- SRO
Single room occupancy
- SSI
Supplemental Security Income
- T2DM
Type 2 diabetes mellitus
- UCSF
University of California, San Francisco
Compliance with Ethical Standards
Financial Support
Project Open Hand (POH) provided funding for this study as an external evaluation, but had no role in data collection or analysis, nor in scientific interpretation of the data. Funding from Burke Global Health supported student and intern participation in data collection. The authors acknowledge the following sources of salary support: NIH/NIDDK K01DK107335 (Dr. Palar) and NIH/NIMH R01MH095683 (Dr. Weiser).
Conflict of Interest
K Palar, T Napoles, LL Hufstedler, H Seligman, FM Hecht, EA Frongillo, and SD Weiser report no conflicts of interest. M Ryle and K Madsen are current POH employees; S Pitchford was a POH employee at the time of the study.
Footnotes
Kartika Palar and Tessa Napoles share co-first authorship.
References
- 1.Ivers LC, ed. Food Insecurity and Public Health. Boca Raton, FL: CRC Press, Taylor & Francis Group; 2015.
- 2.Seligman HK, Schillinger D. Hunger and socioeconomic disparities in chronic disease. N Engl J Med. 2010;363(1):6–9. doi: 10.1056/NEJMp1000072. [DOI] [PubMed] [Google Scholar]
- 3.Coleman-Jensen A, Rabbitt M, Gregory C, Singh A. Household food security in the United States in 2015. ERR-215: United States Department of Agriculture, Economic Research Service 2016.
- 4.Seligman HK, Bindman AB, Vittinghoff E, et al. Food insecurity is associated with diabetes mellitus: results from the National Health Examination and Nutrition Examination Survey (NHANES) 1999–2002. J Gen Intern Med. 2007;22(7):1018–1023. doi: 10.1007/s11606-007-0192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seligman HK, Laraia BA, Kushel MB. Food insecurity is associated with chronic disease among low-income NHANES participants. J Nutr. 2010;140(2):304–310. doi: 10.3945/jn.109.112573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palar K, Laraia B, Tsai AC, Johnson M, Weiser SD. Food insecurity is associated with HIV, sexually transmitted infections and drug use among men in the United States. AIDS. 2016; 30(9):1457–1465. [DOI] [PMC free article] [PubMed]
- 7.Vogenthaler NS, Kushel MB, Hadley C, et al. Food Insecurity and Risky Sexual Behaviors Among Homeless and Marginally Housed HIV-Infected Individuals in San Francisco. AIDS and Behavior. 2012:1–6. [DOI] [PMC free article] [PubMed]
- 8.Shannon K, Kerr T, Milloy M-J, et al. Severe food insecurity is associated with elevated unprotected sex among HIV-seropositive injection drug users independent of HAART use. AIDS. 2011;25(16):2037–2042. doi: 10.1097/QAD.0b013e32834b35c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalichman S, Cherry C, Amaral C, et al. Health and treatment implications of food insufficiency among people living with HIV/AIDS, Atlanta. Georgia. J Urban Health. 2010;87(4):1–11. doi: 10.1007/s11524-010-9446-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiser SD, Yuan C, Guzman D, et al. Food insecurity and HIV clinical outcomes in a longitudinal study of homeless and marginally housed HIV-infected individuals in San Francisco. AIDS. 2013;27(18):2953–2958. doi: 10.1097/01.aids.0000432538.70088.a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMahon JH, Wanke CA, Elliott JH, et al. Repeated assessments of food security predict CD4 change in the setting of antiretroviral therapy. JAIDS J Acquir Immune Defic Syndromes. 2011;58(1):60–63. doi: 10.1097/QAI.0b013e318227f8dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiser S, Frongillo EA, Ragland K, et al. Food insecurity is associated with incomplete HIV RNA suppression among homeless and marginally housed HIV-infected individuals in San Francisco. J Gen Intern Med. 2009;24(1):14–20. doi: 10.1007/s11606-008-0824-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang EA, McGinnis KA, Fiellin DA, et al. Food Insecurity is associated with poor virologic response among HIV-infected patients receiving antiretroviral medications. J Gen Intern Med. 2011;26(9):1012–1018. doi: 10.1007/s11606-011-1723-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiser S, Tsai AC, Gupta R, et al. Food insecurity is associated with morbidity and patterns of healthcare utilization among HIV-infected individuals in rural Uganda. AIDS. 2012;26(1):67–75. doi: 10.1097/QAD.0b013e32834cad37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiser S, Fernandes K, Brandson E, et al. The association between food insecurity and mortality among HIV-infected individuals on HAART. J Acquir Immune Defic Syndr. 2009;52(3):342–349. doi: 10.1097/QAI.0b013e3181b627c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seligman HK, Davis TC, Schillinger D, et al. Food insecurity is associated with hypoglycemia and poor diabetes self-management in a low-income sample with diabetes. J Health Care Poor Underserved. 2010;21(4):1227. doi: 10.1353/hpu.2010.0921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seligman HK, Jacobs EA, López A, et al. Food insecurity and glycemic control among low-income patients with type 2 diabetes. Diabetes Care. 2012;35(2):233–238. doi: 10.2337/dc11-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nelson K, Brown ME, Lurie N. Hunger in an adult patient population. JAMA. 1998;279(15):1211–1214. doi: 10.1001/jama.279.15.1211. [DOI] [PubMed] [Google Scholar]
- 19.Weiser SD, Young SL, Cohen CR, et al. Conceptual framework for understanding the bidirectional links between food insecurity and HIV/AIDS. Am J Clin Nutr. 2011;94(suppl):1729S–1739S. doi: 10.3945/ajcn.111.012070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiser SD, Palar K, Hatcher A, Young S, Frongillo EA, Laraia B. Food Insecurity and Health: A Conceptual Framework. In: Ivers LC, ed. Food Insecurity and Public Health: CRC Press; 2015:23–50.
- 21.Smith R, Rossetto K, Peterson B. A meta-analysis of disclosure of one’s HIV-positive status, stigma and social support. AIDS Care. 2008;20(10):1266–1275. doi: 10.1080/09540120801926977. [DOI] [PubMed] [Google Scholar]
- 22.Darmon N, Drewnowski A. Contribution of food prices and diet cost to socioeconomic disparities in diet quality and health: a systematic review and analysis. Nutr Rev. 2015;73(10):643–660. doi: 10.1093/nutrit/nuv027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DiSantis KI, Grier SA, Odoms-Young A, et al. What “price” means when buying food: insights from a multisite qualitative study with Black Americans. Am J Public Health. 2013;103(3):516–522. doi: 10.2105/AJPH.2012.301149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drewnowski A, Aggarwal A, Hurvitz PM, et al. Obesity and supermarket access: proximity or price? Am J Public Health. 2012;102(8):e74–80. doi: 10.2105/AJPH.2012.300660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weinfield NS, Mills G, Borger C, et al. Hunger in America 2014: National Report Prepared for Feeding America. Westat and the Urban Institute for Feeding America 2014.
- 26.California – 2015 State Health Profile: HIV/AIDS Epidemic: CDC National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention; 2015.
- 27.Trenkamp B, Wiseman M. The Food Stamp Program and Supplemental Security Income, Social Security Bulletin, Vol. 67, No. 4. Office of Disability and Income Assistance Policy, Office of Retirement and Disability Policy, Social Security Administration 2007. [PubMed]
- 28.Ellwood M, Downer S, Broad Leib E, et al. Food Is Medicine: Opportunities in Public and Private Health Care for Supporting Nutritional Counseling and Medically-Tailored. Home-Delivered Meals, Harvard Law School, Center For Health Law & Policy Innovation. 2014.
- 29.Seligman HK, Lyles C, Marshall MB, et al. A pilot food bank intervention featuring diabetes-appropriate food improved glycemic control among clients in three states. Health Aff (Millwood) 2015;34(11):1956–1963. doi: 10.1377/hlthaff.2015.0641. [DOI] [PubMed] [Google Scholar]
- 30.Martin KS, Wu R, Wolff M, et al. A novel food pantry program: food security, self-sufficiency, and diet-quality outcomes. Am J Prev Med. 2013;45(5):569–575. doi: 10.1016/j.amepre.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 31.Habicht JP, Victora CG, Vaughan JP. Evaluation designs for adequacy, plausibility and probability of public health programme performance and impact. Int J Epidemiol. 1999;28(1):10–18. doi: 10.1093/ije/28.1.10. [DOI] [PubMed] [Google Scholar]
- 32.Bickel G, Nord M, Price C, Hamilton W, Cook J. Guide to Measuring Household Food Security, Revised 2000. Alexandria, VA: U.S. Department of Agriculture, Food and Nutrition Service; 2000.
- 33.Thompson FE, Midthune D, Subar AF, et al. Performance of a short tool to assess dietary intakes of fruits and vegetables, percentage energy from fat and fibre. Public Health Nutr. 2004;7(8):1097–1105. doi: 10.1079/PHN2004642. [DOI] [PubMed] [Google Scholar]
- 34.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bradley KA, DeBenedetti AF, Volk RJ, et al. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcohol Clin Exp Res. 2007;31(7):1208–1217. doi: 10.1111/j.1530-0277.2007.00403.x. [DOI] [PubMed] [Google Scholar]
- 36.Bunn JY, Solomon SE, Miller C, et al. Measurement of stigma in people with HIV: a reexamination of the HIV Stigma Scale. AIDS Educ Prev. 2007;19(3):198–208. doi: 10.1521/aeap.2007.19.3.198. [DOI] [PubMed] [Google Scholar]
- 37.Berger BE, Ferrans CE, Lashley FR. Measuring stigma in people with HIV: psychometric assessment of the HIV stigma scale. Res Nurs Health. 2001;24(6):518–529. doi: 10.1002/nur.10011. [DOI] [PubMed] [Google Scholar]
- 38.Garcia de Olalla P, Knobel H, Carmona A, et al. Impact of adherence and highly active antiretroviral therapy on survival in HIV-infected patients. J Acquir Immune Defic Syndr. 2002;30(1):105–110. doi: 10.1097/00042560-200205010-00014. [DOI] [PubMed] [Google Scholar]
- 39.Bangsberg DR, Perry S, Charlebois ED, et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS. 2001;15(9):1181–1183. doi: 10.1097/00002030-200106150-00015. [DOI] [PubMed] [Google Scholar]
- 40.Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28(3):626–631. doi: 10.2337/diacare.28.3.626. [DOI] [PubMed] [Google Scholar]
- 41.Wallston KA, Rothman RL, Cherrington A. Psychometric properties of the perceived diabetes self-management scale (PDSMS) J Behav Med. 2007;30(5):395–401. doi: 10.1007/s10865-007-9110-y. [DOI] [PubMed] [Google Scholar]
- 42.Center of Excellence for Transgender Health. Recommendations for Inclusive Data Collection of Trans People in HIV Prevention, Care & Services. University of California, San Francisco. Available at: http://transhealth.ucsf.edu/trans?page=lib-data-collection. Accessed July 22, 2016.
- 43.Food is Medicine: Achieving the Triple Aim through Medically Tailored Nutrition Congressional Briefing. The Food is Medicine Coalition. Washington, DC; March 16, 2016.
- 44.Martinez H, Palar K, Linnemayr S, et al. Tailored nutrition education and food assistance improve adherence to HIV antiretroviral therapy: evidence from Honduras. AIDS Behav. 2014;18(5S):566–577. doi: 10.1007/s10461-014-0786-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cantrell R, Sinkala M, Megazinni K, et al. A pilot study of food supplementation to improve adherence to antiretroviral therapy among food-insecure adults in Lusaka, Zambia. JAIDS J Acquir Immune Defic Syndr. 2008;49(2):190–195. doi: 10.1097/QAI.0b013e31818455d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aidala A, Yomogida M, Vardy Y. Food and Nutrition Services, HIV Medical Care, and Health Outcomes: Community Health Advisory & Information Network (CHAIN), Mailman School of Public Health, Columbia University.
- 47.Gurvey J, Rand K, Daugherty S, et al. Examining health care costs among MANNA clients and a comparison group. J Prim Care Community Health. 2013;4(4):311–317. doi: 10.1177/2150131913490737. [DOI] [PubMed] [Google Scholar]
- 48.Amorosa V, Synnestvedt M, Gross R, et al. A tale of 2 epidemics: the intersection between obesity and HIV infection in Philadelphia. JAIDS J Acquir Immune DeficSyndr. 2005;39(5):557. [PubMed] [Google Scholar]
- 49.Crum-Cianflone N, Roediger MP, Eberly L, et al. Increasing rates of obesity among HIV-infected persons during the HIV epidemic. PLoS One. 2010;5(4):e10106. doi: 10.1371/journal.pone.0010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palar K, Derose K, Linnemayr S, et al. Impact of food support on food security and body weight among HIV antiretroviral recipients in Honduras: a pilot intervention trial AIDS Care. 2015; 27(4): 409–415. [DOI] [PMC free article] [PubMed]
- 51.Li R, Bilik D, Brown MB, et al. Medical costs associated with type 2 diabetes complications and comorbidities. Am J Manag Care. 2013;19(5):421–430. [PMC free article] [PubMed] [Google Scholar]
- 52.Weiser SD, Hatcher A, Frongillo EA, et al. Food insecurity is associated with greater acute care utilization among HIV-infected homeless and marginally housed individuals in San Francisco. J Gen Intern Med. 2013;28(1):91–98. doi: 10.1007/s11606-012-2176-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaiser Family Foundation. 2014 Hospital Adjusted Expenses per Inpatient Day by Ownership. Available at: http://kff.org/other/state-indicator/expenses-per-inpatient-day-by-ownership/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. Accessed Sept. 14, 2016.
- 54.Whittle HJ, Palar K, Hufstedler LL, et al. Food insecurity, chronic illness, and gentrification in the San Francisco Bay Area: an example of structural violence in United States public policy. Soc Sci Med. 2015;143:154–161. doi: 10.1016/j.socscimed.2015.08.027. [DOI] [PubMed] [Google Scholar]