Abstract
Many scholars have studied the effect of nicorandil on perioperative myocardial protection in patients undergoing elective percutaneous coronary intervention (PCI), but results are inconsistent. Therefore, we performed this meta-analysis. Finally, 16 articles, including 1616 patients, were included into this meta-analysis. Meta-analysis results showed that: (1) Nicorandil can reduce the level of CK-MB after PCI, including at 6 hours, 12 hours, 18 hours and 24 hours. (2) Nicorandil can reduce the level of TnT after PCI, including at 6 hours, 12 hours, 18 hours and 24 hours. (3) Nicorandil can reduce the incidence of adverse reactions after PCI. (4) Nicorandil cannot reduce the level of MVP after PCI, including at 12 hours and 24 hours. (5) Subgroup analysis showed that nicorandil can reduce CK-MB and TnT level at 24 hours after PCI for Chinese’s population (P < 0.05), but can not reduce CK-MB and TnT level at 24 hours after PCI for non Chinese’s population (P > 0.05). Our meta-analysis indicate that nicorandil can reduce myocardial injury and reduce the incidence of adverse reaction caused by PCI for Chinese’s population, but is not obvious for non Chinese’s population. However, this conclusion still needs to be confirmed in the future.
In recent years, percutaneous coronary intervention (PCI)1,2,3 has become the principal means of revascularization in patients with coronary heart disease(CHD). According to new research, PCI can obviously improve the myocardial ischemia symptoms and reduce the incidence of cardiovascular events in patients by the revascularization of the ischemic area4,5. However, some complications will inevitably happen during PCI6. Perioperative myocardial injury (PMI) is one of the common complications to the PCI process, and the incidence rate is approximately 5~30%7,8. Many research results have indicated that the mechanism of myocardial injury after PCI include vascular endothelial injury, distal microvascular thrombosis, surgical occlusion of the blood vessels, coronary artery spasm, plaque displacement leading to side branch occlusion and reperfusion injury9,10. Once occurred PMI, the incidence of long-term adverse cardiovascular events will be significantly increased, and result in poor prognosis. Therefore, early identification, prevention and treatment of PMI have an important clinical significance.
At present, a number of studies11,12,13 about how to prevent perioperative myocardial injury after PCI had been carried out, and the majority of researches are focused on how to prevent myocardial injury after PCI through drug therapy. So far, the commonly used drugs include aspirin, clopidogrel, heparin, statins, beta blockers and calcium antagonists and so on, and statins is commonly used in clinical. Studies have indicated that long-term use of statins can improve the initial clinical symptoms of ACS patients and reduce the incidence of ST segment elevation myocardial infarction14,15. Different retrospective studies and meta-analysis have shown that for patients undergoing elective PCI, statin therapy can effectively reduce the incidence of postoperative PMI16,17,18. A large number of studies have shown that, in addition to stable plaque, statins can improve endothelial function and anti-inflammatory and improve the vascular wall inflammation induced by PCI. Meanwhile, it can effectively reduce the level of c-reactive protein, which can increase the survival rate of the patients after operation, and reduce the inflammatory reaction after PCI19,20.
However, statins also have some adverse drug reaction, such as liver toxicity and muscle toxicity21,22. The mechanism of liver toxicity induced by statin is not entirely clear, it may be related to the inhibition of HMG-CoA reductase and HMG-CoA pathway, leading to a reduction of the HMG-CoA reductase and metallic acid. In vitro, studies have revealed that statins can lead to apoptosis of liver cells, and fenofibrate can aggravate apoptosis. The typical clinical manifestations of muscle toxicity induced by statin are fatigue, muscle pain, muscle weakness, convulsions and tendon pain. From 1987 to 2001 years, the United States FDA recorded a total of 42 death cases of rhabdomyolysis induced by statins. Due to the incidence of adverse drug reaction induced by statins is increasing, many scholars are exploring new drugs to prevent the occurrence of perioperative myocardial injury after PCI.
Nicorandil is an anti-anginal agent with a dual mechanism of action. It is the only potassium channel opener which has the effect of antiangina pectoris, playing an effective role in the expansion of artery, vein and coronary artery23,24,25. In addition, there was no serious effect on heart rate, myocardial contractility and conduction system. However, the conclusion, whether nicorandil has a myocardial protective effect is inconsistent. Hwang J26 and Kim J27 have found that nicorandil had no significant effect on PMI and cardiac enzymes after PCI in patients with stable or unstable angina, but Kim S28, Murakami M29 and Shehata M30 have carried out studies about the clinical effect of nicorandi, and their conclusion is that nicorandil can significantly reduce myocardial enzymes in patients after PCI, which was different from Hwang J and Kim J. Therefore, the purpose of our meta-analysis is to evaluate the myocardial protective effect of nicorandil in the perioperative period of patients after PCI, and to provide a reference for clinical applications.
Results
Study Selection and Characteristics
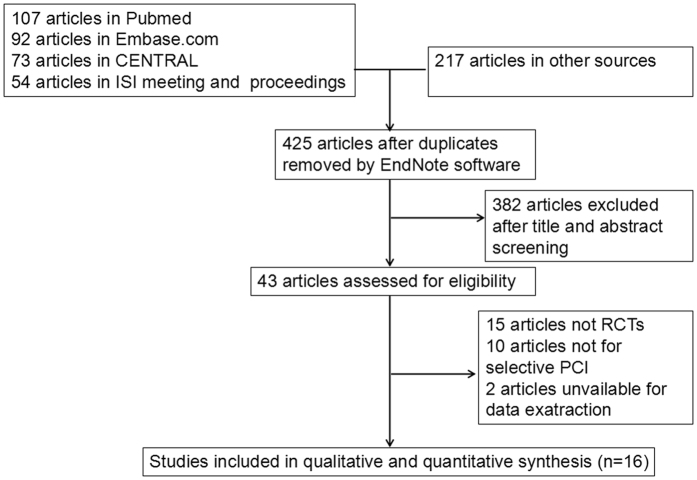
Finally, 543 articles were retrieval by searching the electronic databases and references of relevant articles. After excluding duplicate articles, 425 articles were left. By screening titles and abstracts of remaining articles, 382 apparently irrelevant articles were excluded. Then, the full texts of 43 articles were downloaded to evaluate in detail. Eventually, data from 16 articles26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41 include 1616 patients were listed into this meta-analysis. Among these patients, 804 patients in the nicorandil group and 812 patients in the control group. The flow diagram of study selection is shown in Fig. 1. The basic information of each included literature is shown in Table 1.
Figure 1. Flowchart of the selection strategy and inclusion/exclusion criteria in the current meta-analysis.
Table 1. Characteristics of studies included in meta-analysis.
| Author | year | country | Sample number |
Average age (years) |
Use method of Nicorandil (dose) | preoperative method of Control group | outcome Measures | ||
|---|---|---|---|---|---|---|---|---|---|
| N | C | C | N | ||||||
| XM Ceng21 | 2015 | china | 100 | 100 | 59.4 ± 10.2 | 59.4 ± 10.2 | Venous pump (12 mg/day) | Conventional therapy | CK-MB,TnT |
| WN Pei22 | 2014 | china | 40 | 32 | 56.97 ± 9.83 | 56.97 ± 9.83 | Oral (15 mg/day) | Conventional therapy | CK-MB,TnT |
| JZ Lv23 | 2015 | china | 25 | 20 | 55.41 ± 9.82 | 55.13 ± 9.62 | Oral (15 mg/day) | Conventional therapy | CK-MB,TnT |
| FR Mo24 | 2016 | china | 26 | 31 | 60.35 ± 12.45 | 61. 37 ± 10. 98 | Oral (15–30 mg/day) | Conventional therapy | TnT |
| XP Wu25 | 2013 | china | 48 | 44 | 61.3 ± 8.4 | 61.3 ± 8.4 | Oral (15 mg/day) | Conventional therapy | CK-MB,TnT |
| YH Li26 | 2012 | china | 52 | 48 | 66.4 ± 10.2 | 64.8 ± 10.5 | Oral (15 mg/day) | Conventional therapy | CK-MB,TnT |
| Y Zhang27 | 2012 | china | 30 | 30 | 61.0 ± 5.8 | 63.0 ± 6.2 | Oral (15 mg/day) | Conventional therapy | CK-MB,TnT |
| HY Han28 | 2015 | china | 45 | 49 | 58.64 ± 8.53 | 56.93 ± 9.74 | Oral (15 mg/day) | Conventional therapy | CK-MB,TnT |
| SH Yang29 | 2014 | china | 37 | 38 | 65.37 ± 8.24 | 64.27 ± 8.66 | Oral (15 mg/day) | Conventional therapy | CK-MB,TnT |
| XC Wang30 | 2012 | china | 42 | 48 | 63.47 ± 9.24 | 63.37 ± 8.06 | Oral (15 mg/day) | Conventional therapy | CK-MB,TnT |
| Shehata M20 | 2014 | Egypt | 50 | 50 | 59.4 ± 7.4 | 60.2 ± 4.3 | Oral (20 mg/day) | Conventional therapy | CK-MB,TnT |
| Murakami M19 | 2006 | Japan | 91 | 101 | 65.0 ± 9.7 | 66.1 ± 10.3 | intravenous (2 μg/kg/min) | Conventional therapy | CK-MB,TnT |
| Kim S18 | 2012 | Korea | 54 | 55 | 65.5 ± 7.4 | 63.2 ± 9.2 | Intracoronary (4 mg) | Conventional therapy | CK-MB,TnT |
| Isono T31 | 2008 | Japan | 23 | 26 | 66.3 ± 7.9 | 66.5 ± 9.4 | intravenous (6 mg/h for 24 h) | Conventional therapy | CK-MB,TnT |
| Kim J17 | 2005 | korea | 100 | 100 | 60.4 ± 11.7 | 61.7 ± 8.2 | Intravenous (10–15 mg/day) | Conventional therapy | CK-MB,TnT |
| Hwang J16 | 2013 | Japan | 41 | 40 | 66.2 ± 9 | 65.3 ± 10 | Intracoronary (4 mg) | Conventional therapy | CK-MB,TnT |
N: Nicorandil group, C: control group.
Literature quality evaluation
Of the 16 articles, four articles31,32,33,39 used a random number method, three articles28,30,41 used a block randomization, eight articles26,27,29,34,35,36,37,38 refer to the random method, but did not give a specific description, and the random method of the rest article40 is not clear. The hidden distribution of the sixteen articles26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41 is low; Fifteen articles26,27,28,29,30,31,32,33,34,35,36,37,38,39,41 used a random single blind method, but the blind method of one article40 is not clear. The incomplete outcome data, selective reporting of results and other biases of the sixteen articles26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41 are low. The literature quality score is shown in Table 2.
Table 2. Assessment of Methodological Quality of Included Studies.
| Study | Random allocation | Hidden distribution | Blind method | Incomplete Outcome Data | Selective reporting of results | Other bias | quality grade |
|---|---|---|---|---|---|---|---|
| XM Ceng21 | Random number method | Low | Single-blind | Low | Low | Low | A |
| WN Pei22 | Random number method | Low | Single-blind | Low | Low | Low | A |
| JZ Lv23 | Random number method | Low | Single-blind | Low | Low | Low | A |
| FR Mo24 | mentioned random | Low | Single-blind | Low | Low | Low | A |
| XP Wu25 | mentioned random | Low | Single-blind | Low | Low | Low | A |
| YH Li26 | mentioned random | Low | Single-blind | Low | Low | Low | A |
| Y Zhang27 | mentioned random | Low | Single-blind | Low | Low | Low | A |
| HY Han28 | mentioned random | Low | Single-blind | Low | Low | Low | A |
| SH Yang29 | Random number method | Low | Single-blind | Low | Low | Low | A |
| XC Wang30 | No clear | Low | No clear | Low | Low | Low | B |
| Shehata M20 | block randomization | Low | Single-blind | Low | Low | Low | A |
| Murakami M19 | mentioned random | Low | Single-blind | Low | Low | Low | A |
| Kim S18 | block randomization | Low | Single-blind | Low | Low | Low | A |
| Isono T31 | block randomization | Low | Single-blind | Low | Low | Low | A |
| Kim J17 | mentioned random | Low | Single-blind | Low | Low | Low | A |
| Hwang J16 | mentioned random | Low | Single-blind | Low | Low | Low | A |
CK-MB after PCI
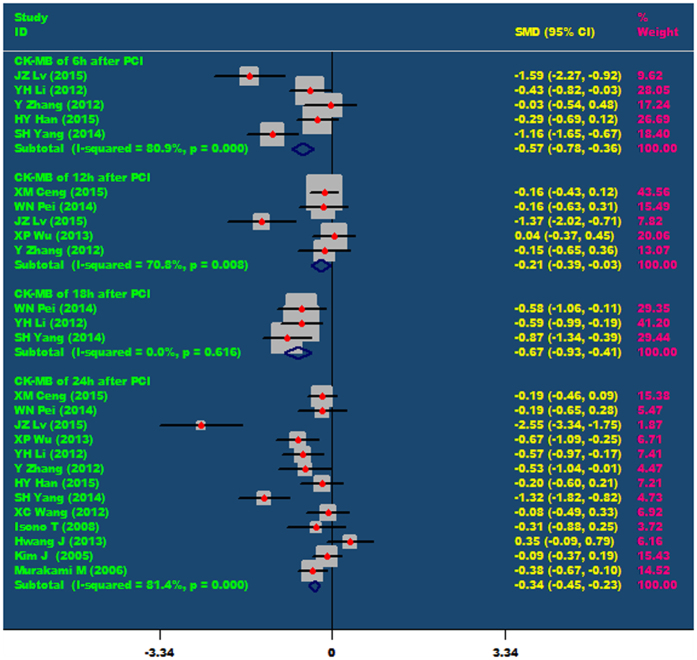
Nicorandil can significantly reduce the level of CK-MB in patients after PCI, including at 6hours (SMD = −0.57, 95% CI −0.78 ~ −0.36, P < 0.001), 12 hours (SMD = −0.21, 95% CI −0.39 ~ −0.03, P = 0.024), 18 hours (SMD = −0.67, 95% CI −0.93 ~ −0.41, P < 0.001) and 24 hours (SMD = −0.34, 95% CI −0.45 ~ −0.23, P < 0.001) after PCI. As showed in Fig. 2.
Figure 2. The comparisons of CK-MB at 6 h, 12 h, 18 h and 24 h after PCI between the nicorandil group and the control group.
Subgroup analysis
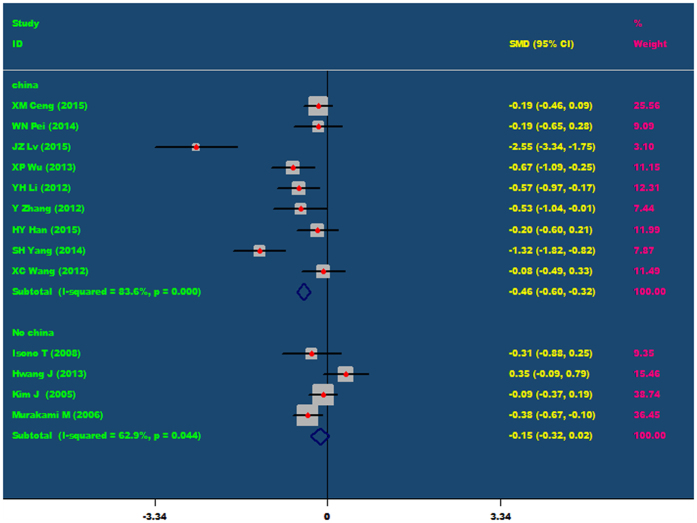
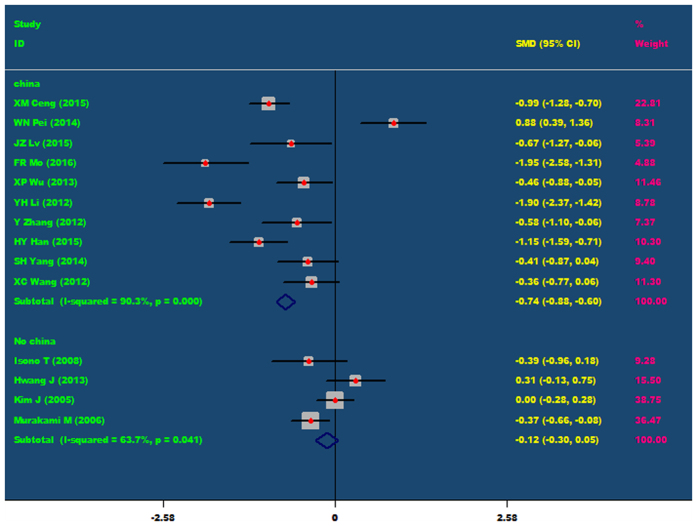
Because the population included in our meta-analysis is mainly coming from China, Japan and Korea, and most of the research objects belong to Chinese population, thus, the population was divided into the Chinese population and non Chinese population, and then conducted a subgroup analysis. Subgroup analysis showed that for Chinese population, Nicorandil can obviously decrease CK-MB level at 24 hours after PCI (SMD = −0.46, 95% CI −0.60 ~ −0.32, P = 0.001). However, for non Chinese population, nicorandil cannot decrease CK-MB level at 24 hours after PCI (P = 0.440), As showed in Fig. 3.
Figure 3. The comparisons of CK-MB at 24 h after PCI between the nicorandil group and the control group (Subgroup analysis: Chinese’s population and non Chinese’s population).
TnT after PCI
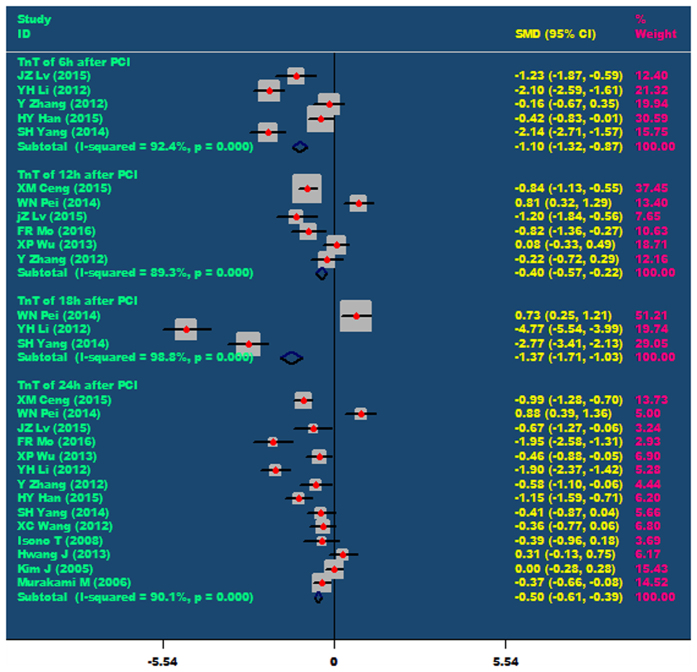
Nicorandil can significantly reduce the level of TnT in patients after PCI, including at 6 hours (SMD = −1.10, 95% CI −1.32 ~ −0.87, P < 0.001), 12 hours (SMD = −0.40, 95% CI −0.57 ~ −0.22, P < 0.001), 18 hours (SMD = −1.37, 95% CI −1.71 ~ −1.03, P < 0.001) and 24 hours(SMD = 0.50, 95% CI −0.61 ~ −0.39, P < 0.001). As showed in Fig. 4.
Figure 4. The comparisons of TnT at 6 h, 12 h, 18 h and 24 h after PCI between the nicorandil group and the control group.
Subgroup analysis
In addition, all patients were divided into Chinese’s population and non Chinese population, and then conducted a subgroup analysis. Subgroup analysis showed that for Chinese’s people, nicorandil can obviously decrease TnT level at 24 hours after PCI (SMD = −0.74, 95% CI −0.88 ~ −0.60, P = 0.001). However, for non Chinese’s population, nicorandil cannot remarkable decrease TnT level at 24 hours after PCI (P = 0.487), As showed in Fig. 5.
Figure 5. The comparisons of TnT at 24 h after PCI between the nicorandil group and the control group (Subgroup analysis: Chinese’s population and non Chinese’s population).
Incidence of adverse reactions
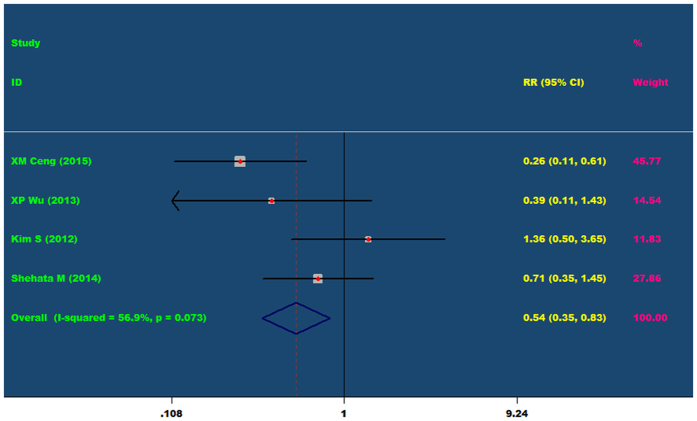
Four articles evaluating the incidence of adverse reactions of nicorandil undergoing elective PCI. Meta-analysis showed that I2 = 56.9%, the heterogeneity is large, so random effects model was used to analyze. Meta-analysis (random effect’s model) results showed that nicorandil can reduce the incidence of adverse reactions in patients after elective PCI (RR = 0.54, 95% CI 0.35 ~ 0.83, P = 0.005). As showed in Fig. 6.
Figure 6. The incidence of adverse reactions in the nicorandil group and the control group (RR = 0.54, 95% CI 0.35~0.83, P = 0.073).
MPV after PCI
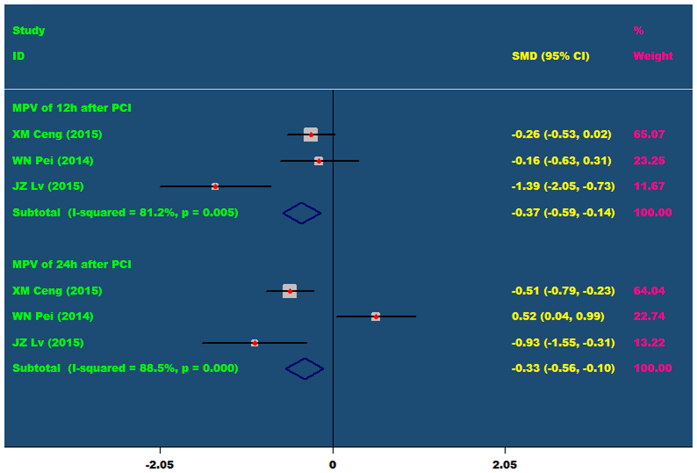
Nicorandil cannot significantly reduce the level of MVP in patients after PCI, including at 12 hours (P = 0.078) and 24 hours (P = 0.445). As showed in Fig. 7.
Figure 7. The comparisons of MPV at 12 h and 24 h after PCI between the nicorandil group and the control group.
Publication bias
Egger regression analysis was used to evaluate the publication bias. There was no publication bias of CK-MB at 24 hours after PCI (Egger’s test: P = 0.214) (Fig. 8A). There was also no publication bias of TnT at 24 hours after PCI (Egger’s test: P = 0.978) (Fig. 8B).
Figure 8. Egger regression analysis was used to evaluate the publication bias.
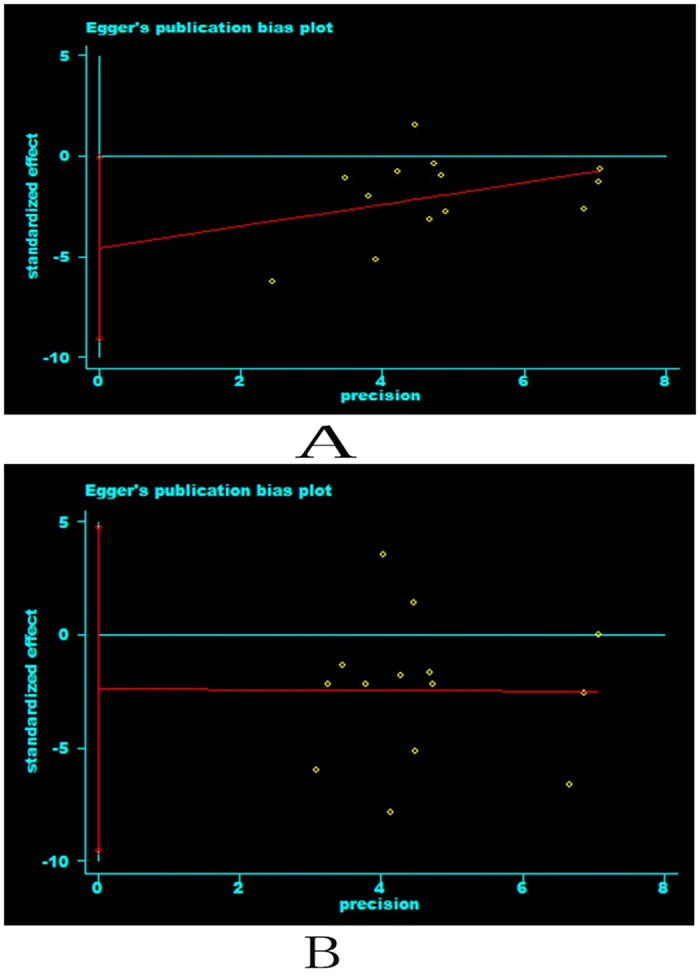
There was no publication bias of CK-MB at 24 hours after PCI (Egger’s test: P = 0.214) (Fig. 8A). There was also no publication biasof TnT at 24 hours after PCI (Egger’s test: P = 0.978) (Fig. 8B).
Discussion
Percutaneous coronary intervention (PCI) is one of the most effective methods for the treatment of myocardial infarction (MI) or unstable angina42,43,44. Compared with traditional drug therapy, PCI can more effectively restore the blood supply to the myocardium and improve the prognosis of patients. However, a lot of studies have shown that there are still approximately 3% of patients failing to benefit from the treatment of PCI. On the contrary, the phenomenon of myocardial injury was appeared in a patient after PCI, which seriously affects the patient’s heart function and prognosis. Therefore, how to improve the blood flow perfusion of the ischemic myocardium after PCI and reduce the occurrence of myocardial injury is of particular importance. Research has shown that the K+ -ATP channel opener (such as nicorandil) can significantly reduce arrhythmia, chest pain and slow reflow phenomenon caused by PCI45,46. However, the conclusion, whether nicorandil has a myocardial protective effect is inconsistent. Thus, we conduct this meta-analysis.
In 2012, Kim S28 enrolled 213 consecutive patients with stable or unstable angina, which were expected in non-urgent PCI for de-novo coronary lesions. 54 patients in the nicorandil group and 55 patients in the control group. The purpose of the study is to assess whether nicorandil has a myocardial protective effect or not. Finally, they found that there were no significant differences in the incidence of post-procedural myocardial necrosis among the two groups (10.9% vs 14.8%, respectively, p = 0.9) and there were no significant differences in the incidence of post-procedural MI among two groups (p = 0.6). Meanwhile, in 2013, Hwang J26 conducted a clinical experiment includes 41 patients in the nicorandil group (n = 41) and 40 patients in the control group (n = 40). In the nicorandil group before PCI, four mg of intracoronary nicorandil was infused prior, and the results showed that the post-PCI peak CK-MB and troponin I levels were not significantly different between the two groups. However, Isono T41 conducted an experiment, including 29 patients undergoing elective PCI. And they found that nicorandil can suppress elevations of cardiac enzymes after elective PCI, suggesting that nicorandil enhances the myocardial protective effect of PCI against angioplasty-related myocardial injury. Kim S28 and Shehata M29 have also carried out a trial, and they results showed that nicorandil can protect the myocardium, which was different from Hwang J and Kim J. Therefore, it is necessary to make a systematic evaluation of this conclusion. In our meta-analysis, a total of 16 randomized controlled trials (RCTs) were included, and 1616 patients entered our study. Our results showed that nicorandil can reduce the levels of CK-MB and TnT after elective PCI and the incidence of adverse reaction caused by PCI for Chinese’s population, but the clinical benefit of nicorandil is not obvious for non Chinese’s population. Through this meta-analysis, we speculated that nicorandil has a cardioprotective effect for Chinese’s population, but may not be appropriate for non Chinese’s population.
Nicorandil is an ATP sensitive potassium channel (KATP) open agent with the effect of nitrate47,48. At the present, research on the myocardial protective effect of nicorandil after PCI is intravenous administration. Results have shown that intravenous nicorandil before PCI can significantly reduce the incidence of coronary slow flow after postoperative49,50. The mechanism may be related with the decrease of infiltration of neutrophils into the ischemic area, which leading to the decrease of neutrophil mediated microcirculation. Our meta analysis results showed that when compared with the control group, the myocardial enzyme levels were significantly decreased in the nicorandil group after PCI, and the myocardial enzyme level of some time point was lower than that of the control group (P < 0.05). Acting as a KATP agent, nicorandil can shorten the action potential duration, inhibit calcium overload. Furthermore, nicorandil can antagonize ADP induced platelet aggregation, improve microcirculation in ischemia area, decrease no reflow phenomenon. In addition, nicorandil can inhibit the formation of active oxygen, which is one of the mechanisms implicated in the protective effect on the myocardium51.
The limitations of our meta-analysis include the following aspects: ① The included studies are mainly coming from China, Japan and Korea, lacking of randomized controlled trials from North America and Europe. ② The method of drug delivery is uniform. Some articles adopted the method of oral administration, and some articles adopted the method of intravenous administration, even some articles using the method of coronary administration, which may lead to a certain bias in implementation. In addition, there is no unity for the dosage and using time for Nicorandil. The possibility of implementation bias is further increased. ③Although the articles included in our meta-analysis were RCT, but most of the studies were single-blind RCT, bias will be inevitable appear. ④Short-term and long-term index need to be observed to evaluate the myocardial protective effects of nicorandil in patients after PCI. Because CK-MB, TnT, MPV and adverse reactions all belongs to short-term index, lacking the long-term index (For example, heart failure, myocardial infarction and arrhythmia, etc.) to estimate the effect of nicorandil, the representation is poor.
Our study also suggests that some aspects should be paid attention to in the future when carrying out RCT: ① Due to the difference of nation and race, it needs to conduct RCT of multi-regional and multi-center, in order to evaluate the clinical efficacy of a drug. ② To regulate the use method of drugs, including usage, dosage and use of time, as far as possible to reduce the occurrence of confounding bias. ③ Describe the method of random grouping, single-blind or double-blind and the implementation of the method in detail, so as to reduce confounding bias. ④Long-term indicators should be increased to estimate the clinical effect after PCI, and then increase the reliability of results.
Conclusion
Our systematic review and meta-analysis indicate that nicorandil can reduce myocardial injury and reduce the incidence of adverse reaction caused by PCI for Chinese’s population, but the clinical benefit of Nicorandil is not obvious for Non Chinese’s population. However, due to the limitations of the quality and quantity of the articles, this conclusion still needs to be confirmed by multi-center, double-blind, randomized controlled trials.
Methods
Literature search
According to the statement of the preferred reporting items for Systematic Reviews and Meta-Analyses, two researchers independently searched published randomized controlled trial(RCT) that investigated the clinical effect of nicorandil on prevention of perioperative myocardial injury in patients undergoing elective PCI. The retrieved database includes PubMed, Embase, the Cochrane Library, Web of Science, CBM, CNKI, VIP database and Wang Fang database, the retrieval time was limited from inception to October 7, 2016. Relevant keywords related to nicorandil in combination as MeSH terms and text words (“Nicorandil” or “2-Nicotinamidoethyl Nitrate” or “2 Nicotinamidoethyl Nitrate” or “Nitrate, 2-Nicotinamidoethyl” or “2-Nicotinamidethyl Nitrate” or “2 Nicotinamidethyl Nitrate” or “Nitrate, 2-Nicotinamidethyl” or “SG-75” or “SG 75” or “SG75” or “Ikorel” or “Aventis Pharma Brand of Nicorandil” or “Rhône-Poulenc Rorer Brand of Nicorandil” or “Rhône Poulenc Rorer Brand of Nicorandil” or “Aventis Brand of Nicorandil” or “Nicorandil Aventis Brand” or “Adancor”) were used in combination with words related to percutaneous coronary intervention and myocardial reperfusion injury(“Coronary Intervention, Percutaneous” or “Coronary Interventions, Percutaneous” or “Intervention, Percutaneous Coronary” or “Interventions, Percutaneous Coronary” or “Percutaneous Coronary Interventions” or “Percutaneous Coronary Revascularization” or “Injuries, Myocardial Reperfusion” or “Myocardial Reperfusion Injuries” or “Reperfusion Injuries, Myocardial” or “Myocardial Ischemic Reperfusion Injury” or “Reperfusion Injury, Myocardial” or “Injury, Myocardial Reperfusion”). The retrieval language was limited to Chinese and English. In addition, reference articles of the extracted articles were also retrieved. When multiple reports of the same study were present, we used the most recent publication and supplemented it. All analyses were based on previously published studies, and thus no ethical approval or patient consent was required.
Study selection
We identified studies that prospectively evaluated the clinical effect of nicorandil on prevention of perioperative myocardial injury in patients undergoing elective PCI. Inclusion criteria:① The study was limited to randomized controlled trials (randomized controlled trials, RCTs), and the purpose of the study was to evaluate the effect of nicorandil on prevention of perioperative myocardial injury in patients undergoing elective PCI; ② At least one of the observation group was applied nicorandil in the experiment; ③The does and usage of nicorandil is not limited; ④The article should provide sufficient data for analysis; ⑤ The study subjects were patients undergoing elective PCI, and coronary angiography was performed; ⑥ The retrieval language is limited to Chinese and English.
Exclusion criteria: ① Retrospective, non-randomized trial; ② Semi randomized controlled trial, in which the grouping method of the participants in the experiment was not strictly random; ③Patients with acute myocardial infarction (AMI) or with AMI within the last 6 months; ④ Articles with incomplete or erroneous data.
Data extraction
The contents of the retrieved articles were reviewed by two researchers (ZY and QS) in accordance with the prior search methods. Data to be extracted including basic data of subjects (First author, publication year, country, sample number, average age, use method of nicorandil (dose), preoperative method of control group and outcome measures). If there was a lack of necessary data or some content to be clarified in the articles, an effort was made to try to make contact with the study authors, and if the necessary data to analyze was still unavailable, this article was excluded.
Statistical analyses
We used the Stata software, version 11.0 (Stata Corp, College Station, Tex) to pool and analyze results from the individual studies. Pooled results were reported as relative risks (RRs) and standardized mean difference (SMD), and presented with 95% confidence interval (CI) with two-sided P-values. P < 0.05 indicates that the difference was statistically significant. Heterogeneity of the inclusion study was assessed by I2 test, which assessed the appropriateness of pooling the individual study results. When I2 < 50%, the heterogeneity of the study was considered small; When I2 > 50%, the heterogeneity of the study was considered substantial, and then subgroup analysis and sensitivity analysis were performed to investigate the sources of heterogeneity. If necessary, meta-regression analysis was performed to explore heterogeneity.
Additional Information
How to cite this article: Ye, Z. et al. The clinical effect of nicorandil on perioperative myocardial protection in patients undergoing elective PCI: A Systematic Review and Meta-Analysis. Sci. Rep. 7, 45117; doi: 10.1038/srep45117 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
This study was supported by the First Affiliated Hospital of Guangxi Medical University. We also thank Dr Peng Liu for his statistical guidance and thank authors who contributed data of their studies.
Footnotes
The authors declare no competing financial interests.
Author Contributions All authors’ responsibilities were as follows: Y.Z. designed the subject and revised the article, S.Q. and L.L. developed inclusion and exclusion criteria, developed and performed the search strategy, Y.Z. and S.Q. conducted the statistical analysis and wrote the article. Y.Z. and S.Q. screened relevant literature, made decisions according to inclusion and exclusion criteria. All authors participated in the interpretation of data and reviewed the manuscript.
References
- Capodanno D. et al. Percutaneous coronary intervention with everolimus-eluting bioresorbable vascular scaffolds in routine clinical practice: early and midterm outcomes from the European multicentre GHOST-EU registry. Eurointervention Journal of Europcr in Collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology 10, 1144–1153 (2014). [DOI] [PubMed] [Google Scholar]
- Han Y. et al. Bivalirudin vs heparin with or without tirofiban during primary percutaneous coronary intervention in acute myocardial infarction: the BRIGHT randomized clinical trial. Jama the Journal of the American Medical Association 313, 1336–1346 (2015). [DOI] [PubMed] [Google Scholar]
- White H. D. et al. Outcomes with cangrelor versus clopidogrel on a background of bivalirudin: insights from the CHAMPION PHOENIX (A Clinical Trial Comparing Cangrelor to Clopidogrel Standard Therapy in Subjects Who Require Percutaneous Coronary Intervention [PCI]). Jacc Cardiovascular Interventions 8, 424–433 (2015). [DOI] [PubMed] [Google Scholar]
- Fihn S. D. et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease. Journal of Thoracic & Cardiovascular Surgery 149, e5–e23 (2015). [DOI] [PubMed] [Google Scholar]
- Hess C. N. et al. Comparison of quality-of-life measures after radial versus femoral artery access for cardiac catheterization in women: Results of the Study of Access Site for Enhancement of Percutaneous Coronary Intervention for Women quality-of-life substudy. American heart journal 170, 371–379 (2015). [DOI] [PubMed] [Google Scholar]
- Hammoudeh A. J. & Izraiq M. In CARDIOALEX.
- Gillies M. A. et al. Perioperative myocardial injury in patients receiving cardiac output-guided haemodynamic therapy: a substudy of the OPTIMISE Trial. British Journal of Anaesthesia 115, 227–233 (2015). [DOI] [PubMed] [Google Scholar]
- Larsen M. H., Ekeløf S. & Gögenur I. [Myocardial injury and infarction is an overlooked complication after non-cardiac surgery]. Ugeskrift for Laeger 177 (2015). [PubMed] [Google Scholar]
- Kambara T. et al. C1q/Tumor Necrosis Factor-Related Protein 9 Protects against Acute Myocardial Injury through an Adiponectin Receptor I-AMPK-Dependent Mechanism. Molecular & Cellular Biology 35, MCB.01518–01514 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- N R. et al. Inhibition of TGF-β by a novel PPAR-γ agonist, chrysin, salvages β-receptor stimulated myocardial injury in rats through MAPKs-dependent mechanism. Nutrition & Metabolism 12, 1–11 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudetz J. A., Patterson K. M., Iqbal Z., Gandhi S. D. & Pagel P. S. Remote Ischemic Preconditioning Prevents Deterioration of Short-Term Postoperative Cognitive Function After Cardiac Surgery Using Cardiopulmonary Bypass: Results of a Pilot Investigation. Journal of Cardiothoracic & Vascular Anesthesia 29, 382–388 (2014). [DOI] [PubMed] [Google Scholar]
- Duceppe E., Mrkobrada M., Thomas S. & Devereaux P. J. Role of aspirin for prevention and treatment of perioperative cardiovascular events. Journal of Thrombosis & Haemostasis Jth 13, S297–S303 (2015). [DOI] [PubMed] [Google Scholar]
- Filippone S. M., Roh S. K., Salloum F. N., Kukreja R. C. & Das A. Abstract 13498: Reperfusion Therapy With Rapamycin Prevents Myocardial Ischemic Injury, Through Activation of AKT and ERK.(2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hann N. et al. Lipid Markers Associated with Recurrent ACS in Patients Treated with Statins: LDL- Versus Non-HDL-cholesterol. Heart Lung & Circulation 25, S88 (2016). [Google Scholar]
- Maggioni A. P. et al. Outcomes, Health Costs And Use Of Statins In 6,226 Patients Admitted In 2011 For An Acute Coronary Syndrome (Acs) Occurring In A Large Community Setting Of 2,989,512 Subjects. Value in Health 18, A134 (2015). [Google Scholar]
- Herrington W. Impact of renal function on the effects of LDL cholesterol lowering with statin-based regimens: a meta-analysis of individual participant data from 28 randomised trials. Lancet Diabetes & Endocrinology 4, 829–839 (2016). [DOI] [PubMed] [Google Scholar]
- Pradelli D. et al. Statins use and the risk of all and subtype hematological malignancies: a meta-analysis of observational studies. Cancer Medicine 4, 770–780 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin D. et al. Comparison of the Risk of Gastrointestinal Bleeding among Different Statin Exposures with Concomitant Administration of Warfarin: Electronic Health Record-Based Retrospective Cohort Study. Plos One 11, e0158130 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuma K. Abstract T P65: Early Statin Intervention Can Reduce the Early Neurological Deterioration and Recurrence in Acute Lacunar Stroke. Stroke; a journal of cerebral circulation(2015). [Google Scholar]
- Stroes E. S. et al. Statin-associated muscle symptoms: impact on statin therapy—European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. European Heart Journal 36, 1012–1022 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A. T., Johnson P. C. D., Hall G. C., Ford I. & Mills P. R. High Dose Atorvastatin Associated with Increased Risk of Significant Hepatotoxicity in Comparison to Simvastatin in UK GPRD Cohort. Plos One 11, e0151587 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedenmalm K., Granberg A. G. & Dahl M. L. Statin-induced muscle toxicity and susceptibility to malignant hyperthermia and other muscle diseases: a population-based case-control study including 1st and 2nd degree relatives. European Journal of Clinical Pharmacology 71, 117–124 (2015). [DOI] [PubMed] [Google Scholar]
- Ito N. et al. Beneficial effects of intracoronary nicorandil on microvascular dysfunction after primary percutaneous coronary intervention: demonstration of its superiority to nitroglycerin in a cross-over study. Cardiovascular Drugs and Therapy 27, 279–287 (2013). [DOI] [PubMed] [Google Scholar]
- Jang H. J. et al. Safety and efficacy of a novel hyperaemic agent, intracoronary nicorandil, for invasive physiological assessments in the cardiac catheterization laboratory. European Heart Journal 34, 2055 (2013). [DOI] [PubMed] [Google Scholar]
- Garg V. & Hu K. Protein kinase C isoform-dependent modulation of ATP-sensitive K+ channels in mitochondrial inner membrane. American Journal of Physiology Heart & Circulatory Physiology 293, H322–H332 (2007). [DOI] [PubMed] [Google Scholar]
- Hwang J. et al. The effect on periprocedural myocardial infarction of intra-coronary nicorandil prior to percutaneous coronary intervention in stable and unstable angina. Journal of Cardiology 62, 77–81 (2013). [DOI] [PubMed] [Google Scholar]
- Kim J. H. et al. Myocardial protective effects of nicorandil during percutaneous coronary intervention in patients with unstable angina. Circulation Journal 69, 306–310 (2005). [DOI] [PubMed] [Google Scholar]
- Kim S. J. et al. Effect of myocardial protection of intracoronary adenosine and nicorandil injection in patients undergoing non-urgent percutaneous coronary intervention: a randomized controlled trial. International journal of cardiology 158, 88–92 (2012). [DOI] [PubMed] [Google Scholar]
- Murakami M. et al. Nicorandil reduces the incidence of minor cardiac marker elevation after coronary stenting. International journal of cardiology 107, 48–53 (2006). [DOI] [PubMed] [Google Scholar]
- Mohamed S. M. D. Cardioprotective Effects of Oral Nicorandil Use in Diabetic Patients Undergoing Elective Percutaneous Coronary Intervention. Journal of Interventional Cardiology 27, 472–481 (2014). [DOI] [PubMed] [Google Scholar]
- Ceng Ximing & Liu Donglin Nicorandil for Injection observation in treatment of unstable angina pectoris patients undergoing PCI. Capital food and medicine 111–112 (2015). [Google Scholar]
- Weina Pei et al. Sibutramine comparison of trimetazidine and nicorandil on percutaneous coronary intervention related myocardial injury intervention. Chinese Circulation Journal 29, 256–260 (2014). [Google Scholar]
- Lv Jianzhuang, Zhang Minjuan & Ge Xingli Compared with Sibutramine trimetazidine and nicorandil on myocardial injury PCI treatment intervention. Anhui Medical Journal 36, 1103–1105 (2015). [Google Scholar]
- Mo Fanrui & Li Juan. The protective effect of nicorandil on patients undergoing PCI surgery in patients with coronary heart disease and myocardial vascular endothelial. China gerontology 36, 1619–1621 (2016). [Google Scholar]
- Wu Xiaopeng, Wang Xuanqi & Li Weijie. Effect of nicorandil on no reflow after percutaneous coronary intervention in coronary artery. Shanxi Medical Journal, 1538–1540 (2013). [Google Scholar]
- Li Yanhui. Nicorandil on the effect of percutaneous coronary intervention in treatment of myocardial injury. Chinese Journal of evidence based cardiovascular medicine 04, 460–461 (2012). [Google Scholar]
- Zhang Yan, Sun Daoyuan & Tian Jianhui The protective effect of nicorandil on patients with unstable angina undergoing PCI. Medical Journal of Qilu 27, 238–240 (2012). [Google Scholar]
- Han Hongyan, Jia Haizhen, Zhou Qi et al. Protective effects of nicorandil on myocardial in patients with unstable angina pectoris after PCI operation. Journal of difficult diseases, 16–19 (2015). [Google Scholar]
- Yang Shuhan, Wang Cheng, Liu Yanbin et al. Effect of nicorandil on PCI related myocardial injury and recurrence of angina symptoms. Tianjin Medical Journal, 1026–1028 (2014). [Google Scholar]
- Wang Xiaochen, Ma Rui, Xu Banglong et al. Effect of nicorandil on Tp-Te interval after PCI. shandong medical journal 52, 69–71 (2012). [Google Scholar]
- Isono T. et al. Nicorandil suppressed myocardial injury after percutaneous coronary intervention. International journal of cardiology 123, 123–128 (2008). [DOI] [PubMed] [Google Scholar]
- Kampinga M. A., Vlaar P. J., Fokkema M., Gu Y. L. & Zijlstra F. Thrombus Aspiration during Percutaneous coronary intervention in Acute non-ST-elevation myocardial infarction Study (TAPAS II)-Study design. Netherlands Heart Journal 17, 409–413 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalescot G. et al. Prasugrel compared with clopidogrel in patients undergoing percutaneous coronary intervention for ST-elevation myocardial infarction (TRITON-TIMI 38): double-blind, randomised controlled trial. Lancet (London, England) 373, 723–731 (2009). [DOI] [PubMed] [Google Scholar]
- Whitlow P. L. & Clinic C. 2009 Focused Updates: ACC/AHA Guidelines for the Management of Patients With ST‐ Elevation Myocardial Infarction (Updating the 2004 Guideline and 2007 Focused Update) and ACC/AHA/SC.
- Ono H. et al. Nicorandil improves cardiac function and clinical outcome in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention: role of inhibitory effect on reactive oxygen species formation. American heart journal 148, E15 (2004). [DOI] [PubMed] [Google Scholar]
- Tonino P. A. et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. New England Journal of Medicine 360, 213–224 (2009). [DOI] [PubMed] [Google Scholar]
- Taira N. Nicorandil as a hybrid between nitrates and potassium channel activators. American Journal of Cardiology 63, J18–J24 (1989). [DOI] [PubMed] [Google Scholar]
- Group I. S. Effect of nicorandil on coronary events in patients with stable angina: the Impact Of Nicorandil in Angina (IONA) randomised trial. Acc Current Journal Review 11, 13 (2002). [DOI] [PubMed] [Google Scholar]
- Sadamatsu K. et al. Acute effects of isosorbide dinitrate and nicorandil on the coronary slow flow phenomenon. American Journal of Cardiovascular Drugs 10, 203–208 (2010). [DOI] [PubMed] [Google Scholar]
- Tsubokawa A., Ueda K., Sakamoto H., Iwase T. & Tamaki S. Effect of intracoronary nicorandil administration on preventing no-reflow/slow flow phenomenon during rotational atherectomy. Circulation Journal Official Journal of the Japanese Circulation Society 66, 1119–1123 (2002). [DOI] [PubMed] [Google Scholar]
- Kawai Y., Hisamatsu K. & Matsubara H. et al. Intravenous administration of nicorandil immediately before percutaneous coronary intervention can prevent slow coronary flow phenomenon. European Heart Journal 30, 765–772 (2009). [DOI] [PubMed] [Google Scholar]