Abstract
Study Objectives:
Obesity and regional fat distribution, measured by neck fat mass percentage using dual-energy X-ray absorptiometry (DXA), correlate with obstructive sleep apnea (OSA) severity in adults. In obese children, neck-to-waist-circumference ratio predicts OSA. This study examined associations between body fat percentage and distribution and sleep-disordered breathing (SDB) severity in obese youth, measured with DXA.
Methods:
Cross-sectional retrospective study conducted at a tertiary children's hospital. Participants were aged 6 to 18 years with obesity (body mass index [BMI] > 99th percentile [BMI z-score 2.35] or > 95th percentile with comorbidity). They underwent polysomnography and DXA to quantify body fat percentage and distribution ratios (neck-to-abdominal fat percentage [NAF % ratio]). SDB was defined as apnea-hypopnea index (AHI) > 5 and OSA as obstructive AHI (OAHI) > 1 event/h. Relationships of BMI z-score and NAF % ratio to log AHI and log OAHI were evaluated.
Results:
Thirty individuals participated; 18 male; median age 14.1 years. Twenty-four individuals had BMI z-scores > 2.35. Ten had AHI > 5 events/h. NAF % ratio was significantly associated with log AHI in males and with log OAHI in all, whereas total fat mass percent was not. The association between log OAHI and NAF % ratio was significant in males, but not females. NAF % ratio was significantly associated with log OAHI in those with BMI z-score above 2.35.
Conclusions:
NAF % ratio was associated with OSA severity in males and youth with BMI > 99th percentile; however, total fat mass percentage was not, suggesting that body fat distribution is associated with OSA risk in youth.
Citation:
Glicksman A, Hadjiyannakis S, Barrowman N, Walker S, Hoey L, Katz SL. Body fat distribution ratios and obstructive sleep apnea severity in youth with obesity. J Clin Sleep Med. 2017;13(4):545–550.
Keywords: DXA, obesity, obstructive sleep apnea, ratio, youth
INTRODUCTION
Obstructive sleep apnea (OSA) is characterized by recurrent episodes of upper airway resistance and obstruction in sleep, leading to hypopneas and apneas, disturbed sleep pattern, and impaired gas exchange.1,2 When untreated, OSA in children can lead to failure to thrive and cor pulmonale, in addition to behavioral difficulties, hyperactivity, and poor academic performance.3,4 Questionnaires and clinical assessment are insufficient to predict OSA, and polysomnography (PSG) is needed to determine presence and severity of OSA.4,5 Unfortunately although OSA is the most common indication for PSG assessment,4 resources are often inadequate to deal with the large volume of referrals for testing and wait times for pediatric PSG may be up to 2 years in some centers.6 Because of high demand for a limited resource, screening tools for prioritization for PSG testing are needed.6,7
OSA in older children8 and adults is associated with obesity and particularly with increased visceral adipose tissue.9 Increased neck size in adults, reflective of increased volume of neck adipose tissue,10 predicts OSA, particularly in males. Furthermore, in adults, increased fat mass at the level of the neck region and neck fat mass percentage as assessed by dual X-ray absorptiometry (DXA), a noninvasive method of accurately quantifying tissue mass and type in regional body areas,11 is associated with OSA severity.12 In children aged 7 years and older, neck-to-waist circumference ratio predicts OSA, particularly in those who are overweight or obese.13 These findings suggest that relative body fat distribution is likely of importance in understanding OSA risk in youth, although this has not been well studied. The objectives of this study are therefore to determine if total body fat percentage and body fat distribution, as examined by body fat ratios determined with DXA, are associated with OSA severity in youth.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Recent investigations have found that OSA risk in adults is associated with distribution of body fat directly measured using dual-energy X-ray absorptiometry. Whether a similar connection exists in obese youth is not yet well understood.
Study Impact: These study results suggest that distribution of fat rather than total adiposity is important to the development of OSA in obese youth, suggesting a pathophysiology of disease similar to that in adults. These findings also support the utility of neck-to-waist circumference ratios as a surrogate marker of body fat distribution and OSA risk.
METHODS
Study Population
The study included participants in the Center for Healthy Active Living program at the Children's Hospital of Eastern Ontario, which manages children with complex medical obesity aged 5 to 16 years, with a body mass index (BMI) above the 99th percentile (extreme obesity, corresponding to a BMI z-score of > 2.3514) or BMI z-score greater than the 95th percentile with associated severe medical or psychosocial comorbidity or chronic illness affected by obesity. Those who had undergone both PSG and DXA as part of their evaluation through the Center for Healthy Active Living program from 2008 to 2012 were included in this study, provided the time interval between PSG and DXA was less than 1 year. Data for this cross-sectional study were collected retrospectively. Patients were excluded if they had neuromuscular, craniofacial, or genetic illnesses that could affect the results of the PSG or body fat distribution or if the time period between PSG and DXA was greater than 1 year. This study was approved by the Research Ethics Board at the Children's Hospital of Eastern Ontario Research Institute.
Data Abstraction
Polysomnograms
Polysomnograms were obtained and scored according to American Association of Sleep Medicine pediatric standards.15 This included monitoring of 4 electroencephalogram leads, electrooculogram, and submental and tibial electromyogram, in addition to chest and abdominal wall inductance plethysmography, airflow measurements (nasal pressure), oxygen saturation, transcutaneous and end-tidal carbon dioxide measurements, and video and audio recordings. Studies were scored by a sleep technologist, then reviewed and interpreted by one of two physicians. The polysomnogram closest to the date of the initial DXA was selected, provided there was a recording of sleep without intervention. Any sleep studies that included time with positive airway pressure treatment were reanalyzed such that only data from sleep without respiratory support were included in the PSG data collection. If no such data were available, the next closest PSG with information about sleep without intervention was selected. The following parameters were abstracted from the polysomnogram: age, sex, height, weight, total sleep time, and number of central, obstructive, and mixed apneas and hypopneas. The following indices were recorded: central apnea index, central hypopnea index, obstructive apnea-hypopnea index (OAHI), apnea index (AI), apnea-hypopnea index (AHI), and hypopnea index. When hypopnea was not otherwise differentiated in the report it was considered as an obstructive event. Mixed hypopneas were included in calculation of OAHI when recorded in the sleep study. Additional data included lowest oxygen saturation and highest carbon dioxide level measured by both transcutaneous and end-tidal carbon dioxide probes. Sleep-disordered breathing (SDB) was defined as AHI > 5 events/h and OSA as OAHI > 1 event/h.
Body Mass Index
Height and weight for all patients were obtained from PSG, in order to calculate a BMI; if not available from PSG, then parameters were obtained from the closest clinical encounter. BMI values were translated into a z-score based on Centers for Disease Control and Prevention norms for age and sex.14
DXA Scan
The GE Lunar Prodigy scanner (GE Medical Systems Lunar, Madison, Wisconsin, United States) was used along with the enCORE 2008 version 12.30 software (GE Medical Systems Lunar, Madison, Wisconsin, United States). The standard data was extracted from the composition results tab using standard body composition analysis.
Using the model for region of interest (ROI) created by Bruno et al.,12 custom composition results were obtained from all the manually drawn ROI. Data from novel regions were extracted from the custom composition results tab using manual body composition analysis.
If a patient was unable to fit into the acquisition field, the patient would be shifted to his or her left so that all of the patient's right side could be imaged. The data for the missing left side would then be estimated based on the right sided data, as per standard protocol.
Body Fat Percentage
From the DXA scan, both fat mass and percentage were recorded for the following areas: total body, total body without head, and trunk. Fat volume mass and fat percentage were calculated for the following ROI:
Neck: the neck region was bounded superiorly by the lower margin of the mandible, inferiorly to the upper margin of the sternum, and laterally by the outer margins of the neck's soft tissue.
Abdomen: the abdominal region was bounded superiorly by the upper margin of L1, inferiorly by the lower margin of L4, and laterally by the outer margins of the abdomen's soft tissue.
The composition tab then displayed the percentage of fat (fat per tissue) and grams of tissue (fat and lean) for each region.
From these regions, a ratio of neck fat percentage to abdominal fat percentage (NAF % ratio) was created for each study subject.
Statistical Analysis
A log transformation of AHI and OAHI was used to facilitate parametric statistical analysis. In cases where no apneas or hypopneas were detected, it was necessary to impute a small value, representing a very low event rate, prior to log transformation. On the assumption that over an 8-hour time period, a single event could have been missed, a value of one-eighth was imputed in these cases. Subsequent sensitivity analyses indicated that results were robust to the specific value imputed. Pearson correlation was then calculated to examine relationships between log of AHI and BMI z-score, percentage total body fat, and NAF % ratio. The same statistical method was used to examine log of OAHI in comparison with BMI-z, and NAF % ratio. For minimum oxygen saturation and maximum carbon dioxide level, Spearman rank correlation coefficient was used because of the presence of extreme values.
Multivariate association of log AHI with BMI z-score and NAF % ratio, adjusting for sex, a known contributor to risk of SDB,16,17 was evaluated using linear regression. A similar analysis was performed for log OAHI. Multicollinearity was evaluated using the variance inflation factor. Performance of the regression models was evaluated using R.
RESULTS
Forty-six individuals were identified for whom both PSG and DXA scan were obtained. Individuals with underlying disease affecting SDB or body weight distribution were excluded from the analysis; 2 subjects with spina bifida, 2 with trisomy 21, and 1 with Prader-Willi syndrome were excluded. One individual was excluded because of a discrepancy between date of birth listed on PSG and DXA. Finally, 10 individuals were excluded because the time interval between PSG and DXA was longer than 1 year. Ultimately 30 children with obesity ages 8 to 18 years for whom PSG and DXA were performed were included in the analysis.
Demographics are highlighted in Table 1. Median age at DXA was 14.1 years (interquartile range [IQR] 11.8–15.9). The median time between PSG and DXA was 4.5 months (IQR 3.2–6.3). The median BMI change between assessments was an increase of 0.16 units of BMI (IQR −0.47 to 1.82; P = .10). One patient had a significant weight loss of 9 kg in the 9-month interval between the sleep study and DXA, corresponding to a decrease of 4 units of BMI. No other individuals had weight decreases exceeding 1 kg.
Table 1.
Demographic and clinical characteristics of study population (n = 30).
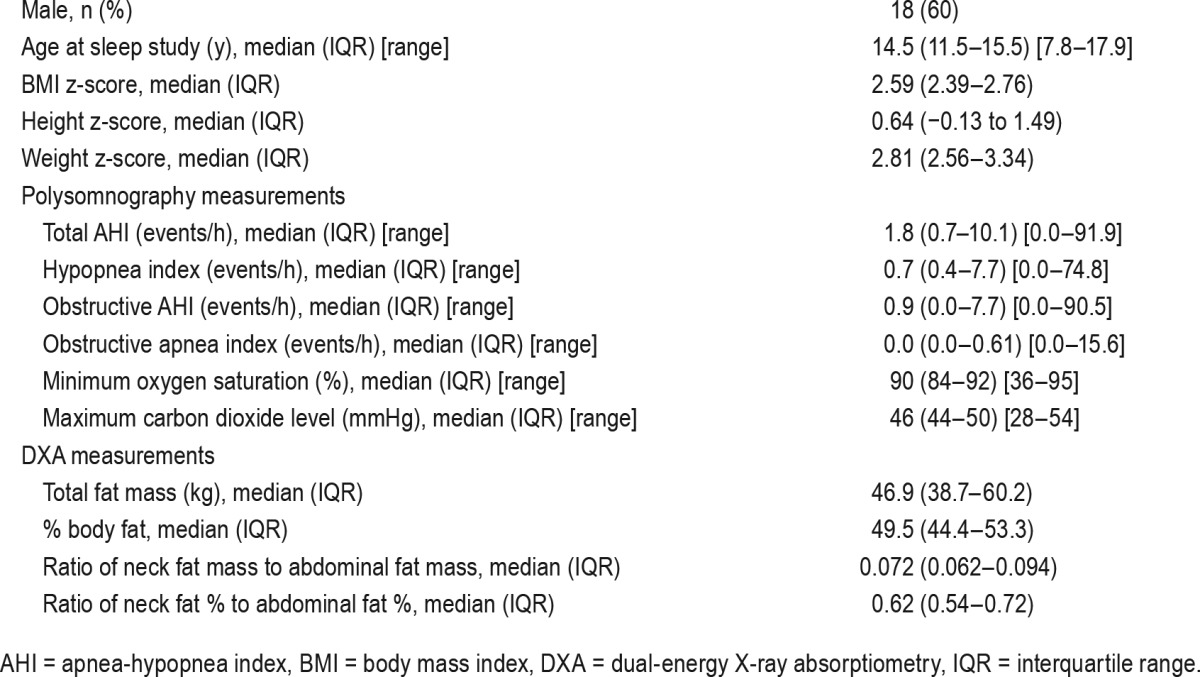
Ten participants had AHI > 5 events/h; 8 (80%) were male. All participants were obese (BMI above 95th percentile); 24 (80%) had a BMI z-score above 2.35, of whom 17 were male (71%).
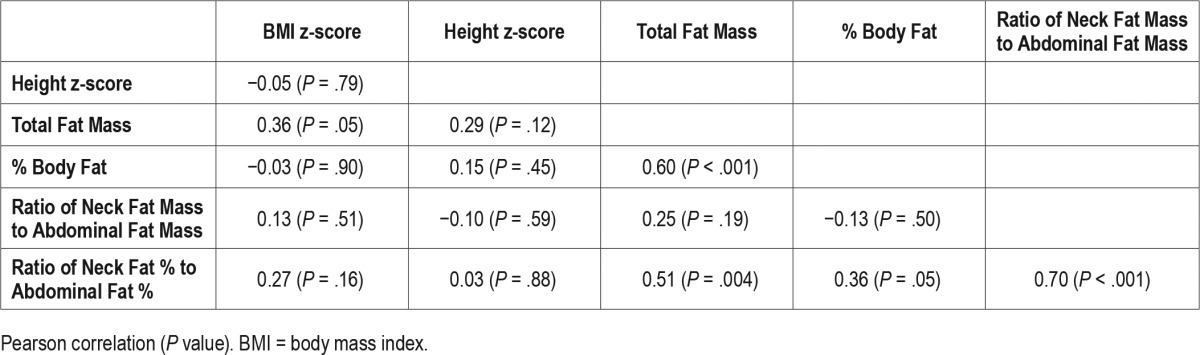
Associations between measurements of body fat and its distribution are reported in Table 2. The NAF % ratio was significantly associated with total fat mass (P = .004), percent body fat (P = .05), and ratio of neck-to-abdominal fat mass (P < .001), but not with BMI z-score (P = .16). BMI z-score was significantly associated with total fat mass (P = .05) but not with measures of body fat distribution.
Table 2.
Association between body fat measurements.
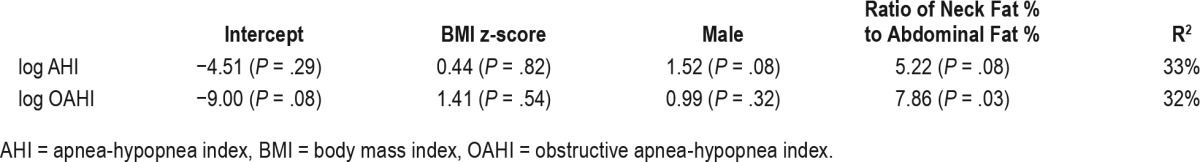
BMI z-score was significantly associated with both log AHI and log OAHI (Table 3; P = .01 and P = .02 respectively). The NAF % ratio was significantly associated with log OAHI (P = .02) but not with log AHI (P = .08). Other measures of body fat distribution were not significantly associated with either log OAHI or log AHI. Minimum oxygen saturation and maximum carbon dioxide were not significantly associated with any of these measures (data not shown).
Table 3.
Association between log AHI, log OAHI, and anthropometric variables.

Multiple linear regressions of log AHI and log OAHI on BMI z-score and NAF % ratio, adjusting for sex, demonstrated an independent association between log OAHI and NAF % ratio (Table 4). Among obese youth, greater fat mass and percent body fat are correlated with NAF % ratio, suggesting that those who have higher fat mass tend to distribute fat in the neck preferentially over the abdomen (Table 2).
Table 4.
Regression model coefficients and R2.
DISCUSSION
Although studies exist within the adult literature examining body fat distribution and SDB, there are limited reports investigating this relationship in youth with obesity. To the best of our knowledge, this is the first study to examine the connection between OSA and body fat distribution in this population specifically by using DXA to assess the ratio of neck-to-abdominal fat.
The most striking finding of this study was an independent association of NAF % ratio and log OAHI. Specifically, in a regression model for log OAHI, with adjustment for sex, NAF % ratio was statistically significant but BMI z-score was not. This supports our hypothesis that body fat distribution is a more important predictor of OSA than total adiposity. The predictive utility of NAF % ratio for OSA remains to be determined in larger prospective studies.
Novel to our study, DXA was used to assess regional differences in adipose tissue in obese youth in order to create ratios to examine these differences. DXA is a noninvasive method used primarily to measure bone mineral density. It can also be used to accurately quantify tissue mass including bone, lean mass, and fat, as well as the percentage of each tissue type in different regional areas.11 Values of fat mass obtained through DXA have proven to be highly correlated to values obtained through magnetic resonance imaging (MRI) and computed tomography (CT), which are considered to be the gold standard methods for volumetric analysis of adipose tissue.18–20 In children, particularly in those who are overweight or obese, visceral adipose tissue determined by DXA correlates with CT measurements.21 DXA has advantages over MRI and CT in children. Measurements created by DXA provide a lower effective dose of radiation in comparison with CT. Whole body DXA is faster to perform and more cost effective than whole body MRI.22 Additionally, the enclosed space of an MRI scanner is contrasted by the open design of a DXA scanner, which enhances tolerability in both youth and adults predisposed to claustrophobia.
Previous research has shown that neck-to-waist circumference ratio predicts OSA, particularly in youth who are over-weight or obese.13 Our study provides additional evidence that body fat distribution is important and more sensitive than BMI alone as a predictor of OSA severity in this population.
Our findings are consistent with previous reports of increased pharyngeal fat pad size in obese children with OSA compared to counterparts without OSA. A study of 22 obese youth with OSA with age-matched obese controls by Arens et al. discovered a 28% and 30% increase in pharyngeal fat pads and abdominal visceral fat, respectively, in the OSA cohort.23 BMI z-score and OSA severity were not found to be effect modifiers. Restriction of airway size secondary to both pharyngeal fat pad size and increased lymphoid tissue were related to OSA risk, but comparisons of the ratio of regional fat percent differences was not examined; as per our findings, however, this does appear to contribute to OSA risk. Similarly, height z-score, a surrogate measure of airway length and size,24 was not associated with body fat distribution nor OSA.
Our study used the same regions of interest via DXA as those used in a study comparing adults with obesity, with and without OSA, in whom body fat percentage overall was similar between groups, as was fat-free mass at the abdominal region.12 Those with OSA and obesity, however, were found to have a decrease in fat-free mass at the level of the oral region, as well as an increase in soft tissue and greater fat mass at the neck region. It was proposed that the specific fat mass distribution played an important role in the development of OSA. Our study of youth rather than adults found similar regional distribution effects of fat in association with log OAHI, suggesting that body fat distribution in youth with obesity plays a similar role in risk for OSA as it does in adults with obesity.
Another recently published study also examined the correlation between body fat composition in youth with obesity and parameters of SDB; as in our study DXA was used as the mode of body composition analysis. Unlike in our study, however, log OAHI (OAHI including mixed apneas) in that study was not correlated significantly with BMI across the entire cohort, but rather only associated in males aged 10 to 12 years.25 Trunk fat percentage, however, did correlate with OAHI severity in the older male subgroup. This suggests that obesity alone cannot account for OSA severity.
Fat percentage ratios may be better methods than BMI or single regional fat percentage values for risk assessment and understanding the mechanism of OSA in youth.25 Obese youth often have neck and waist circumference values that plot in the upper percentiles of a normal curve, making it more challenging to use such measurements alone to help distinguish differences between those with and without OSA. Ratios, however, provide an objective measurement of the relative body fat distribution between two areas. When examined in this way, it is easier to discriminate and identify differences in fat distribution between those with and without OSA.
Limitations of our study include its small sample size. Although the regression model for log OAHI demonstrated a significant independent association with NAF % ratio, the model for log AHI came close (P = .08) to our threshold of statistical significance. We speculate that a larger study may show that log AHI is also independently associated with NAF % ratio. In the model for log AHI, male sex was also close to statistical significance (P = .08) and this variable is known to be associated with SDB.16.17 Furthermore, the sample size is insufficient to evaluate the predictive power of NAF % ratio and to determine which other variables should be included in a predictive model. For example, pubertal status, which was not assessed in this study, could be considered in future studies. Our study included participants with DXA and PSG evaluations as much as 1 year apart, although 75% percent of studies were conducted at an interval of less than 6 months. It is unlikely that body fat distribution or BMI z-score would change dramatically over such a short time period. Finally, the population in our study was highly selected, because these individuals were part of a tertiary care clinical obesity program. However, all participants in the program routinely undergo PSG and DXA; therefore, the study population was not selected based on suspicion of SDB and may therefore approximate a representative sample of obese youth who would meet criteria for entry in such a clinical program.
CONCLUSIONS
This study showed an independent association of NAF % ratio with log OAHI using DXA to quantify body fat. These findings give us insight into the mechanism of obesity-associated OSA in youth, suggesting that the distribution of fat is important in the development of OSA. These findings also support the clinical utility of use of neck-to-waist circumference ratio as a surrogate marker of body fat distribution and OSA risk, a measurement that can be quickly, reliably, and inexpensively obtained in a health practitioner's office.13,26
DISCLOSURE STATEMENT
This project was completed at the Children's Hospital of Eastern Ontario in Ottawa, Canada. This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the participants and their families, the Children's Hospital of Eastern Ontario Centre for Healthy Active Living team, and the Children's Hospital of Eastern Ontario Research Institute.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- DXA
dual X-ray absorptiometry
- NAF % ratio
neck-to-abdominal fat percentage ratio
- NC
neck circumference
- OAHI
obstructive apnea-hypopnea index
- OSA
obstructive sleep apnea
- PSG
polysomnography
- WC
waist circumference
REFERENCES
- 1.Kendig EL, Wilmott RW. Kendig and Chernick's Disorders of the Respiratory Tract in Children. Philadelphia, PA: Saunders/Elsevier; 2012. [Google Scholar]
- 2.Marcus CL, Moore RH, Rosen CL, et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368(25):2366–2376. doi: 10.1056/NEJMoa1215881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marcus CL, Loughlin GM. Obstructive sleep apnea in children. Semin Pediatr Neurol. 1996;3(1):23–28. doi: 10.1016/s1071-9091(96)80025-8. [DOI] [PubMed] [Google Scholar]
- 4.Beck SE, Marcus CL. Pediatric polysomnography. Sleep Med Clin. 2009;4(3):393–406. doi: 10.1016/j.jsmc.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schechter MS Section on Pediatric Pulmonology, Subcommittee on Obstructive Sleep Apnea Syndrome. Technical report: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2002;109(4):e69. doi: 10.1542/peds.109.4.e69. [DOI] [PubMed] [Google Scholar]
- 6.Katz SL, Witmans M, Barrowman N, et al. Paediatric sleep resources in Canada: the scope of the problem. Paediatr Child Health. 2014;19(7):367–372. doi: 10.1093/pch/19.7.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Certal V, Camacho M, Winck JC, Capasso R, Azevedo I, Costa-Pereira A. Unattended sleep studies in pediatric OSA: a systematic review and meta-analysis. Laryngoscope. 2015;125(1):255–262. doi: 10.1002/lary.24662. [DOI] [PubMed] [Google Scholar]
- 8.Bhattacharjee R, Kheirandish-Gozal L, Spruyt K, et al. Adenotonsillectomy outcomes in treatment of obstructive sleep apnea in children: a multicenter retrospective study. Am J Respir Crit Care Med. 2010;182(5):676–683. doi: 10.1164/rccm.200912-1930OC. [DOI] [PubMed] [Google Scholar]
- 9.Canapari CA, Hoppin AG, Kinane TB, Thomas BJ, Torriani M, Katz ES. Relationship between sleep apnea, fat distribution, and insulin resistance in obese children. J Clin Sleep Med. 2011;7(3):268–273. doi: 10.5664/JCSM.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan H, Schwab RJ, Kim C, et al. Relationship between body fat distribution and upper airway dynamic function during sleep in adolescents. Sleep. 2013;36(8):1199–1207. doi: 10.5665/sleep.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shuster A, Patlas M, Pinthus JH, Mourtzakis M. The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis. Br J Radiol. 2012;85(1009):1–10. doi: 10.1259/bjr/38447238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruno E, Alessandrini M, Napolitano B, De Padova A, Di Daniele N, De Lorenzo A. Dual-energy X-ray absorptiometry analysis of body composition in patients affected by OSAS. Eur Arch Otorhinolaryngol. 2009;266(8):1285–1290. doi: 10.1007/s00405-008-0844-0. [DOI] [PubMed] [Google Scholar]
- 13.Katz SL, Vaccani JP, Barrowman N, Momoli F, Bradbury CL, Murto K. Does neck-to-waist ratio predict obstructive sleep apnea in children? J Clin Sleep Med. 2014;10(12):1303–1308. doi: 10.5664/jcsm.4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention website. [Accessed January 3, 2017]. http://www.cdc.gov/growthcharts/who_charts.htm. Updated September 9, 2010.
- 15.Iber C, Ancoli-Israel S, Chesson A, Quan SF for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 16.Millman RP, Carlisle CC, McGarvey ST, Eveloff SE, Levinson PD. Body fat distribution and sleep apnea severity in women. Chest. 1995;107(2):362–366. doi: 10.1378/chest.107.2.362. [DOI] [PubMed] [Google Scholar]
- 17.Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):242–252. doi: 10.1513/pats.200708-135MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scherzer R, Shen W, Bacchetti P, et al. Comparison of dual-energy X-ray absorptiometry and magnetic resonance imaging-measured adipose tissue depots in HIV-infected and control subjects. Am J Clin Nutr. 2008;88(4):1088–1096. doi: 10.1093/ajcn/88.4.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor AE, Kuper H, Varma RD, et al. Validation of dual energy X-ray absorptiometry measures of abdominal fat by comparison with magnetic resonance imaging in an Indian population. PLoS One. 2012;7:e51042. doi: 10.1371/journal.pone.0051042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bredella MA, Gill CM, Keating LK, et al. Assessment of abdominal fat compartments using DXA in premenopausal women from anorexia nervosa to morbid obesity. Obesity (Silver Spring) 2013;21(12):2458–2464. doi: 10.1002/oby.20424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bosch TA, Dengel DR, Kelly AS, Sinaiko AR, Moran A, Steinberger J. Visceral adipose tissue measured by DXA correlates with measurement by CT and is associated with cardiometabolic risk factors in children. Pediatr Obes. 2015;10(3):172–179. doi: 10.1111/ijpo.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benfield LL, Fox KR, Peters DM, et al. Magnetic resonance imaging of abdominal adiposity in a large cohort of British children. Int J Obes (Lond) 2008;32(1):91–99. doi: 10.1038/sj.ijo.0803780. [DOI] [PubMed] [Google Scholar]
- 23.Arens R, Sin S, Nandalike K, et al. Upper airway structure and body fat composition in obese children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2011;183(6):782–787. doi: 10.1164/rccm.201008-1249OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arens R, McDonough JM, Corbin AM, et al. Upper airway size analysis by magnetic resonance imaging of children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2003;167(1):65–70. doi: 10.1164/rccm.200206-613OC. [DOI] [PubMed] [Google Scholar]
- 25.Bhatia R, Lesser DJ, Oliveira FG, et al. Body fat composition: a predictive factor for sleep related breathing disorder in obese children. J Clin Sleep Med. 2015;11(9):1039–1045. doi: 10.5664/jcsm.5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LaBerge RC, Vaccani JP, Gow RM, Gaboury I, Hoey L, Katz SL. Inter- and intra-rater reliability of neck circumference measurements in children. Pediatr Pulmonol. 2009;44(1):64–69. doi: 10.1002/ppul.20944. [DOI] [PubMed] [Google Scholar]


