Abstract
Disruption of continuous retention in care (discontinuity) is associated with HIV disease progression. We examined sex, race, and HIV risk disparities in discontinuity after antiretroviral therapy (ART) initiation among patients in North America. Adults (≥18 years of age) initiating ART from 2000 to 2010 were included. Discontinuity was defined as first disruption of continuous retention (≥2 visits separated by >90 days in the calendar year). Relative hazard ratio (HR) and times from ART initiation until discontinuity by race, sex, and HIV risk were assessed by modeling of the cumulative incidence function (CIF) in the presence of the competing risk of death. Models were adjusted for cohort site, baseline age, and CD4+ cell count within 1 year before ART initiation; nadir CD4+ cell count after ART, but before a study event, was assessed as a mediator. Among 17,171 adults initiating ART, median follow-up time was 3.97 years, and 49% were observed to have ≥1 discontinuity of care. In adjusted regression models, the hazard of discontinuity for patients was lower for females versus males [HR: 0.84; 95% confidence interval (CI): 0.79–0.89] and higher for blacks versus nonblacks (HR: 1.17; 95% CI: 1.12–1.23) and persons with injection drug use (IDU) versus non-IDU risk (HR: 1.33; 95% CI: 1.25–1.41). Sex, racial, and HIV risk differences in clinical retention exist, even accounting for access to care and known competing risks for discontinuity. These results point to vulnerable populations at greatest risk for discontinuity in need of improved outreach to prevent disruptions of HIV care.
Keywords: : discontinuity, injection drug use, NA-ACCORD, racial, retention, sex, disparity
Introduction
Retention in clinical care is important for achieving HIV treatment success.1–4 Despite these benefits, disparities in retention have been noted across race, sex, and HIV acquisition risk groups by multiple researchers.2–7 As such, the US National HIV/AIDS Strategy (NHAS) has set a goal of diminishing these disparities, focusing on racial/ethnic minorities.8,9 Retention is part of the HIV continuum of care.10 Once patients access antiretroviral therapy (ART) after linkage to care, they may subsequently progress toward virologic suppression and improved outcomes or, conversely, toward AIDS and/or death. Throughout this process, patients may remain in continuous care or cycle in and out of care (a process described as churn).11 Patients may meet retention definitions, but not be placed on ART due to patient/provider preference, lack of insurance coverage, housing instability, or drug use; or if on ART, they may not be adherent with therapy.12
In the same studies noting race, sex, and HIV acquisition risk associations with retention in care, poorer immune health has also been a powerful predictor of retention independent of other characteristics, partly because sicker patients may require more frequent care.13–16 However, studies examining sex differences in retention or interruption of therapy have been limited in the generalizability of their findings due to being performed in the context of clinical trials, in resource-limited settings, or at single clinical sites.17–20 Where racial and HIV risk disparities in retention and HIV outcomes are concerned, many of the aforementioned studies have also noted significant differences for persons of black race and those with injection drug use (IDU),4,5,9,12,14,16 although at least one clinical cohort has noted a resolution of racial disparities in HIV outcomes in its recent history.21 These studies have also been limited by lack of information on ART use or have used different methods to adjust for the ART status of their participants while examining retention outcomes.
While race, sex, and HIV acquisition risk factor cannot be modified, the identification of disparities can be helpful in focusing policy, programs, and funding choices toward those groups that require more intensive interventions.22,23 We therefore examined disparities in discontinuity of care after ART initiation by sex, race, and HIV risk factor among patients in a large collaborative cohort in the United States and Canada.5,10 We chose to focus on males, black patients, and those with IDU HIV risk factors as they constitute highly vulnerable populations with poorer HIV outcomes, including retention, both nationally and within our cohort.1–3,5–7,9
Methods
Population and study design
The North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) is a multisite collaboration of cohort studies of HIV-infected individuals receiving care in the United States and Canada.24 The NA-ACCORD comprises a large patient population that is demographically similar to persons living with HIV/AIDS (PLWHA) in the United States.25 The Institute of Medicine (IOM) endorsed the NA-ACCORD as one of 12 data systems appropriate to assess quality of care measures (including retention in care) in monitoring progress in the NHAS and Affordable Care Act.5 Details on the NA-ACCORD collaboration and participating cohort studies have been published previously.26 Briefly, at scheduled intervals, these cohorts submit data regarding enrolled participants' demographic characteristics, vital status, prescribed antiretrovirals (ART), clinical diagnoses, and dates and results of laboratory tests, including HIV-1 RNA and CD4+ cell count. Among clinical cohorts, only patients with ≥2 HIV primary care visits within 12 months are included in the NA-ACCORD; the patient's enrollment date is the date of the first visit after which they meet this criterion. Death is determined by each contributing cohort using the National Death Index, Social Security Death Index, state, and local sources, including death certificates and electronic medical records. The human subjects' activities of NA-ACCORD have been approved by the institutional review boards of all participating sites.
Inclusion criteria and variables of interest
Participants in clinical cohorts of the NA-ACCORD with primary care visit, race/ethnicity, sex, and HIV acquisition risk data were included in this analysis. Clinical retention is not reflected in the visit structure of interval cohorts, whose participants were thus excluded. The 13 included cohorts comprise patients residing in all 50 US states, Washington DC, Puerto Rico, and 5 Canadian provinces.
We analyzed data from ART-initiating HIV-infected adults (≥18 years of age) who had ≥1 HIV primary care encounter between January 2000 and December 2010 and ≥1 CD4+ count after ART initiation before death or first discontinuity of retention. This allowed us to focus on persons who likely had equivalent access to HIV care.
Retention in care was defined using the IOM definition: ≥2 HIV primary care visits within 12 months, but at least 90 days apart, beginning with the year following the patient's ART initiation during the study period.5 We have previously evaluated the consistency of this definition with the Department of Health and Human Services (DHHS) indicator as well as with laboratory-based proxies.27,28 This retention measure has also been noted to correlate strongly with several other metrics and to be independently associated with negative clinical outcomes such as lack of viral suppression.1,5,29–31 This retention definition was anchored to calendar time to create estimates comparable with other studies and clinic-level reports applying the indicator in regular reporting intervals. Disruption of continuous retention (discontinuity) was defined as the first instance of failing to meet the IOM indicator definition in any calendar year after ART initiation and before study follow-up ended. This definition of discontinuity was chosen to highlight risks and disparities for failure to meet the benchmark measure of retention contained in the NHAS, strengthening the ability of inferences from this study to inform policy and future interventions to improve retention. Therefore, the first calendar year following the year of ART initiation in which an individual failed to have ≥2 visits, >90 days apart (before death or the end of the study), was deemed the year of discontinuity. ART initiation was defined as the first recorded prescription of ≥3 antiretroviral agents from ≥2 classes, or a triple nucleoside/nucleotide reverse transcriptase inhibitor (NRTI) regimen, following entry to care and with no prior HIV-1 RNA <200 copies/mL.
Event of interest, competing event, and follow-up
The event of interest was first discontinuity before end of study period (December 31, 2010), with all-cause mortality treated as a competing event. In particular, we were concerned that groups at higher risk of death would appear to be poorly retained simply due to increased mortality. Individuals who neither died nor experienced discontinuity before the end of the study period were censored at their final primary care visit before December 31, 2010. Baseline (T0) was ART initiation, and the time of origin for the analysis was 1 year after ART initiation (denoted as ART initiation +1). Discontinuity was assessed as the last visit date in the first year with disruption of retention after ART initiation (described above).
Exposures of interest and factors associated with death and discontinuity in retention
Birth year, race, HIV transmission risk group, sex, closest CD4+ count measured within 1 year before ART initiation, CD4+ count nadir after ART initiation, but before study exit, and median CD4+ count in each year after ART initiation were identified as potential confounders. Race was categorized as black or nonblack (nonblack included white race, Hispanic/Latino ethnicity, and other/unknown race). HIV transmission risk group was divided into IDU or non-IDU (non-IDU included men who have sex with men [MSM], heterosexual contact, and other/unknown risk factors). Patients with both sexual and IDU transmission risk were categorized as IDU. We chose not to focus on Hispanic/Latino ethnicity or MSM HIV risk in our primary analyses due to superior retention among these groups in our cohort; our intent here was to explore the timing and risk of inferior retention outcomes.4,8 Race and HIV transmission risk were dichotomized as described to create categories so that relative risks and times to discontinuity could be more easily visualized and to highlight disparities by high burden and high incidence groups with poorer HIV outcomes in the United States and Canada.1–3,8,9 CD4+ counts were categorized as <200, 200–349, 350–499, ≥500 cells/mm3, or missing. Patients were classified as using ART if they were prescribed ART for ≥1 month in a calendar period. Cohort site was treated as a confounding factor due to potential differences in clinical practice between sites that may have influenced discontinuity.
Statistical analyses
Bivariate comparisons of patient characteristics and event types by exposure status (female vs. male, black vs. nonblack, and IDU vs. non-IDU) were conducted using the χ2 test for categorical variables or the Kruskal–Wallis test for continuous variables.
A competing risks analysis of time from ART initiation to first discontinuity or death by sex, race, and HIV risk group was conducted by regression modeling of the cumulative incidence function (CIF). This approach yielded the subdistribution hazard ratios (HRs) for failure due to discontinuity in the presence of the competing event of death.32,33 A bootstrap procedure with 1000 replicates was used to generate 95% confidence intervals (CIs) for the CIF.
All models were adjusted for age at ART initiation (scaled by 10 years), baseline CD4+ count, sex, black race, and IDU as HIV acquisition risk factor. Stabilized inverse probability of selection weights (IPWs) was used to minimize confounding and produce stratified adjusted hazards in addition to estimates produced through direct adjustment for covariates in regression modeling of the CIF. Details of the construction, truncation, and distribution of weights may be found in the Appendix.32–36 All weights were constructed and models adjusted using cohort site as a covariate.
Nadir CD4+ count after ART initiation and median CD4+ count in each year after ART initiation were used in additional analyses to reveal disparities after accounting for mediation by CD4+ count. Separate adjusted models were also fitted among males with MSM HIV risk, non-MSM males, females, white patients, and black patients so that the impacts of sex, race, and IDU could be isolated within these strata; these models were also adjusted by age, baseline CD4+ count, nadir CD4+ count after ART initiation, and cohort site. Finally, a secondary analysis adjusting for year of ART initiation in addition to all other demographic, HIV risk, and clinical factors, to account for secular trends by ART era, was conducted, modeling ART initiation year using a restricted cubic spline with four knots.37,38
Analyses were conducted and graphics produced using R v. 3.0.3 (www.r-project.org).
Results
Among 17,171 adults initiating ART from January 2000 to December 2010, the median follow-up time was 3.97 years (interquartile range: 1.85–6.21 years) (Table 1). During the study period, 49% of participants experienced discontinuity, 9% died before discontinuity, and 42% were retained in care without experiencing either event. In addition, the distribution of times until censoring was shorter comparing females with males (median 5.44 vs. 5.87 years, p < 0.001) and longer for death while in care comparing IDU with non-IDU persons (median 3.16 vs. 2.66 years, p = 0.001) (Tables 2–4). Females were younger than males (p < 0.001), and patients with IDU risk were older than non-IDU patients (p < 0.001). Baseline CD4+ count at ART initiation was lower for black than nonblack patients, males than females, and non-IDU than IDU patients (each p < 0.05, Tables 2–4). Nadir CD4+ count after ART initiation, but before first discontinuity, death, or censoring, was lower among black compared with nonblack patients (144 vs. 176 cells/mm3, p < 0.01).
Table 1.
Distribution of Demographic, Risk, and Clinical Characteristics and Endpoints Among 17,171 Antiretroviral Therapy Initiators in the North American AIDS Cohort Collaboration on Research and Design, 2000–2010
| Characteristica | N (%) |
|---|---|
| Total | 17,171 (100) |
| Age (years) | 47.1 (39.7, 55.1) |
| Sex | |
| Male | 14,371 (84) |
| Female | 2800 (16) |
| Race | |
| Nonblack | 9606 (56) |
| Black | 7565 (44) |
| HIV risk | |
| Non-IDU | 13,959 (81) |
| IDU | 3212 (19) |
| CD4+ countb (cells/mm3) | 253 (88, 435) |
| Nadir CD4+ countc (cells/mm3) | 162 (43, 288) |
| HIV-1 RNAd (log10 copies/mL) | 4.525 (3.29, 5.15) |
| Time in follow-up (years) | 3.97 (1.9, 6.2) |
| Events during follow-up | |
| Death | 1511 (9) |
| Discontinuity in care | 8367 (49) |
| Event free | 7293 (42) |
Numbers presented as N (%) if categorical, as median (IQR) if continuous.
At ART initiation, available for N = 14,356 individuals.
After ART initiation, available for N = 17,171 individuals.
At ART initiation, available for N = 14,261 individuals.
ART, antiretroviral therapy; IDU, injection drug use; IQR, interquartile range.
Table 2.
Characteristic Differences and Endpoint Distributions Among 17,171 Antiretroviral Therapy Initiators in the North American AIDS Cohort Collaboration on Research and Design, By Sex, 2000–2010
| Characteristica | Female | Male | p |
|---|---|---|---|
| Total | 2800 (16) | 14,371 (84) | |
| Time in follow-up (years) | 3.47 (1.7, 5.7) | 4.07 (1.9, 6.3) | <0.001 |
| Age (years) | 42 (35, 50) | 48 (40, 56) | <0.001 |
| Race | <0.001 | ||
| Nonblack | 1050 (11) | 8556 (89) | |
| Black | 1750 (23) | 5815 (77) | |
| HIV risk | 0.66 | ||
| Non-IDU | 2268 (16) | 11,691 (84) | |
| IDU | 532 (17) | 2680 (83) | |
| CD4+ countb (cells/mm3) | 264 (102, 448) | 251 (86, 432) | 0.02 |
| Nadir CD4+ countc (cells/mm3) | 163 (45, 284) | 162 (43, 289) | 0.83 |
| HIV-1 RNAd (log10 copies/mL) | 4.398 (3.45, 5.05) | 4.548 (3.25, 5.17) | 0.01 |
| Event | N | Years in FU | N | Years in FU | p |
|---|---|---|---|---|---|
| Death | 177 | 2.65 (1.49, 3.95) | 1334 | 2.80 (1.51, 4.71) | 0.13 |
| Discontinuity in care | 1525 | 2.38 (1.40, 4.00) | 6842 | 2.45 (1.42, 4.24) | 0.10 |
| Event free | 1098 | 5.44 (3.88, 7.63) | 6195 | 5.87 (4.30, 7.96) | <0.001 |
p-Value calculated by χ2 for categorical variables, Kruskal–Wallis for continuous variables.
Numbers presented as N (%) if categorical, as median (IQR) if continuous.
At ART initiation, available for N = 14,356 individuals.
After ART initiation, available for N = 17,171 individuals.
At ART initiation, available for N = 14,261 individuals.
ART, antiretroviral therapy; FU, follow-up; IDU, injection drug use; IQR, interquartile range.
Table 3.
Characteristic Differences and Endpoint Distributions Among 17,171 Antiretroviral Therapy Initiators in the North American AIDS Cohort Collaboration on Research and Design, By Black Race, 2000–2010
| Characteristica | Black | Nonblack | p |
|---|---|---|---|
| Total | 7565 (44) | 9606 (56) | |
| Time in follow-up (years) | 3.82 (1.8, 6.0) | 4.08 (1.9, 6.3) | <0.001 |
| Age (years) | 47 (40, 55) | 47 (39, 55) | 0.62 |
| Sex | <0.001 | ||
| Male | 5815 (40) | 8556 (60) | |
| Female | 1750 (62) | 1050 (38) | |
| HIV risk | <0.001 | ||
| Non-IDU | 5731 (41) | 8228 (59) | |
| IDU | 1834 (57) | 1378 (43) | |
| CD4+ countb (cells/mm3) | 231 (66, 404) | 270 (103, 456) | <0.001 |
| Nadir CD4+ countc (cells/mm3) | 144 (30, 272) | 176 (57, 302) | <0.001 |
| HIV-1 RNAd (log10 copies/mL) | 4.521 (3.42, 5.13) | 4.531 (3.21, 5.17) | 0.99 |
| Event | N | Years in FU | N | Years in FU | p |
|---|---|---|---|---|---|
| Death | 738 | 2.66 (1.51, 4.32) | 773 | 2.88 (1.51, 4.82) | 0.17 |
| Discontinuity in care | 3830 | 2.41 (1.42, 4.19) | 4537 | 2.46 (1.40, 4.22) | 0.75 |
| Event free | 2997 | 5.79 (4.23, 7.88) | 4296 | 5.85 (4.26, 7.92) | 0.77 |
p-Value calculated by χ2 for categorical variables, Kruskal–Wallis for continuous variables.
Numbers presented as N (%) if categorical, as median (IQR) if continuous.
At ART initiation, available for N = 14,356 individuals.
After ART initiation, available for N = 17,171 individuals.
At ART initiation, available for N = 14,261 individuals.
ART, antiretroviral therapy; FU, follow-up; IDU, injection drug use; IQR, interquartile range.
Table 4.
Characteristic Differences and Endpoint Distributions Among 17,171 Antiretroviral Therapy Initiators in the North American AIDS Cohort Collaboration on Research and Design, By Injection Drug Use as HIV Risk Factor, 2000–2010
| Characteristica | IDU | Non-IDU | p |
|---|---|---|---|
| Total | 3212 (19) | 13,959 (81) | |
| Time in follow-up (years) | 3.85 (1.8, 6.1) | 3.99 (1.9, 6.2) | 0.30 |
| Age (years) | 50 (44, 55) | 46 (39, 55) | <0.001 |
| Sex | 0.66 | ||
| Male | 2680 (19) | 11,691 (81) | |
| Female | 532 (19) | 2268 (81) | |
| Race | <0.001 | ||
| Nonblack | 1378 (14) | 8228 (86) | |
| Black | 1834 (24) | 5731 (76) | |
| CD4+ countb (cells/mm3) | 266 (106, 455) | 251 (84, 430) | <0.001 |
| Nadir CD4+ countc (cells/mm3) | 160 (48, 281) | 163 (42, 290) | 0.65 |
| HIV-1 RNAd (log10 copies/mL) | 4.418 (3.28, 5.05) | 4.547 (3.30, 5.17) | <0.001 |
| Event | N | Years in FU | N | Years in FU | p |
|---|---|---|---|---|---|
| Death | 340 | 3.16 (1.81, 4.86) | 1171 | 2.66 (1.47, 4.45) | 0.001 |
| Discontinuity in care | 1623 | 2.37 (1.40, 4.20) | 6744 | 2.46 (1.42, 4.21) | 0.48 |
| Event free | 1249 | 5.91 (4.24, 7.99) | 6044 | 5.81 (4.25, 7.88) | 0.26 |
p-Value calculated by χ2 for categorical variables, Kruskal–Wallis for continuous variables.
Numbers presented as N (%) if categorical, as median (IQR) if continuous.
At ART initiation, available for N = 14,356 individuals.
After ART initiation, available for N = 17,171 individuals.
At ART initiation, available for N = 14,261 individuals.
ART, antiretroviral therapy; FU, follow-up; IDU, injection drug use; IQR, interquartile range.
In univariate regression, there was decreased risk of discontinuity for older individuals (HR: 0.57; 95% CI: 0.56–0.58 per 10-year increase in age), while there was increased risk for females versus males (HR: 1.26; 95% CI: 1.19–1.33) and black versus nonblack (HR: 1.09; 95% CI: 1.05–1.14) patients. There was no significant unadjusted association between IDU and discontinuity (Table 5).
Table 5.
Hazard Ratios for and Median Times to Discontinuity of Retention, from Unadjusted, Adjusted, and Weighted Competing Risks Regression Models for the Cumulative Incidence Function with 95% Confidence Intervals
| Characteristic | Discontinuity: unadjusted HR (95% CI) | Discontinuity: adjustedaHR (95% CI) | Discontinuity: weightedbHR (95% CI) | Median timea,b(years) (95% CI) | Reaching discontinuity by 5 years (%) |
|---|---|---|---|---|---|
| Age (per 10 years) | 0.57 (0.56–0.58) | 0.61 (0.59–0.62) | |||
| Sex | Difference = 1.08 | ||||
| Male | Ref. | Ref. | Ref. | 5.72 (5.40–5.87) | 45 |
| Female | 1.26 (1.19–1.33) | 0.84 (0.79–0.89) | 1.03 (0.96–1.12) | 6.80 (6.29–7.01) | 40 |
| Race | Difference = −0.99 | ||||
| Nonblack | Ref. | Ref. | Ref. | 6.74 (6.27–7.07) | 40 |
| Black | 1.09 (1.05–1.14) | 1.17 (1.12–1.23) | 1.12 (1.06–1.18) | 5.75 (5.42–5.94) | 45 |
| HIV risk factor | Difference = −1.77 | ||||
| Non-IDU | Ref. | Ref. | Ref. | 7.21 (6.73–7.46) | 38 |
| IDU | 1.05 (0.99–1.10) | 1.33 (1.25–1.41) | 1.12 (1.06–1.20) | 5.44 (5.08–5.64) | 47 |
| CD4+ cell count | |||||
| <200 Cells/mm3 | Ref. | Ref. | |||
| 200–349 Cells/mm3 | 1.12 (1.05–1.19) | 1.00 (0.94–1.08) | |||
| 350–499 Cells/mm3 | 1.10 (1.03–1.18) | 0.97 (0.90–1.05) | |||
| ≥500 Cells/mm3 | 0.97 (0.91–1.04) | 0.84 (0.77–0.92) | |||
| Nadir CD4+ cell countc (per 100 cells/mm3) | 1.06 (1.05–1.07) | 1.09 (1.07–1.10) | |||
Bold results are significant (p < 0.05). Estimates of median time differences are from predictions of the cumulative incidence function, stratified on the characteristic of interest, adjusting, and weighting by all other variables, including cohort site, and holding covariates at their mean values. All variables are measured at ART initiation, except for Nadir CD4+ count.
Adjusted model includes all factors described in the table and cohort site.
Inverse probability of selection weights for sex, race, and HIV risk mode, constructed using all other covariates with the addition of cohort subsite (to account for potential differences in clinical practice between sites).
After ART initiation, before event, or censoring.
95% CI, 95% confidence interval; ART, antiretroviral therapy; HR, hazard ratio; IDU, injection drug use.
In adjusted regression models accounting for demographic factors (Table 5), baseline CD4+ count, and nadir CD4+ count after ART initiation, the hazard of discontinuity was significantly lower for older adults (HR: 0.61, per 10-year increase in age) and females versus males (HR: 0.84), but was higher for black versus nonblack (HR: 1.17) and for IDU versus non-IDU risk (HR: 1.33). The hazard for mortality (the competing event; Table 6) was not significantly different for females versus males or for black versus nonblack or IDU versus non-IDU risk, but was significantly higher among older adults, as would be expected (HR: 1.29). In a secondary analysis using the fully adjusted models and simultaneously adjusting for year of ART initiation, HRs for discontinuity by demographic factors and IDU risk were substantively unchanged, with point estimates only altered from the primary results in the second or third decimal place; the statistical significance of all estimates was the same as in the primary analysis (results not shown).
Table 6.
Hazard Ratios for and Median Times to Death Before Discontinuity, from Unadjusted, Adjusted, and Weighted Competing Risks Regression Models for the Cumulative Incidence Function with 95% Confidence Intervals
| Characteristic | Death: unadjusted HR (95% CI) | Death: adjustedaHR (95% CI) | Death: weightedbHR (95% CI) | Median timea,b(years, 95% CI) | Reaching death by 5 years (%) |
|---|---|---|---|---|---|
| Age (per 10 years) | 1.34 (1.29–1.40) | 1.29 (1.23–1.35) | |||
| Sex | |||||
| Male | Ref. | Ref. | Ref. | >10 | 4.8 |
| Female | 0.68 (0.58–0.80) | 0.85 (0.72–1.01) | 0.74 (0.60–0.90) | 4.5 | |
| Race | |||||
| Nonblack | Ref. | Ref. | Ref. | >10 | 4.4 |
| Black | 1.22 (1.10–1.35) | 1.09 (0.97–1.21) | 1.16 (1.04–1.30) | 4.8 | |
| HIV risk factor | |||||
| Non-IDU | Ref. | Ref. | Ref. | >10 | 4.4 |
| IDU | 1.25 (1.11–1.41) | 1.11 (0.98–1.26) | 1.18 (1.03–1.36) | 4.9 | |
| CD4+ cell count | |||||
| <200 Cells/mm3 | Ref. | Ref. | |||
| 200–349 Cells/mm3 | 0.54 (0.46–0.62) | 0.89 (0.75–1.04) | |||
| 350–499 Cells/mm3 | 0.46 (0.39–0.55) | 0.91 (0.74–1.10) | |||
| ≥500 Cells/mm3 | 0.44 (0.37–0.52) | 1.09 (0.90–1.32) | |||
| Nadir CD4+ cell countc (per 100 cells/mm3) | 0.68 (0.64–0.71) | 0.68 (0.64–0.72) | |||
Bold results are significant (p < 0.05). Estimates of median time differences are from predictions of the cumulative incidence function, stratified on the characteristic of interest, adjusting, and weighting by all other variables, including cohort site, and holding covariates at their mean values. All variables are measured at ART initiation, except for Nadir CD4+ count.
Adjusted model includes all factors described in the table and cohort site.
Inverse probability of selection weights for sex, race, and HIV risk mode, constructed using all other covariates with the addition of cohort subsite (to account for potential differences in clinical practice between sites).
After ART initiation, before event, or censoring.
95% CI, 95% confidence interval; ART, antiretroviral therapy; HR, hazard ratio; IDU, injection drug use.
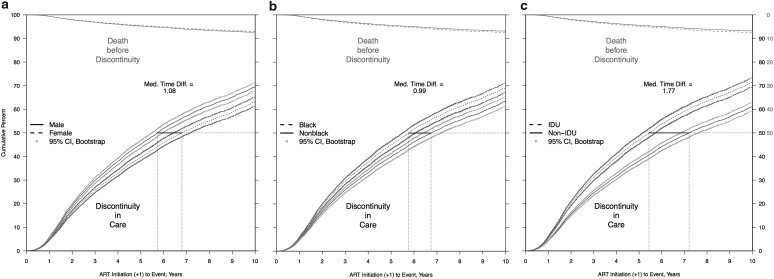
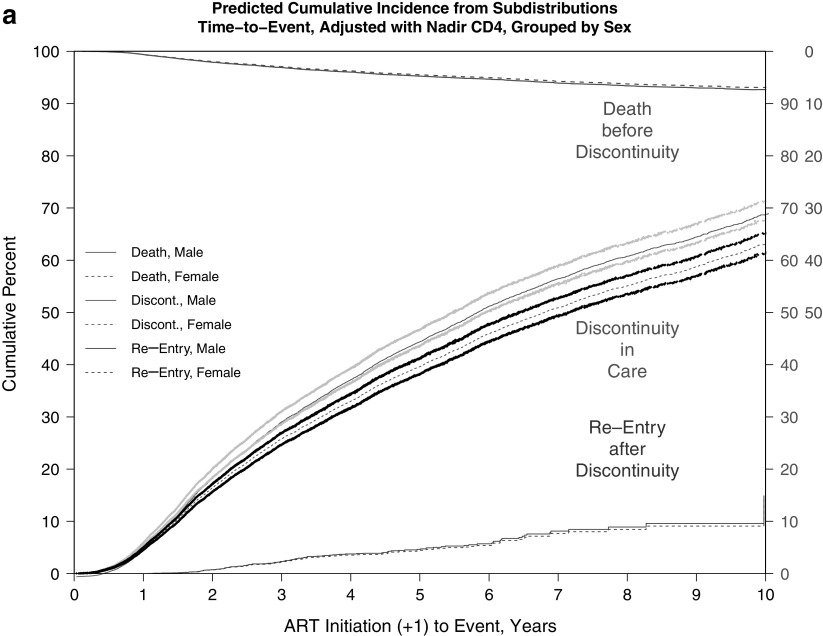
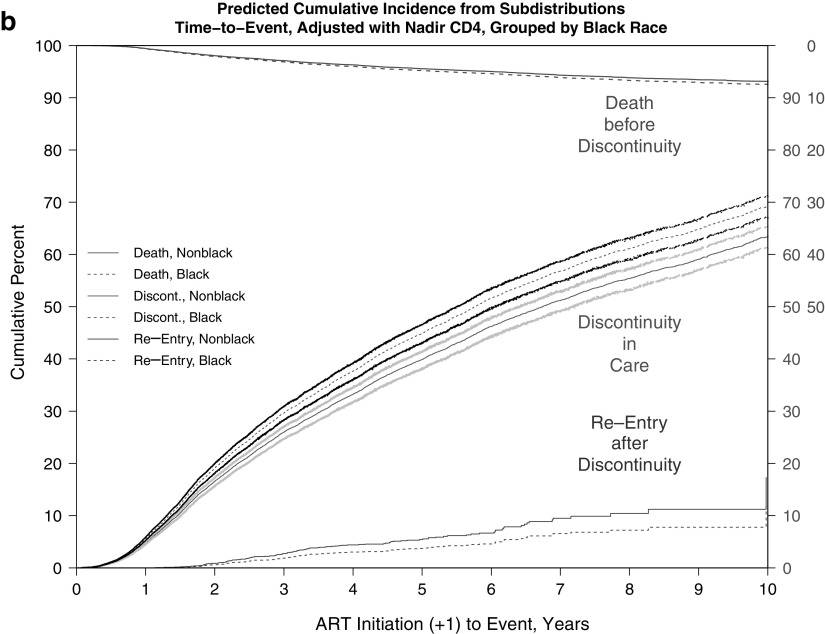
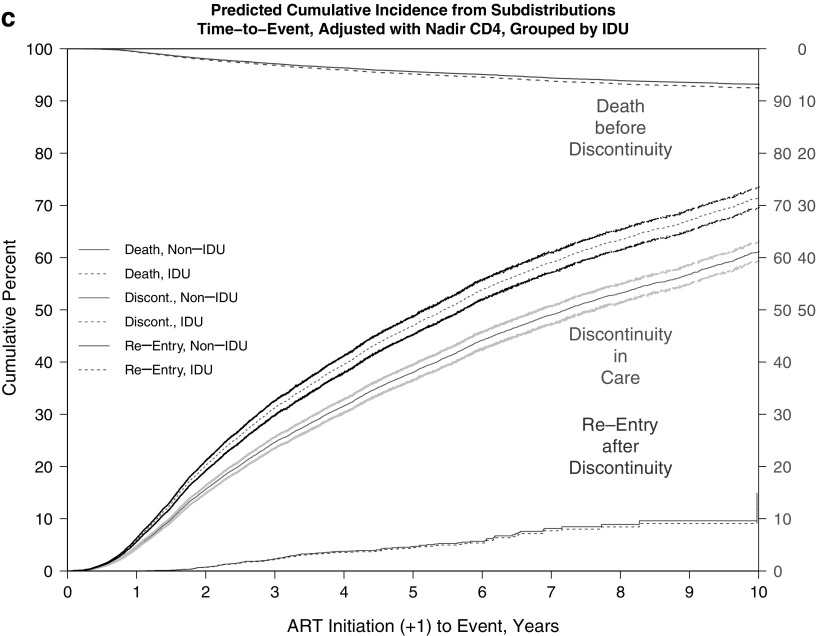
The adjusted cumulative incidence of discontinuity was 0.67 (67%) and for death before discontinuity was 0.09 (9%) at the end of 10 years of follow-up. Estimated median times to discontinuity from the adjusted CIFs (Table 5 and Fig. 1a–c) were significantly longer for females versus males (6.80 vs. 5.72 years) and were shorter for black versus nonblack patients (5.75 vs. 6.74 years) and for persons with IDU versus non-IDU risk (5.44 vs. 7.21 years).
FIG. 1.
(a–c) Predicted CIF of discontinuity of retention and death before discontinuity, adjusted and weighted by available demographic, HIV risk, and site characteristics, stratified by (a) sex, (b) black race, and (c) IDU as HIV risk factor. (a) Adjusted and weighted CIFs, stratified by sex. (b) Adjusted and weighted CIFs, stratified by black race. (c) Adjusted and weighted CIFs, stratified by IDU as HIV risk factor. CIFs, cumulative incidence functions; IDU, injection drug use.
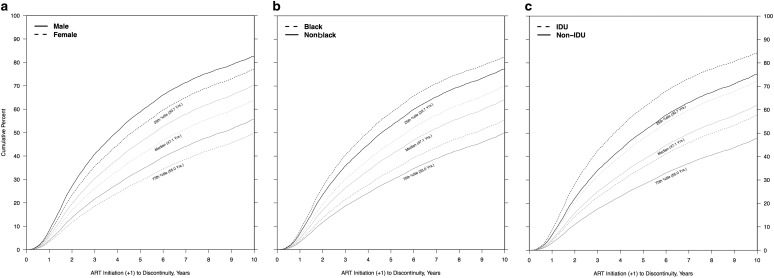
Accounting for median CD4+ count in each year between ART initiation and first discontinuity or death showed similar patterns of association between sex, race, risk, and the event of interest (and competing event) (data not shown). The stratified adjusted cumulative incidence curves describe the sex, race, and risk differences in median times to discontinuity (Table 5 and Fig. 2a–c).
FIG. 2.
(a–c) Predicted CIF of discontinuity of retention, stratified and adjusted by scaled baseline age, estimated at quartiles of sample age distribution, by (a) sex, (b) black race, and (c) IDU as HIV risk factor. (a) Age-adjusted CIFs at quartiles of age distribution, stratified by female sex. (b) Age-adjusted CIFs at quartiles of age distribution, stratified by black race. (c) Age-adjusted CIFs at quartiles of age distribution, stratified by IDU as HIV risk factor. CIFs, cumulative incidence functions; IDU, injection drug use.
In separate models fitted within strata of vulnerable populations, black patients had higher hazards for discontinuity versus nonblack patients, whether with MSM or non-MSM HIV risks, and IDU patients had higher hazards for discontinuity versus non-IDU, whether they were non-MSM men or women. Black race was not significantly associated with discontinuity among women after adjusting for IDU status, although black non-MSM males had a higher hazard of discontinuity compared with black non-IDU females. (Appendix Table A1). The magnitude, direction, and significance of these estimates were similar to those in the overall population (Tables 5 and 6).
Finally, additional analyses addressing return to care or reentry after first discontinuity found no significant differences in times to reentry by sex, race, or IDU status (Appendix Fig. 3a–c); despite the visible separation in CIFs by race, with black patients apparently reentering later than nonblack patients, this difference did not achieve statistical significance (Appendix Fig. 3b).
Discussion
In this study, disparities in the risk and timing of disruptions in continuous retention in care were identified by characteristics investigated: sex, black race, and IDU risk. These groups were chosen for their relatively high incidence, burden, or risk of progression of HIV in the United States and Canada. In contrast to prior studies attempting to address these issues, we explored the associations between these groups and discontinuity while appropriately accounting for the competing risk of death and focusing on ART initiators alone (suggesting equivalent access to care). Our adjusted analyses revealed no disparities in death associated with our factors of interest, accounting for contemporaneous CD4+ count. After adjustment for age and other factors, apparent differences in discontinuity after ART initiation by sex (females interrupting continuous care before males) were reversed (males experiencing discontinuity before females), and differences in death before discontinuity by female sex, black race, and IDU risk were all attenuated (Fig. 2a–c). Results from additional analyses conducted solely within populations of concern underscored the conclusions that male sex, black race, and IDU risk were characteristics by which disparities in retention discontinuity were most pronounced (Appendix Table A1). These models allowed us to focus on vulnerable groups that have been cited as priority populations in improving HIV care continuum outcomes while supporting inferences from the primary models.8,9
There were several limitations to this analysis. First, we did not have data on income, education, employment, insurance coverage, or other contextual and social factors that have been associated with both retention and HIV disease outcomes in other studies and that may differ by demographic factors or HIV transmission risk group.39,40 Some studies with single-sex populations have noted that poverty and housing instability reduce retention and ART adherence.41,42 We maintain that the absence of these data has been mitigated by the fact that all patients under study were successfully engaged in care (a prerequisite for the NA-ACCORD) and had access to ART. Immune status of individuals at ART start was also included in adjusted models, accounting for complications due to late care initiation (i.e., low CD4+ count).
Second, by using a deidentified study population drawing on several independent cohorts, we are not able to exhaustively account for all sources where participants received HIV care. This could lead to negative misclassification of retention if care occurred outside of the NA-ACCORD (apparent discontinuity of care, despite actually receiving care). A potential mitigating factor for this limitation is that the large geographic dispersion of sites makes it unlikely that individuals access care at disparate locations simultaneously. Improved linkage between medical records or state health department surveillance records could reduce this misclassification.43 In contrast, ascertainment of death (the competing event) is likely complete due to use of various death indices.
Despite these limitations, the NA-ACCORD provides a large, geographically diverse study population demographically representative of PLWHA in the United States and Canada, making our results more generalizable.25 We also used models that help account for the competing risk of death when estimating the hazard of discontinuity, evaluating information over a decade following the initiation of ART. Retention in care and HIV disparities research have been linked through numerous studies describing the relationship between clinical engagement, access to and initiation of therapy, continuity of care, and viral suppression and other markers of HIV disease progression in various populations and settings, both in resource-rich and resource-limited settings.12,13,15,16,18,29–31 In fact, the relationship between retention disparities and lack of viral suppression alone merits close attention as viral suppression is a priority outcome in the NHAS, implicating improved individual outcomes and reduced HIV transmission.5,8,29–31 Few studies, however, have followed a cohort as large as the NA-ACCORD population over such a long period of time after ART initiation, a long enough period to directly observe the median times of first discontinuity of retention in care.
Disparities in clinical retention by sex, race, and HIV transmission risk persisted after accounting for the competing risk of death, age, access to care and therapy, and CD4+ cell count changes over time. These results highlight the fact that ART use, clinical retention, and disease outcomes (including death) are dynamically linked, and successful linkage to and engagement in care and treatment initiation do not necessarily imply successful retention in care or improved disease progression. Multiple public health agencies have recommended that clinical retention must be actively pursued and made a priority by clinicians, communities, and public health officials.44 These entities should focus resources on the groups repeatedly identified as being at higher risk for discontinuities of care.
Beyond clinic-level interventions aimed at improving overall clinical retention, individual-level interventions such as enhanced medical case management, peer navigation, transportation subsidies, and mental health evaluation and treatment should be offered with greater vigilance and consistency to the identified vulnerable groups, particularly after the initiation of ART and even after many years in care.45 There have also been studies associating improved trust in clinicians and disclosure of disease status with better clinical retention, evaluated immediately after linkage to care.46,47 However, as ART initiation is now universally recommended, the events of linkage and ART initiation are converging, making these conclusions germane to a broader population. It has also been shown that patients feeling well or experiencing a loss of interest in their own healthcare are more likely to encounter discontinuities in care.48 Evidence from each of these studies implies the need for novel interventions to alter more complicated social and behavioral factors to improve retention outcomes.
The fact that disparities in the clinical experience of HIV patients remain, even after accounting for demographic traits, HIV disease factors, and potential differences in access to care, implies that many factors differentially influencing clinical retention and disease progression remain. It is those factors (including the social and structural determinants) that should be the focus of future research and targeted interventions to provide equally high-quality HIV care for all HIV-infected individuals.
Appendix
Assessment of proportional hazards assumption
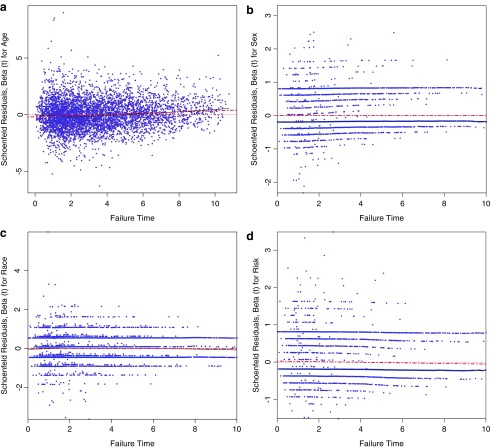
Regression models used to estimate the cumulative incidence function (CIF) in the context of competing events assumes a constant cause-specific subhazard and proportionality of hazards for the events. This proportionality assumption can be assessed by fit of the scaled Schoenfeld residuals to an estimated ratio coefficient of 1 (or a log difference of 0) for the covariate of interest plotted against the event times.(33) The plots of these residuals should exhibit a flat horizontal fitted line close to a log value of 0 to demonstrate no violations of this assumption. The weighted Schoenfeld residual plots for discontinuity of retention by age, sex, race, and risk are presented below (Appendix Fig. 1). They demonstrate no violations of the proportional hazard subdistribution assumption.
APPENDIX FIG. 1.
Plots of weighted Schoenfeld residuals and fitted values for 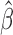 over event times by (a) age, (b) female sex, (c) black race, and (d) IDU risk for the event of interest (discontinuity of retention). Residuals may be used to assess the proportional hazard subdistribution assumption by locally constant mean of the residuals across failure times; the heavy dotted red line is a locally weighted regression smoother.
over event times by (a) age, (b) female sex, (c) black race, and (d) IDU risk for the event of interest (discontinuity of retention). Residuals may be used to assess the proportional hazard subdistribution assumption by locally constant mean of the residuals across failure times; the heavy dotted red line is a locally weighted regression smoother.
Construction of stabilized inverse probability of selection weights
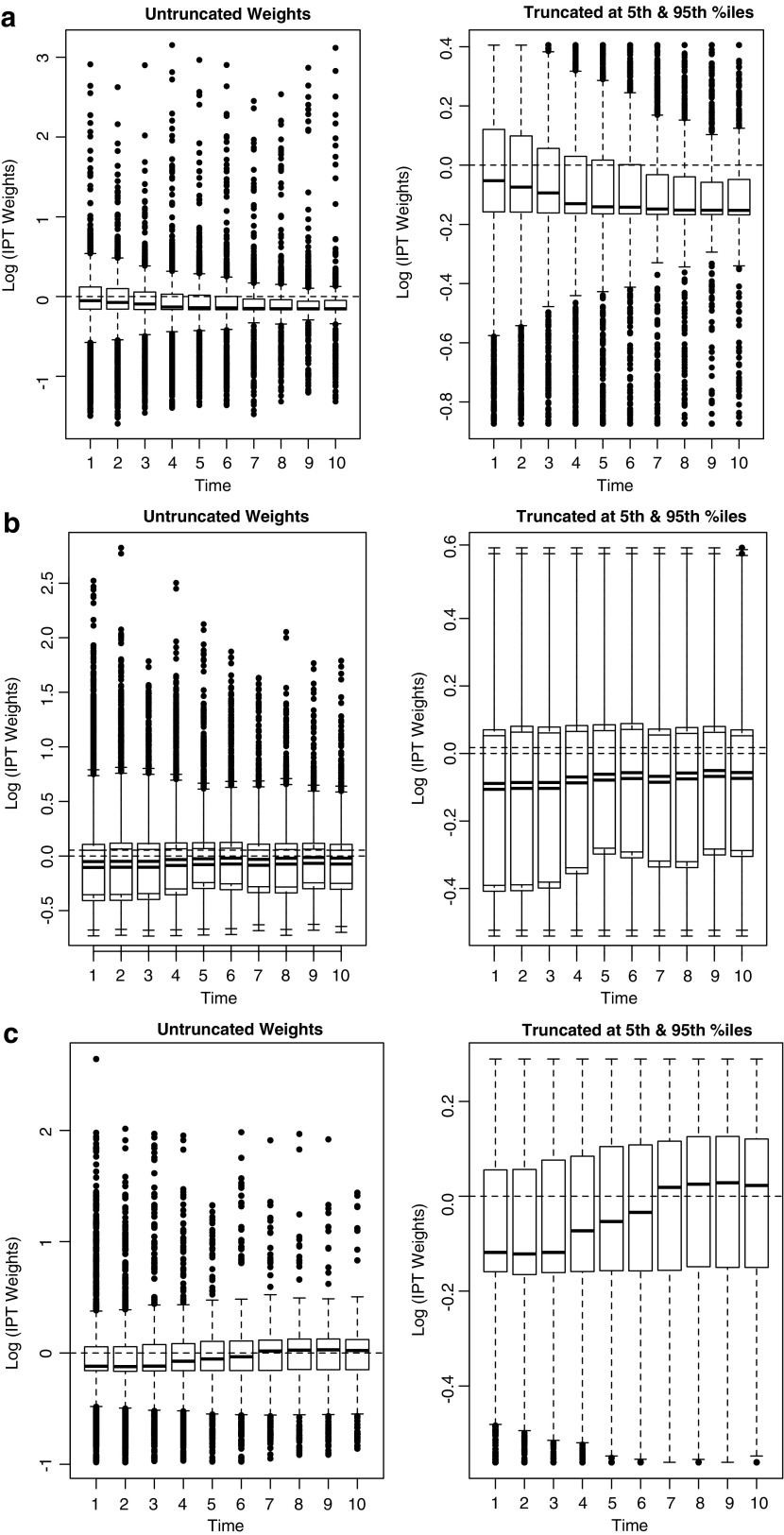
Inverse Probability of Selection Weights (IPW) were used in this context to eliminate confounding by balancing populations with respect to the exposures of interest (sex, race, and HIV risk) conditional on confounders such as baseline age and CD4+ count, and to assess the direct effect of these exposures on the event of interest by reweighting according to the mediating factor of nadir CD4+ count after ART initiation. In this analysis, the potential confounders of baseline age, sex, race, HIV risk factor, and cohort site were used to construct IPW, excluding sex, race, or HIV risk from the denominator when using that respective factor as the exposure. These weights were applied to the marginal models and, in robust estimation, to the conditional fully adjusted models. Survival curves were created using predictions from both the weighted and adjusted estimates, noting that the stratified adjusted curves used covariate values fixed at their means across strata of the exposure of interest.(30,32) Because these were time-fixed confounders, single weights across all timepoints for an individual were used. The weights were also stabilized and truncated at the 5th and 95th percentiles to improve balance (Appendix Fig. 2).(31)
APPENDIX FIG. 2.
Distribution of constructed IPW for the probability of (a) female sex, (b) black race, and (c) IDU risk, both untruncated and truncated at the 5th and 95th percentiles, including adjustment for clinic site, baseline age, baseline CD4+ count, nadir CD4+ count after ART initiation, and the other exposures not under estimation in that model.
The weights were constructed with regression models estimating the probability of exposure received, conditioning on potential confounding or mediating factors. Because the exposures of interest were binary (sex was male vs. female, race was black vs. nonblack, and risk was IDU vs. non-IDU), a logistic regression model was used to create the IPW. For sex, the regression model for the stabilized weights was specified as:
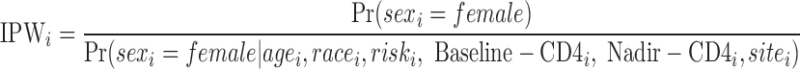 |
Similar models were used to construct weights by race and risk. The truncated weights for female sex had a mean value of 0.94 (IQR: 0.85–1.04) and a range of 0.42–1.50. The truncated weights for black race had a mean value of 0.95 (IQR: 0.69–1.06) and a range of 0.58–1.81. The truncated weights for IDU risk had a mean value of 0.96 (IQR: 0.85–1.09) and a range of 0.57–1.34. These distributions indicate a lack of extreme values which could lead to unstable effect estimates. The untruncated and truncated distribution of weights accounting for sex, race, and HIV risk are illustrated below (Appendix Fig. 2).
Appendix Table 1.
Adjusted Subdistribution Hazard Ratios (HR) and 95% Confidence Intervals (95% CI) for Discontinuity, Isolating the Impacts of Sex, Race, and IDU HIV Risk Within Vulnerable Populations
| Vulnerable population | HR | (95% CI) |
|---|---|---|
| Among MSM males alone | ||
| Black vs. nonblack | 1.16 | (1.05, 1.27) |
| Among non-MSM males alone | ||
| Black vs. nonblack | 1.21 | (1.13, 1.29) |
| IDU vs. non-IDU | 1.31 | (1.22, 1.4) |
| Among females alone | ||
| Black vs. nonblack | 1.05 | (0.94, 1.17) |
| IDU vs. non-IDU | 1.16 | (1.01, 1.34) |
| Among black, non-IDU patients alone | ||
| Non-MSM male vs. non-IDU female | 1.26 | (1.14, 1.4) |
| MSM male vs. non-IDU female | 1.08 | (0.97, 1.2) |
| Among white, non-IDU patients alone | ||
| Non-MSM male vs. non-IDU female | 1.18 | (0.98, 1.43) |
| MSM male vs. non-IDU female | 1.08 | (0.92, 1.26) |
Each model accounted for competing risk of death before discontinuity and adjusted for age, baseline CD4+ count, nadir CD4+ count after ART initiation, and cohort site.
Bold estimates are statistically significant (p < 0.05).
APPENDIX FIG. 3.
Disparities in modeled cumulative incidence functions for first discontinuity of retention after ART initiation and re-entry to care after first discontinuity, treating death as a competing risk by (a) female sex, (b) black race, and (c) IDU risk.
Contributor Information
Collaborators: for the North American AIDS Cohort Collaboration On Research and Design (NA-ACCORD)
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was supported by National Institutes of Health grants U01AI069918, F31DA037788, G12MD007583, K01AI093197, K23EY013707, K24AI065298, K24AI118591, K24DA000432, KL2TR000421, M01RR000052, N01CP01004, N02CP055504, N02CP91027, P30AI027757, P30AI027763, P30AI027767, P30AI036219, P30AI050410, P30AI094189, P30AI110527, P30MH62246, R01AA016893, R01CA165937, R01DA011602, R01DA012568, R24AI067039, U01AA013566, U01AA020790, U01AI031834, U01AI034989, U01AI034993, U01AI034994, U01AI035004, U01AI035039, U01AI035040, U01AI035041, U01AI035042, U01AI037613, U01AI037984, U01AI038855, U01AI038858, U01AI042590, U01AI068634, U01AI068636, U01AI069432, U01AI069434, U01AI103390, U01AI103397, U01AI103401, U01AI103408, U01DA03629, U01DA036935, U01HD032632, U10EY008057, U10EY008052, U10EY008067, U24AA020794, U54MD007587, UL1RR024131, UL1TR000004, UL1TR000083, UL1TR000454, UM1AI035043, Z01CP010214, and Z01CP010176; contracts CDC-200-2006-18797 and CDC-200-2015-63931 from the Centers for Disease Control and Prevention, United States; contract 90047713 from the Agency for Healthcare Research and Quality, United States; contract 90051652 from the Health Resources and Services Administration, United States; grants CBR-86906, CBR-94036, HCP-97105, and TGF-96118 from the Canadian Institutes of Health Research, Canada; Ontario Ministry of Health and Long-Term Care; and the Government of Alberta, Canada. Additional support was provided by the National Cancer Institute, National Institute for Mental Health, and National Institute on Drug Abuse. The authors would like to thank all collaborators, staff, and participants of the NA-ACCORD, particularly those cohorts contributing data to these analyses:
■ Fenway Health HIV Cohort: Stephen Boswell, Chris Grasso, and Kenneth H. Mayer.
■ HIV Outpatient Study: John T. Brooks and K.B.
■ HIV Research Network: Kelly A. Gebo and Richard D. Moore.
■ Johns Hopkins HIV Clinical Cohort: Richard D. Moore.
■ John T. Carey Special Immunology Unit Patient Care and Research Database, Case Western Reserve University: Benigno Rodriguez.
■ Kaiser Permanente Northern California: Michael J. Silverberg.
■ Southern Alberta Clinic Cohort: M. John Gill.
■ University of Alabama at Birmingham 1917 Clinic Cohort: Michael S. Saag, Michael J. Mugavero, and James Willig.
■ University of North Carolina at Chapel Hill HIV Clinic Cohort: Joseph J. Eron and Sonia Napravnik.
■ University of Washington HIV Cohort: Mari M. Kitahata and Heidi M. Crane.
■ Veterans Aging Cohort Study: Amy C. Justice, Robert Dubrow, and David Fiellin.
■ Vanderbilt-Meharry Centers for AIDS Research Cohort: T.R.S., David Haas, Sally Bebawy, and Megan Turner.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Giordano TP, Gifford AL, White AC, et al. Retention in care: A challenge to survival with HIV infection. Clin Infect Dis 2007;44:1493–1499 [DOI] [PubMed] [Google Scholar]
- 2.Althoff KN, Rebeiro P, Brooks JT, et al. Disparities in the quality of HIV care when using US department of health and human services indicators. Clin Infect Dis 2014;58:1185–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. HIV Surveillance Report. 2011, vol. 23 Available at: http://www.cdc.gov/hiv/topics/surveillance/resources/reports/ Published February 2013 (Last accessed August8, 2016) [Google Scholar]
- 4.Ford MA, Spicer CM. Monitoring HIV Care in the United States: Indicators and Data Systems. Washington, DC: National Academies Press, 2012 [PubMed] [Google Scholar]
- 5.Mugavero MJ, Lin HY, Allison JJ, et al. Racial disparities in HIV virologic failure: Do missed visits matter? J Acquir Immune Defic Syndr 2009;50:100–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rebeiro P, Althoff KN, Buchacz K, et al. Retention among North American HIV-infected persons in clinical care, 2000–2008. J Acquir Immune Defic Syndr 2013;62:356–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stone VE. HIV/AIDS in women and racial/ethnic minorities in the U.S. Curr Infect Dis Rep 2012;14:53–60 [DOI] [PubMed] [Google Scholar]
- 8.National HIV/AIDS Strategy for the United States. Washington, DC: White House Office of National AIDS Policy; 2010. Available at: http://purl.access.gpo.gov/GPO/LPS124282 (Last accessed August8, 2016) [Google Scholar]
- 9.National HIV/AIDS Strategy for the United States: Updated to 2020. Washington, DC: White House Office of National AIDS Policy; 2015. Available at: www.aids.gov/federal-resources/national-hiv-aids-strategy/nhas-update.pdf (Last accessed August8, 2016) [Google Scholar]
- 10.Bradley H, Hall HI, Wolitski RJ, et al. Vital signs: HIV diagnosis, care, and treatment among persons living with HIV—United States, 2011. MMWR Morb Mortal Wkly Rep 2014;63:1113–1117 [PMC free article] [PubMed] [Google Scholar]
- 11.Gill MJ, Krentz HB. Unappreciated epidemiology: The churn effect in a regional HIV care programme. Int J STD AIDS 2009;20:540–544 [DOI] [PubMed] [Google Scholar]
- 12.Beer L, Valverde EE, Raiford JL, Weiser J, White BL, Skarbinski J. Clinician perspectives on delaying initiation of antiretroviral therapy for clinically eligible HIV-infected patients. J Int Assoc Provid AIDS Care 2015;14:245–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howe CJ, Cole SR, Napravnik S, Eron JJ. Enrollment, retention, and visit attendance in the University of North Carolina Center for AIDS Research HIV clinical cohort, 2001–2007. AIDS Res Hum Retroviruses 2010;26:875–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giordano TP, Hartman C, Gifford AL, Backus LI, Morgan RO. Predictors of retention in HIV care among a national cohort of US veterans. HIV Clin Trials 2009;10:299–305 [DOI] [PubMed] [Google Scholar]
- 15.Wilson LE, Korthuis T, Fleishman JA, et al. HIV-related medical service use by rural/urban residents: A multistate perspective. AIDS Care 2011;23:971–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horberg MA, Hurley LB, Silverberg MJ, Klein DB, Quesenberry CP, Mugavero MJ. Missed office visits and risk of mortality among HIV-infected subjects in a large healthcare system in the United States. AIDS Patient Care STDS 2013;27:442–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ochieng-Ooko V, Ochieng D, Sidle JE, et al. Influence of gender on loss to follow-up in a large HIV treatment programme in western Kenya. Bull World Health Organ 2010;88:681–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor-Smith K, Tweya H, Harries A, Schoutene E, Jahn A. Gender differences in retention and survival on antiretroviral therapy of HIV-1 infected adults in Malawi. Malawi Med J 2010;22:49–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krishnan S, Wu K, Smurzynski M, et al. Incidence rate of and factors associated with loss to follow-up in a longitudinal cohort of antiretroviral-treated HIV-infected persons: An AIDS Clinical Trials Group (ACTG) Longitudinal Linked Randomized Trials (ALLRT) analysis. HIV Clin Trials 2011;12:190–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murri R, Lepri AC, Phillips AN, et al. Access to antiretroviral treatment, incidence of sustained therapy interruptions, and risk of clinical events according to sex: Evidence from the I.Co.N.A. study. J Acquir Immune Defic Syndr 2003;34:184–190 [DOI] [PubMed] [Google Scholar]
- 21.Moore RD, Keruly JC, Bartlett JG. Improvement in the health of HIV-infected persons in care: Reducing disparities. Clin Infect Dis 2012;55:1242–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamblyn R, Eguale T, Huang A, Winslade N, Doran P. The incidence and determinants of primary nonadherence with prescribed medication in primary care: A cohort study. Ann Intern Med 2014;160:441–450 [DOI] [PubMed] [Google Scholar]
- 23.Thompson MA, Mugavero MJ, Amico KR, et al. Guidelines for improving entry into and retention in care and antiretroviral adherence for persons with HIV: Evidence-based recommendations from an International Association of Physicians in AIDS Care panel. Ann Intern Med 2012;156:817–833, W-284, W-285, W-286, W-287, W-288, W-289, W-290, W-291, W-292, W-293, W-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lau B, Gange SJ, Moore RD. Interval and clinical cohort studies: Epidemiological issues. AIDS Res Hum Retroviruses 2007;23:769–776 [DOI] [PubMed] [Google Scholar]
- 25.Althoff KN, Buchacz K, Hall HI, et al. U.S. trends in antiretroviral therapy use, HIV RNA plasma viral loads, and CD4 T-lymphocyte cell counts among HIV-infected persons, 2000 to 2008. Ann Intern Med 2012;157:325–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gange SJ, Kitahata MM, Saag MS, et al. Cohort profile: The North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD). Int J Epidemiol 2007;36:294–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rebeiro PF, Horberg MA, Gange SJ, et al. Strong agreement of nationally recommended retention measures from the Institute of Medicine and Department of Health and Human Services. PLoS One 2014;9:e111772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rebeiro PF, Althoff KN, Lau B, et al. Laboratory measures are imperfect proxies of primary care encounters: Implications for quantifying clinical retention among HIV-infected adults in North America. Am J Epidemiol 2015;182:952–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yehia BR, Fleishman JA, Metlay JP, et al. Comparing different measures of retention in outpatient HIV care. AIDS 2012;26:1131–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yehia BR, Rebeiro P, Althoff KN, et al. Impact of age on retention in care and viral suppression. J Acquir Immune Defic Syndr 2015;68:413–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colasanti J, Kelly J, Pennisi E, et al. Continuous retention and viral suppression provide further insights into the HIV care continuum compared to the cross-sectional HIV care cascade. Clin Infect Dis 2015;62:648–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol 2009;170:244–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cole SR, Hernán MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed 2004;75:45–49 [DOI] [PubMed] [Google Scholar]
- 34.Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol 2008;168:656–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nandi A, Glymour MM, Kawachi I, VanderWeele TJ. Using marginal structural models to estimate the direct effect of adverse childhood social conditions on onset of heart disease, diabetes, and stroke. Epidemiology 2012;23:223–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994;81:515–526 [Google Scholar]
- 37.Shepherd BE, Rebeiro PF; Caribbean, Central, South America network for HIV epidemiology (CCASAnet). Assessing and interpreting the association between continuous covariates and outcomes in observational studies of HIV using splines. J Acquir Immune Defic Syndr 2016. October 27 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harrell FE. Regression Modeling Strategies with Applications to Linear Models, Logistic Regression, and Survival Analysis. New York: Springer, 2001 [Google Scholar]
- 39.Cunningham CO, Buck J, Shaw FM, Spiegel LS, Heo M, Agins BD. Factors associated with returning to HIV care after a gap in care in New York State. J Acquir Immune Defic Syndr 2014;66:419–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cunningham WE, Sohler NL, Tobias C, et al. Health services utilization for people with HIV infection: Comparison of a population targeted for outreach with the U.S. population in care. Med Care 2006;44:1038–1047 [DOI] [PubMed] [Google Scholar]
- 41.Haley DF, Lucas J, Golin CE, et al. Retention strategies and factors associated with missed visits among low income women at increased risk of HIV acquisition in the US (HPTN 064). AIDS Patient Care STDS 2014;28:206–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Squires K, Feinberg J, Bridge DA, et al. Insights on GRACE (gender, race, and clinical experience) from the patient's perspective: GRACE participant survey. AIDS Patient Care STDS 2013;27:352–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greenberg AE, Purcell DW, Gordon CM, et al. NIH support of Centers for AIDS Research and Department of Health Collaborative Public Health Research: Advancing CDC's enhanced comprehensive HIV prevention planning project. J Acquir Immune Defic Syndr 2013;64 Suppl 1:S1–S6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Centers for Disease Control and Prevention, Health Resources and Services Administration, National Institutes of Health, American Academy of HIV Medicine, Association of Nurses in AIDS Care, International Association of Providers of AIDS Care, the National Minority AIDS Council, Urban Coalition for HIV/AIDS Prevention Services. Recommendations for HIV Prevention with Adults and Adolescents with HIV in the United States, 2014; 2014. Available at: http://stacks.cdc.gov/view/cdc/26062 (Last accessed August8, 2016)
- 45.Mugavero MJ, Amico KR, Horn T, Thompson MA. The state of engagement in HIV care in the United States: From cascade to continuum to control. Clin Infect Dis 2013;57:1164–1171 [DOI] [PubMed] [Google Scholar]
- 46.Elopre L, Hook EW, Westfall AO, et al. The role of early HIV status disclosure in retention in HIV care. AIDS Patient Care STDS 2015;29:646–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Graham JL, Shahani L, Grimes RM, Hartman C, Giordano TP. The influence of trust in physicians and trust in the healthcare system on linkage, retention, and adherence to HIV care. AIDS Patient Care STDS 2015;29:661–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gill VC, Krentz HB. Patient perspectives on leaving, disengaging, and returning to HIV care. AIDS Patient Care STDS 2015;29:400–407 [DOI] [PubMed] [Google Scholar]