Abstract
Tuberculosis (TB) causes disease worldwide, and multidrug resistance is an increasing problem. Matrix metalloproteinases (MMPs), particularly the collagenase MMP-1, cause lung extracellular matrix destruction, which drives disease transmission and morbidity. The role in such tissue damage of the stromelysin MMP-10, a key activator of the collagenase MMP-1, was investigated in direct Mycobacterium tuberculosis (Mtb)–infected macrophages and in conditioned medium from Mtb-infected monocyte–stimulated cells. Mtb infection increased MMP-10 secretion from primary human macrophages 29-fold, whereas Mtb-infected monocytes increased secretion by 4.5-fold from pulmonary epithelial cells and 10.5-fold from fibroblasts. Inhibition of MMP-10 activity decreased collagen breakdown. In two independent cohorts of patients with TB from different continents, MMP-10 was increased in both induced sputum and bronchoalveolar lavage fluid compared with control subjects and patients with other respiratory diseases (both P < 0.05). Mtb drove 3.5-fold greater MMP-10 secretion from human macrophages than the vaccine strain bacillus Calmette–Guerin (P < 0.001), whereas both mycobacteria up-regulated TNF-α secretion equally. Using overlapping, short, linear peptides covering the sequence of early secretory antigenic target-6, a virulence factor secreted by Mtb, but not bacillus Calmette–Guerin, we found that stimulation of human macrophages with a single specific 15–amino acid peptide sequence drove threefold greater MMP-10 secretion than any other peptide (P < 0.001). Mtb-driven MMP-10 secretion was inhibited in a dose-dependent manner by p38 and extracellular signal–related kinase mitogen-activated protein kinase blockade (P < 0.001 and P < 0.01 respectively), but it was not affected by inhibition of NF-κB. In summary, Mtb activates inflammatory and stromal cells to secrete MMP-10, and this is partly driven by the virulence factor early secretory antigenic target-6, implicating it in TB-associated tissue destruction.
Keywords: matrix metalloproteinases, early secretory antigenic target-6, tuberculosis
Clinical Relevance
This study reports on the regulation of matrix metalloproteinase (MMP)-10 gene expression and secretion by early secretory antigenic target-6, an important virulence determinant of Mycobacterium tuberculosis. MMP-10 activates the principal collagenase, MMP-1, to drive immunopathology and, hence, morbidity and mortality in human tuberculosis infection. With the rise in drug resistance, such increased understanding of innate immune responses causing clinical disease is required to develop novel host-directed treatments for tuberculosis.
There were 9.6 million cases and 1.5 million deaths attributed worldwide to tuberculosis (TB) in 2014, and rising drug resistance is of great concern (1). To develop new therapeutic approaches, it is critical to understand underlying mechanisms of disease. In pulmonary TB, extracellular matrix (ECM) breakdown causes cavitation and facilitates Mtb transmission (2) and drug resistance (3), and also increases morbidity and mortality (4). Matrix metalloproteinases (MMPs) are zinc-dependent proteolytic enzymes, which are involved in pulmonary ECM turnover and have diverse immunological roles, such as chemokine processing (5). These enzymes may be secreted by leukocytes and stromal cells during Mtb infection (6, 7). Triple helical collagens (types I–III) are the primary structural fibrils of the lung, and MMP-1 and MMP-8 are the predominant secreted collagenases in TB (8, 9). Accumulating evidence from murine and human studies implicates the interstitial collagenase or MMP-1 as a key effector in tissue destruction and cavity formation in pulmonary TB (10, 11).
MMP-10, also known as stromelysin-2, may promote breakdown of collagen within the ECM by regulating MMP-1 collagenase activity at both the gene expression level and via zymogen activation (12–14). MMP-10 has also been shown to cleave fibronectin, laminin, and type IV collagen in in vitro studies (15). A pathological role for MMP-10 in other pulmonary diseases involving ECM degradation has been reported, including lung malignancy (16) and emphysema (17). MMP-10 activation of MMP-1 is of importance in disease models of angiogenesis (14), and MMP-10 gene expression has been shown to be induced by some bacterial infections (18). Besides ECM breakdown, MMPs may have additional functional roles in the immune response to infections by modulating cytokine processing, defensin activation (5), or leukocyte influx (19). Recently, a novel role for MMP-10 on macrophage polarization was identified in Pseudomonas aeruginosa infection (20). However, MMP-10 has not been systematically investigated in TB.
The region of difference 1 (RD1) is a key determinant of mycobacterial pathogenicity and is absent from all avirulent strains of Mycobacterium bovis bacillus Calmette–Guerin (BCG) and M. microti. Deletion of RD1 from Mtb results in significant strain attenuation (21). The early secretory antigenic target (ESAT)-6 and the culture filtrate protein-10 are encoded by RD1 genes, Rv3874 and Rv3875, are secreted by Mtb, and are highly immunogenic. They have pleiotropic effects on both innate and adaptive immune responses. For example, ESAT-6 induces macrophage apoptosis and interacts with Toll-like receptor (TLR) 2 to decrease IL-12 secretion, which can favor a Th2 phenotype (22). ESAT-6 and ESAT-6:culture filtrate protein-10 complexes may cause pneumocyte cell lysis facilitating Mtb dissemination in the lung (23). ESAT-6 also regulates migratory gradients via MMP-9 (24), but the effect of ESAT-6 on MMP-derived tissue destruction has not been investigated.
In this study, we investigated MMP-10 secretion from human macrophages and stromal cells in TB. We measured MMP-10 concentrations in patients, studying sputum and bronchoalveolar lavage fluid (BALF) from two geographically distinct groups, and analyzed the mechanisms regulating MMP-10 gene expression and secretion, identifying a novel role for ESAT-6 in driving MMP secretion.
Materials and Methods
Clinical TB Studies
Studies were approved by the University of Cape Town (Cape Town, South Africa; HREC Refs 509/2009 and 516/2011) and Nalanda University Hospitals Research Ethics Committees (Patna, India; REC SS/0810/TB). Informed consent was obtained in all cases. In Ubuntu clinic (Cape Town, South Africa), TB and control patient recruitment, recording of data, sputum induction, and processing was performed as described previously (25). In the Nalanda University Hospital, TB and respiratory symptomatic patient recruitment and BALF collection and processing were performed as described previously (26).
Reagents
SB203580, PD98059, and SC-514 (Millipore, Beeston, UK) were used to block activity of p38 mitogen-activated protein kinase (MAPK), extracellular signal–related kinase (ERK), and NF-κB, respectively.
Anti-human MMP-10 (Abcam, Cambridge, UK) and horse radish peroxidase-conjugated anti-rabbit IgG (Cell Signaling, Boston, MA) antibodies were used for Western blot. MMP-10–neutralizing antibody and IgG2B isotype control antibody (R&D Systems, Abingdon, UK) were used for functional assays.
Lipoarabinomannan (LAM; Colorado State University, Fort Collins, CO) and peptides covering the entire ESAT-6 sequence (Pepceuticals, Enderby, UK) were used to stimulate macrophages. Peptides sequences are shown in Table 1. Peptide purity was greater than 90% by mass spectrometry.
Table 1.
Early Secretory Antigenic Target-6 Overlapping Peptide Sequences
| Peptide No. | Sequence |
|---|---|
| 1 | MTEQQWNFAGIEAAA |
| 2 | WNFAGIEAAASAIQG |
| 3 | IEAAASAIQGNVTSI |
| 4 | SAIQGNVTSIHSLLD |
| 5 | NVTSIHSLLDEGKQS |
| 6 | HSLLDEGKQSLTKLA |
| 7 | EGKQSLTKLAAAWGG |
| 8 | LTKLAAAWGGSGSEA |
| 9 | AAWGGSGSEAYQGVQ |
| 10 | SGSEAYQGVQQKWDA |
| 11 | YQGVQQKWDATATEL |
| 12 | QKWDATATELNNALQ |
| 13 | TATELNNALQNLART |
| 14 | NNALQNLARTISEAG |
| 15 | NLARTISEAGQAMAS |
| 16 | ISEAGQAMASTEGNV |
| 17 | QAMASTEGNVTGMFA |
Cell Culture
Human monocytes were matured over 5 days in RPMI with 100 ng/ml macrophage-colony stimulating factor (R&D Systems), 2 mM glutamine, 10 μg/ml ampicillin, and 10% heat-inactivated FBS. Macrophages in serum-free media were infected with Mtb H37Rv or BCG at multiplicity of infection (MOI) 1, which were cultured in Middlebrook 7H9 medium (BD Biosciences, Oxford, UK) and used at mid-log growth.
Normal human bronchial epithelial cells (Lonza, Slough, UK) were cultured according to suppliers’ instructions. Medical Research Council cell strain 5 (MRC-5) lung fibroblasts were cultured in minimum essential medium (MEM) with 2 mM glutamine, 10 μg/ml ampicillin, 1% sodium pyruvate, nonessential amino acids, and 10% FBS. Experiments commenced at 80% confluence.
Conditioned Medium from Mtb-Infected Monocytes
Mtb-infected monocyte (CoMtb) was prepared as previously described (27). Briefly, monocytes were infected with Mtb H37Rv at an MOI of 1, incubated for 24 hours, and supernatants sterile filtered. Medium from uninfected monocytes was termed CoMCont.
MMP-10, TNF-α ELISA, and Luminex Assays
ELISA Duoset kits (R&D Systems) were used to measure MMP-10 and TNF-α concentrations in cell supernatants. Lower detection limits were 31.2 pg/ml and 15.6 pg/ml, respectively. MMP-10 Fluorokine beads (R&D Systems) were used to measure concentrations of MMP-10 in induced sputum and BALF samples on the Luminex 200 platform (Bio-Rad, Hertfordshire, UK). Lower detection limit was 3.2 pg/ml. Assays were performed per manufacturer’s instructions.
RT-PCR
Total RNA was extracted using the Purelink RNA Mini Kit (Life Technologies, Paisley, UK) and reverse transcribed with the Quantitec Reverse Transcription Kit (Qiagen, Manchester, UK). RT-PCR was performed on a Stratagene Mx3000Pro using MMP-10 and β-actin primers and probes (Applied Biosystems, Warrington, UK). Analysis was performed using the ΔΔCt method.
Collagenase Activity
MMP-1 collagenolytic activity was measured using EnzChek fluorescent dye-quenched (DQ) type I collagen assay (Life Technologies) performed per the manufacturer’s instructions. DQ collagen–coated slides were used for confocal microscopy.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism (GraphPad Software Inc., San Diego, CA). Mann–Whitney U, Student’s unpaired t test, or one-way ANOVA with Tukey’s post hoc analysis were used, as appropriate. A P value less than 0.05 was considered significant.
Results
Mtb Infection Drives MMP-10 Secretion from Human Macrophages, Respiratory Epithelial Cells, and Fibroblasts
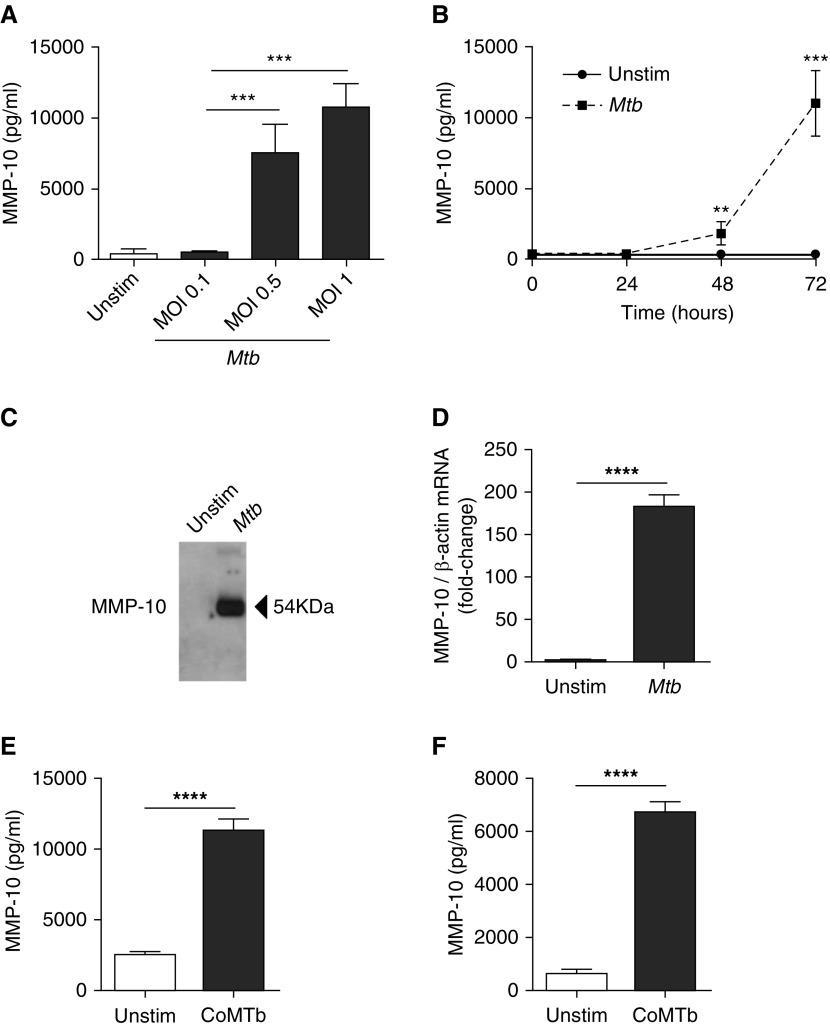
First, MMP-10 secretion from Mtb-infected macrophages was investigated. Mtb caused a dose-dependent up-regulation in MMP-10 secretion, causing a 20.2-fold increase (370 ± 331.6 pg/ml to 7,461.7 ± 2,073.7 pg/ml) at an MOI of 0.5 and a 29-fold increase (10,719.3 ± 1713.2 pg/ml) at an MOI of 1 compared with uninfected macrophages (both P < 0.001). Low-dose infection at an MOI of 0.1 did not up-regulate MMP-10 (Figure 1A). Kinetic studies showed a 5.1-fold up-regulation of MMP-10 secretion from Mtb-infected macrophages at 48 hours (P < 0.01) increasing to 31.5-fold at 72 hours (P < 0.001; Figure 1B). Western blotting confirmed that Mtb stimulation drives MMP-10 secretion from macrophages at 72 hours (Figure 1C). Increased secretion was secondary to increased gene expression, as Mtb increased MMP-10 mRNA accumulation by 182-fold compared with uninfected macrophages at 24 hours (P < 0.0001; Figure 1D).
Figure 1.
Mycobacterium tuberculosis (Mtb) infection drives matrix metalloproteinase (MMP)-10 secretion from macrophages, respiratory epithelial cells, and fibroblasts. (A) MMP-10 secretion from macrophages infected with increasing multiplicity of infection (MOI) of Mtb. (B) Kinetics of MMP-10 secretion from Mtb-infected macrophages (MOI 1). (C) Western blots on cell supernatants were performed to analyze secreted MMP-10 by macrophages after 72 hours of Mtb infection. MMP-10 corresponds to a band of 54 kD. (D) MMP-10 gene expression in Mtb-infected macrophages. MMP-10 transcript abundance was measured by RT-PCR and was normalized to β-actin. Copy numbers were calculated from standard curves with known concentrations of MMP-10 and β-actin. (E) MMP-10 secretion from Mtb-infected monocyte (CoMtb; 1:5 dilution)-stimulated pulmonary epithelial cells at 72 hours. (F) MMP-10 secretion from CoMtb (1:10 dilution)-stimulated pulmonary fibroblasts at 72 hours. Data correspond to mean ± SD and are representative of at least two independent experiments performed in triplicate. **P < 0.01, ***P < 0.001, ****P < 0.0001. Unstim, unstimulated.
Next, we investigated MMP-10 secretion from CoMtb-simulated pulmonary epithelial cells and fibroblasts to investigate the effects of monocyte-dependent intercellular networks. CoMtb stimulation increased MMP-10 secretion by 4.5-fold (from 2525 ± 208.5pg/ml to 11,250 ± 886.1 pg/ml; P < 0.0001) from epithelial cells (Figure 1E) and 10.5-fold from fibroblasts (from 641.7 ± 152.9 pg/ml to 6,715.7 ± 391.9 pg/ml; P < 0.0001; Figure 1F) compared with unstimulated cells.
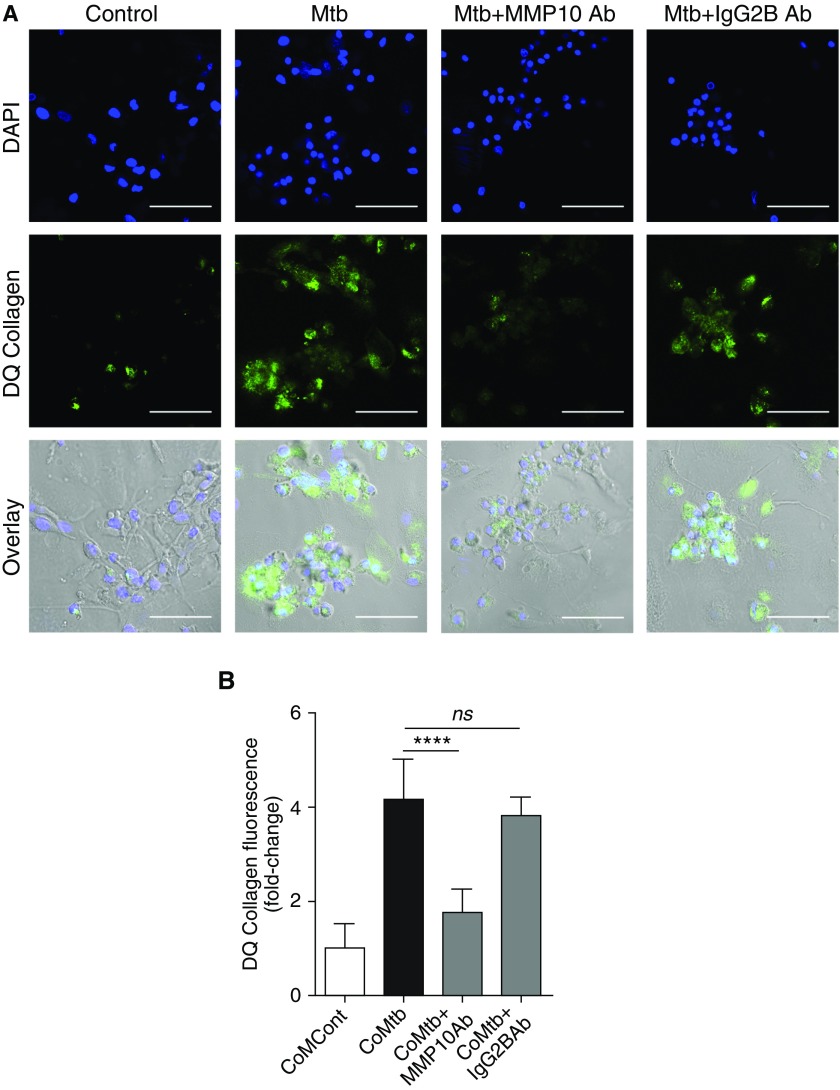
To confirm that MMP-10 was functionally active and affected MMP-1 collagenolytic activity, 15 μg/ml MMP-10 neutralizing antibody was used to block MMP-10 activity. Collagenolytic activity was analyzed using DQ type I collagen, which fluoresces in areas of collagen degradation. A significant increase in collagen breakdown was observed in macrophages infected with Mtb by confocal microscopy, compared with uninfected control subjects. Inhibition of MMP-10 activity led to inhibition of DQ collagen degradation by Mtb-infected macrophages (Figure 2A). Similarly, in CoMtb-stimulated normal human bronchial epithelial cells, addition of 15 μg/ml MMP-10–neutralizing antibody significantly decreased collagen breakdown (P < 0.0001; Figure 2B).
Figure 2.
Mtb-driven MMP-10 is required for MMP-1 collagenolytic activity in macrophages and respiratory epithelial cells. (A) Confocal microscopy figures of collagen breakdown in control macrophages, infected with Mtb (MOI 1) and where MMP-10 activity was inhibited by 15 μg/ml neutralizing antibody (Ab); 15 μg/ml of an IgG2B isotype was used as control. Nucleic acids were stained with 4′,6-diamidino-2-phenylindole (DAPI; blue), whereas dye-quenched (DQ) type I collagen breakdown is shown by release of FITC fluorescence (green). Overlay of DAPI, FITC, and bright field is also shown in the last row of panels. Scale bars: 50 μm. (B) Supernatants of normal human bronchial epithelial cells stimulated for 72 hours with conditioned medium from monocyte controls (CoMCont) or CoMtb, and where MMP-10 activity was inhibited by 15 μg/ml neutralizing antibody where used on a DQ type I collagen assay; 15 μg/ml of an IgG2B isotype was used as control. Increase in fluorescence corresponds to increase in DQ collagen breakdown. Data correspond to mean ± SD and are representative of at least two independent experiments performed in triplicate. ****P < 0.0001; ns, not significant.
MMP-10 Is Increased at the Site of Infection in Patients with Pulmonary TB
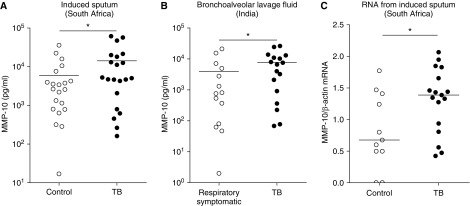
To investigate the relevance of MMP-10 in clinical disease, we measured MMP-10 levels in the respiratory secretions of two separate patient cohorts. We studied induced sputum of patients with TB (mixed human immunodeficiency virus serostatus) and non-TB control subjects from South Africa, and BALF of patients with TB without human immunodeficiency virus compared with patients with nontuberculous respiratory disease from India. MMP-10 concentrations were increased in patients with TB compared with control groups for both of these cohorts (Figure 3; P < 0.05). In the induced sputum study of South African patients, MMP-10 concentrations were 5,067 pg/ml (1,440–19,077 pg/ml) in patients with TB compared with 2,648 pg/ml (797–5,914 pg/ml) in control subjects. In BALF, MMP-10 concentrations were 6,531pg/ml (637–11,929 pg/ml) in patients with TB compared with 783 pg/ml (76–5,473 pg/ml) in respiratory symptomatic control subjects. MMP-10 mRNA accumulation was also increased by 60% in induced sputum of patients with TB compared with control subjects, normalized to β-actin (Figure 3B; P < 0.05). Therefore, these findings show that MMP-10 is present at the site of disease in pulmonary TB, and that concentrations are higher in TB compared with other respiratory diseases.
Figure 3.
MMP-10 is elevated in pulmonary tuberculosis (TB) in two independent cohorts of distinct ethnicity. (A) Induced sputum samples were collected prospectively from patients with active pulmonary TB (n = 21) and non-TB control subjects (n = 21) in Cape Town, South Africa. (B) Bronchoalveolar lavage fluid samples were collected prospectively from patients with active pulmonary TB (n = 14) and patients with other nontuberculous pulmonary diseases (n = 17) in Patna, India. MMP-10 concentrations were measured by Luminex, and, in each cohort, MMP-10 was elevated in patients with TB. (C) Induced sputum samples were collected prospectively from human immunodeficiency virus–negative patients with active pulmonary TB (n = 11) and healthy control subjects (n = 17) in Cape Town for RNA extraction. MMP-10 mRNA accumulation was analyzed by RT-PCR using β-actin mRNA as control. Statistical analysis was performed using a Mann–Whitney U test (*P < 0.05).
MMP-10 Expression in Human Macrophages Is Driven by Virulent Mtb, but Not by LAM or the Vaccine Strain, BCG
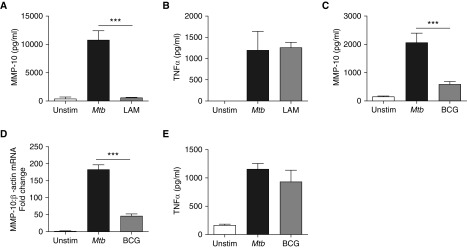
Next, we investigated whether MMP-10 secretion was driven specifically by virulent Mtb or was a nonspecific response to infection. LAM is a mycobacterial cell wall component and principally a TLR2 agonist known to be important in inflammatory responses to Mtb (28). Stimulation of macrophages with 10 μg/ml LAM resulted in MMP-10 secretion, which was 5% of that observed after stimulation with live Mtb (Figure 4A). In contrast, TNF-α secretion from macrophages in response to LAM and direct Mtb infection were similar (Figure 4B). LAM stimulated MMP-1 secretion, which was 30% of that driven by direct Mtb infection (data not shown).
Figure 4.
MMP-10 secretion in human macrophages is driven by Mtb but not by mycobacterial lipoarabinomannan (LAM) or vaccine bacillus Calmette–Guerin (BCG) strain. MMP-10 and TNF-α secretion from macrophages in response to Mtb infection (MOI 1) or stimulation with LAM (10 µg/ml). (A) MMP-10 secretion from macrophages at 72 hours after stimulation with LAM or Mtb. (B) TNF-α secretion from macrophages at 72 hours after stimulation with LAM or Mtb. (C) MMP-10 secretion at 72 hours from Mtb (MOI 1)-infected macrophages compared with BCG-infected macrophages (MOI 1). (D) MMP-10 gene expression at 24 hours from Mtb-infected macrophages compared with BCG-infected macrophages (normalized to β-actin). (E) TNF-α secretion from Mtb-infected macrophages and BCG-infected macrophages at 72 hours. Data correspond to mean ± SD and are representative of three independent experiments performed in triplicate. ***P < 0.001.
Tissue destruction is not usually a feature of pulmonary infection with the vaccine strain, M. bovis BCG (29). MMP-10 concentrations after macrophage infection with BCG were 586.3 (±96.7) pg/ml compared with 2,047.7 (±347) pg/ml from Mtb-infected macrophages at 72 hours (Figure 4C; P < 0.001). Mtb infection of macrophages up-regulated MMP-10 gene expression fourfold more than BCG infection at 24 hours (Figure 4D; P < 0.001), demonstrating that the divergent effects of BCG and Mtb on MMP-10 secretion are transcriptionally regulated. In contrast, Mtb and BCG stimulated similar TNF-α secretion from macrophages at 72 hours (Figure 4E). Colony counting confirmed that equal infectious doses of Mtb and BCG were used in these experiments (data not shown).
MMP-10 Secretion Is Dependent on a Specific 15–Amino Acid Peptide Sequence in ESAT-6 of Mtb
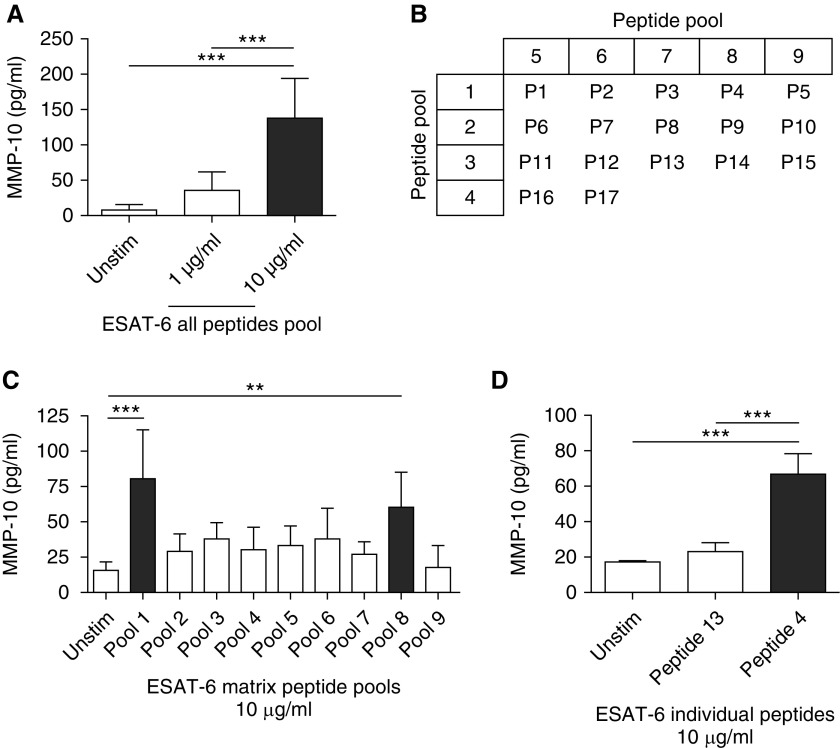
Because BCG did not drive MMP-10 secretion and lacks the RD1 region in its genome (30), we investigated ESAT- 6, a protein encoded by the RD1 region, which is implicated in Mtb virulence (31) and modulation of the host immune response (32, 33). Macrophages were stimulated with a series of short linear peptide sequences from ESAT-6 (Table 1). Stimulation of macrophages with the total pool of all ESAT-6 peptides at a concentration of 10 μg/ml caused a 17.6-fold increase in MMP-10 secretion (from 7.8 ± 7.9 pg/ml to 137.2 ± 56.5 pg/ml), whereas 1 μg/ml ESAT-6 peptides increased MMP-10 by 4.7-fold (36.3 ± 25.9 pg/ml) compared with unstimulated macrophages at 72 hours (Figure 5A; P < 0.001). Next, overlapping pools containing three to four peptides at a total concentration of 10 μg/ml, defined by the matrix shown in Figure 5B, were used to stimulate macrophages. Pools 1 and 8 were the only pools that caused significantly higher MMP-10 secretion at 72 hours than unstimulated macrophages, with 5.2-fold and 3.9-fold up-regulation, respectively (Figure 5C; P < 0.001 and P < 0.01). The single peptide common to both these pools is ESAT-6 peptide 4, which has the sequence SAIQGNVTSIHSLLD (Table 1). Stimulation of macrophages with 10 μg/ml ESAT-6 peptide 4 alone drove a threefold increase in MMP-10 secretion of 66.7 (±11.7) pg/ml compared with the randomly selected ESAT-6 peptide 13 (present in nonstimulatory peptide matrix pools 3 and 7) and a fourfold increase in MMP-10 when compared with control, unstimulated macrophages at 72 hours (Figure 5D; P < 0.001), confirming that peptide 4 was the dominant regulator of MMP-10.
Figure 5.
A 15–amino acid peptide sequence of early secretory antigenic target (ESAT)-6 drives MMP-10 secretion from human macrophages. (A) MMP-10 secretion from macrophages stimulated with a complete pool of 17 overlapping ESAT-6 peptides spanning the entire protein sequence (see Table 1). (B) Matrix indicating peptides present in pools 1–9 containing all peptides (P1–P17). (C) MMP-10 secretion from macrophages stimulated with the ESAT-6 peptide pools from the matrix. (D) MMP-10 secretion from macrophages stimulated with ESAT-6 peptide 4 or ESAT-6 peptide 13 individually. Data correspond to mean ± SD and are representative of three independent experiments performed in triplicate. **P < 0.01, ***P < 0.001.
MMP-10 Secretion from Mtb-Stimulated Macrophages Is MAPK Dependent, but NF-κB Independent
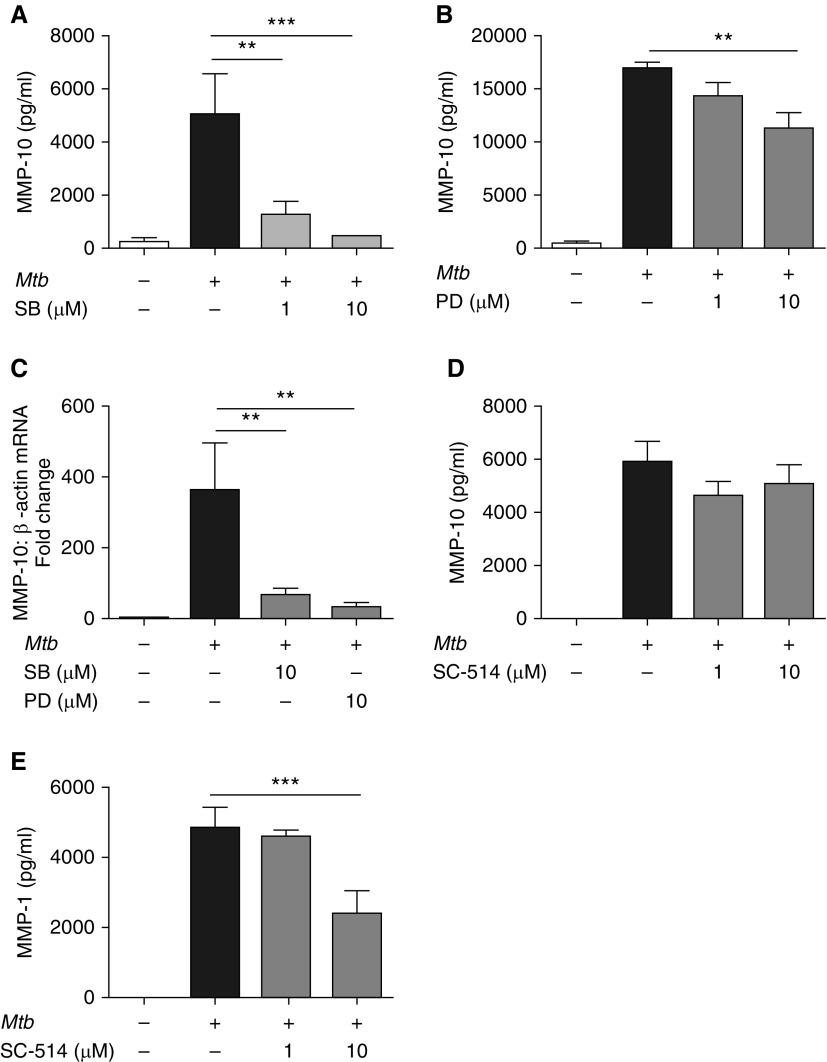
Next, we investigated the role of key signaling pathways in control of Mtb-driven MMP-10 secretion. p38 MAPK inhibition caused a significant dose-dependent decrease in Mtb-driven MMP-10 secretion from macrophages at 72 hours (Figure 6A). ERK MAPK inhibition caused a less marked, but significant, decrease in MMP-10 secretion with 10 μM PD98059 (Figure 6B; P < 0.01). Similarly, blockade of both the p38 and ERK MAPKs inhibited MMP-10 gene expression (Figure 6C; P < 0.01). No significant cell death was observed with the concentrations of inhibitors used.
Figure 6.
MMP-10 expression in Mtb-infected human macrophages is p38 and extracellular signal–related kinase (ERK) mitogen-activated protein kinase (MAPK) dependent, but NF-κB independent. (A and B) MMP-10 secretion at 72 hours after Mtb infection of human macrophages treated with chemical inhibitors of (A) p38 and (B) ERK MAPK macrophages were preincubated with the p38 MAPK inhibitor SB203580 (SB) or the ERK MAPK inhibitor PD98059 (PD) at 1 µM or 10 µM for 2 hours before Mtb infection (MOI 1). (C) MMP-10 gene expression normalized to β-actin in Mtb-infected macrophages SB (p38) or PD (ERK) chemical inhibitors. (D and E) MMP-10 (D) and MMP-1 (E) secretion from Mtb-infected human macrophages with chemical inhibition of NF-ĸB signaling. Macrophages were preincubated with SC-514 at 1 or 10 µM for 2 hours before Mtb infection. Data correspond to mean ± SD and are representative of three independent experiments performed in triplicate. **P < 0.01, ***P < 0.001.
NF-κB regulates gene expression of diverse MMPs, including collagenases in TB (34, 35). MMP-10 does not have a consensus NF-κB binding site in its promoter, although NF-κB noncanonical promoter binding was shown to occur in the promoter of the related stromelysin MMP-3 (36). NF-κB inhibition by the IKK-2 inhibitor SC-514 did not alter Mtb-driven monocyte-derived macrophage MMP-10 secretion (Figure 6D). As a control, we confirmed that 10 μM SC-514 reduced Mtb-driven MMP-1 secretion by 51% (Figure 6E), demonstrating that MMP-10 regulation is NF-κB independent.
Discussion
The stromelysin MMP-10 regulates activity of the collagenase MMP-1, which plays a central role in TB pathogenesis (8), but has not previously been investigated in TB. In the present study, we showed that Mtb infection drives macrophage MMP-10 gene expression and secretion, and investigated its regulation. MMP-10 secretion was also increased by CoMtb stimulation of pulmonary epithelial cells and fibroblasts, which emphasizes a key role for monocyte-dependent networks in stimulating stromal cells, thereby amplifying the matrix-degrading response. Furthermore, MMP-10 may be an important driver of type I collagen breakdown via its regulation of MMP-1 activity, because we demonstrated that inhibition of MMP-10 decreases collagen breakdown with Mtb stimulation. Our data are consistent with other studies on the role of MMP-10 on procollagenase activation (13, 14), although in vivo studies that address these findings are still limited, possibly due to experimental challenges in providing in vivo correlates.
Networking effects are similarly important in driving MMP-1 expression in TB (27, 35, 37). ESAT-6, a secreted virulence factor not expressed by the attenuated vaccine strain, M. bovis BCG, up-regulated MMP-10 secretion, implicating this pathway in mycobacterial pathology. Furthermore, increased MMP-10 expression has been shown to drive macrophage polarization toward an M2-like phenotype (20), an alternatively activated form of macrophage that is present within TB granulomas and associated with Mtb persistence (38).
In clinical studies, increased MMP-10 concentrations were detected in the respiratory secretions of patients with pulmonary TB relative to non-TB control subjects, and compared with patients with nontuberculous pulmonary diseases. These data were consistent between two populations from different continents. To understand tissue destruction in human TB, it is important to study patients with TB, as most animal models do not accurately mimic the cavitatory disease seen in man (39). Our data are consistent with the observation that MMP-10 gene expression was 14-fold higher in human TB granulomas compared with normal lung tissue on microarray analysis (40). Our finding of increased levels of MMP-10 by Luminex bead array in induced sputum and BALF of patients with TB shows that such increased gene expression translates to secreted protein, which may augment pathology in clinical disease. Furthermore, the finding in the Indian cohort of patients that MMP-10 is increased in the BALF of patients with TB compared with patients with other respiratory diseases indicates that there is some specificity in the increased MMP-10 concentrations in TB. MMP-10 activity has been implicated in other destructive pulmonary pathology, such as chronic obstructive pulmonary disease (41), and was associated with disease severity and prognosis in patients with idiopathic pulmonary fibrosis (42). Although our study implicated MMP-10 in the protease network up-regulated in TB, as MMP-10 was increased in respiratory secretions of patients with TB and at the gene expression level, we did not precisely define the cellular source. However, it is most likely due to an MMP-amplification network involving multiple cell types within the granuloma, including macrophages, respiratory epithelial cells, and fibroblasts.
Tissue destruction and cavitation are features of pulmonary TB, whereas administration of vaccine BCG does not cause lung pathology in immunocompetent individuals (29). Mtb and BCG caused similar up-regulation in TNF-α secretion, showing that BCG causes a proinflammatory reaction, but did not up-regulate MMP-10. This finding suggested that specific factors present in Mtb, but not BCG, were responsible for driving MMP-10 expression. RD1 is a region absent in the genome of all BCG strains and avirulent mycobacteria, but present in virulent M. bovis and Mtb (30). Mice infected with BCG containing RD antigens showed an increased bacterial growth rate and inflammatory cell infiltration with granuloma formation, confirming its role in pathogenesis, but an emerging paradigm is that RD1 has multiple causal effects on pathology (24, 31). We employed an overlapping pool of peptides spanning the entire ESAT-6 sequence to identify a single linear 15–amino acid sequence, which alone was sufficient to drive MMP-10 secretion from macrophages. Thus, ESAT-6–dependent MMP-10 secretion is an additional pathological mechanism through which this protein promotes Mtb virulence. This peptide, along with other ESAT-6 fragments, has been shown previously to induce specific IFN-γ–secreting T cells (43). Therefore, it is possible that, in macrophages, this peptide may act as a pathogen-associated molecular pattern to activate pattern recognition receptors (44, 45).
There are multiple signaling pathways involved in the innate immune response to Mtb. TLR activation by Mtb infection drives activation of signaling molecule myeloid differentiation primary response protein, p38, ERK, phosphoinositide 3-kinase, and NF-κB pathways (46, 47). These pathways have been shown to regulate diverse MMPs in Mtb infection and other diseases, but their importance in controlling MMP-10 expression has not been assessed. MMP-10 secretion from Mtb-infected macrophages was regulated by both p38 and ERK MAPK signaling. ESAT-6, which we showed activated MMP-10 secretion, is also known to increase p38 and ERK MAPK phosphorylation in macrophages. MAPKs often activate inflammatory mediators in an NF-κB–dependent manner (37, 48). Both Mtb and LAM stimulation activated TLR2, leading to similar TNF-α induction via MAP/NF-κB signaling. However, our data show that MMP-10 up-regulation appears independent of NF-κB. It is possible that the independence of MMP-10 expression from the NF-κB/TLR2 signaling pathway may be part of a regulatory network to limit inflammation, as less MMP-10 at the site of disease may prevent excessive tissue destruction. Activator protein 1 and signal transducer and activator of transcription, both of which have consensus binding sites in the MMP-10 promoter (49), may be more important factors and are known to regulate ERK-dependent MMP-10 expression in cardiomyocytes (50).
In summary, MMP-10 is up-regulated by macrophage Mtb infection and by TB-dependent networks acting on respiratory epithelial cells and fibroblasts. MMP-10 is elevated in induced sputum and BALF of patients with TB. MMP-10 expression during Mtb infection is specifically driven by a central peptide sequence within ESAT-6, which is absent in BCG, causing ERK- and p38 MAPK–dependent expression of MMP-10. In pulmonary TB, MMP-10 may play a key role in promoting tissue destruction and cavitation, acting at the apex of a proteolytic cascade, resulting in matrix degradation.
Acknowledgments
Acknowledgments
The authors are grateful to Rene Goliath, Ronnett Seldon, and all the staff and patients at Ubuntu clinic (Cape Town, South Africa) for their assistance with this study.
Footnotes
This work was supported by the Portuguese Foundation for Science and Technology (S.B.), the Rosetrees Trust and Breathing Matters charities (S.B. and J.S.F.), Medical Research Council (UK) Clinical Research Training Fellowships (T.S. and S.S.), Wellcome Trust Clinical Research Training Fellowship in Tropical Medicine and Public Health no. 094,000 (N.F.W.), a Wellcome Trust Clinical Research Training Fellowship (R.C.M.), the Francis Crick Institute (R.J.W.), which receives its core funding from Cancer Research UK grant FC00110218, United Kingdom Medical Research Council grant FC00110218, Wellcome Trust grants FC00110218 and 104,803, the South African Medical Research Council Strategic Health Innovation Partnerships, and National Research Foundation of South Africa grant 96,841, as well as the Imperial Biomedical Research Centre (J.S.F.).
Author Contributions: P.T.E. and J.S.F. conceived the study; T.S., N.F.W., R.J.W., and S.S. organized the clinical studies, recruited patients, and collected data and samples; S.B., T.S., L.H.S., and R.C.M. performed all in vitro experiments; S.B., T.S., P.T.E., and J.S.F. analyzed all data; all authors participated in drafting and revising the manuscript and have approved the final version of the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2016-0162OC on September 21, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.WHO. Global tuberculosis report 2015. Geneva: World Health Organization; 2015. [Google Scholar]
- 2.Kline SE, Hedemark LL, Davies SF. Outbreak of tuberculosis among regular patrons of a neighborhood bar. N Engl J Med. 1995;333:222–227. doi: 10.1056/NEJM199507273330404. [DOI] [PubMed] [Google Scholar]
- 3.Kempker RR, Rabin AS, Nikolaishvili K, Kalandadze I, Gogishvili S, Blumberg HM, Vashakidze S. Additional drug resistance in Mycobacterium tuberculosis isolates from resected cavities among patients with multidrug-resistant or extensively drug-resistant pulmonary tuberculosis. Clin Infect Dis. 2012;54:e51–e54. doi: 10.1093/cid/cir904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rhee CK, Yoo KH, Lee JH, Park MJ, Kim WJ, Park YB, Hwang YI, Kim YS, Jung JY, Moon JY, et al. Clinical characteristics of patients with tuberculosis-destroyed lung. Int J Tuberc Lung Dis. 2013;17:67–75. doi: 10.5588/ijtld.12.0351. [DOI] [PubMed] [Google Scholar]
- 5.Elkington PT, D’Armiento JM, Friedland JS. Tuberculosis immunopathology: the neglected role of extracellular matrix destruction. Sci Transl Med. 2011;3:71ps6. doi: 10.1126/scitranslmed.3001847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elkington PT, Green JA, Emerson JE, Lopez-Pascua LD, Boyle JJ, O’Kane CM, Friedland JS. Synergistic up-regulation of epithelial cell matrix metalloproteinase-9 secretion in tuberculosis. Am J Respir Cell Mol Biol. 2007;37:431–437. doi: 10.1165/rcmb.2007-0011OC. [DOI] [PubMed] [Google Scholar]
- 7.Elkington PT, Ugarte-Gil CA, Friedland JS. Matrix metalloproteinases in tuberculosis. Eur Respir J. 2011;38:456–464. doi: 10.1183/09031936.00015411. [DOI] [PubMed] [Google Scholar]
- 8.Elkington P, Shiomi T, Breen R, Nuttall RK, Ugarte-Gil CA, Walker NF, Saraiva L, Pedersen B, Mauri F, Lipman M, et al. MMP-1 drives immunopathology in human tuberculosis and transgenic mice. J Clin Invest. 2011;121:1827–1833. doi: 10.1172/JCI45666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ong CW, Elkington PT, Brilha S, Ugarte-Gil C, Tome-Esteban MT, Tezera LB, Pabisiak PJ, Moores RC, Sathyamoorthy T, Patel V, et al. Neutrophil-derived MMP-8 drives AMPK-dependent matrix destruction in human pulmonary tuberculosis. PLoS Pathog. 2015;11:e1004917. doi: 10.1371/journal.ppat.1004917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al Shammari B, Shiomi T, Tezera L, Bielecka MK, Workman V, Sathyamoorthy T, Mauri F, Jayasinghe SN, Robertson BD, D’Armiento J, et al. The extracellular matrix regulates granuloma necrosis in tuberculosis. J Infect Dis. 2015;212:463–473. doi: 10.1093/infdis/jiv076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ugarte-Gil CA, Elkington P, Gilman RH, Coronel J, Tezera LB, Bernabe-Ortiz A, Gotuzzo E, Friedland JS, Moore DA. Induced sputum MMP-1, -3 & -8 concentrations during treatment of tuberculosis. PLoS One. 2013;8:e61333. doi: 10.1371/journal.pone.0061333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rohani MG, McMahan RS, Razumova MV, Hertz AL, Cieslewicz M, Pun SH, Regnier M, Wang Y, Birkland TP, Parks WC. MMP-10 regulates collagenolytic activity of alternatively activated resident macrophages. J Invest Dermatol. 2015;135:2377–2384. doi: 10.1038/jid.2015.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barksby HE, Milner JM, Patterson AM, Peake NJ, Hui W, Robson T, Lakey R, Middleton J, Cawston TE, Richards CD, et al. Matrix metalloproteinase 10 promotion of collagenolysis via procollagenase activation: implications for cartilage degradation in arthritis. Arthritis Rheum. 2006;54:3244–3253. doi: 10.1002/art.22167. [DOI] [PubMed] [Google Scholar]
- 14.Saunders WB, Bayless KJ, Davis GE. MMP-1 activation by serine proteases and MMP-10 induces human capillary tubular network collapse and regression in 3D collagen matrices. J Cell Sci. 2005;118:2325–2340. doi: 10.1242/jcs.02360. [DOI] [PubMed] [Google Scholar]
- 15.Nicholson R, Murphy G, Breathnach R. Human and rat malignant-tumor–associated mRNAs encode stromelysin-like metalloproteinases. Biochemistry. 1989;28:5195–5203. doi: 10.1021/bi00438a042. [DOI] [PubMed] [Google Scholar]
- 16.Gill JH, Kirwan IG, Seargent JM, Martin SW, Tijani S, Anikin VA, Mearns AJ, Bibby MC, Anthoney A, Loadman PM. MMP-10 is overexpressed, proteolytically active, and a potential target for therapeutic intervention in human lung carcinomas. Neoplasia. 2004;6:777–785. doi: 10.1593/neo.04283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gosselink JV, Hayashi S, Elliott WM, Xing L, Chan B, Yang L, Wright C, Sin D, Paré PD, Pierce JA, et al. Differential expression of tissue repair genes in the pathogenesis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181:1329–1335. doi: 10.1164/rccm.200812-1902OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenner RG, Young RA. Insights into host responses against pathogens from transcriptional profiling. Nat Rev Microbiol. 2005;3:281–294. doi: 10.1038/nrmicro1126. [DOI] [PubMed] [Google Scholar]
- 19.Manicone AM, Birkland TP, Lin M, Betsuyaku T, van Rooijen N, Lohi J, Keski-Oja J, Wang Y, Skerrett SJ, Parks WC. Epilysin (MMP-28) restrains early macrophage recruitment in Pseudomonas aeruginosa pneumonia. J Immunol. 2009;182:3866–3876. doi: 10.4049/jimmunol.0713949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McMahan RS, Birkland TP, Smigiel KS, Vandivort TC, Rohani MG, Manicone AM, McGuire JK, Gharib SA, Parks WC. Stromelysin-2 (MMP10) moderates inflammation by controlling macrophage activation. J Immunol. 2016;197:899–909. doi: 10.4049/jimmunol.1600502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Etna MP, Giacomini E, Pardini M, Severa M, Bottai D, Cruciani M, Rizzo F, Calogero R, Brosch R, Coccia EM. Impact of Mycobacterium tuberculosis RD1-locus on human primary dendritic cell immune functions. Sci Rep. 2015;5:17078. doi: 10.1038/srep17078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pathak SK, Basu S, Basu KK, Banerjee A, Pathak S, Bhattacharyya A, Kaisho T, Kundu M, Basu J. Direct extracellular interaction between the early secreted antigen ESAT-6 of Mycobacterium tuberculosis and TLR2 inhibits TLR signaling in macrophages. Nat Immunol. 2007;8:610–618. doi: 10.1038/ni1468. [DOI] [PubMed] [Google Scholar]
- 23.Kinhikar AG, Verma I, Chandra D, Singh KK, Weldingh K, Andersen P, Hsu T, Jacobs WR, Jr, Laal S. Potential role for ESAT6 in dissemination of M. tuberculosis via human lung epithelial cells. Mol Microbiol. 2010;75:92–106. doi: 10.1111/j.1365-2958.2009.06959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Volkman HE, Pozos TC, Zheng J, Davis JM, Rawls JF, Ramakrishnan L. Tuberculous granuloma induction via interaction of a bacterial secreted protein with host epithelium. Science. 2010;327:466–469. doi: 10.1126/science.1179663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker NF, Clark SO, Oni T, Andreu N, Tezera L, Singh S, Saraiva L, Pedersen B, Kelly DL, Tree JA, et al. Doxycycline and HIV infection suppress tuberculosis-induced matrix metalloproteinases. Am J Respir Crit Care Med. 2012;185:989–997. doi: 10.1164/rccm.201110-1769OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh S, Kubler A, Singh UK, Singh A, Gardiner H, Prasad R, Elkington PT, Friedland JS. Antimycobacterial drugs modulate immunopathogenic matrix metalloproteinases in a cellular model of pulmonary tuberculosis. Antimicrob Agents Chemother. 2014;58:4657–4665. doi: 10.1128/AAC.02141-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh S, Saraiva L, Elkington PT, Friedland JS. Regulation of matrix metalloproteinase-1, -3, and -9 in Mycobacterium tuberculosis–dependent respiratory networks by the rapamycin-sensitive PI3K/p70(S6K) cascade. FASEB J. 2014;28:85–93. doi: 10.1096/fj.13-235507. [DOI] [PubMed] [Google Scholar]
- 28.Jo EK, Yang CS, Choi CH, Harding CV. Intracellular signalling cascades regulating innate immune responses to Mycobacteria: branching out from Toll-like receptors. Cell Microbiol. 2007;9:1087–1098. doi: 10.1111/j.1462-5822.2007.00914.x. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez OY, Musher DM, Brar I, Furgeson S, Boktour MR, Septimus EJ, Hamill RJ, Graviss EA. Spectrum of bacille Calmette-Guérin (BCG) infection after intravesical BCG immunotherapy. Clin Infect Dis. 2003;36:140–148. doi: 10.1086/344908. [DOI] [PubMed] [Google Scholar]
- 30.Behr MA, Wilson MA, Gill WP, Salamon H, Schoolnik GK, Rane S, Small PM. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science. 1999;284:1520–1523. doi: 10.1126/science.284.5419.1520. [DOI] [PubMed] [Google Scholar]
- 31.Hsu T, Hingley-Wilson SM, Chen B, Chen M, Dai AZ, Morin PM, Marks CB, Padiyar J, Goulding C, Gingery M, et al. The primary mechanism of attenuation of bacillus Calmette–Guerin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proc Natl Acad Sci USA. 2003;100:12420–12425. doi: 10.1073/pnas.1635213100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koo IC, Wang C, Raghavan S, Morisaki JH, Cox JS, Brown EJ. ESX-1–dependent cytolysis in lysosome secretion and inflammasome activation during mycobacterial infection. Cell Microbiol. 2008;10:1866–1878. doi: 10.1111/j.1462-5822.2008.01177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mishra BB, Moura-Alves P, Sonawane A, Hacohen N, Griffiths G, Moita LF, Anes E. Mycobacterium tuberculosis protein ESAT-6 is a potent activator of the NLRP3/ASC inflammasome. Cell Microbiol. 2010;12:1046–1063. doi: 10.1111/j.1462-5822.2010.01450.x. [DOI] [PubMed] [Google Scholar]
- 34.Green JA, Elkington PT, Pennington CJ, Roncaroli F, Dholakia S, Moores RC, Bullen A, Porter JC, Agranoff D, Edwards DR, et al. Mycobacterium tuberculosis upregulates microglial matrix metalloproteinase-1 and -3 expression and secretion via NF-κB and activator protein-1 dependent monocyte networks. J Immunol. 2010;184:6492–6503. doi: 10.4049/jimmunol.0903811. [DOI] [PubMed] [Google Scholar]
- 35.O’Kane CM, Elkington PT, Jones MD, Caviedes L, Tovar M, Gilman RH, Stamp G, Friedland JS. STAT3, p38 MAPK, and NF-κB drive unopposed monocyte-dependent fibroblast MMP-1 secretion in tuberculosis. Am J Respir Cell Mol Biol. 2010;43:465–474. doi: 10.1165/rcmb.2009-0211OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borghaei RC, Gorski G, Javadi M Mariah Chambers. NF-κB and ZBP-89 regulate MMP-3 expression via a polymorphic site in the promoter. Biochem Biophys Res Commun. 2009;382:269–273. doi: 10.1016/j.bbrc.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elkington PT, Emerson JE, Lopez-Pascua LD, O’Kane CM, Horncastle DE, Boyle JJ, Friedland JS. Mycobacterium tuberculosis up-regulates matrix metalloproteinase-1 secretion from human airway epithelial cells via a p38 MAPK switch. J Immunol. 2005;175:5333–5340. doi: 10.4049/jimmunol.175.8.5333. [DOI] [PubMed] [Google Scholar]
- 38.Marino S, Cilfone NA, Mattila JT, Linderman JJ, Flynn JL, Kirschner DE. Macrophage polarization drives granuloma outcome during Mycobacterium tuberculosis infection. Infect Immun. 2015;83:324–338. doi: 10.1128/IAI.02494-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Helke KL, Mankowski JL, Manabe YC. Animal models of cavitation in pulmonary tuberculosis. Tuberculosis (Edinb) 2006;86:337–348. doi: 10.1016/j.tube.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Kim MJ, Wainwright HC, Locketz M, Bekker LG, Walther GB, Dittrich C, Visser A, Wang W, Hsu FF, Wiehart U, et al. Caseation of human tuberculosis granulomas correlates with elevated host lipid metabolism. EMBO Mol Med. 2010;2:258–274. doi: 10.1002/emmm.201000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ostridge K, Williams N, Kim V, Bennett M, Harden S, Welch L, Bourne S, Coombs NA, Elkington PT, Staples KJ, et al. Relationship between pulmonary matrix metalloproteinases and quantitative CT markers of small airways disease and emphysema in COPD. Thorax. 2016;71:126–132. doi: 10.1136/thoraxjnl-2015-207428. [DOI] [PubMed] [Google Scholar]
- 42.Sokai A, Handa T, Tanizawa K, Oga T, Uno K, Tsuruyama T, Kubo T, Ikezoe K, Nakatsuka Y, Tanimura K, et al. Matrix metalloproteinase-10: a novel biomarker for idiopathic pulmonary fibrosis. Respir Res. 2015;16:120. doi: 10.1186/s12931-015-0280-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pathan AA, Wilkinson KA, Klenerman P, McShane H, Davidson RN, Pasvol G, Hill AV, Lalvani A. Direct ex vivo analysis of antigen-specific IFN-γ-secreting CD4 T cells in Mycobacterium tuberculosis–infected individuals: associations with clinical disease state and effect of treatment. J Immunol. 2001;167:5217–5225. doi: 10.4049/jimmunol.167.9.5217. [DOI] [PubMed] [Google Scholar]
- 44.Ogawa T, Asai Y, Hashimoto M, Uchida H. Bacterial fimbriae activate human peripheral blood monocytes utilizing TLR2, CD14 and CD11a/CD18 as cellular receptors. Eur J Immunol. 2002;32:2543–2550. doi: 10.1002/1521-4141(200209)32:9<2543::AID-IMMU2543>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 45.Myneni SR, Settem RP, Sojar HT, Malone JP, Loimaranta V, Nakajima T, Sharma A. Identification of a unique TLR2-interacting peptide motif in a microbial leucine-rich repeat protein. Biochem Biophys Res Commun. 2012;423:577–582. doi: 10.1016/j.bbrc.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rand L, Green JA, Saraiva L, Friedland JS, Elkington PT. Matrix metalloproteinase-1 is regulated in tuberculosis by a p38 MAPK–dependent, p-aminosalicylic acid–sensitive signaling cascade. J Immunol. 2009;182:5865–5872. doi: 10.4049/jimmunol.0801935. [DOI] [PubMed] [Google Scholar]
- 47.Sánchez D, Rojas M, Hernández I, Radzioch D, García LF, Barrera LF. Role of TLR2- and TLR4-mediated signaling in Mycobacterium tuberculosis–induced macrophage death. Cell Immunol. 2010;260:128–136. doi: 10.1016/j.cellimm.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 48.Green JA, Dholakia S, Janczar K, Ong CW, Moores R, Fry J, Elkington PT, Roncaroli F, Friedland JS. Mycobacterium tuberculosis–infected human monocytes down-regulate microglial MMP-2 secretion in CNS tuberculosis via TNFα, NFκB, p38 and caspase 8 dependent pathways. J Neuroinflammation. 2011;8:46. doi: 10.1186/1742-2094-8-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clark IM, Swingler TE, Sampieri CL, Edwards DR. The regulation of matrix metalloproteinases and their inhibitors. Int J Biochem Cell Biol. 2008;40:1362–1378. doi: 10.1016/j.biocel.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 50.Cui C, Shi Q, Zhang X, Liu X, Bai Y, Li J, Liu S, Hu S, Wei Y. CRP promotes MMP-10 expression via c-Raf/MEK/ERK and JAK1/ERK pathways in cardiomyocytes. Cell Signal. 2012;24:810–818. doi: 10.1016/j.cellsig.2011.11.019. [DOI] [PubMed] [Google Scholar]