Abstract
Background and Objective: Sleep disturbance is a common problem among adults with obesity. Mindfulness interventions have been shown to improve sleep quality in various populations but have not been investigated in adults with obesity. The aim of this study was to compare the effects of a mindfulness-based weight-loss intervention with an active control on self-reported sleep quality among adults with obesity.
Method: This study was a secondary analysis of a randomized controlled trial and included 194 adults with a body mass index in the range 30–45 kg/m2. The treatment intervention included mindfulness-based eating and stress-management practices, and the active control intervention included training in progressive muscle relaxation (PMR). Both groups received identical diet and exercise guidelines in 17 group sessions conducted over 5.5 months that were matched for time, attention, and social support. The primary outcome of this analysis was between-group change in self-reported sleep quality, which was assessed using the Pittsburgh Sleep Quality Index (PSQI) global score at baseline and at 6, 12, and 18 months.
Results: Between-group differences in mean PSQI change scores in the mindfulness group (n = 100) compared to the control group (n = 94) were −0.27 (−0.68, 1.22; p = 0.58) at 6 months, −0.57 (−0.35, 1.50; p = 0.22) at 12 months, and −0.50 (−0.53, 1.53; p = 0.34) at 18 months, all in the direction of more sleep improvement in the mindfulness group but none reaching statistical significance. In the mindfulness group, average weekly minutes of meditation practice time was associated with improved sleep quality from baseline to 6 months.
Conclusions: No statistically significant evidence was found that a weight-loss program that incorporates mindfulness improves self-reported sleep quality compared to a control diet/exercise intervention that included PMR. Within the mindfulness group, average weekly minutes of mindfulness practice was associated with improved sleep quality.
Keywords: : mindfulness, PSQI, sleep, obesity
Introduction
Poor sleep is a common medical complaint and is associated with poor health outcomes and significant economic impact.1,2 Obesity, which affects approximately 35% of U.S. adults,3 is associated with increased sleep disturbance.4–8 Recent evidence suggests that better sleep quality may increase the likelihood of weight loss,9 and interventions that improve sleep quality in this population may have important health benefits.
Pharmacotherapy for sleep disturbance is limited by side effects, tolerance, and dependence.10–12 Mindfulness-based interventions represent a potentially accessible, long-term alternative and have been shown to improve sleep quality among various adult populations, including those with primary chronic insomnia, cancer, and older adults.13–15 Mindfulness interventions aim to strengthen skills for coping with stress and difficult emotions by building nonjudgmental awareness and decreasing ruminative thinking. Mindfulness trains “metacognitive awareness,” a state of detached and inclusive awareness in which thoughts and emotions are seen as transient events.16 This may have a calming effect on cognition, affect, and physiologic arousal and thereby improve sleep.17
Despite evidence of promise, previous research on the effects of mindfulness on sleep quality has limitations, including small sample sizes and short follow-up. There has also been little investigation of mindfulness-based interventions on sleep among people with obesity. Data from a randomized controlled trial of a mindfulness-based weight-loss intervention were used to examine the effects of mindfulness training on self-reported sleep quality among adults with obesity. It was hypothesized that the mindfulness group would show greater improvements in self-reported sleep quality relative to the active control group.
Prior research suggests that time spent practicing mindfulness as well as increases in mindfulness as measured by the Five Facet Mindfulness Questionnaire (FFMQ) are associated with calming effects on cognition and arousal.18 Therefore, the hypothesis that both FFMQ scores and home mindfulness practice time would be associated with improved sleep in the intervention group was also examined.
Additionally, recent literature has established an association between losing ≥5% of body weight and improvement in sleep.19,20 Therefore, the hypothesis that reductions in body mass index (BMI) would be associated with improvements in self-reported sleep quality was tested.
Materials and Methods
Study design
This study was a secondary analysis from the Supporting Health by Integrating Nutrition and Exercise (SHINE) clinical trial (Clinicaltrials.gov: NCT00960414). The University of California, San Francisco (UCSF) Institutional Review Board approved study procedures, and all participants provided written informed consent.
Adults with obesity were randomized to a 5.5-month diet/exercise intervention with or without mindfulness components in a 1:1 ratio. Participants were assessed at baseline and at 6 (post intervention), 12, and 18 months from intervention initiation. At each time point, participants self-reported sleep quality using the Pittsburgh Sleep Quality Index (PSQI). The primary outcome of this analysis was the difference in changes in mean PSQI global score between groups from baseline to 6, 12, and 18 months.
Participants and participant flow
Eligibility required participants to have a BMI between 30–45 kg/m2 and to be aged ≥18 years. Participants were recruited for a weight-loss study using newspaper advertisements, online postings, and fliers in the community and at UCSF clinics. Participants were randomized to the mindfulness or active control intervention using a computer-generated random allocation sequence (see main outcomes paper21 for additional study details).
Intervention
Both interventions included sixteen 2–2.5 h sessions (12 weekly, three biweekly, and one monthly) and one all-day session over approximately 5.5 months. Both groups received identical diet/exercise guidelines, emphasizing modest calorie reduction (500 kcal/day) and increasing daily activity. Sleep content, such as sleep hygiene education or instruction, was not included in either group.
The intervention arm included meditation practices modeled on Mindfulness-Based Stress Reduction (MBSR),22 mindful eating practices modeled on the Mindfulness-Based Eating Awareness Training program,23 mindful walking, gentle yoga, and loving-kindness meditation.24 Home practice included mindfulness practice for up to 30 min a day, including sitting meditation, eating mindfully, gentle yoga, and brief “mini-meditations” during daily life. Participants received handouts of course material and audio CDs with guided mindfulness practices. Registered dietitians and experienced mindfulness instructors led the sessions.
In the active control group, a mindfulness approach to stress management was controlled for by including training in progressive muscle relaxation (PMR), although at a lower dose than in the mindfulness intervention. PMR instruction was taught in 4/16 sessions (session 4, session 6, the all-day session, and session 14). The control group also received nutritional and physical activity information, strength training with exercise bands, discussion of societal issues concerning weight loss, snacks, and home activities in order to control for the time, attention, social support, and expectation of benefit that the mindfulness participants may have experienced. Participants were provided handouts as well as an audio CD with PMR instruction for optional home practice. Registered dietitians led the control-arm sessions.
Measurements
Self-reported sleep quality was measured using the PSQI, a widely used and validated 19-item questionnaire of clinical sleep behavior and quality.25 The total Global Sleep Score can range from 0 to 21. Higher scores reflect more sleep disturbance, and scores >5 represent clinically significant sleep disturbance.29 The PSQI has high sensitivity (99%) and specificity (84%) for identifying sleep disturbance and has demonstrated good test–retest reliability and validity among adults with sleep disturbance.26,27
Participants in the mindfulness group completed home practice logs on the number of minutes spent each day in home mindfulness practice, which included sitting mindfulness meditation (breath-centered meditation with attention to thoughts, feelings, and other body sensations); an extended-exhalation breathing exercise; gentle yoga; and loving-kindness meditation. Mindfulness practice was assessed after the intervention period ended using retrospective report. All meditation practices were examined together as well as sitting meditation specifically. Practice variables were defined for both as average minutes per week during the intervention phase, as well as average weekly minutes during the final intervention week. Average minutes of weekly practice was chosen rather than total minutes of practice in order to be as informative as possible for clinical practice. It was assumed that an association between sleep quality and weekly minutes of practice would be easier to discuss in patient counseling than an association would between sleep quality and total minutes of practice over 5.5 months. Practice variables were examined during the final week of the intervention in order to explore longevity of the impact of mindfulness practice. As the first follow-up PSQI was administered about 2 weeks after the end of the intervention (6 months), the study sought to examine the impact of practice close to the time of PSQI survey completion.
In the control group, home PMR practice was assessed. For comparison purposes, PMR home practice variables were chosen that were consistent with home mindfulness practice variables: average minutes of home PMR practice per week throughout the intervention and during the final week of the intervention.
To assess mindfulness, the FFMQ,28 a 39-item self-report questionnaire assessing five dimensions of mindfulness (observing, describing, acting with awareness, non-judging, and non-reactivity), was administered at 0, 6, 12, and 18 months. The FFMQ has demonstrated sensitivity to change in mindfulness, increasing as individuals learn and practice mindfulness.28 For the present analysis, total FFMQ scores were examined.
Sleep apnea was assessed using the Berlin Sleep Questionnaire (BSQ), a validated self-report tool for determining risk of sleep apnea,29 in order to examine differences in sleep apnea risk (high vs. low risk) between groups.
Statistical analysis
Intention-to-treat analyses were performed. Observed data were analyzed with STATA v14.1 using t-tests to analyze between- and within-group changes in PSQI from baseline to post intervention (6, 12, and 18 months). These analyses of observed data were compared to mixed models that used multiple imputation to replace missing data, based on guidelines for reporting and interpreting results of multiple imputation analyses.30 Missing data were handled using SAS v9.4 procedures PROC MI and MIANALYZE. Imputation models for each outcome variable included values at other time points, attendance (counting the all-day session as two) and its interaction with arm, and an arm–round interaction term to adjust for clustering effects.
Additionally, linear regression was used to examine variables that were hypothesized to be associated with change in PSQI score from baseline to 6 months (post intervention), including change in mindfulness as measured by the FFMQ, change in BMI, home mindfulness practice (mindfulness group), and home PMR practice (active control group). Time-lagged linear regression of the change in PSQI from 6 to 18months was used to examine if practice variables during the intervention period predicted subsequent changes in sleep quality during long-term follow-up.
Results
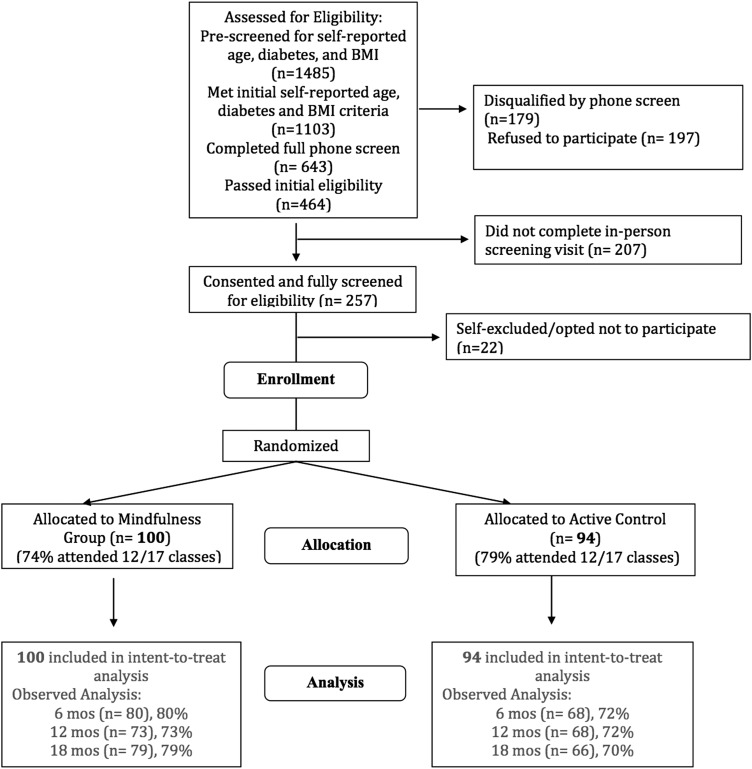
The trial enrolled 194 adults, 100 of whom were randomized to the mindfulness group and 94 to the control group (Fig. 1). Participants in the two groups did not significantly differ on socio-demographic or clinical characteristics at baseline (Table 1). The majority of participants were female (80%), white (59%), and well-educated (64% with a bachelor's or advanced degree). At baseline, the mean (SD) age of participants was 47 years (13 yeras), and the mean (SD) BMI was 35.5 kg/m2 (3.62 kg/m2). The mean (SD) PSQI score was 5.95 (3.00), above the cutoff for clinically significant sleep disturbance.
FIG. 1.
Recruitment, randomization, and follow-up of participants.
Table 1.
Baseline Characteristics of Study Participants
| Characteristic | Mindfulness group (N = 100) | Control group (N = 94) |
|---|---|---|
| Age, years ± SD | 47 ± 13 | 47 ± 12 |
| Female, n (%) | 79 (79%) | 76 (81%) |
| Ethnicity, n (%) | ||
| White | 64 (65%) | 50 (53%) |
| Black | 13 (13%) | 12 (12%) |
| Latina/o | 7 (7%) | 16 (17%) |
| Asian/Pacific Islander | 8 (8%) | 11 (11%) |
| Education attained, n (%) | ||
| High school | 1 (1%) | 6 (6%) |
| Some college | 15 (15%) | 21 (22%) |
| Bachelor's | 41 (41%) | 34 (36%) |
| Advanced degree | 28 (28%) | 22 (23%) |
| Baseline global PSQI score, mean ± SD | 5.81 ± 3.4 | 6.1 ± 2.6 |
| Baseline BMI, mean ± SD | 35.4 ± 3.5 | 35.6 ± 3.8 |
| Berlin Sleep Questionnaire, % high riska | 66% | 69% |
| Average hours of sleep per night, mean ± SD | 7.22 ± 1.22 | 7.35 ± 1.57 |
| Sleep aid use | 4.00% | 2.86% |
| Antidepressant use | 17.00% | 17.02% |
| Diuretic use | 7.00% | 7.45% |
| Baseline FFMQb score | 134.09 ± 18.75 | 134.46 ± 20.24 |
According to the Berlin Sleep Questionnaire, a patient is considered high risk if there are two or more of three categories in which the score is positive (categories are: snoring/sleep behavior, daytime sleepiness, and BMI/history of hypertension).
Five Facet Mindfulness Questionnaire, total score from sum of five subcomponents. These baseline FFMQ scores are comparable to other total FFMQ scores among adults shown in recent studies. A 2016 study of 80 American adults with osteoarthritis showed a baseline total FFMQ score of 142.8 ± 16.6.31 Another 2016 study of 305 Dutch adults aged 16–65 years showed a baseline total FFMQ score of 128.24 ± 15.58.32
SD, standard deviation; PSQI, Pittsburg Sleep Quality Index; BMI, body mass index.
Of the 194 adults enrolled in the study, 148 (75%) were retained to the final 18-month assessment. Retention was similar for the mindfulness and control participants at 6 months (84% and 77%, respectively), 12 months (79% and 74%, respectively), and 18 months (81% and 71%, respectively). There were no statistically significant between-group differences in use of sleep medications, antidepressants, or diuretics (medications known to affect sleep) at any time points. There were no adverse events throughout the study.
Analyses of observed data showed that between-group differences in mean PSQI change scores were −0.27 at 6 months (−0.68, 1.22; p = 0.58), −0.57 at 12 months (−0.35, 1.50; p = 0.22), and −0.50 at 18 months (−0.53, 1.53; p = 0.34), all in the direction of more improvement in the mindfulness group but none reaching statistical significance (Table 2). Mixed-effects models with multiple imputation yielded similar results. Between-group analysis tended to show more sleep improvement in the mindfulness group but with small differences in effect size that were not statistically significant (between-group effect sizes of 0.09 at 6 months, 0.20 at 12 months, and 0.15 at 18 months; see Table 2).
Table 2.
Change in PSQI Score
| Observed | Multiple imputation | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean change (95% CI) | Mean change (95% CI), [effect size]b | |||||||
| Measure | M, Ca | Mindfulness | Control | Between-group difference, mean (95% CI) | p-Value | Mindfulness | Control | Between-group difference, mean (95% CI), [effect size]c | p-Value |
| PSQI score | |||||||||
| 6 months | 80, 68 | −1.55 (−2.26, −0.84)* | −1.28 (−1.91, −0.65)* | −0.27 (−0.68, 1.22) | 0.58 | −1.48 (−2.18, −0.77),* [0.49] | −1.46 (−2.21, −0.71),* [0.49] | −0.02 (−1.05, 1.02), [0.09] | 0.97 |
| 12 months | 73, 68 | −0.99 (−1.61, −0.36)* | −0.42 (−1.11, 0.28) | −0.58 (−0.35, 1.50) | 0.22 | −0.98 (−1.74, −0.23),* [0.37] | −0.63 (−1.40, 0.13), [0.14] | −0.35 (−1.43, 0.73), [0.20] | 0.52 |
| 18 months | 79, 66 | −0.47 (−1.22, 0.28) | −0.30 (−0.73, 0.67) | −0.50 (−0.53, 1.53) | 0.34 | −0.18 (−0.96, 0.59), [0.14] | −0.36 (−1.16, 0.43), [0.01] | 0.18 (−0.92, 1.28), [0.15] | 0.75 |
Decrease in mean PSQI score (negative change score) indicates improved sleep quality from baseline. Independent t-tests were used to compare means between arms using observed data (left columns). These analyses were compared with mixed models using multiple imputation to replace missing data (right columns). Imputation models for each outcome variable included values at other time points, attendance (counting the all-day session as two) and its interaction with arm, and an arm–round interaction term (ROUNDARM) to adjust for clustering effects.
“M” indicates mindfulness group; “C” indicates control group.
Within-group effect sizes are Cohen's d.
Between-group effect sizes are bias-corrected Cohen's d, as done in Black et al.14
p < 0.05.
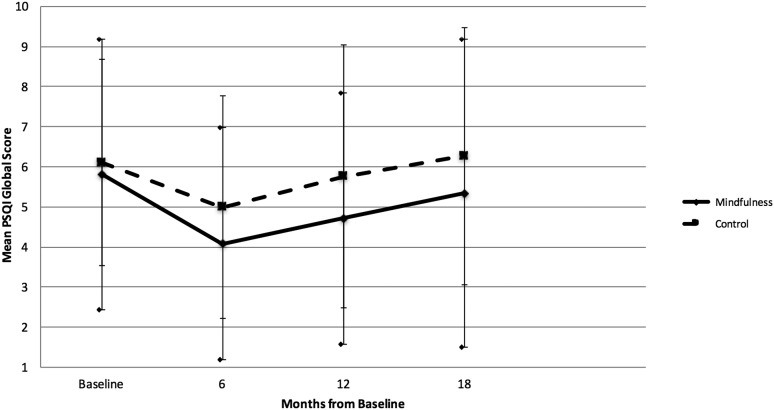
The mindfulness group experienced a statistically significant decrease in mean PSQI scores at 6 and 12 months compared with baseline, consistent with improved sleep quality. The active control group also experienced improved sleep quality at 6 and 12 months, but the change was of lower magnitude than the mindfulness group and was not statistically significant at 12 months (Fig. 2). Neither group had mean changes in PSQI score statistically significantly different from 0 at 18 months.
FIG. 2.
Mean Pittsburgh Sleep Quality Index (PSQI) global score by group at each time point. Mean PSQI global score for mindfulness group (solid line) and control group (dotted line) at baseline and at 6, 12, and 18 months. Error bars represent standard error (SE bars for the mindfuless group are bounded by diamond shapes). Decrease in mean PSQI score indicates improved sleep quality. Intervention began at baseline and lasted 5.5 months.
Within the mindfulness group, during the intervention phase, the mean (SD) number of minutes of practice per week was 42.3 min (30.1 min) for sitting practice and 71.3 min (50.9 min) for all meditation practices. Univariate linear regression showed that average minutes per week of all meditation practices as well as sitting meditation during the intervention phase was statistically significantly associated with improvement in sleep quality from baseline to 6 months (i.e., post-intervention; Table 3). Sitting practice during the final week of the 5.5-month intervention was also significantly associated with improved sleep. Increased FFMQ scores were associated with improved sleep quality. In time-lagged linear regression, meditation practice during the intervention period (0 to 5.5 months) was not a statistically significant predictor of improvement in sleep quality from 6 to 18 months. However, the average minutes of meditation per week from 15 to 18 months was associated with statistically significantly improved sleep quality from 6 to 18 months (regression coefficient −0,071; 95% confidence interval −0.129, −0.013; p = 0.017).
Table 3.
Predictors of Change in PSQI Score
| Change in PSQI from 0 to 6 months | Time-lagged: change in PSQI from 6 to 18 months | |||
|---|---|---|---|---|
| Dependent variable | Regression coefficient(95% CI) | p-Value | Regression coefficient (95% CI) | p-Value |
| Mindfulness group | ||||
| Change in total average FFMQ score, 0–6 months | −0.45 (−0.83, −0.66) | 0.02 | −0.03 (−0.40, 0.33) | 0.86 |
| Change in BMI, 0–6 months | 0.13 (−0.19, 0.45) | 0.43 | 0.01 (−0.30, 0.31) | 0.96 |
| Average minutes of home sitting mindfulness meditation practicea per week | −0.04 (−0.06, −0.02) | 0.001 | 0.0001 (−0.02, 0.02) | 0.99 |
| Average minutes of home total meditation practiceb per week | −0.02 (−0.03, −0.002) | 0.03 | 0.0001 (−0.01, 0.01) | 0.98 |
| Average minutes of home sitting mindfulness meditation practice during final intervention week (week 22) | −0.02 (−0.03, −0.01) | 0.005 | −0.003 (−0.02, 0.01) | 0.67 |
| Average minutes of home total meditation practice during final intervention week (week 22) | −0.01 (−0.01, 0.00) | 0.17 | −0.01 (−0.01, 0.00) | 0.18 |
| Active control group | ||||
| Change in total FFMQ score, 0–6 months | 0.08 (−0.35, 0.51) | 0.73 | 0.01 (−0.52, 0.54) | 0.97 |
| Change in BMI, 0–6 months | 0.06 (−0.26, 0.38) | 0.70 | −0.13 (−0.51, 0.24) | 0.49 |
| Average minutes of home PMR practice per week | −0.02 (−0.09, 0.04) | 0.48 | 0.03 (−0.05, 0.10) | 0.47 |
| Average minutes of home PMR practice during final intervention week (week 22) | −0.04 (−0.07, −0.20) | 0.001 | 0.01 (−0.03, 0.04) | 0.74 |
Analyses used univariate linear regression. Note that negative regression coefficient generally denotes that the independent variable predicts improved sleep quality (decrease in PSQI score indicates improved sleep quality). Statistically significant p-values (<0.05) are shown in bold.
Total meditation practice denotes the sum of minutes of any intervention meditation activities, including abdominal breathing, loving kindness meditation, meditation, and gentle yoga.
Sitting mindfulness meditation practice denotes the sum of minutes spent in sitting breath-centered meditation. This practice invited participants to bring attention to breathing sensations as the recurring focus of attention and to notice without judgment other physical sensations, sounds, thoughts, and feelings that arise in awareness with an attitude of kindness towards oneself.
FFMQ, Five Facet Mindfulness Questionnaire; PMR, progressive muscle relaxation.
Within the active control group, the mean (SD) minutes of PMR practiced at home per week was 7.9 min (10.0 min). Univariate linear regression showed that average minutes of PMR practice per week during the intervention was not statistically significantly associated with improved sleep quality from baseline to 6 months (Table 3). However, average minutes of PMR practice during the last week of the intervention period was statistically significantly associated with improvement in sleep quality. Time-lagged regression showed that PMR practice did not predict improved sleep from 6 to 18 months.
Both groups experienced reduction in BMI, but change in BMI from 0 to 6 months was not associated with change in sleep quality from 0 to 6 months or from 6 to 18 months (time-lagged analysis) in either group (Table 3).
Discussion
Data were analyzed from a randomized controlled trial of a mindfulness-based weight-loss intervention to examine the effects of mindfulness training on self-reported sleep quality among adults with obesity. The main findings showed no significant improvement in sleep quality between the mindfulness group and the active control group, despite a statistically significant improvement in sleep within the mindfulness group from baseline to 6 and 12 months.
While the improvements observed in sleep quality from baseline in the mindfulness group are generally similar to those observed in other studies, the between-group differences in sleep quality observed are smaller than some other reports of mindfulness and behavioral interventions.33 For example, in a randomized controlled trial among older adults, Black et al. showed that participants in a 6-week mindfulness intervention experienced significant improvement in sleep quality compared with a control group receiving sleep hygiene education, with a between-group effect size of 0.89.14 The larger effect seen suggests that sustained mindfulness practice over the period being assessed may be important for effects on sleep. The Black et al. study incorporated weekly 2 h mindfulness sessions plus daily home practice and assessed change in sleep quality within 10 days after the 6-week period. Participants had a mean meditation practice time of about 172 min per week. In contrast, the present intervention was spread out over 5.5 months, and sleep quality was assessed at 6 months at the earliest, providing a potentially important lapse in mindfulness practice between intervention and assessment. Additionally, participants in the mindfulness group practiced mindfulness at home for an average of 71 min per week. This less-concentrated dose of meditation may have contributed to the smaller effect. Indeed, most other studies suggesting the benefits of mindfulness for improving sleep quality are modeled after MBSR, and thus are usually 6 or 8 weeks in duration and more concentrated than the present intervention.13,34 More research to elucidate the relationship between the length, concentration, and dose of mindfulness and impact on sleep quality would clarify this relationship. In addition, the current results show a waning of benefits over time within the mindfulness group, suggesting the importance of long-term follow-up to assess durability of effects in future studies.
This potential relationship between the concentration of the dose of mindfulness practice and the effect is especially pertinent in light of the findings on the association between mindfulness as measured by the FFMQ and mindfulness practice time with improved sleep. It was found that increased self-reported mindfulness as well as average minutes of weekly meditation practice and sitting mindfulness meditation practice during the first 6 months of the study was associated with improvements in sleep quality from baseline to 6 months. It was also found that the average minutes of sitting meditation in the last week of the 5.5-month intervention was associated with improved self-reported sleep quality at 6 months. However, meditation practice during the first 6 months was not clearly associated with improved sleep from 6 to 18 months. In contrast, average minutes of meditation practice per week from 15 to 18 months after intervention initiation was significantly associated with improved sleep quality at 18 months. Taken together, these findings suggest that the benefits of mindfulness training for sleep quality depend on continued practice.
Some studies have observed a modest association between weight loss and improved sleep.19 An association between reduction in BMI and improved sleep was not found in the present study. Given the theoretical model linking mindfulness to sleep, in which mindfulness cultivates nonjudgmental awareness and creates a calming effect on cognition and physiologic arousal,17 it follows that BMI may not have a significant role in the mechanism by which mindfulness might improve sleep. Further research on the interplay between mindfulness, weight, arousal, and sleep may help to clarify the relationship between mindfulness, BMI, and sleep.
Despite the lack of clear differences between groups, the mindfulness group reported statistically significant improvements in sleep quality from baseline to 6 and 12 months. While the effect was modest, the improvement was clinically meaningful, with the mean PSQI dropping from 5.81 at baseline to 4.08 at 6 months (i.e., below the cutoff for clinically significant sleep disturbance).
In light of the lack of significant differences between groups, it is important to note that the active control group included several components that may have contributed to improvements in sleep quality, particularly the incorporation of PMR. PMR practice during the last week of the intervention period was significantly associated with improvement in sleep quality from baseline to 6 months. This suggests that, similar to meditation practice, PMR may improve sleep quality when it is practiced, but only with a short-term effect. The potential benefit of PMR on sleep quality is consistent with prior studies showing improved sleep with PMR compared to no or sham treatment.35,36
This study has several limitations. First, changes in sleep may have been complemented by polysomnography or actigraphy. Second, this was primarily a weight-loss, not sleep, intervention. A mindfulness intervention aimed at improving sleep and powered to detect differences in sleep quality may have shown stronger effects. Third, the trial lacked a no-treatment arm that did not receive any stress-reduction or relaxation training. A no-treatment arm may have identified the extent to which sleep improvement in either group was due to more general effects of group participation or behavior changes for weight loss, rather than specifically due to mindfulness or PMR practice. Finally, while the study sample was fairly diverse, the predominance of white, well-educated, and female participants places some limits on the generalizability of the results.
Conclusions
This study did not find statistically significant evidence that a mindfulness-based weight-loss program improved self-reported sleep quality compared to a control diet/exercise intervention that included PMR. However, increases in mindfulness and average weekly minutes of mindfulness practice were associated with improved sleep quality.
Acknowledgments
This study was supported by NIH grants from the National Center for Complementary and Integrative Health (NCCIH) P01AT005013 (F.M.H.), K24AT007827 (F.M.H.), T32AT003997 (F.M.H./E.A.) and K01AT004199 (J.D.), as well as the National Center for Advancing Translational Sciences, UCSF-CTSI Grant Number UL1 TR000004.
Author Disclosure Statement
F.M.H. is on the Scientific Advisory Board for Virta. The other authors have no conflicts of interest to disclose and no competing financial interests exist.
References
- 1.Colten HR, Altevogt BM, eds. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. Washington DC: National Academy of Sciences, 2006 [PubMed] [Google Scholar]
- 2.Ozminkowski RJ, Wang S, Walsh JK. The direct and indirect costs of untreated insomnia in adults in the United States. Sleep 2007;30:263–273 [DOI] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Kit BK, et al. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 2014;311:806–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wallander MA, Johansson S, Ruigomez A, et al. Morbidity associated with sleep disorders in primary care: A longitudinal cohort study. Prim Care Companion J Clin Psychiatry 2007;9:338–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Theorell-Haglow J, Lindberg E, Janson C. What are the important risk factors for daytime sleepiness and fatigue in women? Sleep 2006;29:751–757 [DOI] [PubMed] [Google Scholar]
- 6.Fogelholm M, Kronholm E, Kukkonen-Harjula K, et al. Sleep-related disturbances and physical inactivity are independently associated with obesity in adults. Int J Obes (Lond) 2007;31:1713–1721 [DOI] [PubMed] [Google Scholar]
- 7.Foley D, Ancoli-Israel S, Britz P, et al. Sleep disturbances and chronic disease in older adults: Results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res 2004;56:497–502 [DOI] [PubMed] [Google Scholar]
- 8.Vgontzas AN, Bixler EO, Tan TL, et al. Obesity without sleep apnea is associated with daytime sleepiness. Arch Intern Med 1998;158:1333–1337 [DOI] [PubMed] [Google Scholar]
- 9.Thomson CA, Morrow KL, Flatt SW, et al. Relationship between sleep quality and quantity and weight loss in women participating in a weight-loss intervention trial. Obesity (Silver Spring) 2012;20:1419–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morin CM, Colecchi C, Stone J, et al. Behavioral and pharmacological therapies for late-life insomnia: A randomized controlled trial. JAMA 1999;281:991–999 [DOI] [PubMed] [Google Scholar]
- 11.Nowell PD, Mazumdar S, Buysse DJ, et al. Benzodiazepines and zolpidem for chronic insomnia: A meta-analysis of treatment efficacy. JAMA 1997;278:2170–2177 [PubMed] [Google Scholar]
- 12.Silber MH. Clinical practice. Chronic insomnia. N Engl J Med 2005;353:803–810 [DOI] [PubMed] [Google Scholar]
- 13.Gross CR, Kreitzer MJ, Reilly-Spong M, et al. Mindfulness-based stress reduction versus pharmacotherapy for chronic primary insomnia: A randomized controlled clinical trial. Explore (NY) 2011;7:76–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Black DS, O'Reilly GA, Olmstead R, et al. Mindfulness meditation and improvement in sleep quality and daytime impairment among older adults with sleep disturbances: A randomized clinical trial. JAMA Intern Med 2015;175:494–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlson LE, Garland SN. Impact of mindfulness-based stress reduction (MBSR) on sleep, mood, stress and fatigue symptoms in cancer outpatients. Int J Behav Med 2005;12:278–285 [DOI] [PubMed] [Google Scholar]
- 16.Teasdale JD, Segal ZV, Williams JM, et al. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. J Consult Clin Psychol 2000;68:615–623 [DOI] [PubMed] [Google Scholar]
- 17.Garland SN, Willoughby B, Agagianian N, et al. Mindfulness, affect, and sleep: Current perspectives and future directions. In: Babson K, Feldner M, eds. Sleep and Affect: Assessment, Theory, and Clinical Implications. London: Academic Press, 2015:339–373 [Google Scholar]
- 18.Carmody J, Baer RA. Relationships between mindfulness practice and levels of mindfulness, medical and psychological symptoms and well-being in a mindfulness-based stress reduction program. J Behav Med 2008;31:23–33 [DOI] [PubMed] [Google Scholar]
- 19.Alfaris N, Wadden TA, Sarwer DB, et al. Effects of a 2-year behavioral weight loss intervention on sleep and mood in obese individuals treated in primary care practice. Obesity (Silver Spring) 2015;23:558–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaput JP, Drapeau V, Hetherington M, et al. Psychobiological impact of a progressive weight loss program in obese men. Physiol Behav 2005;86:224–232 [DOI] [PubMed] [Google Scholar]
- 21.Daubenmier J, Moran PJ, Kristeller J, et al. Effects of a mindfulness-based weight loss intervention in adults with obesity: A randomized clinical trial. Obesity (Silver Spring) 2016;24:794–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kabat-Zinn J. Full Catastrophe Living. New York: Dell Publishing, 1990 [Google Scholar]
- 23.Kristeller JL, Wolever RQ. Mindfulness-based eating awareness training for treating binge eating disorder: The conceptual foundation. Eat Disord 2011;19:49–61 [DOI] [PubMed] [Google Scholar]
- 24.Dreyer D, Dreyer K. Chi Walking: The Five Mindful Steps for Lifelong Health and Energy. New York: Simon and Schuster, 2006 [Google Scholar]
- 25.Buysse DJ, Reynolds CF, 3rd, Monk TH, et al. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213 [DOI] [PubMed] [Google Scholar]
- 26.Mariman A, Vogelaers D, Hanoulle I, et al. Validation of the three-factor model of the PSQI in a large sample of chronic fatigue syndrome (CFS) patients. J Psychosom Res 2012;72:111–113 [DOI] [PubMed] [Google Scholar]
- 27.Backhaus J, Junghanns K, Broocks A, et al. Test–retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res 2002;53:737–740 [DOI] [PubMed] [Google Scholar]
- 28.Baer RA, Smith GT, Lykins E, et al. Construct validity of the five facet mindfulness questionnaire in meditating and nonmeditating samples. Assessment 2008;15:329–342 [DOI] [PubMed] [Google Scholar]
- 29.Netzer NC, Stoohs RA, Netzer CM, et al. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med 1999;131:485–491 [DOI] [PubMed] [Google Scholar]
- 30.Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: Potential and pitfalls. BMJ 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee AC, Harvey WF, Price LL, et al. Mindfulness is associated with psychological health and moderates pain in knee osteoarthritis. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society; 2016. http://dx.doi.org/10.1016/j.joca.2016.06.017 (accessed August30, 2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsafou KE, Lacroix JP, van Ee R, et al. The relation of trait and state mindfulness with satisfaction and physical activity: A cross-sectional study in 305 Dutch participants. J Health Psychol 2016. http://journals.sagepub.com/doi/full/10.1177/1359105315624748 (accessed August30, 2016) [DOI] [PubMed]
- 33.Irwin MR, Cole JC, Nicassio PM. Comparative meta-analysis of behavioral interventions for insomnia and their efficacy in middle-aged adults and in older adults 55+ years of age. Health Psychol 2006;25:3–14 [DOI] [PubMed] [Google Scholar]
- 34.Klatt MD, Buckworth J, Malarkey WB. Effects of low-dose mindfulness-based stress reduction (MBSR-ld) on working adults. Health Educ Behav 2009;36:601–614 [DOI] [PubMed] [Google Scholar]
- 35.Means MK, Lichstein KL, Epperson MT, et al. Relaxation therapy for insomnia: Nighttime and day time effects. Behav Res Ther 2000;38:665–678 [DOI] [PubMed] [Google Scholar]
- 36.Edinger JD, Wohlgemuth WK, Radtke RA, et al. Cognitive behavioral therapy for treatment of chronic primary insomnia: A randomized controlled trial. JAMA 2001;285:1856–1864 [DOI] [PubMed] [Google Scholar]