ABSTRACT
Improvement in the osmotolerance of Escherichia coli is essential for the production of high titers of various bioproducts. In this work, a cusS mutation that was identified in the previously constructed high-succinate-producing E. coli strain HX024 was investigated for its effect on osmotolerance. CusS is part of the two-component system CusSR that protects cells from Ag(I) and Cu(I) toxicity. Changing cusS from strain HX024 back to its original sequence led to a 24% decrease in cell mass and succinate titer under osmotic stress (12% glucose). When cultivated with a high initial glucose concentration (12%), introduction of the cusS mutation into parental strain Suc-T110 led to a 21% increase in cell mass and a 40% increase in succinate titer. When the medium was supplemented with 30 g/liter disodium succinate, the cusS mutation led to a 120% increase in cell mass and a 492% increase in succinate titer. Introducing the cusS mutation into the wild-type strain ATCC 8739 led to increases in cell mass of 87% with 20% glucose and 36% using 30 g/liter disodium succinate. The cusS mutation increased the expression of cusCFBA, and gene expression levels were found to be positively related to osmotolerance abilities. Because high osmotic stress has been associated with deleterious accumulation of Cu(I) in the periplasm, activation of CusCFBA may alleviate this effect by transporting Cu(I) out of the cells. This hypothesis was confirmed by supplementing sulfur-containing amino acids that can chelate Cu(I). Adding methionine or cysteine to the medium increased the osmotolerance of E. coli under anaerobic conditions.
IMPORTANCE In this work, an activating Cus copper efflux system was found to increase the osmotolerance of E. coli. In addition, new osmoprotectants were identified. Supplementation with methionine or cysteine led to an increase in osmotolerance of E. coli under anaerobic conditions. These new strategies for improving osmotolerance will be useful for improving the production of chemicals in industrial bioprocesses.
KEYWORDS: osmotolerance, CusS, copper efflux, Cu(I), methionine, Escherichia coli
INTRODUCTION
Resistance to high osmotic pressure is a desirable phenotype for engineered bacterial strains for the efficient production of fuels and chemicals in industrial bioprocesses (1–6). Batch fermentation with a high initial glucose concentration is also desirable in industrial production, as it eliminates the need for glucose feeding and simplifies the bioprocess. However, high glucose concentrations lead to high osmotic pressure, which inhibits cell growth and metabolic activity. The osmotic pressure problem is more pronounced in the production of organic acids by Escherichia coli. E. coli growth is reduced significantly in low-pH environments. The common fermentation pH for E. coli is around 7, and alkali is usually added during organic acid fermentation to maintain a neutral pH. Organic acids are in a dissociated form, increasing the osmotic pressure by 2- or 3-fold. High osmotic pressure is one of the main problems for improving the production efficiency of organic acids, such as succinic acid (7), pyruvic acid (1), and lactic acid (8).
In our previous work, high-succinate-producing E. coli strain HX024 was obtained by a combination of genetic engineering and metabolic evolution (9). Using sodium salt (2.4 M Na2CO3 and 1.2 M NaOH) as the neutralizer to maintain the pH, strain HX024 produced 813 mM succinate. Because the pH was held at 7, succinate remained in the disodium form, resulting in a high osmotic pressure of 2,439 milliosmoles (mosmol). The optimal osmotic pressure for E. coli to maintain its physiological activity is about 300 mosmol, and growth arrest occurs when the external osmotic pressure is above 1,700 mosmol (10). This suggests that strain HX024 developed its osmotolerant capacity through metabolic evolution. Through genome sequencing of strain HX024, a mutation in cusS (G629T), which encodes the CusS sensory histidine kinase, was identified (9).
Two-component systems (TCSs) are widely distributed in microorganisms that encounter hostile environments. CusSR is one TCS that helps protect cells from high concentrations of Ag(I) and Cu(I) (11, 12). CusS is the histidine kinase, and CusR is the response regulator that senses elevated metal ion concentrations and regulates the expression of the cusCFBA gene operon (13). CusCFBA are efflux proteins that export Ag(I) and Cu(I) outside cells (11–14). At least a 2-fold increase in the transcriptional level of cusS was required for expression of the cusCFBA efflux pump to export the excess metal ion (14). A strain in which cusS has been deleted showed no expression of cusCFBA and exhibited higher susceptibility to Cu(I), whereas overexpression of cusS on a plasmid restored cusCFBA expression and efflux ability (11).
In this work, the effect of mutated cusS on osmotolerance was investigated, and the mechanism underlying this effect was investigated.
RESULTS AND DISCUSSION
The cusS mutation increased E. coli osmotolerance.
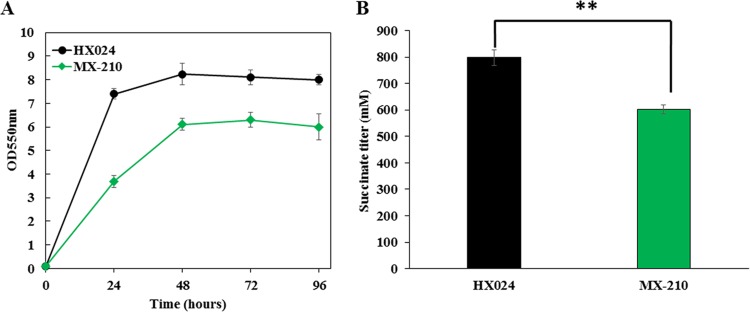
High-succinate-producing E. coli strain HX024 was previously obtained through a combination of genetic engineering and metabolic evolution (9). A mutation in cusS (G629T) was found in strain HX024 (9) that we speculated was related to osmotolerance. The mutated cusS in strain HX024 was restored to its original sequence, resulting in strain MX-210 (Table 1). Cell growth and succinate production decreased significantly in strain MX-210 compared with those in strain HX024 when cultivated in 12% glucose (Fig. 1A and B). The maximum cell mass of strain MX-210 was 76% of that of strain HX024 (Fig. 1A). The succinate titer from strain MX-210 decreased 24% compared to that of strain HX024 (Fig. 1B).
TABLE 1.
E. coli strains used in this study
| Strain | Characteristic(s)a | Source or reference |
|---|---|---|
| ATCC 8739 | Wild type | Lab collection |
| Suc-T110 | ATCC 8739, ΔldhA, ΔpflB, ΔptsI, Ppck*-galP, Ppck*-pck | 9 |
| HX024 | Suc-T110, ΔackA-pta, Ppck*-aceBA, Ppck*-dcuC, ΔmgsA, cusS (G629T), adaptively evolved for 1,440 generations | 9 |
| MX-210 | HX024, cusS (G629T) mutation recovered to the original sequence | This study |
| NZ-504 | Suc-T110, cusS (G629T) mutation | This study |
| MX-204 | ATCC 8739, cusS (G629T) mutation | This study |
| NZ-521 | Suc-T110, ΔcusS | This study |
| NZ-523 | Suc-T110, ΔcusCFBA | This study |
| MX-217 | Suc-T110, FRT-Km-FRT-mRSL1-cusCFBA | This study |
| MX-218 | Suc-T110, FRT-Km-FRT-mRSL2-cusCFBA | This study |
| MX-219 | Suc-T110, FRT-Km-FRT-mRSL3-cusCFBA | This study |
| MX-220 | Suc-T110, FRT-Km-FRT-mRSL4-cusCFBA | This study |
| MX-221 | Suc-T110, FRT-Km-FRT-mRSL5-cusCFBA | This study |
| MX-222 | Suc-T110, FRT-Km-FRT-mRSL6-cusCFBA | This study |
| MX-223 | Suc-T110, FRT-Km-FRT-mRSL7-cusCFBA | This study |
| MX-224 | Suc-T110, FRT-Km-FRT-mRSL8-cusCFBA | This study |
| MX-225 | Suc-T110, FRT-Km-FRT-mRSL9-cusCFBA | This study |
| MX-226 | Suc-T110, FRT-Km-FRT-mRSL10-cusCFBA | This study |
Ppck* represents a mutated promoter of the E. coli pck gene, which had a G to A transition at position −64 relative to the ATG start codon.
FIG 1.
Effects of the cusS mutation in strain HX024 on cell growth and succinate production under high osmotic stress. Fermentations of strain HX024 and its derivative, with the mutated cusS restored to its original sequence, were performed in NBS medium containing 12% (weight per volume [wt/vol]) glucose. The pH was maintained at 7.0 by the automatic addition of a base containing 2.4 M potassium carbonate and 1.2 M potassium hydroxide. The fermentations were performed three times, and the error bars represent standard deviations. (A) Cell growth curve. (B) Succinate titer at 96 h. Strain MX-210 had the restored cusS. Significant differences were determined by t test. The asterisk indicates a significant difference from the control (**, P value of <0.01).
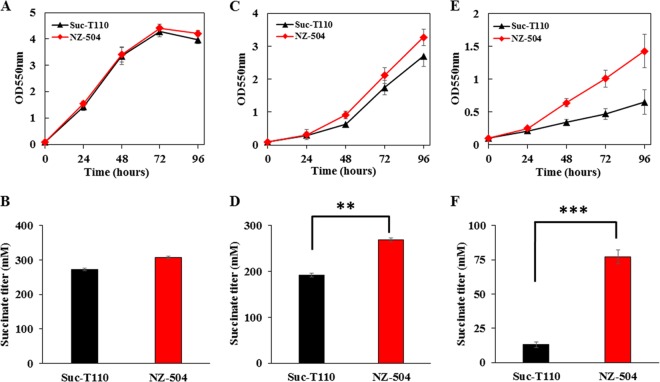
In order to verify that the cusS mutation led to osmotolerance in E. coli, this mutation was introduced into parent strain Suc-T110, which was not adaptively evolved (9), and strain NZ-504 was obtained (Table 1). To compare the osmotolerance capacities of strains NZ-504 and Suc-T110, both a high initial glucose concentration (12%) and disodium succinate supplementation (30 g/liter) were used as osmotic inhibitors. Because the optimal osmotic pressure for E. coli to maintain its physiological activity is about 300 mosmol (10), an initial glucose concentration of 5% (278 mosmol) was used as the normal osmotic condition (5% glucose, 278 mosmol). Strain NZ-504 showed growth similar to that of parent strain Suc-T110 under the normal osmotic condition (Fig. 2A). The succinate titer of strain NZ-504 was 307 mM, which was 12% higher than that of strain Suc-T110 (Fig. 2B). When cultivated with a high initial glucose concentration (12%, 667 mosmol), the maximum cell mass and succinate titer of strain Suc-T110 were 37% and 30% lower, respectively, than those obtained in the normal osmotic condition. There was an obvious inhibitory effect of the high concentration of glucose. The maximum cell mass of strain NZ-504 was 21% greater than that of strain Suc-T110 (Fig. 2C), and the titer of succinate produced from strain NZ-504 was 40% higher than that of strain Suc-T110 (Fig. 2D). When cultivated in 5% glucose and 30 g/liter disodium succinate, the maximum cell mass of strain NZ-504 was 120% greater than that of strain Suc-T110 (Fig. 2E), and the titer of succinate produced from strain NZ-504 was 492% higher than that of strain Suc-T110 (Fig. 2F).
FIG 2.
Reverse metabolic engineering of mutated cusS in the parent strain Suc-T110. Fermentations of strain Suc-T110 and its derivative, with the mutated cusS restored to its original sequence, were performed in NBS medium containing 5% (wt/vol) glucose, 12% (wt/vol) glucose, and 5% (wt/vol) glucose supplemented with 30 g/liter disodium succinate. The pH was maintained at 7.0 by the automatic addition of a base containing 2.4 M potassium carbonate and 1.2 M potassium hydroxide. The fermentations were performed three times, and the error bars represent standard deviations. (A, B) Cell growth curve and succinate titer in 5% (wt/vol) glucose. (C, D) Cell growth curve and succinate titer in 12% (wt/vol) glucose. (E, F) Cell growth curve and succinate titer in 5% (wt/vol) glucose and 30 g/liter disodium succinate. Strain NZ-504 had the mutated cusS. Significant differences were determined by t test; the asterisks indicate a significant difference from the control (**, P value of <0.01; ***, P value of <0.001).
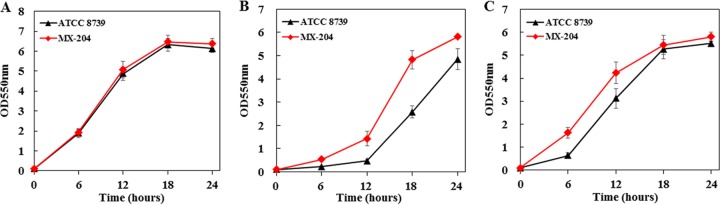
The cusS mutation was also introduced into wild-type strain ATCC 8739, resulting in strain MX-204 (Table 1). In the normal osmotic condition (5% glucose, 278 mosmol), strain MX-204 showed growth that was similar to that of wild-type strain ATCC 8739 (Fig. 3A). When strain ATCC 8739 was cultivated with a high initial glucose concentration (20% glucose, 1,112 mosmol), there was a long growth delay phase, and the cell mass at 18 h was 59% less than that obtained in the normal osmotic condition (5% glucose). Strain MX-204 showed a shorter delay phase, and its cell mass at 18 h was 87% higher than that of strain ATCC 8739 (Fig. 3B). When cultivated in 5% glucose and 30 g/liter disodium succinate, the cell mass of strain ATCC 8739 at 12 h was 51% less than that obtained in the normal osmotic condition. The cell mass of strain MX-204 at 12 h was 36% higher than that of strain ATCC 8739 (Fig. 3C). These reverse metabolic engineering results further confirmed that the cusS mutation led to greater osmotolerance in E. coli.
FIG 3.
Reverse metabolic engineering of mutated cusS in wild-type strain ATCC 8739. Fermentations of strain ATCC 8739 and its derivative, with the mutated cusS restored to its original sequence, were performed in NBS medium containing 5% (wt/vol) glucose, 20% (wt/vol) glucose, and 5% (wt/vol) glucose supplemented with 30 g/liter disodium succinate. The pH was maintained at 7.0 by the automatic addition of a base containing 6 M potassium hydroxide. The fermentations were performed three times, and the error bars represent standard deviations. (A) 5% (wt/vol) glucose. (B) 20% (wt/vol) glucose. (C) 5% (wt/vol) glucose and 30 g/liter disodium succinate. Strain MX-204 had the mutated cusS.
Activation of cusCFBA increased osmotolerance in E. coli.
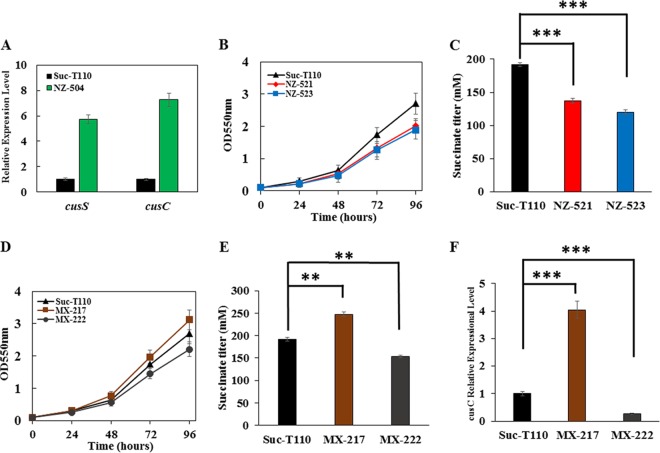
Through real-time PCR (RT-PCR) analysis, the expression levels of cusS and cusC in strain NZ-504, which had the mutated cusS, were found to be 5.7- and 7.3-fold higher, respectively, than those of strain Suc-T110 under a 12% glucose condition (Fig. 4A). cusS and cusCFBA were also deleted in strain Suc-T110, resulting in strains NZ-521 and NZ-523, respectively (Table 1). Both strains exhibited similar cell growth and succinate production as Suc-T110 in 5% glucose (data not shown), whereas both strains exhibited reduced cell growth and succinate production compared to those of Suc-T110 under 12% glucose (Fig. 4B and C). This suggested that cusCFBA expression levels were related to osmotolerance.
FIG 4.
Characterization of the osmotolerance mechanism of the mutated cusS. Fermentations of strain Suc-T110 and its derivatives were performed in NBS medium containing 12% (wt/vol) glucose. The pH was maintained at 7.0 by automatic addition of a base containing 2.4 M potassium carbonate and 1.2 M potassium hydroxide. The fermentations were performed three times, and the error bars represent standard deviation. (A) The relative expression levels of cusS and cusC in strain Suc-T110 and its derivative NZ-504, which had the cusS mutation, in 12% (wt/vol) glucose. (B, C) Cell growth curves and succinate titers of strains Suc-T110 and its derivatives NZ-521 and NZ-523, which had cusS and cusC deleted, respectively, in 12% (wt/vol) glucose. (D, E) Cell growth curves and succinate titers of strains Suc-T110 and its derivatives MX-217 and MX-222, which had cusC modulated, in 12% (wt/vol) glucose. (F) The relative expression levels of cusC in strain Suc-T110 and its derivatives MX-217 and MX-222 in 12% (wt/vol) glucose. Significant differences were determined by one-way ANOVA; the asterisks indicate a significant difference from the control (***, P value of <0.001; **, P value of <0.01).
In order to further test this hypothesis, an artificial promoter library was used to modulate the expression of the cusCFBA operon in strain Suc-T110. Ten recombinants were randomly chosen for osmotolerance testing in 12% glucose. These 10 recombinants exhibited a wide range of osmotolerance capacities (see Fig. S1 in the supplemental material). Strain MX-217 showed the greatest osmotolerance, whereas strain MX-222 showed the least (Fig. 4D). The succinate titer produced by strain MX-217 was 28% higher than that of Suc-T110, whereas the succinate titer of strain MX-222 decreased by 15% (Fig. 4E). Both strains exhibited similar cell growth and succinate production as that of Suc-T110 in 5% glucose (data not shown). RT-PCR analyses were also performed with these two strains. The cusC expression level in strain MX-217 was 4.0-fold higher than that of Suc-T110, whereas the expression level in strain MX-222 decreased by 73% (Fig. 4F). These results indicated that cusCFBA expression levels were positively correlated with the osmotolerance of a strain.
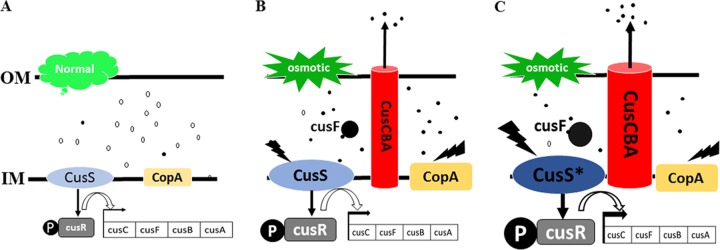
Copper is an essential trace metal in bacteria. However, copper, especially Cu(I), is potentially cytotoxic. In addition to eliciting hydroxyl radicals during aerobic metabolism that can oxidize essential cellular components and damage DNA, cytotoxicity was more extreme under anaerobic conditions because of damage to the Fe-S cluster biogenesis machinery and important Fe-S cluster-containing metabolic enzymes (15, 16). These Fe-S cluster-containing enzymes are the primary targets of copper toxicity, which leads to a global defect in the physiological and metabolic processes of E. coli, including energy conservation and amino acid biosynthesis (15). E. coli has developed several copper homeostasis systems to deal with copper toxicity (15, 17). Under aerobic condition, Cu(I) is exported from the cytosol to the periplasmic space by the cytoplasmic copper transporter CopA, which is then oxidized by the periplasmic multicopper oxidase CueO to the less toxic Cu(II) (15, 17). Under anaerobic amino acid-limiting conditions, CopA is still functional, whereas CueO is inactivated. The amount of free Cu(I) increased in the periplasmic space because of the reduced environment and lack of methionine, which can chelate Cu(I).
Based on our strain Suc-T110 transcriptome sequencing data, the expression levels of cusS, cusR, cusCFBA, and copA under osmotic stress (12% glucose) were 2- to 7-fold higher than those under normal conditions (5% glucose) (see Fig. S2 in the supplemental material). Conversely, the CusCFBA efflux system was induced by elevated Cu(I) in the periplasmic space for export of toxic Cu(I) (11–14). It was thus speculated that osmotic stress led to an elevated Cu(I) concentration in the periplasmic space, after which the CusCFBA efflux system was induced to export Cu(I) out of the cells and protect cells from copper toxicity. The expression levels of the methionine biosynthesis genes metA, metB, metE, and metF and cysteine biosynthesis genes serA and serC under osmotic stress conditions (12% glucose) were 2- to 4-fold higher than those under normal conditions (5% glucose) (Fig. S2). This was good evidence that osmotic stress led to elevated intracellular Cu(I) and that more methionine and cysteine needed to be synthesized to chelate intracellular Cu(I). The CopA and Cus systems were self-activated in parent strain Suc-T110 to protect E. coli from copper toxicity (Fig. 5A and B). The higher expression levels of mutated cusS and the cusCFBA operon may have further increased the activity of the CusCFBA efflux pump (Fig. 5C) to expel more Cu(I), thus resulting in better cell growth under osmotic stress.
FIG 5.
Working model of the CopA and Cus system for copper homeostasis in parent strain Suc-T110 and the cusS mutant. (A) For strain Suc-T110 under normal conditions (5% glucose), copper exists mostly in the form of less-toxic Cu(II), and the CopA and Cus system was not induced. (B) For strain Suc-T110 under high osmotic pressure (12% glucose), increasing free Cu(I) in the periplasmic space activates the expression of copA, cusS, cusR, and cusCFAB to expel toxic Cu(I). (C) For strain NZ-504 with the mutated cusS under high osmotic pressure (12% glucose), expression levels of cusS and cusCFAB are increased to expel more Cu(I) from the periplasmic space. Closed circles represent toxic Cu(I), and open circles represent less-toxic Cu(II).
Methionine or cysteine supplementation led to increased osmotolerance in E. coli.
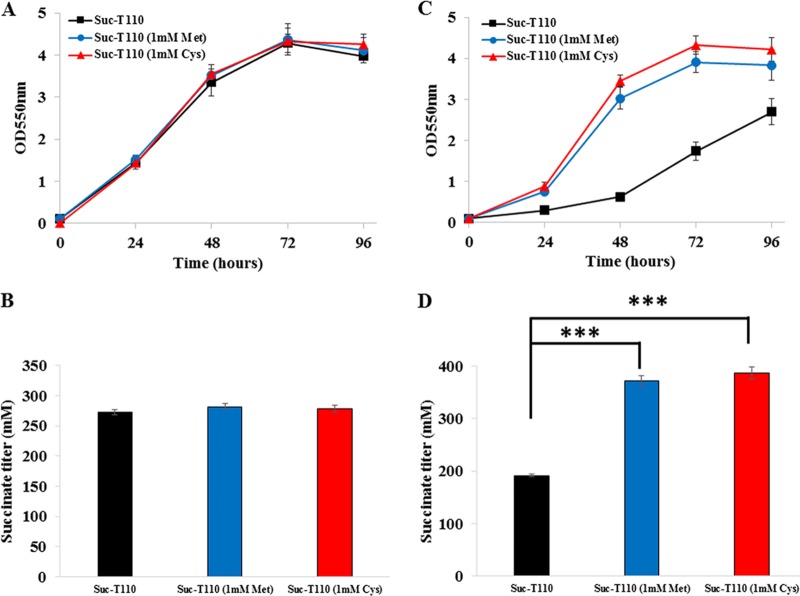
Sulfur-containing amino acids, such as methionine and cysteine, can chelate Cu(I) (15). Thus, we speculated that methionine or cysteine supplementation could increase osmotolerance in E. coli. In order to confirm this hypothesis, 1 mM methionine was added to the medium at the beginning of the anaerobic fermentation of strain Suc-T110. When cultivated in the normal osmotic condition (5% glucose), methionine supplementation had no effect on cell growth and succinate production (Fig. 6A and B). When cultivated in a high initial glucose concentration (12%), cell mass and succinate titer increased 42% and 93%, respectively (Fig. 6C and D). Supplementation with 1 mM cysteine was also tested. When cultivated in the normal osmotic condition (5% glucose), cysteine supplementation had no effect on cell growth and succinate production (Fig. 6A and B). When cultivated in a high initial glucose concentration (12%), cell mass and succinate titer increased 56% and 102%, respectively (Fig. 6C and D). These results confirmed that methionine or cysteine supplementation led to increased osmotolerance in E. coli.
FIG 6.
Effects of methionine or cysteine supplementation on cell growth and succinate production of strain Suc-T110. Fermentations were performed in NBS medium containing 5% (wt/vol) or 12% (wt/vol) glucose. The pH was maintained at 7.0 by the automatic addition of a base containing 2.4 M potassium carbonate and 1.2 M potassium hydroxide. The fermentations were performed three times, and the error bars represent standard deviations. (A, B) Cell growth curve and succinate titer at 96 h under 5% (wt/vol) glucose. (C, D) Cell growth curve and succinate titer at 96 h under 12% (wt/vol) glucose. Significant differences were determined by one-way ANOVA; the asterisks indicate a significant difference from the control (***, P value of <0.001).
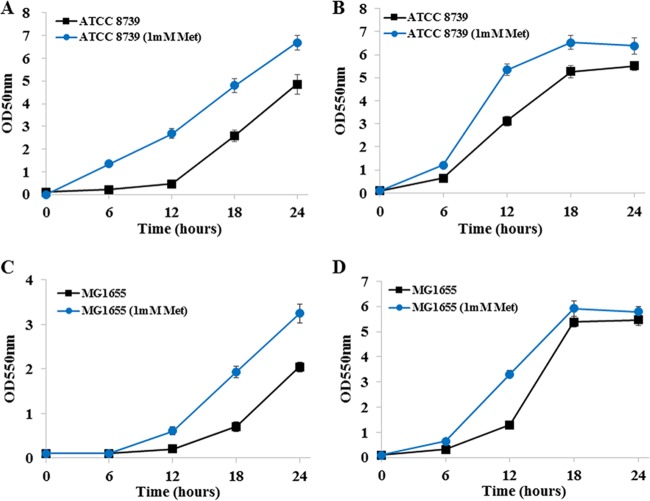
Methionine (1 mM) supplementation was also tested in wild-type E. coli strains ATCC 8739 and MG1655. For ATCC 8739 cultivated in a high initial glucose concentration (20%), cell mass at 12 h was 470% higher than that of the control (Fig. 7A). When cultivated in 5% glucose and 30 g/liter disodium succinate, cell mass at 12 h was 71% higher than that of the control (Fig. 7B). For MG1655 cultivated in a high initial glucose concentration (20%), cell mass at 18 h was 174% higher than that of the control (Fig. 7C). When cultivated in 5% glucose and 30 g/liter disodium succinate, cell mass at 12 h was 154% higher than that of the control (Fig. 7D).
FIG 7.
Effects of methionine or cysteine supplementation on the growth of wild-type E. coli strains ATCC 8739 and MG1655. Fermentations were performed in NBS medium containing 20% (wt/vol) glucose or 5% (wt/vol) glucose supplemented with 30 g/liter disodium succinate. The pH was maintained at 7.0 by automatic addition of a base containing 6 M potassium hydroxide. The fermentations were performed three times, and the error bars represent standard deviations. (A) Cell growth of strain ATCC 8739 under 20% (wt/vol) glucose. (B) Cell growth of strain ATCC 8739 under 5% (wt/vol) glucose supplemented with 30 g/liter disodium succinate. (C) Cell growth of strain MG1655 under 20% (wt/vol) glucose. (D) Cell growth of strain MG1655 under 5% (wt/vol) glucose supplemented with 30 g/liter disodium succinate.
Intracellular accumulation of compatible solutes to balance the external hypertonic broth is regarded as a major mechanism to regulate osmotolerance. Because compatible solutes carry a net charge at physiological pH and are highly soluble, they do not only accumulate intracellularly to a relatively high concentration but also play a protective role for macromolecular substances, such as DNA and proteins, preventing disturbances in their vital bioprocesses under hyperosmotic stress (10, 18). These osmoprotectants, including glycine betaine, trehalose, proline, and glutamate, are well conserved as the major solution to cope with osmotic stress, and increasing the intracellular concentration of these compatible solutes is a major strategy to increase osmotolerance. Supplementation with betaine increased the cell growth rate, glucose consumption rate, and succinate productivity of engineered E. coli strains in a high initial glucose concentration (150 g/liter) (19). Supplementation with proline increased the succinate titer by 22% from engineered Actinobacillus succinogenes under high osmotic pressure (20). The expression of exogenous betaine biosynthesis genes from the extreme halophile Ectothiorhodospira halochloris resulted in a recombinant E. coli with double the cellular yield compared to that of its control strain in 0.5 M NaCl (21). Increasing the production of endogenous trehalose by increasing the expression of trehalose biosynthesis genes (ostBA) improved the growth of E. coli under osmotic stress (3).
In this work, new osmoprotectants, including methionine and cysteine, were identified. These sulfur-containing amino acids can chelate Cu(I) in the periplasmic space and protect E. coli from copper toxicity under high osmotic stress. It should be noted that these new osmoprotectants only functioned under anaerobic conditions and that they did not promote osmotolerance under aerobic conditions (see Fig. S3 in the supplemental material). This was reasonable because few Cu(I) molecules can accumulate in the periplasmic space under aerobic conditions (17, 22). These new strategies to improve osmotolerance will be useful for increasing the production of chemicals under high osmotic stress conditions. Activation of cusCFBA or supplementation with sulfur-containing amino acids increased the cell growth and productivity of target compounds in batch fermentation with a high initial glucose concentration. Higher product titers can also be obtained during production of organic acid salts at a neutral pH.
MATERIALS AND METHODS
Strains, media, and growth conditions.
The mutants constructed in this study are listed in Table 1. During construction, strains were cultured aerobically at 30°C or 37°C in Luria broth (10 g liter−1 Difco tryptone, 5 g liter−1 Difco yeast extract, and 10 g liter−1 NaCl). Ampicillin (100 mg liter−1), kanamycin (25 mg liter−1), or chloramphenicol (17 mg liter−1) were used as appropriate.
Genetic methods.
Two-step recombination methods were used for markerless gene deletion (23, 24), and one-step recombination methods were used for gene modulation (25). Red recombinase technology (Gene Bridges GmbH, Dresden, Germany) was used to facilitate chromosomal gene deletion and modulation (23). All primers are listed in Table 2.
TABLE 2.
Primers used in this study
| Description | Sequence |
|---|---|
| Replacing cusS mutation with the wild type or vice versa | |
| cusS-QC-cat-up | GGCAAACAGCAGAATTATGTCATGCTGACTGGCCTCTCCATTAATTTCCATCTCCATTACTGTGACGGAAGATCACTTCGCA |
| cusS-QC-sacB-down | ATTGGTGATGGGCGTTCTGATCTCATGCGCGATATCGGCAGAGAAATTGGCCTGGCGGGTTTATTTGTTAACTGTTAATTGTCCT |
| cusS-up | TGCTGTGAGTTCAGCCGATT |
| cusS-down | TGGTTATTGTCCGCCTGTG |
| cusS gene deletion | |
| cusS-QC2-cat-up | TACGAGACAAAACTGATCCAGACAGTGCGGGGCGTGGGCTACATGCTGGAGATCCCGGTGTGACGGAAGATCACTTCGCA |
| cusS-QC2-sacB-down | ATCGAATGGCTTCTGTTTGCTGCATGACAGGCTAATGACATCTTTGTCATTTACATCTTTATTTTTATTTGTTAACTGTTAATTGTCCT |
| cusS-QC-1 | GGTTCGTAGTGGATCACGCT |
| cusS-QC-2 | GCTAGGTACCCCGGGATCTCCAGCATGTA |
| cusS-QC-3 | GCTAGGTACCGATGTCATTAGCCTGTCATGCA |
| cusS-QC-4 | ACGGTCGTGGATACCGTGT |
| cusCFBA gene deletion | |
| cusC-QC-cat-up | TGCTGGCAGGAACAAAGCCAATCTGAAGCTGGCTGAAATTCGCCAGCAACAGTGTGACGGAAGATCACTTCGCA |
| cusC-QC-sacB-down | CCCTGAACCTGATTCAGGTATTCCAGCACGCGCGAACGGGCCCAGTACAGATCGTTATTTGTTAACTGTTAATTGTCCT |
| cusC-QC-1 | CCGAAATAGCCAAACGAG |
| cusC-QC-2 | GCTAGGTACCCTGTTGCTGGCGAATTTC |
| cusC-QC-3 | GCTAGGTACCCGATCTGTACTGGGCCC |
| cusC-QC-4 | TACTCCGCTTCGGCTATTTC |
| Modulation of cusCFBA genes | |
| cusC-FRT-up | CTGGACTTCGATTGAACCATTTACCAGGTCTGCCTGTACGAGAAGCGTTGTGTAGGCTGGAGCTGCTTC |
| cusC-mRSL-down | GAAACACAACCTGCCAGGATGAATATGGTACTGATGCTAAGTAATTTTAATTTGAACATAGCTGTTTCCTGGTTTAAAC(N18)GGCTCAATTATATCAACG |
| cusC-down-200 | CTCAAATCACGGTTATTATTCAGG |
| Kan-F | CCGTGATATTGCTGAAGAG |
| RT-PCR analysis of cusS gene | |
| RT-cusS-F | GCCCATCACCAATCTGGTGA |
| RT-cusS-R | TGACCATTTTGGTCATCCGG |
| RT-PCR analysis of cusC gene | |
| RT-cusC-F | AACGAGAAGGCGATCTGGCT |
| RT-cusC-R | TGACGACAGATTTGGTGGCA |
| 16S-F | CATGCCGCGTGTATGAAGAA |
| 16S-R | TGCGCTTTACGCCCAGTAAT |
Replacement of the mutated cusS in strain HX024 with the original sequence.
A two-step recombination method was used to replace the cusS mutation in strain HX024 with its original sequence (24). In the first recombination, the cat-sacB cassette was amplified from plasmid pXZ-CS (26) with primer set cusS-QC-cat-up/cusS-QC-sacB-down and used to replace mutated cusS in the strain HX024 chromosome. In the second recombination, wild-type cusS was amplified from E. coli ATCC 8739 with primer set cusS-up/cusS-down and used to replace the cat-sacB cassette by selecting for sucrose resistance.
Introduction of the cusS mutation into strains Suc-T110 and ATCC 8739.
A two-step recombination method was used to introduce the cusS mutation into strains Suc-T110 and ATCC 8739 (24). In the first recombination, the cat-sacB cassette was amplified from plasmid pXZ-CS (26) with primer set cusS-QC-cat-up/cusS-QC-sacB-down and used to replace native cusS in the chromosomes of strains Suc-T110 and ATCC 8739. In the second recombination, mutated cusS was amplified from strain HX024 with primer set cusS-up/cusS-down and used to replace the cat-sacB cassette by selecting for sucrose resistance.
Deletion of cusS and cusCFBA.
cusS in strain Suc-T110 was deleted using a two-step recombination method as described previously (24). In the first recombination, the cat-sacB cassette was amplified from plasmid pXZ-CS (26) with primer set cusS-QC2-cat-up/cusS-QC2-sacB-down and used to replace the coding region of cusS in the strain Suc-T110 chromosome. Fusion PCR was performed to prepare the DNA fragment for the second recombination. The upstream and downstream regions (about 700 bp) of cusS were amplified from E. coli ATCC 8739 genomic DNA using primer sets cusS-QC-1/cusS-QC-2 and cusS-QC-3/cusS-QC-4. These two DNA fragments were then used as the templates to perform fusion PCR using primer set cusS-QC-1/cusS-QC-4. The resulting DNA fragment (about 1,400 bp) was used to replace the cat-sacB cassette by selecting for sucrose resistance. The cusCFBA operon was deleted in the same manner.
Modulation of cusCFBA in strain Suc-T110 with an mRS library.
A one-step recombination method was used to modulate cusCFBA with an mRNA-stabilizing region (mRS) library (25, 27). DNA fragments containing the mRS library were amplified from the genomic DNA of recombinant E. coli M1-93 (27) using primer set cusC-up-FRT/cusC-mRSL-down and inserted before the ATG translation start site of cusC in the strain Suc-T110 chromosome. Recombinant E. coli M1-93 was selected from a previously constructed promoter library, with a promoter strength of five times that of the induced E. coli lacZ promoter (27). Colonies with kanamycin resistance were picked and verified by PCR using primer set Kan-F/cusC-down-200. Ten right colonies were selected for fermentation.
Fermentation.
Fresh colonies were picked from New Brunswick Scientific (NBS) (28) mineral salt plates containing 20 g liter−1 glucose, inoculated into 250-ml flasks containing 100 ml of NBS mineral salts medium with 50 g liter−1 glucose, and grown at 37°C and 100 rpm for 12 h. Cultures were then transferred to a 500-ml fermentation vessel containing 250 ml of NBS mineral salts medium with different concentrations of glucose or disodium succinate as an osmotic inhibitor. The initial optical density at 550 nm (OD550) was 0.1.
For strains HX024 and MX-210, fermentation was carried out in NBS mineral salts medium containing 12% glucose. For strains Suc-T110 and NZ-504, fermentation was carried out in NBS mineral salts medium containing 5% glucose as the normal condition. Both 12% glucose and 5% glucose with 30 g/liter disodium succinate were used as osmotic inhibitors. The pH was maintained at 7.0 by automatic addition of a base containing 2.4 M potassium carbonate and 1.2 M potassium hydroxide.
For strains ATCC 8739 and MX-204, fermentation was carried out in NBS mineral salts medium containing 5% glucose as the normal condition. Both 20% glucose and 5% glucose with 30 g/liter disodium succinate were used as osmotic inhibitors. The pH was maintained at 7.0 by automatic addition of a base containing 6.0 M potassium hydroxide.
When testing the effects of supplementation with sulfur-containing amino acids, either 1 mM methionine or 1 mM cysteine was added to the NBS mineral salts medium at the beginning of fermentation.
RNA extraction and RT-PCR analysis.
Cells were grown to the mid-exponential phase (based on cell mass) and harvested for total RNA isolation. Extraction and on-column DNase I treatment were performed using an RNeasy minikit (Qiagen) according to the manufacturer's recommendations. The purified RNA was assessed with an Agilent 2100 Bioanalyzer (Agilent) and quantified using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies). RNA integrity scores ranged between 9.1 and 9.5. For RT-PCR analysis, reverse transcription was performed using an iScript cDNA synthesis kit (Bio-Rad). RT-PCR was performed with an iQ SYBR green supermix (Bio-Rad) on a CFX Connect real-time system (Bio-Rad). The relative transcript level of each gene was calculated by the 2−ΔΔCT method. Data were normalized to the level of endogenous control 16S rRNA, and expression levels of strain Suc-T110 grown under 12% glucose were used as references. Forward and reverse primers used in this study are listed in Table 2.
Analysis.
The dry weight of cells was calculated by measuring the optical density value at 550 nm (1 OD550 = 0.33 g dry cell weight liter−1). Organic acids and residual glucose in the fermentation broth were measured by high-performance liquid chromatography (28). When 30 g/liter disodium succinate was used as the osmotic inhibitor, succinate titer was calculated by subtracting 30 g/liter of succinate at the beginning of the medium from the final succinate concentration at the end of the fermentation. Product titers were normalized by arithmetically factoring out the volume of the base solution that was added to the fermentors for pH control: product titer = (the real product titer) × (starting volume + added volume)/(starting volume). All experiments were performed in triplicate to obtain the average values; error bars represent standard deviations. Percentages were calculated from average values. Statistical tests for significance were determined by one-way analysis of variance (ANOVA) or t test using R (version 3.1.1).
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by grants from the Key Deployment Project of the Chinese Academy of Sciences (ZDRW-ZS-2016-3), the National High Technology Research and Development Program of China (2014AA021205), the National Natural Science Foundation of China (31522002), and the Tianjin Key Technology R&D Program of the Tianjin Municipal Science and Technology Commission (13ZCZDSY05300).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.03050-16.
REFERENCES
- 1.Liu L, Xu Q, Li Y, Shi Z, Zhu Y, Du G, Chen J. 2007. Enhancement of pyruvate production by osmotic-tolerant mutant of Torulopsis glabrata. Biotechnol Bioeng 97:825–832. doi: 10.1002/bit.21290. [DOI] [PubMed] [Google Scholar]
- 2.Miller EN, Ingram LO. 2007. Combined effect of betaine and trehalose on osmotic tolerance of Escherichia coli in mineral salts medium. Biotechnol Lett 29:213–217. doi: 10.1007/s10529-006-9226-0. [DOI] [PubMed] [Google Scholar]
- 3.Purvis JE, Yomano LP, Ingram LO. 2005. Enhanced trehalose production improves growth of Escherichia coli under osmotic stress. Appl Environ Microbiol 71:3761–3769. doi: 10.1128/AEM.71.7.3761-3769.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sévin DC, Sauer U. 2014. Ubiquinone accumulation improves osmotic-stress tolerance in Escherichia coli. Nat Chem Biol 10:266–272. doi: 10.1038/nchembio.1437. [DOI] [PubMed] [Google Scholar]
- 5.Winkler JD, Garcia C, Olson M, Callaway E, Kao KC. 2014. Evolved osmotolerant Escherichia coli mutants frequently exhibit defective N-acetylglucosamine catabolism and point mutations in cell shape-regulating protein MreB. Appl Environ Microbiol 80:3729–3740. doi: 10.1128/AEM.00499-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang H, Chong H, Ching CB, Jiang R. 2012. Random mutagenesis of global transcription factor cAMP receptor protein for improved osmotolerance. Biotechnol Bioeng 109:1165–1172. doi: 10.1002/bit.24411. [DOI] [PubMed] [Google Scholar]
- 7.Kwon YD, Kim S, Lee SY, Kim P. 2011. Long-term continuous adaptation of Escherichia coli to high succinate stress and transcriptome analysis of the tolerant strain. J Biosci Bioeng 111:26–30. doi: 10.1016/j.jbiosc.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Tian X, Wang Y, Chu J, Zhuang Y, Zhang S. 2016. Enhanced l-lactic acid production in Lactobacillus paracasei by exogenous proline addition based on comparative metabolite profiling analysis. Appl Microbiol Biotechnol 100:2301–2310. doi: 10.1007/s00253-015-7136-6. [DOI] [PubMed] [Google Scholar]
- 9.Zhu X, Tan Z, Xu H, Chen J, Tang J, Zhang X. 2014. Metabolic evolution of two reducing equivalent-conserving pathways for high-yield succinate production in Escherichia coli. Metab Eng 24:87–96. doi: 10.1016/j.ymben.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Record MT Jr, Courtenay ES, Cayley DS, Guttman HJ. 1998. Responses of E. coli to osmotic stress: large changes in amounts of cytoplasmic solutes and water. Trends Biochem Sci 23:143–148. doi: 10.1016/S0968-0004(98)01196-7. [DOI] [PubMed] [Google Scholar]
- 11.Gudipaty SA, Larsen AS, Rensing C, McEvoy MM. 2012. Regulation of Cu(I)/Ag(I) efflux genes in Escherichia coli by the sensor kinase CusS. FEMS Microbiol Lett 330:30–37. doi: 10.1111/j.1574-6968.2012.02529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamamoto K, Ishihama A. 2005. Transcriptional response of Escherichia coli to external copper. Mol Microbiol 56:215–227. doi: 10.1111/j.1365-2958.2005.04532.x. [DOI] [PubMed] [Google Scholar]
- 13.Franke S, Grass G, Rensing C, Nies DH. 2003. Molecular analysis of the copper-transporting efflux system CusCFBA of Escherichia coli. J Bacteriol 185:3804–3812. doi: 10.1128/JB.185.13.3804-3812.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamamoto K, Hirao K, Oshima T, Aiba H, Utsumi R, Ishihama A. 2005. Functional characterization in vitro of all two-component signal transduction systems from Escherichia coli. J Biol Chem 280:1448–1456. doi: 10.1074/jbc.M410104200. [DOI] [PubMed] [Google Scholar]
- 15.Fung DK, Lau WY, Chan WT, Yan A. 2013. Copper efflux is induced during anaerobic amino acid limitation in Escherichia coli to protect iron-sulfur cluster enzymes and biogenesis. J Bacteriol 195:4556–4568. doi: 10.1128/JB.00543-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macomber L, Imlay JA. 2009. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc Natl Acad Sci U S A 106:8344–8349. doi: 10.1073/pnas.0812808106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Outten FW, Huffman DL, Hale JA, O'Halloran TV. 2001. The independent cue and cus systems confer copper tolerance during aerobic and anaerobic growth in Escherichia coli. J Biol Chem 276:30670–30677. doi: 10.1074/jbc.M104122200. [DOI] [PubMed] [Google Scholar]
- 18.Galinski EA. 1995. Osmoadaptation in bacteria. Adv Microb Physiol 37:272–328. [PubMed] [Google Scholar]
- 19.Andersson C, Helmerius J, Hodge D, Berglund KA, Rova U. 2009. Inhibition of succinic acid production in metabolically engineered Escherichia coli by neutralizing agent, organic acids, and osmolarity. Biotechnol Prog 25:116–123. doi: 10.1002/btpr.127. [DOI] [PubMed] [Google Scholar]
- 20.Fang X, Li J, Zheng X, Xi Y, Chen K, Wei P, Ouyang PK, Jiang M. 2011. Influence of osmotic stress on fermentative production of succinic acid by Actinobacillus succinogenes. Appl Biochem Biotechnol 165:138–147. doi: 10.1007/s12010-011-9239-6. [DOI] [PubMed] [Google Scholar]
- 21.von Weymarn N, Nyyssölä A, Reinikainen T, Leisola M, Ojamo H. 2001. Improved osmotolerance of recombinant Escherichia coli by de novo glycine betaine biosynthesis. Appl Microbiol Biotechnol 55:214–218. doi: 10.1007/s002530000515. [DOI] [PubMed] [Google Scholar]
- 22.Macomber L, Rensing C, Imlay JA. 2007. Intracellular copper does not catalyze the formation of oxidative DNA damage in Escherichia coli. J Bacteriol 189:1616–1626. doi: 10.1128/JB.01357-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jantama K, Haupt MJ, Svoronos SA, Zhang X, Moore JC, Shanmugam KT, Ingram LO. 2008. Combining metabolic engineering and metabolic evolution to develop nonrecombinant strains of Escherichia coli C that produce succinate and malate. Biotechnol Bioeng 99:1140–1153. doi: 10.1002/bit.21694. [DOI] [PubMed] [Google Scholar]
- 25.Shi A, Zhu X, Lu J, Zhang X, Ma Y. 2013. Activating transhydrogenase and NAD kinase in combination for improving isobutanol production. Metab Eng 16:1–10. doi: 10.1016/j.ymben.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Tan Z, Zhu X, Chen J, Li Q, Zhang X. 2013. Activating phosphoenolpyruvate carboxylase and phosphoenolpyruvate carboxykinase in combination for improvement of succinate production. Appl Environ Microbiol 79:4838–4844. doi: 10.1128/AEM.00826-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu J, Tang J, Liu Y, Zhu X, Zhang T, Zhang X. 2012. Combinatorial modulation of galP and glk gene expression for improved alternative glucose utilization. Appl Microbiol Biotechnol 93:2455–2562. doi: 10.1007/s00253-011-3752-y. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Jantama K, Moore JC, Jarboe LR, Shanmugam KT, Ingram LO. 2009. Metabolic evolution of energy-conserving pathways for succinate production in Escherichia coli. Proc Natl Acad Sci U S A 106:20180–20185. doi: 10.1073/pnas.0905396106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.