Abstract
Oxidative stress resulting from inflammatory responses that occur during acute lung injury and sepsis can initiate changes in mitochondrial function. Autophagy regulates cellular processes in the setting of acute lung injury, sepsis, and oxidative stress by modulating the immune response and facilitating turnover of damaged cellular components. We have shown that mesenchymal stromal cells (MSCs) improve survival in murine models of sepsis by also regulating the immune response. However, the effect of autophagy on MSCs and MSC mitochondrial function during oxidative stress is unknown. This study investigated the effect of depletion of autophagic protein microtubule–associated protein 1 light chain 3B (LC3B) and beclin 1 (BECN1) on the response of MSCs to oxidative stress. MSCs were isolated from wild-type (WT) and LC3B−/− or Becn1+/− mice. MSCs from the LC3B−/− and Becn1+/− animals had increased susceptibility to oxidative stress–induced cell death as compared with WT MSCs. The MSCs depleted of autophagic proteins also had impaired mitochondrial function (decreased intracellular ATP, reduced mitochondrial membrane potential, and increased mitochondrial reactive oxygen species production) under oxidative stress as compared with WT MSCs. In WT MSCs, carbon monoxide (CO) preconditioning enhanced autophagy and mitophagy, and rescued the cells from oxidative stress–induced death. CO preconditioning was not able to rescue the decreased survival of MSCs from the LC3B−/− and Becn1+/− animals, further supporting the tenet that CO exerts its cytoprotective effects via the autophagy pathway.
Keywords: mesenchymal stromal cells, autophagy, mitochondria, oxidative stress, carbon monoxide
Clinical Relevance
This work supports the idea that autophagic protein–depleted mesenchymal stromal cells (MSCs) are more severely affected by exogenous oxidative stress as compared to wild-type MSCs as a result of mitochondrial dysfunction. These findings indicate the importance of autophagy in regulating the response of MSCs to oxidative stress. We also describe carbon monoxide as a novel modulator of autophagy in MSCs. These findings will provide the basis for future studies to investigate autophagy-modulated MSCs in in vivo models of disease that are associated with increased oxidative stress and help shape MSC-based therapy for acute respiratory distress syndrome and sepsis.
Investigators have begun to explore cell-based therapies for numerous disease processes, including sepsis and lung injury (1). Mesenchymal stromal cells (MSCs) are known to have immunomodulatory properties and are thought to be immune privileged, making them an attractive candidate for this type of therapy. In fact, there is currently an ongoing clinical trial evaluating the use of MSCs for acute respiratory distress syndrome (ARDS) (2).
MSCs are a heterogeneous population of cells that have been identified in numerous organs and tissues. They are plastic-adherent, spindle-shaped, multipotent adult stem cells that were originally described in the 1960s (3). Since their discovery, MSCs have been shown to play important roles in mediating the immune response and homing to sites of injury to contribute to tissue repair (4). It appears that a critical property of MSCs is regulation of the immune response. Our laboratory and other groups have demonstrated that MSCs improve outcomes in a murine sepsis model by modulating the immune response (5). In addition to sepsis, other studies have demonstrated the beneficial effects of MSCs given in lung injury, myocardial infarction, tissue injury, graft-versus-host disease, and autoimmune disorders (6). Despite their potential as a cell-based therapy, a limitation to the use of MSCs in clinical applications is their poor viability at the site of injury (7). This may be due to the harsh microenvironment into which they are introduced.
The disease processes in which MSCs are being tested for transplantation, such as ARDS, are characterized by highly oxidative microenvironments. This results in oxidative stress and the secondary cellular production of reactive oxygen species (ROS). In this context, ROS refers mainly to hydroxyl radical, superoxide anion, and hydrogen peroxide (H2O2) (8). In MSCs, excessive ROS has been shown to directly damage cell membranes, protein, and DNA, promote cell senescence, compromise cell function, and threaten cell survival (9). ROS have also been shown to decrease MSC cell adhesion, migration, and proliferation, and to impact the mitochondrial function of MSCs (10). As a result, an oxidizing exogenous environment likely plays a role in controlling the immune-regulatory function and survival of MSCs.
One of the protective processes that could explain MSC-mediated immunomodulation and response to oxidative stress is autophagy. The process of autophagy is tightly linked with normal immune function. Autophagy also regulates cellular function under conditions of oxidative stress. Autophagy regulates immune responses by facilitating the turnover of damaged proteins and organelles through a lysosome-dependent degradation pathway (11). Selective sequestration and subsequent degradation of dysfunctional mitochondria is known as mitochondrial autophagy or mitophagy (12). In the absence of autophagy and mitophagy, damaged mitochondria accumulate oxidized macromolecules and generate excessive ROS, often leading to release of mitochondrial DNA into the cytoplasm of cells. This can result in further oxidative damage and, ultimately, activation of cell death (13). Autophagy and mitophagy play a role in stabilizing the cell’s functional mitochondrial population (14). In addition, it has been reported that ROS induce autophagy, and that autophagy serves to reduce oxidative damage (15). As a result, autophagy has a significant impact on the pathogenesis of many diseases, and defects in autophagy have been associated with systemic and lung pathology (16).
The autophagy pathway involves the concerted action of evolutionarily conserved gene products involved in the initiation of autophagy, elongation and closure of the autophagosome, and lysosomal fusion (17). Among the numerous autophagy-related genes that have been identified, beclin 1 (Becn1), plays a critical regulatory function in the initiation of the autophagic pathway (18). It is important for the nucleation of autophagosomes (19). The biallelic loss of Becn1 results in early embryonic lethality (20). The conversion of microtubule-associated protein-1 light chain 3B (LC3B) from LC3B-I to LC3B-II represents another major step in autophagosome formation (21). Damaged mitochondria can be sequestered by autophagosomes and degraded before they trigger cell death. The phosphatase and tensin homolog–induced putative kinase 1 (PINK) 1 pathway is important in regulating mitophagy in cells. PINK1 is found at very low levels on intact mitochondria, because it is rapidly imported and cleaved by mitochondrial proteases. Upon collapse of the mitochondrial membrane potential (MMP), PINK1 accumulates on the outer mitochondrial membrane and targets the mitochondria for autophagic degradation (12). Despite the important functions autophagy plays in modulating cell survival, very little is known about the role of autophagy in MSCs.
Autophagic pathways can be activated by different stimuli, including starvation, DNA damage, ROS, and multiple pharmaceutical agents (22). Based on our prior studies in MSCs, we chose to investigate carbon monoxide (CO) as a regulator of autophagy in MSCs. This low-molecular-weight diatomic gas that is endogenously produced (23) has been shown to have cytoprotective effects when applied at low doses in animal models of inflammation (24). In addition, when cultured human epithelial cells were exposed to CO, they showed evidence of increased autophagy (15). However, the effect of CO administration on the level of autophagy in MSCs has never been reported.
In this study, we describe the effect of autophagy protein deficiency on the response of MSCs to exogenous oxidative stress and the impact on their mitochondrial function. We demonstrate that CO can increase autophagy and mitophagy in wild-type (WT) MSCs, and that this protects them from oxidative stress–induced injury.
Materials and Methods
See the online supplement for further Materials and Methods, including chemicals, animals, isolation, and characterization of murine adipose-derived MSCs, CO exposure, and statistical analysis.
Treatment with H2O2
MSC survival in response to oxidative stress was measured after treatment with various concentrations of H2O2. A dose–response curve was generated to determine dose amount. Timing of H2O2 treatment was based upon the assay being performed. For lactate dehydrogenase (LDH) treatment, an 8-hour time point was chosen, as the half-life of LDH in media is 8–9 hours. For survival experiments, 24 hours was used. For mitochondrial function, a time point before death was desired, so these treatments were performed at 6 hours.
3-(4,5-Dimethylthiazol-2-yl)2,5-Diphenyl-Tetrazolium Bromide Cell Viability Assay
Cell death was quantified by the 3-(4,5-dimethylthiazol-2-yl)2,5-diphenyl-tetrazolium bromide (thiazolyl blue tetrazolium bromide; MTT) assay (25). The reduction of MTT to produce a dark blue formazan product was assayed, corresponding to viable cells. MTT was added to each well 24 hours after the beginning of the insult. After a 2-hour incubation, media were removed, and formazan crystals were solubilized in DMSO. The formation of formazan was quantified by measuring absorbance using a microplate reader (Molecular Devices, Sunnyvale, CA) at 590 nm. The percentage of cell viability was determined by normalizing the optical density value of the treated group to the corresponding control group.
Tetraethylbenzimidazolylcarbocyanine Iodide Assay for MMP
MMP was measured using the tetraethylbenzimidazolylcarbocyanine iodide (JC-1) assay (Life Technologies, Carlsbad, CA). Cells were treated with 2 μmol/L JC-1 dye for 20 minutes. A positive control group was treated with 50 μmol/L carbonyl cyanide 3-chlorophenylhydrazone (CCCP) (a known uncoupling agent) for 5 minutes before the addition of the JC-1 dye. Cells were harvested and analyzed on a flow cytometer (BD Biosciences, San Jose, CA). The ratio of green (depolarization) to red (polarized) fluorescence, indicating loss of MMP, was calculated.
ATP Assay
Cells were plated and then lysed, and intracellular ATP was measured using the luminescent ATPlite assay system (Perkin Elmer, Santa Clara, CA) using a luminescent plate reader (Perkin Elmer).
Measurement of Mitochondrial ROS Generation
After respective treatments, cells were incubated with the MitoSOX red mitochondrial oxidant indicator (Invitrogen, Carlsbad, CA) at a final concentration of 5 μmol/L. After a 30-minute incubation, cells were washed with PBS, and then harvested and MitoSox fluorescence analyzed on a flow cytometer. MitoSOX is very selective for the detection of superoxide (the main mitochondrial ROS) in the mitochondria of live cells. It is readily oxidized by superoxide, but not by other ROS- or reactive nitrogen species-generating systems (26).
Fluorescent Microscopy for Green Fluorescent Protein-LC3B
MSCs harvested from the green fluorescent protein (GFP)-LC3 mice were visualized by fluorescent light microscopy, and digital images were acquired for analysis. The percentage of cells exhibiting punctated GFP-LC3 fluorescence was calculated relative to all GFP-positive cells. Images from 15 representative 20× fields were used for analysis.
Confocal Microscopy
Images were acquired using the Zeiss LSM700 laser scanning confocal microscope equipped with the charge coupled device camera (Zeiss, Dublin, CA). Optical Z sections (ΔZ = 1.5 μm) spanning the sample thickness were projected into a single plane for each color channel and merged. Pixel size was 0.14 μm. Zen 2009 software (Zeiss) was used to compile images. Original magnification was ×40.
Results
WT MSCs and MSCs Deficient in Autophagic Proteins Are Phenotypically Similar
MSCs were harvested from the adipose tissue of WT or autophagy protein–deficient (LC3B−/− or Becn1+/−) mice. The MSCs were phenotyped by their ability to adhere to plastic, to express specific cell surface markers, and to demonstrate multipotent differentiation potential (see Figures E1 and E2 in the online supplement). WT MSCs and autophagic protein–depleted MSCs showed expression of mesenchymal markers, including CD90.2, CD73, CD105, CD29, CD44, and CD140b (Figure E1). They also expressed stem cells antigen-1, but not the receptor of stem cell factor, tyrosine-protein kinase Kit (c-kit or CD117). There was no evidence for expression of hematopoietic lineage markers, such as CD45 and CD11b. A low percentage of cells also expressed major histocompatibility complex II. There was no difference in the cell surface markers of WT MSCs and MSCs deficient in autophagic proteins. In addition, both WT and autophagic protein–depleted MSCs were able to differentiate in vitro into osteoblasts, adipocytes, and chondrocytes (Figure E2).
Autophagic Protein–Depleted MSCs Show Increased Susceptibility to Oxidative Stress–Induced Cell Death
As oxidative stress plays a large part in sepsis, lung injury, and other disease processes in which MSCs may potentially be given as a therapeutic, we wanted to determine the effect of oxidative stress on WT and autophagy protein–depleted MSCs. The ROS that are generated by mitochondrial respiration, including H2O2, are potentially potent inducers of oxidative damage (27). Therefore, we used exogenous H2O2 as a source of oxidative stress, and exposed WT MSCs to various concentrations of H2O2 for 24 hours to generate a dose–response curve (Figure E3). Cell survival was measured using the tetrazolium MTT assay. This dose–response curve is shown in Figure E3. After 24 hours, 50% cell death in WT MSCs is achieved at a concentration between 125 and 150 μmol/L. As a result, we used these concentrations for the remainder of our experiments.
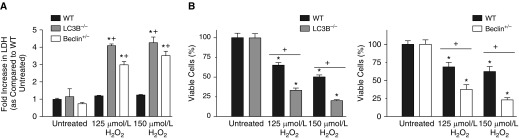
One method of assaying loss of membrane integrity is measuring release into the medium of the cytosolic enzyme, LDH. It has been shown to be an accurate marker of cell death (25). WT and autophagic protein–depleted MSCs were treated with H2O2 for 8 hours. This time point was chosen, as the half-life of LDH in media is 8–9 hours (28). When compared with untreated cells, the WT cells treated with either 125 or 150 μmol/L H2O2 did not have a significant increase in LDH release into the media (Figure 1A). These cells exhibit some cell death at the 24-hour time point (Figure E3), but, at 8 hours into treatment (time point chosen based on LDH half-life), they did not have significant LDH release. However, MSCs deplete in autophagic proteins, LC3B and BECN1, had a significant LDH release compared with untreated cells after being treated with either 125 or 150 μmol/L H2O2. In addition, when compared with the WT cells treated with the same dose of H2O2, the MSCs deplete in autophagic proteins, LC3B and BECN1, had increased LDH release after 8-hour treatment with 125 and 150 μmol/L H2O2 (Figure 1A).
Figure 1.
Autophagy protein–depleted mesenchymal stromal cells (MSCs) show increased susceptibility to oxidative stress–induced cell death. (A) Passage 3 MSCs were plated in 96-well plates. At 24 hours after plating, cells were treated with different concentrations of hydrogen peroxide (H2O2) for 8 hours. A control group was left untreated. After H2O2 treatment, media from each sample was aliquoted. The Sigma TOX7 assay kit (Sigma Aldrich, St. Louis, MO) was used to quantify lactate dehydrogenase (LDH). Data are shown as the fold increase in the measured LDH of the H2O2 groups as compared with wild-type (WT) untreated cells and are expressed as mean ± SEM (n = 3). *P < 0.001 as compared with WT untreated; +P < 0.001 as compared with WT H2O2 of the same concentration. (B) Passage 3 MSCs were plated in 12-well plates, and 24 hours after plating, cells were treated with different concentrations of H2O2 for 24 hours. A control group was left untreated. 3-(4,5-dimethylthiazol-2-yl)2,5-diphenyl-tetrazolium bromide (MTT) assay for living cells was performed. MTT was quantified by reading absorbance at 590 nm. Data are shown as MTT absorbance compared with the absorbance of the untreated cells of each group and expressed as a percentage. Data are expressed as mean ± SEM (n = 3–4). *P < 0.001 as compared with its own untreated group. +P < 0.001 as compared with WT H2O2 of the same concentration. LC3B, microtubule-associated protein-1 light chain 3B.
We also used another method for assaying cell death: the MTT assay. The MTT assay quantifies cell viability by measuring the amount of formation of formazan product formed by the reduction of MTT (25). WT MSCs and MSCs from the LC3B−/− and Becn1+/− animals were treated with 125 and 150 μmol/L H2O2 for 24 hours. The MTT assay was then performed to determine cell survival (Figure 1B). WT MSCs did show cell death after exposure to 125 or 150 μmol/L H2O2. After treatment with 125 μmol/L H2O2, 68.8 (±6.3)% of the WT cells survived at 24 hours. However, the MSCs depleted in autophagic proteins, LC3B and BECN1, were more susceptible to H2O2 induced cell death as compared with WT cells. After treatment with 125 μmol/L H2O2, MSCs from the LC3B−/− and Becn1+/− animals showed decreased survival (33.3 ± 1.4% and 37.5 ± 6.5%, respectively) as compared with WT MSCS (Figure 1B). This same pattern was seen when cells were treated with 150 μmol/L H2O2. As a result, for future experiments, we focused on one concentration of H2O2: 125 μmol/L.
Autophagic Protein–Depleted MSCs Show Increased Loss of MMP under Oxidative Stress
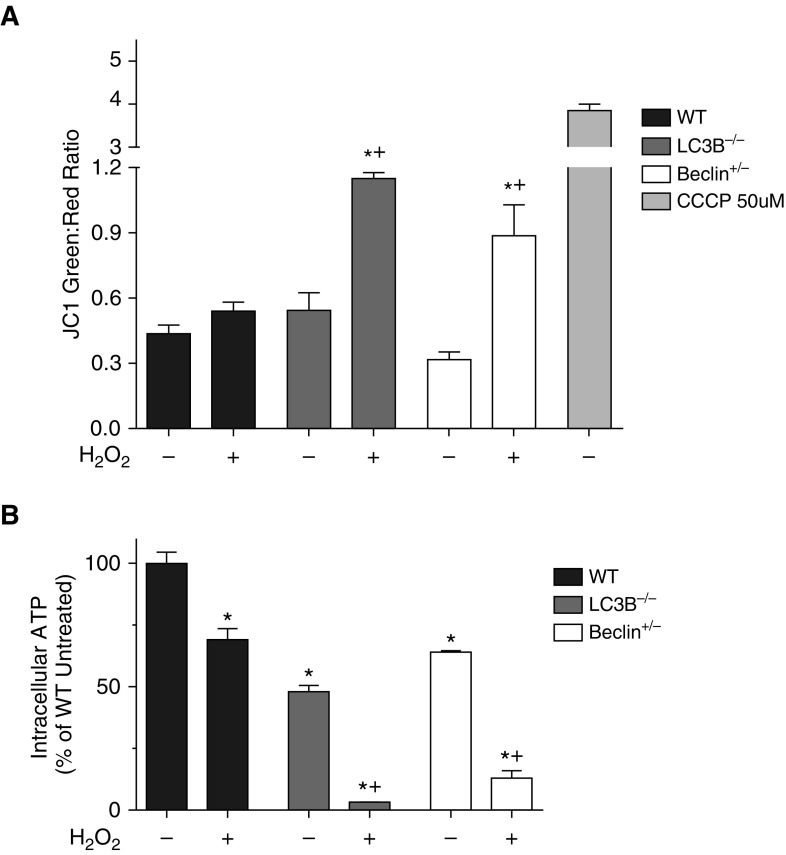
As the reduction of MTT is thought to mainly occur in the mitochondria (29, 30), we wanted to evaluate the mitochondrial function in the WT MSCs and MSCs deficient in autophagic proteins, LC3B and BECN1, in response to oxidative stress. The main event in mitochondrial signaling and control of cell death is loss of MMP. To study mitochondrial function before cell death, assays evaluating mitochondria were performed after 6 hours of 125 μmol/L H2O2 treatment. The JC-1 assay is a well accepted method of studying MMP (31).
JC-1 is a cationic dye that accumulates in energized mitochondria. At low concentrations (due to low MMP), JC-1 is predominantly a monomer that yields green fluorescence. At high concentrations (due to high MMP), the dye aggregates yielded a red-to-orange-colored emission that can be studied using flow cytometry. Using the JC-1 assay, we were able to study MMP after 6 hours of 125 μmol/L H2O2 treatment. At this dose of H2O2, there was no significant change in the MMP of WT cells after 6 hours. In contrast, both the MSCs deplete in autophagic proteins LC3B and BECN1 had increased loss of MMP after the same treatment with H2O2 (Figure 2A).
Figure 2.
Autophagic protein–depleted MSCs show increased loss of mitochondrial membrane potential (MMP) and impaired intracellular ATP generation under oxidative stress. (A) Passage 3 MSCs were plated in 6-cm dishes and, 24 hours after plating, cells were treated with 125 μmol/L H2O2 for 6 hours. A control group was left untreated. Cells were then treated with 2 μmol/L tetraethylbenzimidazolylcarbocyanine iodide (JC-1) dye for 20 minutes. A positive control group was treated with 50 μmol/L carbonyl cyanide 3-chlorophenylhydrazone (CCCP) (a known MMP disruptor) for 5 minutes before the addition of the JC-1 dye. Cells were then harvested and analyzed on a flow cytometer. Data are shown as the ratio of green to red fluorescence, indicating loss of MMP. They are expressed as mean ± SEM (n = 3). *P < 0.05 as compared with its own untreated group; +P < 0.05 versus WT H2O2. (B) Passage 3 MSCs were plated in 96-well plates. At 24 hours after plating, cells were treated with 125 μmol/L H2O2 for 6 hours. A control group was left untreated. After H2O2 treatment, cells were lysed and intracellular ATP was measured using the luminescent ATPlite assay system. Data are expressed as the amount of luminescence of each group compared with the luminescence of the WT untreated cells and expressed as a percentage. Data are shown as mean ± SEM (n = 3). *P < 0.001 as compared with WT untreated; +P < 0.001 as compared with WT H2O2.
MSCs Deficient in Autophagic Proteins Show Impaired Intracellular ATP Generation under Oxidative Stress
The consequences of loss of MMP include depletion of ATP. This led us to study the levels of ATP in WT MSCs and MSCs from the LC3B−/− and Becn1+/− animals. After 6-hour H2O2 treatment, cells were lysed and intracellular ATP was measured. The WT MSCs did show a significant decrease in ATP level compared with untreated cells. However, when compared with WT MSCs treated with H2O2 (69 ± 2.6%), the MSCs from LC3B−/− and Becn1+/− animals had a much lower level of intracellular ATP after H2O2 treatment (3.3 ± 0.3% and 13 ± 3% ATP, respectively) (Figure 2B). It is interesting to note that the MSCs deficient in autophagic proteins, LC3B and BECN1, had lower ATP levels, even at baseline, when compared with the WT untreated MSCs (48 ± 2.6% and 64 ± 1% of WT ATP level, respectively).
MSCs Deficient in Autophagic Proteins Show Increased Mitochondrial ROS under Oxidative Stress
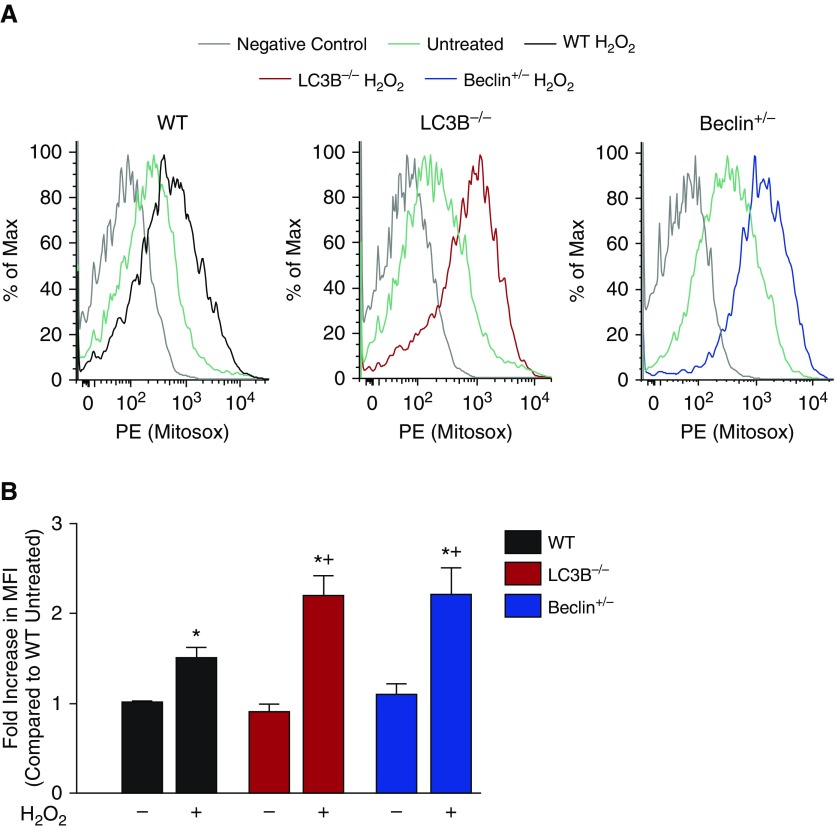
Another consequence of loss of MMP includes increased generation of ROS. As a result, we wanted to study the mitochondrial ROS levels in the WT MSCs and MSCs deficient in autophagic proteins. MitoSOX Red is a fluorogenic dye developed and validated for detection of mitochondrial superoxide anion radical (O2−) that can be detected by flow cytometry (15). FACS analysis demonstrated a slight increase of the fluorescence intensity of MitoSOX Red in the WT MSCs after treatment with H2O2. The MSCs from the LC3B−/− and Becn1+/− animals showed a much greater increase in intensity of MitoSOX after H2O2 treatment when compared with autophagy protein–deficient cells not receiving H2O2 and WT MSCs treated with the same concentration of oxidant (Figure 3). Figure 3A shows representative histograms, and Figure 3B shows the composite of several experiments. These data indicate that H2O2 exposure in the MSCs deficient in autophagic proteins leads to increased mitochondrial ROS production compared with WT cells.
Figure 3.
MSCs deficient in autophagic proteins show increased mitochondrial reactive oxygen species under oxidative stress. Passage 3 MSCs were plated in 6-cm dishes and, 24 hours after plating, cells were treated with 125 μmol/L H2O2 for 6 hours. A control group was left untreated. After treatment, cells were incubated with the MitoSOX red mitochondrial superoxide indicator. Cells were then harvested and analyzed on a flow cytometer. (A) Representative samples are shown as histograms. Phycoerythrin (PE) fluorescence represents MitoSox. (B) The composite data are expressed as the increase in mean fluorescence intensity (MFI) as compared with WT untreated. Data are expressed as mean ± SEM (n = 3). *P < 0.001 as compared with WT untreated; +P < 0.001 as compared with WT H2O2.
MSCs Deficient in Autophagic Proteins Are Rescued from Cell Death by a Mitochondrial-Specific Antioxidant
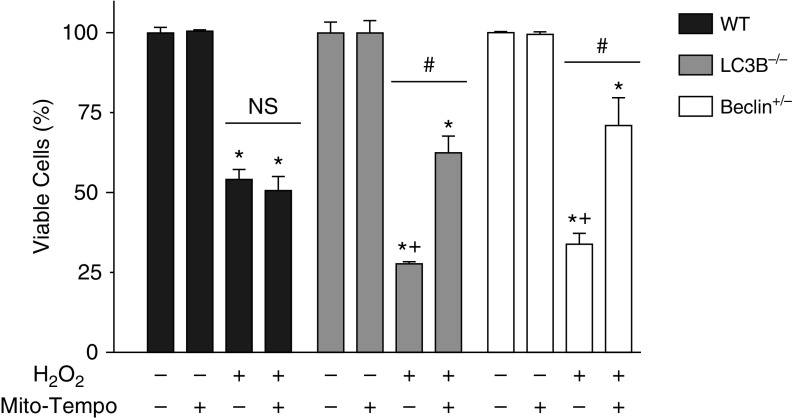
We hypothesized that the mitochondrial ROS production of MSCs deficient in autophagic proteins, LC3B and BECN1, would be significant enough to cause the increased cell death of these autophagy protein–deficient MSCs exposed to H2O2. To test this hypothesis, we pretreated WT MSCs and MSCs from the LC3B−/− and Becn1+/− mice with the mitochondria-targeted triphenylphosphonium-conjugated antioxidant, MitoTEMPO. We then exposed cells to 24 hours of 125 μmol/L H2O2 and used the MTT assay to assess survival. Consistent with the results in Figure 1B, we again saw that the MSCs deficient in autophagic proteins had decreased survival (27 ± 2.7% for MSCs deficient in LC3B and 33 ± 3.4% for MSCs deficient in BECN1) as compared with WT cells (55 ± 3%) after treatment with 125 μmol/L H2O2 (Figure 4). When the WT cells were pretreated with MitoTEMPO, there was no change in their viability after H2O2 treatment. In contrast, when the MSCs from the LC3B−/− and Becn1+/− animals were pretreated with MitoTEMPO, they were protected from cell death, and cell viability increased to a level comparable to WT cells (62 ± 5.2% for LC3B−/− and 70 ± 7.1% for Becn1+/− MSCs) (Figure 4). As pretreatment of WT MSCs with MitoTEMPO did not result in a change in cell viability after H2O2 treatment, we chose to investigate the effect of a general ROS scavenger, N-acetylcysteine (NAC) on WT cells. When pretreated with NAC, the WT cells had full rescue in viability to the level of the WT MSCs not treated with H2O2 (Figure E4). MSCs from the LC3B−/− and Becn1+/− animals showed partial protection from cell death with pretreatment with NAC (Figure E4).
Figure 4.
MSCs deficient in autophagic proteins are rescued from cell death by a mitochondrial-specific antioxidant. Passage 3 MSCs were plated in 12-well plates and, 24 hours after plating, cells were pretreated with 500 μmol/L MitoTEMPO, a mitochondrial-specific antioxidant, or control substance for 1 hour. Cells were then treated with 125 μmol/L H2O2 for 24 hours. A control group was left untreated. MTT assay for living cells was performed. MTT was quantified by reading absorbance at 590 nm. Data are shown as MTT absorbance compared with the absorbance of the untreated cells of each group and expressed as a percentage. Data are expressed as mean ± SEM (n = 3–4). *P < 0.001 as compared with its own untreated group; +P < 0.001 as compared with WT H2O2; #P < 0.001 compared with its own H2O2 group without MitoTEMPO. NS, not significant.
Oxidative Stress Increases Autophagy and Mitophagy in Cultured Murine WT MSCs
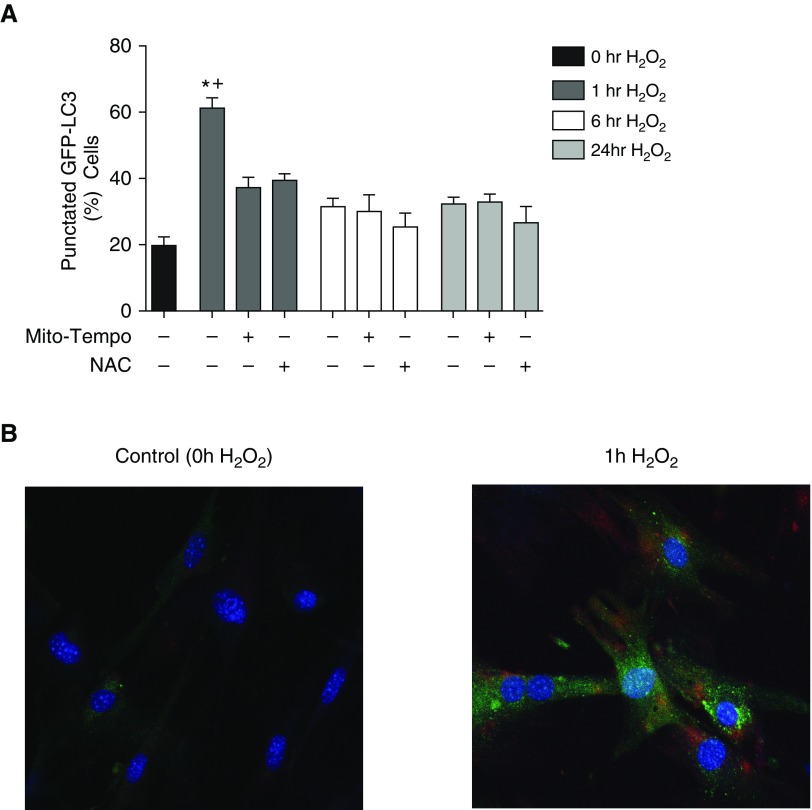
As mild oxidative stress is known to increase autophagy in cells (32), we wanted to study the effect of H2O2 treatment on autophagy in WT MSCs. To study the effect of H2O2 treatment on WT MSCs, we employed MSCs harvested from the well described GFP-LC3 reporter mice (33). These MSCs allowed us to examine a time course of the level of autophagy in MSCs in response to H2O2 treatment. H2O2 treatment induced the formation of GFP-LC3 puncta (Figure 5), a powerful marker of autophagosome formation (34), in a time-dependent manner (Figure 5A). Although there was an initial increase in autophagy 1 hour after treatment with H2O2, the level of autophagy quickly returned to baseline with more prolonged H2O2 treatment (Figure 5A). Quantification of these results revealed a threefold increase in the percentage of cells with GFP puncta after 1-hour H2O2 treatment (Figure 5A). To identify whether general ROS or mitochondrial ROS was contributing to the autophagy response, cells were pretreated with NAC or MitoTEMPO for 1 hour before H2O2 treatment. The cells that were pretreated with an antioxidant did not show an increase in autophagy after H2O2 treatment (Figure 5A).
Figure 5.
Oxidative stress increases autophagy and mitophagy in cultured murine WT MSCs. MSCs harvested from the LC3B–green fluorescent protein (GFP) reporter mice were plated. At 24 hours after plating, the cells were pretreated with 500 μmol/L MitoTEMPO, 5 mmol/L N-acetylcysteine (NAC), or vehicle for 1 hour. They were then exposed to 125 μmol/L H2O2 for 0 (control), 1, 6, or 24 hours. Cells were visualized by fluorescence microscopy. (A) The percentage of cells exhibiting punctated GFP-LC3 fluorescence was calculated relative to all GFP-positive cells. Cells were counted from 15 randomly selected fields per condition. Data are presented as mean ± SEM. *P < 0.001 as compared with 0 hours of H2O2 and +P < 0.001 compared with 1 hour H2O2 with MitoTEMPO or NAC. (B) A representative field is shown after 1 hour of H2O2 exposure. Confocal imaging of phosphatase and tensin homolog–induced putative kinase 1 staining (red) is shown, along with the puncta formation (green), and compared with a cell exposed to 0 hours of H2O2. The cells were costained with 4′,6-diamidino-2-phenylindole (blue) to identify nuclei. Original magnification, ×40.
As loss of MMP and increased production of mitochondrial ROS are believed to represent initiating signals for mitophagy (32), we wanted to evaluate mitophagy in the WT MSCs after H2O2 treatment. As the increase in autophagy occurred at the 1-hour time point, we exposed MSCs harvested from the GFP-LC3 reporter mouse to 1 hour of H2O2 and then used immunohistochemistry to evaluate the expression of PINK1. As previously demonstrated in Figure 5A, treatment with 1 hour of H2O2 induced the formation of GFP-LC3 puncta. In addition, the MSCs treated with 1 hour of H2O2 showed increased staining for PINK1, a marker of mitophagy (Figure 5B).
CO Increases Autophagy and Mitophagy in Cultured Murine WT MSCs
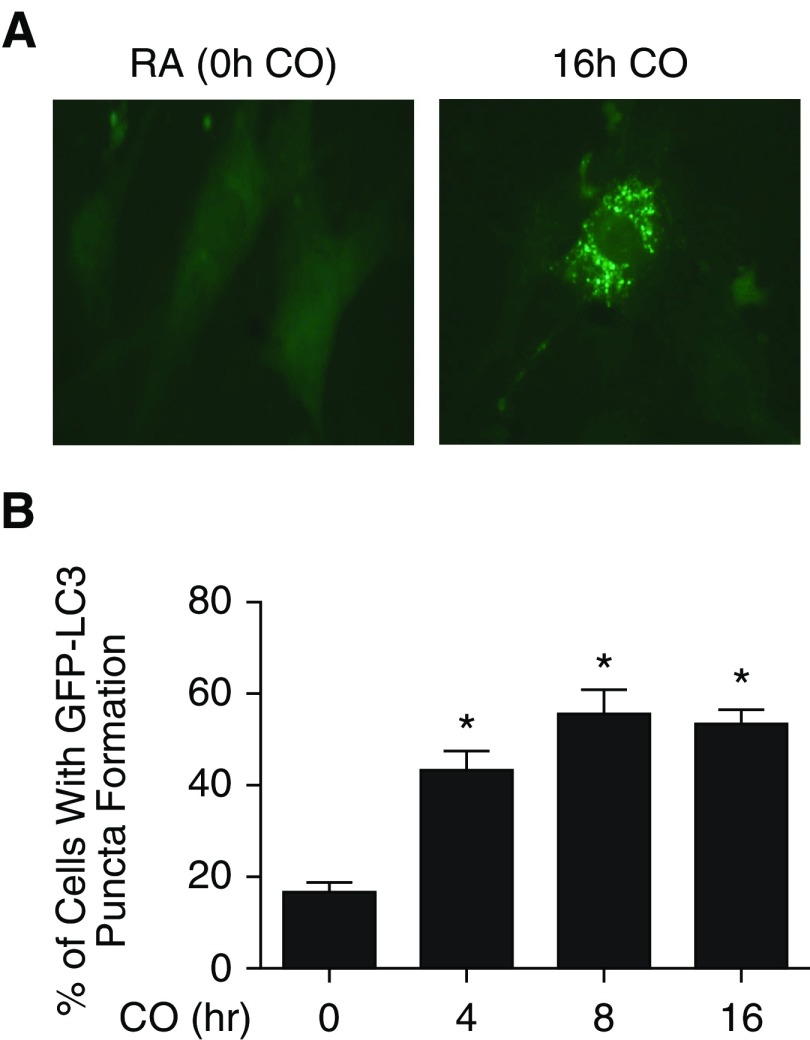
We then wanted to study whether increasing autophagy in MSCs in a more sustained way than with H2O2 treatment would increase survival under oxidative stress–induced cell death. We chose to investigate CO as a potential regulator of autophagy in MSCs, because it has been shown to have cytoprotective effects when applied at low doses in animal models of acute inflammation and lung injury (35). In addition, low-dose CO exposure induces biochemical markers of autophagy in vivo and in cultured epithelial cells (15). To study the effect of CO preconditioning on MSCs, we employed MSCs harvested from GFP-LC3 reporter mice (33). These MSCs allowed us to examine a time course of the level of autophagy in MSCs in response to CO (250 ppm) exposure. CO exposure induced the formation of GFP-LC3 puncta (Figure 6A) in a time-dependent manner (Figure 6B). Although there was increase in autophagy as early as 4 hours after exposure to CO, the peak increase from baseline occurred at 8 hours after exposure, and persisted with 16-hour exposure. Quantification of these results revealed a threefold increase in the percentage of cells with GFP puncta after 8- and 16-hour CO exposure (Figure 6B). In addition, MSCs treated with 16 hours of CO showed increased staining for PINK1, a marker of mitophagy (Figure E5).
Figure 6.
Carbon monoxide (CO) increases autophagy in cultured murine MSCs. MSCs harvested from the LC3B-GFP reporter mice were exposed to 250 ppm CO for 0–16 hours. Cells were visualized by fluorescence microscopy. (A) A representative cell is shown after CO exposure to illustrate puncta formation and compared with a cell exposed to room air (RA). Original magnification, ×20. (B) The percentage of cells exhibiting punctated GFP-LC3 fluorescence was calculated relative to all GFP-positive cells. Cells were counted from 15 randomly selected fields per condition. Data are presented as mean ± SEM. *P < 0.001 as compared with 0 hours of CO.
CO Pretreatment Rescues WT, but Not MSCs Deficient in Autophagic Proteins, from Oxidative Stress–Induced Cell Death
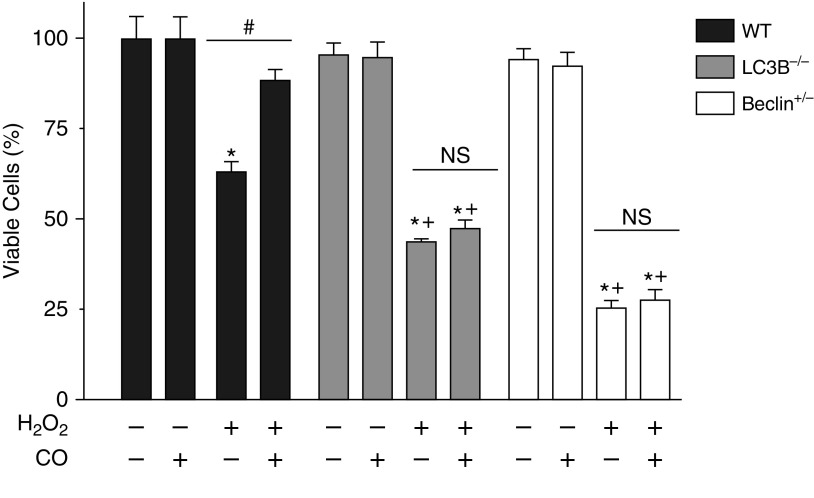
Finally, we tested our hypothesis that MSCs with induced autophagy would have increased survival under oxidative stress. WT and autophagy protein–deficient MSCs exposed to ambient air or preconditioned with CO (250 ppm for 16 h) were treated with 125 μmol/L H2O2. As seen previously (Figures 1B and 4), although WT cells had a level of cell death, the MSCs deficient in autophagic proteins had decreased survival as compared with WT cells. The WT MSCs preconditioned with CO had significantly increased survival (88.3 ± 1.3%) under oxidative stress compared with WT cells exposed to ambient air (63 ± 2%) (Figure 7). In fact, there was no significant difference in their survival as compared with untreated cells. In contrast, when we pretreated the MSCs from the LC3B−/− and Becn1+/− animals with CO, we were unable to produce improvements in their survival under oxidative stress.
Figure 7.
CO pretreatment does not rescue MSCs deficient in autophagic proteins from oxidative stress–induced cell death. Passage 3 MSCs were plated in 12-well plates. At 24 hours after plating, cells were pretreated with 250 ppm CO for 16 hours. Cells were then treated with 125 μmol/L H2O2 for 24 hours. A control group was left untreated. MTT assay for living cells was performed. MTT was quantified by reading absorbance at 590 nM. Data are shown as MTT absorbance compared with the absorbance of the untreated cells of each group and expressed as a percentage. Data are expressed as mean ± SEM (n = 3–4). *P < 0.001 as compared with its own untreated group; +P < 0.001 as compared with WT H2O2; #P < 0.001 compared with its own H2O2 group without CO.
Discussion
Mitochondria play an important role in many cellular processes, including production of ATP, fatty acid oxidation, control of cell death, and regulation of cytosolic Ca+2 homeostasis (36). Mitochondria are the primary source of ROS production and also a target of oxidative stress (33). This study showed a fundamental difference in the mitochondria of WT MSCs and MSCs deficient in autophagic proteins when exposed to oxidative stress. MSCs from autophagy protein–deficient animals showed increased mitochondrial dysfunction under oxidative stress.
This study used exogenous H2O2 as a source of oxidative stress. H2O2 has been demonstrated in numerous cells to cause cell death by both apoptosis and necrosis (37). Although there are many shared pathways between autophagy and cell death, it is believed that autophagy has a survival role in halting the occurrence of death after oxidative exposure. This was seen in our MSCs, as autophagy protein depletion led to increased death after exposure to oxidative stress. However, autophagy can be a defense mechanism or a programmed cell death pathway, depending on the conditions (38).
We used a mitochondrial specific antioxidant, MitoTEMPO, to further support the idea that oxidant-induced mitochondrial dysfunction plays an important role in the increased death response of autophagic protein–depleted MSCs under conditions of oxidative stress. MitoTEMPO has been identified to have a preferential mitochondrial location for its ROS-scavenging effect (39). The fact that autophagic protein–depleted MSCs could be rescued from cell death by MitoTEMPO supports the strong impact that mitochondrial ROS and mitochondrial dysfunction have on the survival of these cells under oxidative stress. WT cells had minimal effects from MitoTEMPO pretreatment, likely because they only had mild mitochondrial dysfunction with exposure to H2O2 treatment (no change in MMP, minimal decrease in ATP, and minimal increase in mitochondrial ROS). However, WT MSC survival after exposure to oxidative stress was increased by a nonspecific antioxidant, NAC (Figure E4). Intracellular ROS generation consists of membrane, cytosolic, and/or mitochondrial sources. Our results suggest that membrane and cytosolic sources play a role in WT MSC injury to oxidative stress at these doses of H2O2, whereas mitochondrial ROS has a significant impact on the survival of autophagy protein–depleted MSCs.
Our findings also suggest that basal adenine nucleotide turnover may be different in the mitochondria of WT MSCs and MSCs deficient in autophagic proteins. At baseline, the MSCs from the LC3B−/− and Becn1+/− mice had lower intracellular ATP as compared with the untreated WT cells (Figure 2B). The levels in MSCs deficient in autophagic proteins, LC3B and BECN1, were 40–60% that of WT ATP levels. This decrease in ATP was not enough to cause cell death, as ATP levels need to drop significantly lower than 50% to cause cell death (40). However, when a stressor, such as H2O2, was added to the MSCs deficient in autophagic proteins, ATP levels further plummeted and caused cell death. Disruption of MMP alone cannot explain a decrease in ATP in the autophagic protein–depleted MSCs. Mitochondrial ATP generation is a crucial cellular function, so mitochondria have evolved powerful feedback loops to maintain it in the face of dysfunction and loss of MMP. Mitochondria generate ATP by using the proton electrochemical gradient potential, which is made up of the MMP and the mitochondrial pH gradient. As a result, MMP is only a part of the bioenergetic driving force for ATP production and cannot be used to make direct inferences regarding ATP production (41). However, taken together, MMP, as well as ATP production and mitochondrial ROS levels, provide valuable information about mitochondrial function. In this study, these assays demonstrated that autophagy protein–deficient MSCs had significant mitochondrial dysfunction under oxidative stress.
In MSCs deficient in autophagic proteins in control conditions, there was only a difference in ATP. This finding has been seen in other cell lines. T cells harvested from patients with rheumatoid arthritis had lower levels of autophagy and ATP (42). There are several explanations for the relationship between autophagy and ATP. The first is that depletion of ATP might also be due to inhibition of ATP synthesis (40). For example, retinal pigment epithelium cells from aged donors have lower levels of autophagy than their younger controls, and there is a decline in the activity of enzymes involved in ATP synthesis in these cells (40). Another possibility is that autophagic degradation of mitochondria, other organelles, and proteins can yield significant substrate to provide energy to cells (43). There were increased levels of glycolysis in the macrophages from mice deficient in autophagic protein (autophagy-related protein 7) suggesting an increased requirement for ATP to compensate for ATP that is normally produced from autophagy (44).
The LC3B−/− mice display the ability to form residual autophagosomes. This suggests that LC3B function is compensated by other endogenous proteins, as there are over 13 mammalian autophagy proteins, in regard to basal homeostatic processes. As the biallelic loss of BECN results in embryonic lethality, we used MSCs from BECN1 heterozygous knockout mice. These mice have previously been validated to have impaired autophagy (18, 20). Both LC3B and BECN1 represent core autophagy proteins, even though they differ in their role in autophagy regulation. The deficiency of both of these proteins has shown a clear phenotype in our work and numerous other studies (45).
MSCs are currently being trialed in experimental and human diseases, including ARDS. It was recently reported that a single intravenous infusion of allogeneic, bone marrow–derived human MSCs was well tolerated in patients with moderate to severe ARDS (2). However, despite their impressive therapeutic potential, the poor survival and engraftment of MSCs is a major obstacle in MSC-based therapy (10). The inflammatory microenvironment that can be seen in ARDS and sepsis plays important roles in regulating the proliferation, differentiation, and immunomodulatory properties of MSCs (46). However, much remains to be learned about the mechanism by which the oxidizing potential of the inflammatory microenvironment into which MSCs are given actually modulates the immunoregulatory function and fate of MSCs (46). Our study showed that autophagic proteins play a critical role in regulating MSC survival under oxidative stress. Survival of MSCs after transplantation in damaged myocardium can be enhanced by autophagy-activating drugs (47). The process of autophagy could contribute to the engraftment of transplanted MSCs used in therapy, and lends itself to an application of prolonging survival of MSCs (47, 48).
WT MSCs demonstrate the ability to induce autophagy under stress. This was demonstrated by increase in autophagosome formation after H2O2 treatment (Figure 5) and CO conditioning (Figure 6). Pretreating cells with antioxidants before H2O2 treatment blocked the induction of autophagy, indicating that both general and mitochondrial ROS serve to activate autophagy. Induction of autophagy by CO via ROS formation was previously described, although not in MSCs (15). Several other studies have implicated intracellular ROS in the regulation of autophagy. Starvation, a well known activator of autophagy, is associated with increased intracellular ROS production (15). Autophagy is crucial for removal of damaged mitochondria by mitophagy, and low levels of ROS are known to enhance mitophagy (49). This was also seen in our study, as H2O2 and CO treatment both increased mitophagy in WT MSCs. The data support the concept that autophagy is primarily a survival mechanism in response to ROS (8).
Our study is also the first to examine the effects of CO on the autophagy response of MSCs. We found that CO preconditioning enhances autophagy and mitophagy (Figure 6 and Figure E5) and protects WT MSCs from oxidative stress–induced cell death (Figure 7). In addition, when we pretreated MSCs deficient in autophagic proteins with CO, we could not make any improvements in their survival under oxidative stress (Figure 7), further supporting the idea that CO may exert its cytoprotective benefits through the autophagy pathway (18).
At longer treatment times of H2O2, autophagy returned to basal levels (Figure 5). This indicates that oxidative stress rapidly enhanced autophagy at an early stage, but that prolonged oxidative exposure reduced autophagy and likely increased cell injury and death (37). The nonsustained induction of autophagy in WT MSCs after a short treatment of H2O2 illustrates an important point that sufficient levels of ROS are needed to activate autophagy, but excess production can lead to cell death. Intracellular ROS at low, controlled levels can act as important signals for cell protection and autophagy activation. If autophagy is not able to be activated, as in our autophagy protein–deficient MSCs, uncontrolled ROS generation eventually overwhelms cells and causes structural damage, particularly to mitochondria (33). This study illustrates that low levels of CO can act as an inducer of autophagy. This concept is useful for the idea that MSCs can be preconditioned to have enhanced autophagy by a steady inducer, such as CO, before their injection (50).
In conclusion, this work supports the idea that autophagic protein–depleted MSCs are more severely affected by exogenous oxidative stress as compared with WT MSCs as a result of mitochondrial dysfunction. These findings indicate the importance of autophagy in regulating the response of MSCs to oxidative stress. We also describe CO as a novel modulator of autophagy in MSCs. These findings will provide the basis for future studies to investigate autophagy-modulated MSCs in in vivo models of disease that are associated with increased oxidative stress.
Acknowledgments
Acknowledgments
The authors acknowledge and thank Dr. Polina Goihberg (Division of Pulmonary and Critical Care Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA) for her assistance with confocal microscopy image acquisition.
Footnotes
This work was supported by National Institutes of Health grants HL108801 (M.A.P., A.M.K.C., C.A.P., and L.E.F.), HL102897 (M.A.P.), T32HL 007633-29 (S.G.), T32HD 007466-17 (S.G.), and GM 102,695 (J.A.E.), and by a Peabody Foundation grant (S.G.).
Author Contributions: Conception and design—S.G., K.T., X.L., K.N., C.A.P., A.M.K.C., and M.A.P.; acquisition, analysis, or interpretation of data—S.G., K.T., X.L., K.N., B.I., A.A.C., L.E.F., J.A.E., C.A.P., A.M.K.C., and M.A.P.; drafting the manuscript for important intellectual content—S.G., K.T., X.L., K.N., L.E.F., J.A.E., C.A.P., A.M.K.C., and M.A.P.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2016-0061OC on September 16, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Walter J, Ware LB, Matthay MA. Mesenchymal stem cells: mechanisms of potential therapeutic benefit in ARDS and sepsis. Lancet Respir Med. 2014;2:1016–1026. doi: 10.1016/S2213-2600(14)70217-6. [DOI] [PubMed] [Google Scholar]
- 2.Wilson JG, Liu KD, Zhuo H, Caballero L, McMillan M, Fang X, Cosgrove K, Vojnik R, Calfee CS, Lee JW, et al. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med. 2015;3:24–32. doi: 10.1016/S2213-2600(14)70291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues: cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell. 2012;10:709–716. doi: 10.1016/j.stem.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 5.Hall SR, Tsoyi K, Ith B, Padera RF, Jr, Lederer JA, Wang Z, Liu X, Perrella MA. Mesenchymal stromal cells improve survival during sepsis in the absence of heme oxygenase-1: the importance of neutrophils. Stem Cells. 2013;31:397–407. doi: 10.1002/stem.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei X, Yang X, Han ZP, Qu FF, Shao L, Shi YF. Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol Sin. 2013;34:747–754. doi: 10.1038/aps.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee S, Choi E, Cha MJ, Hwang KC. Cell adhesion and long-term survival of transplanted mesenchymal stem cells: A prerequisite for cell therapy. Oxid Med Cell Longev. 2015;2015:632902. doi: 10.1155/2015/632902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scherz-Shouval R, Elazar Z. Regulation of autophagy by ROS: physiology and pathology. Trends Biochem Sci. 2011;36:30–38. doi: 10.1016/j.tibs.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Valle-Prieto A, Conget PA. Human mesenchymal stem cells efficiently manage oxidative stress. Stem Cells Dev. 2010;19:1885–1893. doi: 10.1089/scd.2010.0093. [DOI] [PubMed] [Google Scholar]
- 10.Song H, Cha MJ, Song BW, Kim IK, Chang W, Lim S, Choi EJ, Ham O, Lee SY, Chung N, et al. Reactive oxygen species inhibit adhesion of mesenchymal stem cells implanted into ischemic myocardium via interference of focal adhesion complex. Stem Cells. 2010;28:555–563. doi: 10.1002/stem.302. [DOI] [PubMed] [Google Scholar]
- 11.Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kubli DA, Gustafsson AB. Mitochondria and mitophagy: the yin and yang of cell death control. Circ Res. 2012;111:1208–1221. doi: 10.1161/CIRCRESAHA.112.265819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rathinam VA, Vanaja SK, Fitzgerald KA. Regulation of inflammasome signaling. Nat Immunol. 2012;13:333–342. doi: 10.1038/ni.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryter SW, Mizumura K, Choi AM. The impact of autophagy on cell death modalities. Int J Cell Biol. 2014;2014:502676. doi: 10.1155/2014/502676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SJ, Ryter SW, Xu JF, Nakahira K, Kim HP, Choi AM, Kim YS. Carbon monoxide activates autophagy via mitochondrial reactive oxygen species formation. Am J Respir Cell Mol Biol. 2011;45:867–873. doi: 10.1165/rcmb.2010-0352OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryter SW, Choi AM. Autophagy in lung disease pathogenesis and therapeutics. Redox Biol. 2015;4:215–225. doi: 10.1016/j.redox.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee S, Lee SJ, Coronata AA, Fredenburgh LE, Chung SW, Perrella MA, Nakahira K, Ryter SW, Choi AM. Carbon monoxide confers protection in sepsis by enhancing beclin 1–dependent autophagy and phagocytosis. Antioxid Redox Signal. 2014;20:432–442. doi: 10.1089/ars.2013.5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Virgin HW, Levine B. Autophagy genes in immunity. Nat Immunol. 2009;10:461–470. doi: 10.1038/ni.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci USA. 2003;100:15077–15082. doi: 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 22.Pan H, Cai N, Li M, Liu GH, Izpisua Belmonte JC. Autophagic control of cell ‘stemness’. EMBO Mol Med. 2013;5:327–331. doi: 10.1002/emmm.201201999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryter SW, Choi AM. Carbon monoxide: present and future indications for a medical gas. Korean J Intern Med. 2013;28:123–140. doi: 10.3904/kjim.2013.28.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fredenburgh LE, Kraft BD, Hess DR, Harris SR, Wolf MA, Suliman HB, Roggli VL, Davies JD, Baron RM, Thompson BT, et al. Inhaled carbon monoxide administration and resolution of acute lung injury in baboons with pneumococcal pneumonia. Am J Physiol Lung Cell Mol Physiol. 2015;309:834–846. doi: 10.1152/ajplung.00240.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lobner D. Comparison of the LDH and MTT assays for quantifying cell death: validity for neuronal apoptosis? J Neurosci Methods. 2000;96:147–152. doi: 10.1016/s0165-0270(99)00193-4. [DOI] [PubMed] [Google Scholar]
- 26.Tal MC, Sasai M, Lee HK, Yordy B, Shadel GS, Iwasaki A. Absence of autophagy results in reactive oxygen species–dependent amplification of RLR signaling. Proc Natl Acad Sci USA. 2009;106:2770–2775. doi: 10.1073/pnas.0807694106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yagi H, Tan J, Tuan RS. Polyphenols suppress hydrogen peroxide–induced oxidative stress in human bone-marrow derived mesenchymal stem cells. J Cell Biochem. 2013;114:1163–1173. doi: 10.1002/jcb.24459. [DOI] [PubMed] [Google Scholar]
- 28.Moser LA, Pollard AM, Knoll LJ. A genome-wide siRNA screen to identify host factors necessary for growth of the parasite Toxoplasma gondii. PLoS One. 2013;8:e68129. doi: 10.1371/journal.pone.0068129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slater TF, Sawyer B, Straeuli U. Studies on succinate-tetrazolium reductase systems. III. Points of coupling of four different tetrazolium salts. Biochim Biophys Acta. 1963;77:383–393. doi: 10.1016/0006-3002(63)90513-4. [DOI] [PubMed] [Google Scholar]
- 30.Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem J. 2011;435:297–312. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Troiano L, Ferraresi R, Lugli E, Nemes E, Roat E, Nasi M, Pinti M, Cossarizza A. Multiparametric analysis of cells with different mitochondrial membrane potential during apoptosis by polychromatic flow cytometry. Nat Protoc. 2007;2:2719–2727. doi: 10.1038/nprot.2007.405. [DOI] [PubMed] [Google Scholar]
- 32.Ryter SW, Choi AM. Regulation of autophagy in oxygen-dependent cellular stress. Curr Pharm Des. 2013;19:2747–2756. doi: 10.2174/1381612811319150010. [DOI] [PubMed] [Google Scholar]
- 33.Chang AL, Ulrich A, Suliman HB, Piantadosi CA. Redox regulation of mitophagy in the lung during murine Staphylococcus aureus sepsis. Free Radic Biol Med. 2015;78:179–189. doi: 10.1016/j.freeradbiomed.2014.10.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mizushima N. Methods for monitoring autophagy using GFP-LC3 transgenic mice. Methods Enzymol. 2009;452:13–23. doi: 10.1016/S0076-6879(08)03602-1. [DOI] [PubMed] [Google Scholar]
- 35.Ryter SW, Alam J, Choi AMK. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 36.Galley HF. Oxidative stress and mitochondrial dysfunction in sepsis. Br J Anaesth. 2011;107:57–64. doi: 10.1093/bja/aer093. [DOI] [PubMed] [Google Scholar]
- 37.Saito Y, Nishio K, Ogawa Y, Kimata J, Kinumi T, Yoshida Y, Noguchi N, Niki E. Turning point in apoptosis/necrosis induced by hydrogen peroxide. Free Radic Res. 2006;40:619–630. doi: 10.1080/10715760600632552. [DOI] [PubMed] [Google Scholar]
- 38.Song C, Song C, Tong F. Autophagy induction is a survival response against oxidative stress in bone marrow–derived mesenchymal stromal cells. Cytotherapy. 2014;16:1361–1370. doi: 10.1016/j.jcyt.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 39.Jiang J, Stoyanovsky DA, Belikova NA, Tyurina YY, Zhao Q, Tungekar MA, Kapralova V, Huang Z, Mintz AH, Greenberger JS, et al. A mitochondria-targeted triphenylphosphonium-conjugated nitroxide functions as a radioprotector/mitigator. Radiat Res. 2009;172:706–717. doi: 10.1667/RR1729.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schütt F, Aretz S, Auffarth GU, Kopitz J. Moderately reduced ATP levels promote oxidative stress and debilitate autophagic and phagocytic capacities in human RPE cells. Invest Ophthalmol Vis Sci. 2012;53:5354–5361. doi: 10.1167/iovs.12-9845. [DOI] [PubMed] [Google Scholar]
- 41.Perry SW, Norman JP, Barbieri J, Brown EB, Gelbard HA. Mitochondrial membrane potential probes and the proton gradient: a practical usage guide. Biotechniques. 2011;50:98–115. doi: 10.2144/000113610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang Z, Fujii H, Mohan SV, Goronzy JJ, Weyand CM. Phosphofructokinase deficiency impairs ATP generation, autophagy, and redox balance in rheumatoid arthritis T cells. J Exp Med. 2013;210:2119–2134. doi: 10.1084/jem.20130252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kelekar A. Autophagy. Ann N Y Acad Sci. 2005;1066:259–271. doi: 10.1196/annals.1363.015. [DOI] [PubMed] [Google Scholar]
- 44.Stranks AJ, Hansen AL, Panse I, Mortensen M, Ferguson DJ, Puleston DJ, Shenderov K, Watson AS, Veldhoen M, Phadwal K, et al. Autophagy controls acquisition of aging features in macrophages. J Innate Immun. 2015;7:375–391. doi: 10.1159/000370112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee SJ, Smith A, Guo L, Alastalo TP, Li M, Sawada H, Liu X, Chen ZH, Ifedigbo E, Jin Y, et al. Autophagic protein LC3B confers resistance against hypoxia-induced pulmonary hypertension. Am J Respir Crit Care Med. 2011;183:649–658. doi: 10.1164/rccm.201005-0746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dang S, Xu H, Xu C, Cai W, Li Q, Cheng Y, Jin M, Wang RX, Peng Y, Zhang Y, et al. Autophagy regulates the therapeutic potential of mesenchymal stem cells in experimental autoimmune encephalomyelitis. Autophagy. 2014;10:1301–1315. doi: 10.4161/auto.28771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Phadwal K, Watson AS, Simon AK. Tightrope act: autophagy in stem cell renewal, differentiation, proliferation, and aging. Cell Mol Life Sci. 2013;70:89–103. doi: 10.1007/s00018-012-1032-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herberg S, Shi X, Johnson MH, Hamrick MW, Isales CM, Hill WD. Stromal cell–derived factor-1β mediates cell survival through enhancing autophagy in bone marrow–derived mesenchymal stem cells. PLoS One. 2013;8:e58207. doi: 10.1371/journal.pone.0058207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frank M, Duvezin-Caubet S, Koob S, Occhipinti A, Jagasia R, Petcherski A, Ruonala MO, Priault M, Salin B, Reichert AS. Mitophagy is triggered by mild oxidative stress in a mitochondrial fission dependent manner. Biochim Biophys Acta. 2012;1823:2297–2310. doi: 10.1016/j.bbamcr.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 50.Tsoyi K, Hall SR, Dalli J, Colas RA, Ghanta S, Ith B, Coronata A, Fredenburgh LE, Baron RM, Choi AM, et al. Carbon monoxide improves efficacy of mesenchymal stromal cells during sepsis by production of specialized proresolving lipid mediators. Crit Care Med. In press doi: 10.1097/CCM.0000000000001999. [DOI] [PMC free article] [PubMed] [Google Scholar]