Abstract
Epidemiologic studies have linked gestational vitamin D deficiency to respiratory diseases, although mechanisms have not been defined. We hypothesized that antenatal vitamin D deficiency would impair airway development and alveolarization in a mouse model. We studied the effect of antenatal vitamin D deficiency by inducing it in pregnant mice and then compared lung development and function in their offspring to littermate controls. Postnatal vitamin D deficiency and sufficiency models from each group were also studied. We developed a novel tracheal ultrasound imaging technique to measure tracheal diameter in vivo. Histological analysis estimated tracheal cartilage total area and thickness. We found that vitamin D–deficient pups had reduced tracheal diameter with decreased tracheal cartilage minimal width. Vitamin D deficiency increased airway resistance and reduced lung compliance, and led to alveolar simplification. Postnatal vitamin D supplementation improved lung function and radial alveolar count, a parameter of alveolar development, but did not correct tracheal narrowing. We conclude that antenatal vitamin D deficiency impairs airway and alveolar development and limits lung function. Reduced tracheal diameter, cartilage irregularity, and alveolar simplification in vitamin D–deficient mice may contribute to increased airways resistance and diminished lung compliance. Vitamin D supplementation after birth improved lung function and, potentially, alveolar simplification, but did not improve defective tracheal structure. This mouse model offers insight into the mechanisms of vitamin D deficiency–associated lung disease and provides an in vivo model for investigating preclinical preventive and therapeutic strategies.
Keywords: vitamin D deficiency, tracheal ultrasound, pulmonary function, murine model, lung development
Clinical Relevance
This murine investigation details structural and physiologic aberrances resulting from prenatal and postnatal vitamin D deficiency. In addition to modeling consequences on lung development, our findings emphasize differential benefit of prenatal versus postnatal vitamin D supplementation. These data provide mechanistic insight into clinical consequences of vitamin D deficiency–induced lung disease and the utility of a mouse model to test preventive and therapeutic interventions.
Vitamin D deficiency is common during pregnancy. Among African Americans, the prevalence of vitamin D deficiency is estimated to be more than 40% (1). Similarly, 45.6% of African American neonates are vitamin D insufficient at birth (2). Vitamin D deficiency during pregnancy is associated with low birth weight and reduced fetal bone mineral content (3), with an increased risk of preterm labor, preterm birth, and infections (2). Prenatal vitamin D deficiency is also associated with increased wheezing during the first years of life (4), increased risk of lower respiratory tract infections (5), asthma severity (6), reduced lung function, and chronic use of inhaled steroids (7). A recent prospective study of patients with asthma found that increased vitamin D concentrations were associated with better forced expiratory volume in 1 second and forced vital capacity measures (8, 9).
Vitamin D is essential to normal development and the structure of bone and cartilage. Deficiency in vitamin D leads to hypertrophy and softening of the growth plate. As trachea and bronchi share similar cartilaginous structure, vitamin D deficiency during airway development may have deleterious effects on the supportive structures of these airways. Tracheomalacia and bronchomalacia are common diseases of infancy, due to defective airway cartilage. In children undergoing bronchoscopy for respiratory distress or chronic wet cough, lower airway malacia is present in approximately 30% of patients (10). This increases to approximately 50% in premature infants (11), and in almost all infants who require chronic mechanical ventilation (12). The specific cause for airway malacia remains undefined, and few preventive or therapeutic options are available for the clinician. In severe cases, surgical approaches aimed at improving the caliber of the airway are the only available interventions, and carry substantial morbidity (13). No studies have quantified the effect of vitamin D deficiency on the development of airway malacia.
Human data demonstrate an association between vitamin D deficiency and increased respiratory symptoms without an obvious mechanistic explanation. Animal studies indicate that vitamin D deficiency alters pulmonary immune mechanisms (14), decreases surfactant production (15), accelerates airway smooth muscle proliferation (16), and impedes epithelial–mesenchymal interactions during lung development (17). Vitamin D deficiency regulates chondrocyte function through increased vitamin D receptor (VDR) activity (18–20). VDR has been identified in human airway epithelial cells (21), but no previous studies have determined the effect of vitamin D deficiency on airway development and function.
In this study, we hypothesized that prenatal vitamin D deficiency impedes respiratory development. We investigated the effects of prenatal vitamin D deficiency on lung structure and function using tracheal ultrasound, airway casting, histopathology, and pulmonary function studies. We compared these findings with those of postnatal vitamin D deficiency and the response to postnatal vitamin D supplementation.
Materials and Methods
Institutional Approval
The protocols were approved by the Institutional Animal Care and Use Committee of University of Alabama at Birmingham (Birmingham, AL), and were consistent with the Public Health Service policy on humane care and use of laboratory animals and guidelines for the care and use of laboratory animals.
Animal Model
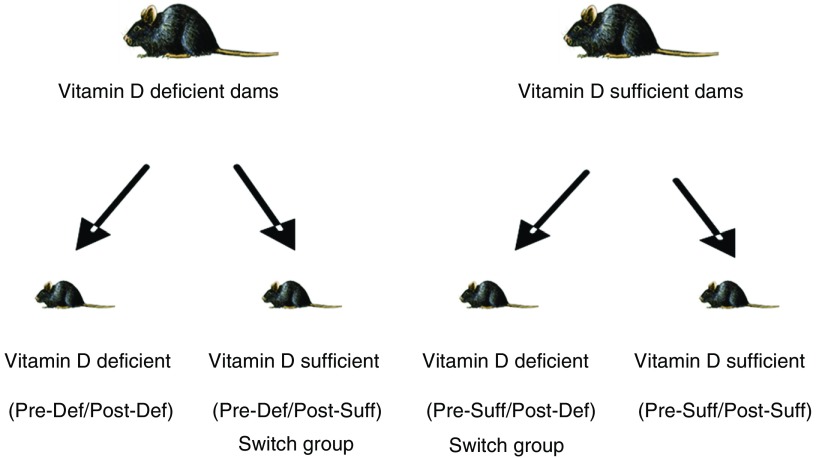
C57BL/6J littermate female mice were randomly assigned to vitamin D–deficient and –sufficient groups at the age of 3 weeks and mated at the age of 7 weeks. The vitamin D–deficient group was housed in ultraviolet (UV) light–free surroundings using UV-blocking films (F007-005NA; UV Process Supply, Inc., Chicago, IL) and received a vitamin D–depleted diet (Vitamin D–deficient diet, TD.89123; Harlan Laboratories, Inc., Madison, WI) as previously described (22). The vitamin D–sufficient mice were exposed to normal light and their food was supplemented with 2,200 IU/kg vitamin D (Vitamin D Control Diet, TD.89124; Harlan Laboratories Inc.) with the same caloric content as the deficient diet. At weaning, we further divided the pups in each group into vitamin D–deficient and –sufficient subgroups, resulting in four subgroups with different combinations of prenatal and postnatal vitamin D status: prenatal deficiency/postnatal deficiency (Pre-Def/Post-Def), prenatal deficiency/postnatal sufficiency (Pre-Def/Post-Suff), prenatal sufficiency/postnatal deficiency (Pre-Suff/Post-Def), and prenatal sufficiency/postnatal sufficiency (Pre-Suff/Post-Suff) (Figure 1). “Switch” groups refers to offspring with different group assignments from their dams (e.g., Pre-Suff/Post-Def and Pre-Def/Post-Suff). The experiments were repeated three times on the original groups and twice on the switch groups using 8–25 animals in each subgroup.
Figure 1.
Schematic representation of the mouse groups. After weaning at the age of 3 weeks, female mice were randomized into the vitamin D–sufficient or –deficient groups and mated with vitamin D–sufficient males at the age of 7 weeks. The offspring were kept in the same environment as their dams until the age of 4 weeks when some of them were studied. The remaining pups were randomized into vitamin D–sufficient and –deficient subgroups and then studied at 8 weeks of age. The resultant four subgroups were as follows: prenatal deficiency/postnatal deficiency (Pre-Def/Post-Def); prenatal deficiency/postnatal sufficiency (Pre-Def/Post-Suff; switch group); prenatal sufficiency/postnatal deficiency (Pre-Suff/Post-Def; switch group); and prenatal sufficiency/postnatal sufficiency (Pre-Suff/Post-Suff).
In Vivo Tracheal Ultrasound
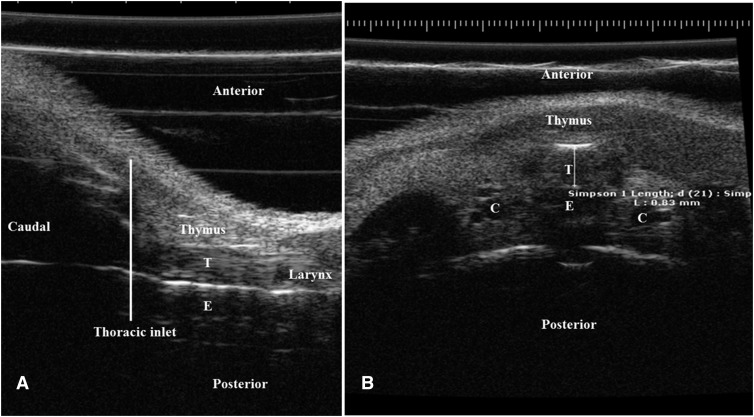
Ultrasound imaging was done at 4 and 8 weeks using a high-frequency ultrasound imaging instrument (Vevo-6600; VisualSonics, Toronto, ON, Canada). We refined the ultrasound settings (focus of 4 mm, frequency of 55 MHz, master gain of 14 dB, and frame rate of 30 Hz over 10 s) to obtain optimal resolution of the tracheal image. Tracheal diameter in both axial and transverse views was assessed 1 mm proximal to the thoracic inlet, with measurement from mid-anterior to midposterior wall reported in millimeters (Figure 2). The study was repeated two to three times on the different subgroups.
Figure 2.
Tracheal ultrasound images showing sagittal (A) and axial (B) views with the anatomic landmarks. (A) The sagittal left lateral view of the neck and upper chest of an 8-week-old mouse shows in a cephalocaudal sequence: the larynx followed by the trachea (T) with the thymus anteriorly and the esophagus (E) posteriorly. The thoracic inlet is represented by the line at the level of the manubrium sterni. (B) The cross-sectional ultrasound view 1 mm above the thoracic inlet reveals the trachea in the middle of the image with the thymus anteriorly and esophagus posteriorly and the carotid arteries (indicated by “C”) at both sides. The anteroposterior diameter is measured in millimeters from the midanterior to the midposterior walls of the trachea 1 mm proximal to the thoracic inlet, as shown.
Airway Casting
Silicone casts of mouse airways were performed at 8 weeks using negative-pressure chambers modified from previously published techniques used for larger animals (airway casting method is described in the online supplement) (23). Tracheal casting at earlier time points was not technically feasible. Casting was repeated on two batches of animals.
Pulmonary Function Test
Lung function was measured with the flexiVent apparatus (Scireq, Montreal, PQ, Canada) at 4 and 8 weeks for the original groups and at 8 weeks for the switch groups (pulmonary function tests [PFTs] in the online supplement) (24). PFTs were repeated three times on the original groups and twice on the switch subgroups.
Histological Studies
After isoflurane anesthesia, mice were killed and exsanguinated. The lungs were inflation fixed at 25 cm water pressure using 10% formalin for 30 minutes. Morphometric analysis of lung development was assessed at 8 weeks—to include the switch groups—using standard mean linear intercept (MLI) and radial alveolar count (RAC) techniques (25).
Tracheal histology was performed at 7 days using neck block (detailed in the online supplement). Processing the isolated trachea at greater ages resulted in significant deformity that rendered measurements inaccurate. Mid-tracheal sections were analyzed using Bio-ImageXD software (Free Software Foundation, Inc., Boston, MA). The following dimensions of the tracheal cartilage were measured: total cartilage area (mm2), and average, maximum, and minimum tracheal cartilage thickness (mm). The histologic study was repeated twice.
Statistical Analysis
Results are expressed as mean (±SEM). Data were analyzed using a t test for two-group comparisons and ANOVA for multiple comparisons, with statistical significance defined as P less than 0.05. Regression analysis was used to evaluate the relationship between different variables.
Results
General Characteristics
The study was performed three times, with 8–25 pups studied in each subgroup over 2 years (May 2013 to April 2015). The results presented here represent the collective data of the different animal batches with the number of animals included in each analysis.
Vitamin D 25-OH level was lower in the vitamin D–deficient group compared with the vitamin D–sufficient group (Pre-Def/Post-Def, 0.19 ± 0.11 nmol/dl [n = 5] versus Pre-Suff/Post-Suff, 0.60 ± 0.10 nmol/dl [n = 5]; P < 0.05). No significant difference in vitamin D level was observed between the two switch groups and other groups (Pre-Suff/Post-def, 0.28 ± 0.08 nmol/dl [n = 5] versus Pre-Def/Post-Suff, 0.43 ± 0.12 nmol/dl [n = 5]; ANOVA P > 0.05). Vitamin D–deficient dams had decreased fertility compared with controls (1.7 ± 0.6 pups/dam [n = 8] versus 4.5 ± 1.0 pups/dam [n = 9]; P < 0.05). Vitamin D–deficient pups weighed less than control littermates, both at 4 weeks (10.8 ± 0.8 g [n = 19] versus 16.4 ± 0.5 g [n = 15]) and 8 weeks (P < 0.0001; and 13.0 ± 0.9 g [n = 14] versus 21.1 ± 0.7 g [n = 14] P < 0.0001). No sex differences were found in weight at 4 weeks. With vitamin D sufficiency at 8 weeks, males outweighed females (22.6 ± 0.4 g [n = 15] versus 18.4 ± 0.2 g [n = 14]; P < 0.0001). With vitamin D deficiency at 8 weeks, males continued to have the same weight as females (12.8 ± 1.2 [n = 10] versus 13.3 ± 0.8 [n = 4]; P = 0.8). Vitamin D supplementation improved the weight of pups born to vitamin D–deficient dams at 8 weeks (Pre-Def/Post-Suff, 18.8 ± 0.6 g [n = 30]; Pre-Def/Post-Def, 13.0 ± 0.9 g [n = 14]; P < 0.0001), but did not fully correct malnutrition when compared with the control group at the same age (Pre-Suff/Post-Suff, 21.1 ± 0.7 g [n = 14]; P < 0.05). Sex distribution was the same in these groups.
Effect of Prenatal Vitamin D Deficiency on Pulmonary Structure and Development
Tracheal ultrasound
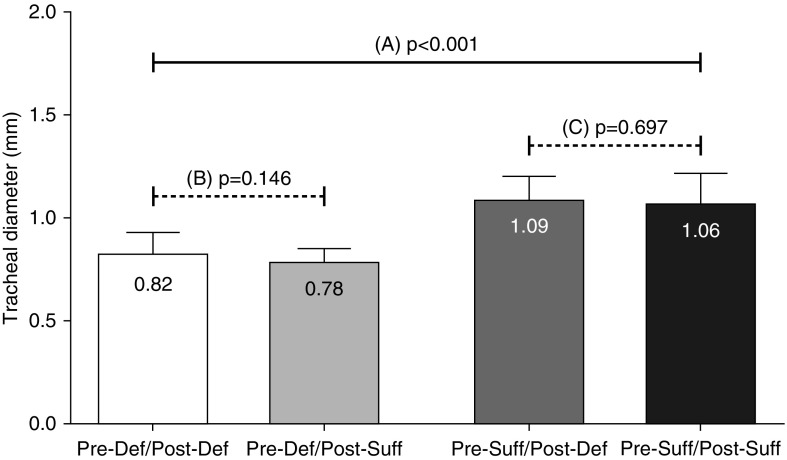
The anteroposterior tracheal diameter measurements using both sagittal and axial views on the same animals were moderately correlated (r = 0.61; P < 0.0001). Axial view measurements were used for our data analysis. Tracheal diameter measured by ultrasound was correlated to that measured by tracheobronchial cast (r = 0.75; P < 0.05). Tracheal diameter correlated with weight (r = 0.72; P < 0.0001) and was larger in the 8-week-old versus the 4-week-old mice (1.01 ± 0.01 mm [n = 53] versus 0.75 ± 0.03 mm [n = 13]; P < 0.0001). Tracheal diameter was smaller in offspring of vitamin D–deficient dams (Pre-Def/Post-Def) compared with vitamin D–sufficient controls (Pre-Suff/Post-Suff) (0.82 ± 0.02 mm [n = 22] versus 1.06 ± 0.04 mm [n = 18]; P < 0.0001; Figure 3A). Multiple regression analysis indicated this difference was independent of animal weight (r = −0.56; P < 0.0001). Tracheal ultrasound was performed on 22 (Pre-Def/Post-Def) and 18 (Pre-Suff/Post-Suff) mice, including the 3 batches studied.
Figure 3.
Tracheal diameter measured with ultrasound at the age of 8 weeks. Comparison “A”: prenatal vitamin D deficiency (Pre-Def/Post-Def) resulted in narrowing of the trachea compared with prenatally sufficient (Pre-Suff/Post-Suff) mice (P < 0.0001). Comparison “B”: postnatal vitamin D supplementation (Pre-Def/Post-Suff) did not improve tracheal diameter in prenatally deficient mice (P = 0.146). Comparison “C”: similarly, postnatal vitamin D deficiency alone (Pre-Suff/Post-Def) did not affect tracheal diameter with measures similar to their littermates with postnatal sufficiency (Pre-Suff/Post-Suff) (P = 0.697). Results are expressed as mean (±SEM).
Pulmonary function
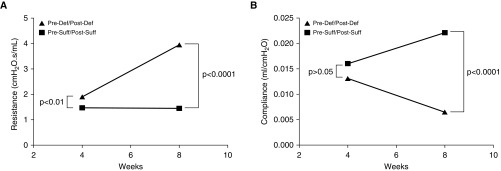
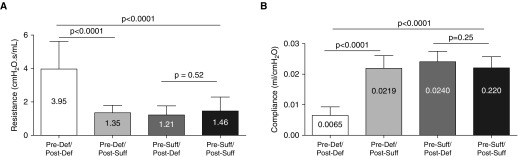
Lung function measures in vitamin D–deficient (Pre-Def/Post-Def) mice showed increased resistance at 4 weeks (1.91 ± 0.18 cm H2O ⋅ s/ml [n = 12] versus 1.35 ± 0.10 cm H2O ⋅ s/ml [n = 14]; P = 0.008) compared with the vitamin D–sufficient group (Pre-Suff/Post-Suff). This difference in airway resistance widened further at the age of 8 weeks (3.95 ± 0.59 cm H2O ⋅ s/ml [n = 8] versus 1.46 ± 0.22 cm H2O ⋅ s/ml [n = 14]; P = 0.0001; Figure 4A).
Figure 4.
Impact of vitamin D deficiency on pulmonary function. (A) Airway resistance measured with flexiVent was significantly higher in mice born to vitamin D–deficient dams (Pre-Def/Post-Def) compared with those born to vitamin D–sufficient dams (Pre-Suff/Post-Suff) at 4 weeks (P < 0.01), and continued to increase at 8 weeks (P < 0.0001) (brackets represent the differences). (B) Pulmonary compliance was initially not significantly different in Pre-Def/Post-Def and Pre-Suff/Post-Suff mice at the 4-week measurement (P > 0.05) but became significantly decreased (worse) in the deficient (Pre-Def/Post-Def) group by 8 weeks of age (P < 0.0001).
Pulmonary compliance was not different in the vitamin D–deficient (Pre-Def/Post-Def) compared with the vitamin D–sufficient (Pre-Suff/Post-Suff) pups at 4 weeks (0.013 ± 0.001 ml/cm H2O [n = 12] versus 0.016 ± 0.001 ml/cm H2O [n = 15]; P = 0.068). By 8 weeks, lung compliance became significantly decreased in the vitamin D–deficient (Pre-Def/Post-Def) group in comparison to the (Pre-Suff/Post-Suff) mice (0.006 ± 0.001 ml/cm H2O [n = 9] versus 0.022 ± 0.001 ml/cm H2O [n = 16]; P < 0.0001; Figure 4B). Lung elastance also was not significantly different at the earlier time point (81.5 ± 6.4 cm H2O/ml [n = 12] versus 68.2 ± 6.0 cm H2O/ml [n = 15]; P = 0.1490), but by 8 weeks had become significantly higher in the deficient (Pre-Def/Post-Def) compared with vitamin D–sufficient (Pre-Suff/Post-Suff) animals (189.4 ± 36.6 cm H2O/ml [n = 16] versus 46.6 ± 1.9 cm H2O/ml [n = 9]; P < 0.0001). A total of 8–16 animals included in each group analysis in this experiment during the 3 rounds of repetition.
Tracheal histology
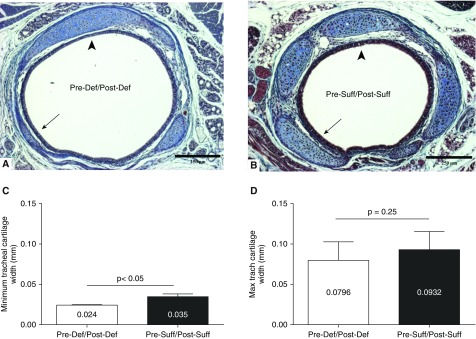
Cross-sectional tracheal histology on 7-day-old pups revealed vitamin D–deficient (Pre-Def/Post-Def) mice to have irregular tracheal cartilage deposition, with focal areas of thinning (Figures 5A and 5B). The minimum tracheal cartilage width was less in the deficient (Pre-Def/Post-Def) group (0.024 ± 0.003 mm [n = 8] versus 0.035 ± 0.002 mm [n = 8]; P < 0.05; Figure 5C), but there was no difference in the average and maximum cartilage thickness (average, 0.057 ± 0.005 mm [n = 8] versus 0.066 ± 0.005 mm [n = 8]; P = 0.21; maximum, 0.080 ± 0.008 mm [n = 8] versus 0.093 ± 0.008 mm [n = 8]; P = 0.25; Figure 5D). Total cartilage surface area was similar between the two groups (0.125 ± 0.039 mm2 [n = 8] versus 0.141 ± 0.053 mm2 [n = 8]; P = 0.81). Eight pups were studied in each group during the two repetitions of the histologic study on those two groups.
Figure 5.
Effect of prenatal vitamin D deficiency on midtrachea cartilage morphology at 7 days. (A) Cross-section of the tracheal ring for a pup of a vitamin D–deficient dam (Pre-Def/Post-Def) shows segmental narrowing of the tracheal cartilage in the deficient mouse (arrow) when compared with (B) that of a pup from a sufficient dam (Pre-Suff/Post-Suff). The thickest part of trachea is comparable between the two tracheas (arrowhead; TriChrome stain [EMD Millipore, Billerica, MA], 100×). Scale bars, 250 μm. (C) Morphometric analysis showed thinner tracheal cartilage at its minimum dimension in the vitamin D–deficient (Pre-Def/Post-Def) group (P < 0.05). (D) Maximal cartilage thickness was similar between Vitamin D groups, emphasizing the heterogeneity of collagen deposition in the deficient mice. Results are expressed as mean (±SEM).
Lung morphometry
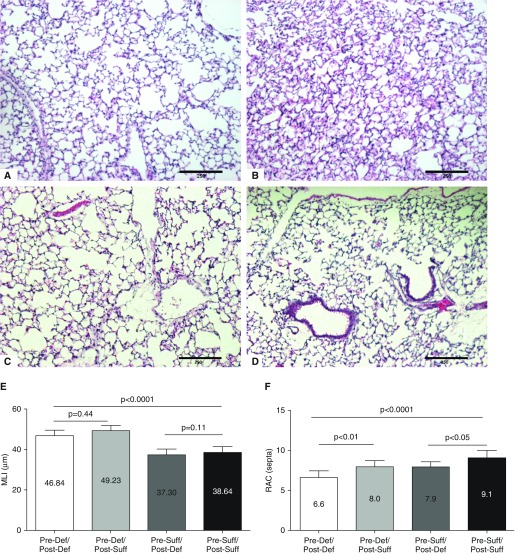
Morphometric study of the lungs showed that offspring of vitamin D–deficient (Pre-Def/Post-Def) dams developed alveolar simplification when compared with those of vitamin D–sufficient (Pre-Suff/Post-Suff) dams (Figures 6A and 6B). Reduced alveolarization in the deficient (Pre-Def/Post-Def) mice was confirmed by increased MLI (46.8 ± 1.04 μm [n = 7] versus 38.6 ± 1.05 μm [n = 7]; P < 0.0005) and decreased RACs (6.6 ± 0.3 septa [n = 7] versus 9.1 ± 0.3 septa [n = 7]; P < 0.0005) when compared with the vitamin D–sufficient (Pre-Suff/Post-Suff) group (Figures 6E and 6F). Lung morphometry was repeated three times to compare the two prenatal groups.
Figure 6.
Effect of vitamin D deficiency on alveolar development. (A) Pups born to vitamin D–deficient dams (Pre-Def/Post-Def) have alveolar simplification, with larger and fewer alveoli when compared with (B) pups born to vitamin D–sufficient dams (Pre-Suff/Post-Suff) (hematoxylin and eosin stain [Fisher Scientific, Pittsburgh, PA], 100×). (C) Mice of postnatal supplementation and (D) deficiency showed intermediary alveolar size and number. (E) Morphometric analysis showed that Pre-Def/Post-Def mice had increased intra-alveolar spacing, as quantified by mean linear intercept (MLI; P < 0.0001), with no effect of postnatal supplementation (P = 0.44) or postnatal deficiency (P = 0.11). (F) Pre-Def/Post-Def showed decreased alveolar septation measured by radial alveolar count (RAC; P < 0.0001) when compared with the Pre-Suff/Post-Suff group. RAC improved with postnatal supplementation (P < 0.01) and deteriorated with postnatal deficiency (P < 0.05). Scale bars, 250 μm. Results are expressed as mean (±SEM).
Effect of Postnatal Vitamin D Deficiency and Supplementation on Pulmonary Structure and Development
Tracheal ultrasound
Postnatal vitamin D deficiency or supplementation did not affect tracheal diameter measured with ultrasound at 8 weeks of age. Postnatal vitamin D sufficiency did not improve tracheal diameter in prenatal vitamin D–deficient (Pre-Def/Post-Suff) mice when compared with their deficient (Pre-Def/Post-Def) littermates (0.78 ± 0.02 mm [n = 21] versus 0.82 ± 0.02 mm [n = 22]; P = 0.146; Figure 3B). Mice with only postnatal deficiency (and that were prenatally sufficient) had wider tracheas than either of the two prenatal deficient groups (Pre-Suff/Post-Def, 1.09 ± 0.04 mm [n = 10]; Pre-Def/Post-Def, 0.82 ± 0.02 mm [n = 22]; Pre-Def/Post-Suff, 0.78 ± 0.02 mm [n = 21]; P < 0.0001). Tracheal diameter in the postnatal vitamin D–deficient (Pre-Suff/Post-Def) group was similar to that of their littermates with postnatal sufficiency (Pre-Suff/Post-Suff) (1.09 ± 0.04 mm [n = 10] versus 1.06 ± 0.04 mm [n = 18]; P = 0.697; Figure 3C). Tracheal ultrasound was performed on 22 (Pre-Def/Post-Def), 21 (Pre-Def/Post-Suff), 10 (Pre-Suff/Post-Def), and 18 (Pre-Suff/Post-Suff) mice, including the 3 batches studied.
Pulmonary function
In contrast to structural data, postnatal supplementation with vitamin D improved lung function in mice. For airway resistance, postnatal supplementation of pups born to deficient (Pre-Def/Post-Suff) dams significantly improved pulmonary resistance, and was comparable to the airway resistance of the sufficient (Pre-Suff/Post-Suff) group (Pre-Def/Post-Def, 3.95 ± 0.59 cm H2O ⋅ s/ml [n = 8]; Pre-Def/Post-Suff, 1.23 ± 0.12 cm H2O ⋅ s/ml [n = 18]; Pre-Suff/Post-Def, 0.99 ± 0.13 cm H2O ⋅ s/ml [n = 12]; Pre-Suff/Post-Suff, 1.46 ± 0.22 cm H2O ⋅ s/ml [n = 14]; P < 0.0001; Figure 7A). When corrected for weight, airway resistance still improved significantly with vitamin D supplementation (Pre-Def/Post-Def, 45.09 ± 8.94 cm H2O ⋅ s/ml.g [n = 8]; Pre-Def/Post-Suff, 23.61 ± 2.56 cm H2O ⋅ s/ml.g [n = 18]; P < 0.005), and was still higher in Pre-Def/Post-Def than the sufficient (Pre-Suff/Post-Suff) group (23.07 ± 3.43 cm H2O ⋅ s/ml.g [n = 8]; P < 0.05). The postnatal deficient (Pre-Suff/Post-Def) group had similar resistance to the sufficient (Pre-Suff/Post-Suff) (P = 0.82) group.
Figure 7.
Effect of postnatal vitamin D supplementation and deficiency on mice pulmonary function. (A) Postnatal vitamin D rescue resulted in significant reduction in airway resistance at 8 weeks in prenatal deficient (Pre-Def/Post-Suff) mice compared with that of the persistently deficient (Pre-Def/Post-Def) littermates (P < 0.0001). Airway resistance decreased to levels similar to control (Pre-Suff/Post-Suff) animals (P = 0.34). Postnatal deficiency (Pre-Suff/Post-Def) did not affect airway resistance when compared with Pre-Suff/Post-Suff controls (P = 0.52). (B) Pulmonary compliance improved after postnatal vitamin D supplementation (Pre-Def/Post-Suff) compared with values from persistently deficient (Pre-Def/Post-Def) mice at 8 weeks (P < 0.0001). No difference in pulmonary compliance was observed between the vitamin D rescue group compared with the persistently sufficient (Pre-Suff/Post-Suff) controls (P = 0.93). Postnatal deficiency alone (Pre-Suff/Post-Def) had no effect on compliance (P = 0.25). Results are expressed as mean (±SEM).
Lung compliance similarly improved after postnatal vitamin D rescue, and became similar to that of sufficient mice (Pre-Def/Post-Def, 0.007 ± 0.001 ml/cm H2O [n = 8]; Pre-Def/Post-Suff, 0.022 ± 0.001 ml/cm H2O [n = 18]; Pre-Suff/Post-Def, 0.028 ± 0.002 ml/cm H2O [n = 12]; Pre-Suff/Post-Suff, 0.022 ± 0.001 ml/cm H2O [n = 14]; P < 0.0001; Figure 7B). A total of 8–18 animals was included in each group analysis of the PFT during the three rounds of repetition on the original groups and the two rounds on the switch groups.
Lung morphometry
Postnatal vitamin D supplementation increased RAC (Pre-Def/Post-Def, 6.6 ± 0.31 septa, [n = 7]; Pre-Def/Post-Suff, 8.0 ± 0.27 septa, [n = 8]; P < 0.01) and postnatal deficiency reduced RAC (Pre-Def/Post-Suff, 7.9 ± 0.29 septa, [n = 5]; Pre-Suff/Post-Suff, 9.10 ± 0.34 septa [n = 7]; P < 0.05). However, both postnatal supplementation and deficiency did not have any impact on MLI (Pre-Def/Post-Def, 46.84 ± 1.04 μm, [n = 7]; Pre-Def/Post-Suff, 49.23 ± 0.95 μm [n = 8]; P = 0.11; and Pre-Suff/Post-Def: 37.3 ± 1.31 μm, [n = 5]; Pre-Suff/Post-Suff: 38.64 ± 1.05 μm, [n = 7]; P = 0.44) (Figures 6C and 6D). The group comparison was repeated three times for the Pre-Def/Post-Def and Pre-Suff/Post-Suff subgroups and twice for the Pre-Def/Post-Suff and Pre-Suff/Post-Def subgroups.
Discussion
This study is the first to document that antenatal vitamin D deficiency impairs airway development in a mouse model. Affected pups showed narrowing of the trachea and thinning of the tracheal ring cartilage when compared with control pups born to vitamin D–sufficient dams. Gestational vitamin D deficiency also resulted in impaired pulmonary development with alveolar simplification. These changes manifested in abnormal lung function with increased airway resistance and decreased pulmonary compliance. Comparison of prenatal to postnatal vitamin D deficiency groups emphasized the importance of normal vitamin D levels during gestation, and that postnatal supplementation cannot entirely reverse sequelae of in utero deficiency. Our mouse model is an attractive alternative to the VDR null construct, as the mechanism of impairment physiologically represents consequences of nutritional vitamin D deficiency rather than terminal receptor blockade. These studies indicate the importance of the timing of vitamin D deficiency (and repletion) to pulmonary functional and structural outcome.
We developed a novel tracheal ultrasound technique that can be used to study airway structure in animal models with systemic or pulmonary diseases. To our knowledge, this is the first study of in vivo ultrasound to assess the mouse trachea. We used tracheobronchial casting to validate ultrasound measure, modifying techniques used in larger animals by increasing the dilution of silicone and prolonging the time of negative pressure application (see the online supplement). The airway cast was produced using a negative-pressure chamber that facilitated passive passage of silicone into the airways and avoided airway distension (26). Current use of airway ultrasound is limited to a few indications, such as percutaneous tracheostomy (27) and confirmation of endotracheal position during intubation (28). Our findings extend previous use of tracheal ultrasound in lambs to suggest a role for tracheal ultrasound in the evaluation of tracheomalacia in human newborns (29).
Reduced minimal tracheal cartilage thickness without difference in maximal thickness may point to irregularity of tracheal cartilage deposition. Similar findings have been shown in cystic fibrosis animal models (30). Cartilaginous thinning in vitamin D–deficient pups may be due to a reduction in vitamin D–induced deposition of proteoglycans by mature chondrocytes, as shown in cell culture data from rabbits (31). Reduced cartilage thickness in vitamin D deficiency has been similarly observed in human femoral cartilage (32). Thinner tracheal cartilage, therefore, may be a risk factor for tracheomalacia in the vitamin D–deficient mouse model. In humans, pliable tracheal cartilage is known to adversely affect flow dynamics during lower respiratory tract diseases (33, 34).
Our data also indicate that gestational vitamin D deficiency induces alveolar simplification. These findings support previously published epidemiologic data that have linked vitamin D–responsive genes to early lung development and asthma phenotypes (35, 36). Vitamin D3 at the cellular level functions as a growth factor for alveolar type II cells, which are important in promoting alveolarization during lung growth and development (37). Diminished alveolar septation may result in increased work of breathing and energy expenditure and greater susceptibility to lower airway diseases in young children. The improvement of RAC with postnatal supplementation and the worsening with postnatal deficiency suggests a postnatal role for vitamin D. Our results imply the significance of preventing vitamin D deficiency during pregnancy to interrupt the impact of vitamin D deficiency on childhood respiratory disease.
The increased airway resistance in pups of vitamin D–deficient dams may be partially explained by reduced tracheal diameter, as measured with ultrasound. The improvement of airway resistance after postnatal vitamin D supplementation in the absence of significant change in tracheal diameter suggests the importance of airway dimensions distal to the trachea. Altered pulmonary compliance and elastance may be due to decreased alveolarization shown with lung morphometry, changes in tissue structures (especially elastin and collagen), or heterogeneity of regional airway resistance (38).
An important finding of our study is the beneficial effect of postnatal vitamin D supplementation on lung function. Previous reports have indicated that vitamin D deficiency has deleterious effects on airway structure secondary to subepithelial collagen deposition, increased airway smooth muscle mass, and goblet cell hyperplasia (39). These defects could be partially reversed with vitamin D supplementation (40). Although vitamin D supplementation improved weight, this did not fully explain improvements in airway resistance. The positive effects of postnatal vitamin D supplementation on lung function in our model suggest possible benefit of vitamin D supplementation to mitigate the consequences of prenatal deficiency.
We acknowledge several limitations of this study, including a focus on anatomical and lung function measure without significant biochemical or mechanistic assays. The complexity and multiple endpoints of the study precluded simultaneous completion of the multiple arms of the experiment. We have detailed the experimental numbers and repetitions required for each endpoint to clarify the rigor of the experiment. Further repetition of the experiments may increase the accuracy of the study. However, the time and resources required to develop the model and do multiple studies on the different subgroups precluded repeating the studies more than two or three times. The emphasis of this study was to develop a mouse model of vitamin D deficiency that correlates with human disease. Future studies will further use this model to interrogate aberrant pathways and signaling. Our model investigates the effect of severe vitamin D deficiency on lung development compared with complete restoration. Dose–response studies, both of deficiency and repletion, will be useful complements to these data to delineate thresholds for disease and therapeutic intervention. Detailed evaluation of lung development at earlier time points will also be of interest. We evaluated tracheal structure at 7 days, but did not perform tracheal ultrasound and PFTs for technical reasons. Therefore, we used the 4-week-old time point to represent the prenatal effect of vitamin D deficiency. Postnatal development may have altered findings observed at 4 weeks. Further information is to be obtained from studying younger pups and fetuses that experience vitamin D deficiency. Improvement of lung function with vitamin D supplementation could not be explained fully by structural changes in trachea and alveoli. Therefore, we hypothesize that our findings point to abnormalities in small airway development that is distal to the trachea, but proximal to the alveoli. Lung function may be affected by functional dynamics of the airways (in addition to the structural changes), such as smooth muscle contractility, small airway malacia, and bronchial autonomic nervous system output. Finally, the potential confounding effect of vitamin D deficiency on weight is a significant consideration. We have attempted to control for this statistically, but prospective evaluation with weight-matched controls would confirm the independent action of vitamin D deficiency on tracheal rather than somatic growth.
In conclusion, prenatal vitamin D deficiency resulted in significant abnormalities in lung function and structure. Diminished tracheal diameter and tracheal cartilage thickness, and alveolar simplification, led to impaired respiratory function, as measured by increased airway resistance and decreased pulmonary compliance. These findings may explain the greater predisposition to and severity of lower airway diseases (such as bronchiolitis, asthma, and pneumonia) observed in human epidemiologic studies. Postnatal vitamin D supplementation improved lung function in our mouse model, but did not normalize tracheal abnormalities. Development of both the mouse model and measurement techniques described in this article will permit further mechanistic investigation of vitamin D–associated pulmonary disease and preclinical application of therapeutic interventions.
Acknowledgments
Acknowledgments
The authors thank the Translational Research in Normal and Disordered Development (University of Alabama at Birmingham, Birmingham, AL) for offering the infrastructure to perform the research; Sharon Samuel and Marie Taylor from Advanced Medical Imaging Research (University of Alabama at Birmingham, Birmingham, AL) for making tracheal ultrasound possible; and Patricia Lott, Dezhi Wang, and Adam Martin from the Bone Metabolism Laboratory at University of Alabama at Birmingham (Birmingham, AL) for their help with the tracheal histology studies.
Footnotes
This work was supported by a grant from the Kaul Pediatric Research Institute (A.S. and W.T.H.) and National Institutes of Health grants R01 HL092906 (N.A.), R01 HD066982 (N.A.), and U01 HL122626 (N.A.).
Author Contributions: conception and design: A.S., N.A., and W.T.H.; analysis and interpretation: A.S., N.A., K.Z., A.P.A., M.M., T.N., M.V.F., and W.T.H.; drafting the manuscript for important intellectual content: A.S., N.A., A.P.A., and W.T.H.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2014-0482OC on November 21, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Nesby-O’Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, Allen C, Doughertly C, Gunter EW, Bowman BA. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2002;76:187–192. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- 2.Bodnar LM, Simhan HN, Powers RW, Frank MP, Cooperstein E, Roberts JM. High prevalence of vitamin D insufficiency in black and white pregnant women residing in the northern United States and their neonates. J Nutr. 2007;137:447–452. doi: 10.1093/jn/137.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scholl TO, Chen X. Vitamin D intake during pregnancy: association with maternal characteristics and infant birth weight. Early Hum Dev. 2009;85:231–234. doi: 10.1016/j.earlhumdev.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Devereux G, Litonjua AA, Turner SW, Craig LC, McNeill G, Martindale S, Helms PJ, Seaton A, Weiss ST. Maternal vitamin D intake during pregnancy and early childhood wheezing. Am J Clin Nutr. 2007;85:853–859. doi: 10.1093/ajcn/85.3.853. [DOI] [PubMed] [Google Scholar]
- 5.Camargo CA, Jr, Ingham T, Wickens K, Thadhani R, Silvers KM, Epton MJ, Town GI, Pattemore PK, Espinola JA, Crane J New Zealand Asthma and Allergy Cohort Study Group. Cord-blood 25-hydroxyvitamin D levels and risk of respiratory infection, wheezing, and asthma. Pediatrics. 2011;127:e180–e187. doi: 10.1542/peds.2010-0442. [DOI] [PubMed] [Google Scholar]
- 6.Brehm JM, Celedón JC, Soto-Quiros ME, Avila L, Hunninghake GM, Forno E, Laskey D, Sylvia JS, Hollis BW, Weiss ST, et al. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am J Respir Crit Care Med. 2009;179:765–771. doi: 10.1164/rccm.200808-1361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu AC, Tantisira K, Li L, Fuhlbrigge AL, Weiss ST, Litonjua A Childhood Asthma Management Program Research Group. The effect of vitamin D and inhaled corticosteroid treatment on lung function in children. Am J Respir Crit Care Med. 2012;186:508–513. doi: 10.1164/rccm.201202-0351OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tolppanen AM, Sayers A, Granell R, Fraser WD, Henderson J, Lawlor DA. Prospective association of 25-hydroxyvitamin d3 and d2 with childhood lung function, asthma, wheezing, and flexural dermatitis. Epidemiology. 2013;24:310–319. doi: 10.1097/EDE.obo13e318280dd5e. [DOI] [PubMed] [Google Scholar]
- 9.Price PA, Buckley JR, Williamson MK. The amino bisphosphonate ibandronate prevents vitamin D toxicity and inhibits vitamin D–induced calcification of arteries, cartilage, lungs and kidneys in rats. J Nutr. 2001;131:2910–2915. doi: 10.1093/jn/131.11.2910. [DOI] [PubMed] [Google Scholar]
- 10.Mair EA, Parsons DS. Pediatric tracheobronchomalacia and major airway collapse. Ann Otol Rhinol Laryngol. 1992;101:300–309. doi: 10.1177/000348949210100403. [DOI] [PubMed] [Google Scholar]
- 11.Vijayasekaran D, Kalpana S, Ramachandran P, Nedunchelian K. Indications and outcome of flexible bronchoscopy in neonates. Indian J Pediatr. 2012;79:1181–1184. doi: 10.1007/s12098-011-0595-6. [DOI] [PubMed] [Google Scholar]
- 12.Mok Q, Negus S, McLaren CA, Rajka T, Elliott MJ, Roebuck DJ, McHugh K. Computed tomography versus bronchography in the diagnosis and management of tracheobronchomalacia in ventilator dependent infants. Arch Dis Child Fetal Neonatal Ed. 2005;90:F290–F293. doi: 10.1136/adc.2004.062604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masters IB, Chang AB. Interventions for primary (intrinsic) tracheomalacia in children. Cochrane Database Syst Rev. 2005;(4):CD005304. doi: 10.1002/14651858.CD005304.pub2. [DOI] [PubMed] [Google Scholar]
- 14.Taher YA, van Esch BC, Hofman GA, Henricks PA, van Oosterhout AJ. 1α,25-dihydroxyvitamin D3 potentiates the beneficial effects of allergen immunotherapy in a mouse model of allergic asthma: role for IL-10 and TGF-β. J Immunol. 2008;180:5211–5221. doi: 10.4049/jimmunol.180.8.5211. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen M, Trubert CL, Rizk-Rabin M, Rehan VK, Besançon F, Cayre YE, Garabédian M. 1,25-Dihydroxyvitamin D3 and fetal lung maturation: immunogold detection of VDR expression in pneumocytes type II cells and effect on fructose 1,6 bisphosphatase. J Steroid Biochem Mol Biol. 2004;89-90:93–97. doi: 10.1016/j.jsbmb.2004.03.054. [DOI] [PubMed] [Google Scholar]
- 16.Damera G, Fogle HW, Lim P, Goncharova EA, Zhao H, Banerjee A, Tliba O, Krymskaya VP, Panettieri RA., Jr Vitamin D inhibits growth of human airway smooth muscle cells through growth factor–induced phosphorylation of retinoblastoma protein and checkpoint kinase 1. Br J Pharmacol. 2009;158:1429–1441. doi: 10.1111/j.1476-5381.2009.00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brunvand L, Quigstad E, Urdal P, Haug E. Vitamin D deficiency and fetal growth. Early Hum Dev. 1996;45:27–33. doi: 10.1016/0378-3782(95)01719-4. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Zhu J, Deluca HF. Identification of the vitamin D receptor in osteoblasts and chondrocytes but not osteoclasts in mouse bone. J Bone Min Res. 2014;29:685–692. doi: 10.1002/jbmr.2081. [DOI] [PubMed] [Google Scholar]
- 19.Masuyama R, Stockmans I, Torrekens S, Van Looveren R, Maes C, Carmeliet P, Bouillon R, Carmeliet G. Vitamin D receptor in chondrocytes promotes osteoclastogenesis and regulates FGF23 production in osteoblasts. J Clin Invest. 2006;116:3150–3159. doi: 10.1172/JCI29463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lam NN, Triliana R, Sawyer RK, Atkins GJ, Morris HA, O’Loughlin PD, Anderson PH. Vitamin D receptor overexpression in osteoblasts and osteocytes prevents bone loss during vitamin D–deficiency. J Steroid Biochem Mol Biol. 2014;144:128–131. doi: 10.1016/j.jsbmb.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Coughlan CA, Chotirmall SH, Renwick J, Hassan T, Low TB, Bergsson G, Eshwika A, Bennett K, Dunne K, Greene CM, et al. The effect of Aspergillus fumigatus infection on vitamin D receptor expression in cystic fibrosis. Am J Respir Crit Care Med. 2012;186:999–1007. doi: 10.1164/rccm.201203-0478OC. [DOI] [PubMed] [Google Scholar]
- 22.Giulietti A, Gysemans C, Stoffels K, van Etten E, Decallonne B, Overbergh L, Bouillon R, Mathieu C. Vitamin D deficiency in early life accelerates type 1 diabetes in non-obese diabetic mice. Diabetologia. 2004;47:451–462. doi: 10.1007/s00125-004-1329-3. [DOI] [PubMed] [Google Scholar]
- 23.Lee D, Fanucchi MV, Plopper CG, Fung J, Wexler AS. Pulmonary architecture in the conducting regions of six rats. Anat Rec (Hoboken) 2008;291:916–926. doi: 10.1002/ar.20726. [DOI] [PubMed] [Google Scholar]
- 24.Chavhan SG, Brar RS, Banga HS, Sandhu HS, Sodhi S, Gadhave PD, Kothule VR, Kammon AM. Clinicopathological studies on vitamin D(3) toxicity and therapeutic evaluation of Aloe vera in rats. Toxicol Int. 2011;18:35–43. doi: 10.4103/0971-6580.75851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooney TP, Thurlbeck WM. The radial alveolar count method of Emery and Mithal: a reappraisal 2—intrauterine and early postnatal lung growth. Thorax. 1982;37:580–583. doi: 10.1136/thx.37.8.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Einstein DR, Neradilak B, Pollisar N, Minard KR, Wallis C, Fanucchi M, Carson JP, Kuprat AP, Kabilan S, Jacob RE, et al. An automated self-similarity analysis of the pulmonary tree of the Sprague-Dawley rat. Anat Rec (Hoboken) 2008;291:1628–1648. doi: 10.1002/ar.20771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rezende-Neto JB, Oliveira AJ, Neto MP, Botoni FA, Rizoli SB. A technical modification for percutaneous tracheostomy: prospective case series study on one hundred patients. World J Emerg Surg. 2011;6:35. doi: 10.1186/1749-7922-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gottlieb M, Bailitz JM, Christian E, Russell FM, Ehrman RR, Khishfe B, Kogan A, Ross C. Accuracy of a novel ultrasound technique for confirmation of endotracheal intubation by expert and novice emergency physicians. West J Emerg Med. 2014;15:834–839. doi: 10.5811/westjem.22550.9.22550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller TL, Zhu Y, Altman AR, Dysart K, Shaffer TH. Sequential alterations of tracheal mechanical properties in the neonatal lamb: effect of mechanical ventilation. Pediatr Pulmonol. 2007;42:141–149. doi: 10.1002/ppul.20549. [DOI] [PubMed] [Google Scholar]
- 30.Bonvin E, Le Rouzic P, Bernaudin JF, Cottart CH, Vandebrouck C, Crié A, Leal T, Clement A, Bonora M. Congenital tracheal malformation in cystic fibrosis transmembrane conductance regulator–deficient mice. J Physiol. 2008;586:3231–3243. doi: 10.1113/jphysiol.2008.150763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartz Z, Bonewald LF, Caulfield K, Brooks B, Boyan BD. Direct effects of transforming growth factor-β on chondrocytes are modulated by vitamin D metabolites in a cell maturation–specific manner. Endocrinology. 1993;132:1544–1552. doi: 10.1210/endo.132.4.8462452. [DOI] [PubMed] [Google Scholar]
- 32.Malas FU, Kara M, Aktekin L, Ersoz M, Ozcakar L. Does vitamin D affect femoral cartilage thickness? An ultrasonographic study. Clin Rheumatol. 2014;33:1331–1334. doi: 10.1007/s10067-013-2432-y. [DOI] [PubMed] [Google Scholar]
- 33.Fischer AJ, Singh SB, Adam RJ, Stoltz DA, Baranano CF, Kao S, Weinberger MM, McCray PB, Jr, Starner TD. Tracheomalacia is associated with lower FEV and Pseudomonas acquisition in children with CF. Pediatr Pulmonol. 2014;49:960–970. doi: 10.1002/ppul.22922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Baets F, De Schutter I, Aarts C, Haerynck F, Van Daele S, De Wachter E, Malfroot A, Schelstraete P. Malacia, inflammation and bronchoalveolar lavage culture in children with persistent respiratory symptoms. Eur Respir J. 2012;39:392–395. doi: 10.1183/09031936.00035111. [DOI] [PubMed] [Google Scholar]
- 35.Kho AT, Sharma S, Qiu W, Gaedigk R, Klanderman B, Niu S, Anderson C, Leeder JS, Weiss ST, Tantisira KG. Vitamin D related genes in lung development and asthma pathogenesis. BMC Med Genomics. 2013;6:47. doi: 10.1186/1755-8794-6-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hart PH, Lucas RM, Walsh JP, Zosky GR, Whitehouse AJ, Zhu K, Allen KL, Kusel MM, Anderson D, Mountain JA. Vitamin D in fetal development: findings from a birth cohort study. Pediatrics. 2015;135:e167–e173. doi: 10.1542/peds.2014-1860. [DOI] [PubMed] [Google Scholar]
- 37.Edelson JD, Chan S, Jassal D, Post M, Tanswell AK. Vitamin D stimulates DNA synthesis in alveolar type-II cells. Biochim Biophys Acta. 1994;1221:159–166. doi: 10.1016/0167-4889(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 38.Irvin CG, Bates JH. Measuring the lung function in the mouse: the challenge of size. Respir Res. 2003;4:4. doi: 10.1186/rr199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foong RE, Shaw NC, Berry LJ, Hart PH, Gorman S, Zosky GR. Vitamin D deficiency causes airway hyperresponsiveness, increases airway smooth muscle mass, and reduces TGF-β expression in the lungs of female BALB/c mice. Physiol Rep. 2014;2:e00276. doi: 10.1002/phy2.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lai G, Wu C, Hong J, Song Y. 1,25-Dihydroxyvitamin D(3) (1,25-(OH)(2)D(3)) attenuates airway remodeling in a murine model of chronic asthma. J Asthma. 2013;50:133–140. doi: 10.3109/02770903.2012.738269. [DOI] [PubMed] [Google Scholar]