Abstract
Background: An elevated plasma total homocysteine (tHcy) level is an independent risk factor for vascular events. The aim of the present study was to investigate the association between tHcy levels in the acute phase of cerebral infarction and functional outcome among elderly patients.
Methods: Between October 2009 and December 2012, we recruited 594 elderly patients (age > 75) with first-onset acute cerebral infarction who were consecutively admitted to the Department of Neurology of Tianjin Huanhu Hospital, China. Levels of tHcy and other biochemical values were measured within 24 h after admission. tHcy values were classified according to quartiles (<9.94; 9.94 to <12.7; 12.7 to <16.8; and ≥16.8 μmol/L). We examined the relationship between tHcy levels at admission and modified Rankin Scale scores (mRS) using univariate and multivariate analyses. Patients were followed up at 3 months and 1 year after stroke.
Results: Within 3 months after stroke, 64 patients died, 37 had recurrent ischemic stroke, and 22 were lost to follow-up; thus, 471 patients were reviewed and analyzed. By the time of the 1-year follow-up, an additional 48 patients had died, 44 had recurrent ischemic stroke, and 40 had been lost to follow-up; the remaining 339 patients were thus reviewed and analyzed. Elevated tHcy levels were not associated with functional outcome among elderly patients with acute cerebral infarction (p > 0.05). Only the National Institutes of Health Stroke Scale score was associated with a poor outcome after adjusting for confounders at 3 months and 1 year (adjusted odds ratio, 1.38; 95% CI, 1.28–1.49; p < 0.01; adjusted odds ratio, 1.34; 95% CI, 1.25–1.44; p < 0.01, respectively).
Conclusion: Among elderly patients with acute cerebral infarction, elevated tHcy at admission was not a predictive factor of outcome at 3 months and 1 year after stroke onset.
Keywords: homocysteine, cerebral infarction, functional outcome, elderly, predictor
Introduction
Stroke is the second most common cause of death and the leading cause of disability worldwide, especially among aging patients (Lloyd-Jones et al., 2010). An estimated two million strokes occur each year in China, and the Chinese population is aging, with 200 million residents aged ≥65 years (Liu et al., 2011).
Some serum biomarkers, such as homocysteine (Hcy) have a predictive value in evaluating vascular events (Boushey et al., 1995; Eikelboom et al., 1999; Collaboration, 2002; Klerk et al., 2002; Wald et al., 2002). Hcy is a sulfhydryl-containing amino acid that is derived from the essential amino acid methionine, which is found in animal sources of protein (Hankey and Eikelboom, 1999).
A recent study shows that elevated tHcy levels could be associated with poor outcomes after stroke onset in women, but not in men (Zhong et al., 2016). However, little is known about the relationship between elevated tHcy and functional outcome among elderly patients with a cerebral infarction.
Therefore, the aim of the present study was to investigate the association between tHcy levels in the acute phase of cerebral infarction and functional outcome among elderly patients.
Materials and Methods
The present study received the approval of the Ethics Committee of Tianjin Huanhu Hospital, and all procedures were in accordance with the ethical standards set forth by the committee. Informed consent was obtained from all individual participants included in the study. The present study is observational studies, without giving extra intervention on the patients.
Patient Selection
We used a hospital-based registry of consecutive patients with first-onset acute cerebral infarction from October 2009 to December 2012. All patients were admitted into the Department of Neurology of Tianjin Huanhu Hospital, which is a specialized neurology hospital in Tianjin, China. Diagnosis of acute cerebral infarction was made according to the criteria of the World Health Organization (Schlegel et al., 2003). The inclusion criteria included a first ischemic stroke within 7 days, which was confirmed by imaging studies of the head (magnetic resonance imaging or computed tomography). Exclusion criteria for the present study were an age ≤75 years, B vitamin and/or folic acid therapy within 2 weeks of hospital admission, renal failure, or an unwillingness to participate.
Detailed baseline demographics and clinical characteristics were collected and recorded during the patient hospitalization. Patients or their authorized proxies were followed up at 3 months and 1 year after stroke onset through either telephone or face-to-face interviews. Follow-up results were immediately recorded into the stroke database.
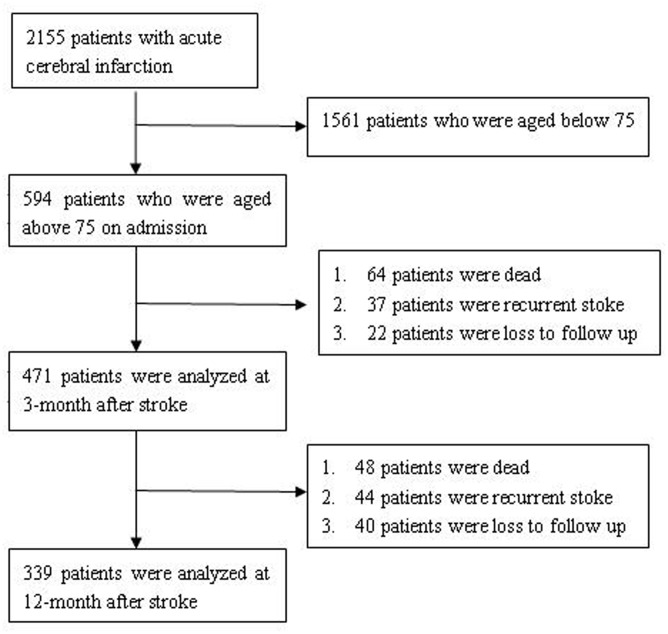
We retrospectively reviewed and extracted data from the registry. Among 2,155 patients with acute cerebral infarction who were admitted to the hospital within 7 days of stroke onset, 1,561 patients who were aged ≤75 years were excluded from the analysis. A total of 594 patients aged >75 years with acute cerebral infarction were included in the study. Finally, 471 patients were followed up at 3 months, and 339 patients were followed up at 1 year (Figure 1).
FIGURE 1.
How chart of patient selection.
Demographic and Clinical Characteristics
Baseline information and stroke risk factors for all patients were collected within 24 h of admission. The baseline information included age, sex, height, and weight data. Stroke risk factors included a clinical medical history of hypertension, diabetes mellitus, atrial fibrillation, dyslipidemia, or cerebral artery stenosis. We also assessed patients’ modifiable lifestyle factors, including current smoking status (≥1 cigarette per day for 1 year) and current alcohol drinker status (≥1 drink per week for 1 year). Body mass index was measured according to body weight and height, and obesity was defined as a body mass index ≥30 kg/m2.
The stroke subtypes were classified according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria, and were defined as atherothrombotic, cardioembolic, lacunar, other causes, and undetermined (Adams et al., 1993). The National Institutes of Health Stroke Scale (NIHSS) was evaluated at the time of admission. The modified Rankin Scale (mRS) was evaluated at 3 months and 1 year after stroke onset.
Laboratory Methods
Blood biochemical variables were measured following a fast of at least 8 h on the first day after admission. Levels of tHcy were measured using nephelometric technology, which was conducted on a BNII system (SIEMENS, Erlangen, Germany). Serum high-sensitivity C-reaction protein (hsCRP) concentration was measured using an immunoturbidimetric assay. Fasting glucose was determined using the glucose oxidase method. All variables were analyzed in a certified central laboratory.
Quartile classification is the most common statistical layering method; therefore tHcy values were classified into four groups according to quartiles (<9.94; 9.94 to <12.7; 12.7 to <16.8; and ≥16.8 μmol/L).
Study Outcome
To evaluate functional outcome in elderly patients, functional impairment was graded using the mRS. A favorable outcome was defined as an mRS score of 0–2 at 3 months and 1 year after stroke onset. A poor outcome was defined as an mRS score of 3–5, which indicates that patients cannot live independently.
Statistical Analysis
In the present study, categorical variables are reported as counts and percentages, and continuous variables are reported as median values or means ± standard deviations. Normality of distribution was assessed with frequency histograms and normal probability plots. The univariate associations between variables of interest were evaluated using a Student’s t-test, Kruskal–Wallis test, and chi-square test, as appropriate. Covariate-adjusted associations between the variables of interest were assessed using a logistic regression analyses. The selection of the potentially confounding covariates was based on the existing knowledge about their potential relationship with the variable in focus. Two-tailed tests of significance were performed, and p-values < 0.05 were considered statistically significant. The software package SPSS version 21.0 was used to perform statistical analyses.
Results
Baseline Demographics and Clinical Characteristics According to Quartiles of tHcy Levels
Baseline demographics and clinical characteristics according to quartiles of tHcy levels are listed in Table 1. A total of 594 elderly patients (323 men [54.4%]; median age, 78 years) were included in the present study. The mean tHcy level of all elderly patients was 15.61 ± 10.77 mmol/L (range, 2.42–105.00 μmol/L); a total of 437 (73.6%) patients had hypertension, 172 (29.0%) had diabetes mellitus, 91 (15.3%) had atrial fibrillation, 125 (21.0%) had dyslipidemia, and 130 (21.9%) had cerebral arterial stenosis. Regarding the modifiable lifestyle factors, 123 (20.7%) patients were current smokers, 36 (6.1%) were alcohol drinkers, and 101 (17.0%) were obese. Elderly patients with elevated tHcy levels were more likely to be male than patients with lower tHcy levels. Stroke subtypes were equally distributed among the Hcy quartiles. There was no statistically significant difference among the stratifications with regard to stroke severity and the mean time from stroke onset (p = 0.693, p = 0.888, respectively).
Table 1.
Baseline characteristics of all patients according to quartiles of tHcy levels.
| Q1 (<9.94), n = 150 | Q2 (9.94–12.7), n = 149 | Q3 (12.7–16.8), n = 147 | Q4 (>16.8), n = 148 | P | |
|---|---|---|---|---|---|
| Age, year∗ | 78 | 78 | 79 | 79 | 0.768 |
| Male, n (%) | 68 (45.3) | 68 (45.3) | 91 (61.9) | 96 (64.9) | 0.000 |
| Hypertension, n (%) | 111 (74.0) | 106 (71.1) | 102 (69.4) | 118 (79.7) | 0.197 |
| DM, n (%) | 49 (32.7) | 53 (35.6) | 36 (24.5) | 34 (23.0) | 0.043 |
| AF, n (%) | 30 (20.0) | 22 (14.8) | 23 (15.6) | 16 (10.8) | 0.179 |
| Dyslipidemia, n (%) | 42 (28.0) | 28 (18.8) | 25 (17.0) | 30 (20.3) | 0.097 |
| CAS, n (%) | 32 (21.3) | 23 (15.4) | 35 (26.9) | 40 (27.0) | 0.100 |
| Smoking, n (%) | 27 (18.0) | 28 (18.8) | 28 (19.0) | 40 (27.0) | 0.183 |
| Alcohol drinker, n (%) | 7 (4.7) | 7 (4.7) | 10 (6.8) | 12 (8.1) | 0.526 |
| Obesity, n (%) | 27 (18.0) | 28 (18.8) | 21 (14.3) | 25 (16.9) | 0.750 |
| TOAST subtype | 0.859 | ||||
| LA | 73 (48.7) | 82 (55.0) | 77 (52.4) | 85 (57.4) | |
| CE | 10 (6.7) | 15 (10.1) | 15 (10.2) | 14 (9.5) | |
| SA | 47 (31.3) | 32 (21.5) | 36 (24.5) | 32 (21.6) | |
| OE | 10 (6.7) | 9 (6.0) | 9 (6.1) | 7 (4.7) | |
| UE | 10 (6.7) | 11 (7.4) | 10 (6.8) | 10 (6.9) | |
| CHO, mmol/L∗ | 4.79 (1.29) | 4.84 (1.53) | 4.37 (1.32) | 4.71 (1.21) | 0.147 |
| TG, mmol/L∗ | 1.06 (0.62) | 1.05 (0.75) | 1.03 (0.77) | 1.07 (0.75) | 0.983 |
| HDL-C, mmol/L∗ | 1.01 (0.35) | 1.04 (0.42) | 1.02 (0.31) | 0.98 (0.32) | 0.599 |
| LDL-C, mmol/L∗ | 3.17 (1.20) | 2.95 (1.12) | 2.73 (1.05) | 3.03 (1.08) | 0.268 |
| FG, mmol/L∗ | 5.83 (2.93) | 5.61 (2.30) | 5.50 (2.18) | 5.57 (1.53) | 0.166 |
| hsCRP, mg/L∗ | 4.34 (15.72) | 4.26 (8.31) | 3.97 (12.25) | 5.29 (15.36) | 0.357 |
| Hcy, μmol/L | 8.75 (1.58) | 11.30 (1.25) | 14.50 (2.00) | 22.40 (14.35) | <0.001 |
| NIHSS∗ | 6.50 (10.75) | 6.00 (9.00) | 7.00 (11.00) | 8.00 (9.00) | 0.693 |
| Time # | 83.04 ± 50.94 | 80.38 ± 53.59 | 80.97 ± 49.87 | 84.65 ± 52.70 | 0.888 |
DM, diabetes mellitus; AF, atrial fibrillation; CAS, cerebral artery stenosis; CHO, cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; FG, fasting glucose; hsCRP, high sensitivity C-reactive protein; Hcy, homocysteine. NIHSS, National Institutes of Health Stroke Scale.
Time represents hours from stroke onset.
∗Median (interquartile range); #Mean ± standard deviation.
Results at a 3-Month Follow-up
A total of 64 (10.8%) patients died within 3 months of stroke onset. Among these patients, 17 patients (16 from cerebral infarction and complications, 1 from acute myocardial infarction) died during hospitalization, and 47 patients (36 from cardio-cerebral vascular events and complications, 11 from other causes) died during the first 3 months of follow-up. Thirty-seven patients had recurrent ischemic stroke, and 22 patients were lost to follow-up (Figure 1). At the 3-month follow-up, 471 patients responded by telephone or in-person interview and were included in the analysis. There were 246 patients with mRS scores of 0–2 and 225 patients with mRS scores of 3–5. Univariate analyses demonstrated that total cholesterol, low density lipoprotein cholesterol, hsCRP, fasting blood glucose, and NIHSS score were associated with a poor outcome; however, only NIHSS score was associated with a poor outcome after adjusting for confounders (adjusted odds ratio [OR], 1.38; 95% CI, 1.28–1.49; p < 0.01). Total cholesterol, low-density lipoprotein cholesterol, hsCRP, time from stroke onset, and fasting blood glucose levels had no relationship with a poor outcome after adjusting for confounders. There was no significant difference in outcome among patients grouped by tHcy quartile at a 3-month follow-up (Table 2).
Table 2.
Predictors of poor functional outcome in elderly patients with acute cerebral infarction.
| 3 months | Unadjusted OR (95% CI) | P | Adjusted OR (95% CI) | P |
|---|---|---|---|---|
| CHO, mmol/L | 1.25 (1.05–1.48) | 0.01 | 0.81 (0.39–1.70) | 0.58 |
| LDL-C, mmol/L | 1.37 (1.10–1.69) | <0.01 | 1.74 (0.73–4.15) | 0.70 |
| hsCRP, mg/L | 1.01 (1.00–1.02) | 0.01 | 0.99 (0.98–1.01) | 0.22 |
| FG, mg/L | 1.20 (1.08–1.35) | <0.01 | 1.09 (0.94–1.27) | 0.26 |
| NIHSS | 1.35 (1.27–1.43) | <0.01 | 1.38 (1.28–1.48) | <0.01∗ |
| Time | 1.00 (0.99–1.01) | 0.26 | 0.99 (0.99–1.01) | 0.78 |
| tHcy∗ | ||||
| Q1 | 1 | 1 | ||
| Q2 | 1.50 (0.90–2.50) | 0.12 | 0.50 (0.17–1.50) | 0.21 |
| Q3 | 0.93 (0.55–1.56) | 0.78 | 0.77 (0.28–2.10) | 0.61 |
| Q4 | 1.55 (0.77–2.15) | 0.33 | 0.73 (0.27–1.94) | 0.52 |
| 1 year | ||||
| AF, (%) | 2.04 (1.08–3.87) | 0.03 | 0.96 (0.39–2.33) | 0.92 |
| LDL-C, mmol/L | 1.26 (0.98–1.62) | 0.08 | 1.26 (0.93–1.72) | 0.14 |
| NIHSS | 1.34 (1.25–1.43) | <0.01 | 1.34 (1.25–1.43) | <0.01# |
| Time | 1.00 (1.00–1.01) | 0.068 | 1.00 (0.99–1.01) | 0.28 |
| tHcy# | ||||
| Q1 | 1 | 1 | ||
| Q2 | 1.05 (0.56–1.97) | 0.87 | 1.16 (0.51–2.63) | 0.73 |
| Q3 | 0.85 (0.45–1.62) | 0.63 | 1.25 (0.56–2.79) | 0.59 |
| Q4 | 1.07 (0.57–2.01) | 0.83 | 0.98 (0.43–2.21) | 0.96 |
CI indicates confidence interval; OR, odd ratio. Time represents hours from stroke onset.
∗Adjusted for total cholesterol, low-density lipoprotein cholesterol level, high-sensitivity C-reactive protein level, fasting glucose, NIHSS and time.
#Adjusted for atrial fibrillation, low-density lipoprotein cholesterol, NIHSS and time.
Results at a 1-Year Follow-Up
Among the 471 patients available for follow-up at 3 months, 48 (10.2%) patients died, 44 (9.3%) patients had recurrent ischemic stroke, and 40 (8.5%) patients were lost at a 1-year follow-up at 1 year. Thirty-two patients died from cardio-cerebral vascular events and complications, and 16 died from other causes (Figure 1). A total of 339 patients responded by telephone or in-person interview at 1 year and were included in the analysis. There were 220 patients with mRS scores of 0–2, and 119 patients with mRS scores of 3–5. Univariate analyses demonstrated that atrial fibrillation, low-density lipoprotein cholesterol levels, and NIHSS score were associated with a poor outcome; however, only NIHSS score was associated with a poor outcome after adjusting for confounders (adjusted odds ratio, 1.34; 95% CI, 1.25–1.44; p < 0.01). Atrial fibrillation, time from stroke onset, and low-density lipoprotein cholesterol levels had no relationship with a poor outcome after adjusting for confounding variables. There was no significant difference in outcome among patients grouped by tHcy quartile at a 1-year follow-up (Table 2).
Discussion
Some neurological scales, such as the NIHSS and mRS (Schlegel et al., 2003; Wilson et al., 2005; Bruno et al., 2013; Kim et al., 2013), can predict stroke patient outcomes. However, it is very difficult to evaluate patient outcomes using scales when patients suffer from apraxia, aphasia, or disorientation. In addition, specific serum biomarkers also have predictive value in evaluating stroke outcome (Yang et al., 2014; Yoo et al., 2014). Therefore, it is important for clinicians to evaluate functional impairment and predict patient outcome after ischemic stroke, as this would help in determining early-stage treatment and rehabilitation methods.
Stroke severity is a well-known predictor of stroke outcome (Veerbeek et al., 2011). Furthermore, the clinical predictive validity of the NIHSS score has been shown in several investigations (Schlegel et al., 2003; Kwah et al., 2013). Consistent with other studies (Adams et al., 1999; Kwah et al., 2013), we also found that initial NIHSS score was an independent predictor (OR, 1.38; p < 0.01) of patient outcome at 3 months and (OR, 1.34; p < 0.01) 1 year after stroke.
A previous study reported that serum Hcy is an independent risk factor for stroke (Pang et al., 2014). The mechanisms explaining the relationship between hyperhomocysteinemia and stroke are not yet fully understood. Hyperhomocysteinemia could induce changes in mRNA and protein expression of CRP in vascular smooth muscle cells via the NMDAr-ROS-ERK1/2/p38-NF-κB signal pathway (Kim et al., 2013). Moreover, an increased serum Hcy level is an early atherosclerotic promoter. Hyperhomocysteinemia causes cardiovascular disease through not only the proliferation of vascular smooth muscle cells but also the acceleration of endothelial dysfunction, platelet coagulation, and cholesterol synthesis (Stuhlinger et al., 2001; Ke et al., 2010; Shenoy et al., 2014). The findings on the relationship between hyperhomocysteinemia and outcome after stroke have been inconsistent (Del Ser et al., 2001; Faeh et al., 2006). Moreover, trials investigating the effect of B vitamins, which reduce tHcy levels, on endothelial function and outcome of patients with cardiovascular disease have shown conflicting results (Chambers et al., 2000; Title et al., 2000; Woo et al., 2002; van Dijk et al., 2016). The heterogeneous methodologies included in these studies may partially explain discrepancies in the final results. For example, differences in time of evaluation, age and sex of recruited patients, the localization of the stroke area, outcome measures, and type of stroke may result in different results.
There is debate about whether tHcy is a causative risk factor in stroke or is merely a secondary marker of risk in survivors. An evaluation of tHcy both before and after acute stroke would help to answer this question. Given the difficulty in predicting the onset of stroke, however, these data are unavailable. The measurement of tHcy concentrations immediately after acute stroke is necessary because an observation of elevated tHcy levels at this time would be more suggestive of a causal association than the occurrence of hyperhomocysteinemia in survivors sampled at convalescent phases after stroke. Some studies report that tHcy concentrations are not elevated after acute stroke, but rise significantly at convalescent phases (Lindgren et al., 1995; Meiklejohn et al., 2001). Our findings are in accordance with the Lindgren et al. (1995) study; both studies recruited elderly patients (median age, 78 vs.75 years) at the acute phase (mean of 3 vs. 2 days after stroke onset), and tHcy concentration was comparable (mean of 15.61 vs.13.4 μmol/L).
In the present study, both univariate and multivariate logistic regression analyses indicated that an elevated Hcy level at admission was not a predictive factor among elderly patients with acute cerebral infarction. Similar findings reported that plasma Hcy levels have no value as predictors of functional disability in Asian patients with stroke (Mizrahi et al., 2005; Song et al., 2009). However, the Mizrahi et al. (2005) study assessed patient outcome with Function Independent Measure scores instead of mRS scores, and those two studies recruited both younger and elderly patients. One possible explanation for this finding is that the association between Hcy level and stroke is not causal among elderly patients. Alternatively, the harmful effects of hyperhomocysteinemia could be masked by other vascular risk factors, such as hypertension and diabetes. The patients included in the present study were elderly, therefore the prevalence rates of hypertension and diabetes were 74.9 and 29.5%, respectively, which were much higher than rates than those reported in other studies. However, there may be a causal link between hyperhomocysteinemia and hypertension because elevated Hcy levels may lead to hypertension through the induction of a diastolic dysfunction of vessels and a reduction of vascular wall flexibility. In addition, we failed to find any relationship between the Hcy level and serum hsCRP level, indicating that elevated Hcy does not lead to additional inflammatory responses among elderly patients.
Some studies report that elevated Hcy levels can cause a more severe stroke via multiple mechanisms, such as platelet activation, inflammation, prothrombotic disorders, and fibrinolysis (Stamler et al., 1993; Parnetti et al., 2004; Tay et al., 2006). However, in accordance with the study by Perini et al. (2005), we found no association between plasma tHcy and stroke severity, suggesting that elevated tHcy is not associated with stroke severity in our cohort of patients.
Another finding of the present study was that the percentage of men was much higher in the two highest tHcy quartiles than in the two lowest quartiles (65.7% vs. 44.5%). This result could be explained by the fact that estrogen or estrogen associated with progestin may have a positive role in decreasing cardiovascular risk due to a significant reduction in Hcy levels (Lakryc et al., 2015).
There are a few limitations of the current study, as it was retrospective and used data obtained from a single hospital. Moreover, the present study was performed in hyperhomocysteinemic elderly patients; therefore, our results may not be representative of the general population. This design may have resulted in an inevitable selection bias. Regretfully, another limitation is the lack of data on repeated measurements of Hcy levels during the follow-up period. Future studies with larger patient populations are necessary to assess the prognostic value of tHcy levels after acute cerebral infarction.
Conclusion
In summary, in the present study, we observed that an elevated tHcy level at admission was not a predictive factor of the outcome at 3 months and 1 year after acute cerebral infarction among elderly patients. These results indicate that it is necessary to treat acute cerebral infarction on an individual basis in elderly patients, especially when therapies to normalize plasma tHcy levels are considered.
Author Contributions
JW contributed to the conception and design of the work; WW, CG, CY, SL, DH, YW, CW, and LM contributed the data acquisition; JW and WW contributed the analysis and interpretation of data for the work; WW and CG contributed drafting the work, JW contributed revising the work for important intellectual content. All authors approved of the final version to be published, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This study was generously supported by Grants from General Administration of Sport of China (2015B098), and Tianjin Public Health Bureau (2013KG122), and Program to Establish Scientific Research Resources by Tianjin Municipal Health bureau (Establishment and Quality Control for biobanking of Neurological Diseases).
References
- Adams H., Bendixen B., Kappelle L., Biller J., Love B., Gordon D., et al. (1993). Classification of subtype of acute ischemic stroke:definitions for use in a multicenter clinicaltrial:TOAST:Trial of Org10172 in Acute Stroke Treatment. Stroke 24 35–41. 10.1161/01.STR.24.1.35 [DOI] [PubMed] [Google Scholar]
- Adams H. P., Jr., Davis P. H., Leira E. C., Chang K. C., Bendixen B. H., Clarke W. R., et al. (1999). Baseline NIH stroke scale score strongly predicts outcome after stroke: a report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology 53 126–131. 10.1212/WNL.53.1.126 [DOI] [PubMed] [Google Scholar]
- Boushey C. J., Beresford S. A., Omenn G. S., Motulsky A. G. (1995). A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA 274 1049–1057. 10.1001/jama.1995.03530130055028 [DOI] [PubMed] [Google Scholar]
- Bruno A., Close B., Switzer J. A., Hess D. C., Gross H., Nichols F. T., III, et al. (2013). Simplified modified Rankin Scale questionnaire correlates with stroke severity. Clin. Rehabil. 27 724–727. 10.1177/0269215512470674 [DOI] [PubMed] [Google Scholar]
- Chambers J. C., Ueland P. M., Obeid O. A., Wrigley J., Refsum H., Kooner J. S. (2000). Improved vascular endothelial function after oral B vitamins: an effect mediated through reduced concentrations of free plasma homocysteine. Circulation 102 2479–2483. 10.1161/01.CIR.102.20.2479 [DOI] [PubMed] [Google Scholar]
- Collaboration H. S. (2002). Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA 288 2015–2022. 10.1001/jama.288.16.2015 [DOI] [PubMed] [Google Scholar]
- Del Ser T., Barba R., Herranz A. S., Seijas V., Lopez-Manglano C., Domingo J., et al. (2001). Hyperhomocyst(e)inemia is a risk factor of secondary vascular events in stroke patients. Cerebrovasc. Dis. 12 91–98. 10.1159/000047687 [DOI] [PubMed] [Google Scholar]
- Eikelboom J. W., Lonn E., Genest J., Jr., Hankey G., Yusuf S. (1999). Homocyst(e)ine and cardiovascular disease: a critical review of the epidemiologic evidence. Ann. Intern. Med. 131 363–375. 10.7326/0003-4819-131-5-199909070-00008 [DOI] [PubMed] [Google Scholar]
- Faeh D., Chiolero A., Paccaud F. (2006). Homocysteine as a risk factor for cardiovascular disease: should we (still) worry about? Swiss Med. Wkly. 136 745–756. [DOI] [PubMed] [Google Scholar]
- Hankey G. J., Eikelboom J. W. (1999). Homocysteine and vascular disease. Lancet 354 407–413. 10.1016/S0140-6736(98)11058-9 [DOI] [PubMed] [Google Scholar]
- Ke X. D., Foucault-Bertaud A., Genovesio C., Dignat-George F., Lamy E., Charpiot P. (2010). Homocysteine modulates the proteolytic potential of human arterial smooth muscle cells through a reactive oxygen species dependant mechanism. Mol. Cell. Biochem. 335 203–210. 10.1007/s11010-009-0270-7 [DOI] [PubMed] [Google Scholar]
- Kim J. T., Park M. S., Chang J., Lee J. S., Choi K. H., Cho K. H. (2013). Proximal arterial occlusion in acute ischemic stroke with low NIHSS scores should not be considered as mild stroke. PLoS ONE 8:e70996 10.1371/journal.pone.0070996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klerk M., Verhoef P., Clarke R., Blom H. J., Kok F. J., Schouten E. G. (2002). MTHFR 677C– > T polymorphism and risk of coronary heart disease: a meta-analysis. JAMA 288 2023–2031. 10.1001/jama.288.16.2023 [DOI] [PubMed] [Google Scholar]
- Kwah L. K., Harvey L. A., Diong J., Herbert R. D. (2013). Models containing age and NIHSS predict recovery of ambulation and upper limb function six months after stroke: an observational study. J. Physiother. 59 189–197. 10.1016/S1836-9553(13)70183-8 [DOI] [PubMed] [Google Scholar]
- Lakryc E. M., Machado R. B., Soares J. M., Jr., Fernandes C. E., III, Baracat E. C. (2015). What is the influence of hormone therapy on homocysteine and crp levels in postmenopausal women? Clinics (Sao Paulo) 70 107–113. 10.6061/clinics/2015(02)07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren A., Brattström L., Norrving B., Hultberg B., Andersson A., Johansson B. B. (1995). Plasma homocysteine in the acute and convalescent phases after stroke. Stroke 26 795–800. 10.1161/01.STR.26.5.795 [DOI] [PubMed] [Google Scholar]
- Liu L., Wang D., Wong K. S., Wang Y. (2011). Stroke and stroke care in China: huge burden, significant workload, and a national priority. Stroke 42 3651–3654. 10.1161/STROKEAHA.111.635755 [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones D., Adams R. J., Brown T. M., Carnethon M., Dai S., De Simone G., et al. (2010). Heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation 121 e46–e215. 10.1161/CIRCULATIONAHA.109.192667 [DOI] [PubMed] [Google Scholar]
- Meiklejohn D. J., Vickers M. A., Dijkhuisen R., Greaves M. (2001). Plasma homocysteine concentrations in the acute and convalescent periods of atherothrombotic stroke. Stroke 32 57–62. 10.1161/01.STR.32.1.57 [DOI] [PubMed] [Google Scholar]
- Mizrahi E. H., Fleissig Y., Arad M., Adunsky A. (2005). Plasma homocysteine level and functional outcome of patients with ischemic stroke. Arch. Phys. Med. Rehabil. 86 60–63. 10.1016/j.apmr.2004.01.031 [DOI] [PubMed] [Google Scholar]
- Pang X., Liu J., Zhao J., Mao J., Zhang X., Feng L., et al. (2014). Homocysteine induces the expression of C-reactive protein via NMDAr-ROS-MAPK-NF-kappaB signal pathway in rat vascular smooth muscle cells. Atherosclerosis 236 73–81. 10.1016/j.atherosclerosis.2014.06.021 [DOI] [PubMed] [Google Scholar]
- Parnetti L., Caso V., Santucci A., Corea F., Lanari A., Floridi A., et al. (2004). Mild hyperhomocysteinemia is a risk-factor in all etiological subtypes of stroke. Neurol. Sci. 25 13–17. 10.1007/s10072-004-0219-5 [DOI] [PubMed] [Google Scholar]
- Perini F., Galloni E., Bolgan I., Bader G., Ruffini R., Arzenton E., et al. (2005). Elevated plasma homocysteine in acute stroke was not associated with severity and outcome: stronger association with small artery disease. Neurol. Sci. 26 310–318. 10.1007/s10072-005-0505-7 [DOI] [PubMed] [Google Scholar]
- Schlegel D., Kolb S. J., Luciano J. M., Tovar J. M., Cucchiara B. L., Liebeskind D. S., et al. (2003). Utility of the NIH Stroke Scale as a predictor of hospital disposition. Stroke 34 134–137. 10.1161/01.STR.0000048217.44714.02 [DOI] [PubMed] [Google Scholar]
- Shenoy V., Mehendale V., Prabhu K., Shetty R., Rao P. (2014). Correlation of serum homocysteine levels with the severity of coronary artery disease. Indian J. Clin. Biochem. 29 339–344. 10.1007/s12291-013-0373-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song I. U., Kim J. S., Ryu S. Y., Lee S. B., Lee S. J., Jeong D. S., et al. (2009). Are plasma homocysteine levels related to neurological severity and functional outcome after ischemic stroke in the Korean population? J. Neurol. Sci. 278 60–63. 10.1016/j.jns.2008.11.011 [DOI] [PubMed] [Google Scholar]
- Stamler J. S., Osborne J. A., Jaraki O., Rabbani L. E., Mullins M., Singel D., et al. (1993). Adverse vascular effects of homocysteine are modulated by endothelium-derived relaxing factor and related oxides of nitrogen. J. Clin. Invest. 91 308–318. 10.1172/JCI116187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhlinger M. C., Tsao P. S., Her J. H., Kimoto M., Balint R. F., Cooke J. P. (2001). Homocysteine impairs the nitric oxide synthase pathway: role of asymmetric dimethylarginine. Circulation 104 2569–2575. 10.1161/hc4601.098514 [DOI] [PubMed] [Google Scholar]
- Tay S. Y., Ampil E. R., Chen C. P., Auchus A. P. (2006). The relationship between homocysteine, cognition and stroke subtypes in acute stroke. J. Neurol. Sci. 250 58–61. 10.1016/j.jns.2006.06.028 [DOI] [PubMed] [Google Scholar]
- Title L. M., Cummings P. M., Giddens K., Genest J. J., Jr., Nassar B. A. (2000). Effect of folic acid and antioxidant vitamins on endothelial dysfunction in patients with coronary artery disease. J. Am. Coll. Cardiol. 36 758–765. 10.1016/S0735-1097(00)00809-3 [DOI] [PubMed] [Google Scholar]
- van Dijk S. C., Enneman A. W., Swart K. M., van Wijngaarden J. P., Ham A. C., de Jonge R., et al. (2016). Effect of vitamin B12 and folic acid supplementation on biomarkers of endothelial function and inflammation among elderly individuals with hyperhomocysteinemia. Vasc. Med. 21 91–98. 10.1177/1358863X15622281 [DOI] [PubMed] [Google Scholar]
- Veerbeek J. M., Kwakkel G., van Wegen E. E., Ket J. C., Heymans M. W. (2011). Early prediction of outcome of activities of daily living after stroke: a systematic review. Stroke 42 1482–1488. 10.1161/STROKEAHA.110.604090 [DOI] [PubMed] [Google Scholar]
- Wald D. S., Law M., Morris J. K. (2002). Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ 325 1202 10.1136/bmj.325.7374.1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. T., Hareendran A., Hendry A., Potter J., Bone I., Muir K. W. (2005). Reliability of the modified Rankin Scale across multiple raters: benefits of a structured interview. Stroke 36 777–781. 10.1161/01.STR.0000157596.13234.95 [DOI] [PubMed] [Google Scholar]
- Woo K. S., Chook P., Chan L. L., Cheung A. S., Fung W. H., Qiao M., et al. (2002). Long-term improvement in homocysteine levels and arterial endothelial function after 1-year folic acid supplementation. Am. J. Med. 112 535–539. 10.1016/S0002-9343(02)01075-6 [DOI] [PubMed] [Google Scholar]
- Yang X. Y., Gao S., Ding J., Chen Y., Zhou X. S., Wang J. E. (2014). Plasma D-dimer predicts short-term poor outcome after acute ischemic stroke. PLoS ONE 9:e89756 10.1371/journal.pone.0089756 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yoo D. S., Chang J., Kim J. T., Choi M. J., Choi J., Choi K. H., et al. (2014). Various blood glucose parameters that indicate hyperglycemia after intravenous thrombolysis in acute ischemic stroke could predict worse outcome. PLoS ONE 9:e94364 10.1371/journal.pone.0094364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong C., Xu T., Xu T., Peng Y., Wang A., Wang J., et al. (2016). Plasma homocysteine and prognosis of acute ischemic stroke: a gender-specific analysis from CATIS randomized clinical trial. Mol. Neurobiol. 10.1007/s12035-016-9799-0 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]