To the Editor:
Amyotrophic lateral sclerosis (ALS) is a devastating neurodegenerative disease characterized by degeneration of motor neurons (1). Respiratory failure is the leading cause of death in ALS, due to progressive respiratory motor neuron degeneration and muscle weakness. This respiratory muscle weakness results in hypoventilation and severe restrictive lung impairment. Breathing support with noninvasive ventilation prolongs survival significantly (2), but invasive mechanical ventilation eventually becomes necessary for survival (3). This work was presented as an abstract at the American Thoracic Society in San Francisco, California, May 2016.
Approximately 5–10% of ALS is familial, and the remaining cases are classified as sporadic. A total of 20% of familial ALS is due to mutations in the gene encoding Cu/Zn superoxide dismutase (SOD) 1 (4). The most commonly used ALS mouse model is the transgenic SOD1G93A mouse, which ubiquitously expresses the human SOD1 gene with the G93A mutation (5). It recapitulates pathophysiology of patients with ALS, including motor neuron loss, axonal degeneration, muscle denervation, and limb paralysis. Although respiratory impairment is a key aspect of ALS, studies of respiratory function in the SOD1G93A rodent models have been limited to examination of minute ventilation and motor neuron output (6, 7). To our knowledge, the role of restrictive lung disease and the impact on the mechanics of the respiratory system have not been examined in the SOD1G93A mouse model. Because patients with ALS eventually succumb to respiratory failure as a result of restrictive lung disease and respiratory muscle weakness, we sought to characterize the pulmonary mechanics in SOD1G93A mice during disease progression. These measurements provide important, clinically translatable outcome measures for future therapies that target the respiratory insufficiency in the ALS animal model.
SOD1G93A mice and nontransgenic (NTG) littermate controls were evaluated before disease (9 wk), at disease onset (13 wk), during overt hind-limb weakness (18 wk), and at end stage (paralysis).
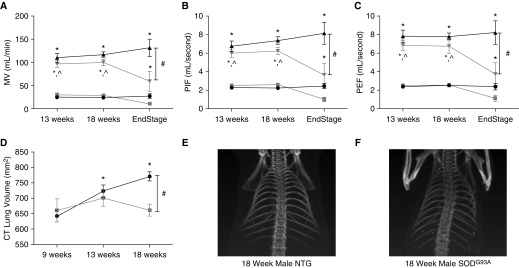
Initially, awake spontaneous breathing was assessed in unrestrained mice using whole-body plethysmography as previously described (8). Whole-body plethysmography in unanesthetized mice is used to quantify minute ventilation, changes in flow, and lung volumes. Unanesthetized, unrestrained mice are placed in a clear Plexiglas chamber, and both ambient and chamber pressure and temperature are used to calculate mouse ventilation. Baseline measurements are taken under conditions of normoxia (fraction of inspired oxygen, 0.21; fraction of inspired carbon dioxide, 0.00) followed by a 10-minute exposure to hypercapnia (fraction of inspired oxygen 0.21; fraction of inspired carbon dioxide, 0.07; nitrogen balance). Hypercapnia is used to assess the ability of the respiratory system to respond to an increased respiratory demand. Our results confirmed previous rodent studies that described a preservation of tidal volume and minute ventilation up until end-stage disease in both the mouse and rat models at baseline and during hypercapnia (6, 7) (Figure 1A). The animals were unable to mount a robust response to hypercapnia at end stage, indicating a lower respiratory reserve. Furthermore, similar to findings by Tankersley and colleagues (6), in end-stage disease, there was an abrupt decline in minute ventilation (Figure 1A; P < 0.05 compared with 13 and 18 wk; P < 0.001 compared with NTG; see figure legends for statistical analyses). No significant changes in breathing frequency were noted between the two groups at baseline or during hypercapnia. In addition, the peak inspiratory flow (PIF) (Figure 1B) and peak expiratory flow (PEF) (Figure 1C) were studied at baseline and during hypercapnia. PIF and PEF reflect inspiratory muscle strength (namely diaphragm) and expiratory muscle strength (internal intercostal and abdominal muscles), respectively. Similar to the minute ventilation (Figure 1A), the SOD1G93A mice were able to maintain PIF and PEF until end stage, after which these flows rapidly declined (Figures 1B and 1C; P < 0.05 compared with 13 and 18 wk; P < 0.001 compared with NTG).
Figure 1.
Cu/Zn superoxide-dismutase (SOD) 1G93A mice at end stage have hypoventilation, reduced lung volumes, and pronounced kyphoscoliosis. Whole-body plethsmography at baseline during normoxia (squares or circles) or during hypercapnic challenge (triangles). SOD1G93A (n = 8 at 13 wk, n = 11 at 18 wk, and n = 4 at end stage) animals are designated by gray lines, and nontransgenic (NTG; n = 8 at 13 wk, n = 11 at 18 wk, and n = 6 at time point of littermate end stage SOD1G93A) controls by black lines. Measurement of minute ventilation (MV) (A), peak inspiratory flow (PIF) (B), and peak expiratory flow (PEF) (C) are reported. NanoSPECT/computed tomography (CT) small animal imaging CT scan quantified lung volumes (D) (SOD1G93A, n = 4; NTG, n = 4). Representative CT imaging of an 18-week NTG control (E) and a littermate SOD1G93A (F) mouse reveal kyphoscoliosis in the SOD1G93A mouse. (A–C) *P < 0.001 compared with baseline; #P < 0.001 compared with SOD1G93A at end stage; ^P < 0.05 compared with SOD1G93A at end stage (two-way ANOVA, SigmaPlot; Systat Software, San Jose, CA). (D) *P < 0.05 compared with 9 weeks; #P < 0.05 compared with SOD1G93A at 18 weeks (two-way repeated measures ANOVA).
Next, lung volumes were measured using computed tomography (NanoSPECT/computed tomography small animal imaging; Boston, MA) at 9, 13, and 18 weeks. At 9 and 13 weeks, there was no significant difference in lung volume between the SOD1G93A mice and their age-matched NTG littermate controls (Figure 1D). However, lung volume was significantly decreased at 18 weeks in the SOD1G93A mice (Figure 1D; P < 0.05). By contrast, the NTG lung volumes continued to increase with age (Figure 1D, P < 0.05). Of note, the SOD1G93A mice had significant kyphoscoliosis, presumably due to weakness of trunk muscles during a period of growth and development (Figures 1E and 1F). Although this kyphoscoliosis is seen in several neuromuscular diseases, it is less common in human ALS, possibly owing to the rapid progression of disease as well as the onset of disease in adulthood. However, in the SOD1G93A mouse model, this kyphoscoliosis is prominent and is a reflection of neuromuscular degeneration that further exacerbates the restrictive lung disease.
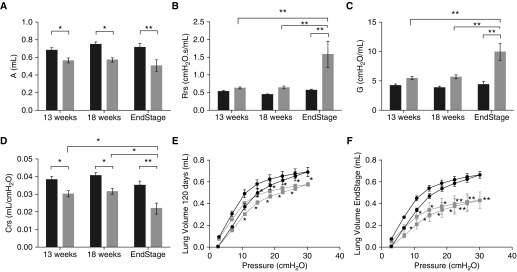
Next, we used forced oscillometry (Flexivent; Scireq, Montreal, PQ, Canada) to further define the impact of this disease on the pulmonary mechanics of the SOD1G93A mice (9). Despite preservation of spontaneous ventilation until 18 weeks, there were significant differences in pulmonary mechanics starting as early as 13 weeks. Examination of quasistatic mechanical properties of the respiratory system revealed a decreased lung capacity in SOD1G93A mice compared with littermate NTG controls (measured by the upper bounds estimate of the difference between total lung capacity and zero volume). This decrease was noted at 13 weeks (P < 0.05), 18 weeks (P < 0.05), and at end stage (P < 0.001) (Figure 2A). In addition, overall respiratory resistance (Figure 2B) was significantly increased at end stage compared with NTG (P < 0.001). As expected, this overall increase in resistance was due to an increase in tissue resistance (Figure 2C; P < 0.001), not central airway resistance (P = 0.21; data not shown) (9). Finally, assessment of respiratory compliance (Figure 2D) confirmed this restrictive pattern with a decrease in compliance as early as 13 weeks (P < 0.05) and a more robust decrease at end stage (P < 0.001). This dramatic shift in compliance is illustrated in the shift of the pressure–volume loops at 18 weeks (Figure 2E) and end stage (Figure 2F), when higher pressures resulted in lower volumes in SOD1G93A compared with NTG (P < 0.05). This shift of the pressure–volume curve down and to the right is pathognomonic for restrictive lung disease.
Figure 2.
SOD1G93A mice have evidence of pulmonary restriction as early as 13 weeks of age. Using forced oscillation, measurements of pulmonary mechanics are shown in (A) lung capacity (A), (B) overall respiratory resistance (Rrs), (C) tissue damping (G), and (D) compliance (Crs) in SOD1G93A (gray bars, n = 7 at 13 wk, n = 8 at 18 wk, and n = 6 at end stage) mice as compared with NTG (black bars, n = 6 at 13 wk, n = 8 at 18 wk, and n = 7 at time point of littermate end-stage SOD1G93A) littermates. Pressure–volume measurements also reveal age-dependent decreases in lung volumes (E and F) in SOD1G93A mice (gray lines, n = 8 for E; n = 6 for F) as compared with NTG (black lines, n = 8 for E; n = 7 for F) littermates. (A–D) *P < 0.05; **P < 0.01 (two-way ANOVA). (E and F) *P < 0.05 compared with NTG lung volume at the same pressure; **P < 0.001 compared with NTG lung volume at the same pressure (two-way repeated measures ANOVA).
This study is the first to show progressive restrictive pulmonary disease in the most commonly used ALS mouse model—the SOD1G93A mouse. This restriction starts as early as 13 weeks, at the approximate time that hind-limb weakness begins. Restrictive lung disease is the hallmark of respiratory pathology in neuromuscular illnesses, including ALS. The exact etiology of restrictive pulmonary disease in the mouse model is unknown but is presumably due to trunk and respiratory muscle weakness, resulting in chest wall restriction of lung expansion. In patients with ALS, restriction of lung expansion is also due to inspiratory muscle weakness and the inability to generate enough negative pressure to expand the lungs. Many studies in ALS animal models use hind-limb weakness and survival to measure therapeutic success, without much emphasis on correction of the respiratory phenotype. Because respiratory failure is the primary cause of death in patients with ALS, we propose that future therapeutic studies using the SOD1G93A mouse model evaluate the impact of therapies on respiratory insufficiency and restrictive lung disease.
Footnotes
This work was supported by National Institutes of Health (NIH) NINDS grant 1R21NS098131-01 (M.K.E.), NIH NICHD 1K08HD077040-01A1 (M.K.E.), an ALS Foundation Starter Grant (M.K.E.), NINDS grants NS088689 and NS079836 (R.H.B.), the ALS Therapy Alliance, the ALS Association, the Angel Fund, the Al-Athel Foundation, the Pierre L. de Bourgknecht ALS Research Foundation, Project ALS, and P2ALS (R.H.B.).
Author Contributions: L.S. was involved in the conception, hypotheses delineation, and study design, and was primarily responsible for acquisition, analysis, and interpretation of data and drafting the manuscript; A.M.K. was involved in the design of the study, and assisted with data collection and interpretation and drafting the manuscript; L.X., M.K., and K.D. were involved in the design of the study, and assisted with data collection and interpretation; R.H.B. and M.S.-E. were involved in the conception of the study, reviewed the data, and helped draft the manuscript; T.R.F. was involved in the conception, the hypotheses delineation, reviewing the data, and helping draft the manuscript; M.K.E. was involved in the conception, hypotheses delineation, and design of the study, data interpretation, and writing and revising the manuscript; M.K.E. approved the final version of the submitted manuscript.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Peters OM, Ghasemi M, Brown RH., Jr Emerging mechanisms of molecular pathology in ALS. J Clin Invest. 2015;125:1767–1779. doi: 10.1172/JCI71601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kleopa KA, Sherman M, Neal B, Romano GJ, Heiman-Patterson T. Bipap improves survival and rate of pulmonary function decline in patients with ALS. J Neurol Sci. 1999;164:82–88. doi: 10.1016/s0022-510x(99)00045-3. [DOI] [PubMed] [Google Scholar]
- 3.Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med. 2001;344:1688–1700. doi: 10.1056/NEJM200105313442207. [DOI] [PubMed] [Google Scholar]
- 4.Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O’Regan JP, Deng HX, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 5.Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 6.Tankersley CG, Haenggeli C, Rothstein JD. Respiratory impairment in a mouse model of amyotrophic lateral sclerosis. J Appl Physiol (1985) 2007;102:926–932. doi: 10.1152/japplphysiol.00193.2006. [DOI] [PubMed] [Google Scholar]
- 7.Nichols NL, Gowing G, Satriotomo I, Nashold LJ, Dale EA, Suzuki M, Avalos P, Mulcrone PL, McHugh J, Svendsen CN, et al. Intermittent hypoxia and stem cell implants preserve breathing capacity in a rodent model of amyotrophic lateral sclerosis. Am J Respir Crit Care Med. 2013;187:535–542. doi: 10.1164/rccm.201206-1072OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.ElMallah MK, Pagliardini S, Turner SM, Cerreta AJ, Falk DJ, Byrne BJ, Greer JJ, Fuller DD. Stimulation of respiratory motor output and ventilation in a murine model of pompe disease by ampakines. Am J Respir Cell Mol Biol. 2015;53:326–335. doi: 10.1165/rcmb.2014-0374OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vanoirbeek JA, Rinaldi M, De Vooght V, Haenen S, Bobic S, Gayan-Ramirez G, Hoet PH, Verbeken E, Decramer M, Nemery B, et al. Noninvasive and invasive pulmonary function in mouse models of obstructive and restrictive respiratory diseases. Am J Respir Cell Mol Biol. 2010;42:96–104. doi: 10.1165/rcmb.2008-0487OC. [DOI] [PubMed] [Google Scholar]