ABSTRACT
Wolbachia is an intracellular endosymbiont present in most arthropod and filarial nematode species. Transmission between hosts is primarily vertical, taking place exclusively through the female germ line, although horizontal transmission has also been documented. The results of several studies indicate that Wolbachia spp. can undergo transfer between somatic and germ line cells during nematode development and in adult flies. However, the mechanisms underlying horizontal cell-to-cell transfer remain largely unexplored. Here, we establish a tractable system for probing horizontal transfer of Wolbachia cells between Drosophila melanogaster cells in culture using fluorescence in situ hybridization (FISH). First, we show that horizontal transfer is independent of cell-to-cell contact and can efficiently take place through the culture medium within hours. Further, we demonstrate that efficient transfer utilizes host cell phagocytic and clathrin/dynamin-dependent endocytic machinery. Lastly, we provide evidence that this process is conserved between species, showing that horizontal transfer from mosquito to Drosophila cells takes place in a similar fashion. Altogether, our results indicate that Wolbachia utilizes host internalization machinery during infection, and this mechanism is conserved across insect species.
IMPORTANCE Our work has broad implications for the control and treatment of tropical diseases. Wolbachia can confer resistance against a variety of human pathogens in mosquito vectors. Elucidating the mechanisms of horizontal transfer will be useful for efforts to more efficiently infect nonnatural insect hosts with Wolbachia as a biological control agent. Further, as Wolbachia is essential for the survival of filarial nematodes, understanding horizontal transfer might provide new approaches to treating human infections by targeting Wolbachia. Finally, this work provides a key first step toward the genetic manipulation of Wolbachia.
KEYWORDS: Drosophila, Wolbachia, endocytosis, entry, horizontal, invasion, phagocytosis, transfer, transmission
INTRODUCTION
Wolbachia spp. are intracellular bacteria that are transmitted through the female germ lines of arthropods and filarial nematodes (1, 2). In arthropods, Wolbachia spp. function as either a mutualist or a parasite, while in filarial nematodes, Wolbachia spp. are essential for host survival. Efficient maternal transmission of Wolbachia cells in Drosophila melanogaster requires their localization to the posterior cortex of the developing embryo, as this is the future site of the germ line (3). In filarial nematodes, Wolbachia cells undergo a precise pattern of migration during host development that involves not only asymmetric mitotic segregation but also the invasion of germ line precursors from somatic cells (4). Thus, the ability of Wolbachia spp. to undergo cell-to-cell transfer plays an important role in maintaining vertical transmission (5).
While Wolbachia spp. are primarily vertically transmitted, horizontal transmission between arthropods has also been documented in nature (6–8). In these cases, the simplest routes of transmission appear to be the hemolymph or the gut, as Wolbachia bacteria present in these tissues can easily exit the host through excretion or injury and come into contact with an uninfected host (9). Support for this route comes from previous studies that found that purified Wolbachia can remain viable in an extracellular environment and infect mosquito cell lines, ovaries, and testes when cocultured (10, 11). Indeed, Wolbachia cells injected into the hemolymph of an uninfected fly can navigate to the germ line after crossing multiple somatic tissues not only in Drosophila (12, 13) but also in parasitoid wasps (14). It remains unclear how Wolbachia achieves this, as it must traverse a number of membrane and extracellular matrix barriers.
Insight into the mechanisms driving horizontal Wolbachia transmission will likely come from work on the well-studied mechanisms by which other pathogenic bacteria invade host cells, which can be categorized as mechanisms that utilize or alter internalization processes, such as pinocytosis, phagocytosis, and endocytosis (15). Pinocytosis involves the invagination of specialized plasma membrane regions to form pockets that allow for the nonspecific entry of extracellular particles (16). Phagocytosis involves the formation of membrane protrusions, driven by actin rearrangements, to engulf large receptor-bound particles (17). However, the use of host cellular pathways for invasion often requires active manipulation by the microbe. Bacterial entry via modification of host cellular machinery is known to be accomplished via two general mechanisms, the clathrin-dependent “zipper method” and the bacterial effector-dependent “trigger method” (18). In the zipper method, bacteria bind to receptors on the cell surface that induce actin extensions of the membrane through a clathrin-dependent pathway and serve to engulf the cell. Bacteria that utilize the trigger method synthesize type III secretion systems through which they secrete effector proteins to restructure the host cytoskeleton in order to facilitate attachment and invasion (18–20). In addition, invasive microbes may also up- or downregulate host cellular signaling pathways to disable host defenses and increase their own survival (21, 22). While viruses primarily utilize the same pathways to enter host cells, some enveloped viruses can enter through passive membrane fusion by simply blending their host-derived envelope with the plasma membrane of a new host cell (23). Within the host cell, Wolbachia bacteria are encompassed by a self-derived membrane and an outer host-derived membrane (24, 25), which potentially play a role in horizontal transfer by membrane fusion.
Given these possibilities, we sought to identify the mechanisms by which Wolbachia bacteria are horizontally transferred and to establish a useful system for the further study of this interesting phenomenon.
RESULTS
Horizontal transfer of Wolbachia is independent of cell-to-cell contact.
Previous studies established that Wolbachia extracted from infected mosquito cell lines can enter uninfected cells and tissues when cocultured (10, 11). By extracting Wolbachia from Drosophila JW18 and LDW1 cells infected with the wMel strain and coculturing this extract with doxycycline-cured JW18 (JW18-DOX) or LDW1 (LDW1-DOX) cells for 1 to 24 h, we confirmed this phenomenon in Drosophila (Fig. 1A and B). That is, free Wolbachia cells entering uninfected JW18-DOX cells were observed through fixed fluorescence imaging (Fig. 1A). In addition, the early and late stages of free Wolbachia cell entry into LDW1-DOX cells were observed using electron microscopy (Fig. 1B). These observations included contact between free Wolbachia cells and the host cell membrane and integration of Wolbachia into the host cytoplasm following entry in a vacuole.
FIG 1.
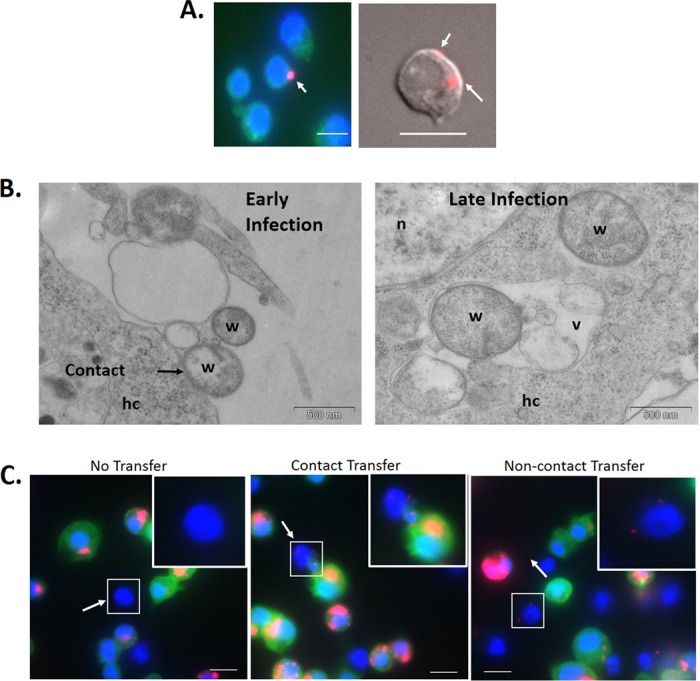
Horizontal transfer of Wolbachia bacteria between Drosophila cells. (A) Wolbachia bacteria extracted from infected JW18 cells were added to JW18-DOX cells and incubated for 24 h. (B) Wolbachia bacteria extracted from infected LDW1 cells were added to LDW1-DOX cells and incubated for 1 h. (C) Uninfected Drosophila S2 cells and Wolbachia-infected JW18 cells were cocultured on a glass coverslip for 24 h. Wolbachia infections in previously uninfected cells can be seen with FISH (A) and DIC (C) imaging or electron microscopy (B) to determine if horizontal transfer of infection took place. Results are typical of the multiple fields of view examined. Red, Wolbachia; blue, nuclei stained with DAPI; green, GFP-Jupiter (JW18 only). hc, host cell; n, nucleus; v, vesicle; w, Wolbachia. Bar, 10 μm.
While significant, these experiments did not reflect the in vivo environment of Wolbachia spp., where they must transfer between living cells. Thus, to determine if Wolbachia can horizontally transfer between intact Drosophila cells, we cocultured uninfected S2 cells and Wolbachia-infected JW18 cells on the same surface (Fig. 1C). JW18 cells carry GFP-Jupiter, a tubulin binding protein, which allows for the distinction of originally infected and uninfected cells by visualization of green fluorescent protein-tagged microtubules (26). Within 24 h of coculturing, transfer of Wolbachia from JW18 to previously uninfected S2 cells was readily apparent (Fig. 1C). While some S2 cells remained uninfected, many in close proximity to infected JW18 cells became infected, perhaps through cell-to-cell contact. We also observed that S2 cells that were not adjacent to JW18 cells became infected. These results suggest that Wolbachia can transfer horizontally from cell to cell in culture. Thus, our next goal was to determine if this phenomenon required contact between infected and uninfected cells.
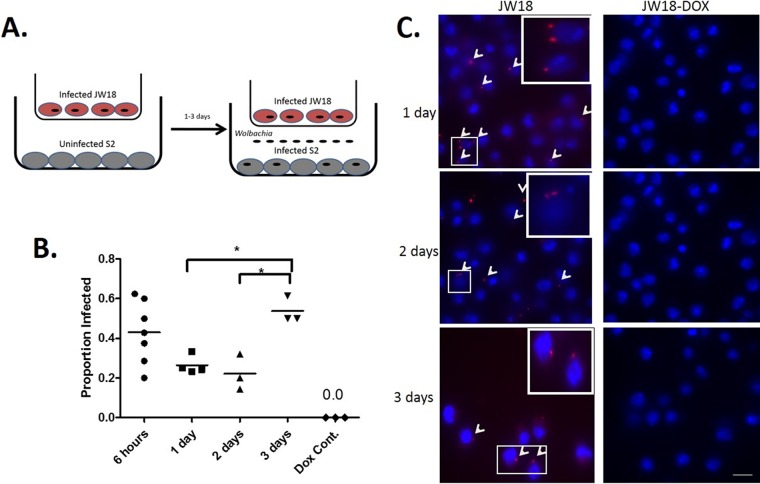
To address this issue, we utilized a transwell system in which infected JW18 cells and uninfected S2 cells were seeded in chambers separated by a polyester membrane that allowed for the sharing of culture medium and passage of bacteria but prevented contact between larger eukaryotic cells (Fig. 2A) (see Materials and Methods). In these assays, transfer of Wolbachia infection was also observed, similar to when cells were cultured on the same surface (Fig. 2B and C). The proportion of newly infected cells after 6 h of coculturing was 43% (n = 56). After the cells were cocultured for 1 day, this number decreased slightly to 26% (n = 90). A similar number, 22% (n = 93), was observed after 2 days of coculturing. The infection rate then rose to 54% by 3 days of coculturing. As a control, JW18-DOX cells were used in place of infected cells in the transwell assay; no Wolbachia infections were detected in the S2 cells. Significantly, Wolbachia infections acquired through coculture in a transwell localized within the host cell (see Fig. S1 in the supplemental material) and were present and abundant 21 days after infection. Thus, the horizontally transferred Wolbachia was stably maintained through multiple division cycles (Fig. 3). These results strongly suggest that Wolbachia can horizontally transfer between infected and uninfected cells in culture, and this ability does not require cell-to-cell contact.
FIG 2.
Horizontal transfer of Wolbachia bacteria between Drosophila cells separated in a transwell. (A) Uninfected Drosophila S2 cells were seeded beneath Wolbachia-infected JW18 cells in a transwell insert. (B) After coculture for 6 h or 1, 2, or 3 days, new Wolbachia infections in previously uninfected S2 cells were visualized by FISH in 3 to 7 fields of view for each group. S2 cells plated underneath doxycycline-cured JW18 cells (JW18-DOX) served as a negative control for FISH staining. Data are presented as proportion of infected cells ± SEM and were analyzed by one-way analysis of variance (ANOVA), followed by Newman-Keul's multiple-comparison test (F = 5.31; R2 = 0.551; df = 16). Differences were deemed significant when the P value was <0.05 (indicated by an asterisk above the bracket). (C) Representative images for 1- to 3-day time points. Red, Wolbachia (arrowheads); blue, nuclei stained with DAPI. Bars, 10 μm.
FIG 3.
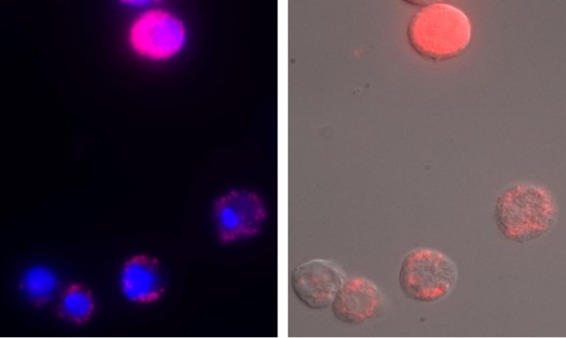
Long-term Wolbachia infection in Drosophila S2 cells after coculture with infected JW18 cells in a transwell chamber. Uninfected Drosophila S2 cells were seeded beneath Wolbachia-infected JW18 cells in a transwell insert. After coculture for 3 days, the transwell insert containing infected cells was removed, and new medium was added to the previously uninfected S2 cells. S2 cells were then cultured for an additional 18 days (21 days total), and Wolbachia infections were visualized by FISH and DIC. Red, Wolbachia; blue, nuclei stained with DAPI.
Horizontal transfer of Wolbachia uses host clathrin and dynamin.
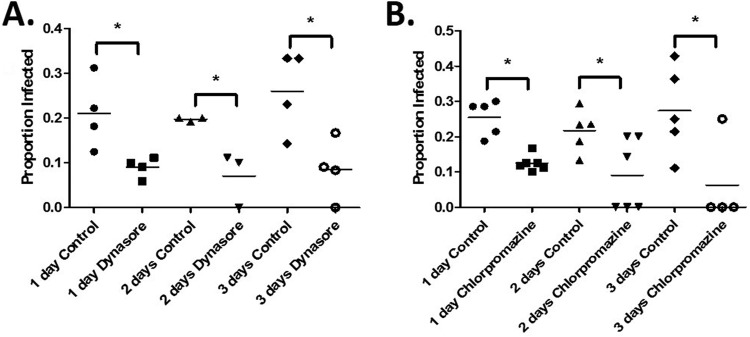
We next sought to investigate the mechanisms involved in the horizontal transfer of Wolbachia. Given that many intracellular bacteria enter host cells by engaging components of the endocytic pathway, we hypothesized that this might also hold true for Wolbachia. We tested this hypothesis by inhibiting host cell dynamin, a GTPase necessary for the pinching and intracellular release of a variety of endocytic vesicles, using the small-molecule inhibitor dynasore (27). We then analyzed cell-to-cell transfer rates between infected JW18 and uninfected S2 cells in our transwell assay. As predicted, treatment with dynasore significantly reduced the efficiency of cell-to-cell transfer relative to dimethyl sulfoxide (DMSO)-treated controls (Fig. 4A). After 1 day, the infection rate in untreated control cells was 21% (n = 105), compared to 9% (n = 47) in dynasore-treated cells. We observed a similar pattern after 2 days of dynasore treatment, with infection decreasing from 20% in controls (n = 66) to 7% in dynasore-treated groups (n = 42). Dynasore produced the strongest effect after 3 days of treatment, reducing infection from 26% in controls (n = 45) to 8% in treated cells (n = 46). The incomplete inhibition of horizontal transmission by dynasore suggests that Wolbachia spp. employ additional mechanisms of internalization (see below).
FIG 4.
Horizontal transfer of Wolbachia is clathrin mediated. (A) Uninfected Drosophila S2 cells were pretreated with 80 μM dynasore or DMSO (control) for 1 h prior to seeding Wolbachia-infected Drosophila JW18 cells in a transwell insert above them. After being cocultured for 1, 2, or 3 days, new Wolbachia infections in previously uninfected S2 cells were visualized by FISH in 3 to 7 fields of view for each group. Data are presented as proportion of infected cells ± SEM and were analyzed by t test to determine differences between control and dynasore-treated groups at each time point (t = 2.96, df = 6 at 1 day; t = 3.58, df = 4 at 2 days; t = 3.05, df = 6 at 3 days). Differences were deemed significant when the P value was <0.05 (indicated by an asterisk above the bracket). (B) Uninfected Drosophila S2 cells were pretreated with 10 μM chlorpromazine or DMSO (control) for 1 h prior to seeding Wolbachia-infected Drosophila JW18 cells in a transwell insert above them. After being cocultured for 1, 2, or 3 days, new Wolbachia infections in previously uninfected cells were visualized by FISH and analyzed as described for panel A (t = 5.73, df = 9 at 1 day; t = 2.44, df = 9 at 2 days; t = 2.51, df = 7 at 3 days).
Nevertheless, these experiments demonstrate that Wolbachia spp. use dynamin for horizontal transfer into new host cells. Using dynamin, Wolbachia cells entered through a clathrin-dependent mechanism. To test this, we used chlorpromazine to inhibit host clathrin (28, 29), a coat protein involved in the formation of vesicles. Similar to dynamin inhibition, inhibition of clathrin reduced infection from 25% in controls (n = 75) to 12% after 1 day of treatment (n = 64) (Fig. 4B). After 2 days of treatment, the infection rate decreased from 22% in controls (n = 95) to 9% in treated cells (n = 35). As with dynasore, chlorpromazine produced the strongest effect after 3 days of treatment, reducing infection from 27% (n = 45) to 6% (n = 17). These results suggest that Wolbachia spp. utilize clathrin-mediated endocytosis pathways for entry during horizontal cell-to-cell transfer. We also used the inhibitors genistein and filipin to test the involvement of caveolin (29). In these experiments, caveolin inhibition did not inhibit cell-to-cell transfer (J. E. Pietri, unpublished data).
Host cells internalize Wolbachia via engulfment.
Finding that clathrin and dynamin are involved in Wolbachia uptake prompted us to examine the interaction between Wolbachia spp. and host cells at the ultrastructural level. Transmission electron microscopy (TEM) reveals that Wolbachia uptake by host cells appears to be accomplished by engulfment via extensions of the cytoplasm similar to those of phagocytic pseudopodia (Fig. 5A and B). The bacteria were observed in contact with putative clathrin-coated pits (Fig. 5C and D), which in some cases were associated with pseudopodia (Fig. 5D). Internalization via membrane fusion may also contribute to transfer rates, as the host-derived membrane of extracellular Wolbachia was often seen in close contact with the host membrane (Fig. 5E and F).
FIG 5.
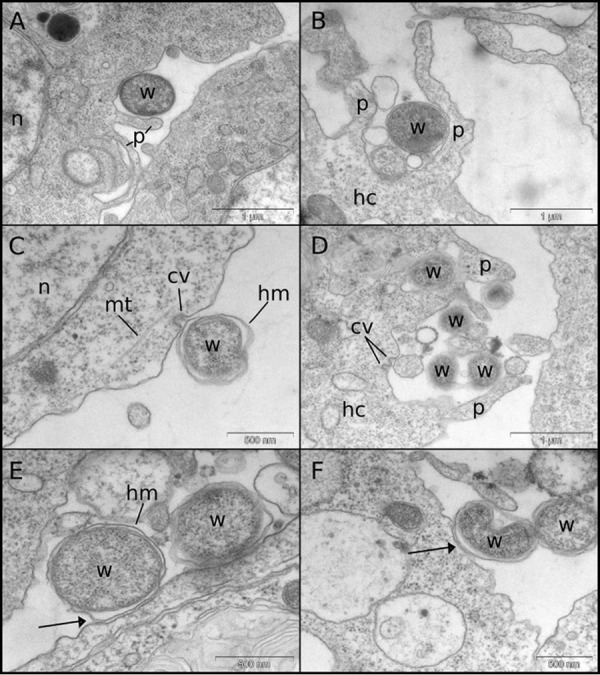
Transmission electron micrographs of LDW/JW18 cells exposed to Wolbachia bacteria from cell lysates or infected JW18 cells. (A and B) Wolbachia bacteria are frequently seen surrounded by phagocytic pseudopodium-like extensions of the host cell. (C and D) Wolbachia bacteria can be seen contacting what appear to be clathrin-coated pits, sometimes coinciding with pseudopodia (D). (E and F) The host-derived membrane surrounding the Wolbachia double membrane can be seen in close contact with the host cell membrane (arrows). cv, clathrin vesicle; hc, host cell; hm, host membrane; mt, microtubules; n, nucleus; p, pseudopodia; w, Wolbachia.
Horizontal transfer of Wolbachia takes place efficiently between cells of divergent hosts.
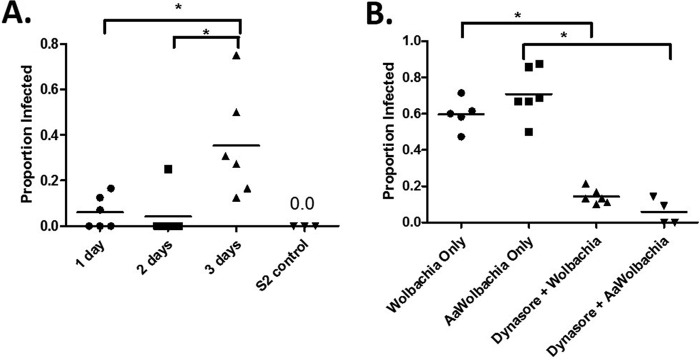
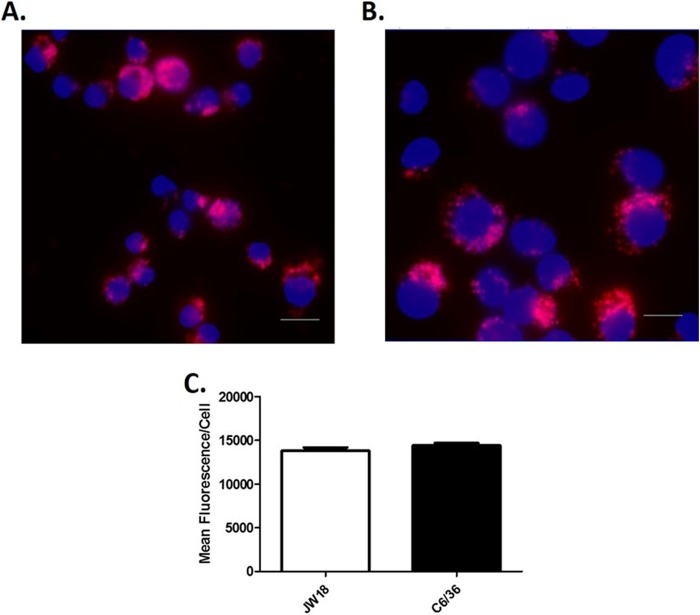
Having implicated components of the host endocytic and phagocytic pathways in horizontal transfer, we sought to determine if a species barrier to horizontal transfer exists. We predicted that if this were the case, horizontal transfer of Wolbachia between cells of different insect species would be reduced or inhibited altogether. We examined this possibility by analyzing horizontal transfer rates between infected C6/36 cells from the mosquito Aedes albopictus and uninfected Drosophila S2 cells in our transwell assay (Fig. 6A). Despite Wolbachia infection rates in C6/36 and Drosophila JW18 cells being equal (Fig. 7), cell-to-cell transfer of Wolbachia from these cells to Drosophila S2 cells was somewhat lower. The proportion of newly infected cells after 1 day of coculturing was a mere 6% and decreased to 4% on day 2. Although new infections increased to 35% after 3 days of coculturing, this rate was lower than that observed between Drosophila cells (Fig. 2B), suggesting that while horizontal transfer takes places between different species, it may be less efficient. To rule out the effect of differences in Wolbachia exocytosis rates in mosquito and Drosophila cells, we pretreated JW18-DOX cells with dynasore and incubated them with crude Wolbachia preparations derived from fly or mosquito cells. In these experiments, Wolbachia infection rates in cells treated with Wolbachia bacteria derived from mosquito cells and with Wolbachia bacteria derived from Drosophila cells were not different, regardless of pretreatment (Fig. 6B). That is, within 24 h of incubation with Wolbachia bacteria from Drosophila cells, 60% of previously uninfected cells became infected (n = 63). This proportion was reduced to 14% by pretreating the cells with dynasore (n = 75). Similarly, when Wolbachia bacteria from A. albopictus cells were used, 71% (n = 63) of previously uninfected cells became infected. After pretreatment of the cells with dynasore, infection was almost completely blocked, as only 6% of the cells became infected (n = 55). Thus, reliance of Wolbachia spp. on components of the endocytic pathway for cell-to-cell transfer appears to be conserved across species.
FIG 6.
Horizontal transfer of Wolbachia bacteria takes places between mosquito and Drosophila cells. (A) Uninfected Drosophila S2 cells were seeded beneath Wolbachia-infected A. albopictus cells (C6/36) in a transwell insert. After being cocultured for 1, 2, or 3 days, new Wolbachia infections in previously uninfected cells were visualized by FISH in 6 fields of view for each group. S2 cells plated in the absence of C6/36 cells served as a control for FISH staining. Data are presented as proportion of infected cells ± SEM and were analyzed by one-way ANOVA, followed by Newman-Keuls multiple-comparison test to determine differences between time points (F = 7.78, R2 = 0.509, df = 17). Values were deemed significant when P < 0.05 (indicated by an asterisk above the bracket). (B) Pretreatment of JW18-DOX cells with dynamin prior to the addition of crude Wolbachia preparations from infected Drosophila JW18 cells or mosquito C6/36 cells (AaWolbachia) for 24 h resulted in a reduced ability of Wolbachia bacteria to invade cells. Data are presented as proportion of infected cells ± SEM and were analyzed by one-way ANOVA, followed by Newman-Keuls multiple-comparison test to determine differences between groups (F = 61.4, R2 = 0.912, df = 20). Values were deemed significant when the P value was <0.05 (indicated an asterisk above the bracket).
FIG 7.
Wolbachia infection in Drosophila and A. albopictus cells. Cells were seeded on glass coverslips for 24 h and subsequently fixed with 8% paraformaldehyde for detection of Wolbachia by FISH (red) in Drosophila JW18 cells (A) and A. albopictus C6/36 cells (B). DAPI was used as a counterstain for cell nuclei (blue). Bar, 10 μm. (C) Wolbachia infection in JW18 and C6/36 cells was quantified by measurement of red fluorescence intensity. Data were analyzed by t test, and no significant differences between the two groups were found (P = 0.223, t = 1.25, df = 18).
DISCUSSION
In our study, we documented the horizontal transfer of Wolbachia bacteria between Drosophila cells in culture and demonstrate that this process occurs through components of the host phagocytic and endocytic pathways. As such, our work directly demonstrates horizontal transfer of Wolbachia bacteria between cells while identifying a potential mechanism.
Our finding that horizontal transfer takes place between infected and uninfected cells when cultured together (Fig. 1) or separated by transwells (Fig. 3) suggests that cell-to-cell contact is not required to achieve efficient transfer. Nonetheless, cell-to-cell contact may play some role in horizontal transfer, as we observed several instances of Wolbachia bacteria transferring between cells in direct contact with each other (Fig. 1C). However, a large proportion of horizontally acquired infections can be accounted for by transfer through the culture medium (Fig. 2). Wolbachia spp. can achieve a >50% infection rate through this route, implicating it as the prevalent mechanism for horizontal transfer. Nonetheless, a fair proportion of bacteria invading through this method may not survive, as reduced infection levels between 6 and 24 h in our transwell assay suggest that perhaps some horizontally acquired Wolbachia bacteria are digested or killed by the host cell.
Transfer through the culture medium likely takes place via uptake after Wolbachia bacteria are exocytosed from infected cells. The release of Wolbachia bacteria after cell lysis may make some minor contribution to horizontal transmission. However, it is unlikely that these infrequent cell death events account for the high rates of infection transfer observed in our short-term assays, given that infected JW18 cells can be maintained in culture without passaging for 7 to 10 days without notable cell lysis occurring (J. E. Pietri, unpublished data).
Our experiments using dynasore and chlorpromazine to block dynamin and clathrin activity in uninfected cells reveal the particular pathways of endocytosis coopted by Wolbachia spp. after their release from infected cells (Fig. 4A). Reduced horizontal transfer following inhibition of dynamin and clathrin, but not caveolin, argues against the possibility that Wolbachia bacteria enter cells exclusively through a process such as passive membrane fusion. Further, while Drosophila S2 and JW18 cells are passively phagocytic to some extent, the use of clathrin and dynamin in transfer suggests a bacterially induced mode of entry, such as the zipper method (18). However, clathrin has been reported to be involved in some forms of phagocytosis in Drosophila (e.g., references 30 to 32), preventing us from excluding this as a mechanism of uptake with these data alone.
It is possible that Wolbachia spp. use an active mode, such as the zipper method, and a passive method, such as phagocytosis, for uptake, as is the case for several other invasive bacteria. For instance, Chlamydia spp. can specifically trigger phagocytosis for entry into in HeLa cells, as demonstrated by experiments comparing the internalization rate of this bacterium with those of Escherichia coli and polystyrene beads (33). However, in the same cell type (i.e., HeLa cells), and in human endometrial gland epithelial cells, Chlamydia can be observed in coated pits and vesicles, indicative of endocytosis (34). Similarly, Listeria has been shown to enter cells through multiple mechanisms depending on the cell type being invaded. For instance, traditional phagocytosis and a formin-dependent phagocytosis-like process (35) have been demonstrated in vascular endothelial cells, while a clathrin-mediated process (33) appears to be critical in HeLa cells.
In addition, we suggest that Wolbachia bacteria may bind to a variety of host cell receptors to gain entry into host cells. This is consistent with results of studies of other invasive intracellular bacteria, which demonstrate that while the machinery for endocytosis is often conserved, a variety of receptors can be used. For instance, although Listeria and Neisseria both enter through clathrin-coated pits (33–38), Listeria utilizes the hepatocyte growth factor receptor (met) (38), while Neisseria uses the asialoglycoprotein receptor (ASGP-R) (37). Similarly, microorganisms may make use of the same receptors but achieve entry through different mechanisms. For example, both Salmonella and Candida bind to the epidermal growth factor receptor (EGFR) (39, 40), but they make use of phagocytosis and clathrin-mediated pathways, respectively (18, 41). The receptor(s) that Wolbachia spp. bind prior to entry remain undetermined. However, the conservation of horizontal transfer across species suggests that this receptor and its ligand(s) may be highly conserved, as Wolbachia derived from the C6/36 and JW18 cells used as Wolbachia donors in our experiments harbored wAlbB and wMel, respectively.
The processes of phagocytosis and endocytosis are intrinsically linked to the actin cytoskeleton (42). Intriguingly, a number of microbes rely on host actin for invasion and are able to manipulate its structure through the use of secreted effectors (19). The same appears to be true for Wolbachia, which was recently shown to rely on host actin for efficient maternal transmission (43). Wolbachia also encodes a secreted effector, WD0830, which interacts with the host cytoskeleton (44). This is particularly important, as it suggests that the horizontal transfer process may not be passive and host driven but, rather, induced by Wolbachia spp. through the secretion of effector proteins that drive cytoskeletal changes for engulfment. This mode of transfer might explain cortical actin rearrangements that are associated with Wolbachia migration during filarial nematode development (4).
Differences in Wolbachia exocytosis rates may play some role in controlling horizontal transmission, as entry of Wolbachia extracted from mosquito cells from crude extractions was not inhibited compared to Wolbachia extracted from Drosophila cells (Fig. 6B), despite our transwell assay in which lower rates of horizontal transfer were found (Fig. 6A). It is unlikely that the reduced titer in mosquito cells plays a role in this discrepancy between assays, as infection levels in mosquito cells were equal to those in Drosophila cells (Fig. 7). Likewise, genotype-specific differences in bacterial surface factors are likely not involved given the different strains of Wolbachia harbored by JW18 and C6/36 cells. However, differences in recipient cell properties, such as the presence or absence of particular receptors, may contribute to differences in the efficiency of horizontal transfer and should be explored further.
Ultimately, the results of our work significantly advance our understanding of how Wolbachia is transmitted both vertically and horizontally. During early embryogenesis in filarial nematodes, Wolbachia segregates exclusively to the lineage producing the hypodermal chords, somatic tissues that provide nutrients to developing germ line cells. Occupation of the germ line for eventual vertical transmission requires cell-to-cell transfer from the chords (4). The relevance of somatic to germ line cell-to-cell transfer for vertical transmission is further illuminated by images of Wolbachia-infected oocytes from recently captured wild Drosophila (45). Egg chambers were identified in which Wolbachia was not present in many of the early developing oocytes, but all of the mature oocytes were infected. The absence of Wolbachia early in oogenesis is likely a direct result of its failure to segregate to the differentiating daughter cell during germ line stem cell division. The fact that these empty oocytes eventually become infected suggests that Wolbachia bacteria present in the surrounding somatic follicle cells eventually enter the oocyte using cell-to-cell transfer as a backup mechanism to ensure vertical transmission (46).
Our findings also shed some light on possible routes of horizontal transmission of Wolbachia infection in nature. Previous work showed that Wolbachia bacteria in the hemolymph of adult flies can migrate to the germ line across multiple somatic tissues (12). This is likely mediated by cell-to-cell transfer between various tissues and suggests that Wolbachia which enters a new host through the gut or a wound may use cell-to-cell transfer to establish both a somatic and stable (germ line) infection.
While more specifics regarding the mechanisms of horizontal transfer remain to be uncovered, our transwell fluorescence in situ hybridization (FISH) assay is a simple and tractable system for further probing cell exit and entry of Wolbachia bacteria, as it allows for the separate manipulation of recipient (uninfected) and donor (infected) cells while providing several advantages over antibody-based staining by increasing specificity and reducing background fluorescence. Our system is also highly biologically relevant, as Wolbachia bacteria that infect through this method can achieve proper localization inside the host cell (Fig. 1; see also Fig. S1 in the supplemental material) and also appear highly stable, surviving for at least 21 days (Fig. 3).
MATERIALS AND METHODS
Cell culture and infections.
Stocks of uninfected Drosophila S2 cells, Wolbachia-infected Drosophila JW18 cells (26), Wolbachia-infected A. albopictus C6/36 cells, and doxycycline-cured JW18 (JW18-DOX) cells were maintained in Shields and Sang M3 insect medium (Sigma-Aldrich) supplemented with 10% fetal bovine serum (FBS) (Gibco) at a temperature of 24 to 26°C. We also created an additional immortalized cell line from primary cultures of Wolbachia-infected D. melanogaster bearing red fluorescent protein (RFP)-histone (47) and green fluorescent protein (GFP)-Jupiter (48). This cell line is called LDW1.
JW18 and LDW1 cells are naturally infected with the wMel strain of Wolbachia (26), while C6/36 cells were artificially infected with the wAlbB strain from Aa23 cells, as previously described (49). For FISH assays, cells were seeded on glass coverslips in untreated 6-well polystyrene plates (Costar). In transwell assays, uninfected cells were seeded in the same manner, while infected cells were seeded on polyester transwell membrane inserts with a pore size of 3.0 μm (Costar). For assays of dynamin inhibition, uninfected cells on coverslips in the bottom transwell were treated with 80 μM dynasore (27) or an equal volume of DMSO (control) for 1 h, and the medium was then changed prior to seeding infected cells on the top well or prior to adding crude Wolbachia preparations directly to the culture medium for an additional 24 h. For assays of clathrin inhibition, uninfected cells on coverslips in the bottom transwell were treated with 10 μM chlorpromazine (28) for 1 h, and the medium was changed prior to seeding infected cells on the top well. Crude Wolbachia extracts were prepared by running infected cells in culture medium through 5.0-μm filter spin columns (Millipore) for lysis to release Wolbachia bacteria and remove large cellular debris.
Primary neuroblast cell culture and infections.
Drosophila stocks homozygous for neuroblast-specific GAL4 expression (OK371, as identified in reference 50) and CD-ChRFP (2) under an upstream activation sequence (UAS) promoter (Bloomington stock 27391) were crossed. Third-instar larvae were collected for brain dissection and primary culture (51), modified to exclude antibiotics from all reagents except for the Shields and Sang medium used to wash the cells, which contained 1:1,000 penicillin-streptomycin. The brain homogenate was plated on concanavalin A-coated glass coverslips as described above and incubated at 25°C overnight. Neuroblasts were tested for cell-to-cell transfer as described above.
Passive uptake of fluorescently labeled dextran.
S2 cells and neuroblasts were incubated with 20 mg/ml 1:1,000 fluorescently labeled dextran (molecular weight, 40,000) overnight at 25°C. Culture medium with beads was aspirated, and cells were processed for detection of Wolbachia bacteria by using FISH (see next section).
FISH detection of Wolbachia.
Wolbachia detection by FISH was performed 1, 2, or 3 days after coculture of uninfected and infected cells and 24 h after the addition of crude Wolbachia preparations to cured cells. Cells on glass coverslips were fixed with 8% paraformaldehyde for 20 min at room temperature, washed 3 times with phosphate-buffered saline (PBS), and treated with prehybridization buffer for 90 min at room temperature. The prehybridization buffer consisted of 50% deionized formamide by volume, 4× saline sodium citrate (SSC), 0.5× Denhardt's solution, 0.1 M dithiothreitol (DTT), and 0.1% Tween 20 in deionized water. After prehybridization, cells were hybridized overnight at 37°C in hybridization buffer (prehybridization buffer minus detergent) containing 500 nM Wolbachia W2 fluorescent DNA probe (5-CTTCTGTGAGTACCGTCATTATC-3) (Bioresearch Technologies) (52). After hybridization, cells were washed 3 times with 1× SSC plus 0.1% Tween 20, 3 times with 0.5× SSC, and 3 times with PBS to remove any free Wolbachia bacteria on the slide. The last step of each wash series was performed at 42°C to eliminate nonspecific binding of the FISH probe. Slides were then mounted and stained using Vectashield fluorescent mounting medium with DAPI (4′,6-diamidino-2-phenylindole) (Vector Laboratories).
Microscopy and image analysis.
All fluorescence and differential interference contrast (DIC) imaging was performed on a Leica DMI 6000 inverted wide-field microscope under equal exposure times and conditions. For quantitation of Wolbachia infection during coculture over time, 3 to 7 fields (technical replicates) for each group from 3 independent experiments (biological replicates) were scored for the proportion of cells displaying red puncta in the ImageJ cell counter tool (http://imagej.nih.gov/ij/). Only cells with Wolbachia puncta in close association with the nucleus were scored as infected to reduce the number of false-positive infections from Wolbachia bacteria on the slides outside the cell, despite them being negligible. Counts from each field were plotted as the proportion infected per field of view ± standard error of the mean (SEM) and were pooled for analysis by one-way ANOVA followed by Newman-Keuls multiple-comparison test or by t test to determine differences in infection over time and between the treated and untreated groups. For electron microscopy, samples were fixed with 2% glutaraldehyde and 0.5% paraformaldehyde in 0.075 M cacodylate buffer and postfixed with 2% osmium tetroxide. Samples were dehydrated through a graded series of ethanol and embedded in epoxy resin. Ultrathin (70-nm) sections (Ultracut UC6, Leica) were collected on Formvar/carbon-coated copper grids. Sections were then poststained with aqueous 4% uranyl acetate and lead citrate. All samples were observed in a Tecnai 12 (FEI, The Netherlands) transmission electron microscope at 80 kV equipped with a 1K-by-1K-resolution Keen View camera.
Supplementary Material
ACKNOWLEDGMENTS
We thank Roy Ng for assistance with and maintenance of cell cultures. We also thank Filnat Yildiz and Martha Zuniga for their advice and guidance and present and past Sullivan lab members for their helpful discussion and suggestions.
Funding for these experiments was provided by National Institutes of Health grant GM104486 and National Science Foundation grant 1456535.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.03425-16.
REFERENCES
- 1.Serbus LR, Sullivan W. 2007. A cellular basis for Wolbachia recruitment to the host germline. PLoS Pathog 3:e190. doi: 10.1371/journal.ppat.0030190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Werren J, Baldo L, Clark ME. 2008. Wolbachia: master manipulator of invertebrate biology. Nat Rev Microbiol 6:741–751. doi: 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]
- 3.Serbus LR, Casper-Lindley C, Landmann F, Sullivan W. 2008. The genetics and cell biology of Wolbachia-host interactions. Annu Rev Genet 42:683–707. doi: 10.1146/annurev.genet.41.110306.130354. [DOI] [PubMed] [Google Scholar]
- 4.Landmann F, Bain O, Martin C, Uni S, Taylor MJ, Sullivan W. 2012. Both asymmetric mitotic segregation and cell-to-cell invasion are required for stable germline transmission of Wolbachia in filarial nematodes. Biol Open 1:536–547. doi: 10.1242/bio.2012737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pietri JE, DeBruhl H, Sullivan W. 2016. The rich somatic life of Wolbachia. Microbiologyopen 5:923–936. doi: 10.1002/mbo3.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dyson EA, Kamath MK, Hurst GDD. 2002. Wolbachia infection associated with all-female broods in Hypolimnas bolina (Lepidoptera: Nymphalidae): evidence for horizontal transmission of a butterfly male killer. Heredity 88:166–171. doi: 10.1038/sj.hdy.6800021. [DOI] [PubMed] [Google Scholar]
- 7.Haine ER, Pickup NJ, Cook JM. 2005. Horizontal transmission of Wolbachia in a Drosophila community. Ecol Entomol 30:464–472. doi: 10.1111/j.0307-6946.2005.00715.x. [DOI] [Google Scholar]
- 8.Morrow JL, Frommer M, Shearman DCA, Riegler M. 2014. Tropical tephritid fruit fly community with high incidence of shared Wolbachia strains as platform for horizontal transmission of endosymbionts. Environ Microbiol 16:3622–3637. doi: 10.1111/1462-2920.12382. [DOI] [PubMed] [Google Scholar]
- 9.Rigaud T, Juchault P. 1995. Success and failure of horizontal transfers of feminizing Wolbachia endosymbionts in woodlice. J Evol Biol 8:249–255. doi: 10.1046/j.1420-9101.1995.8020249.x. [DOI] [Google Scholar]
- 10.Hughes GL, Pike AD, Xue P, Rasgon JL. 2012. Invasion of Wolbachia into Anopheles and other insect germlines in an ex vivo organ culture system. PLoS One 7:e36277. doi: 10.1371/journal.pone.0036277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rasgon JL, Gamston CE, Ren X. 2006. Survival of Wolbachia pipientis in cell-free medium. Appl Environ Microbiol 72:6934–6937. doi: 10.1128/AEM.01673-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frydman HM, Li JM, Robson DN, Wieschaus E. 2006. Somatic stem cell niche tropism in Wolbachia. Nature 441:509–512. doi: 10.1038/nature04756. [DOI] [PubMed] [Google Scholar]
- 13.Van Meer MMM, Stouthamer R. 1999. Cross-order transfer of Wolbachia from Muscidifurax uniraptor (Hymenoptera:Pteromalidae) to Drosophila simulans (Diptera:Drosophilidae). Heredity 82:163–169. doi: 10.1038/sj.hdy.6884610. [DOI] [PubMed] [Google Scholar]
- 14.Huigens ME, de Almeida RR, Boons PA, Luck RF, Stouthamer R. 2004. Natural interspecific and intraspecific transfer of parthenogenesis-inducing Wolbachia in Trichogramma wasps. Proc Biol Sci 271:509–515. doi: 10.1098/rspb.2003.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doherty GJ, McMahon HT. 2009. Mechanisms of endocytosis. Annu Rev Biochem 78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 16.Francis CL, Ryan TA, Jones BD, Smith SJ, Falkow S. 1993. Ruffles induced by Salmonella and other stimuli direct macropinocytosis of bacteria. Nature 364:639–642. doi: 10.1038/364639a0. [DOI] [PubMed] [Google Scholar]
- 17.Clerc P, Sansonetti PJ. 1987. Entry of Shigella flexneri into HeLa cells: evidence for directed phagocytosis involving actin polymerization and myosin accumulation. Infect Immun 55:2681–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veiga E, Guttman JA, Bonazzi M, Boucrot E, Toledo-Arana A, Lin AE, Enninga J, Pizarro-Cerdá J, Finlay BB, Kirchhausen T, Cossart P. 2007. Invasive and adherent bacterial pathogens co-opt host clathrin for infection. Cell Host Microbe 2:340–351. doi: 10.1016/j.chom.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tu X, Nisan Y, Yona C, Rosenshine I. 2003. EspH, a new cytoskeleton-modulating effector of enterohaemorrhagic and enteropathogenic Escherichia coli. Mol Microbiol 47:595–606. doi: 10.1046/j.1365-2958.2003.03329.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhou D, Chen LM, Hernandez L, Shears SB, Galan JE. 2001. A Salmonella inositol phosphate acts in conjunction with other bacterial effectors to promote host cell actin cytoskeleton rearrangements and bacterial internalization. Mol Microbiol 39:248–260. doi: 10.1046/j.1365-2958.2001.02230.x. [DOI] [PubMed] [Google Scholar]
- 21.Cossart P, Pizzaro-Cerda J, Lecuit M. 2003. Invasion of mammalian cells by Listeria monocytogenes: functional mimicry to subvert cellular functions. Trends Cell Biol 13:23–31. doi: 10.1016/S0962-8924(02)00006-5. [DOI] [PubMed] [Google Scholar]
- 22.Navarro L, Alto NM, Dixon JE. 2005. Functions of the Yersinia effector proteins in inhibiting host immune responses. Curr Opin Microbiol 8:21–27. doi: 10.1016/j.mib.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 23.Más V, Melero JA. 2013. Entry of enveloped viruses into host cells: membrane fusion. Subcell Biochem 68:467–487. doi: 10.1007/978-94-007-6552-8_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho KO, Kim GW, Lee OK. 2011. Wolbachia bacteria reside in host Golgi-related vesicles whose position is regulated by polarity proteins. PLoS One 6:e22703. doi: 10.1371/journal.pone.0022703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fischer K, Beatty WL, Weil GJ, Fischer PU. 2014. High pressure freezing/freeze substitution fixation improves the ultrastructural assessment of Wolbachia endosymbiont-filarial nematode host interaction. PLoS One 9:e86383. doi: 10.1371/journal.pone.0086383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serbus L, Landmann F, Bray W, White PM, Ruybal J, Lokey RS, Debec A, Sullivan W. 2012. A cell-based screen reveals that albendazole metabolite, albendazole sulfone, targets Wolbachia. PLoS Pathog 8:e1002922. doi: 10.1371/journal.ppat.1002922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pietila TE, Latvala S, Osterlund P, Julkunen I. 2010. Inhibition of dynamin-dependent endocytosis interferes with type III IFN expression in bacteria-infected human monocyte-derived DCs. J Leukoc Biol 88:665–674. doi: 10.1189/jlb.1009651. [DOI] [PubMed] [Google Scholar]
- 28.Ivanov A. 2014. Pharmacological inhibition of exocytosis and endocytosis: novel bullets for old targets. Methods Mol Biol 1174:3–18. doi: 10.1007/978-1-4939-0944-5_1. [DOI] [PubMed] [Google Scholar]
- 29.Rejman J, Bragonzi A, Conese M. 2005. Role of clathrin and caveolae mediated endocytosis in gene transfer mediated by lipo and polyplexes. Mol Ther 12:468–474. doi: 10.1016/j.ymthe.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 30.Stuart LM, Ezekowitz RA. 2008. Phagocytosis and comparative innate immunity: learning on the fly. Nat Rev Immunol 8:131–141. doi: 10.1038/nri2240. [DOI] [PubMed] [Google Scholar]
- 31.Rocha JJE, Korolchuk VI, Robinson IM, O'Kane CJ. 2011. A phagocytic route for uptake of double-stranded RNA in RNAi. PLoS One 6:e19087. doi: 10.1371/journal.pone.0019087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jha A, Watkins SC, Traub LM. 2012. The apoptotic engulfment protein Ced-6 participates in clathrin-mediated yolk uptake in Drosophila egg chambers. Mol Biol Cell 23:1742–1764. doi: 10.1091/mbc.E11-11-0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Byrne GI, Moulder JW. 1978. Parasite-specified phagocytosis of Chlamydia psittaci and Chlamydia trachomatis by L and HeLa cells. Infect Immun 19:598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wyrick PB, Choong J, Davis CH, Knight ST, Royal MO, Maslow AS, Bagnell CR. 1989. Entry of genital Chlamydia trachomatis into polarized human epithelial cells. Infect Immun 57:2378–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rengarajan M, Hayer A, Theriot JA. 2016. Endothelial cells use a formin-dependent phagocytosis-like process to internalize the bacterium Listeria monocytogenes. PLoS Pathog 12:e1005603. doi: 10.1371/journal.ppat.1005603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Veiga E, Cossart P. 2005. Listeria hijacks the clathrin-dependent endocytic machinery to invade mammalian cells. Nat Cell Biol 7:894–900. doi: 10.1038/ncb1292. [DOI] [PubMed] [Google Scholar]
- 37.Harvey HA, Jennings MP, Campbell CA, Williams R, Apicella MA. 2001. Receptor-mediated endocytosis of Neisseria gonorrhoeae into primary human urethral epithelial cells: the role of the asialoglycoprotein receptor. Mol Microbiol 42:659–672. doi: 10.1046/j.1365-2958.2001.02666.x. [DOI] [PubMed] [Google Scholar]
- 38.Shen Y, Naujokas M, Park M, Ireton K. 2000. InIB-dependent internalization of Listeria is mediated by the Met receptor tyrosine kinase. Cell 103:501–510. doi: 10.1016/S0092-8674(00)00141-0. [DOI] [PubMed] [Google Scholar]
- 39.Galán JE, Pace J, Hayman MJ. 1992. Involvement of the epidermal growth factor receptor in the invasion of cultured mammalian cells by Salmonella Typhimurium. Nature 357:588–589. doi: 10.1038/357588a0. [DOI] [PubMed] [Google Scholar]
- 40.Zhu W, Phan QT, Boontheung P, Solis NV, Loo JA, Filler SG. 2012. EGFR and HER2 receptor kinase signaling mediate epithelial cell invasion by Candida albicans during oropharyngeal infection. Proc Natl Acad Sci U S A 109:14194–14199. doi: 10.1073/pnas.1117676109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moreno-Ruiz E, Galán-Díez M, Zhu W, Fernández-Ruiz E, d'Enfert C, Filler SG, Cossart P, Veiga E. 2009. Candida albicans internalization by host cells is mediated by a clathrin-dependent mechanism. Cell Microbiol 11:1179–1189. doi: 10.1111/j.1462-5822.2009.01319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mooren OL, Galletta BJ, Cooper JA. 2012. Roles for actin assembly in endocytosis. Annu Rev Biochem 81:661–686. doi: 10.1146/annurev-biochem-060910-094416. [DOI] [PubMed] [Google Scholar]
- 43.Newton IL, Savytskyy O, Sheehan KB. 2015. Wolbachia utilize host actin for efficient maternal transmission in Drosophila melanogaster. PLoS Pathog 11:e1004798. doi: 10.1371/journal.ppat.1004798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sheehan KB, Martin M, Lesser CF, Isberg RR, Newton ILG. 2016. Identification and characterization of a candidate Wolbachia pipientis type IV effector that interacts with the actin cytoskeleton. mBio 7:e00622-16. doi: 10.1128/mBio.00622-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Casper-Lindley C, Kimura S, Saxton DS, Essaw Y, Simpson I, Tan V, Sullivan W. 2011. Rapid fluorescence-based screening for Wolbachia endosymbionts in Drosophila germ line and somatic tissues. Appl Environment Microbiol 77:4788–4794. doi: 10.1128/AEM.00215-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toomey M, Panaram K, Fast EM, Beatty C, Frydman HM. 2013. Evolutionarily conserved Wolbachia-encoded factors control pattern of stem-cell niche tropism in Drosophila ovaries and favor infection. Proc Natl Acad Sci U S A 110:10788–10793. doi: 10.1073/pnas.1301524110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Emery G, Hutterer A, Berdnik D, Mayer B, Wirtz-Peitz F, Gaitan MG, Knoblich JA. 2005. Asymmetric Rab11 endosomes regulate delta recycling and specify cell fate in the Drosophila nervous system. Cell 122:763–773. doi: 10.1016/j.cell.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 48.Morin X, Daneman R, Zavortink M, Chia W. 2001. A protein trap strategy to detect GFP-tagged proteins expressed from their endogenous loci in Drosophila. Proc Natl Acad Sci U S A 98:15050–15055. doi: 10.1073/pnas.261408198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clare RH, Cook DA, Johnston KL, Ford L, Ward SA, Taylor MJ. 2015. Development and validation of a high-throughput anti-Wolbachia whole-cell screen: a route to macrofilaricidal drugs against onchocerciasis and lymphatic filariasis. J Biomol Screen 20:64–69. doi: 10.1177/1087057114551518. [DOI] [PubMed] [Google Scholar]
- 50.Mahr A, Aberle H. 2006. The expression pattern of the Drosophila vesicular glutamate transporter: a marker protein for motoneurons and glutamatergic centers in the brain. Gene Expr Patterns 6:299–309. doi: 10.1016/j.modgep.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 51.Egger B, van Giesen L, Moraru M, Sprecher SG. 2013. In vitro imaging of primary neural cell culture from Drosophila. Nat Protoc 8:958–965. doi: 10.1038/nprot.2013.052. [DOI] [PubMed] [Google Scholar]
- 52.Heddi A, Grenier AM, Khatchadourian C, Charles H, Nardon P. 1999. Four intracellular genomes direct weevil biology: nuclear, mitochondrial, principal endosymbiont, and Wolbachia. Proc Natl Acad Sci U S A 96:6814–6819. doi: 10.1073/pnas.96.12.6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.