Highlights
-
•
Pregnancy is considered an important risk factor for severe outcomes from influenza virus infection.
-
•
The World Health Organization recommends that pregnant women be prioritized for vaccination.
-
•
No comprehensive systematic review supporting this recommendation has been conducted to date.
-
•
We found a higher risk for hospital admission following influenza but found no increase in mortality or other outcomes.
-
•
Comparative, population-based studies are needed to best evaluate the attributable risk of pregnancy.
Abbreviations: CI, confidence interval; CENTRAL, Cochrane Central Register of Controlled Trials; ICU, intensive care units; NOS, Newcastle Ottawa Scale; OR, odds ratio; RR, risk ratio; WHO, World Health Organization
Keywords: Influenza, Pregnancy, Risk, Systematic review, Vaccination
Abstract
Background
Pregnancy is considered to be an important risk factor for severe complications following influenza virus infection. As a consequence, WHO recommendations prioritize pregnant women over other risk groups for influenza vaccination. However, the risk associated with pregnancy has not been systematically quantified.
Purpose
Systematic review and meta-analysis of observational studies that reported on pregnancy as a risk factor for severe outcomes from influenza virus infection.
Data source
MEDLINE, EMBASE, CINAHL, and CENTRAL up to April 2014.
Data selection
Studies reporting on outcomes in pregnant women with influenza in comparison to non-pregnant patients with influenza. Outcomes included community-acquired pneumonia, hospitalization, admission to intensive care units (ICU), ventilatory support, and death.
Data extraction
Two reviewers conducted independent screening and data extraction. A random effects model was used to obtain risk estimates. Ecological studies were summarized descriptively.
Data synthesis
A total of 142 non-ecological and 10 ecological studies were included. The majority of studies (n = 136, 95.8%) were conducted during the 2009 influenza A (pH1N1) pandemic. There was a higher risk for hospitalization in pregnant versus non-pregnant patients infected with influenza (odds ratio [OR] 2.44, 95% CI 1.22–4.87), but no significant difference in mortality (OR 1.04, 95% CI 0.81–1.33) or other outcomes. Ecologic studies confirmed the association between hospitalization risk and pregnancy and 4 of 7 studies reported higher mortality rates in pregnant women.
Limitations
No studies were identified in which follow-up began prior to contact with the healthcare system and lack of adjustment for confounding factors.
Conclusions
We found that influenza during pregnancy resulted in a higher risk of hospital admission than influenza infection in non-pregnant individuals, but that the risk of mortality following influenza was similar in both pregnant and non-pregnant individuals.
1. Introduction
It is estimated that three to five million cases of severe influenza illness occur annually worldwide, resulting in 250,000–500,000 deaths [1]. Identifying groups at risk for severe influenza disease is essential to prevention and control efforts.
World Health Organization (WHO) influenza vaccine policy recommendations aim to protect high-risk groups from severe disease. In a 2012 update, WHO recommended for the first time that one risk group, pregnant women, be prioritized over others [2]. This was based on numerous factors, including reports of higher influenza disease risk in pregnant women, the possibility to protect young infants via placental antibody transfer, vaccine safety and effectiveness, and programmatic opportunities [2].
The influenza disease risk posed to pregnant women has never been comprehensively addressed in a systematic review. We conducted a systematic review to quantify the association between pregnancy and severe influenza disease and to summarize the evidence for pregnancy as a risk factor for severe influenza disease.
2. Materials and methods
All the methods outlined below were specified a priori.
2.1. Data sources and searches
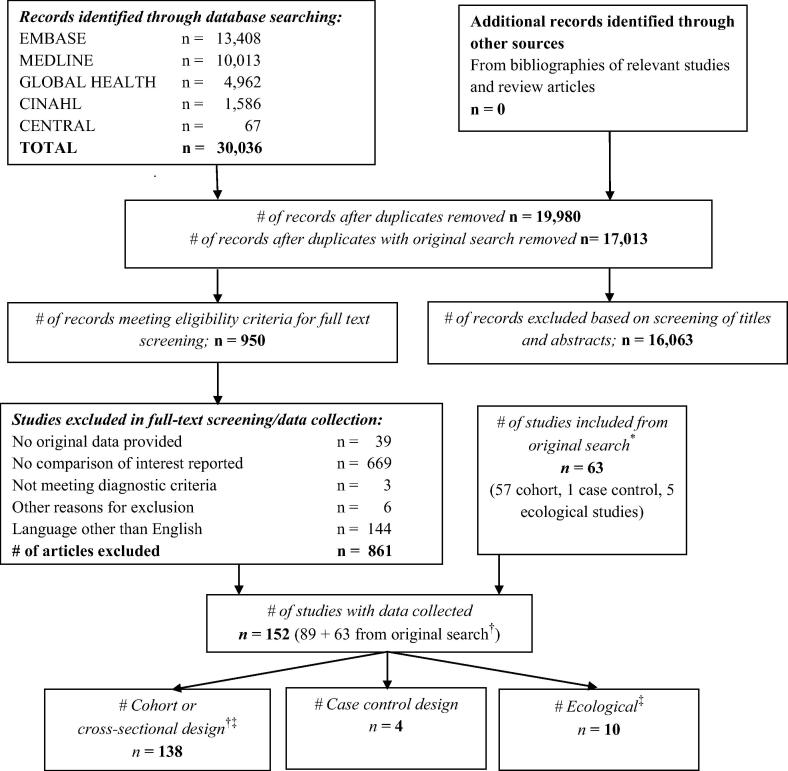
We searched MEDLINE (since 1966; Supplementary Table 1), EMBASE (since 1980), CINAHL (since 1982), and the Cochrane Central Register of Controlled Trials (CENTRAL). We also searched reference lists of identified articles and review articles. We included relevant studies selected in our previous systematic review on risk factors for severe outcomes from influenza (search up to March 25, 2011 [3]), and updated the search using the same search strategy through April 25, 2014 (Fig. 1).
Fig. 1.
Flow sheet of studies included and excluded (search update). Legend: * “Original search” refers to the search for the systematic review conducted in 2011 [3]; † One article reported on two study populations; ‡ One article reported ecological as well as individual-level data.
2.2. Study selection
Studies reporting on pregnancy as a risk factor for the following severe outcomes following influenza: community-acquired pneumonia, death from all causes or related to influenza, hospitalization from all causes or related to influenza, admission to an intensive care unit (ICU) related to influenza, and/or need for mechanical ventilatory support. Study designs included observational studies with a comparator arm of non-pregnant patients with evidence of influenza virus infection. Ecologic studies, also included, were defined as studies that collected data at a group rather than at an individual level, or in which numerators or denominators were imputed or estimated. Non-English language articles were excluded in the search update, based on the limited value demonstrated in the first search [3].
Evidence for influenza virus infection was based on laboratory-confirmed influenza virus infection defined by at least one of the following: serology, viral culture, nucleic acid amplification testing, or antigen detection. Representation of non-laboratory defined evidence, such as influenza-like illness during known influenza circulation, although eligible for the review, was negligible (n = 3 studies, 2.1%). Studies on avian influenza A virus infection in humans were excluded.
2.3. Data extraction and quality assessment
Two reviewers independently screened titles, abstracts and full text articles, extracted data using a standardized and pilot-tested database, and assessed risk of bias. Any disagreement between reviewers was resolved by consensus or arbitration by a third reviewer.
We used the Newcastle Ottawa Scale (NOS) to assess risk of bias for individual-level studies [4]. With this scale, a maximum of 9 points was allocated in four domains: a maximum of 4 points for selection of study groups, 2 points for comparability of groups, and up to 3 points for ascertainment of exposure and outcomes. In order to evaluate publication bias, funnel-plots were made if ten or more studies had been included. The overall quality of evidence was assessed using the recently published GRADE framework for evidence about prognosis [5].
2.4. Data synthesis and analysis
We performed a meta-analyses using a random effects model in Review Manager 5.0 (Cochrane Collaboration) [6] to obtain a summary estimate of the average effect with its 95% confidence interval (CI). Given the small number of non-cohort observational studies, we pooled all design types. Ecologic studies were only synthesized qualitatively.
To compare studies of similar design, we stratified the meta-analyses by type of population at the time of enrollment (community setting, hospitalized patients, and ICU patients). Heterogeneity was evaluated using χ2 and I2 statistics [7]. We considered a χ2 of <0.10 or an I2 statistic of >50% to reflect significant heterogeneity that triggered additional a priori defined subgroup analysis: seasonal versus pandemic influenza, level of risk of bias as judged by the NOS, variable definitions of influenza virus infection, and variable influenza vaccine receipt.
Because the findings may vary depending on the definition of influenza, we planned to conduct a sensitivity analysis excluding all studies with lack of laboratory confirmation. When multiple comparator groups were available, the most appropriate was used, i.e. non-pregnant women of reproductive age was the preferred comparator, followed by non-pregnant women of any age and then all other patients including males. Because non-matching comparator groups may have biased the results of the primary analysis, we conducted a sensitivity analysis including only studies that used non-pregnant women of reproductive age as a comparator.
3. Results
We screened an additional 17,013 records after duplicates were removed to update our previous search [3] yielding an additional 89 publications meeting inclusion criteria in addition to the 63 publications identified from the previous search for a total of 152 included studies, 142 individual-level and 10 ecological studies (Fig. 1; Table 1). Individual-level studies included 134 cohort and four cross-sectional, and four case control studies with data on a combined total of 310,597 patients (Supplementary Tables 2–4). The majority of studies were conducted in Europe (n = 49, 34.5%), followed by Asia (n = 31, 21.8%) and North America (n = 30, 21.1%). A total of 45 studies (31.7%) were conducted in middle-income countries while no studies were conducted in low-income countries (http://data.worldbank.org/).
Table 1.
Characteristics of ecological studies (n = 10).
| Author | Year | Region | Time of study | Strain | Influenza laboratory confirmation | Sample size | Participants | Comparator group | Outcome | 95% confidence interval |
|---|---|---|---|---|---|---|---|---|---|---|
| Baigalmaa [28] | 2012 | Mongolia | 10/2009–1/2010 | pH1N1 | Yes | 29 | Hospital deaths | Nationwide prevalence data for risk factors | Mortality: RR 50 | 21, 118 |
| Campbell [29] | 2011 | England | 4/2009–1/2010 | pH1N1 | Yes | 2416 | Hospitalized patients | Women 15–44 years | Hospitalization: RR 7.8 | 6.4, 9.6 |
| Dede [30] | 2011 | Turkey | 10–12/2009 | pH1N1 | Yes | 599 | pH1N1 confirmed deaths | Non-pregnant general population | Mortality: RR 3.9 | 2.8, 5.4 |
| Jamieson [17] | 2009 | United States | 4–5/2009 | pH1N1 predominant | Yes | 34 | Pregnant women with confirmed influenza | General population | Hospitalization: 0.32/100,000 versus 0.076/100,000 | 0.13, 0.52 |
| 2.3, 7.8 | ||||||||||
| Kelly [31] | 2009 | Australia | 5–10/2009 | pH1N1 | Unclear | 4833 | Pregnant women | Non-pregnant participants | Hospitalization: RR 5.2 | 4.6, 5.8 |
| ICU admission: RR 6.5 | 4.8, 8.8 | |||||||||
| Mortality: RR 1.4 | 0.4–4.5 | |||||||||
| Mytton [32] | 2012 | England | 7/2009–4/2010 | pH1N1 | Yes (not all cases) | 361 | pH1N1 confirmed deaths | Women 16–45 years | Mortality (age standardized): 13 versus 5.1; p < 0.01 | 5.7, 35 |
| 3.8, 6.7 | ||||||||||
| Pebody [33] | 2010 | UK | 4/2009–3/2010 | pH1N1 | Yes (not all cases) | 308 | Confirmed pH1N1 dead | Women 15–44 years | Mortality: RR 7 | 3, 15 |
| Seppelt [34] | 2010 | Australia and New Zealand | 6–8/2009 | pH1N1 | Yes | 64 | Pregnant and postpartum | Non-pregnant women 15–44 years | ICU admission: RR 7.4 | 5.5, 10 |
| Van Kerkhove [21] | 2011 | 19 Countries | 4/2009–1/2010 | pH1N1 | Yes | ∼70,000 | pH1N1 confirmed in ICU | Women 15–49 years | Hospitalization: RR 6.8 | 4.5, 12.3 |
| Mortality: RR 1.9 | 0.0, 2.6 | |||||||||
| Wilking [35] | 2010 | Germany | 4/2009–3/2010 | pH1N1 | Yes (not all cases) | 252 | pH1N1 confirmed deaths | Women 15–49 years | Mortality: RR 2.2 | 0.5, 9.4 |
Legend: RR risk ratio, pH1N1 pandemic 2009 influenza A (H1N1).
3.1. Individual level studies
All but six individual-level studies (n = 136, 95.8%) reported on pandemic influenza either alone or in combination with seasonal influenza. Of these, all but three studies reported on the 2009 influenza A (H1N1) pandemic (pH1N1). A total of 17 studies with 14,366 patients reported on seasonal influenza with 11 studies (64.7%) reporting on post-pandemic H1N1.
All studies enrolled participants that sought medical care. Twenty-four (16.9%) studies enrolled non-hospitalized patients (referred to as community setting below), including seven studies comprised entirely of patients seen at an emergency department, five studies that included patients from emergency departments and outpatient clinics, and 12 studies that did not report the setting in detail. Only three studies provided adjusted risk estimates for pregnancy as a risk factor for hospitalization while controlling for other co-morbidities, influenza vaccination status, and/or treatment with antivirals [8], [9], [10]. A single study reported on other outcomes of interest while adjusting for age [11], but we found no studies that adjusted for any of the other relevant co-morbidities. Furthermore, no attempt was made to measure severity of illness at the time of presentation.
Since there were few non-2009 pandemic studies, no subgroup analyses by type/subtype or pandemic versus seasonal was conducted. Subgroup analyses based on risk of bias was also not feasible given that the vast majority (n = 132, 92.3%) of studies were at some but not severe risk of bias (5–7 NOS points). The same was true for studies that defined influenza virus infection based on laboratory confirmation versus clinical criteria since only three studies (2.1%) lacked laboratory confirmation. Finally, influenza vaccination status and antiviral therapy were not routinely reported in the studies and thus were not analyzed.
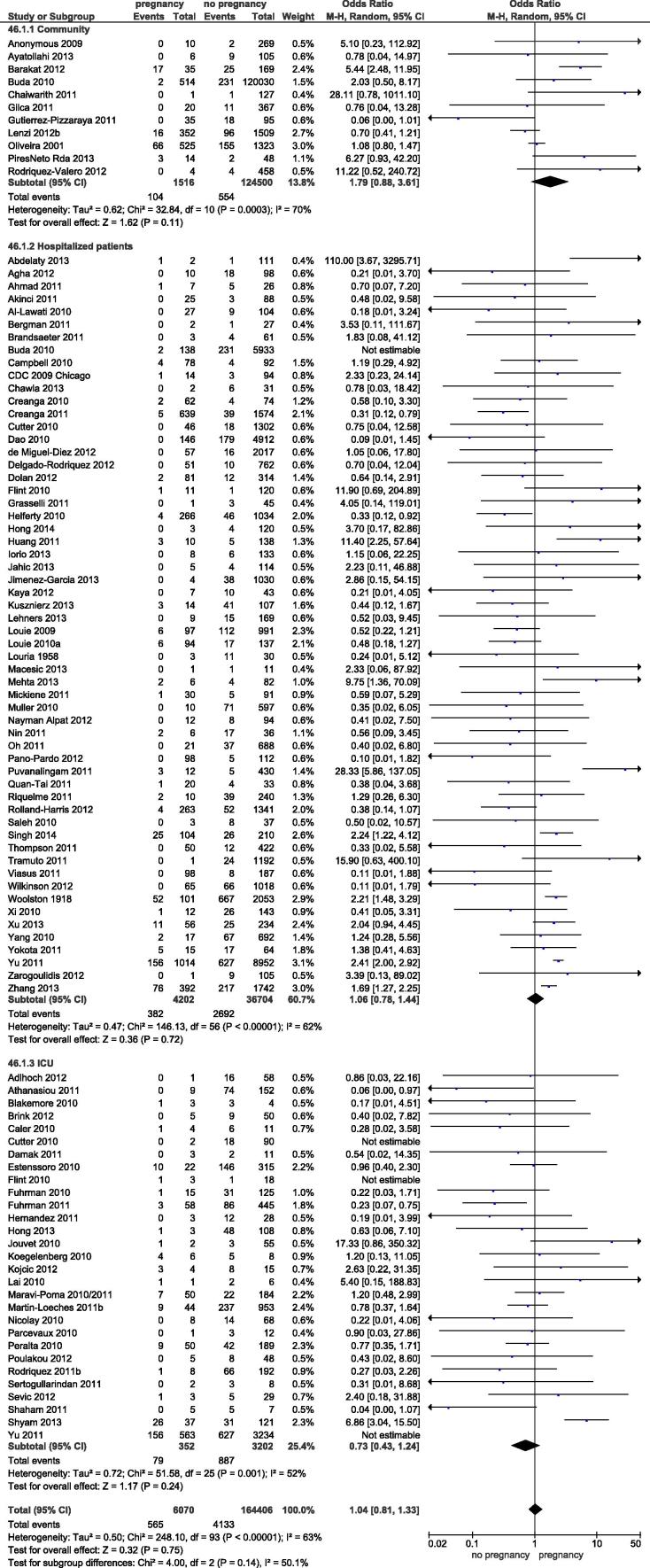
3.1.1. All-cause mortality
A total of 94 studies reported on mortality. All but 11 studies (11.7%) included hospitalized patients, only. Pregnancy was not found to be associated with a higher risk (odds ratio (OR) 1.04, 95% confidence Interval (CI): 0.81–1.33) (Fig. 2). There was significant heterogeneity (I2 = 63%) which could not be explained on the basis of subgrouping by population at risk ((I2 = 50.1%, p = 0.14 for subgroup effect). The risk estimate for pregnancy associated mortality in the community setting was elevated but not significantly (OR 1.79, 95% CI 0.88–3.61) when compared to hospitalized patients. A total of 18 studies allowed for the use of non-pregnant women of reproductive age as the comparator for sensitivity analysis yielding a similar result (OR 1.02, 95% CI 0.57–1.84; I2 = 66%).
Fig. 2.
Forest plot for pregancy as a risk factor for mortality following influenza. Legend: Subgroups: source of population (community-based, hospital-based, ICU-based patient population). Not estimable: each study was only included once in the forest plot with data on the largest patient population available. E.g. the study for Buda et al. was only included in the community subgroup but deemed not estimable in the hospitalized group.
3.1.2. Hospital and ICU admission
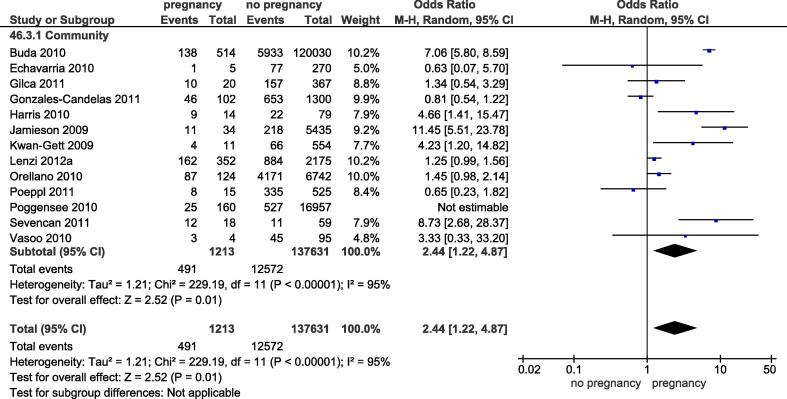
Thirteen studies reported on the risk of hospitalization (Fig. 3). Due to a significant overlap in study populations between two studies [12], [13], the study with the smaller sample size was excluded [13]. Four studies (30.8%) enrolled patients through the emergency department, three studies (23.1%) also included enrolled patients from outpatient clinics, and the enrollment setting was not described in detail in the remaining studies (n = 6, 46.2%).
Fig. 3.
Forest plot for pregancy as a risk factor for hospitalization following influenza.
The risk for hospital admission in pregnant women was significantly higher than for non-pregnant patients (OR 2.44, 95% CI 1.22–4.87; I2 = 95%). Only two studies could be included in the sensitivity analysis restricted to studies with non-pregnant women of reproductive age as the comparator showing a similar effect size (OR 3.28, 95% CI 0.52–20.6; I2 = 84%). A potential explanation for the high heterogeneity between these two studies was related to the inclusion criteria: while one study included patients presenting to the emergency department in Turkey [14], the other study included a mix of patients from a hospital emergency department and from outpatient clinics in Canada [8]. The three studies that reported risk estimates adjusted for other co-morbidities, all found slightly higher risks associated with pregnancy in the adjusted versus unadjusted analysis [8], [9], [10].
ICU admission related to influenza was reported as an outcome in 47 studies (Supplementary Fig. 1). Only four studies (8.5%) included patients from a community setting, with two of these limited to patients presenting to an emergency department. No significant difference between pregnant and non-pregnant patients was found (OR 0.85, 95% CI 0.62–1.17; I2 = 81%), with an I2 of 0% in the subgroup of community-based studies. There was no significant subgroup effect (I2 = 0%, p = 0.45). A sensitivity analysis limited to the 10 studies that reported data on women of reproductive age as the comparator group found a significantly lower risk for ICU admission for pregnant women (OR 0.51, 95% CI 0.42–0.62; I2 = 17%) (Supplementary Fig. 2).
3.1.3. Other outcomes
Eight studies reported on pneumonia following influenza as an outcome (Supplementary Fig. 3) with only one study from a community setting. No significant differences were found (OR 1.80, 95% CI 0.72–4.49; I2 = 75%). There was no significant subgroup effect when stratified by type of population (I2 = 47.7%, p = 0.15). For the three studies used for the sensitivity analysis with women of reproductive age as the comparator, no association was found between pneumonia following influenza and pregnancy (OR 1.09, 95% CI 0.29–4.08; I2 = 84%).
There were a total of 26 studies reporting on receipt of mechanical ventilatory support among patients with evidence of influenza (Supplementary Fig. 4), with only one study from a community setting. No significant difference between pregnant and non-pregnant patients (OR 1.21, 95% CI 0.70–2.08) and no subgroup effect by type of population was found. Heterogeneity was significant with an I2 of 68%, with no statistical heterogeneity in the ICU subgroup. Among eight studies with data on women of reproductive age as comparators, the findings were similar, with no significant association (OR 0.82, 95% CI 0.40–1.67; I2 = 77%).
Twenty-one studies reported a composite outcome of ICU admission and/or all-cause mortality among patients with evidence of influenza, all conducted in a hospital-setting (Supplementary Fig. 5). No association was found between this composite outcome and pregnancy (OR 0.95, 95% CI 0.59–1.52; I2 = 84%). Among four studies with data on women of reproductive age as the comparator, no significant association was seen (OR 0.88, 95% CI 0.62–1.25; I2 = 0%).
3.2. Ecological studies
Ten studies reported group-level data on pregnancy as a risk factor for severe outcomes following influenza, all focused on the 2009 pH1N1 pandemic (Table 1). Of the ecological studies reporting on mortality, 4 of 7 (57%) reported significantly higher mortality rates among pregnant women than among non-pregnant patients (Table 1). All four studies that reported on hospitalization and two studies that reported on ICU-admission found a higher risk for pregnant women than for the comparator.
3.3. Risk of bias and grading the quality of evidence
Individual-level studies achieved an average of 6 out of a maximum of 9 points on the Newcastle-Ottawa Scale (interquartile range 6–7, range 3–8) (Supplementary Table 1). There was no evidence of publication bias in Funnel plots (data not shown).
We identified several biases that may have overestimated risk estimates in ecological studies. For all ecological studies, the number of pregnant women was estimated rather than known. Also, the comparator group included both patients with and without influenza in 8/10 ecological studies (80%). Three ecological studies (30%) included outcomes occurring during the postpartum period. Two studies each (20%) relied on passive surveillance systems or were based on enhanced surveillance for at-risk populations. In one study, lab confirmation was restricted to high risk populations.
Applying the GRADE framework [5], we downgraded the quality of evidence for all outcomes for risk of bias, as well as for inconsistency due to high heterogeneity in the meta-analysis or inconsistencies when comparing the finding in individual-level studies to ecological studies. Additionally, given the wide confidence intervals around the summary estimate that could not rule out patient-important differences, all outcomes other than hospitalization were downgraded further. Hospitalization was upgraded based on the large effect size observed. Therefore, all outcomes other than hospitalization (moderate level of evidence) were deemed to be based on a very low quality of evidence.
4. Discussion
In our systematic review and meta-analysis, we found that pregnant women with influenza have a higher risk of hospitalizations than non-pregnant patients with influenza. Pregnant women with influenza virus infection did not have a greater likelihood of death or other severe outcomes than either the general population or non-pregnant women of reproductive age. These findings contrast with ecological studies which suggest that, in addition to the increase in hospitalization, there is a higher risk of death and ICU admission in pregnancy associated influenza disease. The quality of the evidence was found to be moderate for hospital admission and very low for all other outcomes. To our knowledge, this is the first comprehensive systematic review of outcomes of influenza in pregnancy.
The most unexpected result was for mortality, where the point estimate revealed a risk that is virtually the same when comparing pregnant to non-pregnant patients and the confidence intervals did not suggest a large possible effect size (OR 1.04 (95% CI 0.81–1.33). It remains uncertain whether this represents a true absence of association or whether it is a result of bias. Notably, no studies initiated follow-up before contact with the healthcare system. It is likely that people who present to a health care provider with influenza are a select group enriched for risk factors for a more severe clinical presentation, such as co-morbidities and possibly pregnancy. Consequently, our results may reflect pregnancy conferring no greater risk than the risk associated with conditions such as cardiorespiratory disease, obesity, or advanced age. This hypothesis is supported by the three studies that reported higher odds ratios when adjustments were made for other co-morbidities [9], [10], [15].
An additional potential bias was that none of the community-based studies attempted to assess severity of illness at presentation. If health care providers perceived pregnancy as a particular risk for severe illness, they might have hospitalized pregnant women with less severe illness at presentation for precautionary reasons. This was particularly likely during the 2009 pH1N1 pandemic, when clinicians were warned that pregnant women had increased risk of severe influenza outcomes [16], [17], [18]. To overcome these biases, an ideal study would enroll participants – pregnant and non-pregnant women – in the community prior to the influenza season, and follow all women for different outcomes through the influenza season. While such a study would be robust, it would be complex, involve a large sample size, and be expensive. Also, additional studies are needed to assess the association between pregnancy and severe outcomes resulting from seasonal non-pH1N1 infection, studies that adjust for relevant confounding factors, and studies conducted in low-and middle income country settings.
Our review identified important discrepancies between the results in individual-level and ecological studies. Ecological studies describe a higher risk of death and ICU admission in pregnancy-associated influenza illness. Study designs which estimate risk based on group-level data, are however prone to a number of biases [19], and for this reason are generally regarded as inferior to cohort studies [20]. While the strength of ecological studies is typically a large population size, the ecological studies we found were generally small with the exception of one study [21]. After meta-analysis, the number of patients included in individual-level studies (n = 313,522) was greater than the number of patients from ecological studies (n = 78,896). Use of a population-wide comparator (as opposed to only pregnant women), estimation of pregnancy rates, and lack of tracking of live and still births may have led to overestimation of pregnancy on adverse influenza outcomes. However, it is possible that because the ecologic studies included community-based comparison groups, they detected a signal missed by the individual-level studies.
Strengths of this systematic review include a comprehensive search strategy, risk of bias assessment, sensitivity analyses that showed similar results, and the high proportion of studies using laboratory-confirmation to define a case. Finally, a comprehensive systematic review article has not yet been published on this topic. A review article by Meijer et al. published last year used a systematic approach to identify articles of interest [22]. However, the search strategy was narrow and as such missed relevant studies by including only 32 articles on maternal morbidity in contrast to the 152 studies we identified. Furthermore, it lacked a thorough critical appraisal of the evidence and no meta-analysis was conducted. In our study, an important limitation was significant heterogeneity that could not be explained by the a priori defined subgroup analyses. This may point to clinical heterogeneity in terms of study population and health service resources, but also possibly to different study designs and study conduct [23]. Such differences would not necessarily have been detected by differences in the scores in the NOS, as the risk of bias assessment depends on the level of details known about the included studies as well as potential subjectivity [24], which we aimed to minimize by using independent assessment by two investigators. Despite the 142 individual-level studies we identified, the power to detect clinically relevant differences is still limited, resulting in wide confidence intervals, in particular for the analysis on pneumonia as an outcome. We acknowledge that not considering non-English language articles is a limitation as well as the fact that the search had been completed in spring 2014. In order to make sure that we did not miss any major new studies since, we conducted a targeted search in MEDLINE using the terms “(influenza AND pregnancy) OR (influenza AND risk)”. Of 1588 newly identified studies that were screened by the first author, 53 were screened in full text, and 10 studies provided data that could be added to our meta-analyses. Adding this data did not change the summary estimates significantly (mortality OR 1.04, 95% CI 0.81–1.33 without and 1.05, 0.84–1.30 with update; for ICU admission, 0.85, 0.62–1.17 and 0.76, 0.58–1.00, respectively).
We found that pregnant women are at increased risk for hospitalization but we did not find an increase in risk of death or other severe outcomes. The higher rate of hospitalization supports vaccination for pregnant women, although whether the cause for hospitalization was due to severity of illness versus pre-emptive admission is unclear. Furthermore, influenza vaccines have been found to be safe and effective at preventing influenza virus infection in pregnant women and in their newborn children [25], [26]. This evidence of vaccine performance as well as the programmatic opportunities afforded vaccine delivery during routine antenatal care support public health efforts to immunize pregnant women against influenza illness [27].
5. Conclusions
We found that influenza during pregnancy resulted in a higher risk of hospitalization than in non-pregnant individuals, but that mortality following influenza was similar in pregnant and non-pregnant individuals.
Funding source
This work was funded by a grant from the World Health Organization. We would also like to thank the WHO Taskforce to Evaluate Influenza Data to Inform Vaccine Impact and Economic Modelling for comments and feedback about the study.
DM is a recipient of a Research Early Career Award from Hamilton Health Sciences Foundation (Jack Hirsh Fellowship). ML holds the Michael G DeGroote Chair in Infectious Diseases at McMaster University.
Conflicts of interest
JRO is a staff member of the World Health Organization. The author alone is responsible for the views expressed in this publication and they do not necessarily represent the decisions, policy or views of the World Health Organization.
Contributions
DM, JRO and ML were involved in the conception and design of the study. JG and JW collected the data. DM and ML were responsible for the analysis and drafting of the article. All authors were involved in the interpretation of the data, revised the manuscript critically for important intellectual content, and gave final approval of the version to be published, had full access to all data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2016.12.012.
Appendix A. Supplementary material
References
- 1.World Health Organization (WHO) Fact sheet number 211, influenza (seasonal) 2014. <http://www.who.int/mediacentre/factsheets/fs211/en/> [accessed July 8, 2016]
- 2.World Health Organization Vaccines against influenza. WHO position paper – November 2012. 2012. <http://www.who.int/wer/2012/wer8747.pdf?ua=1> [accessed January 11, 2016]
- 3.Mertz D., Kim T.H., Johnstone J., Lam P.P., Science M., Kuster S.P. Populations at risk for severe or complicated influenza illness: systematic review and meta-analysis. BMJ. 2013;347:f5061. doi: 10.1136/bmj.f5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. <http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp>.
- 5.Iorio A., Spencer F.A., Falavigna M., Alba C., Lang E., Burnand B. Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ. 2015;350:h870. doi: 10.1136/bmj.h870. [DOI] [PubMed] [Google Scholar]
- 6.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 7.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilca R., De Serres G., Boulianne N., Ouhoummane N., Papenburg J., Douville-Fradet M. Risk factors for hospitalization and severe outcomes of 2009 pandemic H1N1 influenza in Quebec, Canada. Influenza Other Respi Viruses. 2011;5:247–255. doi: 10.1111/j.1750-2659.2011.00204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez-Candelas F., Astray J., Alonso J., Castro A., Canton R., Galan J.C. Sociodemographic factors and clinical conditions associated to hospitalization in influenza A (H1N1) 2009 virus infected patients in Spain, 2009–2010. PLoS ONE. 2012;7:e33139. doi: 10.1371/journal.pone.0033139. [Electronic Resource] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwan-Gett T.S., Baer A., Duchin J.S. Spring 2009 H1N1 influenza outbreak in King Country Washington. Disaster Med Publ Health Prep. 2009;3 doi: 10.1097/DMP.0b013e3181c6b818. S109-S16. [DOI] [PubMed] [Google Scholar]
- 11.Barakat A., Ihazmad H., El Falaki F., Tempia S., Cherkaoui I., El Aouad R. 2009 Pandemic influenza A virus subtype H1N1 in Morocco, 2009–2010: epidemiology, transmissibility, and factors associated with fatal cases. J Infect Dis. 2012;206(Suppl. 1):S94–S100. doi: 10.1093/infdis/jis547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buda S., Kopke K., Haas W. Epidemiological characteristics of the influenza pandemic (H1N1) 2009 in Germany based on the mandatory notification of cases. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2010;53:1223–1230. doi: 10.1007/s00103-010-1158-0. [DOI] [PubMed] [Google Scholar]
- 13.Poggensee G., Gilsdorf A., Buda S., Eckmanns T., Claus H., Altmann D. The first wave of pandemic influenza (H1N1) 2009 in Germany: from initiation to acceleration. BMC Infect Dis. 2010;10:155. doi: 10.1186/1471-2334-10-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sevencan F., Ertem M., Ozcullu N., Dorman V., Ormanli A., Kubat N.K. Retrospective evaluation of laboratory-confirmed and recovered cases of influenza (H1N1)v. Turk J Med Sci. 2011;41:647–656. [Google Scholar]
- 15.Harris P.N., Dixit R., Francis F., Buettner P.G., Leahy C., Burgher B. Pandemic influenza H1N1 2009 in north Queensland – risk factors for admission in a region with a large indigenous population. Commun Dis Intell Q Rep. 2010;34:102–109. [PubMed] [Google Scholar]
- 16.Freitas A.R.R. Re: populations at risk for severe or complicated influenza illness: systematic review and meta-analysis. BMJ. 2013:f5061. doi: 10.1136/bmj.f5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jamieson D.J., Honein M.A., Rasmussen S.A., Williams J.L., Swerdlow D.L., Biggerstaff M.S. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374:451–458. doi: 10.1016/S0140-6736(09)61304-0. [DOI] [PubMed] [Google Scholar]
- 18.Louie J.K., Acosta M., Jamieson D.J., Honein M.A., California Pandemic Working G Severe 2009 H1N1 influenza in pregnant and postpartum women in California. N Engl J Med. 2009;2010(362):27–35. doi: 10.1056/NEJMoa0910444. [DOI] [PubMed] [Google Scholar]
- 19.Greenland S., Robins J. Invited commentary: ecologic studies – biases, misconceptions, and counterexamples. Am J Epidemiol. 1994;139:747–760. doi: 10.1093/oxfordjournals.aje.a117069. [DOI] [PubMed] [Google Scholar]
- 20.Oxford Center for Evidence-based Medicine – Level of Evidence (March 2009); 2014. <http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/> [accessed July 8, 2016].
- 21.Van Kerkhove M.D., Vandemaele K.A., Shinde V., Jaramillo-Gutierrez G., Koukounari A., Donnelly C.A. Risk factors for severe outcomes following 2009 influenza A (H1N1) infection: a global pooled analysis. PLoS Med. 2011;8:e1001053. doi: 10.1371/journal.pmed.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meijer W.J., van Noortwijk A.G., Bruinse H.W., Wensing A.M. Influenza virus infection in pregnancy: a review. Acta Obstet Gynecol Scand. 2015;94:797–819. doi: 10.1111/aogs.12680. [DOI] [PubMed] [Google Scholar]
- 23.Darvishian M., Gefenaite G., Turner R.M., Pechlivanoglou P., Van der Hoek W., Van den Heuvel E.R. After adjusting for bias in meta-analysis seasonal influenza vaccine remains effective in community-dwelling elderly. J Clin Epidemiol. 2014;67:734–744. doi: 10.1016/j.jclinepi.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Lo C.K., Mertz D., Loeb M. Newcastle-Ottawa Scale: comparing reviewers' to authors' assessments. BMC Med Res Methodol. 2014;14:45. doi: 10.1186/1471-2288-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madhi S.A., Cutland C.L., Kuwanda L., Weinberg A., Hugo A., Jones S. Influenza vaccination of pregnant women and protection of their infants. New Engl J Med. 2014;371:918–931. doi: 10.1056/NEJMoa1401480. [DOI] [PubMed] [Google Scholar]
- 26.Tapia M.D., Sow S.O., Tamboura B., Teguete I., Pasetti M.F., Kodio M. Maternal immunisation with trivalent inactivated influenza vaccine for prevention of influenza in infants in Mali: a prospective, active-controlled, observer-blind, randomised phase 4 trial. Lancet Infect Dis. 2016;16:1026–1035. doi: 10.1016/S1473-3099(16)30054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meeting of the Strategic Advisory Group of Experts on immunization, April 2015: conclusions and recommendations. Rel Epidemiol Hebdom 2015;90:261–78. [PubMed]
- 28.Baigalmaa J., Tuul T., Darmaa B., Soyolmaa E. Analysis of fatal outcomes from influenza A(H1N1)pdm09 in Mongolia. Western Pac Surveill Response J. 2012;3:43–48. doi: 10.5365/WPSAR.2010.1.1.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campbell C.N., Mytton O.T., McLean E.M., Rutter P.D., Pebody R.G., Sachedina N. Hospitalization in two waves of pandemic influenza A(H1N1) in England. Epidemiol Infect. 2011;139:1560–1569. doi: 10.1017/S0950268810002657. [DOI] [PubMed] [Google Scholar]
- 30.Dede F.S., Celen S., Bilgin S., Ure G., Ozcan A.O., Buzgan T. Maternal deaths associated with H1N1 influenza virus infection in Turkey: a whole-of-population report. Bjog. 2011;118:1216–1222. doi: 10.1111/j.1471-0528.2011.03002.x. [DOI] [PubMed] [Google Scholar]
- 31.Kelly H., Mercer G., Cheng A. Quantifying the risk of pandemic influenza in pregnancy and indigenous people in Australia in 2009. Euro Surveill. 2009;14 [PubMed] [Google Scholar]
- 32.Mytton O.T., Rutter P.D., Mak M., Stanton E.A., Sachedina N., Donaldson L.J. Mortality due to pandemic (H1N1) 2009 influenza in England: a comparison of the first and second waves. Epidemiol Infect. 2012;140:1533–1541. doi: 10.1017/S0950268811001968. [DOI] [PubMed] [Google Scholar]
- 33.Pebody R.G., McLean E., Zhao H., Cleary P., Bracebridge S., Foster K. Pandemic influenza A (H1N1) 2009 and mortality in the United Kingdom: risk factors for death, April 2009 to March 2010. Euro Surveill. 2010;15:20. [PubMed] [Google Scholar]
- 34.Seppelt I. Critical illness due to 2009 A/H1N1 influenza in pregnant and postpartum women: population based cohort study. BMJ. 2010;340:751. doi: 10.1136/bmj.c1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilking H., Buda S., von der Lippe E., Altmann D., Krause G., Eckmanns T. Mortality of 2009 pandemic influenza A(H1N1) in Germany. Euro Surveill. 2010;15:9. doi: 10.2807/ese.15.49.19741-en. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.