Abstract
A majority of people who have sustained spinal cord injury (SCI) experience chronic pain after injury, and this pain is highly resistant to available treatments. Contusive SCI in rats at T10 results in hyperexcitability of primary sensory neurons, which contributes to chronic pain. KCNQ channels are widely expressed in nociceptive dorsal root ganglion (DRG) neurons, are important for controlling their excitability, and their activation has proven effective in reducing pain in peripheral nerve injury and inflammation models. The possibility that activators of KCNQ channels could be useful for treating SCI-induced chronic pain is strongly supported by the following findings. First, SCI, unlike peripheral nerve injury, failed to decrease the functional or biochemical expression of KCNQ channels in DRG as revealed by electrophysiology, real-time quantitative polymerase chain reaction, and Western blot; therefore, these channels remain available for pharmacological targeting of SCI pain. Second, treatment with retigabine, a specific KCNQ channel opener, profoundly decreased spontaneous activity in primary sensory neurons of SCI animals both in vitro and in vivo without changing the peripheral mechanical threshold. Third, retigabine reversed SCI-induced reflex hypersensitivity, adding to our previous demonstration that retigabine supports the conditioning of place preference after SCI (an operant measure of spontaneous pain). In contrast to SCI animals, naïve animals showed no effects of retigabine on reflex sensitivity or conditioned place preference by pairing with retigabine, indicating that a dose that blocks chronic pain-related behavior has no effect on normal pain sensitivity or motivational state. These results encourage the further exploration of U.S. Food and Drug Administration–approved KCNQ activators for treating SCI pain, as well as efforts to develop a new generation of KCNQ activators that lack central side effects.
Keywords: : hyperexcitability, K+ channels, nociceptor, retigabine, spinal contusion
Introduction
Approximately two-thirds of individuals who have spinal cord injury (SCI) experience a slowly developing chronic pain.1,2 Chronic pain in humans after SCI is most commonly at-level and below-level, relative to the lesion.3 Pain is most often spontaneous in human patients, but evoked pain in response to normally innocuous stimuli (allodynia) and exaggerated pain following noxious stimuli (hyperalgesia) also occur.4 Post-SCI pain can be devastating, and is highly resistant to conventional treatments.5 Thus, additional approaches are needed to relieve post-SCI pain.
Multiple sites along the pain pathway are altered after SCI, and both central and peripheral alterations appear to be important for inducing and maintaining SCI pain.5–15 Most research into the mechanisms underlying SCI-induced pain has focused on alterations within the spinal cord associated with reflex hypersensitivity, which is commonly used as an indicator of evoked pain,6–8,15 despite recent indications that hypersensitivity of reflexes below a spinal injury level provides limited information about actual pain.16,17 Few preclinical studies have investigated ongoing spontaneous pain after SCI.18,19
Interestingly, both spontaneous pain and reflex hypersensitivity in a rat model of contusive SCI depend upon hyperactivity in primary afferent neurons.19 This hyperactivity is particularly pronounced in C-fiber nociceptors, and involves spontaneous activity (SA) generated both in peripheral terminals of nociceptors11 and in their cell bodies within dorsal root ganglia (DRGs).10,19,20 The SA in nociceptors is associated with membrane depolarization and increased membrane resistance,10 which strongly suggests that SA is promoted by chronic closing or downregulation of K+ channels.21 Nevertheless, enhancing residual K+ channel function in nociceptors may offer a promising approach for reducing SCI-induced chronic pain.
KCNQ channels (also called M channels) comprise the Kv7 subfamily (Kv7.1-Kv7.5).22,23 The α-subunits tetramerize to form a functional channel, which can be homomeric or heteromeric.23,24 Many neurons in rat DRGs, including small neurons that respond to capsaicin (presumptive nociceptors), express Kv7.2, Kv7.3 and/or Kv7.5.25,26 These channels are important for setting resting membrane potential (RMP) and controlling the excitability of nociceptors.25,27 Retigabine, a KCNQ channel opener, hyperpolarizes primary afferent fibers28 and reduces excitatory transmission from Aδ and C-fibers to dorsal horn neurons.25 Conversely, a KCNQ channel inhibitor, XE991, strongly enhances peripheral Aδ discharge produced by heat or mechanical stimulation in vivo.29 The potential utility of targeting KCNQ channels to treat chronic pain is suggested by pharmacological studies. Intraplantar injection of XE991 induced concentration-dependent nocifensive behavior, whereas administration of the KCNQ opener retigabine reversed behavioral signs of mechanical and thermal hypersensitivity in both inflammatory and neuropathic pain models.25,30–34 Thus, enhancing KCNQ channel activity may be an attractive strategy to help treat intractable chronic pain after SCI. However, if the expression of KCNQ channels decreases substantially after SCI, as has been reported in peripheral neuropathic pain models,26 insufficient KCNQ channels may be available to target for blocking SCI pain. Here, we report that functional KCNQ channel expression is not decreased after SCI, that opening these channels with retigabine suppresses SA in DRG neurons, and that retigabine effectively reduces some signs of chronic pain in a rodent SCI model.
Methods
All procedures conformed to the guidelines of the International Association for the Study of Pain, and were approved by the animal care and use committee of the University of Texas McGovern Medical School at Houston. Male, adult, Sprague-Dawley rats (200–300 g) were used in this study. Animals were housed two per cage in a controlled environment (12 h reversed light/dark cycle, 21 ± 1°C) with standard food and water. The rats were allowed to adjust to their environment for a week before experimentation.
Spinal cord injury
Male, adult, Sprague-Dawley rats (200–300 g) were anesthetized with a mixture of ketamine (80 mg/kg), xylazine (20 mg/kg), and acepromazine (0.75 mg/kg). Animals received a laminectomy at T10 followed by a spinal contusion using the Infinite Horizon impactor (150 kdyne, 1 sec dwell time). Sham animals received a laminectomy and were treated identically except for the spinal impact. The surgical site was flushed with 1 mL of normal saline. The overlying muscles were sutured over the spine and the skin flaps were then stapled with wound clips. Rats were returned to their cages, which were placed on heating pads to maintain temperature at ∼37°C. Animals received twice daily injections (intraperitoneal [i.p.]) of a Lactated Ringer's solution (2 mL) and analgesic (buprenorphine; 0.02 mg/kg, i.p.) for 5 days post-injury and prophylactic antibiotics (Baytril, 2.5 mg/kg) for 10 days. Manual bladder evacuations were performed twice daily until neurogenic bladder voiding returned. Rats were checked for signs of spontaneous pain, which can include marked inactivity, excessive grooming, and/or autotomy. Autotomy is rare in the spinal contusion model, and any animals exhibiting severe autotomy were euthanized immediately.
Behavioral tests
To gauge effects of SCI on locomotor function, animals were placed in an open field (child's pool) and their spontaneous behavior was observed in white light and scored on the 21-point Basso, Beattie, Bresnahan (BBB) locomotor rating scale.35 Animals that had a BBB score of 1 or more than 1 at 1 day after surgery were excluded. Animals received a standard 5-day sequence of tests for hindlimb reflex sensitivity prior to impact, then before and after retigabine treatment, as described previously.10 All reflex data were collected by experimenters blinded to any drug treatment during the animals' active phase under red light. Prior to each test, the animal was habituated for 20 min per day in each of the testing chambers over a 5-day period. Below-level hypersensitivity to heat was tested with the Hargreaves radiant heat method using an IITC Plantar Analgesia Meter (Woodland Hills, CA) to measure the latency for hindpaw withdrawal. If no withdrawal occurred within 20 sec, the stimulus was terminated to prevent possible tissue injury. Each hindpaw was tested, separated by 5 min. This test sequence was repeated three times at 20-min intervals. The mean of six latency measurements (three from each hindpaw) was used for each data point for each animal. Below-level signs of mechanical hypersensitivity were tested with a series of calibrated von Frey filaments (Stoelting, Wood Dale, IL) delivered to the glabrous surface of the hindpaws.36 Thresholds were determined with the “up-down” method.37 Only one test series was applied to each hindpaw.
Conditioned place preference (CPP) tests were performed as previously described.19 Briefly, each rat was habituated to the device on the first day following a 3-day sequence of twice daily conditioning sessions (conditioning phase). In one session, the rat was restricted to the innately non-preferred white chamber for 60 min, starting 5 min after delivery of retigabine (10 mg/kg, i.p.). In the other daily session, the rat was restricted for 60 min to the innately preferred black chamber 5 min after delivery of vehicle. In the testing phase, 1 day after the last conditioning session, the rat was introduced (without any injection) into the open central gray chamber and the time spent in each of the three chambers was recorded for 15 min. Conditioning to retigabine was indicated by greater time spent in the white, compared with in the black chamber, during the testing phase (i.e., if the time in white minus time in black was positive).
Dissociation and culture of DRG neurons
After rats were intracardially perfused with cold phosphate-buffered saline (PBS) under deep anesthesia induced by injection (i.p.) of 75 mg/kg Beuthanasia (Merck Animal Health, Kenilworth, NJ), the thoracic and lumbar segments of the vertebral column were removed and the L4 and L5 DRGs were excised. The ganglia were then minced, and the fragments transferred into Dulbecco's modified eagle medium (DMEM; Invitrogen, Grand Island, NY) containing collagenase (0.6 mg/mL, Roche, Mannheim, Germany) and trypsin (0.4 mg/mL; Worthington, Lakewood, NJ), and incubated for 40 min at 34°C. The cells were spun down and transferred to 35 mm Petri dishes poly-L-lysine (50 mg/mL)-precoated cover glass (8 mm, Warner Instruments, Hamden, CT) and incubated with DMEM (without serum) at 37°C in 5% CO2 overnight. After an 18–24 h incubation, living cells were digitally imaged and recorded.
In vitro recording of DRG neurons
Electrodes with a resistance of ∼2 MΩ were pulled from BF150-86-10 glass capillaries (inner diameter, 0.86 mm; outer diameter, 1.5 mm; Sutter Instrument Co., Novato, CA) using a micropipette puller (P-2000, Sutter Instrument Co, Novato, CA). Neurons were visualized using differential interference contrast (20 × ) optics on an inverted microscope (Axiovert 200M, Zeiss Oberkochen, Germany). Images of cells were taken with a charge-coupled device (CCD) camera. Neurons (soma diameter <30 μm) were recorded in the whole–cell configuration using an EPC-10 amplifier (HEKA Instruments, Lambrecht, Germany). After forming a tight seal (> 1 GΩ), the membrane was ruptured. After the whole–cell configuration was established, the cell membrane capacitance and series resistance were electronically compensated. In current-clamp mode RMP and SA were recorded. All experiments were performed at room temperature (∼23°C). Signals were filtered at 1 kHz, digitized at ≥10 kHz, and acquired using the Pulse software program (HEKA). The pipette solution contained (in mM) 134 KCl, 1.6 MgCl2, 13.2 NaCl, 3 EGTA, 9 HEPES, 4 Mg-ATP, and 0.3 Na-GTP (pH 7.2, 300 mOsM). The bath solution contained (in mM) 140 NaCl, 3 KCl, 1.8 CaCl2, 2 MgCl2, 10 HEPES, 10 glucose (pH 7.4 adjusted with NaOH, osmolarity 320 mOsM).
In vivo recording of DRGs
Somally generated afferent activity was recorded extracellularly from rat dorsal root axons. Under deep anesthesia with sodium pentobarbital (50 mg/kg, i.p.), a laminectomy was performed on vertebrae L3–L6. The dura mater was gently peeled to expose nerve tissue, which was covered with mineral oil. Small filaments were teased from dorsal roots L4 and L5, and individually mounted on gold wire electrodes for the recording using a DAM80 differential amplifier. Units responding to cutaneous stimulation of the hindlimb with a 250 mN von Frey filament were selected. Possible reduction of mechanical hypersensitivity of single units was evaluated by applying a series of graded von Frey filaments (less than 250 mN) to the receptive field before and after i.p. injection of retigabine (10 mg/kg). The SA was recorded before and after disconnecting the DRG from the periphery.
Drug application
Drugs were dissolved in distilled water at 1000 times their final concentration and kept frozen in aliquots except for capsaicin, which was prepared as a stock solution dissolved in ethanol. Stock solutions were diluted in extracellular solution just before use and held in independent syringes connected to an array of fused silica columns (inner diameter, 200 μm). The column mouth was about 100 μm upstream from the cell examined. Rapid solution exchange was achieved by shifting the columns horizontally with a micromanipulator. Cells in the recording chamber were continuously bathed in the extracellular solution. Each drug solution was delivered to the recording chamber by gravity. Drugs and chemicals were purchased from Sigma-Aldrich (St. Louis, MO) except retigabine (Toronto Research Chemicals, Ontario).
Western blotting
One month after surgery, animals were deeply anesthetized with Beuthanasia and perfused with ice-cold PBS. Four DRGs (L4, L5) from each rat were removed and immediately placed on dry ice. DRGs were homogenized in 300 μL lysis buffer (RIPA, Teknova) containing a protease inhibitor cocktail (Sigma). After homogenization, samples were sonicated three times (10 sec pulses), and centrifuged at 14,000 rpm for 10 min at 4°C. The protein concentration of lysates was determined by the BCA method (Pierce BCA Protein Assay Kit). Samples were prepared for SDS-PAGE (Bio-Rad, 4–20% Tris-HCl) by 1:1 dilution with Laemmli buffer and 30 μg of protein was loaded in each well. After electrophoresis, the gel was transferred to a polyvinylidene difluoride membrane and blocked with 10% nonfat dry milk in PBS +0.1% Tween 20 prior to incubation with antibody against KCNQ5 (Alomone, APC-155), or β-actin (Abcam, ab8226) overnight at 4°C. The membrane was incubated with anti-rabbit or anti-mouse IgG for 1 h at room temperature and developed using the ECL kit (Pierce). Protein expression was quantified by optical density using Image J software (NIH). Color molecular weight standards were run on each gel. β-actin was detected as a loading control.
Quantitative RT-PCR analysis
Total RNA was extracted from homogenized DRG with on-column DNase digestion (E.Z.N.A. Total RNA Kit I) and cDNA was synthesized by MMLV reverse transcriptase (Invitrogen) using random primer. Rat KCNQ2 primers were 5′-CCGGCAGAACTCAGAAGAAG-3′ (forward) and 5′-TTTGAGGCCAGGGGTAAGAT-3′ (reverse)26; rat KCNQ3 primers were 5′-ATACACATTTATCTGCTCTTCCTTTTA-3′ (forward) and 5′-TGCTCTCAGTTTATCCGAATCAA-3′ (reverse),38 and rat glyceraldehyde 3-phosphate dehydrogenase (GAPDH) primers were 5′-CCCCCAATGTATCCGTTGTG-3′ (forward) and 5′-TAGCCCAGGATGCCCTTTAGT-3′ (reverse).39 Messenger RNA (mRNA) abundance was determined by real-time PCR (LightCycler 480; Roche) with SYBR Green Master Mix (Sigma). Preincubation at 95°C for 3 min was followed by 45 amplification cycles (95°C for 30 sec, 57°C for 30 sec, and 72°C for 30 sec) and fluorescence collection at 60°C. Gene expression was normalized to GAPDH and averaged over three replicates from each of three to four animals in each group.
Statistical analysis
Analysis was performed with Sigmaplot 11 (Systat Software, San Jose, CA) and Prism 5.0 (Graphpad, La Jolla, CA). Pooled data are presented as means ± standard error of the mean. All comparisons among animal groups were tested for significance using the Student's unpaired t-test or one-way analysis of variance (ANOVA) with repeated measures followed by Bonferroni's post hoc tests. Paired t-tests were used to analyze treatment effects within animals. The SA incidence among different groups of animals was compared using Fisher's exact tests. For all statistical analyses, p < 0.05 was considered to be statistically significant. Significant differences are indicated in each figure (*p < 0.05; **p < 0.01; ***p < 0.001). The n in all experiments indicates the numbers of DRG neurons (electrophysiology) or rats (behavior) tested in each group.
Results
A total of 63 rats (23 SCI, 16 sham, 24 naïve) were used in this study. L4 and L5 ganglia were harvested from sham-operated and SCI rats 1–2 months after injury, as well as from age-matched naïve controls. Small DRG neurons (15–30 μm) were selected for recording in vitro because most of these cells in our preparations are nociceptors.10,19,20
SCI does not change KCNQ channel function in small DRG neurons
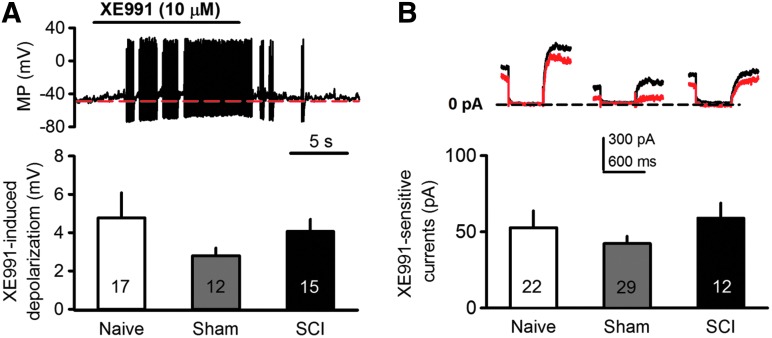
Because KCNQ channel expression in DRG neurons is decreased after peripheral nerve injury,26 we first asked whether sufficient functional KCNQ channels are available in DRG neurons after SCI to permit potential therapeutic targeting. Dissociated DRG neurons were recorded first at RMP using whole–cell current-clamp at 0 pA for 15 sec. Cells were then exposed to the KCNQ channel blocker XE991 (10 μM) for 30 sec, followed by washout. The membrane potentials before and during XE991 application were compared to assess the contribution of KCNQ channels to RMP. XE991 significantly depolarized small DRG neurons dissociated from naïve, sham, and SCI rats, indicating a substantial contribution of KCNQ channels to ongoing RMP in each group (Fig. 1A). XE991 induced the same degree of depolarization in each of the three groups (F2,41 = 1.0, one-way ANOVA), and often produced repetitive firing during the resulting depolarization (Fig. 1A), with eight out of 16 silent naïve DRG neurons, and four of nine silent neurons dissociated from SCI animals showing repetitive firing (the large number of neurons exhibiting SCI-induced SA were not treated with XE991). All of the treated cells became silent again when membrane potential recovered after washout of XE991. These results indicate that SCI does not alter KCNQ channel function at membrane potentials near the RMP.
Fig. 1.
Spinal cord injury (SCI) does not change membrane responses to specific KCNQ channel blocker, XE991. (A) Representative traces (recorded from naïve dorsal root ganglion [DRG] neuron) and summary data showing that bath application of XE991 (10 μM) depolarized and induced repetitive firing in small DRG neurons. No significant differences were found in the depolarization of DRG neurons dissociated from naïve, sham or SCI rats. (B) XE991-sensitive currents are unchanged after SCI. Cells were held at −20 mV and hyperpolarized to −60 mV for 500 ms. KCNQ currents were obtained by measuring the difference in amplitude at the beginning of the hyperpolarizing pulse in the absence (black) and presence (red) of XE991 (10 μM).
We then measured total KCNQ channel currents in small DRG neurons using whole–cell voltage clamp. Because KCNQ channels are reported to be fully opened at −20 mV and closed at −60 mV, cells were held at −20 mV and hyperpolarized to −60 mV for 500 msec.40 KCNQ currents were derived by measuring at the beginning of the hyperpolarizing pulse the difference in current amplitude in the absence (red trace; Fig. 1B) and presence (black trace; Fig. 1B) of XE991 (10 μM).41 SCI had no significant effect on XE991-sensitive currents in small DRG neurons (59.1 ± 9.8 pA), compared with responses in neurons dissociated from naïve (52.7 ± 11.1 pA) and sham-operated (42.3 ± 4.9 pA) rats (F2,60 = 0.96, one-way ANOVA; Fig. 1B). These data provide additional evidence for unaltered KCNQ channel function after SCI, and indicate that a substantial population of functional KCNQ channels remains after SCI that might be targeted to modulate neuronal excitability.
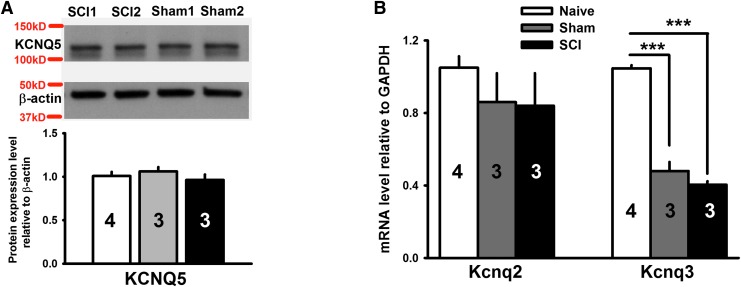
SCI does not change the expression of KCNQ channels in DRGs
If DRG neurons were to respond to the central damage caused by SCI in the same way that they respond to peripheral nerve injury,26 then KCNQ channel expression would be decreased in DRGs. KCNQ2, KCNQ3, and KCNQ5 are the major KCNQ subtypes expressed in DRG neurons.25,26,42 We found that the protein level of KCNQ5 in DRGs was unchanged after SCI or sham treatment as revealed by Western blot analysis (p = 0.184, F2,6 = 2.278, one-way ANOVA; Fig. 2A). Although we were able to use Western blot to measure KCNQ5 protein levels in DRGs, we did not find sufficiently selective antibodies against KCNQ2 and KCNQ3 for reliable assessment of the protein levels of these channels. Instead, we used real-time quantitative polymerase chain reaction (qRT-PCR) analysis to measure KCNQ2 and KCNQ3 mRNA expression in DRGs. We found that KCNQ2 mRNA levels in L4 and L5 DRGs excised 1 month after SCI were not significantly changed, compared with levels from sham and naïve animals (p = 0.548, F2,7 = 0.657, one-way ANOVA; Fig. 2B). While SCI had no effect on KCNQ3 mRNA, compared with the sham group, laminectomy alone decreased KCNQ3 mRNA in DRGs, compared with that from naïve animals (p < 0.001, F2,7 = 160.718, one-way ANOVA).
Fig. 2.
Spinal cord injury (SCI) does not alter KCNQ channel expression in dorsal root ganglions (DRGs). (A) Western blot analysis showing lack of effect of SCI on KCNQ5 protein expression in L4 and L5 DRGs. Upper panel: Sample bands of KCNQ5 and β-actin probed with specific antibodies. Lower panel: relative protein expression levels of KCNQ5, compared with β-actin. (B) Real-time quantitative polymerase chain reaction (qRT-PCR) analysis showing little or no change in KCNQ2 and KCNQ3 mRNA levels in L4 and L5 DRGs 1 month after SCI, compared with that from sham groups. All data are normalized to glyceraldehyde 3-phosphate dehydrogenase. A total of 3–4 animals were used for each group for qRT-PCR and for Western analyses. Experiments for each animal were repeated three times. One-way analysis of variance; ***p < 0.001.
KCNQ channel activation reduces DRG neuron SA in vitro and in vivo
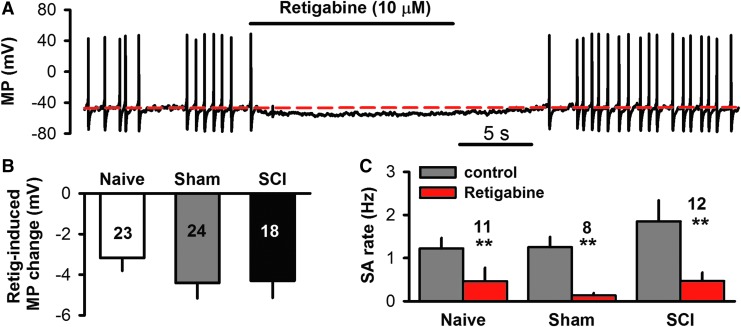
Previously published work indicates that hyperactivity in DRG neurons is essential for maintaining SCI-induced chronic pain, and an important role is played by ongoing SA generated in both the soma10,19 and peripheral processes11 after SCI. Thus, decreasing DRG neuronal excitability sufficiently to block SA may be a useful strategy to treat post-SCI pain. Retigabine, a specific KCNQ channel opener,43,44 was used to test the prediction that increased activity of KCNQ channels can decrease excitability and SA in small DRG neurons after SCI. Basal activity was recorded under whole–cell current-clamp for 15 sec, and then retigabine (10 μM) was applied for 15 sec followed by washout. Retigabine hyperpolarized membrane potentials (Fig. 3A) with similar effects in neurons taken from naïve, sham, and SCI groups (F2,59 = 0.82, one-way ANOVA; Fig. 3B). In the neurons exhibiting SA, retigabine dramatically decreased the spontaneous firing rate in all groups (naïve, sham, and SCI; Fig. 3C), and in many cases completely blocked SA during drug application (Fig. 3A).
FIG. 3.
Enhanced activity of KCNQ channels decreases spontaneous activity (SA) in dorsal root ganglion (DRG) neurons dissociated from spinal cord injury (SCI), sham, and age-matched naïve rats. (A) Representative recording showing suppression of SA by retigabine (10 μM), a specific KCNQ channel opener, in a small DRG neuron 1 month after SCI. (B) Summary of retigabine's effect on resting membrane potential, showing similar hyperpolarization in naïve, sham, and SCI groups. (C) Summary of retigabine's effect on SA firing rate, showing similar reductions in naïve, sham, and SCI groups (comparing baseline rate with rate during retigabine superfusion by paired t-tests).
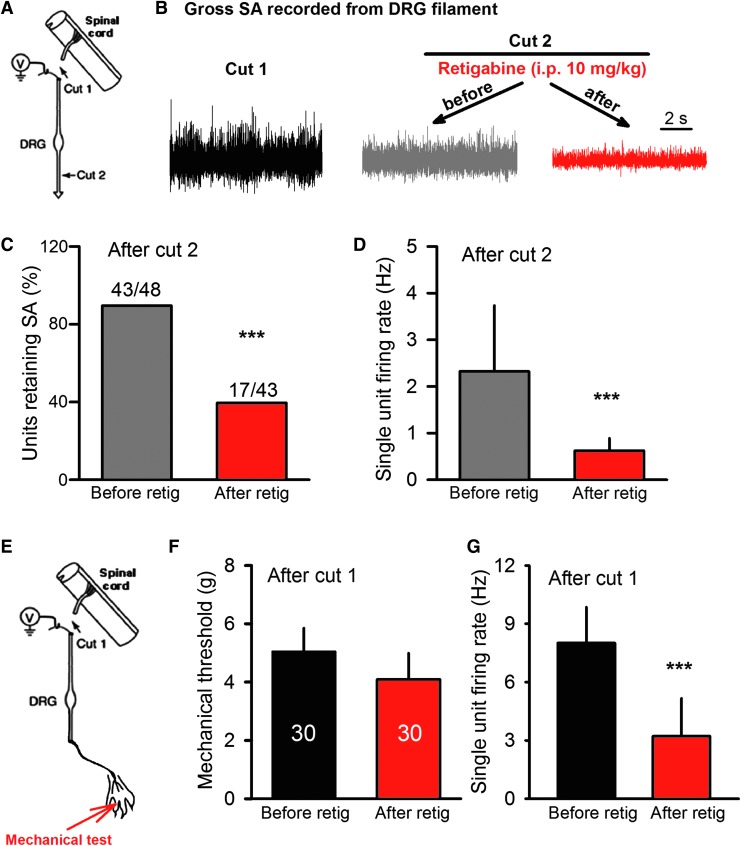
To demonstrate that KCNQ openers may be useful for treating pain maintained by hyperactive DRG neurons, it must be shown that a clinically available KCNQ opener, such as retigabine, can reduce pain-related hyperactivity when applied in vivo. Because of its importance for SCI-induced pain,10,19,20 we asked whether SCI-induced SA generated within lumbar DRGs could be reduced by systemic application of retigabine. Extracellular recordings were made from the axons of primary afferent neurons in teased filaments from dorsal roots of anesthetized rats in vivo. Recordings were made after transecting the dorsal root near the dorsal root entry zone (Fig. 4A, cut 1) to eliminate possible efferent activity. Following a 5-min period in which SA was recorded, single units were identified by cutaneous stimulation of the hindpaw with a stiff (250 mN) von Frey filament. The SA was again recorded after disconnecting (Fig. 4A, cut 2) the DRG from the periphery (3–4 mm peripheral to the DRG), which revealed SA generated within cell bodies in the DRG and/or nearby axonal segments.10 Anesthetized SCI rats then received systemic retigabine (10 mg/kg, i.p.) or vehicle. The retigabine effectively decreased the incidence (p < 0.001, Fisher's exact test; Fig. 4B, 4C) and frequency (p < 0.001, paired t-test; Fig. 4D) of SA (Fig. 4B, 4C) generated in DRG neurons disconnected from both the central nervous system (CNS) and periphery (Fig. 4A). A subset of these animals subsequently received the KCNQ blocker, XE991 (5 mg/kg, i.p), which accelerated the recovery of SA in DRG neurons after retigabine application (data not shown). Thus, systemic injection of retigabine at a concentration known to reduce an operant measure of pain after SCI19 strongly reduced chronic SA generated within the cell bodies of DRG neurons in vivo after SCI.
FIG. 4.
Systemic application of retigabine in vivo inhibits somally generated activity recorded extracellularly from dorsal root (L4/L5) filaments in spinal cord injury (SCI) rats. (A) Schematic showing sites of in vivo dorsal root filament recording and transections. (B) Representative traces of gross activity showing the reduction of SA after intraperitoneal (i.p.) injection of retigabine (following disconnection from the periphery by cut 2). (C) Incidence of single units showing SA (after cut 2) before and 30 min after systemic application of retigabine (10 mg/kg, i.p.) to SCI rats. (D) SA firing rates of single units (after cut 2) before and 30 min after systemic application of retigabine. (E) Schematic showing sites of in vivo dorsal root filament recording, transection, and location of mechanical stimulation. (F) Mechanical thresholds of single units (after cut 1) before and 30 min after systemic application of retigabine. (G) SA firing rates of single units (after Cut 1) before and 30 min after systemic application of retigabine.
Interestingly, a concentration of retigabine that reduced reflexive measures of pain (Fig. 5A, 5E) failed to reduce the threshold for single unit responses to mechanical stimulation when retigabine was injected i.p. without disconnecting the DRG from the periphery (after cut 1; Fig. 4E, 4F). However, it significantly decreased the SA firing frequency in recorded single units when the activity could have been generated in either the peripheral terminals or cell bodies of the DRG neurons (Fig. 4G). These observations show that in vivo delivery of retigabine can reduce SA in DRG neurons without reducing the neurons' sensitivity to peripheral mechanical stimuli.
FIG. 5.
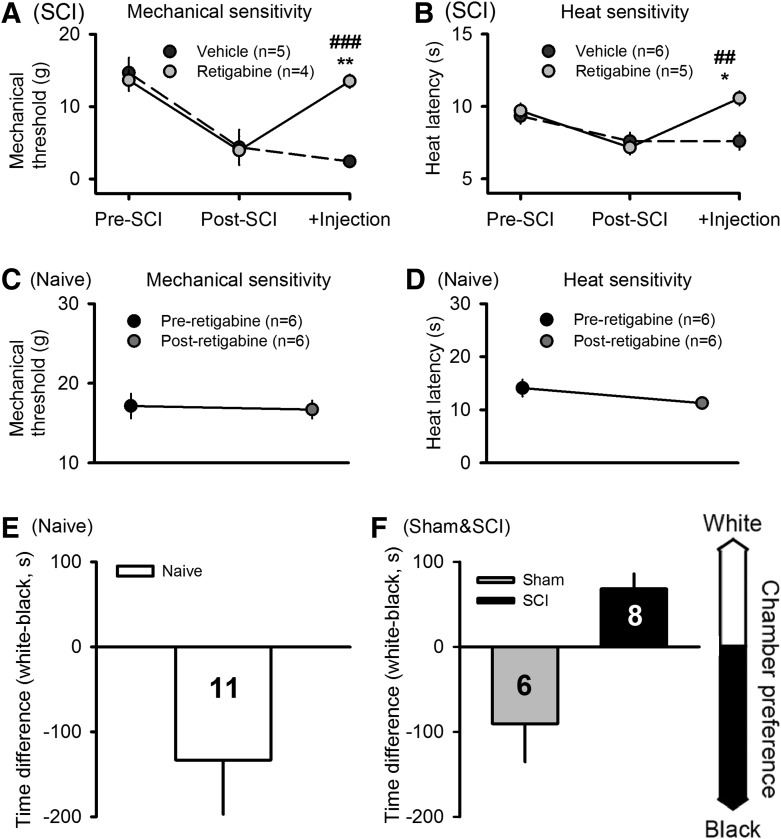
Retigabine reduces pain-related behavior in spinal cord injury (SCI) rats but not in naïve, uninjured rats. (A) Retigabine (10 mg/kg, i.p.), but not vehicle (saline), reversed chronic SCI-induced mechanical hypersensitivity (decreased hindpaw withdrawal threshold) tested 30 min after injections. (B) Retigabine also selectively reversed chronic heat hypersensitivity (decreased latency of hindpaw withdrawal to radiant heat) tested 30 min after injections. *Comparison of postcontusion measures before and after retigabine (paired t-test). #Comparison of post-contusion responses of animals treated with retigabine or vehicle (unpaired t-test). (C) Retigabine had no effect on the sensitivity of naïve rats to Von Frey tests. (D) Retigabine had no effect on the sensitivity of naïve rats to heat stimuli. (E) In naïve rats pairing of retigabine injections with placement in the white chamber failed to alter innate preference for the black chamber in the CPP procedure. (F) For comparison, a reprinted figure shown that in SCI rats, but not sham-operated rats, the same CPP procedure with retigabine produced conditioned preference for the white chamber. Reprinted with permission from Yang and colleagues.19
KCNQ channel activation reduces chronic hypersensitivity of hindpaws after SCI
SA in widespread DRG neurons is likely to help drive central sensitization, promoting hypersensitivity to normally innocuous stimulus (allodynia) and to painful stimuli (hyperalgesia).45 Our findings that systemic application of retigabine decreased SA in DRG neurons in vivo (Fig. 4), along with reports that retigabine reduces measures of allodynia and hyperalgesia after peripheral nerve injury or inflammation (see Introduction), suggested that retigabine also would reduce the reflex hypersensitivity that occurs after SCI.10 Before contusion, each animal received behavioral pretests with mechanical and heat stimuli applied to the plantar surface of each hindpaw. One month after SCI, the same tests were performed 2 days before injection of retigabine or vehicle, at which time reflex sensitivity had increased significantly for both mechanical and heat test stimuli (Fig. 5A, 5B). The same tests were repeated 30 min following i.p. injection of 1 mL of either retigabine (10 mg/kg) or vehicle (saline). Retigabine injection significantly reversed both mechanical and heat hypersensitivity, while vehicle injection failed to reduce the hypersensitivity (Fig. 5A, 5B). Mechanical thresholds were significantly greater after injection of retigabine than vehicle (p < 0.001; t-test) and the latencies for withdrawal to radiant heat were significantly longer after injection of retigabine than vehicle (p = 0.004; t-test). Retigabine-induced effect in SCI animals was observed 2 h after i.p. injection, and disappeared after 18 h (data not shown), which is consistent with the in vivo half-life of about 2 h reported by others.34,46
Retigabine does not alter reflex or operant measures of pain in uninjured animals
If KCNQ activators such as retigabine are to offer promise for treating chronic pain after SCI, it would be useful for them to reduce chronic pain without disrupting normal pain responses or producing intrinsic motivational effects. Therefore, we tested the effects of retigabine in naïve, uninjured animals at the concentration we found to reduce both SCI-induced SA in DRG neurons in vivo (Fig. 4) and SCI-induced reflex hypersensitivity (Fig. 5A, 5B). No significant effect of i.p. injection of retigabine (10 mg/kg) was found on hindlimb reflex withdrawal responses in naïve rats to either mechanical or heat test stimuli (Fig. 5C, 5D).
A lack of intrinsic motivational effects of retigabine in naïve, uninjured animals was shown by using this KCNQ opener (10 mg/kg, i.p.) in the same conditioned place preference (CPP) procedure that we used recently with SCI animals and sham-operated animals to provide evidence for spontaneous pain after SCI.19 No effects on place preference were found in naïve animals when three daily retigabine injections were paired with placement in a white chamber and vehicle injections were paired with the black chamber (Fig. 5E). This result contrasts with the results found with the identical CPP procedure given to SCI rats (Fig. 5F, Yang and colleagues,19 reprinted with permission), where the SCI rats developed a significant preference for the innately less preferred white chamber after pairing retigabine injections with placement in this chamber. The lack of CPP produced by retigabine treatment in naïve rats shows that retigabine is not intrinsically rewarding but instead acts primarily to reduce ongoing pain that may be present.
Discussion
This study has shown that a KCNQ channel activator, retigabine, has electrophysiological and behavioral effects indicating that pharmacological activation of KCNQ channels in DRG neurons could be a useful approach for treating chronic pain after SCI. While retigabine penetrates the blood–brain barrier (BBB), where it might have both analgesic effects and unwanted side effects, it is likely that some and perhaps much of the suppressive effect of retigabine on pain-related behavior in our rat SCI model occurs on the cell bodies of neurons in DRGs. These cell bodies are likely to be exposed to the highest concentrations of peripherally applied retigabine because the DRGs, unlike much of the central and peripheral nervous system, lack an effective vascular permeability barrier.47–49 Moreover, we show here that retigabine potently inhibits SA in DRG neuron cell bodies in vitro and in vivo without changing the peripheral mechanical threshold of DRG neurons. Consistent with this conclusion, retigabine produced significant suppression of behavioral effects known to be correlated with SA in DRG neuron cell bodies10,19 without affecting the same behaviors in naïve, uninjured animals.
KCNQ channels in DRG neurons could only be a useful target for treating SCI pain if sufficient channels remain available chronically after SCI to be activated by pharmacological openers, such as retigabine. This was a potential concern because of evidence that SA in dissociated nociceptors is associated with membrane depolarization and increased membrane resistance,10 consistent with downregulation of K+ channels after SCI. Moreover, a voltage-gated K+ channel, Kv3.4, shows an apparent decrease in surface expression in DRG neurons after SCI.21 Downregulation of KCNQ channels has been demonstrated in rodent models of cancer pain and peripheral nerve injury.26,50 However, using quantitative RT-PCR and Western blot, we failed to detect significant alteration in the expression of KCNQ2 and KCNQ3 mRNA or KCNQ5 protein in DRGs after SCI, compared with the sham control groups. The possibility remained that an SCI-induced reduction in KCNQ channel expression in a subpopulation of DRG neurons particularly important for SCI pain—small diameter nociceptors—might have been offset by increases in KCNQ expression in other cells in DRGs. This possibility was ruled out by our electrophysiological findings, which showed that the maximal KCNQ currents of small DRG neurons from SCI rats were comparable to those from control (sham and naïve) animals.
Surprisingly, laminectomy alone significantly decreased KCNQ3 mRNA level in sham and SCI groups, compared with the naïve group. It has been reported that KCNQ3 is not the major KCNQ subtype in nociceptors.42,51 While mRNA was extracted from whole DRG, the electrophysiological recordings were made on small DRG neurons (presumptive nociceptors). It is possible that KCNQ3 mRNA was decreased after laminectomy in a subgroup of DRG neurons other than the set that we recorded from. Regardless of the population of cells showing the reduction of KCNQ3 transcription after laminectomy, spontaneous pain was not observed in the sham-operated group (Fig. 5F),19 and this reduction was not sufficient to prevent retagabine from reducing SCI-induced chronic pain (Fig. 5). Although XE991 alone doesn't sensitize C-fibers to subsequent heat stimulation,52 it facilitates the response of Aδ-fibers52 and produces a membrane depolarization in small sensory neurons,25 which indicates that KCNQ channels are active at resting potential in these neurons. The lack of a decrease in XE991-induced membrane depolarization after SCI showed that SCI did not reduce the resting activity of KCNQ channels in small DRG neurons. Moreover, there was no significant difference in retigabine-induced membrane hyperpolarization of DRG neurons among naïve, sham, and SCI groups. These data show that numerous KCNQ channels remain functional in DRG neurons after SCI to serve as potential drug targets to decrease nociceptor SA and ameliorate chronic pain after SCI. The continued availability of KCNQ channels after SCI differs from the downregulation of KCNQ channels reported in peripheral nerve injury models.26,50 This difference is probably explained by the fact that nerve injury, but not SCI, axotomizes sensory neurons in the tested DRGs. We investigated DRG neurons taken from L4 and L5 DRGs 5–6 spinal segments below the SCI level (vertebral T10). Most C-fiber nociceptors project only 1–2 segments from their spinal cord entry zone.53 Supporting this conclusion, peripheral nerve injury usually induces expression of activating transcription factor-3 (ATF-3) in axotomized DRG neurons,54–56 but no ATF-3 was detected in DRGs remote from a spinal contusion site.11
Many sensory neurons in rat DRGs, including small neurons that respond to capsaicin (presumptive nociceptors) express KCNQ2, KCNQ3, and KCNQ5.25,26 Retigabine enhances activity of all the KCNQ channels except KCNQ1,57 and was known to reduce the excitability of DRG neurons, hyperpolarizing primary afferent fibers,28 reducing the transmission of Aδ and C-fiber responses to dorsal horn neurons,25 and attenuating Aδ and C-fiber discharge induced by heat stimulation.52,58 Similarly, we show that retigabine hyperpolarizes and blocks SA in small dissociated DRG neurons from SCI animals. In vivo, retigabine produced a significant reduction in SA generated within DRGs, and normalized reflex hypersensitivity induced by SCI. Reversal of reflex hypersensitivity to mechanical and heat stimulation also has been reported for retigabine in other pain models.25,30–34 We found that retigabine reduces SCI-induced hypersensitivity of hindlimb paw withdrawal evoked by mechanical or heat stimulation, with little or no effect on reflex sensitivity in naïve, uninjured animals. Central changes, including lowered thresholds of dorsal horn neurons, are important for many behavioral changes following nerve injury and are thought to be driven at least in part by ongoing activity in primary afferent neurons.59 Following spinal cord injury, central sensitization occurs,14,15 which also may be driven by increased activity in primary afferent neurons.19,60 We have previously shown that spontaneous activity of primary sensory neurons is correlated with mechanical allodynia.10 Retigabine does not change sensory fiber discharge recorded from intact mice in vivo.32,61 However, similar to what has been observed in axotomized sensory fibers,32,61 retigabine decreased the spontaneous firing rate of primary sensory neurons after SCI both in vivo and in vitro, which could reduce central sensitization and consequent behavioral hypersensitivity revealed in reflex tests.
It is possible that below-level nociceptor SA and hindlimb reflex alterations in this model are not directly related to cortically mediated pain, perhaps because of insufficient sparing of ascending axons passing through the injury site.16,17,45 However, SA also induces nociceptor SA in DRGs at and just above the injury level that has been correlated with increased vocalization (mediated by supraspinal circuits) evoked by at- and above-level test stimulation,10 suggesting that retigabine could also reduce pain-related effects of SA in at-level nociceptors. While the actions of retigabine have yet to be tested in at-level nociceptors, the finding that retigabine supports the conditioning of place preference shows that this drug can relieve ongoing, aversive consequences of SCI (likely to include spontaneous pain) that depends upon the ongoing activity of Nav1.8-expressing primary afferent neurons.19 Our demonstration here that retigabine fails to produce conditioned place preference in naïve animals shows that, under our conditions, retigabine is not intrinsically rewarding and adds to our evidence that SCI induces chronic spontaneous pain in rats that can be alleviated by retigabine.19
Drugs targeting KCNQ channels also may exert analgesic effects by central actions. KCNQ channels are present in spinal dorsal horn neurons and brain,22,62 where SCI-induced alterations have been reported.7–9,11,15,63 The involvement of brain KCNQ channels has been reported for retigabine-induced attenuation of mechanical allodynia.33 However, intracerebroventricular administration of XE991 failed to reverse retigabine-mediated reduction of paw withdrawal responses to heat stimulation.34 Both thermal hyperalgesia and mechanical allodynia are reported in mice in which the KCNQ2 gene is deleted from sensory neurons.51 The relative contribution of the CNS to retigabine effects is likely to depend upon the pain model and local concentration of the drug. As discussed above, the maintenance of SCI-induced chronic pain in our rat model depends upon ongoing activity of primary sensory neurons.10,19 The lack of an effective vascular permeability barrier in the DRG47–49 means that systemically applied retigabine has better access to sensory neurons in the DRGs than to neurons within the CNS. Indeed, the lack of significant effects of retigabine on reflex responses and place preference in naïve, uninjured animals in this study suggests that peripheral delivery of relatively low doses of a KCNQ activator may primarily affect SA generated in the nociceptors made hyperexcitable by SCI (or potentially by other insults). This could allow drugs that selectively activate KCNQ channels to preferentially reduce ongoing nociceptor activity and thereby reduce chronic pain driven by such hyperactivity.
Although the therapeutic use of currently available KCNQ activators is limited by centrally mediated side effects, our findings are consistent with evidence that KCNQ activators can suppress pain-related behavior by peripheral actions34 and encourage the investigation of low doses of FDA-approved KCNQ activators such as retigabine to treat SCI pain, as well as the search for new classes of KCNQ activators that do not cross the blood-brain barrier.
Acknowledgments
Supported by grants from the Paralyzed Veterans of America (2791), Mission Connect (a program of TIRR Foundation), Christopher and Dana Reeve Foundation and Department of Defense USAMRAA to Q.Y.; a grant from the Craig H. Neilsen Foundation (E.T.W); and NIH grants (CA172129 to J.A.F; NS 054765 and NS 027910 to S.M.C.).
Author Disclosure Statement
The authors declare no competing financial interests.
References
- 1.Siddall P.J. and Loeser J.D. (2001). Pain following spinal cord injury. Spinal Cord 39, 63–73 [DOI] [PubMed] [Google Scholar]
- 2.Dijkers M., Bryce T., and Zanca J. (2009). Prevalence of chronic pain after traumatic spinal cord injury: a systematic review. J. Rehabil. Res. Dev. 46, 13–29 [PubMed] [Google Scholar]
- 3.Rintala D.H., Hart K.A., and Priebe M.M. (2004). Predicting consistency of pain over a 10-year period in persons with spinal cord injury. J. Rehabil. Res. Dev. 41, 75–88 [DOI] [PubMed] [Google Scholar]
- 4.Finnerup N.B. (2013). Pain in patients with spinal cord injury. Pain 154 Suppl 1, S71–S76 [DOI] [PubMed] [Google Scholar]
- 5.Finnerup N.B. and Baastrup C. (2012). Spinal cord injury pain: mechanisms and management. Curr. Pain Headache Rep. 16, 207–216 [DOI] [PubMed] [Google Scholar]
- 6.Hao J.X., Kupers R.C., and Xu X.J. (2004). Response characteristics of spinal cord dorsal horn neurons in chronic allodynic rats after spinal cord injury. J. Neurophysiol. 92, 1391–1399 [DOI] [PubMed] [Google Scholar]
- 7.Hains B.C. and Waxman S.G. (2006). Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J. Neurosci. 26, 4308–4317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hains B.C., Klein J.P., Saab C.Y., Craner M.J., Black J.A., and Waxman S.G. (2003). Upregulation of sodium channel Nav1.3 and functional involvement in neuronal hyperexcitability associated with central neuropathic pain after spinal cord injury. J. Neurosci. 23, 8881–8892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hains B.C., Saab C.Y., and Waxman S.G. (2005). Changes in electrophysiological properties and sodium channel Nav1.3 expression in thalamic neurons after spinal cord injury. Brain 128, 2359–2371 [DOI] [PubMed] [Google Scholar]
- 10.Bedi S.S., Yang Q., Crook R.J., Du J., Wu Z., Fishman H.M., Grill R.J., Carlton S.M., and Walters E.T. (2010). Chronic spontaneous activity generated in the somata of primary nociceptors is associated with pain-related behavior after spinal cord injury. J. Neurosci. 30, 14870–14882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlton S.M., Du J., Tan H.Y., Nesic O., Hargett G.L., Bopp A.C., Yamani A., Lin Q., Willis W.D., and Hulsebosch C.E. (2009). Peripheral and central sensitization in remote spinal cord regions contribute to central neuropathic pain after spinal cord injury. Pain 147, 265–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jutzeler C.R., Huber E., Callaghan M.F., Luechinger R., Curt A., Kramer J.L., and Freund P. (2016). Association of pain and CNS structural changes after spinal cord injury. Sci. Rep. 6, 18534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wrigley P.J., Press S.R., Gustin S.M., Macefield V.G., Gandevia S.C., Cousins M.J., Middleton J.W., Henderson L.A., and Siddall P.J. (2009). Neuropathic pain and primary somatosensory cortex reorganization following spinal cord injury. Pain 141, 52–59 [DOI] [PubMed] [Google Scholar]
- 14.Tan A.M. and Waxman S.G. (2012). Spinal cord injury, dendritic spine remodeling, and spinal memory mechanisms. Exp. Neurol. 235, 142–151 [DOI] [PubMed] [Google Scholar]
- 15.Walters E.T. (2014). Neuroinflammatory contributions to pain after SCI: roles for central glial mechanisms and nociceptor-mediated host defense. Exp. Neurol. 258, 48–61 [DOI] [PubMed] [Google Scholar]
- 16.Baastrup C., Maersk-Moller C.C., Nyengaard J.R., Jensen T.S., and Finnerup N.B. (2010). Spinal-, brainstem- and cerebrally mediated responses at- and below-level of a spinal cord contusion in rats: evaluation of pain-like behavior. Pain 151, 670–679 [DOI] [PubMed] [Google Scholar]
- 17.van Gorp S., Deumens R., Leerink M., Nguyen S., Joosten E.A., and Marsala M. (2014). Translation of the rat thoracic contusion model; part 1-supraspinally versus spinally mediated pain-like responses and spasticity. Spinal Cord 52, 524–528 [DOI] [PubMed] [Google Scholar]
- 18.Davoody L., Quiton R.L., Lucas J.M., Ji Y., Keller A., and Masri R. (2011). Conditioned place preference reveals tonic pain in an animal model of central pain. J. Pain 12, 868–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Q., Wu Z., Hadden J.K., Odem M.A., Zuo Y., Crook R.J., Frost J.A., and Walters E.T. (2014). Persistent pain after spinal cord injury is maintained by primary afferent activity. J. Neurosci. 34, 10765–10769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Z., Yang Q., Crook R.J., O'Neil R.G., and Walters E.T. (2013). TRPV1 channels make major contributions to behavioral hypersensitivity and spontaneous activity in nociceptors after spinal cord injury. Pain 154, 2130–2141 [DOI] [PubMed] [Google Scholar]
- 21.Ritter D.M., Zemel B.M., Hala T.J., O'Leary M.E., Lepore A.C., and Covarrubias M. (2015). Dysregulation of Kv3.4 channels in dorsal root ganglia following spinal cord injury. J. Neurosci. 35, 1260–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jentsch T.J. (2000). Neuronal KCNQ potassium channels: physiology and role in disease. Nat. Rev. Neurosci. 1, 21–30 [DOI] [PubMed] [Google Scholar]
- 23.Wang H.S., Pan Z., Shi W., Brown B.S., Wymore R.S., Cohen I.S., Dixon J.E., and McKinnon D. (1998). KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science 282, 1890–1893 [DOI] [PubMed] [Google Scholar]
- 24.Schwarz J.R., Glassmeier G., Cooper E.C., Kao T.C., Nodera H., Tabuena D., Kaji R., and Bostock H. (2006). KCNQ channels mediate IKs, a slow K+ current regulating excitability in the rat node of Ranvier. J. Physiol. 573, 17–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Passmore G.M., Selyanko A.A., Mistry M., Al-Qatari M., Marsh S.J., Matthews E.A., Dickenson A.H., Brown T.A., Burbidge S.A., Main M., and Brown D.A. (2003). KCNQ/M currents in sensory neurons: significance for pain therapy. J. Neurosci. 23, 7227–7236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rose K., Ooi L., Dalle C., Robertson B., Wood I.C., and Gamper N. (2011). Transcriptional repression of the M channel subunit Kv7.2 in chronic nerve injury. Pain 152, 742–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Du X., Hao H., Gigout S., Huang D., Yang Y., Li L., Wang C., Sundt D., Jaffe D.B., Zhang H., and Gamper N. (2014). Control of somatic membrane potential in nociceptive neurons and its implications for peripheral nociceptive transmission. Pain 155, 2306–2322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rivera-Arconada I. and Lopez-Garcia J.A. (2006). Retigabine-induced population primary afferent hyperpolarisation in vitro. Neuropharmacology 51, 756–763 [DOI] [PubMed] [Google Scholar]
- 29.Brown D.A. and Passmore G.M. (2009). Neural KCNQ (Kv7) channels. Br. J. Pharmacol. 156, 1185–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blackburn-Munro G. and Jensen B.S. (2003). The anticonvulsant retigabine attenuates nociceptive behaviours in rat models of persistent and neuropathic pain. Eur. J. Pharmacol. 460, 109–116 [DOI] [PubMed] [Google Scholar]
- 31.Linley J.E., Rose K., Patil M., Robertson B., Akopian A.N., and Gamper N. (2008). Inhibition of M current in sensory neurons by exogenous proteases: a signaling pathway mediating inflammatory nociception. Neurosci. 28, 11240–11249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roza C. and Lopez-Garcia J.A. (2008). Retigabine, the specific KCNQ channel opener, blocks ectopic discharges in axotomized sensory fibres. Pain 138, 537–545 [DOI] [PubMed] [Google Scholar]
- 33.Xu W., Wu Y., Bi Y., Tan L., Gan Y., and Wang K. (2010). Activation of voltage-gated KCNQ/Kv7 channels by anticonvulsant retigabine attenuates mechanical allodynia of inflammatory temporomandibular joint in rats. Mol. Pain 6, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayashi H., Iwata M., Tsuchimori N., and Matsumoto T. (2014). Activation of peripheral KCNQ channels attenuates inflammatory pain. Mol. Pain 10, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Basso D.M., Beattie M.S., Bresnahan J.C., Anderson D.K., Faden A.I., Gruner J.A., Holford T.R., Hsu C.Y., Noble L.J., Nockels R., Perot P.L., Salzman S.K., and Young W. (1996). MASCIS evaluation of open field locomotor scores: effects of experience and teamwork on reliability. Multicenter Animal Spinal Cord Injury Study. J. Neurotrauma 13, 343–359 [DOI] [PubMed] [Google Scholar]
- 36.Hulsebosch C.E., Xu G.Y., Perez-Polo J.R., Westlund K.N., Taylor C.P., and McAdoo D.J. (2000). Rodent model of chronic central pain after spinal cord contusion injury and effects of gabapentin. J. Neurotrauma 17, 1205–1217 [DOI] [PubMed] [Google Scholar]
- 37.Chaplan S.R., Bach F.W., Pogrel J.W., Chung J.M., and Yaksh T.L. (1994). Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 53, 55–63 [DOI] [PubMed] [Google Scholar]
- 38.Chadha P.S., Zunke F., Zhu H.L., Davis A.J., Jepps T.A., Olesen S.P., Cole W.C., Moffatt J.D., and Greenwood I.A. (2012). Reduced KCNQ4-encoded voltage-dependent potassium channel activity underlies impaired beta-adrenoceptor-mediated relaxation of renal arteries in hypertension. Hypertension 59, 877–884 [DOI] [PubMed] [Google Scholar]
- 39.Piller N., Decosterd I., and Suter M.R. (2013). Reverse transcription quantitative real-time polymerase chain reaction reference genes in the spared nerve injury model of neuropathic pain: validation and literature search. BMC Res. Notes 6, 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suh B.C., Inoue T., Meyer T., and Hille B. (2006). Rapid chemically induced changes of PtdIns(4,5)P2 gate KCNQ ion channels. Science 314, 1454–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lamas J.A., Selyanko A.A., and Brown D.A. (1997). Effects of a cognition-enhancer, linopirdine (DuP 996), on M-type potassium currents (IK(M)) and some other voltage- and ligand-gated membrane currents in rat sympathetic neurons. Eur. J. Neurosci. 9, 605–616 [DOI] [PubMed] [Google Scholar]
- 42.King C.H. and Scherer S.S. (2012). Kv7.5 is the primary Kv7 subunit expressed in C-fibers. J. Comp. Neurol. 520, 1940–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schenzer A., Friedrich T., Pusch M., Saftig P., Jentsch T.J., Grotzinger J., and Schwake M. (2005). Molecular determinants of KCNQ (Kv7) K+ channel sensitivity to the anticonvulsant retigabine. J. Neurosci. 5051–5060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wickenden A.D., Zou A., Wagoner P.K., and Jegla T. (2001). Characterization of KCNQ5/Q3 potassium channels expressed in mammalian cells. Br. J. Pharmacol. 132, 381–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walters E.T. (2012). Nociceptors as chronic drivers of pain and hyperreflexia after spinal cord injury: an adaptive-maladaptive hyperfunctional state hypothesis. Front. Physiol. 3, 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mazarati A., Wu J., Shin D., Kwon Y.S., and Sankar R. (2008). Antiepileptogenic and antiictogenic effects of retigabine under conditions of rapid kindling: an ontogenic study. Epilepsia 49, 1777–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abram S.E., Yi J., Fuchs A., and Hogan Q.H. (2006). Permeability of injured and intact peripheral nerves and dorsal root ganglia. Anesthesiology 105, 146–153 [DOI] [PubMed] [Google Scholar]
- 48.Hirakawa H., Okajima S., Nagaoka T., Kubo T., Takamatsu T., and Oyamada M. (2004). Regional differences in blood-nerve barrier function and tight-junction protein expression within the rat dorsal root ganglion. Neuroreport 15, 405–408 [DOI] [PubMed] [Google Scholar]
- 49.Jimenez-Andrade J.M., Herrera M.B., Ghilardi J.R., Vardanyan M., Melemedjian O.K., and Mantyh P.W. (2008). Vascularization of the dorsal root ganglia and peripheral nerve of the mouse: implications for chemical-induced peripheral sensory neuropathies. Mol. Pain 4, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng Q., Fang D., Liu M., Cai J., Wan Y., Han J.S., and Xing G.G. (2013). Suppression of KCNQ/M (Kv7) potassium channels in dorsal root ganglion neurons contributes to the development of bone cancer pain in a rat model. Pain 154, 434–448 [DOI] [PubMed] [Google Scholar]
- 51.King C.H., Lancaster E., Salomon D., Peles E., and Scherer S.S. (2014). Kv7.2 regulates the function of peripheral sensory neurons. J. Comp. Neurol. 522, 3262–3280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Passmore G.M., Reilly J.M., Thakur M., Keasberry V.N., Marsh S.J., Dickenson A.H., and Brown D.A. (2012). Functional significance of M-type potassium channels in nociceptive cutaneous sensory endings. Front. Mol. Neurosci. 5, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chung K., Langford L.A., Applebaum A.E., and Coggeshall R.E. (1979). Primary afferent fibers in the tract of Lissauer in the rat. J. Comp. Neurol. 184, 587–598 [DOI] [PubMed] [Google Scholar]
- 54.Peters C.M., Ghilardi J.R., Keyser C.P., Kubota K., Lindsay T.H., Luger N.M., Mach D.B., Schwei M.J., Sevcik M.A., and Mantyh P.W. (2005). Tumor-induced injury of primary afferent sensory nerve fibers in bone cancer pain. Exp. Neurol. 193, 85–100 [DOI] [PubMed] [Google Scholar]
- 55.Seijffers R., Allchorne A.J., and Woolf C.J. (2006). The transcription factor ATF-3 promotes neurite outgrowth. Mol. Cell Neurosci. 32, 143–154 [DOI] [PubMed] [Google Scholar]
- 56.Tsujino H., Kondo E., Fukuoka T., Dai Y., Tokunaga A., Miki K., Yonenobu K., Ochi T., and Noguchi K. (2000). Activating transcription factor 3 (ATF3) induction by axotomy in sensory and motoneurons: a novel neuronal marker of nerve injury. Mol. Cell Neurosci. 15, 170–182 [DOI] [PubMed] [Google Scholar]
- 57.Gribkoff V.K. (2003). The therapeutic potential of neuronal KCNQ channel modulators. Expert Opin. Ther. Targets 7, 737–748 [DOI] [PubMed] [Google Scholar]
- 58.Lang P.M., Fleckenstein J., Passmore G.M., Brown D.A., and Grafe P. (2008). Retigabine reduces the excitability of unmyelinated peripheral human axons. Neuropharmacology 54, 1271–1278 [DOI] [PubMed] [Google Scholar]
- 59.Millan M.J. (1999). The induction of pain: an integrative review. Progress in neurobiology 57, 1–164 [DOI] [PubMed] [Google Scholar]
- 60.Baron R., Hans G., and Dickenson A.H. (2013). Peripheral input and its importance for central sensitization. Ann. Neurol. 74, 630–636 [DOI] [PubMed] [Google Scholar]
- 61.Bernal L., Lopez-Garcia J.A., and Roza C. (2016). Spontaneous activity in C-fibres after partial damage to the saphenous nerve in mice: effects of retigabine. Eur. J. Pain 20, 1335–1345 [DOI] [PubMed] [Google Scholar]
- 62.Cai J., Fang D., Liu X.D., Li S., Ren J., and Xing G.G. (2015). Suppression of KCNQ/M (Kv7) potassium channels in the spinal cord contributes to the sensitization of dorsal horn WDR neurons and pain hypersensitivity in a rat model of bone cancer pain. Oncol. Rep. 33, 1540–1550 [DOI] [PubMed] [Google Scholar]
- 63.Zhao P., Waxman S.G., and Hains B.C. (2007). Modulation of thalamic nociceptive processing after spinal cord injury through remote activation of thalamic microglia by cysteine cysteine chemokine ligand 21. J. Neurosci. 27, 8893–8902 [DOI] [PMC free article] [PubMed] [Google Scholar]