Abstract
Volumetric analysis of the kidney parenchyma provides additional information for the detection and monitoring of various renal diseases. Therefore the purposes of the study were to develop and evaluate a semi-automated segmentation tool and a modified ellipsoid formula for volumetric analysis of the kidney in non-contrast T2-weighted magnetic resonance (MR)-images. Three readers performed semi-automated segmentation of the total kidney volume (TKV) in axial, non-contrast-enhanced T2-weighted MR-images of 24 healthy volunteers (48 kidneys) twice. A semi-automated threshold-based segmentation tool was developed to segment the kidney parenchyma. Furthermore, the three readers measured renal dimensions (length, width, depth) and applied different formulas to calculate the TKV. Manual segmentation served as a reference volume. Volumes of the different methods were compared and time required was recorded. There was no significant difference between the semi-automatically and manually segmented TKV (p = 0.31). The difference in mean volumes was 0.3 ml (95% confidence interval (CI), −10.1 to 10.7 ml). Semi-automated segmentation was significantly faster than manual segmentation, with a mean difference = 188 s (220 vs. 408 s); p < 0.05. Volumes did not differ significantly comparing the results of different readers. Calculation of TKV with a modified ellipsoid formula (ellipsoid volume × 0.85) did not differ significantly from the reference volume; however, the mean error was three times higher (difference of mean volumes −0.1 ml; CI −31.1 to 30.9 ml; p = 0.95). Applying the modified ellipsoid formula was the fastest way to get an estimation of the renal volume (41 s). Semi-automated segmentation and volumetric analysis of the kidney in native T2-weighted MR data delivers accurate and reproducible results and was significantly faster than manual segmentation. Applying a modified ellipsoid formula quickly provides an accurate kidney volume.
Keywords: Clinical application, Evaluation research, Image analysis, Magnetic resonance imaging, Radiology workflow, Segmentation, Semi-automated, Kidney, Ellipsoid
Introduction
The prevalence of chronic kidney disease is rising, especially in developed countries. The costs for treatment are a constant challenge for public healthcare [1–5]. There is an urgent need for fast, reliable, and cheap biomarkers for the evaluation of renal function that can open the gates to new therapies.
It is widely known that the number and volume of glomeruli give information about the individual predisposition for the development of kidney disease and hypertension [6, 7]. Although no in vivo method for determining the number and size of glomeruli currently exists, the volume of the renal cortex or the volume of the entire renal parenchyma could possibly be used as a surrogate marker for the number or the size of the glomeruli. Therefore, kidney volumes are a relevant parameter for epidemiological studies. Segmentation and volumetric analysis of the kidney parenchyma also provides additional information for the detection and monitoring of renal diseases, such as nephritis or hydronephrosis [8]. A simple estimation for the volume of the kidney can be obtained from length measurements of the kidney using different imaging modalities such as sonography, computed tomography (CT), or magnetic resonance (MR) imaging. Previously published articles show that an ellipsoid formula applied to ultrasound measurements tends to underestimate the renal volume [9, 10]. MR examinations provide anatomical images with high spatial resolution and, therefore, are suitable for volumetric assessment. In recent years, dynamic contrast-enhanced sequences have been used for the analysis of renal volume [11, 12]. The use of a contrast agent leads to an improved contrast between the renal cortex and medulla within the first minute after injection. In vivo measurements in animals and humans have demonstrated good differentiation between the entire kidneys and surrounding tissue [13–15]. In patients with severe renal insufficiency, the administration of contrast media is associated with the potential risk to cause nephrogenic systemic fibrosis. Therefore, the proposed segmentation tool was assessed with native MR images that usually have a limited contrast between the kidneys and the surrounding tissue, as well as the inner structures [15–17]. Hence, the potential to develop highly accurate semi-automated kidney segmentation in native MRI sequences is of interest.
The volume of internal organs is seldom determined in clinical practice because manual segmentation is time consuming. Therefore, tools for automated or semi-automated segmentation are needed. Different methods, such as thresholding, clustering, region growing, contour detection, or their combinations have been proposed [13, 18–21]. For identification of the entire kidneys, MR images should offer a high contrast between the kidney parenchyma and the surrounding tissue (liver, spleen, gastrointestinal tract, muscle). However, in clinical practice, MR imaging of the abdomen does not include customized sequences for segmentation purposes.
Therefore, the purposes of this work are to develop a semi-automated segmentation tool and a modified ellipsoid formula for volumetric analysis of the kidney in non-contrast T2-weighted MR images and to evaluate the methods for their accuracy, precision, and time effort.
Materials and Methods
Patient Characteristics
This study included 24 healthy subjects (11 males, 13 females). The mean age of the subjects was 26 years (range 21–41 years). The mean body mass index for subjects was 21.8 kg/m2 (range 18.9–24.8 kg/m2). The study recruitment began on July 2015 and was completed using advertisements in local newspapers. The institutional review board of the University Hospital Erlangen/Germany approved the study. All study procedures that involved human participants were performed in accordance with the ethical standards of the institutional and/or national research committee using the 1964 Helsinki Declaration and its amendments and comparable ethical standards. Informed consent was obtained from all individual participants included in the study. MR examinations were performed in the Department of Radiology of the University Hospital Erlangen, Germany.
MR Imaging
In vivo measurements of healthy subjects were performed with a 1.5 T MR scanner (Magnetom Avanto, Siemens Healthcare GmbH, Erlangen, Germany) with the following parameters: T2 TSE sequence, without fat-suppression, orientation: transversal, TR 6206 ms, TE 88 ms, bandwidth 260 Hz/px, acquisition matrix 328 × 288 px, voxel size 0.98 × 0.98 × 4.4 mm3, spacing between slices 20%.
Manual Image Segmentation
The image segmentation was performed with Photoshop Extended (Version CS6, Adobe Systems, San Jose, CA, USA). Photoshop was chosen as the reference standard because every pixel is displayed as a single pixel with clear margins and not blurred with its surroundings to upscale to a virtual higher resolution. Images and metadata-like dimensions and data resolution were imported using the “DICOM File Import” dialog box. The entire kidney parenchyma was segmented from the surrounding tissues manually on the T2-weighted MR images using knowledge about the shape, location, and structure of the kidney. The contours of both kidneys were carefully drawn manually in each slice for each volunteer. Manual segmentation was performed by a final year medical student. A board-certified radiologist (6 years of work experience (M.H.)) supervised, verified, and corrected the segmentation where necessary. These delineations were considered as the reference volume outline. Manual segmentation was done with an Intel Quad Core at 3.6 GHz CPU.
Semi-Automated Segmentation
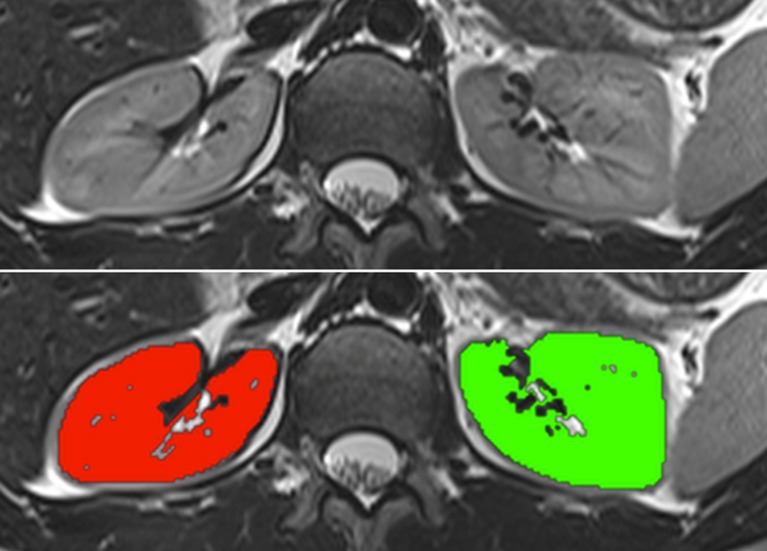
For semi-automated segmentation, dedicated threshold-based software (OsiriX Plugin) was developed by the authors. The software is obtainable from the Chimaera GmbH (Erlangen, Germany, http://www.chimaera.de/chimaera/home.html). The software contains a brush tool which performs a region analysis and determines the mean value inside a pre-defined stencil. Depending on the segmentation mode defined by the user, all pixels within the pre-set threshold above or below the mean value are potentially taken as candidate pixels to be segmented. A “high intensity” segmentation mode expects that the anatomy to be segmented consists of higher intensity values compared to surrounding tissue. A “low intensity” mode works in reverse. In order to process the noise reduction, a morphological filter is applied on the pixels inside the brush stencil in order to close “holes” inside the region of interest. Furthermore, a connected region analysis is performed to ensure that only connected pixels are selected. The computation of these operations is done in real-time. The method operates on DICOM images. An example of a non-contrast T2-weighted MR sequence showing both kidneys before and after semi-automated segmentation is shown in Fig. 1.
Fig. 1.
An example of a non-contrast T2-weighted magnetic resonance sequence showing both kidneys before (upper) and after threshold-based semi-automated segmentation (lower)
Three readers (one final year medical student and two radiology residents with 3 years of work experience) were instructed to segment the entire parenchyma of the kidney (cortex and medulla) in the axial images. The pelvis of the kidney was excluded. The center and window were adjusted by the readers to fit their preferences. For every kidney, a new segmentation was created, stored, and automatically analyzed. The segmentation time was recorded manually by the reader. To assess reproducibility, semi-automated segmentation was performed twice by every reader without knowledge of the results of their first segmentation. There was a 14-day interval between the two evaluations. The initial instruction of the readers took roughly 10 min. There were no test cases. Semi-automated segmentation was done with an Intel Quad Core 2.93 GHz CPU.
Measurement of Kidney Length, Width and Depth
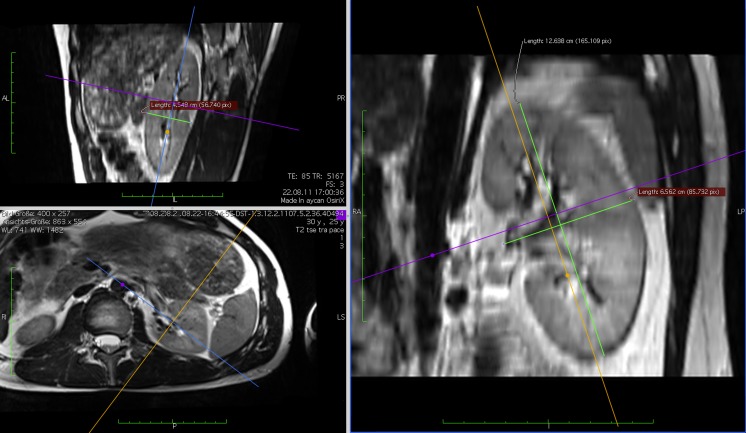
The length (L), width (W), and depth (D) of each kidney were measured in the “3-D MPR” workflow in OsiriX by all three readers. The axes of the multi-planar reconstruction (MPR) were tilted to fit the renal orientation in situ. Measurements were performed parallel to the tilted axes. Exemplary measurements of a left kidney are shown in Fig. 2.
Fig. 2.
The length, width, and depth of each kidney were measured in 3-D MPR. Axes of the multi-planar reconstruction (MPR) were tilted to fit the renal orientation in situ
Evaluation
For evaluation of the agreement of the optimized algorithm of the semi-automated segmentation and the reference volume, the volume error was calculated. The reliability between different readers and repeatability of two measurements performed by the same reader were calculated.
The following formulas for the volumetric analysis of renal dimensions were applied to the measurements.
Ellipsoid volume: V E = π/6 × L × W × D
Spheroid volume: V Sp = π/6 × L × D 2
Modified ellipsoid volume by linear regression: V ME = α + β × (L × W × D)
The agreement between the reference volume and measured volume was calculated.
Statistical Analysis
The data are expressed as mean ± standard deviation. Data were checked for normal distribution using the Shapiro-Wilk test. Measurements were analyzed by two-tailed one-sample Student’s t test. P-values and confidence intervals (95% confidence level) were calculated using SPSS software (SPSS Statistics v 20, IBM, Armonk, USA). Throughout the analysis, a two-sided p value of less than 0.05 was considered statistically significant.
Results
On average, both kidneys were displayed, using 25.6 images (range 20 to 30).
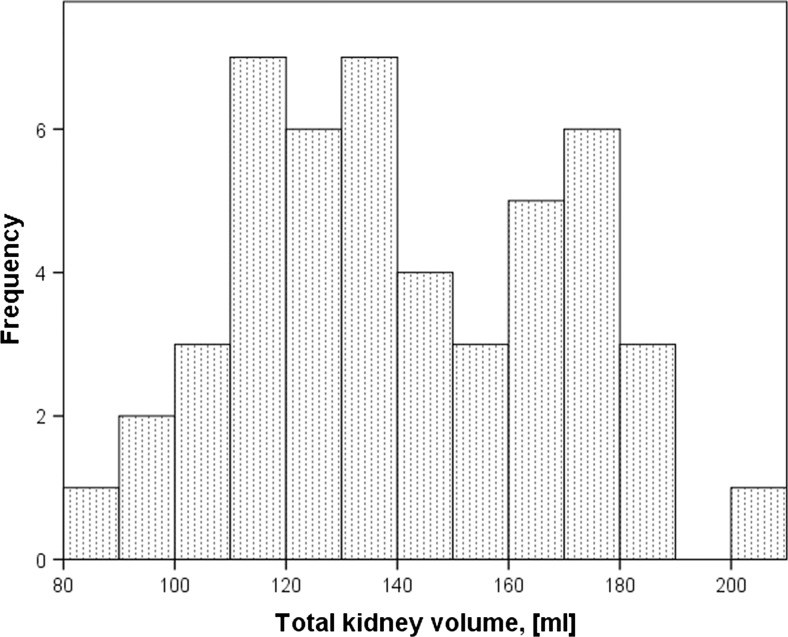
The mean TKV was 141.6 ± 28.5 ml: 143.7 ± 26.9 ml for the left kidney and 139.6 ± 29.3 ml for the right kidney, 129.1 ± 25.9 ml for female and 159.6 ± 29.3 ml for male subjects (Table 1 and Fig. 3).
Table 1.
Results of different methods for volumetric analysis of the total kidney volume
| Method | Mean total kidney volume [ml] | ||||||
|---|---|---|---|---|---|---|---|
| Total | Left | Right | Male | Female | |||
| Manual segmentation (reference volume) | 141.6 ± 28.5 | 143.7 ± 26.9 | 139.6 ± 29.3 | 159.6 ± 23.8 | 129.1 ± 25.9 | ||
| Spheroid volume VSp = π/6 × (L × D 2) | Reader 1 | 343.0 ± 69.8 | 346.9 ± 67.8 | 339.0 ± 70.2 | 367.8 ± 64.0 | 318.9 ± 66.3 | |
| Reader 2 | 206.5 ± 62.5 | 229.7 ± 60.6 | 183.3 ± 53.7 | 217.8 ± 63.0 | 188.4 ± 57.9 | ||
| Reader 3 | 130.7 ± 44.6 | 146.4 ± 43.2 | 115.0 ± 39.3 | 141.3 ± 49.4 | 115.0 ± 34.2 | ||
| Ellipsoid volume VE = π/6 × (L × W × D) | Reader 1 | 161.6 ± 40.0 | 174.3 ± 39.2 | 157.1 ± 32.6 | 174.8 ± 38.0 | 146.9 ± 37.0 | |
| Reader 2 | 165.7 ± 37.5 | 174.1 ± 36.3 | 149.1 ± 38.9 | 183.9 ± 33.3 | 148.8 ± 32.2 | ||
| Reader 3 | 161.2 ± 35.2 | 168.5 ± 34.4 | 153.8 ± 33.8 | 174.4 ± 32.1 | 145.9 ± 31.8 | ||
| Modified ellipsoid volume VME = π/6 × (L × W × D) × 0.85 | Reader 1 | 140.5 ± 34.8 | 151.4 ± 31.5 | 130.2 ± 34.6 | 152.0 ± 33.0 | 127.8 ± 32.2 | |
| Reader 2 | 144.1 ± 32.6 | 151.6 ± 34.1 | 137.6 ± 29.7 | 159.9 ± 28.9 | 129.4 ± 28.0 | ||
| Reader 3 | 140.1 ± 30.7 | 146.5 ± 29.9 | 134.4 ± 30.4 | 151.7 ± 27.9 | 126.9 ± 27.6 | ||
| Semi-automated volume VSe | Reader 1 | 1 | 141.4 ± 26.8 | 142.3 ± 24.9 | 140.6 ± 28.1 | 157.7 ± 23.1 | 130.8 ± 25.6 |
| 2 | 142.7 ± 28.3 | 142.8 ± 25.3 | 142.6 ± 30.5 | 152.2 ± 22.5 | 132.0 ± 27.1 | ||
| Reader 2 | 1 | 141.3 ± 29.1 | 143.9 ± 27.4 | 138.6 ± 29.9 | 151.3 ± 26.3 | 129.7 ± 26.6 | |
| 2 | 141.9 ± 27.7 | 142.7 ± 26.8 | 141.0 ± 27.9 | 151.0 ± 23.9 | 130.7 ± 26.9 | ||
| Reader 3 | 1 | 142.7 ± 30.4 | 145.5 ± 28.3 | 139.9 ± 31.5 | 153.2 ± 27.5 | 129.8 ± 27.3 | |
| 2 | 141.9 ± 29.0 | 143.8 ± 27.0 | 140.1 ± 30.2 | 152.0 ± 26.1 | 130.2 ± 26.4 | ||
Fig. 3.
Distribution of total kidney volumes in the cohort (manual segmentation)
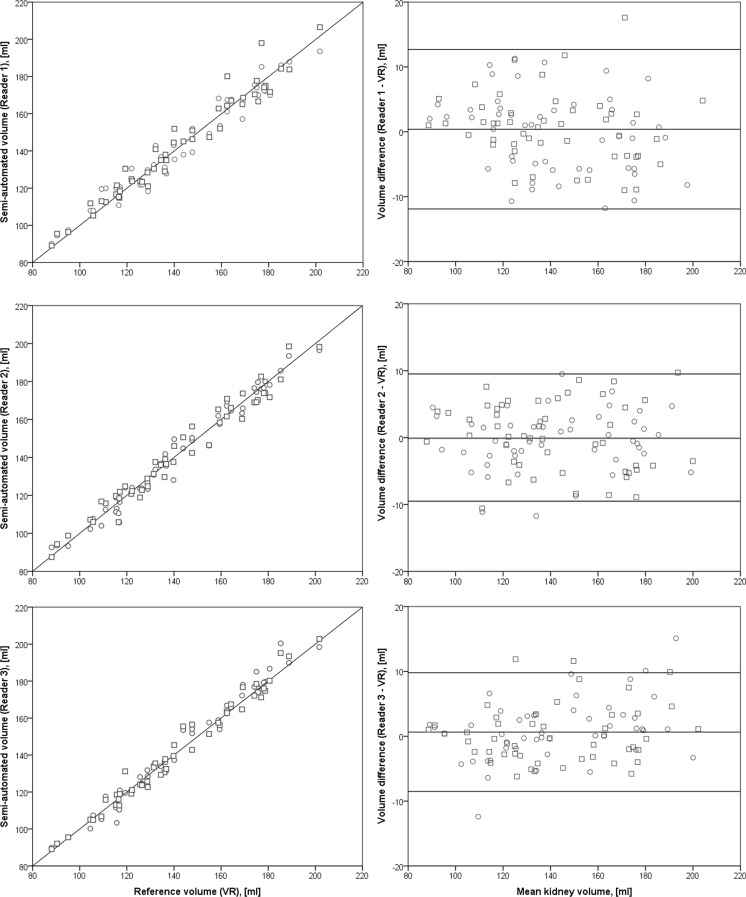
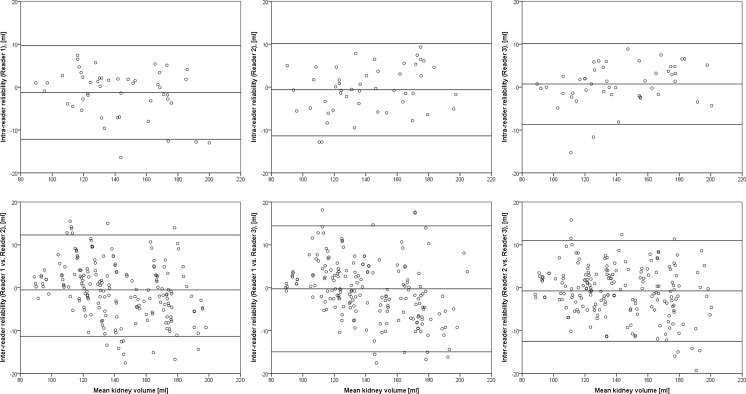
The volumes obtained by semi-automated segmentation did not differ significantly from the reference volume. The difference of mean volumes was 0.3 ml (95% confidence interval (CI): −10.1 to 10.7 ml; N = 288; p > 0.05, Fig. 4). Repeated measurements by the same reader (reproducibility/intra-reader reliability) showed no significant variability. The difference of mean volumes for this case was −0.4 ml (CI −10.8 to 10.1 ml; N = 144; p > 0.05). To check for inter-reader reliability, both segmentations of one reader were compared with the two measurements from the other readers. No significant difference between the measurements was found. The difference of mean volumes for this case was −0.2 ml (CI: −13.0 to 12.7 ml; N = 576; p > 0.05) (Table 2 and Fig. 5).
Fig. 4.
Left: Agreement of total kidney volume determined by semi-automated segmentation and manual segmentation. Results are itemized for the three readers. Right: Bland–Altman plots of the difference of measurements plotted against the mean total kidney volume
Table 2.
Accuracy and reliability of the semi-automated segmentation and volumetry of the kidney parenchyma
| N | Min. | 5th percentile | Mean | 95th percentile | Max. | SD | p value (two-tailed) | ||
|---|---|---|---|---|---|---|---|---|---|
| Accuracy (agreement with reference volume) | Reader 1 | 96 | −11.8 | −10.7 | 0.4 | 11.1 | 21.0 | 6.2 | 0.54 |
| Reader 2 | 96 | −11.7 | −10.0 | −0.1 | 7.7 | 9.7 | 4.8 | 0.84 | |
| Reader 3 | 96 | −12.4 | −6.0 | 0.6 | 9.9 | 15.1 | 4.6 | 0.17 | |
| Total | 288 | −12.4 | −8.4 | 0.3 | 9.5 | 21.0 | 5.2 | 0.31 | |
| Intra-reader reliability | Reader 1 | 48 | −16.4 | −12.9 | −1.2 | 6.1 | 7.4 | 5.5 | 0.12 |
| Reader 2 | 48 | −12.8 | −11.3 | −0.6 | 7.7 | 9.3 | 5.4 | 0.45 | |
| Reader 3 | 48 | −15.3 | −10.0 | 0.7 | 7.1 | 8.9 | 4.7 | 0.29 | |
| Total | 144 | −16.4 | −11.3 | −0.4 | 6.5 | 9.3 | 5.2 | 0.40 | |
| Inter-reader reliability | Reader 1 vs. 2 | 192 | −14.7 | −9.6 | 0.5 | 9.6 | 21.9 | 5.9 | 0.26 |
| Reader 1 vs. 3 | 192 | −17.5 | −11.0 | −0.3 | 12.7 | 26.8 | 7.3 | 0.63 | |
| Reader 2 vs. 3 | 192 | −19.3 | −11.3 | −0.7 | 8.3 | 15.8 | 5.9 | 0.08 | |
| Total | 576 | −19.3 | −10.7 | −0.2 | 9.8 | 26.8 | 6.4 | 0.52 | |
Fig. 5.
Bland–Altman plots showing intra-reader and inter-reader reliability of semi-automated segmentation
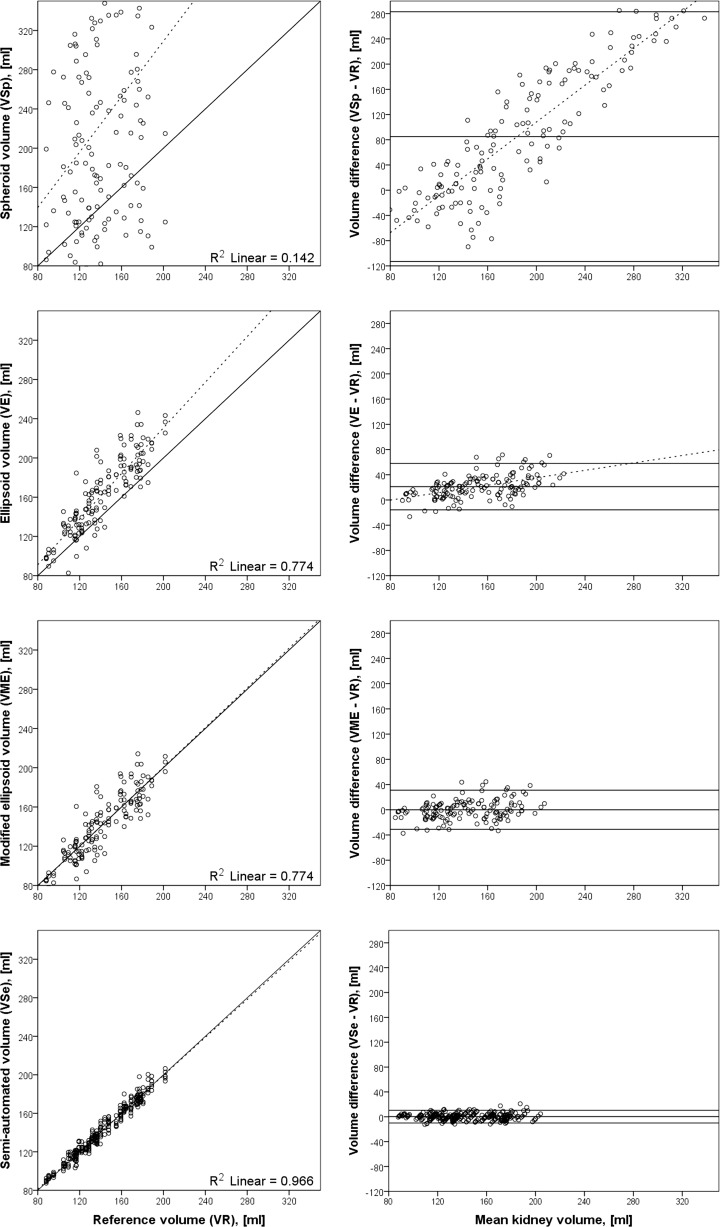
For the calculation of the TKV, three anatomical distances of the kidney were measured (length, width, and depth) and a different formula was applied. The spheroid volume (V Sp = π/6 × L × D 2) formula was used for estimation of the TKV. This formula produced a measurement that was significantly different from the reference volume. The difference of mean volumes for this case was 85.0 ml (CI −113.4 to 283.5 ml; N = 144; p < 0.01). Furthermore, the difference of mean volumes of the readers was heterogeneous. Reader 1: 201.3 ± 48.9 ml, reader 2: 64.8 ± 48.5 ml and reader 3: −11.0 ± 39.7 ml. When we used the ellipsoid formula (V E = π/6 × L × W × D), the variation between measurement was lower; however, the volume was systematically too high. The difference of mean volumes in this case was 21.2 ml (CI −15.6 to 58.0 ml; N = 144; p < 0.01). Inter-reader variability was less in this case when compared with the spheroid volume: Reader 1: 19.9 ± 22.1 ml, reader 2: 24.0 ± 18.0 ml and reader 3: 19.5 ± 14.3 ml. A linear regression of the ellipsoid volume against the reference volume yielded a modified ellipsoid volume equation of V ME = 1.95 + 0.86 × π/6 × L × W × D ml. This is approximately 15% lower than the ellipsoid volume. After correcting the ellipsoid volume by 15%, the mean difference was −0.1 ml (CI −31.1 to 30.9 ml; N = 144; p < 0.01) (Table 3). Detailed information is shown in Fig. 6.
Table 3.
Mean kidney volume differences applying a spheroid, an ellipsoid and a modified ellipsoid (result of the ellipsoid formula minus 15%) formula compared to manual segmentation
| N | Min. | 5th percentile | Mean | 95th percentile | Max. | SD | p value (two-tailed) | ||
|---|---|---|---|---|---|---|---|---|---|
| Spheroid volume VSp = π/6 × (L × D 2) | Reader 1 | 48 | 109.0 | 118.3 | 201.3 | 284.2 | 285.0 | 48.9 | <0.01 |
| Reader 2 | 48 | −26.9 | −10.3 | 64.8 | 175.0 | 189.7 | 48.5 | <0.01 | |
| Reader 3 | 48 | −89.7 | −76.0 | −11.0 | 71.8 | 92.5 | 39.7 | 0.06 | |
| Total | 144 | −89.7 | −48.4 | 85.0 | 257.6 | 285.0 | 99.2 | <0.01 | |
| Ellipsoid volume VE = π/6 × (L × W × D) | Reader 1 | 48 | −26.5 | −17.9 | 19.9 | 66.7 | 70.7 | 22.1 | <0.01 |
| Reader 2 | 48 | −7.6 | 1.6 | 24.0 | 62.4 | 71.6 | 18.0 | <0.01 | |
| Reader 3 | 48 | −13.3 | −2.2 | 19.5 | 47.1 | 50.2 | 14.3 | <0.01 | |
| Total | 144 | −26.5 | −7.0 | 21.2 | 55.9 | 71.6 | 18.4 | <0.01 | |
| Modified ellipsoid volume VME = π/6 × (L × W × D) × 0.85 | Reader 1 | 48 | −37.3 | −32.9 | −1.1 | 38.8 | 43.7 | 18.7 | 0.67 |
| Reader 2 | 48 | −29.8 | −18.1 | 2.4 | 33.5 | 44.5 | 15.1 | 0.27 | |
| Reader 3 | 48 | −29.1 | −19.6 | −1.5 | 19.4 | 27.3 | 11.9 | 0.38 | |
| Total | 144 | −37.3 | −27.8 | −0.1 | 23.9 | 44.5 | 15.5 | 0.95 | |
Fig. 6.
Correlation of different segmentation approaches plotted against the reference volume (left) and the corresponding Bland–Altman plots of difference of volumes plotted against mean total kidney volume (right). The spheroid volume (V Sp) scatters widely without any visible correlation to the reference volume (V R). The ellipsoid volume (V E) shows a better correlation. The volume of the renal pelvis and the hilus of the kidney were included in this geometrical calculation and the volumes were systematically too high. To compensate for this and to estimate only the parenchymal volume, we calculated the modified ellipsoid volume (V ME) by subtracting 15% from V E (V ME = V E × 0.85). Compared with the fast measurements and volume estimations, the semi-automatic volume has the highest correlation and the narrowest confidence interval
For the manual segmentation, the time required per kidney was 408 ± 105 s. The semi-automated segmentation took 220 ± 53 s and, therefore, was significantly faster. The estimation of kidney volume by measuring three distances took 41 ± 11 s and was again significantly faster than the semi-automated segmentation.
Discussion
In this work, we developed a semi-automated segmentation tool and a modified ellipsoid formula for volumetric analysis of the kidney in non-contrast T2-weighted MR images. We also evaluated the methods for accuracy, precision, and time effort.
Semi-automated segmentation delivered accurate and reproducible results. Measuring the kidney in three dimensions and applying different formulae to calculate the volume of the kidney yielded varied results, while a modified ellipsoid formula (result of the ellipsoid formula minus 15%) provided the most accurate results.
In recent years, a number of studies of renal volumetric segmentation have been reported [8, 18, 22]. Because of the fact that different modalities, sequences, and tools were used and that there no reference volume was used, comparison of their results is not always possible or useful. The manually determined volume of the kidney of 141.6 ± 28.5 ml for both kidneys in healthy subjects is slightly lower than the values that were previously reported.
Cheong et al. have shown a single kidney volume of 202 ± 36 ml for men and 154 ± 33 ml for women [23], excluding the pelvis and vasculature. They compared an ellipsoid volume with manual disk summation segmentation. Contrary to our study, their ellipsoid formula underestimated the renal volume. In reverse, one might argue that their manual segmentation overestimated the volume. Gloger et al. recently presented a fully automated kidney segmentation algorithm of customized 3-D non-contrast-enhanced MR images acquired with a T1-w VIBE sequence [24]. They reported a volume error of 7.5% for the right and 10.7% for the left renal parenchyma. Tang et al. reported an agreement between automated and manual segmentation of almost 90% for the renal pelvis [18]. Will et al. presented an automated segmentation algorithm for renal cortex, medulla, and pelvis based on non-contrast-enhanced T1- and T2-weighted MR images [25].
The proposed semi-automated segmentation approach was able to further reduce the volume error to roughly 3.5%. Depending on the setting, i.e., a short-term follow-up of this reduced error might be of clinical importance.
We used axial slice orientation because it provided minor partial volume effects in the kidneys [26]. Nevertheless, axial slice orientation requires the acquisition of more slices to cover the entire kidneys at the given slice thickness. This leads to a longer acquisition time, which potentially causes problems with the breath-hold capacity. It was reported that when using coronal or sagittal slice orientation, breathing movements showed a lower through-plane component compared with axial slice orientation [27]. The reduction of partial volume effects is crucial. It is possible to completely remove breathing artifacts in the routinely used T2-weighted imaging; because of this, axial slice orientation was considered the best choice.
The three readers segmented all kidneys twice. Compared with the reference volume, there was no significant difference in mean volumes (0.3 ml) with an average error of 5.2 ml. Repeated measurements by the same reader and comparison with different readers showed no significant difference in mean volumes (−0.4/−0.2 ml) and had a similar average error of 5.2/6.4 ml. This error is likely the result of inconsistent margin segments because partial volume measurements depend on the readers’ visual perspective. It is unlikely that a refinement of the segmentation tool will be able to compensate for this error. Rather, the MR image quality, contrast, and resolution have to be improved for an even more reliable segmentation of the kidney volume.
To simplify the volumetric analysis of the kidney, we also tried fast measurement of the renal dimensions. Length, width, and depth were measured by three different readers. We tried to avoid potential problems of vague landmarks for the measurement of the width (including the hilus and pelvis) and, therefore, applied a spheroid volume by squaring the depth of the kidney. The range of mean volume differences was 375 ml and therefore was roughly 2.5 times the renal volume. Furthermore, a scatter plot of the spheroid volume (V Sp) over the reference volume (Fig. 6) shows that the calculated volumes appear to be random instead of correlating with the reference. One reason may be the measurement error; the depth is squared in the formula and small errors of this distance have a high impact on the volume. Another reason might be the physiological difference in shape of the kidneys, in some aspects the width and depth were nearly the same (5.2/5.9 cm) while in others the ratio was 4.4/8.1 cm. This approach did not yield reliable results.
Viewing the kidney as an ellipsoid (V E = π/6 × L × W × D) shows a good correlation with the reference volume; however, it overestimates the renal volume systematically by approximately 15%. This was expected because the generated geometrical volume included the pelvis, the bean-like shape of the kidney, and parts of the perirenal fatty tissue. Because the renal pelvis is not part of the metabolic activity of the kidney, it was not included in the segmentation approach.
To compensate for those tissues, we subtracted the correction factor of 15% that was obtained empirically by linear regression. Using this corrected equation, the difference of mean volumes was only −0.1 ml.
Creating a reference volume by manually marking every pixel in every image that included parts of the kidney and calculating the volume was the most time-consuming way to segment the kidney (408 s). Semi-automated segmentation was on average 188 s faster. The time needed for the segmentation depended on the quality of the image, especially on the contrast and moving artifacts. The fastest way to get a rough estimation of renal volume was by measuring the anatomical distances and applying the modified ellipsoid volume equation for the volume (41 s). However, the mean volume error of 15 ml was still three times higher than the mean volume error of the semi-automated segmentation of only 5 ml. Depending on the clinical question, this rough estimation of renal volume might still be sufficient.
An advantage of the presented algorithm is that it works with native MR data. In clinical abdominal MR imaging, T2-weighted images are usually acquired. The proposed tool can be used even retrospectively in the vast majority of MR examinations.
In a clinical practice setting, radiologists may use different software packages to display, evaluate and interpret radiological examinations. OsiriX MD is a stand-alone software and is certified for medical use, FDA cleared, and CE II labeled. Hence, radiologists may use OsiriX MD to interpret radiological examinations. If radiologists use different software for image interpretation, OsiriX can be implemented on the workstation (Mac, Apple, Cupertino, CA, USA). Images can be imported from the PACS (Picture Archiving and Communication System). An additional workstation is needed if you do not use a Mac for image interpretation.
Our study faces some limitations. As mentioned above, partial volume effects due to finite slice thickness and overlapping with other organs lead to an error during the calculation of the volume [26, 28]. Because scan time is limited due to the breath-hold phase, a relatively coarse voxel size of 0.98 × 0.98 × 4.4 mm3 was used. A higher image resolution would improve the volumetric results and diminish the effects of the imprecise volumetric calculation, especially for the first and last slices for both manual and semi-automated segmentation. It is known that a correct determination of the border of the pelvis in native MR images may be challenging. Imaging was performed at 1.5 T. Higher field strength might improve the spatial resolution and contrast of the different tissues, which might improve the segmentation results. The images were obtained with a relatively long TR of 6206 ms. As mentioned above, artifacts due to respiratory motion can influence the accuracy and precision of volumetric measurements. T1-weighted images or gradient echo sequences have a shorter TR and might therefore improve image quality. We segmented the renal parenchyma as a whole. Additional studies could be conducted that focus on the difference between the cortex and the medulla.
The presented semi-automated segmentation algorithm was evaluated with images obtained from healthy volunteers. Therefore, additional research needs to be performed to show how reliable the algorithm works with data from patients with a potentially lower contrast between renal parenchyma and surrounding tissues or with urinary obstruction. Naturally, diseases that lead to changes of the T2 signal may affect the volumetric segmentation of the renal parenchyma. In the investigated cohort, none of the kidneys exhibited a cystic or solid lesion. These lesions may also reduce the reliability of the proposed tool. Further studies could be conducted including patients with solid or cystic kidney lesions.
Conclusions
Semi-automated segmentation and volumetric analysis of the kidney in native T2-weighted MR images deliver accurate and reproducible results in healthy volunteers. This process enables a significantly faster volumetric analysis than manual segmentation, can be easily implemented in the clinical routine, and offers non-invasive assessment and monitoring of the kidney parenchyma volume in routinely acquired MR images. Further research needs to be done to show how well the proposed algorithm works with pathologic kidneys presenting a potentially affected contrast between renal parenchyma and surrounding tissues.
Measuring the kidney in three dimensions and applying different formulas to calculate the volume of the kidney yields heterogeneous results, while a modified ellipsoid formula (result of the ellipsoid formula minus 15%) provides accurate results.
Acknowledgements
The authors thank the participants of this study.
Compliance with Ethical Standards
The institutional review board of the University Hospital Erlangen/Germany approved the study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Funding
This research has been supported by the Smart Data Program in the KDI project of the Federal Ministry for Economic Affairs and Energy, Germany (01MT14001E).
Conflict of Interest
The authors declare that they have no conflict of interest.
Contributor Information
Hannes Seuss, Email: hannes.seuss@uk-erlangen.de.
Rolf Janka, Email: rolf.janka@uk-erlangen.de.
Marcus Prümmer, Email: pruemmer@chimaera.de.
Alexander Cavallaro, Email: alexander.cavallaro@uk-erlangen.de.
Rebecca Hammon, Email: rebecca.hammon@klinikum-nuernberg.de.
Ragnar Theis, Email: ragnar.theis@uk-erlangen.de.
Martin Sandmair, Email: martin.sandmair@gmail.com.
Kerstin Amann, Email: kerstin.amann@uk-erlangen.de.
Tobias Bäuerle, Email: tobias.baeuerle@uk-erlangen.de.
Michael Uder, Email: michael.uder@uk-erlangen.de.
Matthias Hammon, Phone: +49 9131 8536065, Email: matthias.hammon@uk-erlangen.de.
References
- 1.Abraham G, et al. Chronic kidney disease hotspots in developing countries in South Asia. Clinical kidney journal. 2016;9:135–141. doi: 10.1093/ckj/sfv109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grams ME, et al. Trends in the prevalence of reduced GFR in the United States: a comparison of creatinine- and cystatin C-based estimates. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2013;62:253–260. doi: 10.1053/j.ajkd.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coresh J, et al. Prevalence of chronic kidney disease in the United States. Jama. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 4.White SL, Polkinghorne KR, Atkins RC, Chadban SJ. Comparison of the prevalence and mortality risk of CKD in Australia using the CKD Epidemiology Collaboration (CKD-EPI) and Modification of Diet in Renal Disease (MDRD) Study GFR estimating equations: the AusDiab (Australian Diabetes, Obesity and Lifestyle) Study. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2010;55:660–670. doi: 10.1053/j.ajkd.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 5.Matsushita K, Tonelli M, Lloyd A, Levey AS, Coresh J, Hemmelgarn BR. Clinical risk implications of the CKD Epidemiology Collaboration (CKD-EPI) equation compared with the Modification of Diet in Renal Disease (MDRD) Study equation for estimated GFR. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2012;60:241–249. doi: 10.1053/j.ajkd.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 6.Keller G, Zimmer G, Mall G, Ritz E, Amann K. Nephron number in patients with primary hypertension. The New England journal of medicine. 2003;348:101–108. doi: 10.1056/NEJMoa020549. [DOI] [PubMed] [Google Scholar]
- 7.Ritz E, Amann K, Koleganova N, Benz K. Prenatal programming-effects on blood pressure and renal function. Nature reviews Nephrology. 2011;7:137–144. doi: 10.1038/nrneph.2011.1. [DOI] [PubMed] [Google Scholar]
- 8.Jones RA, Easley K, Little SB, Scherz H, Kirsch AJ, Grattan-Smith JD. Dynamic contrast-enhanced MR urography in the evaluation of pediatric hydronephrosis: Part 1, functional assessment. AJR American journal of roentgenology. 2005;185:1598–1607. doi: 10.2214/AJR.04.1540. [DOI] [PubMed] [Google Scholar]
- 9.Bakker J, Olree M, Kaatee R, de Lange EE, Beek FJ. In vitro measurement of kidney size: comparison of ultrasonography and MRI. Ultrasound in medicine & biology. 1998;24:683–688. doi: 10.1016/S0301-5629(97)00212-3. [DOI] [PubMed] [Google Scholar]
- 10.Bakker J, et al. Renal volume measurements: accuracy and repeatability of US compared with that of MR imaging. Radiology. 1999;211:623–628. doi: 10.1148/radiology.211.3.r99jn19623. [DOI] [PubMed] [Google Scholar]
- 11.Li S, et al. Wavelet-based segmentation of renal compartments in DCE-MRI of human kidney: initial results in patients and healthy volunteers. Computerized medical imaging and graphics : the official journal of the Computerized Medical Imaging Society. 2012;36:108–118. doi: 10.1016/j.compmedimag.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Grenier N, Basseau F, Ries M, Tyndal B, Jones R, Moonen C. Functional MRI of the kidney. Abdominal imaging. 2003;28:164–175. doi: 10.1007/s00261-001-0183-8. [DOI] [PubMed] [Google Scholar]
- 13.Coulam CH, Bouley DM, Sommer FG. Measurement of renal volumes with contrast-enhanced MRI. Journal of magnetic resonance imaging : JMRI. 2002;15:174–179. doi: 10.1002/jmri.10058. [DOI] [PubMed] [Google Scholar]
- 14.de Bazelaire CM, Duhamel GD, Rofsky NM, Alsop DC. MR imaging relaxation times of abdominal and pelvic tissues measured in vivo at 3.0 T: preliminary results. Radiology. 2004;230:652–659. doi: 10.1148/radiol.2303021331. [DOI] [PubMed] [Google Scholar]
- 15.Di Leo G, Di Terlizzi F, Flor N, Morganti A, Sardanelli F. Measurement of renal volume using respiratory-gated MRI in subjects without known kidney disease: intraobserver, interobserver, and interstudy reproducibility. European journal of radiology. 2011;80:e212–216. doi: 10.1016/j.ejrad.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Kanki A, et al. Corticomedullary differentiation of the kidney: evaluation with noncontrast-enhanced steady-state free precession (SSFP) MRI with time-spatial labeling inversion pulse (time-SLIP) Journal of magnetic resonance imaging : JMRI. 2013;37:1178–1181. doi: 10.1002/jmri.23909. [DOI] [PubMed] [Google Scholar]
- 17.Cohen BA, Barash I, Kim DC, Sanger MD, Babb JS, Chandarana H. Intraobserver and interobserver variability of renal volume measurements in polycystic kidney disease using a semiautomated MR segmentation algorithm. AJR American journal of roentgenology. 2012;199:387–393. doi: 10.2214/AJR.11.8043. [DOI] [PubMed] [Google Scholar]
- 18.Tang Y, Jackson HA, Filippo RED, Nelson MD, Jr, Moats RA. Automatic renal segmentation applied in pediatric MR Urography. IJIIP. 2010;1:12–19. doi: 10.4156/ijiip.vol1.issue1.2. [DOI] [Google Scholar]
- 19.Borgelt C, Timm H, Kruse R: Using fuzzy clustering to improve naive Bayes classifiers and probabilistic networks. Proc. Fuzzy Systems, 2000 FUZZ IEEE 2000 The Ninth IEEE International Conference on: City, 7-10 May 2000
- 20.Sun Y, et al. Improving spatiotemporal resolution of USPIO-enhanced dynamic imaging of rat kidneys. Magnetic resonance imaging. 2003;21:593–598. doi: 10.1016/S0730-725X(03)00089-4. [DOI] [PubMed] [Google Scholar]
- 21.Vivier PH, Dolores M, Gardin I, Zhang P, Petitjean C, Dacher JN. In vitro assessment of a 3D segmentation algorithm based on the belief functions theory in calculating renal volumes by MRI. AJR American journal of roentgenology. 2008;191:W127–134. doi: 10.2214/AJR.07.3063. [DOI] [PubMed] [Google Scholar]
- 22.Karstoft K, Lodrup AB, Dissing TH, Sorensen TS, Nyengaard JR, Pedersen M. Different strategies for MRI measurements of renal cortical volume. Journal of magnetic resonance imaging : JMRI. 2007;26:1564–1571. doi: 10.1002/jmri.21121. [DOI] [PubMed] [Google Scholar]
- 23.Cheong B, Muthupillai R, Rubin MF, Flamm SD. Normal values for renal length and volume as measured by magnetic resonance imaging. Clinical journal of the American Society of Nephrology : CJASN. 2007;2:38–45. doi: 10.2215/CJN.00930306. [DOI] [PubMed] [Google Scholar]
- 24.Gloger O, Tonies KD, Liebscher V, Kugelmann B, Laqua R, Volzke H. Prior shape level set segmentation on multistep generated probability maps of MR datasets for fully automatic kidney parenchyma volumetry. IEEE transactions on medical imaging. 2012;31:312–325. doi: 10.1109/TMI.2011.2168609. [DOI] [PubMed] [Google Scholar]
- 25.Will S, Martirosian P, Wurslin C, Schick F. Automated segmentation and volumetric analysis of renal cortex, medulla, and pelvis based on non-contrast-enhanced T1- and T2-weighted MR images. Magma (New York, NY) 2014;27:445–454. doi: 10.1007/s10334-014-0429-4. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez Ballester MA, Zisserman AP, Brady M. Estimation of the partial volume effect in MRI. Medical image analysis. 2002;6:389–405. doi: 10.1016/S1361-8415(02)00061-0. [DOI] [PubMed] [Google Scholar]
- 27.Pham D, Kron T, Foroudi F, Schneider M, Siva S. A review of kidney motion under free, deep and forced-shallow breathing conditions: implications for stereotactic ablative body radiotherapy treatment. Technology in cancer research & treatment. 2014;13:315–323. doi: 10.7785/tcrt.2012.500387. [DOI] [PubMed] [Google Scholar]
- 28.Gadeberg P, Gundersen HJ, Tagehoj F. How accurate are measurements on MRI? A study on multiple sclerosis using reliable 3D stereological methods. Journal of magnetic resonance imaging : JMRI. 1999;10:72–79. doi: 10.1002/(SICI)1522-2586(199907)10:1<72::AID-JMRI10>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]