ABSTRACT
Studies on the health-promoting effects of lactic acid bacteria (LAB) are numerous, but few provide examples of the relationship between LAB function and culture conditions. We verified the effect of differences in culture conditions on Lactobacillus plantarum OLL2712 functionality; this strain exhibits anti-inflammatory activity and preventive effects against metabolic disorders. We measured interleukin-10 (IL-10) and IL-12 production in murine immune cells treated with OLL2712 cells prepared under various culture conditions. The results showed that the IL-10-inducing activities of OLL2712 cells on murine immune cells differed dramatically between OLL2712 groups at different culture phases and using different culture medium components, temperatures, and neutralizing pHs. In particular, exponential-phase cells had much more IL-10-inducing activity than stationary-phase cells. We confirmed that the Toll-like receptor 2 (TLR2) stimulation activity of OLL2712 cells depended on culture conditions in conjunction with IL-10-inducing activity. We also demonstrated functional differences by culture phases in vivo; OLL2712 cells at exponential phase had more anti-inflammatory activity and anti-metabolic-disorder effects on obese and diabetic mice than those by their stationary-phase counterparts. These results suggest that culture conditions affect the functionality of anti-inflammatory LAB.
IMPORTANCE While previous studies demonstrated that culture conditions affected the immunomodulatory properties of lactic acid bacteria (LAB), few have comprehensively investigated the relationship between culture conditions and LAB functionality. In this study, we demonstrated several culture conditions of Lactobacillus plantarum OLL2712 for higher anti-inflammatory activity. We also showed that culture conditions concretely influenced the health-promoting functions of OLL2712 in vivo, particularly against metabolic disorders. Further, we characterized a novel mechanism by which changing LAB culture conditions affected immunomodulatory properties. Our results suggest that culture condition optimization is important for the production of LAB with anti-inflammatory activity.
KEYWORDS: culture phase, dendritic cell, interleukin-10, interleukin-12, Lactobacillus plantarum, macrophage, metabolic disorder, mono-oleic acid ester, proinflammatory cytokine, Toll-like receptor
INTRODUCTION
Lactic acid bacteria (LAB) have been reported to modulate immune activities and prevent or ameliorate immune-related disorders, including inflammatory bowel diseases, allergies, and certain metabolic disorders (1–3). Certain LAB strains have been selected as the most suitable strains against each disorder (4–6). In addition to the screening of strains, enhancing the abilities of selected strains improves the health-promoting abilities of LAB. Moreover, the immunomodulatory properties of a given LAB strain are reportedly sensitive to changing culture conditions (7–11). It was also reported that the amount and structure of teichoic acids, which are principal immunomodulatory components in LAB, depended on culture conditions (12–14). These previous studies suggest that it is possible to regulate the immunomodulatory properties of a selected LAB strain by changing culture conditions; however, few studies have comprehensively investigated the relationship between culture conditions and LAB functionality.
In a previous study, we selected Lactobacillus plantarum OLL2712 for its strong ability to induce interleukin-10 (IL-10) production in murine immune cells. Administration of heat-killed OLL2712 cells for 3 weeks alleviated chronic inflammation and improved hyperlipidemia in KKAy mice, an obesity and diabetes model (15). IL-10 is an anti-inflammatory cytokine secreted by immune cells in intestinal and adipose tissues (16, 17). Induction of IL-10 prevented insulin resistance and improved glucose and lipid metabolism in obese mice (18, 19). We therefore hypothesized that the antihyperlipidemic effect of OLL2712 was exerted via stimulation of IL-10 production and suppression of chronic inflammation. However, we did not consider the culture conditions of OLL2712 in the previous study. The anti-inflammatory activity and antihyperlipidemic effects of OLL2712 might be affected by its culture conditions.
In the present study, we evaluated the IL-10-inducing activity and the ratio of IL-10-inducing activity to IL-12-inducing activity (IL-10/IL-12 ratio) by OLL2712 cells, prepared under various culture conditions, on murine immune cells. In addition to IL-10-inducing activity, the IL-10/IL-12 ratio is also considered to be a dominant index for the anti-inflammatory activity of LAB (4, 20, 21); IL-12 is a proinflammatory cytokine which induces chronic inflammation and metabolic disorders (22, 23). Additionally, we analyzed the effect of OLL2712 culture conditions on the ability of this LAB to stimulate murine Toll-like receptor 2 (TLR2) activity. TLR2 expressed on immune cell surfaces recognizes bacterial cell components, including teichoic acids. The activation of TLR2 induced IL-10 production and suppressed IL-12 production (24, 25). Elucidation of the culture conditions that affect these immunomodulatory properties is important not only for developing a process for the production of LAB with anti-inflammatory activity but also for investigating the mechanisms underlying LAB functionality.
The objective of the present study was to investigate the effect of OLL2712 culture conditions on the immunomodulatory properties for murine immune cells. We also sought to verify the preventive effects of OLL2712 cells, prepared under different culture conditions, against metabolic disorders in obese and diabetic mice.
RESULTS
Effects of culture conditions on immunomodulatory properties of OLL2712 for murine immune cells. (i) Culture phase and culture medium components.
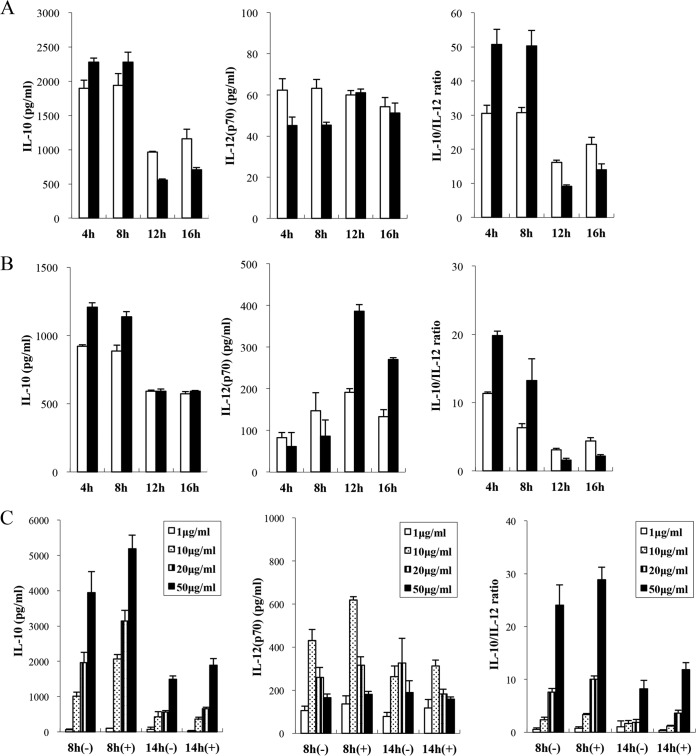
We examined the effect of culture phases and culture medium components on the immunomodulatory properties of OLL2712 cells. In this experiment, we fixed the culture temperature at 33°C and the neutralizing pH at 5.8, because preliminary examinations indicated that the bacterial cell yield was highest under these conditions. Early or late-exponential-phase (cultured for 4 h or 8 h) OLL2712 cells showed higher levels of IL-10-inducing activity in bone marrow-derived dendritic cells (BMDCs) than their early or late-stationary-phase (cultured for 12 h or 16 h) counterparts. Relative to IL-10-inducing activity, the influence of culture phases on IL-12-inducing activity was less pronounced (Fig. 1A). Adding Q-17S, a decaglycerol mono-oleic acid ester, to the culture medium increased the IL-10-inducing activity of exponential-phase OLL2712 cells (Fig. 1A). Adding the other nutrients, including vitamins, minerals, and mono-stearic acid ester, did not affect the IL-10- or IL-12-inducing activity of OLL2712 cells (data not shown). The same experiments using peritoneal macrophages (PMs) produced results similar to those of the BMDC experiments (Fig. 1B). IL-10-inducing activity and the IL-10/IL-12 ratio of OLL2712 cells increased conspicuously when cultured in medium with Q-17S until exponential phase. Then, we cultured OLL2712 again for 8 h (late-exponential phase) or 14 h (mid-stationary phase) with or without Q-17S and confirmed that above-mentioned results were observed regardless of additive concentrations to BMDCs (Fig. 1C). Here, the bacterial cells cultured for 8 h, 14 h, and 16 h with Q-17S are described as 8-h (+), 14-h (+), and 16-h (+) cells.
FIG 1.
Effects of culture phases or culture medium components on immunomodulatory properties of OLL2712 cells for murine immune cells. (A and B) BMDCs (A) or PMs (B) were treated with OLL2712 cells (50 μg/ml), prepared at different culture phases (4 h to 16 h) and in medium with (■) or without (□) mono-oleic acid ester Q-17S, for 24 h. (C) BMDCs were treated with OLL2712 cells at indicated concentrations (1 to 50 μg/ml), prepared at different culture phases (8 h or 14 h) and in medium with (+) or without (−) mono-oleic acid ester Q-17S, for 24 h. The temperature and neutralizing pH in OLL2712 cultures were 33°C and pH 5.8. The IL-10 and IL-12 concentrations in the supernatants were measured. The results are presented as means ± SD (n = 4). Data are representative of 2 independent experiments.
We examined the effect of culture phases and adding Q-17S on the immunomodulatory properties of MEP222801, an L. plantarum strain with high IL-10-inducing activity second to OLL2712 in our LAB library (15), and obtained almost the same result as OLL2712 (Fig. 2).
FIG 2.
Effects of culture phases or culture medium components on immunomodulatory properties of MEP222801 cells for murine immune cells. BMDCs were treated with MEP222801 cells (50 μg/ml), prepared at different culture phases (5 h or 16 h) and in medium with (+) or without (−) mono-oleic acid ester Q-17S, for 24 h. The temperature and neutralizing pH in MEP222801 cultures were 33°C and pH 5.8. The IL-10 and IL-12 concentrations in the supernatants were measured. The results are presented as means ± SD (n = 4). Data are representative of 2 independent experiments.
(ii) Culture temperature and neutralizing pH.
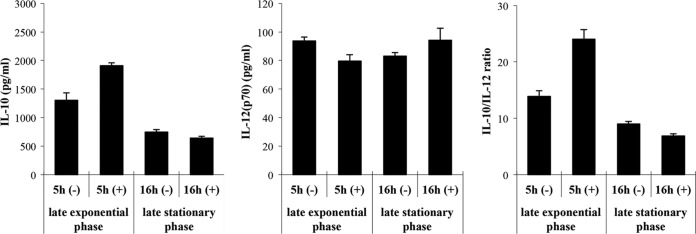
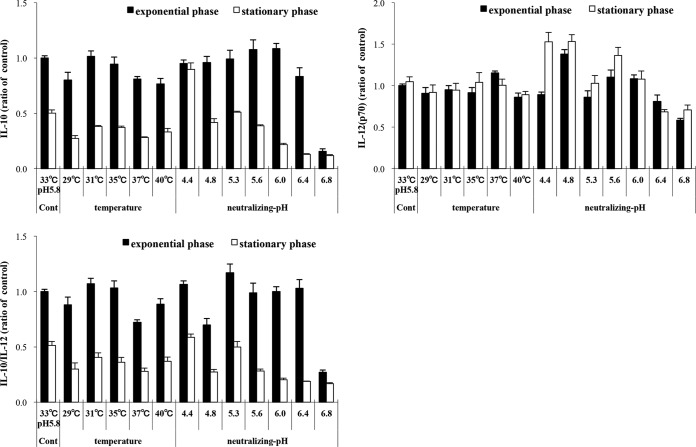
We examined the effect of 29 to 40°C temperatures and neutralizing pH 4.4 to 6.8 on the immunomodulatory properties of late-exponential-phase OLL2712 cells. When changing the temperature, the pH was fixed at 5.8. When changing the pH, the temperature was fixed at 33°C. The IL-10-inducing activity and IL-10/IL-12 ratio were maximal at 31 to 35°C and pH 5.3 to 6.0 (Fig. 3). At any temperature or pH, exponential-phase cells had much more IL-10-inducing activity than stationary-phase cells. The same experiments using PMs produced results similar to those of the BMDC experiments (data not shown).
FIG 3.
Effects of temperature or neutralizing pH on immunomodulatory properties of OLL2712 cells for murine immune cells. BMDCs were treated with late-exponential-phase (■) or late-stationary-phase (□) OLL2712 cells (50 μg/ml) prepared at different temperature or neutralizing pH for 24 h. When changing the temperature, the pH was fixed at 5.8. When changing the pH, the temperature was fixed at 33°C. The IL-10 and IL-12 concentrations in the supernatants were measured. The results are shown as the ratio of control (at 33°C and pH 5.8). The results are presented as means ± SD (n = 4). Data are representative of 2 independent experiments.
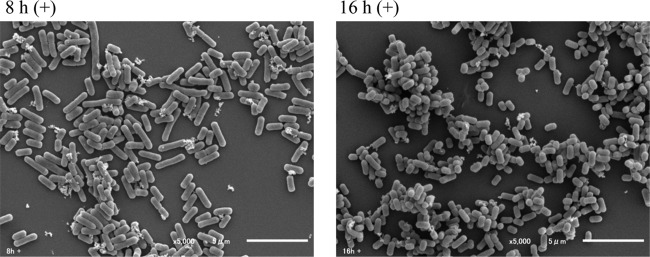
Effects of culture conditions on OLL2712 yield and morphology.
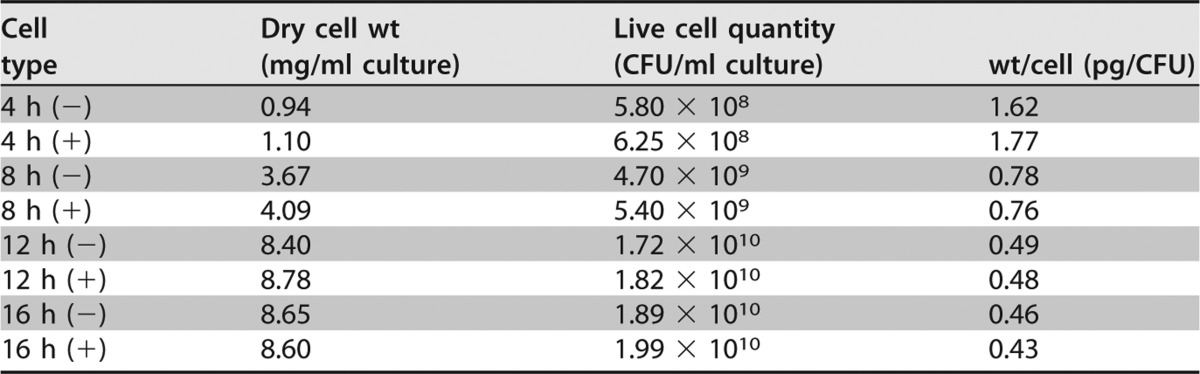
The number of live bacteria increased by approximately 4-fold over 8 h, based on a comparison of 16-h (+) and 8-h (+) data; however, the cell body dry weight increased only by about 2-fold. Within this time frame, the mass per cell fell by half (Table 1). Scanning electron microscope (SEM) images revealed many long bacilli at 8 h (+) and many short bacilli at 16 h (+), but we could not observe any biofilm or extracellular matrix in the SEM images (Fig. 4). Adding Q-17S did not affect OLL2712 growth or morphology.
TABLE 1.
Dry cell weight, live cells, and dry cell weight per CFU of OLL2712
FIG 4.
Effects of culture conditions on OLL2712 morphology.
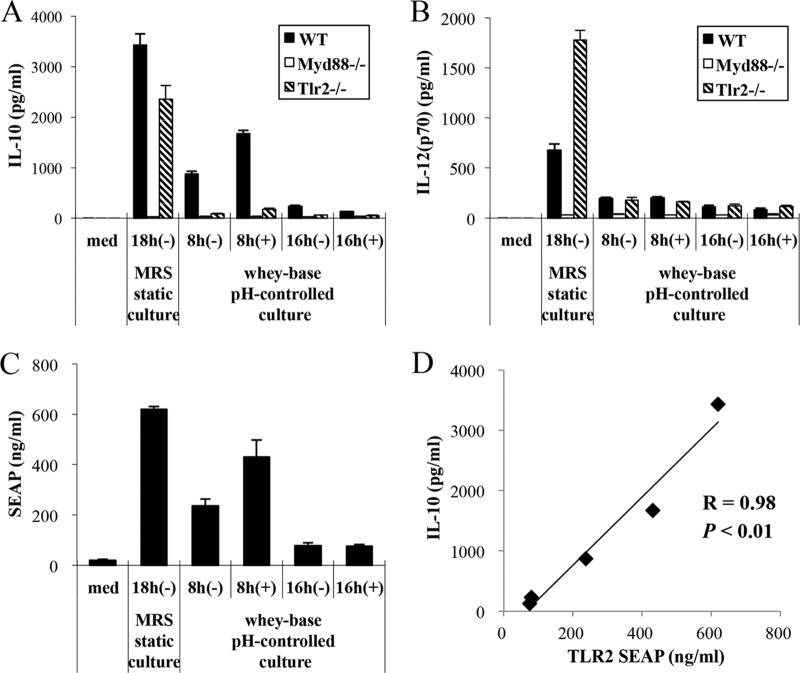
Involvement of MyD88 and TLR2 pathways in immunomodulatory properties of OLL2712 for murine immune cells.
IL-10 and IL-12 production by OLL2712 was completely reduced in BMDCs from MyD88−/− mice. The myeloid differentiation primary response gene 88 (MyD88) is a factor that conveys Toll-like receptor (TLR) signals, including TLR2 (24). In a comparison of BMDCs from Tlr2−/− and wild-type (WT) mice, IL-10 production was reduced, whereas IL-12 production was increased in BMDCs from Tlr2−/− mice (Fig. 5A and B). TLR2 is a receptor that recognizes LAB cell wall components (24). These results suggest that OLL2712 stimulation of immune cell IL-10 production depends on MyD88 and TLR2. In addition, we confirmed that OLL2712 cells cultured statically for 18 h in de Man, Rogosa, and Sharpe (MRS) broth, which we had used in a previous study (15), exhibited higher levels of IL-10 and IL-12 production than 8-h (+) cells.
FIG 5.
The IL-10-inducing activity of OLL2712 cells depended on MyD88 and TLR2 pathways under any culture conditions. med, medium. (A and B) BMDCs (from WT, Myd88−/−, or Tlr2−/− mice) were treated with OLL2712 cells (10 μg/ml) prepared under different culture conditions for 24 h. The IL-10 and IL-12 concentrations in the supernatants were measured. (C) HEK-293 cells expressing TLR2 were treated with OLL2712 cells (10 μg/ml) prepared under different culture conditions for 24 h. The TLR2 stimulation activity was evaluated by quantity of SEAP secreted outside the cells. The results are presented as means ± SD (n = 4). Data are representative of 2 independent experiments. (D) Correlation of IL-10-inducing activity and TLR2 stimulation activity.
Effects of culture conditions on TLR2 stimulation activity of OLL2712.
We checked whether TLR2 stimulation activity would be affected under OLL2712 culture conditions. TLR2 has been reported to affect immune cell production of IL-10 (24, 26–29). We used the HEK 293 cell line, which highly expresses TLR2, and evaluated TLR2 stimulation activity by the quantity of secreted alkaline phosphatase protein (SEAP) secreted outside the cells. We demonstrated that OLL2712 cells statically cultured in MRS broth had the highest activity; TLR2 activities for the other groups, in descending order, were 8-h (+), 8-h (−), 16-h (−), and 16-h (+) cells (Fig. 5C). The IL-10-inducing activity and TLR2 stimulation activity positively correlated (R = 0.98, P < 0.01; Fig. 5D).
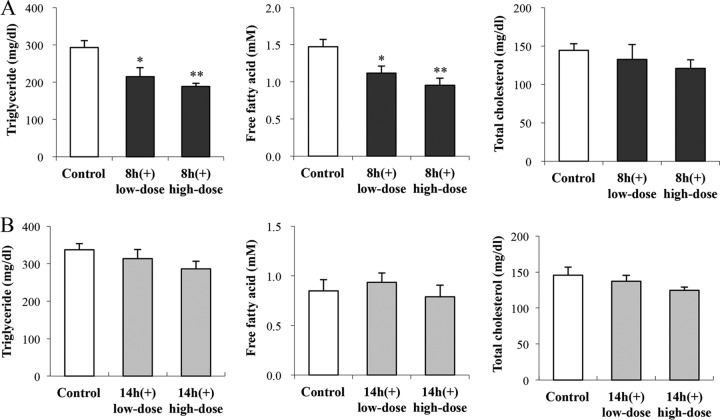
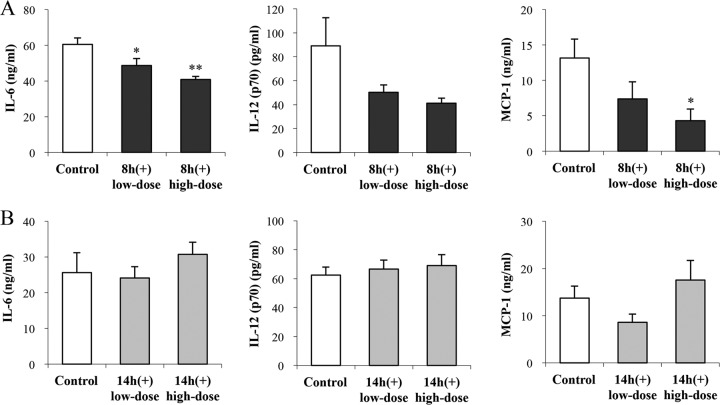
Anti-inflammatory and anti-metabolic-disorder effects of OLL2712 cells on obese and diabetic mice were affected by their culture phases.
In the experiment to administer 8-h (+) cells, we observed no difference between groups in mouse weight change or food intake; however, the final fasting blood glucose levels were significantly lower in the high-dose group than in the control group (Table 2). The blood triglyceride levels and blood free fatty acid levels were significantly lower in the low-dose group and high-dose group than in the control group. The blood total cholesterol levels did not significantly differ between groups (Fig. 6A). Furthermore, the proinflammatory cytokine production by adipose tissue stromal vascular fraction (SVF) cells from KKAy mice was significantly suppressed by administering 8-h (+) cells (Fig. 7A). On the other hand, in the experiment to administer stationary-phase 14-h (+) cells, a meaningful difference was not observed in the above-mentioned measurements (Table 2 and Fig. 6B and 7B).
TABLE 2.
Food intake, body weight, and fasting blood glucose levelsa
| Parameter | Control | Low dose | High dose |
|---|---|---|---|
| 8-h (+) cells | |||
| Food intake (g per 3 weeks) | 121 ± 5 | 124 ± 2 | 123 ± 5 |
| Initial body wt (g) | 32.9 ± 0.6 | 33.3 ± 0.6 | 32.7 ± 0.4 |
| Final body wt (g) | 38.0 ± 1.4 | 38.9 ± 1.1 | 37.3 ± 0.3 |
| Initial blood glucose level (mg/dl) | 297 ± 22 | 310 ± 19 | 290 ± 21 |
| Final blood glucose level (mg/dl) | 359 ± 18 | 342 ± 19 | 274 ± 26* |
| 14-h (+) cells | |||
| Food intake (g per 3 weeks) | 109 ± 3 | 106 ± 5 | 103 ± 4 |
| Initial body wt (g) | 32.3 ± 0.3 | 32.3 ± 0.6 | 31.4 ± 0.4 |
| Final body wt (g) | 37.0 ± 0.5 | 37.0 ± 0.5 | 36.8 ± 0.6 |
| Initial blood glucose level (mg/dl) | 264 ± 8 | 287 ± 29 | 268 ± 25 |
| Final blood glucose level (mg/dl) | 274 ± 32 | 264 ± 17 | 278 ± 34 |
Values are expressed as means with SE (n = 6). Significant differences compared with the control group were determined by Dunnett's test (*, P < 0.05). Data are representative of 2 independent experiments.
FIG 6.
The blood lipid-lowering effects of OLL2712 cells on KKAy mice were affected by their culture phases. The 8-h (+) or 14-h (+) OLL2712 cells were administered to KKAy mice for 3 weeks. After 4 h of fasting, the serum concentrations of triglycerides, free fatty acids, and total cholesterols were measured. The results are presented as means ± SE (n = 6). Significant differences compared with the control group were determined by Dunnett's test (*, P < 0.05; **, P < 0.01). Data are representative of 2 independent experiments.
FIG 7.
The anti-inflammatory effects of OLL2712 cells on the adipose tissues of KKAy mice were affected by their culture phases. SVF cells were prepared from each mouse and were incubated for 48 h. The concentrations of proinflammatory cytokines in the supernatant were measured. The results are presented as means ± SE (n = 6). Significant differences compared with the control group were determined by Dunnett's test (*, P < 0.05; **, P < 0.01). Data are representative of 2 independent experiments. MCP-1, monocyte chemotactic protein 1.
DISCUSSION
LAB may efficaciously prevent and/or treat metabolic disorders (3, 30); the anti-inflammatory effect of LAB is assumed to be an essential mechanism of its action (15, 31). Out of many strains in our LAB library, we had previously selected the optimal OLL2712 strain, which has high IL-10-inducing activity (15). On the other hand, the health-promoting properties of LAB may change in response to their culture conditions, even when the strain remains the same (7–11). With respect to OLL2712, we expected that the anti-inflammatory activity would be changed by modification of the culture conditions. In the present study, we evaluated IL-10 and IL-12 production in murine immune cells treated with OLL2712 cells prepared under various culture conditions. We found that the IL-10-inducing activity and IL-10/IL-12 ratio varied dramatically between OLL2712 groups under different culture conditions. The culture conditions for the highest anti-inflammatory activity were exponential phase, mono-oleic acid ester added to the culture medium, pH 5.3 to 6.0, and 31 to 35°C. We have also demonstrated that the effects of OLL2712 cells on a mouse model of metabolic disorders changed in response to OLL2712 culture phases.
We confirmed that the function of OLL2712 cells differed between culture phases in vitro and in vivo. Prior studies have shown that the immunomodulatory properties of LAB change in response to changes in the culture phases. Sashihara et al. (9) showed that stationary-phase cells of a Lactobacillus gasseri strain stimulated murine splenocytes to secrete a greater amount of IL-12 than exponential-phase cells. van Baarlen et al. (10) showed that exponential-phase and stationary-phase L. plantarum WCFS1 cells elicited distinct human duodenal transcript profiles, which appeared to mainly result from differential modulation of NF-κB-dependent signaling pathways. van Hemert et al. (11) demonstrated that exponential-phase L. plantarum WCFS1 cells stimulated peripheral blood mononuclear cells (PBMCs) to secrete larger amounts of IL-10 and IL-12 than stationary-phase cells. In this study, we showed that exponential-phase MEP222801 cells as well as OLL2712 cells stimulated BMDCs to secrete a greater amount of IL-10 than stationary-phase cells. To obtain maximal cell mass in industrial production, LAB are generally cultivated to the stationary phase in pH-controlled batches. However, our results indicate that exponential-phase OLL2712 cells (probably for MEP222801 as well) showed obviously better performance than their stationary-phase counterparts. Cultivation until stationary phase might not be suitable for producing LAB with anti-inflammatory activity.
Previous studies reported that oleic acid affected the cell growth (32) or survival in gastric juice (33) of some LAB strains by being incorporated into bacterial cell surface membrane lipids, but stearic acid did not. In this study, we found for the first time that adding decaglycerol mono-oleic acid ester Q-17S, normally used as an emulsifier for food, affected the immunomodulatory properties of L. plantarum strains, but decaglycerol monostearic acid ester Q-18S did not. Although Q-17S did not affect the cell growth of OLL2712 or MEP222801, we speculate that Q-17S may be incorporated into bacterial cell surface membrane lipids and affect the structure of bacterial cell components containing fatty acids. Further investigations are needed to clarify the details of the mechanism.
Regarding the bacterial cell components responsible for the immunomodulating activity of LAB, Grangette et al. (34) reported that by genetically extinguishing d-alanine substitution of the teichoic acids (especially lipoteichoic acids), the IL-10-inducing activity of an L. plantarum strain for PBMCs rose remarkably, and the anti-inflammatory effects on model colitis mice were also enhanced. The amount or structure of the teichoic acids was found to strongly influence the IL-10-inducing activity of LAB through the activation of TLR2 (24, 26–29). Moreover, the amount and structure of the teichoic acids were reported to depend on the LAB culture conditions (12–14). It is therefore possible that the amount or structure of the teichoic acids in OLL2712 cells was changed by the culture conditions tested in the present study, resulting in the changes to TLR2 stimulation activity and IL-10-inducing activity. On the other hand, Kotaki et al. (25) reported that TLR2 suppressed TLR9-induced IL-12 production in BMDCs. In this study, we confirmed that the levels of TLR2 stimulation activity under various OLL2712 culture conditions were consistent with those of IL-10-inducing activity, and that Tlr2−/− BMDCs exhibited lower IL-10 production and higher IL-12 production than WT BMDCs (Fig. 5). Furthermore, administration of 8-h (+) cells possessing high TLR2 stimulation activity suppressed proinflammatory cytokine levels in the adipose tissues of KKAy mice, whereas stationary-phase cells, possessing little TLR2 stimulation activity, did not (Fig. 7). These results suggest that the cell surface components that activate TLR2 are the main cause of IL-10-inducing activity by OLL2712 cells. Moreover, TLR2 stimulation activity of OLL2712 cells suppressed chronic inflammation in KKAy mice by inhibiting proinflammatory cytokines, such as IL-12, as well as by inducing IL-10 production.
The modification of cell surface components by genetic recombination reportedly influences the dextran sulfate sodium (DSS) colitis-improving effect of LAB in vivo (34–36). Culture condition changes have been reported previously to influence LAB immunomodulatory properties in vitro and in vivo (8–10). However, to our knowledge, the present study is the first to show that culture conditions concretely influenced health-promoting functions of LAB in vivo, particularly against metabolic disorders. Modification of culture conditions is more applicable than genetic recombination to industrial production. Our results suggest that both strain screening and culture condition optimization are important for the production of LAB with anti-inflammatory activity.
In conclusion, the immunomodulatory properties of OLL2712 cells were dramatically changed by their culture conditions, and culture conditions affected the anti-inflammatory activity of OLL2712 cells. Changes in OLL2712 anti-inflammatory activity in response to culture phase were reflected by this strain's observed antidiabetic and antihyperlipidemic effects in vivo. In addition, we indicated that the change in IL-10-inducing activity of OLL2712 by culture conditions depended on the change of bacterial cell surface molecules affecting TLR2. Investigating the reasons for these changes would further elucidate the mechanisms of LAB function. We intend to investigate the relationship among the amount or structure of OLL2712 cell surface components and the immunomodulatory properties for immune cells by comparing various types of OLL2712 cells prepared under different culture conditions.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
OLL2712 and Lactobacillus plantarum MEP222801 were isolated from the feces from a young Japanese woman volunteer and identified as previously described (15). The preculture of OLL2712 or MEP222801 was grown in de Man, Rogosa, and Sharpe (MRS) broth (Becton Dickinson, Cockeysville, MD) at 37°C under an anaerobic atmosphere for 18 h. In a pH-controlled culture for industrial production, the medium was prepared as follows. Whey powder (Meiji, Odawara, Japan) and whey protein concentrate (WPC472; Fonterra, NZ) were dissolved in distilled water for final concentrations of 6.25% (wt/wt) and 1.75% (wt/wt), respectively; they were digested using protease A (Amano Enzyme, Nagoya, Japan) at 48°C for 3 h. After digestion, yeast extract (Asahi Food and Healthcare, Tokyo, Japan), fish extract [Bacterio N-K(N); Maruha Nichiro, Tokyo, Japan], and MnSO4 were added to final concentrations of 0.5% (wt/wt), 0.5% (wt/wt), and 0.01% (wt/wt), respectively. In addition, each of the nutrients, including vitamins (sodium ascorbate, thiamine hydrochloride, riboflavin, nicotinic acid, nicotinamide, calcium pantothenate, pyridoxine hydrochloride, biotin, and folic acid), minerals (MgSO4, CaCl2, FeSO4, and ferric ammonium sulfate), and fatty acid esters (decaglycerol mono-oleic acid ester [Q-17S; Taiyo Kagaku, Yokkaichi, Japan] and decaglycerol monostearic acid ester [Q-18S; Taiyo Kagaku) was added to final concentrations of 0.05% (wt/wt). The pH was adjusted to 6.7 with NaOH, and the medium was autoclaved at 121°C for 2 min. The preculture was inoculated to the medium at 4% (vol/vol). During incubation, the pH was adjusted to several set points using K2CO3. The culture conditions were 29 to 40°C at pH 4.8 to 6.8, for 4 to 16 h. In a static culture only for the experiment in Fig. 5, the preculture was inoculated in MRS broth at 1% (vol/vol) and incubated at 37°C under an anaerobic atmosphere for 18 h. The cells were harvested by centrifugation (8,000 × g, 15 min, 4°C), washed twice with saline solution, and washed once with distilled water. The cells were resuspended in distilled water, heat killed at 75°C for 60 min, and lyophilized. The lyophilized cells were resuspended in phosphate-buffered saline (PBS) at an adequate concentration and used for in vitro and in vivo assays.
Experiments with murine immune cells.
Bone marrow-derived dendritic cells (BMDCs) and peritoneal macrophages (PMs) were prepared from BALB/c mice, as previously described (15). The immune cells were cultured in RPMI 1640 medium (Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (FBS) and maintained at 37°C in a water-jacketed incubator under 5% carbon dioxide. The immune cells were treated with different preparations of OLL2712 cells for 24 h. The IL-10 and IL-12 (p70) concentrations in the supernatant were measured by enzyme-linked immunosorbent assay (ELISA) (BD Biosciences, San Diego, CA), according to the manufacturer's instructions.
TLR2 SEAP assay.
TLR2 stimulation activity of OLL2712 was evaluated using a secreted alkaline phosphatase protein (SEAP) reporter assay. Activation of TLR2 was determined using a reporter plasmid (pNF-κB/SEAP; Imgenex, San Diego, CA) expressing SEAP under the control of the NF-κB promoter. These plasmids were transfected into HEK 293 cells using Invitrogen Lipofectamine LTX, according to the manufacturer's protocol. Stable cell clones of the transfected cells were obtained by selection using G418 at 500 μg/ml. Further, 2 × 105 transfected HEK 293 cells were seeded into a 24-well plate 1 day prior to treatment. The HEK 293 cells were next treated for 48 h with different preparations of OLL2712 cells. The amount of SEAP in the supernatant was measured using an NF-κB SEAP reporter assay kit (Novus, Littleton, CO).
Handling of mice.
Eight-week-old specific-pathogen-free male BALB/c mice were purchased from Japan SLC (Shizuoka, Japan). Eight-week-old specific-pathogen-free male MyD88−/− or Tlr2−/− BALB/c mice were purchased from Oriental Bioservice (Kyoto, Japan). They were used for the preparation of immune cells. Five-week-old specific-pathogen-free male KKAy mice were purchased from CLEA Japan (Shizuoka, Japan) and used for the in vivo studies. All mice were housed in standard plastic cages in a temperature-controlled (23 ± 2°C) room, using a 12-h light-dark cycle. They were allowed free access to water and a purified diet (AIN-93M; Oriental Yeast, Tokyo, Japan). The KKAy mice were divided into three groups; to each group (n = 6), we administered distilled water, a low dose of OLL2712, or a high dose of OLL2712. Each day, the mice were intragastrically administered 0.5 ml of either distilled water or distilled water containing lyophilized powder of heat-killed OLL2712 cells (0.2 mg or 2 mg per mouse). After 3 weeks of feeding, the mice were subjected to fasting for 4 h and anesthetized by isoflurane inhalation, after which a whole-blood sample was drawn from the axillary artery. The serum was separated from the blood samples and used to measure glucose and lipid levels. The retroperitoneal white adipose tissue was removed from each mouse and used for stromal vascular fraction (SVF) preparation. The Animal Care Committee of the Food Science Research Laboratories approved all study protocols, and animals were maintained in accordance with the Meiji Co., Ltd. guidelines for the care and use of laboratory animals.
Measurement of blood glucose and lipid levels.
Blood glucose concentrations were measured using the Breeze 2 blood glucose monitoring system (Bayer Healthcare, Whippany, NJ). Blood concentrations of triglyceride and total cholesterol were measured using the Fuji Dri-Chem analyzer and special slides (TCHO-P III slide and TG-P III slide; Fujifilm, Tokyo, Japan). Blood concentrations of free fatty acids were measured using the FFA quantification kit (BioVision, Milpitas, CA).
Experiments with SVF cells.
Isolation of SVF was performed using the method proposed by a previous study (17), with minor modifications. Retroperitoneal fat pads from KKAy mice were excised and minced in PBS with calcium chloride and 0.5% BSA. Tissue suspensions were centrifuged at 500 × g for 5 min at 4°C to remove erythrocytes and free leukocytes. Collagenase (Sigma, St. Louis, MO) was added to 1 mg/ml and incubated at 37°C for 20 min with shaking. The cell suspension was filtered through a 100-μm filter and subsequently spun at 300 × g for 5 min at 4°C to separate adipocytes from the SVF pellet. For cell culture, the pellet containing the SVF cells was resuspended in RPMI 1640 medium and incubated at 37°C for 48 h under stimulation of 1 μg/ml lipopolysaccharide (LPS).
Statistical analyses.
Measurements are presented as the means ± standard deviations (SD) or standard errors (SE). Statistical evaluation was performed using the StatView software (SAS, Cary, NC). Statistical differences were analyzed using Dunnett's test. Statistical significance was considered at a P value of <0.05. Each experiment was repeated at least twice, producing similar results; consequently, only one set of results for each experiment is shown.
REFERENCES
- 1.Borchers AT, Selmi C, Meyers FJ, Keen CL, Gershwin ME. 2009. Probiotics and immunity. J Gastroenterol 44:26–46. doi: 10.1007/s00535-008-2296-0. [DOI] [PubMed] [Google Scholar]
- 2.Parvez S, Malik KA, Ah Kang S, Kim HY. 2006. Probiotics and their fermented food products are beneficial for health. J Appl Microbiol 100:1171–1185. doi: 10.1111/j.1365-2672.2006.02963.x. [DOI] [PubMed] [Google Scholar]
- 3.Kadooka Y, Sato M, Imaizumi K, Ogawa A, Ikuyama K, Akai Y, Okano M, Kagoshima M, Tsuchida T. 2010. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur J Clin Nutr 64:636–643. doi: 10.1038/ejcn.2010.19. [DOI] [PubMed] [Google Scholar]
- 4.Foligne B, Nutten S, Grangette C, Dennin V, Goudercourt D, Poiret S, Dewulf J, Brassart D, Mercenier A, Pot B. 2007. Correlation between in vitro and in vivo immunomodulatory properties of lactic acid bacteria. World J Gastroenterol 13:236–243. doi: 10.3748/wjg.v13.i2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snel J, Vissers YM, Smit BA, Jongen JM, van der Meulen ET, Zwijsen R, Ruinemans-Koerts J, Jansen AP, Kleerebezem M, Savelkoul HF. 2011. Strain-specific immunomodulatory effects of Lactobacillus plantarum strains on birch-pollen-allergic subjects out of season. Clin Exp Allergy 41:232–242. doi: 10.1111/j.1365-2222.2010.03650.x. [DOI] [PubMed] [Google Scholar]
- 6.Sashihara T, Sueki N, Ikegami S. 2006. An analysis of the effectiveness of heat-killed lactic acid bacteria in alleviating allergic diseases. J Dairy Sci 89:2846–2855. doi: 10.3168/jds.S0022-0302(06)72557-7. [DOI] [PubMed] [Google Scholar]
- 7.Haller D, Bode C, Hammes WP. 1999. Cytokine secretion by stimulated monocytes depends on the growth phase and heat treatment of bacteria: a comparative study between lactic acid bacteria and invasive pathogens. Microbiol Immunol 43:925–935. doi: 10.1111/j.1348-0421.1999.tb03353.x. [DOI] [PubMed] [Google Scholar]
- 8.Maassen CB, Boersma WJ, van Holten-Neelen C, Claassen E, Laman JD. 2003. Growth phase of orally administered Lactobacillus strains differentially affects IgG1/IgG2a ratio for soluble antigens: implications for vaccine development. Vaccine 21:2751–2757. doi: 10.1016/S0264-410X(03)00220-2. [DOI] [PubMed] [Google Scholar]
- 9.Sashihara T, Sueki N, Furuichi K, Ikegami S. 2007. Effect of growth conditions of Lactobacillus gasseri OLL2809 on the immunostimulatory activity for production of interleukin-12 (p70) by murine splenocytes. Int J Food Microbiol 120:274–281. doi: 10.1016/j.ijfoodmicro.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 10.van Baarlen P, Troost FJ, van Hemert S, van der Meer C, de Vos WM, de Groot PJ, Hooiveld GJ, Brummer RJ, Kleerebezem M. 2008. Differential NF-kappaB pathways induction by Lactobacillus plantarum in the duodenum of healthy humans correlating with immune tolerance. Proc Natl Acad Sci U S A 106:2371–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Hemert S, Meijerink M, Molenaar D, Bron PA, de Vos P, Kleerebezem M, Wells JM, Marco ML. 2010. Identification of Lactobacillus plantarum genes modulating the cytokine response of human peripheral blood mononuclear cells. BMC Microbiol 10:293. doi: 10.1186/1471-2180-10-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knox KW, Campbell LK, Broady KW, Wicken AJ. 1979. Serological studies on chemostat-grown cultures of Lactobacillus fermentum and Lactobacillus plantarum. Infect Immun 24:12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wicken AJ, Broady KW, Ayres A, Knox KW. 1982. Production of lipoteichoic acid by lactobacilli and streptococci grown in different environments. Infect Immun 36:864–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wicken AJ, Evans JD, Campbell LK, Knox KW. 1982. Teichoic acids from chemostat-grown cultures of Streptococcus mutans and Lactobacillus plantarum. Infect Immun 38:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toshimitsu T, Mochizuki J, Ikegami S, Itou H. 2016. Identification of a Lactobacillus plantarum strain that ameliorates chronic inflammation and metabolic disorders in obese and type 2 diabetic mice. J Dairy Sci 99:933–946. doi: 10.3168/jds.2015-9916. [DOI] [PubMed] [Google Scholar]
- 16.Asadullah K, Sterry W, Volk HD. 2003. Interleukin-10 therapy–review of a new approach. Pharmacol Rev 55:241–269. doi: 10.1124/pr.55.2.4. [DOI] [PubMed] [Google Scholar]
- 17.Lumeng CN, Bodzin JL, Saltiel AR. 2007. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cintra DE, Pauli JR, Araújo EP, Moraes JC, de Souza CT, Milanski M, Morari J, Gambero A, Saad MJ, Velloso LA. 2008. Interleukin-10 is a protective factor against diet-induced insulin resistance in liver. J Hepatol 48:628–637. doi: 10.1016/j.jhep.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 19.Hong EG, Ko HJ, Cho YR, Kim HJ, Ma Z, Yu TY, Friedline RH, Kurt-Jones E, Finberg R, Fischer MA, Granger EL, Norbury CC, Hauschka SD, Philbrick WM, Lee CG, Elias JA, Kim JK. 2009. Interleukin-10 prevents diet-induced insulin resistance by attenuating macrophage and cytokine response in skeletal muscle. Diabetes 58:2525–2535. doi: 10.2337/db08-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermúdez-Humarán LG, Gratadoux JJ, Blugeon S, Bridonneau C, Furet JP, Corthier G, Grangette C, Vasquez N, Pochart P, Trugnan G, Thomas G, Blottière HM, Doré J, Marteau P, Seksik P, Langella P. 2008. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A 105:16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kechaou N, Chain F, Gratadoux JJ, Blugeon S, Bertho N, Chevalier C, Le Goffic R, Courau S, Molimard P, Chatel JM, Langella P, Bermúdez-Humarán LG. 2013. Identification of one novel candidate probiotic Lactobacillus plantarum strain active against influenza virus infection in mice by a large-scale screening. Appl Environ Microbiol 79:1491–1499. doi: 10.1128/AEM.03075-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sennello JA, Fayad R, Pini M, Gove ME, Podnemone V, Cabay RJ, Siegmund B, Dinarello CA, Fantuzzi G. 2008. Interleukin-18, together with interleukin-12, induces severe acute pancreatitis in obese but not in nonobese leptin-deficient mice. Proc Natl Acad Sci U S A 105:8085–8090. doi: 10.1073/pnas.0804091105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pini M, Sennello JA, Cabay RJ, Fantuzzi G. 2010. Effect of diet-induced obesity on acute pancreatitis induced by administration of interleukin-12 plus interleukin-18 in mice. Obesity 18:476–481. doi: 10.1038/oby.2009.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaji R, Kiyoshima-Shibata J, Nagaoka M, Nanno M, Shida K. 2010. Bacterial teichoic acids reverse predominant IL-12 production induced by certain Lactobacillus strains into predominant IL-10 production via TLR2-dependent ERK activation in macrophages. J Immunol 184:3505–3513. doi: 10.4049/jimmunol.0901569. [DOI] [PubMed] [Google Scholar]
- 25.Kotaki R, Wajima S, Shiokawa A, Hachimura S. 2015. Toll-like receptor 2 suppresses Toll-like receptor 9 responses in Peyer's patch dendritic cells. Immunobiology 220:734–743. doi: 10.1016/j.imbio.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 26.Sing A, Rost D, Tvardovskaia N, Roggenkamp A, Wiedemann A, Kirschning CJ, Aepfelbacher M, Heesemann J. 2002. Yersinia V-antigen exploits toll-like receptor 2 and CD14 for interleukin 10-mediated immunosuppression. J Exp Med 196:1017–1024. doi: 10.1084/jem.20020908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dillon S, Agrawal A, Van Dyke T, Landreth G, McCauley L, Koh A, Maliszewski C, Akira S, Pulendran B. 2004. A Toll-like receptor 2 ligand stimulates Th2 responses in vivo, via induction of extracellular signal-regulated kinase mitogen-activated protein kinase and c-Fos in dendritic cells. J Immunol 172:4733–4743. doi: 10.4049/jimmunol.172.8.4733. [DOI] [PubMed] [Google Scholar]
- 28.Saraiva M, O'Garra A. 2010. The regulation of IL-10 production by immune cells. Nat Rev Immunol 10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 29.Jeon SG, Kayama H, Ueda Y, Takahashi T, Asahara T, Tsuji H, Tsuji NM, Kiyono H, Ma JS, Kusu T, Okumura R, Hara H, Yoshida H, Yamamoto M, Nomoto K, Takeda K. 2012. Probiotic Bifidobacterium breve induces IL-10-producing Tr1 cells in the colon. PLoS Pathog 8:e1002714. doi: 10.1371/journal.ppat.1002714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takemura N, Okubo T, Sonoyama K. 2010. Lactobacillus plantarum strain no. 14 reduces adipocyte size in mice fed high-fat diet. Exp Biol Med 235:849–856. doi: 10.1258/ebm.2010.009377. [DOI] [PubMed] [Google Scholar]
- 31.Miyoshi M, Ogawa A, Higurashi S, Kadooka Y. 2013. Anti-obesity effect of Lactobacillus gasseri SBT2055 accompanied by inhibition of pro-inflammatory gene expression in the visceral adipose tissue in diet-induced obese mice. Eur J Nutr 53:599–606. [DOI] [PubMed] [Google Scholar]
- 32.Endo Y, Kamisada S, Fujimoto K, Saito T. 2006. Trans fatty acids promote the growth of some Lactobacillus strains. J Gen Appl Microbiol 52:29–35. doi: 10.2323/jgam.52.29. [DOI] [PubMed] [Google Scholar]
- 33.Corcoran BM, Stanton C, Fitzgerald GF, Ross RP. 2007. Growth of probiotic lactobacilli in the presence of oleic acid enhances subsequent survival in gastric juice. Microbiology 153:291–299. doi: 10.1099/mic.0.28966-0. [DOI] [PubMed] [Google Scholar]
- 34.Grangette C, Nutten S, Palumbo E, Morath S, Hermann C, Dewulf J, Pot B, Hartung T, Hols P, Mercenier A. 2005. Enhanced antiinflammatory capacity of a Lactobacillus plantarum mutant synthesizing modified teichoic acids. Proc Natl Acad Sci U S A 102:10321–10326. doi: 10.1073/pnas.0504084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohamadzadeh M, Pfeiler EA, Brown JB, Zadeh M, Gramarossa M, Managlia E, Bere P, Sarraj B, Khan MW, Pakanati KC, Ansari MJ, O'Flaherty S, Barrett T, Klaenhammer TR. 2011. Regulation of induced colonic inflammation by Lactobacillus acidophilus deficient in lipoteichoic acid. Proc Natl Acad Sci U S A 108(Suppl 1):S4623–S4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khan MW, Zadeh M, Bere P, Gounaris E, Owen J, Klaenhammer TR, Mohamadzadeh M. 2012. Modulating intestinal immune responses by lipoteichoic acid-deficient Lactobacillus acidophilus. Immunotherapy 4:151–161. doi: 10.2217/imt.11.163. [DOI] [PMC free article] [PubMed] [Google Scholar]