Abstract
Ample research suggests that social connection reliably generates positive emotions. Oxytocin, a neuropeptide implicated in social cognition and behavior, is one biological mechanism that may influence an individual’s capacity to extract positive emotions from social contexts. Because variation in certain genes may indicate underlying neurobiological differences, we tested whether several SNPs in two genes related to oxytocin signaling would show effects on positive emotions that were context-specific, depending on sociality. For six weeks, a sample of mid-life adults (N = 122) participated in either socially-focused loving-kindness training or mindfulness training. During this timespan they reported their positive emotions daily. Five SNPs within OXTR and CD38 were assayed, and each was tested for its individual effect on daily emotions. The hypothesized three-way interaction between time, training type, and genetic variability emerged: Individuals homozygous for the G allele of OXTR rs1042778 experienced gains in daily positive emotions from loving-kindness training, whereas individuals with the T allele did not experience gains in positive emotions with either training. These findings are among the first to show how genetic differences in oxytocin signaling may influence an individual’s capacity to experience positive emotions as a result of a socially-focused intervention.
Keywords: oxytocin, affective science, positive psychology, genetics, meditation, vantage sensitivity
1. INTRODUCTION
Habitual pleasant emotions forecast mental and physical health (Howell et al., 2007), as well as longevity (Steptoe & Wardle, 2012), even after accounting for the health-diminishing effects of habitual negative emotions (Chida & Steptoe, 2008). The broaden-and-build theory of positive emotions holds that the human capacity to experience pleasant emotions was selectively advantageous, as these states broaden momentary awareness in ways that build personal resources (e.g., resilience, social integration, health), which in turn promoted individual and group survival (Fredrickson, 1998; Fredrickson, 2013). Ample empirical evidence supports this theory (for a review, see Fredrickson, 2013). Given the benefits that stem from positive emotional experiences, a better understanding of their behavioral and biological determinants is vital.
One reliable behavioral determinant of positive emotions is social interaction. Studies of humans (Kahneman et al., 2004) and non-human mammals (Dölen et al., 2013) reveal that interactions with conspecifics predict pleasant or rewarding emotional states. Likewise, adopting a benevolent social focus has been found to increase positive emotions (Otake et al., 2006). The contemplative practice of loving-kindness meditation, which involves cultivating heartfelt wishes for the well-being of others, has been similarly found to increase day-to-day positive emotions (Fredrickson et al., 2008; Kok et al., 2013). In light of robust links between sociality and positive emotions, the present study investigates whether the propensity to extract enjoyment from social connection is further contoured by individual differences in certain biological pathways. In particular, we target the neuropeptide oxytocin (OT), one of the key neuroendocrine components implicated in sociality in humans and other mammals (Carter, 2014).
Increasing evidence from both human and animal data suggest that oxytocin has the potential to specifically heighten the saliency of social information. For example, in rodent mothers oxytocin acts on the oxytocin receptor to alter the firing pattern in the auditory cortex in a manner that heightens the ability to detect vocalizations from pups, but does not impact the ability to discriminate non-social auditory stimuli (Marlin et al., 2015). Similarly, oxytocin acts in the olfactory cortex to enhance responses to social odors, but not general odors (Choe et al., 2015).
Researchers studying the behavioral and social psychological effects of OT in humans frequently employ either pharmacological administration of OT or measures of endogenous OT through saliva, plasma, or urine. However, the mechanism by which intranasal oxytocin impacts neural function is unclear (Leng and Ludwig, 2016), and debate regarding the proper procedures for measuring peripheral oxytocin continues (Carter, 2014; Szeto et al., 2011). Accordingly, multiple approaches are needed to better understand the role of the oxytocin system. Polymorphisms within genes related to oxytocin signaling may create differences in underlying neurobiology that ultimately affect social behavior and experience (Furman et al., 2011). In the present study, five single nucleotide polymorphisms (SNPs) within two genes important to OT function (OXTR rs2254298, rs53576, rs1042778 and CD38 rs6449182, rs3796863) were used to test whether genetic differences in the oxytocin system are related to the affective rewards gained from training in loving-kindness, a contemplative practice with a social focus.
OXTR is the primary receptor for the neurotransmitter OT, and CD38 is a key protein in the secretion of OT within hypothalamic neurons, resulting in measurable downstream endocrinological and behavioral effects (Jin et al., 2007). Alleles of SNPs within OXTR and CD38 have been associated with phenotypes related to prosocial behavior (OXTR 1042778, Israel et al., 2009; OXTR rs53576, Poulin et al., 2012), emotional support seeking (OXTR rs53576, Kim et al., 2010), functional coupling between the hypothalamus and amygdala during emotional cue processing (OXTR rs2254298, Kumsta & Heinrichs, 2013), reduced cortisol after social support (OXTR rs53576, Chen et al., 2011b), and plasma OT levels in new parents (OXTR rs2254298, rs1042778, CD38 rs3796863, Feldman et al., 2012). In a study by Algoe and Way (2014), individual SNPs in CD38 (rs6449182 and rs3796863), as well as a genetic index of CD38 expression, were significantly associated with feeling positive emotions, especially love, after expressing gratitude to a romantic partner, which suggests that CD38 plays a role in positive emotions resulting from other-focused social interactions. Although a recent meta-analysis that examined combined effect sizes across 82 samples for two OXTR SNPs suggested that these polymorphisms failed to explain a significant part of human behavior and experience (Bakermans-Kranenburg & van Ijzendoorn, 2014), the authors note that gene-by-context interactions (the target of investigation here) were not considered. Indeed, behavioral and psychological consequences of oxytocin are likely context-dependent (for reviews, see Shamay-Tsoory & Abu-Akel, 2016; Bartz et al., 2011).
Whereas OT candidate gene studies frequently refer to genetic variants that confer risk (e.g. for psychopathology or social dysfunction, Lerer et al., 2008), we adopt the terminology of vantage sensitivity (Pluess & Belsky, 2013) to support discussion of how certain OT genetic polymorphisms may confer unique benefits in response to benign social opportunities—not simply a lack of vulnerability to adversity. The frameworks of vantage sensitivity and differential susceptibility (Belsky & Pluess, 2009) are not mutually exclusive. Alleles designated here as “vantage” may be sensitive to both positive and negative environments. Because the current investigation only tests the effects of a positive environment (i.e., loving-kindness training), it would be imprecise in this context to use the terminology “sensitivity alleles.” Based on prior research on the risks and benefits associated with specific OT-related genes, we thus tested individual variation across five SNPs from OXTR and CD38 putatively associated with either vantage for prosociality or non-risk for autism and social deficits.
Given the relevance of positive emotions to health, here we investigate two plausible determinants of day-to-day positive emotions: sociality and OT vantage. Substantial evidence has linked (a) sociality to positive emotions, and (b) OT to sociality. However, almost no research has examined OT’s downstream effects on positive emotions (but see Algoe & Way, 2014). To fill this gap, we test whether the effect of OT vantage on positive emotions is context-specific, depending on sociality. A randomized controlled trial of midlife adults included training in either loving-kindness or mindfulness meditation. Both meditation trainings emanate from Buddhist contemplative practices and are often taught together (Salzberg, 2011). Here, for scientific purposes, we cleaved these practices apart and taught them in small groups over six weeks. Both techniques target attention, yet only loving-kindness training (LKT) involves the intentional cultivation of benevolent social focus. Although absent an explicit social focus, mindfulness training (MT) is associated with a diverse array of benefits (Grossman et al., 2004; Seidlmeier et al., 2012) and therefore provides a highly parallel active control condition to capture nonspecific treatment effects such as positive expectations, group contact, and individual practice. Our past work demonstrates that LKT produces gradual, consequential increases in positive emotions over time (Fredrickson et al., 2008; Kok et al., 2013). We suspect that underlying individual differences in oxytocinergic functioning may influence the emotional benefits of LKT. As a proxy for these differences, we hypothesize that variation in one or more of five SNPs in OXTR and CD38 may moderate the positive emotion yield of meditation training, with allelic variants considered vantage (i.e., non-risk) predicting greater positive emotional gains from LKT versus MT.
2. METHODS AND MATERIALS
2.1 Participants
A total of 270 healthy mid-life adults were assessed for eligibility from the counties surrounding Chapel Hill and Durham, North Carolina. Flyers were posted and email advertisements were sent within the community, asking for people interested in learning meditation, stating: “Science has shown that meditation improves people’s health and well-being. Help UNC researchers learn how.” All participants received monetary compensation for completing various aspects of the study, including three 60-minute lab sessions to provide urine and blood samples, participation in a six-week meditation workshop, and completion of daily questionnaires before, during, and after the meditation workshop. Results based on other aspects of this larger NIH-supported study are reported elsewhere.1
All potential participants were first screened with a phone call to assess eligibility. Selection criteria included: within the age range of 35 to 64 years old,2 fluency in English, absence of chronic illness or disability, and not currently engaged in regular meditation practice. Those who met initial selection criteria were formally enrolled and randomized to condition (N = 176; Mage= 48.39 years, SD = 8.82). The sample was mostly female (73%) and Caucasian (79%). Thirteen percent self-identified as African-American, 7% as Asian, 1% as Native American / Pacific Islander, and 1% as another race. Four percent of participants self-identified as Hispanic or Latino.
2.2 Study Design
The study implemented a randomized longitudinal design. The overarching hypothesis that drove this investigation respects the context-dependent nature of OT-related effects (Bartz et al., 2011), predicting that common polymorphisms in OT-related genes would moderate emotional responses within the context of an active training intervention. As such, although the enrolled sample originally included individuals randomized to serve in a waitlist control group (n = 51), the study reported here includes only those randomized to one of the two training contexts: loving-kindness training (LKT) (n = 62) or mindfulness training (MT) (n = 63). Out of the 125 participants randomized to meditation training, 3 were excluded (1 assigned to LKT, 2 assigned to MT) because they did not attend any meditation workshops. All genotypes were successfully assayed, so the final sample size comprised 122 participants. A CONSORT Table is provided in the Supplementary Material. The demographics of the analysis subsample (N = 122; see Table 1) do not appreciably differ from the full sample. For completeness, results comparing LKT to the waitlist control condition are provided in the Supplementary Material and summarized in the Discussion (Section 4).
Table 1.
Participant Characteristics
| Measure | LKT (n = 61) | MT (n = 61) | Statistic | df | p | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| M | SD | % | M | SD | % | ||||
| Age (years) | 48.56 | 8.48 | 48.75 | 9.56 | t = .12 | 120 | .905 | ||
| Sex (female) | 69 | 75 | χ2 = .65 | 1 | .419 | ||||
| Race | χ2 = .45 a | 1 | .501 | ||||||
| Asian | 5 | 8 | |||||||
| Black | 18 | 7 | |||||||
| Other | 0 | 2 | |||||||
| Native Am. / PI | 0 | 2 | |||||||
| White | 77 | 82 | |||||||
| Hispanic Ethnicity | 3 | 7 | χ2 = .70 b | - | .681 | ||||
| Baseline PEs | 1.74 | .66 | 1.47 | .82 | t = −1.98 | 119 c | .050 | ||
Note: The 7 days immediately prior to the first workshop formed the baseline period.
Given the presence of zero cells, the chi-square test is based on White vs. non-White.
Given the presence of low cell sizes, Monte Carlo simulation (2000 replications) was used to calculate the p-value. As such, degrees of freedom are not applicable.
One individual did not provide baseline data for the daily emotion reports.
The two workshops (LKT and MT) centered on ancient Buddhist meditation practices (loving-kindness meditation and mindfulness meditation, respectively) and were led by experienced meditation instructors. For both LKT and MT, participants were instructed to sit quietly, usually with eyes closed and an initial focus on the breath. In MT, participants were trained to pay attention to the present moment in a nonjudgmental, open-minded way. In LKT, participants were trained to self-generate warm, tender, and compassionate feelings and to gradually extend these warm feelings to an ever-increasing circle of social connections. The study design deploys LKT as the experimental intervention to cultivate heightened levels of benevolent other-focus, and uses MT as the active control condition to account for non-specific training effects and general effects of meditation-based training.
2.3 Measures
2.3.1 Daily Emotions
The modified Differential Emotions Scale (mDES) is a 20-item measure that assesses the degree to which respondents experience different emotions (Fredrickson, 2013). It assesses 10 positive emotions (amusement, awe, gratitude, hope, interest, inspiration, joy, love, pride, and serenity) and 10 negative emotions (anger, contempt, disgust, embarrassment, fear, guilt, hate, sadness, shame, and stress). Daily, participants were asked to recall the past 24 hours and indicate the greatest degree to which they experienced a particular emotion using a 5-point Likert-type scale (0 = Not at all, 4 = Extremely). For each participant, we created daily positive emotion (PE) scores by computing the mean across the 10 positive emotion ratings for each day. Similarly, daily negative emotion (NE) scores were calculated by computing the mean across the 10 negative emotions each day. Although not relevant to the current investigation, information on daily NEs can be found in the Supplementary Material. To assess scale reliability for the mDES, McDonald’s omega was calculated using the multilevel confirmatory factor analysis procedure described in Bolger and Laurenceau (2013, p. 138–140, calculated in Mplus version 7.2; Muthén & Muthén, 1998–2015). The reliability estimate was calculated for the within-person model and represents the reliability of within-person changes in positive emotions over time. For daily reports provided during the baseline period, omega was .84; thus, the daily PE scores were considered reliable.3
2.3.2 Genomic DNA
Blood samples from each participant were obtained via venipuncture during the initial lab session. DNA extracted from peripheral-blood leukocytes was assayed for genotypes using a commercial TaqMan Genotyping Assay (Applied Biosystems, Foster City, CA) and an iCycler real-time polymerase chain reaction instrument (BioRad, Hercules, CA) following protocols provided by the manufacturer, as described previously (Bower et al., 2013). The specific genotypes included five different SNPs: OXTR rs2254298, OXTR rs53576, OXTR rs1042778, CD38 rs6449182, and CD38 rs3796863 (see Table S1 in Supplementary Material for context sequences and assay IDs).
To test the independent effect of each SNP, it was necessary to classify genotypes into two categories to create groups of comparable size: homozygous for the major allele vs. carriers of the minor allele (heterozygous and homozygous). Based on existing empirical literature, the two categories of each SNP were classified as being vantage-sensitive (“vantage”)—putatively associated with greater oxytocin functionality—or vantage-resistant (“non-vantage”). Specifically, vantage genotypes included: OXTR rs2254298 A-carriers (functional coupling of amygdala, dorsal anterior cingulate cortex, and hypothalamus during emotional cue processing, Kumsta and Heinrichs, 2013), OXTR rs53576 GG (prosocial temperament and hypothalamic volume, Tost et al., 2010; greater cortisol reduction after social support, Chen et al., 2011b), OXTR rs1042778 GG (plasma OT levels, Feldman et al., 2012; lower negative affectivity and less inhibited sociality, Creswell et al., 2015), CD38 rs6449182 CC (positive emotions after expressing gratitude, Algoe & Way, 2014), and CD38 rs3796863 A-carriers (lower autism risk, Munesue et al., 2010; plasma OT levels, Feldman et al., 2012). Because of moderate linkage disequilibrium among the OXTR SNPs, we also conducted a series of post hoc haplotype analyses to identify any haplotypes that might be driving the observed effects.4 Method and results of the haplotype analyses are also reported in the Supplementary Material.
2.4 Procedure
After providing informed consent, participants first completed a laboratory session, in which several biological measures were taken, including a blood draw (see Section 2.3.2). Prior to the laboratory session, participants completed numerous other questionnaires that are beyond the scope of the present investigation (see Fredrickson et al., 2008, for similar measures). Then, for up to two weeks, participants completed daily emotion reports online from home. Next, participants randomly assigned to either LKT or MT met weekly, in small groups, with an experienced meditation instructor. Attendees were also instructed to cultivate a daily meditation practice outside of the workshops, realistically gauged at 3–5 practice sessions per week. Throughout these six weeks of class instruction, participants continued to complete daily emotion reports; for purposes related to the larger study, participants also completed three weeks of daily emotion reports following the workshop offerings—these reports were excluded from the present analyses as training was no longer active. Participants completed this protocol over two separate study waves conducted between August 2012 and May 2013. Procedures were conducted in accordance with APA regulations regarding the use of human participants and approved by the UNC-CH IRB.
2.5 Analytic Strategy
To analyze the data, PROC MIXED in the SAS 9.2 software was used to fit a series of mixed-effects linear growth curve models for the daily PE reports using restricted maximum likelihood estimation. Analyses proceeded as follows. First, an unconditional linear growth curve model was estimated to test (a) if participants exhibited linear growth in daily PEs over time, and (b) whether significant variation existed in participants’ initial statuses and rates of linear change in daily PEs. In this model, time was measured in weeks elapsed from the first workshop class attended, which began in week 3 of the overall daily reporting period although individual starting dates varied. Thus, the day of each participant’s first workshop class was coded as zero and the two weeks of baseline daily reports were excluded from analysis. At this point, we also tested for improvement in model fit resulting from adding a serial correlation structure to the within-person residuals—specifically, a spatial power structure which accommodates individually-varying time intervals (i.e., due to missing daily dairy reports; Bolger & Laurenceau, 2013, p. 93). Next, training assignment was added to the model as a predictor of initial PE levels and rates of change in PE over time. In the final step, we included each SNP genotype indicator in separate models (non-vantage = 0, vantage = 1) as well as all two- and three-way interactions between time, training condition, and genotype. This final model was re-estimated with the following covariates to determine whether results were sensitive to the inclusion of: (a) participant gender (female = 0, male = 1), because estrogen has been shown to upregulate OT processing (Lim & Young, 2006); (b) a time-varying indicator of weekday vs. weekend status (weekday = 0, weekend = 1) to control for higher levels of PEs typically observed during weekends in daily affect reporting (Stone et al., 2012); (c) age, given the large range of ages in the sample; and (d) a binary variable indicating in which cohort participants completed the study (Wave 1, Fall 2012 = 0; Wave 2, Spring 2013 = 1). Our hypothesis was that a significant three-way interaction would emerge between time, training condition, and SNP genotype, such that relative to those with a non-vantage genotype, participants with a vantage-sensitive genotype would show greater increases in PEs over time from LKT versus MT. To account for multiple testing, we applied a Bonferroni correction, such that the adjusted p-value equaled .01. Additional details regarding the specification and testing of the analysis models can be found in the Supplementary Material.
2.6 Missing Data
Compliance was high for the daily reporting protocol. Out of a total of 9,394 potential daily reports (i.e., 122 participants in final analysis × 77 days), participants provided daily emotion reports on 7,721 days (82.19%). The number of reports varied across participants from a minimum of 19 reports to a maximum of all 77 reports. The average number of emotion reports was 63.29, with 114 participants (93.44%) providing emotion reports on at least half of the days during the daily reporting period. This limited amount of missing data resulted in some cluster imbalance—that is, differing numbers of reports between participants as well as unequal time intervals between observations. Under maximum likelihood estimation, estimates in mixed effects models are unbiased and efficient in the presence of cluster imbalance, assuming missingness is ignorable. Consequently, we assume in the present article that the data meet the missing at random (MAR) assumption such that cause of missing data values for the daily positive emotion reports can be fully accounted for by other variables in the model, and the parameters of the estimated models are independent from those contained in the model governing the missing data process (Black et al., 2012).
3. RESULTS
3.1 Preliminary Analyses
Demographic characteristics of the analysis sample are shown in Table 1. Although the majority of participants identified as White or Caucasian (79%), our sample was ethnically heterogeneous. Because heterogeneous samples can introduce the potential confound of population stratification due to different allelic frequencies across ethnic groups (Cardon & Palmer, 2003), we also conducted the principal analyses separately for the Caucasian subsample, our largest ethnic group. All effects for the Caucasian-only sample patterned those of the full sample (Table S6 in Supplementary Material), and so we report results for the full sample below. In addition, no significant demographic differences emerged between training groups. We note that when comparing baseline PE levels (defined as the seven days prior to the first workshop), participants receiving LKT began with a higher mean compared to those receiving MT. Although the difference was not statistically significant, controlling for baseline PEs did not affect the pattern of results. All SNPs were found to be in Hardy-Weinberg Equilibrium (Table S2 in Supplementary Material) and were evenly distributed between genders.
The intraclass correlation coefficient for the dependent variable during the workshop phase was .70, indicating that the majority of variation in daily PE reports was explained by between-person differences in average levels of PEs over the duration of the study. A random-intercept unconditional growth curve model resulted in a significant effect of time (b = 0.021, p < .001, 95% CI [0.011, 0.031]) such that, on average, participants exhibited linear increases in PEs over the duration of the study. In addition, deviance tests revealed a significant amount of variation in participants’ initial PE levels at the beginning of the 6-week workshop period, intercept variance = 0.482, Δ Deviance (Δdf = 1) = 3990.4, p < .001 as well as their rates of change in daily PEs over time, slope variance = 0.007; Δ Deviance (Δdf = 2) = 118.80, p < .001. The intercept-slope correlation estimate indicated that those with higher initial PE levels increased at slower rates compared to those with lower initial levels, although the magnitude of the negative correlation was small (intercept-slope correlation = −.065). Adding a spatial power covariance structure for the level-1 residuals improved the fit of the model, Δ Deviance (Δdf = 1) = 206.80, p < .001 and was retained for subsequent models. When training assignment (MT vs. LKT) was added to the model, those in the LKT condition had significantly higher PE levels after the first workshop session (b = 0.256, p = .040, 95% CI [0.011, 0.501]) and changed at a significantly higher rate (b = 0.043, p = .020, 95% CI [0.007, 0.079]) compared to those in the MT condition.
3.2 Change in positive emotions depends on condition and OXTR rs1042778 genotype
Because LKT is socially-oriented, we hypothesized that individuals who have a genetic predisposition for putatively greater oxytocin signaling would demonstrate the largest PE gains from LKT. As such, we expected a significant three-way interaction to emerge, in the full model, between time, training condition, and genotype, such that participants in the LKT condition with vantage genotypes would experience greater growth in PEs relative to those with non-vantage genotypes. Because MT does not have a social focus, we did not expect genotype to have an effect on emotions in this condition. Estimates of the fixed effects from the five full models are shown in Table 2. After correcting for multiple testing, the hypothesized three-way interaction emerged as predicted for one SNP, OXTR rs1042778 (b = 0.108, p = .005, 95% CI [0.034, 0.183]).
Table 2.
Model results of daily positive emotion growth, LKT vs. MT.
| Predictor | Fixed effects, b [95% Confidence Interval ] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| OXTR rs2254298a | OXTR rs53576b | OXTR rs1042778c | CD38 rs6449182d | CD38 rs3796863e | ||||||
| Intercept | 1.500* | [1.281, 1.719] | 1.568* | [1.317, 1.818] | 1.406* | [1.178, 1.633] | *1.476 | [1.168, 1.784] | *1.638 | [1.365, 1.911] |
| Week | .006 | [−.026, .038] | −.014 | [−.052, .022] | .024 | [−.009, .056] | .010 | [−.035, .055] | .016 | [−.023, .056] |
| LKT | .277 | [−.027, .580] | .169 | [−.175, .512] | .337 | [.031, .643] | .175 | [−.266, .616] | .119 | [−.290, .528] |
| Genotype | −.032 | [−.396, .333] | −.154 | [−.506, .198] | .202 | [−.153, .557] | .018 | [−.360, .394] | −.253 | [−.608, .102] |
| Week × LKT | .025 | [−.019, .070] | .048 | [−.003, .098] | .004 | [−.039, .048] | .010 | [−.053, .074] | .002 | [−.057, .061] |
| Week × Genotype | −.018 | [−.071, .035] | .026 | [−.025, .078] | −.059 | [−.109, −.008] | −.015 | [−.070, .040] | −.029 | [−.080, .023] |
| LKT × Genotype | −.252 | [−.777, .272] | .047 | [−.451, .546] | −.392 | [−.918, .135] | .036 | [−.498, .571] | .152 | [−.362, .666] |
| Week × Genotype × LKT | .053 | [−.023, .130] | −.006 | [−.078, .066] | .108* | [.034, .183] | .048 | [−.030, .125] | .065 | [−.010, .139] |
|
| ||||||||||
| Random Effects | ||||||||||
|
| ||||||||||
| Level 1 | ||||||||||
| VAR(L1 Residual) | .204 | .204 | .203 | .203 | .203 | |||||
| Spatial Power | .000 | .000 | −.000 | −.000 | −.000 | |||||
| Level 2 | ||||||||||
| VAR(Intercept) | .435 | .439 | .436 | .445 | .436 | |||||
| VAR(Week) | .005 | .005 | .005 | .005 | .005 | |||||
| COR(Intercept/Week) | −.010 | −.015 | .018 | −.045 | −.050 | |||||
|
| ||||||||||
| Model Information | ||||||||||
|
| ||||||||||
| Parameters | 13 | 13 | 13 | 13 | 13 | |||||
| Deviance | 4968.1 | 4970.0 | 4963.0 | 4970.3 | 4966.7 | |||||
Note:
p < .05 after Bonferroni correction. LKT = loving-kindness training. MT = mindfulness training. L1 = Level-1. VAR = variance. COR = correlation.
Vantage/Non-vantage = A-carriers/GG
Vantage/Non-vantage = GG/A-carriers.
Vantage/Non-vantage = GG/T-carriers.
Vantage/Non-vantage = CC/G-carriers.
Vantage/Non-vantage = A-carriers/CC.
To further understand this result, simple intercepts and slopes were calculated and plotted using an online calculator available at http://quantpsy.org/interact/index.html (Preacher et al., 2006). Figure 1 shows the effect of time within each meditation training condition for both non-vantage (T-carriers) and vantage (GG) participants. For participants who had one or two T alleles, small, non-significant increases in PEs emerged over time in the loving-kindness (simple slope = 0.028, SE = 0.014, t = 1.955, p = .053) and mindfulness (simple slope = 0.024, SE = 0.016, t = 1.463, p = .146) training conditions. By contrast, participants who with the GG genotype in the LKT condition showed significant increases in PEs over time (simple slope = 0.078, SE = 0.023, t = 3.392, p = .001), whereas GG individuals in the MT condition showed small, non-significant decreases in PEs (simple slope = −0.035, SE = 0.019, t = −1.793, p = .075). Therefore, loving-kindness training appeared effective in increasing daily PEs, but only for individuals with the rs1042778 GG genotype. Regarding the size of these effects, we calculated Cohen’s d statistics within each level of the moderating variable (non-vantage, vantage) following Feingold (2013, equation 13). The ds represent the difference in PE levels between meditation training types at the end of the study. For individuals who had one or two T alleles, d = 0.024, [−0.340, 0.388]; for individuals with the GG genotype, d = 0.642, [0.278, 1.006]. For completeness, the same analyses were performed on negative emotions, and no significant effects in NEs emerged (Table S6 in Supplementary Material). Finally, inclusion of the set of covariates described in Section 2.5 did not alter the final model results.
Figure 1.
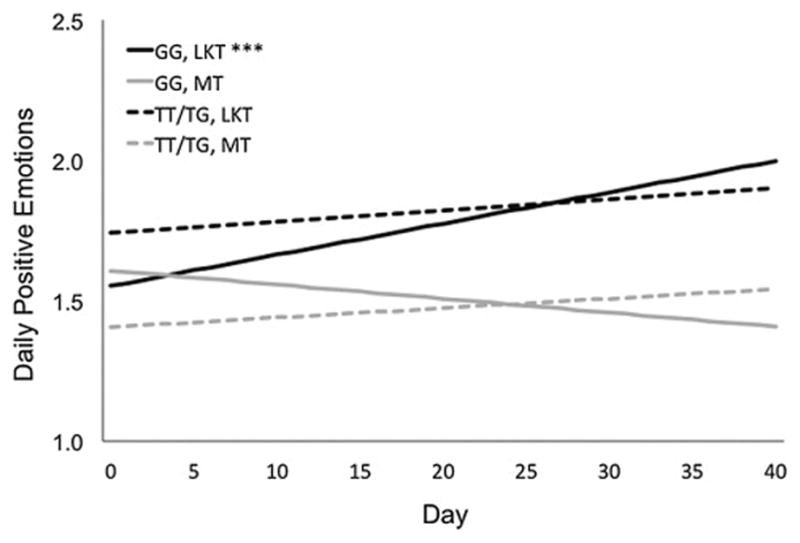
Model estimates of daily positive emotions change, by training condition and OXTR rs1042778 genotype. MT = Mindfulness Training; LKT = Loving-kindness Training. ***p < .001. Day 0 indicates the first day of training.
3.3 Post hoc analyses
To determine if rs1042778 acted in concert with other SNPs in OXTR, haplotypes were generated using the program UNPHASED. The three OXTR SNPs had a moderate degree of linkage disequilibrium (LD): D’ values ranged from .158 (r2 = .004) to .448 (r2=.064). The two CD38 SNPs showed fairly low LD with each other (D′ = .242; r2 = .008). In post hoc analyses, we found that both the three-SNP (rs2254298-rs53576-rs1042778) and the two-SNP (rs53576-rs1042778) OXTR haplotypes containing the rs1042778-T allele (non-vantage) predicted relatively less growth in positive emotions in LKT compared to other haplotypes. These results suggest that rs1042778 was the most influential SNP. Although a significant interaction term was observed for one CD38 haplotype (CT), this did not remain significant after correction for multiple comparisons, with or without covariates. Details on the method and results of the haplotype analysis are included in the Supplementary Material.
4. DISCUSSION
In the present study, we tested how common genetic variability in the oxytocin system was related to positive emotional outcomes of a socially-oriented intervention, namely, training in loving-kindness meditation (with training in mindfulness meditation as the active control condition). The individual effects of genotype in five SNPs—three in OXTR and two in CD38—were tested, and PE growth during meditation training was significantly moderated by one polymorphism, OXTR rs1042778, after correction for multiple testing (Table 2). As hypothesized, individuals with the GG genotype (i.e., vantage genotype) displayed growth in PEs exclusively within the LKT condition, whereas individuals who carried a T allele did not show growth in PEs within either training condition. Our post hoc haplotype analyses also bolster the finding that rs1042778 appears to be the most influential SNP out of the five tested.
These results are consistent with early theorizing (Carter, 1998; Uvnäs-Moberg, 1998) that posits human sociality is, in part, influenced by the oxytocinergic system. Variability in the oxytocin receptor gene may indicate underlying neurobiological differences in the brain, such as the volumes of the amygdala and the anterior cingulate cortex (Furman et al., 2011; Inoue et al., 2010; Tost et al., 2010). Our results are also consistent with previously reported associations between OXTR variants and susceptibility to negative social endophenotypes; our study, however, tested these associations using a vantage sensitivity framework (Pluess & Belsky, 2013). This framing is important because being protected from psychosocial risks does not necessarily imply being advantaged when exposed to positive opportunities. This specific polymorphism within OXTR, which naturally occurs within human populations, may help explain the link between the OT system and individual differences in positive affectivity within social contexts. Because the SNPs in our investigation are located in either the intronic or untranslated regions of OXTR and CD38, their actual functionality is not clear. Further study is needed to uncover how these SNPs affect oxytocin signaling at the molecular level. Future research could also explore whether such individual differences are reflected via morphological and connectivity differences in the extended amygdala network.
Our findings support the idea that oxytocin facilitates the emotional rewards that may arise in social contexts, and contribute to a growing body of research that elucidates the social effects of rs1042778. Consistent with our finding that GG individuals gained positive emotions in a social context, other work has found that this vantage genotype is associated with lower negative affectivity and less inhibited sociality, which in turn, predicted richer social environments (Creswell et al., 2015). The GG genotype has also been associated with greater altruistic behavior (Israel et al., 2009) and higher plasma OT (Feldman et al., 2012), although sometimes the direction of the effect is inconsistent (positive parenting, Michalska et al., 2014) or reversed in different populations (e.g. childhood aggression, Malik et al., 2012). Oxytocin, either administered intranasally or measured peripherally, has been implicated in social processes that involve positive affect, such as attentional bias towards positively-valenced faces (Domes et al., 2013), constructive behaviors during romantic couples’ conflict discussions (Ditzen et al., 2009), and self-reported spirituality, which involves feelings of interconnectedness (Van Cappellen, et al., in press). The present study, however, is one of the first to tie the OT system to experienced positive emotions, and does so after experimentally inducing heightened other-focus, through loving-kindness training. This evidence, taken together, suggests that oxytocin does not affect global positive emotionality, but rather the positive emotional benefits that arise from being benevolently other-focused.
This study has several inherent limitations that we would like to acknowledge. First, inconsistencies can be found in the literature as to the direction of allelic associations, especially across ethnic groups (e.g. Wu et al., 2005; Jacob et al., 2007; Chen et al., 2011a). As such, interpretation of vantage sensitivity is limited by the cultural and ethnic composition of the samples tested. Second, whereas the randomization of participants to training conditions allows us to draw causal inferences about the effects of loving-kindness training on PEs, we note that the genetic data are correlational, and causal inferences cannot be made as to how genetic variation affects positive emotions in this context. Specifically, genetic variation might be responsible for an unmeasured variable (such as greater OT signal transduction) that in turn might account for the patterns of emotionality that emerged here. Additionally, the sample size is modest by genetic standards, and we encourage attempts to replicate these findings in future studies. That said, the predicted and observed three-way interaction—whether based on a single SNP or haplotypes of OXTR—tightly aligns with prior evidence and theory regarding the context-specific effects of OT, suggesting that the current results provide a useful empirical step forward. Finally, although the hypothesized effects emerge when LKT is compared to the active control condition of MT, this study does not help to explain positive emotion growth beyond the training period. Analyses reported in Table S6 of the Supplementary Material show that when LKT is compared to the waitlist control condition, the three-way interaction between time, condition, and genotype is not significant (p = .19). We note, however, that the six-week timespan was not meaningfully equivalent for these two groups, as LKT participants experienced weekly classes, skill learning, and group contact that were not present in the six weeks of daily reporting for the waitlist control group. By contrast, the active control condition of MT provides rigorous experimental control for these contextual factors. In addition, our hypotheses concerned positive emotions while undergoing an intervention (i.e. during a structured, focused workshop) and do not test the long-term effects of learning a type of meditation.
Genetic variation in the oxytocin system remains poorly understood, especially as to how it influences socioemotional processes. For instance, the pathways between variation in OXTR and endogenously circulating OT within the central and peripheral nervous systems have yet to be illuminated. However, future research will be important to uncover how these natural variants alter gene expression and/or the molecular function of proteins critical to OT signaling – a critical step in explaining biobehavioral outcomes related to polymorphisms. Of note, rs1042778 lies in the 3′ untranslated region of OXTR and therefore may affect processes that impact expression of the receptor. Additionally, more research is necessary to determine the psychological mechanisms within rs1042778 T-allele carriers that impede their positive emotion growth when given the opportunity to receive socially-focused training. In light of the current findings, intranasal OT administration during loving-kindness training may be one avenue to pharmacologically manipulate biological support for social orientation with possible consequential changes in PEs (Van Cappellen et al., in press). Finally, the complexity of human sociality suggests the likelihood of biological pathways at multiple levels that influence the emotional benefits of social interaction, and other pathways (e.g. cardiac vagal tone, endocrinological phenotypes) should also be investigated to gain a deeper understanding of the biological basis of sociality.
Despite these limitations, the present study has implications for understanding and tailoring interventions to foster optimal mental health. Insofar as perceived social connection increases positive emotions, it is important to understand how cultivating feelings of social closeness and compassion may be easier for certain individuals relative to others. Our study implies that the oxytocin system may be one of the underlying biological mechanisms that either help or hinder the rewarding qualities of social connection.
In addition, the current study has clinical implications that lend clues for future behavioral interventions that aim to prevent affective disorders, such as depression. Previous research has demonstrated that loving-kindness meditation increases daily positive emotions, raises parasympathetic activity (as indexed by high-frequency heart rate variability, Kok et al., 2013), and decreases depressive symptoms and minor illness symptomatology (Fredrickson et al., 2008). However, the present study demonstrates that certain individuals may not reap these benefits. Knowledge of a person’s oxytocin vantage sensitivity could potentially be used to implement a more personalized approach to selecting behavioral training to improve health and well-being, and may also be useful in pinpointing potential pharmaceutical targets.
In sum, we endeavored to investigate how variability in genes critical to oxytocin reception and secretion influence the positive emotions experienced in response to a socially-focused intervention. G-allele homozygotes of rs1042778 derived positive emotional benefits from loving-kindness training, whereas those with one or two T alleles did not show significant change in positive emotions. Though early considerations of the role of oxytocin in social connection explicitly implicated positive emotions (Carter, 1998; Uvnäs-Moberg, 1998), this is some of the first research on oxytocin and sociality to include day-to-day positive emotions as a dependent measure (see also Algoe & Way, 2014). Moreover, by experimentally manipulating the other-focused sociality of the intervention, we provide strong evidence for the possibility that positive emotions are not simply part of the process of greater sociality, but rather a downstream product. The current evidence illuminates the biological basis of how and for whom the benefits of loving-kindness training emerge. In light of evidence that everyday positive emotions broaden people’s awareness and build their resources, resilience, and physical health, these findings may inform the development of more nuanced interventions to improve health and well-being.
Supplementary Material
Highlights.
OT genetic variability predicts positive emotions for socially-focused meditation training.
OXTR rs1042778 predicted growth in daily positive emotions over 6 weeks.
OXTR rs1042778 GG individuals increased in positive emotions during a socially-focused meditation training.
OXTR rs1042778 T-carriers and GG individuals did not show increased positive emotion growth from non-social meditation training.
Acknowledgments
ROLE OF FUNDING
Barbara L. Fredrickson was supported by grants from the National Institutes of Health (grant Nos. R01NR012899, R01CA170128, and R01AT007884). Suzannah F. Isgett was supported by the National Science Foundation Graduate Research Fellowship (grant No. DGE-1144081). Funding sources had no involvement in any part of conducting research, analyzing data, or writing the manuscript.
The authors thank the lab of Steve W. Cole for providing biological assays and Karen M. Grewen for her comments on an early draft of this manuscript.
Footnotes
Data from this NIH-supported study (R01NR012899) have been reported elsewhere (Fredrickson et al., 2015), and will continue to support other and related investigations.
Two participants inadvertently exceeded this age range and were 65 and 67, respectively, at the study’s beginning.
For analyses reported in this paper, the baseline period was defined as the seven days prior to the beginning of the intervention. In past research we have noted that participants may experience reactivity to the daily measures when first introduced, so we utilized the first week’s reports as an accommodation period. Note, however, that the results reported in this paper are similar regardless of whether all baseline days are included.
We thank the anonymous reviewers for this suggestion.
AUTHOR CONTRIBUTIONS
- Conception and design of study
- Acquisition of data
- Analysis of data
- Interpretation of data
- Drafting of the article
- Revision of the article
- Approved final version of the article
CONFLICTS OF INTEREST None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Algoe SB, Way B. Evidence for a role of the oxytocin system, indexed by genetic variation in CD38, in the social bonding effects of expressed gratitude. Soc Cogn Affect Neurosci. 2014;9(12):1855–1861. doi: 10.1093/scan/nst182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van Ijzendoorn MH. A sociability gene? Meta-analysis of oxytocin receptor genotype effects in humans. Psychiatr Genet. 2014;24:45–51. doi: 10.1097/YPG.0b013e3283643684. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: Context and person matter. Trends Cogn Sci. 2011;15:301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: Differential susceptibility to environmental influences. Psychol Bull. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Black AC, Harel O, Matthews G. Techniques for analyzing intensive longitudinal data with missing values. In: Mehl MR, Conner TS, editors. Handbook of Research Methods for Studying Daily Life. New York, NY: Guilford Press; 2012. pp. 339–356. [Google Scholar]
- Bolger N, Laurenceau JP. An introduction to diary and experience sampling research. New York, NY: Guilford; 2013. Intensive longitudinal methods. [Google Scholar]
- Bower JE, Ganz PA, Irwin MR, Castellon S, Arevalo J, Cole SW. Cytokine genetic variations and fatigue among patients with breast cancer. J Clin Oncol. 2013;31:1656–1661. doi: 10.1200/JCO.2012.46.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardon LR, Palmer LJ. Population stratification and spurious allelic association. The Lancet. 2003;360:598–604. doi: 10.1016/S0140-6736(03)12520-2. [DOI] [PubMed] [Google Scholar]
- Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Carter CS. Oxytocin pathways and the evolution of human behavior. Annu Rev Psychol. 2014;65:10.1–10.23. doi: 10.1146/annurev-psych-010213-115110. [DOI] [PubMed] [Google Scholar]
- Chen FS, Barth ME, Johnson SL, Gotlib IH, Johnson SC. Oxytocin receptor (OXTR) polymorphisms and attachment in human infants. Frontier Psychol. 2011a;2:200. doi: 10.3389/fpsyg.2011.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen FS, Kumsta R, von Dawans B, Monakhov M, Ebstein RP, Heinrichs M. Common oxytocin receptor gene (OXTR) polymorphism and social support interact to reduce stress in humans. Proc Natl Acad Sci U S A. 2011b;108:19937–19942. doi: 10.1073/pnas.1113079108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Positive psychological well-being and mortality: A quantitative review of prospective observational studies. Psychosom Med. 2008;70:741–756. doi: 10.1097/PSY.0b013e31818105ba. [DOI] [PubMed] [Google Scholar]
- Choe HK, Reed MD, Benavidez N, Montgomery D, Soares N, Yim YS, Choi GB. Oxytocin mediates entrainment of sensory stimuli to social cues of opposing valence. Neuron. 2015;87(1):152–163. doi: 10.1016/j.neuron.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creswell KG, Wright AGC, Troxel WM, Ferrell RE, Flory JD, Manuck SB. OXTR polymorphism predicts social relationships through its effects on social temperament. Soc Cogn Affect Neurosci. 2015;10:869–876. doi: 10.1093/scan/nsu132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, Heinrichs M. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biol Psychiatry. 2009;65:728–731. doi: 10.1016/j.biopsych.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Dölen G, Darvishzadeh A, Huang KW, Malenka RC. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 2013;501:179–184. doi: 10.1038/nature12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G, Sibold M, Schulze L, Lischke A, Herpertz SC, Heinrichs M. Intranasal oxytocin increases covert attention to positive social cues. Psychol Med. 2013;43:1747–1753. doi: 10.1017/S0033291712002565. [DOI] [PubMed] [Google Scholar]
- Feingold G. A regression framework for effect size assessments in longitudinal modeling of group differences. Rev Gen Psychol. 2013;17:111. doi: 10.1037/a0030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R, Zagoory-Sharon O, Weisman O, Schneiderman I, Gordon I, Maoz R, et al. Sensitive parenting is associated with plasma oxytocin and polymorphisms in the OXTR and CD38 genes. Biol Psychiatry. 2012;72:175–181. doi: 10.1016/j.biopsych.2011.12.025. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL. What good are positive emotions? Rev Gen Psychol. 1998;2:300–319. doi: 10.1037/1089-2680.2.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL. Positive emotions broaden and build. In: Devine P, Plant A, editors. Adv Exp Soc Psychol. Vol. 47. 2013. pp. 1–53. [Google Scholar]
- Fredrickson BL, Cohn MA, Coffey KA, Pek J, Finkel SM. Open hearts build lives: Positive emotions, induced through loving-kindness meditation, build consequential personal resources. J Pers Soc Psychol. 2008;95:1045–1062. doi: 10.1037/a0013262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman DJ, Chen MC, Gotlib IH. Variant in oxytocin receptor gene is associated with amygdala volume. Psychoneuroendocrinology. 2011;36:891–897. doi: 10.1016/j.psyneuen.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman P, Niemann L, Schmidt S, Walach H. Mindfulness-based stress reduction and health benefits: A meta-analysis. J Psychosom Res. 2004;57:35–43. doi: 10.1016/S0022-3999(03)00573-7. [DOI] [PubMed] [Google Scholar]
- Howell RT, Kern ML, Lyubomirsky S. Health benefits: Meta-analytically determining the impact of well-being on objective health outcomes. Health Psychol Rev. 2007;1:83–136. doi: 10.1080/17437190701492486. [DOI] [Google Scholar]
- Inoue H, Yamasue H, Tochigi M, Abe O, Liu X, Kawamura Y, et al. Association between the oxytocin receptor gene and amygdalar volume in healthy adults. Biol Psychiatry. 2010;68:1066–1072. doi: 10.1016/j.biopsych.2010.07.019. [DOI] [PubMed] [Google Scholar]
- Israel S, Lerer E, Shalev I, Uzefovsky F, Riebold M, Laiba E, et al. The oxytocin receptor (OXTR) contributes to prosocial fund allocations in dictator game and the social value orientations task. PLoS ONE. 2009;4:1–10. doi: 10.1371/journal.pone.0005535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob S, Brune CW, Carter CS, Leventhal BS, Lord C, Cook EH. Association of the oxytocin receptor gene (OXTR) in Caucasian children and adolescents with autism. Neurosci Lett. 2007;417:6–9. doi: 10.1016/j.neulet.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D, Liu HX, Hirai H, Torashima T, Nagai T, Lopatina O, et al. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature. 2007;446:41–45. doi: 10.1038/nature05526. [DOI] [PubMed] [Google Scholar]
- Kahneman D, Krueger AB, Schkade DA, Schwarz N, Stone AA. A survey method for characterizing daily life experience: The day reconstruction method. Science. 2004;306:1776–1780. doi: 10.1126/science.1103572. [DOI] [PubMed] [Google Scholar]
- Kim HS, Sherman DK, Sasaki JY, Xu J, Chu TQ, Ryu C, et al. Culture, distress, and oxytocin receptor polymorphism (OXTR) interact to influence emotional support seeking. Proc Natl Acad Sci U S A. 2010;107:15717–15721. doi: 10.1073/pnas.1010830107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok BE, Coffey KA, Cohn MA, Catalino LI, Vacharkulksemsuk T, Algoe SB, Brantley M, Fredrickson BL. How positive emotions build physical health: Perceived positive social connections account for the upward spiral between positive emotions and vagal tone. Psychol Sci. 2013;24:1123–1132. doi: 10.1177/0956797612470827. [DOI] [PubMed] [Google Scholar]
- Kumsta R, Heinrichs M. Oxytocin, stress and social behavior: Neurogenetics of the human oxytocin system. Curr Opin Neurobiol. 2013;23:11–16. doi: 10.1016/j.conb.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Leng G, Ludwig M. Intranasal oxytocin: Myths and delusions. Biological Psychiatry. 2016;79:243–250. doi: 10.1016/j.biopsych.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Lerer E, Levi S, Salomon S, Darvasi A, Yirmiya N, Ebstein RP. Association between the oxytocin receptor (OXTR) and autism: relationship to Vineland Adaptive Behavior Scales and cognition. Mol Psychiatry. 2008;13:980–988. doi: 10.1038/sj.mp.4002087. [DOI] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Neuropeptide regulation of affiliative behavior and social bonding in animals. Horm Behav. 2006;50:506–517. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Malik AI, Zai CC, Abu Z, Nowrouzi B, Beitchman JH. The role of oxytocin and oxytocin receptor gene variants in childhood-onset aggression. Genes Brain Behav. 2012;11:545–551. doi: 10.1111/j.1601-183X.2012.00776.x. [DOI] [PubMed] [Google Scholar]
- Marlin BJ, Mitre M, D’amour JA, Chao MV, Froemke RC. Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature. 2015;520:499–504. doi: 10.1038/nature14402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalska KJ, Decety J, Liu C, Chen Q, Martz ME, Jacob S, et al. Genetic imaging of the association of oxytocin receptor gene (OXTR) polymorphisms with positive maternal parenting. Front Behav Neurosci. 2014:8. doi: 10.3389/fnbeh.2014.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munesue T, Yokoyama S, Nakamura K, Anitha A, Yamada K, Hayashi K, et al. Two genetic variants of CD38 in subjects with autism spectrum disorder and controls. Neurosci Res. 2010;67:181–191. doi: 10.1016/j.neures.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. 7. Los Angeles, CA: Muthén & Muthén; 1998–2015. [Google Scholar]
- Otake K, Shimai S, Tanaka-Matsumi J, Otsui K, Fredrickson BL. Happy people become happier through kindness: A counting kindnesses intervention. J Happiness Stud. 2006;7:361–375. doi: 10.1007/s10902-005-3650-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluess M, Belsky J. Vantage sensitivity: Individual differences in response to positive experiences. Psychol Bull. 2013;139:901–916. doi: 10.1037/a0030196. [DOI] [PubMed] [Google Scholar]
- Poulin MJ, Holman EA, Buffone A. The neurogenetics of nice: Receptor genes for oxytocin and vasopressin interact with threat to predict prosocial behavior. Psychol Sci. 2012;5:446–452. doi: 10.1177/0956797611428471. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. J Educ Behav Stat. 2006;31:437–448. [Google Scholar]
- Salzberg S. Real Happiness: The Power of Meditation. New York, NY: Workman Publishing Company; 2011. [Google Scholar]
- Seidlmeier P, Eberth J, Schwarz M, Zimmermann D, Haarig F, Jaeger S, Kunze S. The psychological effects of meditation: a meta-analysis. Psychol Bull. 2012;138(6):1129–1171. doi: 10.1037/a0028168. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Abu-Akel A. The social salience hypothesis of oxytocin. Biol Psychiatry. 2016;79:194–202. doi: 10.1016/j.biopsych.2015.07.020. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Wardle J. Enjoying life and living longer. Arch Intern Med. 2012;172:273–275. doi: 10.1001/archinternmed.2011.1028. [DOI] [PubMed] [Google Scholar]
- Stone AA, Schneider S, Harter JK. Day-of-week mood patterns in the United States: On the existence of ‘Blue Monday,’ ‘Thank God It’s Friday’ and weekend effects. J Posit Psychol. 2012;7:306–314. [Google Scholar]
- Szeto A, McCabe PM, Nation DA, Tabak BA, Rossetti MA, McCullough ME, Schneiderman N, Mendez AJ. Evaluation of enzyme immunoassay and radioimmunoassay methods for the measurement of plasma oxytocin. Psychosom Med. 2011;73:393–400. doi: 10.1097/PSY.0b013e31821df0c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tost H, Kolachana B, Hakimi S, Lemaitre H, Verchinski BA, Mattay VS, et al. A common allele in the oxytocin receptor gene (OXTR) impacts prosocial temperament and human hypothalamic-limbic structure and function. Proc Natl Acad Sci U S A. 2010;107:13936–13941. doi: 10.1073/pnas.1003296107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uvnäs-Moberg K. Oxytocin may mediate the benefits of positive social interaction and emotions. Psychoneuroendocrinology. 1998;23:819–835. doi: 10.1016/s0306-4530(98)00056-0. [DOI] [PubMed] [Google Scholar]
- Van Cappellen P, Way B, Isgett SF, Fredrickson BL. Effects of oxytocin administration on spirituality and emotional responses to meditation. Soc Cogn Affect Neurosci. doi: 10.1093/scan/nsw078. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Jia M, Ruan Y, Liu J, Guo Y, Shuang M, et al. Positive association of the oxytocin receptor gene (OXTR) with autism in the Chinese Han population. Biol Psychiatry. 2005;58:74–77. doi: 10.1016/j.biopsych.2005.03.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


