Abstract
Pathogens have evolved mechanisms to modulate host cell functions and avoid recognition and destruction by the host damage response. For many years, researchers have focused on proteins as the main effectors used by pathogens to hijack host cell pathways, but only recently with the development of deep RNA sequencing these molecules were brought to light as key players in infectious diseases. Protozoan parasites such as those from the genera Plasmodium, Toxoplasma, Leishmania, and Trypanosoma cause life-threatening diseases and are responsible for 1000s of deaths worldwide every year. Some of these parasites replicate intracellularly when infecting mammalian hosts, whereas others can survive and replicate extracellularly in the bloodstream. Each of these parasites uses specific evasion mechanisms to avoid being killed by the host defense system. An increasing number of studies have shown that these pathogens can transfer non-coding RNA molecules to the host cells to modulate their functions. This transference usually happens via extracellular vesicles, which are small membrane vesicles secreted by the microorganism. In this mini-review we will combine published work regarding several protozoan parasites that were shown to use non-coding RNAs in inter-kingdom communication and briefly discuss future perspectives in the field.
Keywords: non-coding RNA, miRNA, extracellular vesicles, protozoan parasites, parasitic diseases, infection
Introduction
Protozoan parasites comprise an exceptionally diverse group of unicellular eukaryotic organisms. Some of them are etiological agents of human parasitic diseases leading to significant morbidity and mortality, with high social and economic impacts, particularly in the tropics. In addition to medical and veterinary importance, protozoan parasites are interesting models for studying the evolution of eukaryotic cells, since they diverged early in the evolution of eukaryotes.
Protozoan parasite-host cell interplay can occur directly by physical cell-cell contact or indirectly via secreted/excreted molecules, which can be released into the extracellular medium or packed into extracellular vesicles (EVs) (reviewed in Torrecilhas et al., 2012; Barteneva et al., 2013; Deolindo et al., 2013; Mantel and Marti, 2014; Marcilla et al., 2014; Judice et al., 2016; Marti and Johnson, 2016; Roditi, 2016; Szempruch et al., 2016a; Watanabe Costa et al., 2016). The packaging of proteins and nucleic acids, such as small non-coding RNAs (sncRNAs), in EVs protects them from extracellular degradation until they reach the target recipient cell. Transference of proteins and RNAs within EVs seems to be a safe mechanism for local and systemic intercellular interactions between parasites and host cells, and also between parasites themselves. The exchange of information may take place in both directions, from parasite to host or vice-versa, and may be beneficial or detrimental to the parasite. We herein present the current understanding of sncRNAs in protozoan parasites and provide examples of their role during infections.
Biogenesis of sncRNAs in Human Protozoan Parasites
Small non-coding RNAs range in size from 20 to 300 nucleotides and comprise a large variety of small RNAs, including small interfering RNA (siRNA), microRNA (miRNA), transfer RNA (tRNA), 5S ribosomal RNA (5S rRNA), small nuclear RNA (snRNA), small nucleolar RNA (snoRNA) and 7SL cytoplasmic RNA (7SL RNA). Protozoan parasites show a variety of RNA interference (RNAi) pathways and complex miRNA repertoires, which differ from those in mammalian cells in several features, like the organization and structure of the main components of the RNA-induced silencing complex (RISC) and the nature of the precursor RNA, which could be derived from snoRNA, tRNA, rRNA, satellite DNA, natural antisense transcripts (NATs), or transcripts from transposons and retrotransposons.
Protozoan miRNAs do not share significant homology to known miRNAs of plants and animals. Dicer, Argonaute and Piwi genes (essential components of RISC) of protozoan parasites are grouped in a distinct phylogenetic lineage from metazoan (Braun et al., 2010; Batista and Marques, 2011; Zheng et al., 2013; Garcia-Silva et al., 2014c). In addition, protozoa lack Drosha (a nuclear RNase III) homologs, suggesting that a Drosha-independent pathway is present in these parasites. RNA-dependent RNA polymerase like-genes, which encode an enzyme implicated in the amplification of pre-miRNAs, have been identified in Giardia lamblia, Entamoeba histolytica, and Toxoplasma gondii. The main RISC components (Argonaute, Piwi, Dicer, RNAse III) and miRNAs have been identified in Trypanosoma brucei, Trypanosoma congolense, Leishmania (V.) braziliensis, T. gondii, Neospora caninum, G. lamblia, Trichomonas vaginalis and E. histolytica, suggesting that these organisms have a classical RNAi pathway (Militello et al., 2008; Atayde et al., 2011, 2013; Hakimi and Cannella, 2011; Kolev et al., 2011; Zheng et al., 2013).
Bioinformatic and functional analyses showed that Trypanosoma rangeli, Leishmania donovani, Leishmania major, Trypanosoma cruzi, and Plasmodium falciparum are RNAi-deficient organisms (Robinson and Beverley, 2003; DaRocha et al., 2004; Baum et al., 2009; Atayde et al., 2011; Zheng et al., 2013; Stoco et al., 2014). Orthologs of RNAi machinery components are present only as pseudogenes in T. rangeli, L. major, Leishmania infantum, and Leishmania mexicana (Zheng et al., 2013; Stoco et al., 2014). Although, T. cruzi is not able to respond to dsRNA (DaRocha et al., 2004), it expresses an Argonaute/Piwi protein (Garcia Silva et al., 2010b, Garcia-Silva et al., 2014c). It has been suggested that some sncRNAs that have been conserved during evolution, such as snoRNA and tRNA, could belong to the most primitive small RNA pathways from which the canonical RNA silencing pathways have evolved (Garcia-Silva et al., 2012; Zheng et al., 2013). It is worth highlighting that a failure to identify protein homologs using bioinformatics in genome databases does not imply that the protein function was in fact lost. In these cases, it is possible that primary sequences diverged too much to be detected by sequence homology.
The hypothesis that RNAi-deficient parasitic protozoa may have non-classical RNAi pathways is very attractive. However, we cannot rule out the possibility that these organisms may have regulatory genetic mechanisms mediated by other RNAs than small interfering RNAs. For instance, long non-coding RNAs (lncRNAs) which can be transcribed as whole or partial NATs to coding genes. NATs have been detected in many protozoan parasites such as P. falciparum, T. gondii, T. parvum, T. brucei, Leishmania spp., and G. lamblia (Militello et al., 2008). Finally, high-throughput sequencing of RNAs bound to miRNP (micro RNA ribonucleoproteins) and other RNPs (small nuclear RNP, heterogeneous nuclear RNP, cytoplasmic RNP) would be necessary to determine the full RNA repertoire of these protozoa. These studies may reveal new classes of non-coding regulatory RNAs.
Interplay Between Protozoan Parasites, Mammalian Cells And Vectors: Possible Roles For sncRnas
Descriptions of non-coding RNAs in host–protozoan parasites interactions are discussed below and a summary of references with main findings is shown in Table 1.
Table 1.
Examples of non-coding RNAs in host–protozoan parasites interactions.
| Protozoan | Host | Experimental evidences | Reference |
|---|---|---|---|
| Trypanosoma cruzi | Human | T. cruzi extracellular vesicles (EVs) deliver sncRNA cargo into HeLa cells conferring susceptibility to infection and changing expression of genes related to cytoskeleton, extracellular matrix, and immune responses pathways | Bayer-Santos et al., 2013, 2014; Garcia-Silva et al., 2014a,b; Fernandez-Calero et al., 2015 |
| Human and murine | Dysregulation of miRNAs of myocardial tissue in human chronic Chagasic cardiomyopathy (CCC) and in murine T. cruzi acute infection Overexpression of lncRNA-myocardial infarction-associated transcript (MIAT) in human CCC and murine T. cruzi acute infection | Ferreira et al., 2014; Navarro et al., 2015; Frade et al., 2016 | |
| Murine | Up-regulation of miRNAs of thymic epithelial cells in T. cruzi-induced thymic atrophy | Linhares-Lacerda et al., 2015 | |
| Leishmania spp. | Murine and human | Dysregulation of miRNA expression in L. major-infected murine and human primary macrophages. Up-regulation of miRNAs targeting MAP kinase, JAK-STAT and TGFβ signaling pathways in human monocyte derived dendritic cells and macrophages | Lemaire et al., 2013; Frank et al., 2015; Geraci et al., 2015 |
| Murine | Extracellular vesicles secreted by L. donovani downregulated miR-122 activity in hepatic cells. Leishmania metalloprotease gp63 targets pre-miRNA processor Dicer1 to prevent miRNP formation in hepatic cells | Ghosh et al., 2013 | |
| Human | L. donovani and L. braziliensis EVs deliver specific sncRNAs into human macrophages | Lambertz et al., 2015 | |
| Human | Autophagic machinery of bone marrow-derived macrophages (BMDM) is activated in L. major infection. Transfection of BMDM with specific siRNAs against autophagy-related genes or inhibitors of autophagy-associated miRNAs inhibited autophagic digestion of L. major | Singh et al., 2016 | |
| Murine and human | Downregulation of vacuolar sorting protein HRS in L. donovani-infected macrophages prevents uncoupling of mRNA-AGO2 interaction, blocking degradation of translationally repressed messages. let-7a miRNPs fail to repress newly formed IL-6 mRNA. Translation of IL-6 helps Leishmania to suppress host macrophage activation and promote infection | Bose et al., 2017 | |
| Plasmodium ssp. | Anopheles gambiae | Depletion of Argonaute I and Dicer I in the mosquito A. gambiae during P. berghei infection led to a two-fold increase in the number of oocysts | Winter et al., 2007 |
| Human | In the Plasmodium intraerythrocytic cycle, miRNAs (let-7i and miR-451) are transferred from sickle cell erythrocytes to the P. falciparum inhibiting the parasite growth | LaMonte et al., 2012 | |
| Human and murine | P. falciparum-infected erythrocyte releases EVs carrying functional miRNAs, which are internalized by endothelial cells altering gene expression and barrier properties in endothelial cells | Mantel et al., 2016 | |
| Human and murine | Blocking miR-155 function in an experimental mouse model of cerebral malaria by gene knockout or pre-treatment with miR-155 antagomir enhances endothelial quiescence, blood-brain-barrier integrity and host survival | Barker et al., 2017 | |
| Toxoplasma gondii | Human and murine | Alteration of miRNA profiles in T. gondii-infected human fibroblasts. miR-146a and miR-155, involved in response to T. gondii challenge, were induced in mouse brain during T. gondii infection | Zeiner et al., 2010; Cannella et al., 2014 |
| Cryptosporidium parvum | Human | C. parvum infection dysregulates miRNA expression in human cholangiocytes. Downregulation of some miRNAs (e.g., let-7 miRNA and miR-221) increases infiltration of lymphocytes into the intestinal mucosa and reinforces epithelial defense response against C. parvum. Inhibition of other miRNAs (mir-125b-1, mir-21, mir-30b, and mir-23b-27b-24-1 cluster genes) increases C. parvum burden | Chen et al., 2007; Zhou et al., 2009; Gong et al., 2011 |
| Trichomonas vaginalis | Human | T. vaginalis trophozoites release exosomes carrying small RNAs that fuse with human ectocervical cells to deliver their cargo. Exosomes modulate secretion of proinflammatory cytokines IL-6 and IL-8, which are regulated by endogenous miRNAs | Twu et al., 2013 |
Intracellular Protozoan Pathogens
Invasion and replication inside a mammalian cell is a common strategy used by protozoan pathogens to evade the host immune system. Protozoan parasites may modulate the expression of host miRNAs associated with different biological processes in order to survive in the intracellular environment. Conversely, host miRNAs can inhibit the proliferation of microorganisms by targeting virulence and essential genes of the parasite (Asgari, 2011).
Plasmodium spp., T. gondii, and Cryptosporidium parvum are medically important protozoa that belong to the Apicomplexa phylum. As obligate intracellular parasites, they use different mechanisms to reorganize host cell functions to allow their survival and replication inside host cells (Plattner and Soldati-Favre, 2008). Apicomplexans were reported to change the host cell miRNA expression profile. Changes in miRNA expression might be induced by the parasite or be a defensive response from the host cell (Hakimi and Cannella, 2011). Although it was reported that members of let-7 family were downregulated during infection by different apicomplexan parasites, most changes detected in miRNA expression profile are species-specific. miR-155, which regulates the development and function of immune cells, is upregulated in T. gondii infection and downregulated in Cryptosporidium infection (Lindsay, 2008; Zhou et al., 2009; Zeiner et al., 2010). There is a decrease in expression of miRNAs let-7 and mir-221 in epithelial cells infected with C. parvum, leading to upregulation of ICAM-1 (intercellular adhesion molecule-1) (Chen et al., 2007). Expression of ICAM-1 allows the adhesion and recognition of immune cells, modulating the response against infection (Gong et al., 2011). Another example of host cell miRNome (the full spectrum of miRNAs expressed in a specific genome) alterations was observed in T. gondii-infected fibroblasts in which the expression of about 14% of miRNAs was affected (Zeiner et al., 2010). Different studies showed changes in a subset of miRNAs, including miR-146a and miR-155, which are important in host cell responses to T. gondii infection (Vigorito et al., 2013; Cannella et al., 2014; Saba et al., 2014).
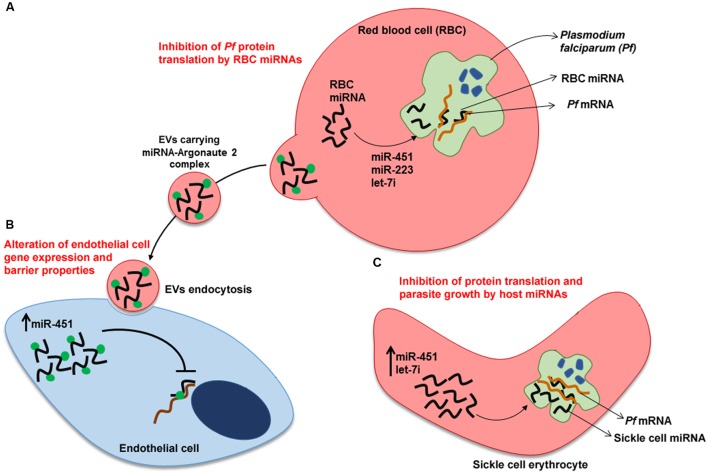
Sickle cell erythrocytes show hemoglobin polymorphism (HbS) in one or both alleles resulting in red blood cells with a sickle shape. Populations living in malaria endemic areas have a high frequency of this gene probably as a result of its protective effect during Plasmodium infection (Aidoo et al., 2002). LaMonte et al. (2012) elegantly showed that about 100 human miRNAs are transferred to the P. falciparum during the intraerythrocytic cycle (Figure 1). Two miRNAs (let-7i and miR-451) that negatively modulate parasite growth were shown to be enriched in sickle cell erythrocytes. These miRNAs bind to specific Plasmodium mRNAs, inhibiting translation by blockage of ribosomal loading. Thus, resistance of sickle cell erythrocytes to malaria infection may be in part due to a protective dysregulated miRNA expression in these cells.
FIGURE 1.
Human miRNAs response to Plasmodium falciparum infection. (A) Plasmodium-infected red blood cells (RBC). Translocation of miRNAs from RBC to P. falciparum inhibit translation of specific mRNAs of the parasite. (B) Transfer of miRNAs from Plasmodium-infected RBC to endothelial cells. Endocytosis by endothelial cell of EVs released by Plasmodium-infected RBC. miRNA-Argonaute 2 complexes alter gene expression and barrier properties in endothelial cells, contributing to malaria resistance. (C) Plasmodium-infected sickle cell erythrocyte. Translation inhibition of parasite mRNAs by sickle cell erythrocyte-miRNAs. miR-223, miR-451and let-7i inhibit parasite growth. Upward arrows denote an increase in expression of miR-451 and let-7i. EV, extracellular vesicle.
Erythrocyte miRNAs also play an important role in communication between host cells during Plasmodium infection. Infected erythrocyte releases EVs carrying functional miRNAs (Argonaute 2 complexes, enriched with miR-451 and let-7b) in the bloodstream (Figure 1). These EVs are internalized by endothelial cells and the delivered miRNAs change recipient cell gene expression, culminating in altered endothelial cell barrier properties (Mantel et al., 2016).
miR-155 seems to have an important role in the pathogenesis of cerebral malaria via negative regulation of blood-brain-barrier integrity and T cell function. In a mouse experimental model of cerebral malaria, Barker et al. (2017) demonstrated that blocking miR-155 function by gene knockout or pre-treatment with miR-155 antagomir enhances endothelial quiescence, blood-brain-barrier integrity and host survival. Further investigation is needed to explore the potential therapeutic use of miR-155 in the treatment of cerebral malaria.
Another example of the importance of miRNA in the communication between host–parasite can be observed between the insect vector Anopheles gambiae and Plasmodium. Winter et al. (2007) identified differences in expression levels of four miRNA in the midgut of A. gambiae infected with Plasmodium. Depletion of Argonaute I and Dicer I of the insect vector during Plasmodium infection led to a two-fold increase in the number of oocysts in the mosquito. It was suggested that these enzymes play an important role in the mosquito’s resistance to P. berghei infection, probably due to post-transcriptional regulation mediated by miRNA genes involved in defense mechanisms.
The trypanosomatids T. cruzi and Leishmania spp. are transmitted by insect vectors to mammalian hosts. L. braziliensis has been reported to have a functional siRNA pathway, while T. cruzi, L. major, and L. amazonensis do not have a functional RNAi machinery (Atayde et al., 2011; Kolev et al., 2011). However, T. cruzi expresses an AGO/PIWI protein (TcPIWI-tryp) that colocalizes with tRNA-derived small RNAs (stRNA) in specific cytoplasmic granules which could represent secretory organelles (Garcia-Silva et al., 2010a). It is known that T. cruzi releases different populations of EVs (Bayer-Santos et al., 2013) that contain different sets of small RNAs, including stRNAs (Bayer-Santos et al., 2014; Garcia-Silva et al., 2014a; Fernandez-Calero et al., 2015). EVs containing stRNAs and TcPIWI-tryp were shown to be transferred between T. cruzi cells, increasing their differentiation rate, and between parasites and mammalian host cells, inducing changes in gene expression (Garcia-Silva et al., 2014b).
Inflammatory lesions not directly related to the presence of T. cruzi have been reported in patients with chronic Chagas disease cardiomyophaty (CCC) and in a murine model (Pinho et al., 2002). Treatment of mice with T. cruzi EVs before the infection induced severe heart pathology and inflammation, and higher levels of proinflammatory cytokines and nitric oxide, suggesting that EVs are involved in the pathology (Torrecilhas et al., 2012; Nogueira et al., 2015). miRNAs were significantly altered in CCC as compared to idiopathic dilated cardiomyopathy, suggesting that miRNA may regulate gene expression in CCC pathogenesis (Ferreira et al., 2014; Navarro et al., 2015; Linhares-Lacerda and Morrot, 2016). The long ncRNA MIAT (myocardial infarction-associated transcript) was overexpressed in patients with CCC and in mice, indicating that MIAT could be a specific biomarker of CCC (Frade et al., 2016). An additional work showed that T. cruzi infection of thymic epithelial cells induce changes in miRNAs levels of host cells (Linhares-Lacerda et al., 2015; Linhares-Lacerda and Morrot, 2016). It was also shown that mammalian cells incubated with T. cruzi can release EVs which bound to trypomastigotes and protect them against lysis by the complement system (Cestari et al., 2012; Ramirez et al., 2017).
Leishmania donovani and L. braziliensis were also reported to release EVs containing RNAs (Lambertz et al., 2015). In agreement with data from T. cruzi EVs, sequencing L. donovani and L. braziliensis exosomal RNA indicated that the majority of cargo sequences were derived from sncRNA species such as rRNA and tRNA, suggesting that the packaging of specific RNA sequences into EVs may be a conserved phenomenon (Lambertz et al., 2015). In L. braziliensis, which is an RNAi-competent organism, it was also found sequences derived from siRNA-coding regions in both sense and anti-sense, suggesting that these molecules might be packaged in EVs as double-stranded RNAs (Lambertz et al., 2015). Additional work showed that infection of macrophages (Lemaire et al., 2013; Frank et al., 2015; Geraci et al., 2015) and dendritic cells (Geraci et al., 2015) by Leishmania changes host cell miRNA expression profile. However, the mechanism regulating such changes were not determined. Conversely, mechanistic insight was given in regard of the Leishmania-induced reduction of miR-122 in host cells. Delivery of Zn-metalloprotease surface glycoprotein GP63 via EVs to mammalian host cells, allow GP63 to target pre-miRNA processor Dicer1 and prevent miRNP formation, resulting in reduction of miR-122 activity and an increase in parasite burden (Ghosh et al., 2013).
Recently, Bose et al. (2017) proposed a new mechanism to explain the dysregulation of host cell miRNA expression by L. donovani. It involves the blocking of maturation of endosomes to late endosome and MVBs by targeting the endosomal protein HRS (hepatocyte growth factor regulated tyrosine kinase substrate), a vacuolar protein sorting (VPS) protein. Downregulation of HRS in L. donovani-infected macrophages prevents uncoupling of mRNA-AGO2 interaction, blocking degradation of translationally repressed messages and recycling of miRNPs. let-7a miRNPs remain bound to target mRNAs and fail to repress IL-6 mRNA. Enhanced translation of IL-6 in host cells helps Leishmania to suppress host macrophage activation and promote infection.
Autophagic machinery of bone marrow-derived macrophages (BMDMs) is activated in L. major infection. Singh et al. (2016) have provided mechanistic details on the regulation of this process by host miRNAs. Expression of MIR30A-3p is enhanced during L. donovani infection, suggesting a regulatory role of this miRNA in the modulation of host cell autophagy. Transfection of BMDM with specific siRNAs against autophagy-related genes or inhibitors of autophagy-associated miRNAs inhibited autophagic digestion of L. major. BECN1/Beclin 1, a key autophagy-promoting protein, is a potential target of MIR30A-3p which negatively regulates BECN1 expression. The regulation of L. donovani autophagy by targeting BECN1 may have impact on the treatment of visceral leishmaniasis.
Extracellular Protozoan Parasites
Interaction between extracellular protozoan parasites and host cells is critical for tissue adhesion, colonization and damage. E. histolytica and T. vaginalis are extracellular parasites that live in the gastrointestinal and urogenital tracts, respectively. These parasites must adhere to epithelial cells to establish a focus of infection. T. vaginalis trophozoites express adherence factors that allow attachment to ectocervical epithelial cells. Twu et al. (2013) showed that trophozoites release exosomes carrying small RNAs and parasite-specific proteins that fuse with host cells to deliver their cargo. EVs released from a highly adherent strain of T. vaginalis, with tissue-tropic adherence to ectocervical cells, are able to enhance attachment of a poorly adherent strain to both vaginal and prostate epithelial cells. T. vaginalis exosomes also modulate the secretion of proinflammatory cytokines IL-6 and IL-8, possibly allowing the parasite to sustain a chronic infection (Twu et al., 2013). Providing that the production of IL-6 and IL-8 is regulated by endogenous miRNAs, it is possible that host miRNA dysregulation control the expression of these cytokines during T. vaginalis infection.
Modulation of host cells miRNA profile by the pathogen also occurs in amebic infections. E. histolytica kills intestinal epithelial cells after attachment of trophozoite to host cell membranes, leading to a Ca2+-induced signaling that culminates in cell death. This is followed by a potent inflammatory response and invasion of colonic crypts by trophozoites (Ralston and Petri, 2011). Whole genome-scale RNAi screening has proved useful for identifying human host factors involved in cell invasion and intracellular growth of T. cruzi or host factors required for amebic cytotoxicity (Genovesio et al., 2011; Caradonna et al., 2013; Marie et al., 2015). Marie et al. (2015) used a genome-wide miRNA library to screen human epithelial cells susceptible to killing by E. histolytica. After successive rounds of selection with trophozoites, they identified resistant cells to the parasite, showing that RNAi library had increased resistance to E. histolytica cytotoxicity when compared to the control library.
Trypanosoma brucei and related subspecies are transmitted between mammalian hosts by an insect vector (tsetse fly). In both hosts, they are extracellular and migration to specific host tissues is essential for parasite development and pathogenesis (Ralston et al., 2009). Bloodstream trypomastigotes can move out of blood vessels and invade extravascular tissues, including the central nervous system. In tsetse fly, epimastigotes attach to epithelial cells in the salivary gland and differentiate into metacyclic trypomastigotes, which are infective for humans. Recently, T. brucei was reported to produce EVs that are mainly originated from membrane nanotubes (Szempruch et al., 2016b). EVs produced by T. brucei rhodesiense contain the serum resistance-associated protein (SRA), which is necessary for human infectivity. T. brucei rhodesiense can transfer SRA to non-human infectious trypanosomes via EVs, allowing these parasites to evade the human innate immunity. In addition, T. brucei EVs can also fuse with mammalian erythrocytes, altering the physical properties of the membrane and causing erythrocyte lysis and anemia (Szempruch et al., 2016b). Despite this interesting work, there is no report of transference of non-coding RNAs in T. brucei to date.
Concluding Remarks
There is growing number of evidence showing that ncRNAs actively change the outcome of infections. Host miRNA dysregulation has been associated with impaired immune response and increased host colonization by the pathogen. Conversely, miRNAs can be employed by the host as a mechanism of defense against the parasite. EVs seem to intermediate this communication, but in most cases it is not known precisely which molecules are involved. Future investigation is required to identify all ncRNAs molecules and the possible mechanism by which they can be transferred to mammalian cells in different conditions. Likewise, the role of human host-derived EVs on infection is another important field to be explored. Besides its potential as diagnostic and prognostic tools, miRNAs and other ncRNAs are potential targets for chemo- and immunotherapies for parasitic diseases.
Author Contributions
All authors (EB-S, MM, JdS) conceived and wrote the manuscript. They have made substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (11/51475-3); EB-S was awarded a FAPESP fellowship (15/25381-2), MM (157637/2015-8) and JdS (306591/2015-4) were awarded fellowships from Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq).
References
- Aidoo M., Terlouw D. J., Kolczak M. S., McElroy P. D., Ter Kuile F. O., Kariuki S., et al. (2002). Protective effects of the sickle cell gene against malaria morbidity and mortality. Lancet 359 1311–1312. 10.1016/S0140-6736(02)08273-8279 [DOI] [PubMed] [Google Scholar]
- Asgari S. (2011). Role of micrornas in insect host-microorganism interactions. Front. Physiol. 2:48 10.3389/fphys.2011.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atayde V. D., Shi H., Franklin J. B., Carriero N., Notton T., Lye L.-F., et al. (2013). The structure and repertoire of small interfering RNAs in Leishmania (Viannia) braziliensis reveal diversification in the trypanosomatid RNAi pathway. Mol. Microbiol. 87 580–593. 10.1111/mmi.12117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atayde V. D., Tschudi C., Ullu E. (2011). The emerging world of small silencing RNAs in protozoan parasites. Trends Parasitol. 27 321–327. 10.1016/j.pt.2011.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker K. R., Lu Z., Kim H., Zheng Y., Chen J., Conroy A. L., et al. (2017). miR-155 modifies inflammation, endothelial activation and blood-brain barrier dysfunction in cerebral malaria. Mol. Med. 10.2119/molmed.2016.00139 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barteneva N. S., Maltsev N., Vorobjev I. A. (2013). Microvesicles and intercellular communication in the context of parasitism. Front. Cell. Infect. Microbiol. 3:49 10.3389/fcimb.2013.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista T. M., Marques J. T. (2011). RNAi pathways in parasitic protists and worms. J. Proteom. 74 1504–1514. 10.1016/j.jprot.2011.02.032 [DOI] [PubMed] [Google Scholar]
- Baum J., Papenfuss A. T., Mair G. R., Janse C. J., Vlachou D., Waters A. P., et al. (2009). Molecular genetics and comparative genomics reveal RNAi is not functional in malaria parasites. Nucleic Acids Res. 37 3788–3798. 10.1093/nar/gkp239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer-Santos E., Aguilar-Bonavides C., Rodrigues S. P., Cordero E. M., Marques A. F., Varela-Ramirez A., et al. (2013). Proteomic analysis of Trypanosoma cruzi secretome: characterization of two populations of extracellular vesicles and soluble proteins. J. Proteome Res. 12 883–897. 10.1021/pr300947g [DOI] [PubMed] [Google Scholar]
- Bayer-Santos E., Lima F. M., Ruiz J. C., Almeida I. C., da Silveira J. F. (2014). Characterization of the small RNA content of Trypanosoma cruzi extracellular vesicles. Mol. Biochem. Parasitol. 193 71–74. 10.1016/j.molbiopara.2014.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose M., Barman B., Goswami A., Bhattacharyya S. N. (2017). Spatiotemporal uncoupling of microRNA-mediated translational repression and target RNA degradation controls microRNP recycling in mammalian cells. Mol. Cell. Biol. 37:e00464–16 10.1128/MCB.00464-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun L., Cannella D., Ortet P., Barakat M., Sautel C. F., Kieffer S., et al. (2010). A complex small RNA repertoire is generated by a plant/fungal-like machinery and effected by a metazoan-like argonaute in the single-cell human parasite Toxoplasma gondii. PLoS Pathog. 6:e1000920 10.1371/journal.ppat.1000920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannella D., Brenier-Pinchart M.-P., Braun L., van Rooyen J. M., Bougdour A., Bastien O., et al. (2014). miR-146a and miR-155 delineate a MicroRNA fingerprint associated with Toxoplasma persistence in the host brain. Cell Rep. 6 928–937. 10.1016/j.celrep.2014.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caradonna K. L., Engel J. C., Jacobi D., Lee C.-H., Burleigh B. A. (2013). Host metabolism regulates intracellular growth of Trypanosoma cruzi. Cell Host Microbe 13 108–117. 10.1016/j.chom.2012.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cestari I., Ansa-Addo E., Deolindo P., Inal J. M., Ramirez M. I. (2012). Trypanosoma cruzi immune evasion mediated by host cell-derived microvesicles. J. Immunol. 188 1942–1952. 10.4049/jimmunol.1102053 [DOI] [PubMed] [Google Scholar]
- Chen X.-M., Splinter P. L., O’Hara S. P., LaRusso N. F. (2007). A cellular micro-RNA, let-7i, regulates toll-like receptor 4 expression and contributes to cholangiocyte immune responses against Cryptosporidium parvum infection. J. Biol. Chem. 282 28929–28938. 10.1074/jbc.M702633200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DaRocha W. D., Otsu K., Teixeira S. M. R., Donelson J. E. (2004). Tests of cytoplasmic RNA interference (RNAi) and construction of a tetracycline-inducible T7 promoter system in Trypanosoma cruzi. Mol. Biochem. Parasitol. 133 175–186. 10.1016/j.molbiopara.2003.10.005 [DOI] [PubMed] [Google Scholar]
- Deolindo P., Evans-Osses I., Ramirez M. I. (2013). Microvesicles and exosomes as vehicles between protozoan and host cell communication. Biochem. Soc. Trans. 41 252–257. 10.1042/BST20120217 [DOI] [PubMed] [Google Scholar]
- Fernandez-Calero T., Garcia-Silva R., Pena A., Robello C., Persson H., Rovira C., et al. (2015). Profiling of small RNA cargo of extracellular vesicles shed by Trypanosoma cruzi reveals a specific extracellular signature. Mol. Biochem. Parasitol. 199 19–28. 10.1016/j.molbiopara.2015.03.003 [DOI] [PubMed] [Google Scholar]
- Ferreira L. R. P., Frade A. F., Santos R. H. B., Teixeira P. C., Baron M. A., Navarro I. C., et al. (2014). MicroRNAs miR-1, miR-133a, miR-133b, miR-208a and miR-208b are dysregulated in Chronic Chagas disease Cardiomyopathy. Int. J. Cardiol. 175 409–417. 10.1016/j.ijcard.2014.05.019 [DOI] [PubMed] [Google Scholar]
- Frade A. F., Laugier L., Ferreira L. R. P., Baron M. A., Benvenuti L. A., Teixeira P. C., et al. (2016). Myocardial infarction–associated transcript, a long noncoding RNA, is overexpressed during dilated cardiomyopathy due to chronic chagas disease. J. Infect. Dis. 214 161–165. 10.1093/infdis/jiw095 [DOI] [PubMed] [Google Scholar]
- Frank B., Marcu A., de Oliveira Almeida Petersen A. L., Weber H., Stigloher C., Mottram J. C., et al. (2015). Autophagic digestion of Leishmania major by host macrophages is associated with differential expression of BNIP3, CTSE, and the miRNAs miR-101c, miR-129, and miR-210. Parasit. Vectors 8 404 10.1186/s13071-015-0974-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Silva M. R., Cabrera-Cabrera F., das Neves R. F., Souto-Padrón T., de Souza W., Cayota A. (2014a). Gene expression changes induced by Trypanosoma cruzi shed microvesicles in mammalian host cells: relevance of tRNA-derived halves. Biomed. Res. Int. 2014:305239 10.1155/2014/305239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Silva M. R., Cabrera-Cabrera F., Güida M. C., Cayota A. (2012). Hints of tRNA-derived small RNAs role in RNA silencing mechanisms. Genes 3 603–614. 10.3390/genes3040603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Silva M. R., das Neves R. F., Cabrera-Cabrera F., Sanguinetti J., Medeiros L. C., Robello C., et al. (2014b). Extracellular vesicles shed by Trypanosoma cruzi are linked to small RNA pathways, life cycle regulation, and susceptibility to infection of mammalian cells. Parasitol. Res. 113 285–304. 10.1007/s00436-013-3655-3651 [DOI] [PubMed] [Google Scholar]
- Garcia-Silva M. R., Frugier M., Tosar J. P., Correa-Dominguez A., Ronalte-Alves L., Parodi-Talice A., et al. (2010a). A population of tRNA-derived small RNAs is actively produced in Trypanosoma cruzi and recruited to specific cytoplasmic granules. Mol. Biochem. Parasitol. 171 64–73. 10.1016/j.molbiopara.2010.02.003 [DOI] [PubMed] [Google Scholar]
- Garcia-Silva M. R., Sanguinetti J., Cabrera-Cabrera F., Franzén O., Cayota A. (2014c). A particular set of small non-coding RNAs is bound to the distinctive Argonaute protein of Trypanosoma cruzi: insights from RNA-interference deficient organisms. Gene 538 379–384. 10.1016/j.gene.2014.01.023 [DOI] [PubMed] [Google Scholar]
- Garcia Silva M. R., Tosar J. P., Frugier M., Pantano S., Bonilla B., Esteban L., et al. (2010b). Cloning, characterization and subcellular localization of a Trypanosoma cruzi argonaute protein defining a new subfamily distinctive of trypanosomatids. Gene 466 26–35. 10.1016/j.gene.2010.06.012 [DOI] [PubMed] [Google Scholar]
- Genovesio A., Giardini M. A., Kwon Y.-J., Dossin F., de M., Choi S. Y., et al. (2011). Visual genome-wide RNAi screening to identify human host factors required for Trypanosoma cruzi infection. PLoS ONE 6:e19733 10.1371/journal.pone.0019733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraci N. S., Tan J. C., McDowell M. A. (2015). Characterization of microRNA expression profiles in Leishmania -infected human phagocytes. Parasite Immunol. 37 43–51. 10.1111/pim.12156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh J., Bose M., Roy S., Bhattacharyya S. N. (2013). Leishmania donovani targets dicer1 to downregulate miR-122, lower serum cholesterol, and facilitate murine liver infection. Cell Host Microbe 13 277–288. 10.1016/j.chom.2013.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong A.-Y., Hu G., Zhou R., Liu J., Feng Y., Soukup G. A., et al. (2011). MicroRNA-221 controls expression of intercellular adhesion molecule-1 in epithelial cells in response to Cryptosporidium parvum infection. Int. J. Parasitol. 41 397–403. 10.1016/j.ijpara.2010.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakimi M., Cannella D. (2011). Apicomplexan parasites and subversion of the host cell microRNA pathway. Trends Parasitol. 27 481–486. 10.1016/j.pt.2011.07.001 [DOI] [PubMed] [Google Scholar]
- Judice C. C., Bourgard C., Kayano A. C. A. V., Albrecht L., Costa F. T. M. (2016). MicroRNAs in the host-apicomplexan parasites interactions: a review of immunopathological aspects. Front. Cell. Infect. Microbiol. 6:5 10.3389/fcimb.2016.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolev N. G., Tschudi C., Ullu E. (2011). RNA interference in protozoan parasites: achievements and challenges. Eukaryot. Cell 10 1156–1163. 10.1128/EC.05114-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambertz U., Oviedo Ovando M. E., Vasconcelos E., Unrau P. J., Myler P. J., Reiner N. E. (2015). Small RNAs derived from tRNAs and rRNAs are highly enriched in exosomes from both old and new world Leishmania providing evidence for conserved exosomal RNA Packaging. BMC Genomics 16:151 10.1186/s12864-015-1260-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMonte G., Philip N., Reardon J., Lacsina J. R., Majoros W., Chapman L., et al. (2012). Translocation of sickle cell erythrocyte microRNAs into Plasmodium falciparum inhibits parasite translation and contributes to malaria resistance. Cell Host Microbe 12 187–199. 10.1016/j.chom.2012.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire J., Mkannez G., Guerfali F. Z., Gustin C., Attia H., Sghaier R. M., et al. (2013). MicroRNA expression profile in human macrophages in response to leishmania major infection. PLoS Negl. Trop. Dis. 7:e2478 10.1371/journal.pntd.0002478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay M. A. (2008). microRNAs and the immune response. Trends Immunol. 29 343–351. 10.1016/j.it.2008.04.004 [DOI] [PubMed] [Google Scholar]
- Linhares-Lacerda L., Morrot A. (2016). Role of small RNAs in trypanosomatid infections. Front. Microbiol. 7:367 10.3389/fmicb.2016.00367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linhares-Lacerda L., Palu C. C., Ribeiro-Alves M., Paredes B. D., Morrot A., Garcia-Silva M. R., et al. (2015). Differential expression of microRNAs in thymic epithelial cells from Trypanosoma cruzi acutely infected mice: putative role in thymic atrophy. Front. Immunol. 6:428 10.3389/fimmu.2015.00428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantel P.-Y., Hjelmqvist D., Walch M., Kharoubi-Hess S., Nilsson S., Ravel D., et al. (2016). Infected erythrocyte-derived extracellular vesicles alter vascular function via regulatory Ago2-miRNA complexes in malaria. Nat. Commun. 7:12727 10.1038/ncomms12727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantel P.-Y., Marti M. (2014). The role of extracellular vesicles in Plasmodium and other protozoan parasites. Cell. Microbiol. 16 344–354. 10.1111/cmi.12259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcilla A., Martin-Jaular L., Trelis M., de Menezes-Neto A., Osuna A., Bernal D., et al. (2014). Extracellular vesicles in parasitic diseases. J. Extracell. Vesicles 3:25040 10.3402/jev.v3.25040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie C., Verkerke H. P., Theodorescu D., Petri W. A., Kotloff K. L., Petri W. A., et al. (2015). A whole-genome RNAi screen uncovers a novel role for human potassium channels in cell killing by the parasite Entamoeba histolytica. Sci. Rep. 5:13613 10.1038/srep13613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti M., Johnson P. J. (2016). Emerging roles for extracellular vesicles in parasitic infections. Curr. Opin. Microbiol. 32 66–70. 10.1016/j.mib.2016.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Militello K. T., Refour P., Comeaux C. A., Duraisingh M. T. (2008). Antisense RNA and RNAi in protozoan parasites: working hard or hardly working? Mol. Biochem. Parasitol. 157 117–126. 10.1016/j.molbiopara.2007.10.004 [DOI] [PubMed] [Google Scholar]
- Navarro I. C., Ferreira F. M., Nakaya H. I., Baron M. A., Vilar-Pereira G., Pereira I. R., et al. (2015). MicroRNA transcriptome profiling in heart of Trypanosoma cruzi-infected mice: parasitological and cardiological outcomes. PLoS Negl. Trop. Dis. 9:e0003828 10.1371/journal.pntd.0003828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira P. M., Ribeiro K., Silveira A. C. O., Campos J. H., Martins-Filho O. A., Bela S. R., et al. (2015). Vesicles from different Trypanosoma cruzi strains trigger differential innate and chronic immune responses. J. Extracell. Vesicles 4:28734 10.3402/jev.v4.28734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinho R. T., Vannier-Santos M. A., Alves C. R., Marino A. P. M. P., Castello Branco L. R. R., Lannes-Vieira J. (2002). Effect of Trypanosoma cruzi released antigens binding to non-infected cells on anti-parasite antibody recognition and expression of extracellular matrix components. Acta Trop. 83 103–115. 10.1016/S0001-706X(02)00062-1 [DOI] [PubMed] [Google Scholar]
- Plattner F., Soldati-Favre D. (2008). Hijacking of host cellular functions by the Apicomplexa. Annu. Rev. Microbiol. 62 471–487. 10.1146/annurev.micro.62.081307.162802 [DOI] [PubMed] [Google Scholar]
- Ralston K. S., Kabututu Z. P., Melehani J. H., Oberholzer M., Hill K. L. (2009). The Trypanosoma brucei flagellum: moving parasites in new directions. Annu. Rev. Microbiol. 63 335–362. 10.1146/annurev.micro.091208.073353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston K. S., Petri W. A. (2011). Tissue destruction and invasion by Entamoeba histolytica. Trends Parasitol. 27 254–263. 10.1016/j.pt.2011.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez M. I., Deolindo P., de Messias-Reason I. J., Arigi E. A., Choi H., Almeida I. C., et al. (2017). Dynamic flux of microvesicles modulate parasite-host cell interaction of Trypanosoma cruzi in eukaryotic cells. Cell. Microbiol. 19:e12672 10.1111/cmi.12672 [DOI] [PubMed] [Google Scholar]
- Robinson K. A., Beverley S. M. (2003). Improvements in transfection efficiency and tests of RNA interference (RNAi) approaches in the protozoan parasite Leishmania. Mol. Biochem. Parasitol. 128 217–228. 10.1016/S0166-6851(03)00079-3 [DOI] [PubMed] [Google Scholar]
- Roditi I. (2016). The languages of parasite communication. Mol. Biochem. Parasitol. 208 16–22. 10.1016/j.molbiopara.2016.05.008 [DOI] [PubMed] [Google Scholar]
- Saba R., Sorensen D. L., Booth S. A. (2014). MicroRNA-146a: a dominant, negative regulator of the innate immune response. Front. Immunol. 5:578 10.3389/fimmu.2014.00578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A. K., Pandey R. K., Shaha C., Madhubala R. (2016). MicroRNA expression profiling of Leishmania donovani-infected host cells uncovers the regulatory role of MIR30A-3p in host autophagy. Autophagy 12 1817–1831. 10.1080/15548627.2016.1203500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoco P. H., Wagner G., Talavera-Lopez C., Gerber A., Zaha A., Thompson C. E., et al. (2014). Genome of the avirulent human-infective trypanosome—Trypanosoma rangeli. PLoS Negl. Trop. Dis. 8:e3176 10.1371/journal.pntd.0003176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szempruch A. J., Dennison L., Kieft R., Harrington J. M., Hajduk S. L. (2016a). Sending a message: extracellular vesicles of pathogenic protozoan parasites. Nat. Rev. Microbiol. 14 669–675. 10.1038/nrmicro.2016.110 [DOI] [PubMed] [Google Scholar]
- Szempruch A. J., Sykes S. E., Kieft R., Dennison L., Becker A. C., Gartrell A., et al. (2016b). Extracellular vesicles from Trypanosoma brucei mediate virulence factor transfer and cause host anemia. Cell 164 246–257. 10.1016/j.cell.2015.11.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrecilhas A. C., Schumacher R. I., Alves M. J. M., Colli W. (2012). Vesicles as carriers of virulence factors in parasitic protozoan diseases. Microbes Infect. 14 1465–1474. 10.1016/j.micinf.2012.07.008 [DOI] [PubMed] [Google Scholar]
- Twu O., de Miguel N., Lustig G., Stevens G. C., Vashisht A. A., Wohlschlegel J. A., et al. (2013). Trichomonas vaginalis exosomes deliver cargo to host cells and mediate host:parasite interactions. PLoS Pathog. 9:e1003482 10.1371/journal.ppat.1003482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigorito E., Kohlhaas S., Lu D., Leyland R. (2013). miR-155: an ancient regulator of the immune system. Immunol. Rev. 253 146–157. 10.1111/imr.12057 [DOI] [PubMed] [Google Scholar]
- Watanabe Costa R., da Silveira J. F., Bahia D. (2016). Interactions between Trypanosoma cruzi secreted proteins and host cell signaling pathways. Front. Microbiol. 7:388 10.3389/fmicb.2016.00388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter F., Edaye S., Hüttenhofer A., Brunel C. (2007). Anopheles gambiae miRNAs as actors of defence reaction against Plasmodium invasion. Nucleic Acids Res. 35 6953–6962. 10.1093/nar/gkm686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiner G. M., Norman K. L., Thomson J. M., Hammond S. M., Boothroyd J. C. (2010). Toxoplasma gondii infection specifically increases the levels of key host microRNAs. PLoS ONE 5:e8742 10.1371/journal.pone.0008742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Cai X., Bradley J. E. (2013). microRNAs in parasites and parasite infection. RNA Biol. 10 371–379. 10.4161/rna.23716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R., Hu G., Liu J., Gong A.-Y., Drescher K. M., Chen X.-M. (2009). NF-kappaB p65-dependent transactivation of miRNA genes following Cryptosporidium parvum infection stimulates epithelial cell immune responses. PLoS Pathog. 5:e1000681 10.1371/journal.ppat.1000681 [DOI] [PMC free article] [PubMed] [Google Scholar]